AmBisome® Formulations for Pediatrics: Stability, Cytotoxicity, and Cost-Effectiveness Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Reconstitution and Dilution of Ambisome® in the Hospital Pharmacy
2.3. Sample Preparation
2.4. UV–Visible Spectrophotometry for Qualitative Analysis
2.5. In Vitro Colloidal Stability of Liposomal AmB Formulations
2.6. HPLC Assessment
2.7. Cytotoxicity Studies
2.8. AmBisome® Therapy Optimization: Cost-Saving Analysis
2.9. Statistical Analysis
3. Results and Discussion
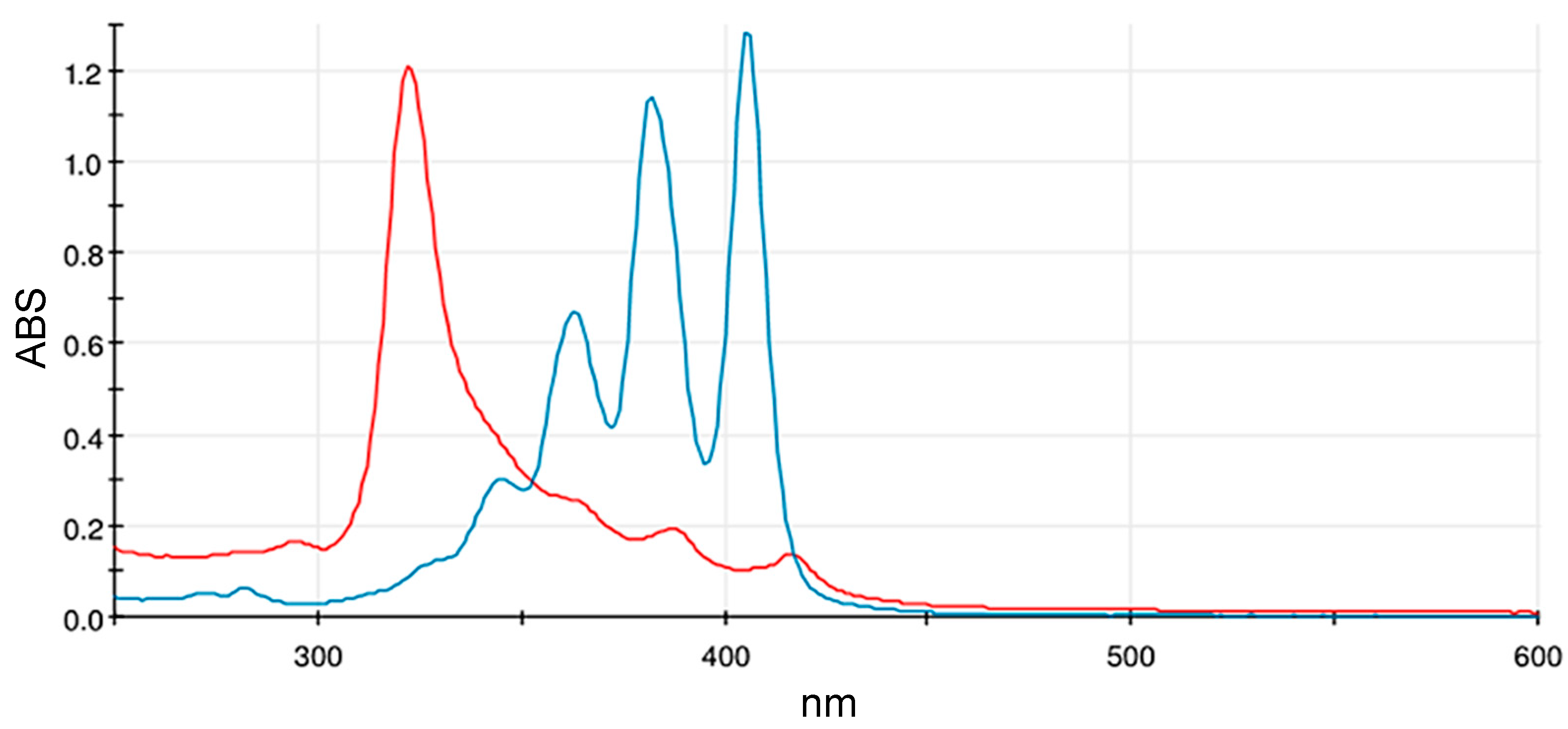
3.1. UV–Visible Spectrophotometry
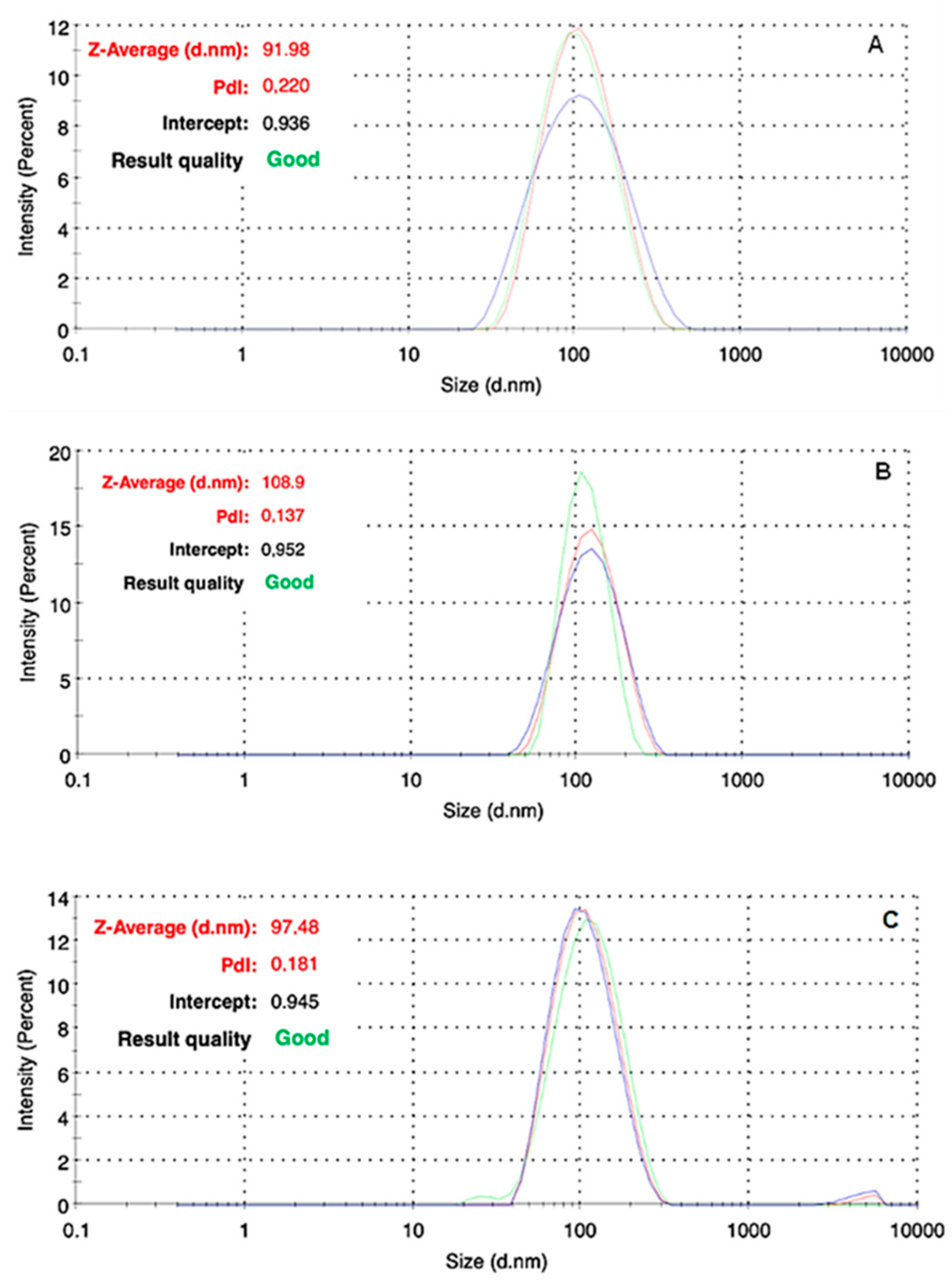
3.2. In Vitro Colloidal Stability of Nanoformulations
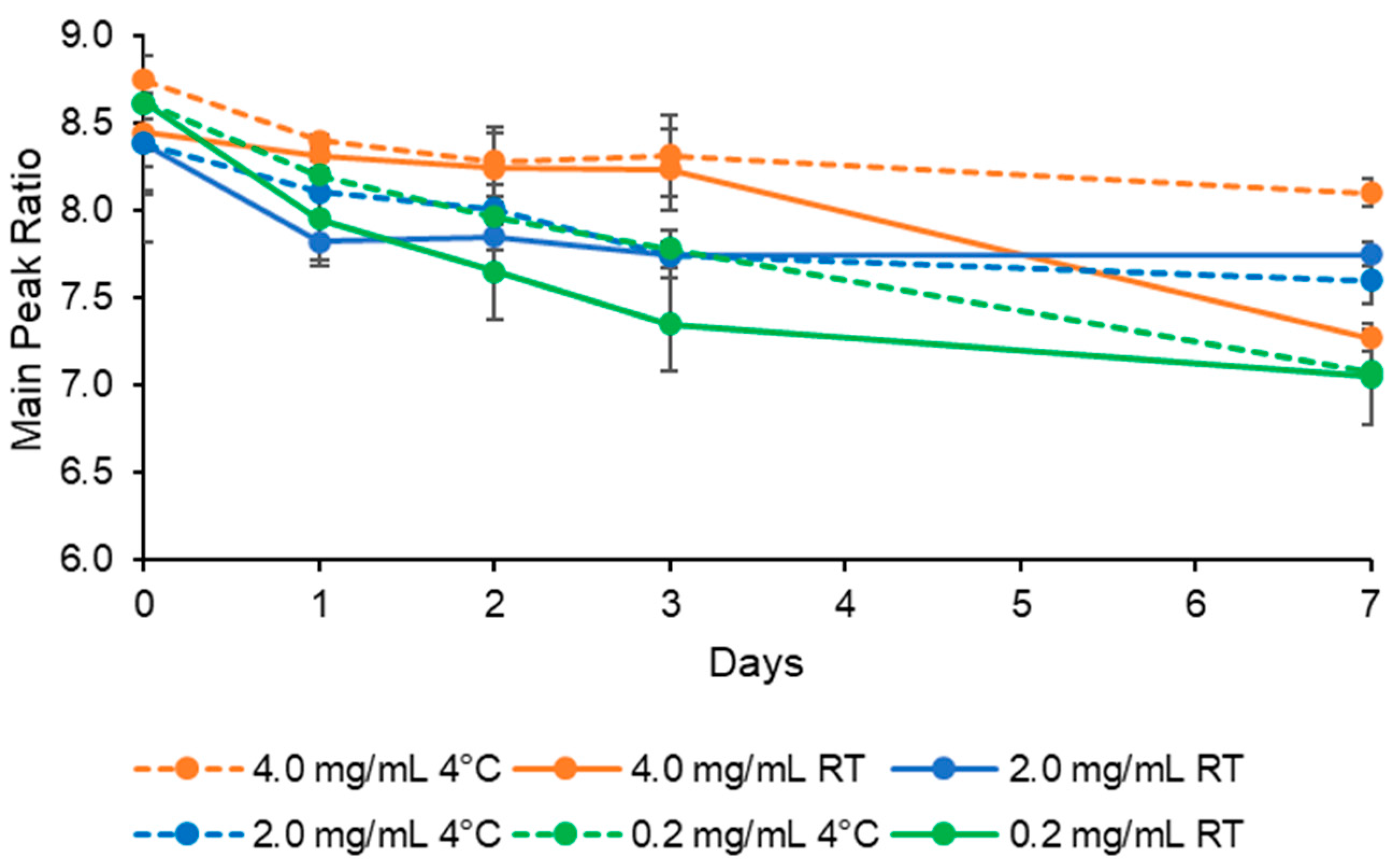
3.3. HPLC Analysis
3.4. In Vitro Toxicity Evaluation of AmB Formulations
3.5. The Impact of the Centralized Compounding of AmBisome® at the G. Gaslini Children Hospital Pharmacy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, L.; Zhang, L.; Xie, Y.; Wang, Y.; Tian, X.; Fang, W.; Xue, X.; Wang, L. Confronting Antifungal