Abstract
Background: Drug combinations with complementary mechanisms of action are able to achieve effective analgesia at lower doses, thereby reducing the risk of adverse effects (AEs). This study evaluated the analgesic efficacy and tolerability of two fixed-dose combinations (FDCs) of ibuprofen/tramadol (IBU/TRA) compared with tramadol and a placebo. Methods: This multicenter, randomized, double-blind, dose-finding, pilot clinical trial compared IBU/TRA (400/37.5 mg and 400/75 mg) with 100 mg of tramadol and a placebo in patients with moderate-to-severe pain following dental surgery. The primary endpoints were pain intensity at 6 h (PI6h) and the pain intensity difference from baseline to 6 h (PID6h). PID7h, the sum of pain intensity differences from baseline to 7 h (SPID0–7h), pain relief (PAR7h), total pain relief (TOTPAR7h), the use of rescue medication and AEs were also assessed. Results: Seventy-two patients were randomized and evaluated. Both FDCs showed superiority over the placebo for PI6h and PID6h (p < 0.05) but were not significantly different from 100 mg of tramadol. The statistical superiority of FDCs over the placebo was observed for PID7h, SPID0–7h, PAR7h and TOTPAR7h. The percentage of patients receiving rescue medication was higher in the placebo (94.1%) and tramadol (52.6%) groups than the FDC groups (35.3% and 36.8% for 400/37.5 mg and 400/75 mg, respectively). A post hoc analysis showed that the FDCs had a superior analgesic efficacy to 100 mg of tramadol in the SPID0–4h (p < 0.005). The incidence of AEs was comparable between treatment groups. Conclusions: Both FDCs of IBU/TRA provided superior analgesic efficacy compared to the placebo. We propose using SPID0–4h as the preferred variable for evaluating the efficacy of this type of drug combination.
1. Introduction
Acute pain is common in hospitalized patients, whether due to an acute illness or surgical procedures. Most patients undergoing surgery report acute postoperative pain, but evidence suggests that less than half receive adequate postoperative pain relief [1]. Postoperative pain is often associated with increased healthcare costs, poor patient satisfaction, and a higher risk of developing chronic pain syndromes [2,3].
Pain is transmitted through multiple neural pathways, with each involving distinct mechanisms [4]. Due to this complexity, combining different classes of analgesics that each target specific pain mechanisms can enhance pain control. This approach, known as multimodal analgesia, improves pain management while reducing the required dosage of monotherapy drugs and consequently minimizing side effects [5,6].
Multimodal analgesia offers several advantages in the postoperative setting. First and foremost, the combination of agents with different analgesic mechanisms can lead to synergistic effects, thereby increasing their overall efficacy. In addition, this synergism allows individual drug doses to be reduced, minimizing dose-related adverse effects, particularly by reducing the need for opioids [7,8,9].
For many years, opioid analgesics have been used to manage pain and provided significant therapeutic benefits when administered appropriately [10,11,12]. Emerging evidence suggests that prescribing lower doses correlates with a reduced incidence of long-term opioid use and a decreased risk of developing opioid use disorder [13,14]. Nonsteroidal anti-inflammatory drugs (NSAIDs) serve as fundamental components of opioid-sparing strategies within multimodal pain management protocols. Several studies have shown that the combination of NSAIDs with low-dose tramadol significantly reduces pain scores and provides superior analgesic efficacy compared to analgesic drugs when used as monotherapy [15,16,17,18,19].
Ibuprofen is an NSAID, a derivate of propionic acid with analgesic, antipyretic and anti-inflammatory activity. Its mechanism of action is based on the inhibition of cyclooxygenase, resulting in decreased prostaglandin synthesis [20,21].
Tramadol, a centrally acting analgesic that is structurally related to codeine and morphine, consists of two enantiomers, both of which contribute to analgesic activity via different mechanisms. (+)-Tramadol and the metabolite (+)-O-desmethyl-tramadol (M1) act as agonists of the μ-opioid receptor. (+)-Tramadol inhibits serotonin reuptake, while (−)-tramadol inhibits norepinephrine reuptake, both contributing to the enhanced inhibitory modulation of pain transmission within the spinal cord [22,23]. Its pharmacological profile contributes to its efficacy and tolerability in the treatment of pain caused by various aetiologies (e.g., post-traumatic, biliary colic, obstetric or postoperative pain) [22,24,25].
The present pilot study was designed to evaluate the preliminary analgesic efficacy and tolerability of two fixed-dose combinations (FDCs) of intravenous ibuprofen arginate/tramadol HCl (IBU/TRA), each containing different low doses of tramadol, compared with 100 mg of tramadol HCl and a placebo for postoperative pain following the extraction of impacted third molars; this was in order to facilitate the selection of an optimal dose of tramadol for combination. In addition, given the complexity of the evaluation of the effects of painkillers with different mechanisms, doses and rates of action, this study aimed to test the method used (variables, measurement times, doses) for the design of future studies. This study was designed in compliance with the recommendations of the European Medicines Agency (EMA) [26].
2. Materials and Methods
This study was conducted at five centres in Spain (Hospital Clínico San Carlos, Hospital Universitario La Princesa, Hospital Universitario La Paz, Hospital Universitario Ramón y Cajal and Hospital Universitario Fundación Jiménez Díaz).
The protocol was authorized by the AEMPS (https://reec.aemps.es/reec/public/list.html (accessed on 20 June 2025), EudraCT Number: 2018-001412-30, see Supplementary File S1) and approved by the Ethics Committee of the Hospital Clínico San Carlos (Internal Code: 18/243-R_M). The study was conducted in compliance with the ethical principles originating in or derived from the more recent Declaration of Helsinki, International Council for Harmonization Good Clinical Practice guidelines, and local regulatory requirements in force.
An informed consent form was signed by each subject prior to inclusion in the study, after receiving comprehensive information about the study design, objectives and risks.
2.1. Study Design
This was a multicentre, randomized, double-blind, dose-finding, parallel design, placebo and active-controlled, single-dose, pilot study that aimed to determine the appropriate dose of tramadol in combination with ibuprofen (IBU/TRA 400/37.5 mg or IBU/TRA 400/75 mg)—compared to a full dose of tramadol (100 mg) and the placebo when administered intravenously—for moderate-to-severe acute pain following the extraction of an impacted third molar tooth. This study also aimed to explore a more appropriate method for evaluating responses in this kind of study.
The study period was structured into three phases for each patient. First, a screening phase was conducted during the four weeks prior to randomization, which included pre-surgical procedures and concluded within four hours post-surgery, once the eligibility criteria had been confirmed. This was followed by the randomization and treatment administration visit, after which a seven-hour assessment period began; during this, patients recorded data using a diary. Finally, an end-of-study visit was carried out between five and nine days after randomization (7 ± 2 days), completing the clinical follow-up.
For randomization into the treatment groups, subjects rated their pain intensity (PI) using a Visual Analogue Scale (VAS) ranging from 0 to 100 mm. Subjects experiencing moderate-to-severe pain (VAS ≥ 55 mm) within 4 h after surgery were randomized to receive an intravenous single dose of any of the following study treatments:
- ▪
- IBU/TRA 400/37.5 mg, intravenous, single dose;
- ▪
- IBU/TRA 400/75 mg, intravenous, single dose;
- ▪
- Tramadol HCl 100 mg, intravenous, single dose;
- ▪
- Placebo, intravenous, single dose.
The drugs were infused via a pump over 30 min. All treatments were supplied by Farmalider S.A. (Madrid, Spain), and were administered under double-blinded conditions. The administration was standardized for all subjects according to a specific medication guide.
Subjects who did not experience adequate pain relief from the study medication were allowed to take rescue medication (RM), which consisted of 1 g paracetamol (if a second RM was required, 575 mg metamizole was administered). Subjects were allowed to take RM at any time, but were advised to wait at least 30 min after receiving the study medication to allow its effect to start.
2.2. Subjects
Healthy subjects ≥18 years of age scheduled to undergo the removal of ≥2 impacted third molars requiring bone remotion under local anesthesia, and who experienced moderate-to-severe pain after the extraction, were randomized to receive an intravenous single dose of the assigned study treatment. Other inclusion criteria included the ability to record necessary information on the diary, and the ability to comply with the study requirements.
The exclusion criteria included patients with a history of allergy or hypersensitivity to the study medication, rescue medication or any of its excipients; patients with a history of asthma; patients with a history of peptic ulceration, gastrointestinal disorders, gastrointestinal bleeding or other active bleeding; patients with a history of moderate-to-severe renal, hepatic or cardiac failure; patients with a history of active bleeding or coagulation disorders; patients with a history of epilepsy; patients with Crohn’s disease or ulcerative colitis; patients with a history of drug or alcohol dependence; patients with a history of any disease or disorder that, at the investigator’s discretion, could pose a risk to the patient or alter the results of the study; patients who had taken any analgesic or medicine that should not be administered due to the risk of interactions; patients who had received an experimental drug or used an experimental medical device within 30 days prior to the screening process; and pregnant or breastfeeding women. Additionally, patients were excluded if any complication of surgery that could interfere with the study’s procedures or assessments occurred.
Although the main objective of this pilot study was exploratory, a sample size of 72 patients was considered enough to assess the analgesic efficacy of FDCs compared with tramadol and the placebo, assuming a power of 80% and an overall significance level of 5% (two-sided). The expected difference in PI 6 h was assumed to be about 10 (SD 11) based on previous trials conducted by the sponsor.
A balanced per-centre computer-generated randomization list was prepared prior to the study, and sealed opaque envelopes were used to ensure that allocations were concealed. Randomization codes were given directly to the individuals responsible for dispensing the medication at the site and not otherwise involved in any part of the clinical trial.
2.3. Efficacy Assessment
The analgesic efficacy evaluation was based on patient assessments of PI using the VAS, ranging from 0 mm (“no pain”) to 100 mm (“worst imaginable pain”), and their assessments of pain relief (PAR), also using the VAS, ranging from 0 mm (“no relief”) to 100 mm (“complete relief”). Data were collected at the following specific time points: baseline and 0.25, 0.5, 0.75, 1, 1.25, 1.5, 1.75, 2, 2.5, 3, 3.5, 4, 4.5, 5, 5.5, 6, 6.5, and 7 h post administration. The percentage of responders and percentage of patients requiring RM were also evaluated. Patients who received RM remained at the study site for the full duration of the study.