Resistance, Tolerance, and Persistence: Advances in Drug Target Discovery and Delivery Systems. Adv. Drug Deliv. Rev. 2023, 200, 115007. [Google Scholar] [CrossRef] [PubMed]
- Groll, A.H.; Piscitelli, S.C.; Walsh, T.J. Clinical Pharmacology of Systemic Antifungal Agents: A Comprehensive Review of Agents in Clinical Use, Current Investigational Compounds, and Putative Targets for Antifungal Drug Development. Adv. Pharmacol. 1998, 44, 343–500. [Google Scholar] [PubMed]
- Sangalli-Leite, F.; Scorzoni, L.; Mesa-Arango, A.C.; Casas, C.; Herrero, E.; Soares Mendes Gianinni, M.J.; Rodríguez-Tudela, J.L.; Cuenca-Estrella, M.; Zaragoza, O. Amphotericin B Mediates Killing in Cryptococcus Neoformans through the Induction of a Strong Oxidative Burst. Microbes Infect. 2011, 13, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, J.; Wieczór, M.; Bączek, T.; Gruszecki, M.; Czub, J. Thermodynamics and kinetics of amphotericin B self-association in aqueous solution characterized in molecular detail. Sci. Rep. 2016, 6, 1910. [Google Scholar] [CrossRef] [PubMed]