2.4. Primary and Secondary Variables
The primary efficacy variables were PI at 6 h (PI6h) and pain intensity difference from baseline through 6 h post treatment (PID6h). Secondary efficacy variables included PID7h, the sum of pain intensity, differences from baseline to 7 h (SPID0–7h), PAR7h, total pain relief over 7 h (TOTPAR7h), the percentage of responders in terms of PI reduction (subjects who achieved at least a 20% PI reduction vs. baseline at 6 h) and the percentage of patients using RM. The inclusion of these secondary variables enhances the interpretation and consistence of the observed effects.
2.5. Safety
AEs were documented throughout the study based on spontaneous patient reports and indirect questioning. Each AE was classified by the investigator according to its relationship with the study medication (unrelated, unlikely, possible, probable, or definite), its intensity (mild, moderate, or severe) and its seriousness.
Safety assessments were conducted by evaluating clinically relevant changes from baseline following drug administration. These evaluations included physical examinations, vital signs (body temperature, blood pressure and heart rate) and laboratory safety parameters such as hematology, biochemistry and coagulation profiles.
2.6. Population and Statistics
The main analysis was performed in the intention-to-treat (ITT) population, which included all the randomized patients. The analysis was also carried out in the per-protocol (PP) population, which included all patients from the ITT population who did not experience any relevant protocol deviation with regard to the efficacy endpoints of the primary objective.
The primary efficacy variables, PI6h and PID6h, were used to assess the analgesic efficacy and safety of the FDCs in comparison to tramadol and the placebo using the analysis of covariance (ANCOVA). The ANCOVA included the baseline VAS score to control for initial differences in pain levels between the groups. In addition, the intervention groups were compared with the control group to determine the efficacy of the treatment while accounting for baseline variability.
The secondary efficacy variables PID7h, PAR7h, SPID0–7h and TOTPAR7h were analyzed descriptively and supported the results of the primary efficacy endpoint. The percentage of patients achieving a PI reduction of at least 20% and the percentage of patients who required RM were tested using a chi-squared test. A descriptive analysis was conducted for the variable SPID0–4h to explore the effect of the treatment at an intermediate time point, using a parameter that may be more sensitive to variations in the onset of drug action.
The last observation carried forward (LOCF) method was used for the imputation of missing data, including all observations made after patients received rescue medication. This procedure was applied to all the efficacy outcomes.
All statistical analyses were performed using R (version 3.5.2). A p-value less than 0.05 was considered statistically significant.
Safety variables were analyzed using descriptive statistics and were run on the safety population; in addition, the chi-2 was used to test the difference in frequencies. AEs were coded using the Medical Dictionary for Regulatory Activities (MedDRA) dictionary.
3. Results
3.1. Study Participants
A total of 72 patients (48 female and 24 male) were randomized and received the study treatment (Figure 1). The main efficacy analysis was run on the ITT population, which included 72 patients. The PP population comprised a total of 66 randomized patients, as six participants were excluded due to major protocol deviations related to efficacy endpoints of primary interest (±5% variation in dose received or discontinuation of the infusion of the study drug due to an adverse event).
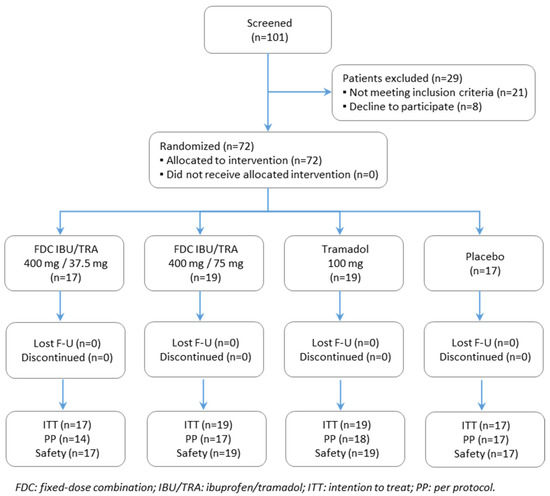
Figure 1.
Participant flow chart of study. The ITT (intention-to-treat) population consisted of all randomized patients; safety population of all patients who received study drug; PP (per-protocol) population of all patients of the ITT who did not experience relevant protocol deviations related to efficacy endpoints of primary interest.
In accordance with the exclusion criteria, no participants reported any relevant comorbidities or concomitant medications that could interfere with their perception of pain.
The demographic and baseline characteristics of different treatment groups were balanced between groups (Table 1). The mean (SD) age was 24.7 (5.29) years (range 18–44). The majority of patients were white (91.7%). The percentage of women was 66.7%. The baseline PI values per group are shown in Table 1. A total of 33 (45.8%) patients completed the study without the use of rescue analgesia.

Table 1.
Patient demographic and baseline characteristics by treatment group (ITT population).
3.2. Efficacy Results
3.2.1. Primary Variables
The evolution of PI over time is shown in Figure 2. The PI6h and PID6h values in the IBU/TRA 400/37.5 mg and the IBU/TRA 400/75 mg groups were statistically different from those of the placebo group in the ITT population (see Table 2), showing a lower PI6h and higher PID6h for both FDCs vs. placebo. The analysis of the PP population showed the same results. However, no statistically significant differences were observed between the two FDCs and 100 mg tramadol.
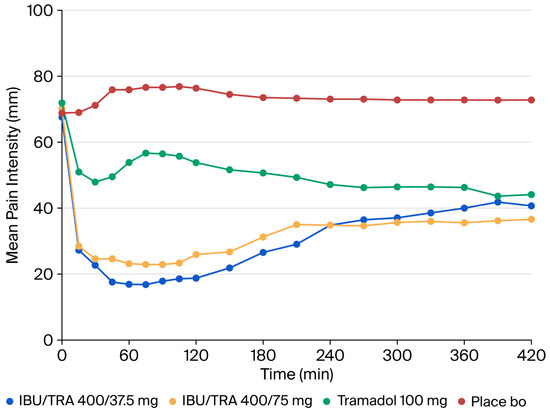
Figure 2.
ITT population. Primary efficacy variable: pain intensity. IBU/TRA37.5: ibuprofen 400 mg/tramadol 37.5 mg; IBU/TRA75: ibuprofen 400 mg/tramadol 75 mg; Tramadol: Tramadol 100 mg.

Table 2.
Summary of efficacy endpoints (ITT population).
3.2.2. Secondary Variables
As shown in Table 2, the analysis of the secondary variables PID7h, SPID0–7h, PAR7h, and TOTPAR0–7h, evaluated at 7 h, corroborated the superior effect of both FDCs in comparison to the placebo after a dosing interval of 6 h. No significant differences were found for any secondary variable when comparing the FDCs vs. tramadol.
A total of 39 (54.2%) patients received RM (see Table 3). Regarding the percentage of responders at 7 h, the most effective results were observed in patients treated with the FDCs IBU/TRA: 400/37.5 mg (64.7%), 400/75 mg (68.4%). The tramadol group showed a 57.9% response rate, while the placebo group had a 17.3% response rate (see Table 4).

Table 3.
Use of rescue medication and patient responders within the evaluation period (7 h) after drug intake.

Table 4.
Post hoc SPID0–4h analysis (ITT and PP populations).
A post hoc descriptive analysis of SPID0–4h showed the superior analgesic efficacy of the FDCs IBU/TRA compared to tramadol (400/37.5 mg, p 0.034; 400/75 mg, p < 0.001) (see Table 4).
3.3. Safety
Safety analysis was performed on 72 randomized patients. Overall, 24 patients (33.3%) experienced one or more adverse events, one of which was considered serious but not related to the study medication. No clinically relevant differences were identified in the incidence of AEs between treatment groups. The most common AEs were dizziness (6.9%), nausea (6.9%), and vomiting (5.6%) (Table 5). No deaths occurred. There were no clinically relevant changes in the vital signs or physical examination vs. baseline. Overall, FDCs were safe and well tolerated.

Table 5.
Summary of adverse events by MedDRA preferred term (PT).
4. Discussion
Pain is a complex phenomenon involving multiple pathophysiological mechanisms, including peripheral nociceptor activation, central sensitization, inflammation and descending pain modulation [27,28]. Because of this complexity, effective pain management requires a multimodal approach that targets the different pathways involved in the generation and maintenance of pain. The current paradigm of drug combination therapy permits the use of low doses of individual agents, thereby diminishing the likelihood of AEs while achieving the intended analgesic efficacy [15,29,30,31,32,33].
Multiple datasets and clinical evidence support the assertion that rapid analgesia is essential to preventing sensitization and neuronal plasticity, which follow all nociceptive signals and are responsible for the maintenance of pain. Specifically, it has been demonstrated that administering effective and fast analgesia during the acute phase can prevent the development of permanent hyperalgesia and therefore reduce the risk of pain chronification [34].
Opioids are potent analgesics and are available in a wide variety of formulations, including IV, oral, and transdermal, which is useful in the postoperative setting. The current crisis of opioid abuse and misuse underscores the imperative for rigorous risk mitigation and precise dose optimization [35]. Opioid monotherapy remains a widely used and effective method for post-operative analgesia. However, monotherapy with opioids has various idiosyncratic or dose-limiting side effects that curtail their practical efficacy, and also exposes patients to serious adverse events.
Several studies have supported the use of analgesic combinations, such as diclofenac/tramadol, dexketoprofen/tramadol, and acetaminophen/tramadol, as an effective strategy for managing acute postsurgical pain, particularly within the framework of multimodal analgesia [2,10,31,36,37,38,39,40]. Clinical guidelines likewise endorse the rational use of drug combinations to optimize analgesia and minimize opioid consumption [13]. In the specific context of postoperative dental pain management, particularly following procedures such as third molar extractions, the combination of NSAIDs and opioids has proven to be an effective strategy [18,41,42]. This multimodal approach not only enhances analgesic efficacy but also enables the use of lower opioid dosages, thereby reducing the likelihood of adverse effects.
The synergy between ibuprofen and opiate drugs is due to their complementary mechanisms of action: NSAIDs act peripherally, and opioids act centrally. This analgesic efficacy may be greater than expected when their individual effects are added, enhancing the efficacy while enabling the required doses of each drug to be reduced.
Although this study focused on dental extraction, this analgesic strategy could be applicable to other acute pain conditions. Nevertheless, further research is needed to confirm its efficacy in different surgical contexts.
After an extensive review, this is the first published study to evaluate the analgesic effect of a combination of ibuprofen with low doses of tramadol administered intravenously. This pilot study provides essential insights into the efficacy and safety of a novel drug combination, thereby supporting the design of future clinical trials. Furthermore, the availability of intravenous formulations provides a valuable alternative when the oral route is not feasible.