- Bolard, J.; Legrand, P.; Heitz, F.; Cybulska, B. One-Sided Action of Amphotericin B on Cholesterol-Containing Membranes Is Determined by Its Self-Association in the Medium. Biochemistry 1991, 30, 5707–5715. [Google Scholar] [CrossRef]
- Barwicz, J.; Tancrède, P. The Effect of Aggregation State of Amphotericin-B on Its Interactions with Cholesterol- or Ergosterol-Containing Phosphatidylcholine Monolayers. Chem. Phys. Lipids 1997, 85, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Haynes, M.P.; Chong, P.L.-G.; Buckley, H.R.; Pieringer, R.A. Fluorescence Studies on the Molecular Action of Amphotericin B on Susceptible and Resistant Fungal Cells. Biochemistry 1996, 35, 7983–7992. [Google Scholar] [CrossRef]
- Yamamoto, T.; Umegawa, Y.; Tsuchikawa, H.; Hanashima, S.; Matsumori, N.; Funahashi, K.; Seo, S.; Shinoda, W.; Murata, M. The Amphotericin B–Ergosterol Complex Spans a Lipid Bilayer as a Single-Length Assembly. Biochemistry 2019, 58, 5188–5196. [Google Scholar] [CrossRef]
- Rivnay, B.; Wakim, J.; Avery, K.; Petrochenko, P.; Myung, J.H.; Kozak, D.; Yoon, S.; Landrau, N.; Nivorozhkin, A. Critical Process Parameters in Manufacturing of Liposomal Formulations of Amphotericin B. Int. J. Pharm. 2019, 565, 447–457. [Google Scholar] [CrossRef]
- Hamill, R.J. Amphotericin B formulations: A comparative review of efficacy and toxicity. Drugs 2013, 73, 919–934. [Google Scholar] [CrossRef]
- Wang, X.; Mohammad, I.S.; Fan, L.; Zhao, Z.; Nurunnabi, M.; Sallam, M.A.; Wu, J.; Chen, Z.; Yin, L.; He, W. Delivery strategies of amphotericin B for invasive fungal infections. Acta Pharm Sin B 2021, 11, 2585–2604. [Google Scholar] [CrossRef]
- Stone, N.R.; Bicanic, T.; Salim, R.; Hope, W. Liposomal Amphotericin B (AmBisome(®)): A Review of the Pharmacokinetics, Pharmacodynamics, Clinical Experience and Future Directions. Drugs 2016, 76, 485–500. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.gilead.com/news-and-press/press-room/press-releases/2022/2/gilead-sciences-announces-fourth-quarter-and-full-year-2021-financial-results (accessed on 18 January 2024).