The study was designed in accordance with the EMA guidelines on the clinical development of medicinal products for pain treatment and fixed-dose drug combinations, providing methodological consistency [26,43]. The study was a randomized and double-blinded trial, minimizing potential sources of experimental bias.
The drug doses tested were based on a prior pharmacodynamics study that employed oral administration and one pharmacokinetics study [44] that indicated that intravenous tramadol doses should not be reduced compared to oral ones; this study also noted the expected synergy of ibuprofen and tramadol.
This pilot study demonstrates that the tested FDCs (IBU/TRA 400/37.5 mg and 400/75 mg) provided better analgesia than the placebo at 6 h in patients with moderate-to-severe acute dental pain after a single dose. No statistically significant differences were observed between the FDCs and tramadol for either the main or secondary variables. However, given the small sample size, the analysis lacked sufficient statistical power to conclusively rule out potential differences. Given the potential relevance of the different pharmacokinetic profiles of the drugs involved in the study (i.e., the expected Tmax for Ibuprofen is 0.5 h, while it is 1 h for tramadol and 2 to 3 h for its main active metabolite [44]), a post hoc analysis of SPID0–4h was conducted. This analysis revealed the significantly greater efficacy of both FDCs compared to tramadol monotherapy, suggesting that SPID may serve as a more suitable endpoint for comparing drugs with different mechanisms of action, onset times, and effect durations. The LOCF method was used to handle missing data, including all the pain evaluations after patients received rescue analgesia. Since tramadol reaches peak plasma concentrations approximately 2 h after a single dose in healthy adults, and more than 50% of patients in the tramadol group required rescue analgesia before this time, many of them were recorded as having no change in pain intensity. This may have underestimated the efficacy of tramadol. In contrast, FDCs IBU/TRA were significantly superior to placebo across all endpoints, despite the use of LOCF, in this case rescue medication use was significantly less frequent in the FDCs groups.
Although no statistically significant differences (p = 0.491) were observed between the two FDCs, the frequency of adverse events was lower with the 400/37.5 mg dose, suggesting a more favourable safety profile. Given that the difference in the analgesic effect between both FDCs is not considered clinically significant, this finding could support the use of lower tramadol doses in combination therapy to minimize the risk of AEs. Currently, there are no commercially available intravenous formulations combining ibuprofen with tramadol doses below 50 mg, and this combination is protected by patent [45]. Given the dose-dependent nature of tramadol-related AEs and the widespread use of opioid analgesics, further research is needed to evaluate the efficacy and safety of more finely tuned intravenous combinations of such drugs.
The main limitation of this study is its single-dose design, with its evaluation of the short-term analgesic efficacy of FDCs IBU/TRA compared to the placebo and tramadol. Additionally, the sample size and duration of the study may have limited the detection of rare or infrequent adverse events. Additionally, the use of result imputation by LOCF in patients who received RM represents an inherent limitation in the assessment of analgesia for a slower action drug; this could be partly mitigated through the evaluation of secondary variables.
5. Conclusions
The results of this study show that both IBU/TRA combinations are effective and safe compared to the placebo in patients with moderate-to-severe pain following dental surgery.
This study provided estimates of both the effect size and variability, as well as the selected efficacy parameters, confirming the consistent and sustained analgesic efficacy of the IBU/TRA combinations throughout the observation period. Therefore, fixed-dose combinations of ibuprofen and tramadol may provide a good opportunity to achieve effective analgesia at lower opiate doses.
We also suggest using the variable SPID for the evaluation of the analgesic effects of these kinds of drugs and their combinations.
6. Patents
Portolés A, Santé L, Salas M, Vargas E, Calandria C et al., inventors; Farmalider S.A. assignee. Combination of ibuprofen and tramadol for relieving pain, WO/2021/005129, 14 January 2021 [45].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pharmaceutics17101248/s1, Supplementary File S1: Synopsis protocol; Supplementary File S2: Study team.
Author Contributions
Conceptualization, A.P.-P. and E.V.-C.; Design (Methodology), A.P.-P., E.V.-C., R.M.-G., M.R.S.-B., N.S., C.C., A.B.R.-P. and L.L.-V.; Data curation, M.R.S.-B.; Formal analysis, I.S.-G., M.R.S.-B.; Acquisition of data (Investigation), M.R.S.-B., L.L.-V., A.B.R.-P., A.G.-C., M.F.M.-G., A.M.B., J.J.A.-S., C.P.-I., F.A.-S., J.-L.C., M.Á.G.-M., C.P.-D., L.L., D.M., R.M.-G. and A.P.-P.; Resources, C.C. and N.S.; Writing—original draft, M.R.S.-B.; Writing—review and editing, M.R.S.-B., L.L.-V., A.B.R.-P., A.G.-C., M.F.M.-G., A.M.B., J.J.A.-S., C.P.-I., F.A.-S., J.-L.C., M.Á.G.-M., I.S.-G., C.P.-D., L.L., D.M., N.S., C.C., E.V.-C., R.M.-G. and A.P.-P.; Visualization, M.R.S.-B. and A.P.-P.; Supervision, E.V.-C. and A.P.-P.; Project Administration, A.P.-P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Farmalíder SA (code: 18/243-R-M); the APC was funded by Health Research Institute of the Hospital Clínico San Carlos, Madrid, Spain.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Health Research Institute of the Hospital Clínico San Carlos, Madrid, Spain (Internal Code: 18/243-R_M).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data are available on reasonable request from the corresponding author due to confidentiality reasons.
Acknowledgments
The authors acknowledge for the collaboration of all the study unit’s teams (see Composition of Febetradi Study Team in Supplementary File S2), as well as Ester Duart, Raquel Horcajada and Ananda Gómez from Farmalíder, and Natalia Pérez-Macías, Susana García-Ramos and Sara Muñoz for her efficient work in project, data and quality management. This study has counted with the collaboration of UICEC-IdISSC, which is affiliated to the Platform SCReN-ISCIII.
Conflicts of Interest
Carlos Calandria and Nuria Sanz are employees of Farmalíder SA, funder of the study; Antonio Portolés-Pérez Emilio Vargas-Castrillón, M Rosario Salas-Butrón, Carlos Calandria and Nuria Sanz are authors of the PCT referenced as 45. The rest of the authors declare no conflicts of interest. The funder had no role in the collection, analyses, or interpretation of data or in the writing of the manuscript, and agreed to the publication of the study.
Abbreviations
The following abbreviations are used in this manuscript:
| AEs | Adverse effects |
| ANCOVA | Analysis of covariance |
| EMA | European Medicines Agency |
| FDC | Fixed-dose combination |
| IBU/TRA | Ibuprofen/Tramadol |
| ITT | Intention-to-treat |
| LOCF | Last observation carried forward |
| NSAIDs | Nonsteroidal anti-inflammatory drugs |
| PAR | Pain relief |
| PI | Pain intensity |
| PID | Pain intensity difference from baseline at specific time points. |
| PP | Per-protocol |
| SPID | Sum of pain intensity differences |
| TOTPAR | Total pain relief |
| VAS | Visual Analogue Scale |
References
- Chou, R.; Gordon, D.B.; de Leon-Casasola, O.A.; Rosenberg, J.M.; Bickler, S.; Brennan, T.; Carter, T.; Cassidy, C.L.; Chittenden, E.H.; Degenhardt, E.; et al. Management of Postoperative Pain: A Clinical Practice Guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists. Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J. Pain 2016, 17, 131–157. [Google Scholar] [CrossRef]
- Shah, D.D.; Sorathia, Z.H. Tramadol/Diclofenac Fixed-Dose Combination: A Review of Its Use in Severe Acute Pain. Pain Ther. 2020, 9, 113–128. [Google Scholar] [CrossRef]
- Joshi, G.P.; Ogunnaike, B.O. Consequences of inadequate postoperative pain relief and chronic persistent postoperative pain. Anesthesiol. Clin. N. Am. 2005, 23, 21–36. [Google Scholar] [CrossRef]
- White, P.F. Multimodal analgesia: Its role in preventing postoperative pain. Curr. Opin. Investig. Drugs 2008, 9, 76–82. [Google Scholar]
- Raffa, R.B.; Pergolizzi, J.V., Jr.; Tallarida, R.J. The determination and application of fixed-dose analgesic combinations for treating multimodal pain. J. Pain 2010, 11, 701–709. [Google Scholar] [CrossRef]
- Raffa, R.B.; Tallarida, R.J.; Taylor, R., Jr.; Pergolizzi, J.V., Jr. Fixed-dose combinations for emerging treatment of pain. Expert. Opin. Pharmacother. 2012, 13, 1261–1270. [Google Scholar] [CrossRef]
- Raffa, R.B. Pharmacology of oral combination analgesics: Rational therapy for pain. J. Clin. Pharm. Ther. 2001, 26, 257–264. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, A.; Lirk, P. Multimodal Analgesia. Anesthesiol. Clin. 2022, 40, 455–468. [Google Scholar] [CrossRef]
- Moore, R.A.; Derry, C.J.; Derry, S.; Straube, S.; McQuay, H.J. A conservative method of testing whether combination analgesics produce additive or synergistic effects using evidence from acute pain and migraine. Eur. J. Pain 2012, 16, 585–591. [Google Scholar] [CrossRef]
- Filitz, J.; Ihmsen, H.; Günther, W.; Tröster, A.; Schwilden, H.; Schüttler, J.; Koppert, W. Supra-additive effects of tramadol and acetaminophen in a human pain model. Pain 2008, 136, 262–270. [Google Scholar] [CrossRef]
- Gaskell, H.; Derry, S.; Moore, R.A.; McQuay, H.J.; Moore, M. Single dose oral oxycodone and oxycodone plus paracetamol (acetaminophen) for acute postoperative pain in adults. Cochrane Database Syst. Rev. 2009, 2009, CD002763. [Google Scholar] [CrossRef] [PubMed]