- Available online: https://www.ema.europa.eu/en/documents/comments/overview-comments-received-liposomal-amphotericin-b-powder-dispersion-infusion-50-mg-product/chmp/559889/2021_en.pdf (accessed on 18 January 2024).
- Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/liposomal-amphotericin-b-powder-dispersion-infusion-50-mg-product-specific-bioequivalence-guidance_en.pdf (accessed on 18 January 2024).
- Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/reflection-paper-data-requirements-intravenous-liposomal-products-developed-reference-innovator_en.pdf (accessed on 18 January 2024).
- Fleury, M.; Fonzo-Christe, C.; Normand, C.; Bonnabryet, P. Confusion between Two Amphotericin B Formulations Leading to a Paediatric Rehospitalisation. Drug Saf. Case Rep. 2016, 3, 4. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stemple, K.; Schnoor, J.; Ahern, J.; Bunnell, A. Utilization of an Order Panel to Encourage Safe Ordering and Administration of Amphotericin B. Hosp. Pharm. 2019, 54, 212–216. [Google Scholar] [CrossRef]
- Available online: https://farmaci.agenziafarmaco.gov.it/aifa/servlet/PdfDownloadServlet?pdfFileName=footer_007212_028581_RCP.pdf&sys=m0b1l3 (accessed on 18 January 2024).
- Liu, Y.; Mei, Z.; Mei, L.; Tang, J.; Yuan, W.; Srinivasan, S.; Ackermann, R.; Schwendeman, A.S. Analytical Method Development and Comparability Study for AmBisome® and Generic Amphotericin B Liposomal Products. Eur. J. Pharm. Biopharm. 2020, 157, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Raggi, F.; Cangelosi, D.; Consolaro, A.; Rossi, C.; Pelassa, S.; Cortese, K.; Gagliani, M.C.; Morini, M.; Segalerba, D.; Brignole, C.; et al. Extracellular Vesicle-derived microRNAs as Potential Biomarkers in Oligoarticular Juvenile Idiopathic Arthritis Patients: Methodological Challenges and New Perspectives. Clin. Transl. Med. 2022, 12, e1067. [Google Scholar] [CrossRef] [PubMed]
- Espada, R.; Valdespina, S.; Alfonso, C.; Rivas, G.; Ballesteros, M.P.; Torrado, J.J. Effect of Aggregation State on the Toxicity of Different Amphotericin B Preparations. Int. J. Pharm. 2008, 361, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Svirkin, Y.; Lee, J.; Marx, R.; Yoon, S.; Landrau, N.; Kaisar, M.A.; Qin, B.; Park, J.H.; Alam, K.; Kozak, D.; et al. Amphotericin B Release Rate Is the Link between Drug Status in the Liposomal Bilayer and Toxicity. Asian J. Pharm. Sci. 2022, 17, 544–556. [Google Scholar] [CrossRef]
- Díaz De León–Ortega, R.; D’Arcy, D.M.; Lamprou, D.A.; Fotaki, N. In Vitro—In Vivo Relations for the Parenteral Liposomal Formulation of Amphotericin B: A Clinically Relevant Approach with PBPK Modeling. Eur. J. Pharm. Biopharm. 2021, 159, 177–187. [Google Scholar] [CrossRef]
- Ye, J.; Li, R.; Cheng, J.; Liu, D.; Yang, Y.; Wang, H.; Xu, X.; Li, L.; Ma, P.; Liu, Y. Comparative Colloidal Stability of Commercial Amphotericin B Nanoformulations Using Dynamic and Static Multiple Light Scattering Techniques. Int. J. Nanomed. IJN 2022, 17, 6047–6064. [Google Scholar] [CrossRef]