- Oldfield, V.; Perry, C.M. Oxycodone/ibuprofen combination tablet: A review of its use in the management of acute pain. Drugs 2005, 65, 2337–2354. [Google Scholar] [CrossRef]
- Dowell, D.; Ragan, K.R.; Jones, C.M.; Baldwin, G.T.; Chou, R. CDC Clinical Practice Guideline for Prescribing Opioids for Pain-United States, 2022. MMWR Recomm. Rep. 2022, 71, 1–95. [Google Scholar] [CrossRef]
- Decker, H.; Wu, C.L.; Wick, E. Multimodal Pain Control in Surgery 2020. Adv. Surg. 2021, 55, 147–157. [Google Scholar] [CrossRef]
- Varrassi, G.; Hanna, M.; Macheras, G.; Montero, A.; Perez, A.M.; Meissner, W.; Perrot, S.; Scarpignato, C. Multimodal analgesia in moderate-to-severe pain: A role for a new fixed combination of dexketoprofen and tramadol. Curr. Med. Res. Opin. 2017, 33, 1165–1173. [Google Scholar] [CrossRef]
- Varrassi, G.; Yeam, C.T.; Rekatsina, M.; Pergolizzi, J.V.; Zis, P.; Paladini, A. The Expanding Role of the COX Inhibitor/Opioid Receptor Agonist Combination in the Management of Pain. Drugs 2020, 80, 1443–1453. [Google Scholar] [CrossRef]
- Wainwright, T.W.; Gill, M.; McDonald, D.A.; Middleton, R.G.; Reed, M.; Sahota, O.; Yates, P.; Ljungqvist, O. Consensus statement for perioperative care in total hip replacement and total knee replacement surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations. Acta Orthop. 2020, 91, 3–19. [Google Scholar] [CrossRef]
- Desjardins, P.; Alvarado, F.; Gil, M.; González, M.; Guajardo, R. Efficacy and safety of two fixed-dose combinations of tramadol hydrochloride and diclofenac sodium in postoperative dental pain. Pain Med. 2020, 21, 2447–2457. [Google Scholar] [CrossRef]
- Moore, R.A.; McQuay, H.J.; Tomaszewski, J.; Raba, G.; Tutunaru, D.; Lietuviete, N.; Galad, J.; Hagymasy, L.; Melka, D.; Kotarski, J.; et al. Dexketoprofen/tramadol 25 mg/75 mg: Randomised double-blind trial in moderate-to-severe acute pain after abdominal hysterectomy. BMC Anesthesiol. 2016, 16, 9. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, D.; Clementi, E. Clinical Pharmacokinetics of Ibuprofen Arginine. Curr. Clin. Pharmacol. 2010, 5, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Scarpignato, C.; Lanas, A.; Blandizzi, C.; Lems, W.F.; Hermann, M.; Hunt, R.H.; International NSAID Consensus Group. Safe prescribing of non-steroidal anti-inflammatory drugs in patients with osteoarthritis—An expert consensus addressing benefits as well as gastrointestinal and cardiovascular risks. BMC Med. 2015, 13, 55. [Google Scholar] [CrossRef]
- Grond, S.; Sablotzki, A. Clinical Pharmacology of Tramadol. Clin. Pharmacokinet. 2004, 43, 879–923. [Google Scholar] [CrossRef]
- Raffa, R.B.; Friderichs, E.; Reimann, W.; Shank, R.P.; E Codd, E.; Vaught, J.L.; I Jacoby, H.; Selve, N. Complementary and synergistic antinociceptive interaction between the enantiomers of tramadol. J. Pharmacol. Exp. Ther. 1993, 267, 331–340. [Google Scholar] [CrossRef]
- Subedi, M.; Bajaj, S.; Kumar, M.S.; Yc, M. An overview of tramadol and its usage in pain management and future perspective. Biomed. Pharmacother. 2019, 111, 443–451. [Google Scholar] [CrossRef]
- Vazzana, M.; Andreani, T.; Fangueiro, J.; Faggio, C.; Silva, C.; Santini, A.; Garcia, M.; Silva, A.; Souto, E. Tramadol hydrochloride: Pharmacokinetics, pharmacodynamics, adverse side effects, co-administration of drugs and new drug delivery systems. Biomed. Pharmacother. 2015, 70, 234–238. [Google Scholar] [CrossRef]
- European Medicines Agency. Guideline on the Clinical Development of Medicinal Products Intended for the Treatment of Pain. 15 December 2016. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-clinical-development-medicinal-products-intended-treatment-pain-first-version_en.pdf (accessed on 26 June 2025).
- Cao, B.; Xu, Q.; Shi, Y.; Zhao, R.; Li, H.; Zheng, J.; Liu, F.; Wan, Y.; Wei, B. Pathology of pain and its implications for therapeutic interventions. Signal Transduct. Target. Ther. 2024, 9, 155. [Google Scholar] [CrossRef]
- Melzack, R.; Wall, P.D. Pain mechanisms: A new theory. Science 1965, 150, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Raffa, R.B.; Clark-Vetri, R.; Tallarida, R.J.; Wertheimer, A.I. Combination strategies for pain management. Expert. Opin. Pharmacother. 2003, 4, 1697–1708. [Google Scholar] [CrossRef]
- Moore, R.A.; Gay-Escoda, C.; Figueiredo, R.; Tóth-Bagi, Z.; Dietrich, T.; Milleri, S.; Torres-Lagares, D.; Hill, C.M.; García-García, A.; Coulthard, P.; et al. Dexketoprofen/tramadol: Randomised double-blind trial and confirmation of empirical theory of combination analgesics in acute pain. J. Headache Pain 2015, 16, 541. [Google Scholar] [CrossRef]
- Biswas, S.; Chandanwale, A.S.; Sundar, S.; Gabhane, M.; Naik, M.; Patel, K.; Latchoumibady, V.; Latchoumibady, K. Efficacy and safety profile of combination of tramadol-diclofenac vs. tramadol-paracetamol in patients with acute musculoskeletal conditions, postoperative pain, and acute flare of osteoarthritis and rheumatoid arthritis: A Phase III; 5-day open-label study. J. Pain Res. 2014, 7, 455–463. [Google Scholar] [CrossRef]
- Paladini, A.; Rawal, N.; Coca Martinez, M.; Trifa, M.; Montero, A.; Pergolizzi, J., Jr.; Pasqualucci, A.; Tamayo, M.A.N.; Varrassi, G.; De Leon Casasola, O. Advances in the Management of Acute Postsurgical Pain: A Review. Cureus 2023, 15, e42974. [Google Scholar] [CrossRef]
- Smith, H.S. Combination opioid analgesics. Pain Physician 2008, 11, 201–214. [Google Scholar] [CrossRef]
- Aasvang, E.; Kehlet, H. Chronic postoperative pain: The case of inguinal herniorrhaphy. Br. J. Anaesth. 2005, 95, 69–76. [Google Scholar] [CrossRef]
- Volkow, N.D.; Blanco, C. The changing opioid crisis: Development, challenges and opportunities. Mol. Psychiatry 2021, 26, 218–233. [Google Scholar] [CrossRef]
- Kehlet, H.; Dahl, J.B. The value of “multimodal” or “balanced analgesia” in postoperative pain treatment. Anesth. Analg. 1993, 77, 1048–1056. [Google Scholar] [CrossRef]
- Memtsoudis, S.G.; Poeran, J.; Zubizarreta, N.; Cozowicz, C.; Mörwald, E.E.; Mariano, E.R.; Mazumdar, M. Association of Multimodal Pain Management Strategies with Perioperative Outcomes and Resource Utilization: A Population-based Study. Anesthesiology 2018, 128, 891–902. [Google Scholar] [CrossRef]
- Singla, N.; Pong, A.; Newman, K. Combination oxycodone 5 mg/ibuprofen 400 mg for the treatment of pain after abdominal or pelvic surgery in women: A randomized, double blind, placebo and active-controlled parallel-group study. Clin. Ther. 2005, 27, 45–57. [Google Scholar] [CrossRef]
- Langford, R.; Morte, A.; Sust, M.; Cebrecos, J.; Vaqué, A.; Ortiz, E.; Fettiplace, J.; Adeyemi, S.; Raba, G.; But-Husaim, L.; et al. Efficacy and safety of co-crystal of tramadol-celecoxib (CTC) in acute moderate-to-severe pain after abdominal hysterectomy: A randomized, double-blind, phase 3 trial (STARDOM2). Eur. J. Pain 2022, 26, 2083–2096. [Google Scholar] [CrossRef]
- McQuay, H.; Moore, R.; Berta, A.; Gainutdinovs, O.; Fülesdi, B.; Porvaneckas, N.; Petronis, S.; Mitkovic, M.; Bucsi, L.; Samson, L.; et al. Randomized clinical trial of dexketoprofen/tramadol 25 mg/75 mg in moderate-to-severe pain after total hip arthroplasty. Br. J. Anaesth. 2016, 116, 269–276. [Google Scholar] [CrossRef]
- Vallecillo, C.; Vallecillo-Rivas, M.; Gálvez, R.; Vallecillo-Capilla, M.; Olmedo-Gaya, M.V. Analgesic efficacy of tramadol/dexketoprofen vs ibuprofen after impacted lower third molar extraction: A randomized controlled clinical trial. J. Evid. Based Dent. Pract. 2021, 21, 101618. [Google Scholar] [CrossRef]
- Van Dyke, T.; Litkowski, L.J.; Kiersch, T.A.; Zarringhalam, N.M.; Zheng, H.; Newman, K. Combination oxycodone 5 mg/ibuprofen 400 mg for the treatment of postoperative pain: A double-blind, placebo- and active-controlled parallel-group study. Clin. Ther. 2004, 26, 2003–2014. [Google Scholar] [CrossRef]
- European Medicines Agency. Guideline on Clinical Development of Fixed Combination Medicinal Products. 3 March 2017. Available online: https://www.ema.europa.eu/en/clinical-development-fixed-combination-medicinal-products-scientific-guideline (accessed on 5 March 2018).
- Portolés-Díez, C.; Salas-Butrón, M.R.; Ascaso-del-Rio, A.; Rivas-Paterna, A.B.; Laredo-Velasco, L.; Calandria, C.; Sanz, S.; Bergeron, A.; Santé, L.; Vargas-Castrillón, E.; et al. Intravenous vs. Oral Dose Comparison of Ibuprofen and Tramadol Combinations-Enantiomers, Metabolite, Linearity, and Sex-Related Effects: A Pharmacokinetics Randomized Clinical Trial. Pharmaceuticals 2025, 18, 331. [Google Scholar] [CrossRef]
- Portolés, A.; Santé, L.; Salas, M.; Vargas, E.; Calandria, C.; Peréz, C.C.; Cordóba, R.H.; Ruiz, Á.J.M.; De La Torre, M.V.; Menéndez, N.S.; et al. inventors; Farmalider, S.A., assignee. Combination of Ibuprofen and Tramadol for Relieving Pain. WO/2021/005129, 14 January 2021. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).