- Imran, A.; Popescu, D.; Movileanu, L. Cyclic Activity of an Osmotically Stressed Liposome in a Finite Hypotonic Environment. Langmuir 2020, 36, 3659–3666. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, M.; De Souza, S.; Leão, R.; Rocha, H.; De Rezende, C.; De Souza, R. Methodology Development and Validation of Amphotericin B Stability by HPLC-DAD. J. Braz. Chem. Soc. 2020, 31, 916–926. [Google Scholar] [CrossRef]
- Sawangchan, P.; Alexandrino, F., Jr.; Alencar, É.N.; Egito, E.S.T.; Kirsch, L.E. The Role of Aggregation and Ionization in the Chemical Instability of Amphotericin B in Aqueous Methanol. Int. J. Pharm. 2023, 632, 122586. [Google Scholar] [CrossRef] [PubMed]
- Espada, R.; Josa, J.M.; Valdespina, S.; Dea, M.A.; Ballesteros, M.P.; Alunda, J.M.; Torrado, J.J. HPLC Assay for Determination of Amphotericin B in Biological Samples. Biomed. Chromatogr. 2008, 22, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Gurudevan, S.; Francis, A.P.; Jayakrishnan, A. Amphotericin B-Albumin Conjugates: Synthesis, Toxicity and Anti-Fungal Activity. Eur. J. Pharm. Sci. 2018, 115, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Grainger, D.W. Regulatory Considerations Specific to Liposome Drug Development as Complex Drug Products. Front. Drug. Deliv. 2022, 2, 901281. [Google Scholar] [CrossRef]
- Fernández-García, R.; Muñoz-García, J.C.; Wallace, M.; Fabian, L.; González-Burgos, E.; Gómez-Serranillos, M.P.; Raposo, R.; Bolás-Fernández, F.; Ballesteros, M.P.; Healy, A.M.; et al. Self-Assembling, Supramolecular Chemistry and Pharmacology of Amphotericin B: Poly-Aggregates, Oligomers and Monomers. J. Control. Release 2022, 341, 716–732. [Google Scholar] [CrossRef] [PubMed]
- Shibata, H.; Izutsu, K.; Yomota, C.; Okuda, H.; Goda, Y. Investigation of Factors Affecting in Vitro Doxorubicin Release from PEGylated Liposomal Doxorubicin for the Development of in Vitro Release Testing Conditions. Drug Dev. Ind. Pharm. 2015, 41, 1376–1386. [Google Scholar] [CrossRef]
- Van Der Schors, T.; Amann, S.; Makridaki, D.; Kohl, S. Pharmacy Preparations and Compounding. Eur. J. Hosp. Pharm. 2021, 28, 190–192. [Google Scholar] [CrossRef]
- Ministers’ Deputies. Resolution CM/Res(2016)2 on Good Reconstitution Practices in Health Care Establishments for Medicinal Products for Parenteral Use (Adopted by the Committee of Ministers on 1 June 2016 at the 1258th Meeting of the Ministers’ Deputies. Available online: https://www.edqm.eu/sites/default/files/resolution_cm_res_2016_2_good_reconstitution_practices_in_health_care_establishments_for_medicinal_products_for_parenteral_use_.pdf (accessed on 18 January 2024).
- Seibel, N.L.; Shad, A.T.; Bekersky, I.; Groll, A.H.; Gonzalez, C.; Wood, L.V.; Jarosinski, P.; Buell, D.; Hope, W.W.; Walsh, T.J. Safety, Tolerability, and Pharmacokinetics of Liposomal Amphotericin B in Immunocompromised Pediatric Patients. Antimicrob. Agents Chemother. 2017, 61, e01477-16. [Google Scholar] [CrossRef]
- Maertens, J.; Pagano, L.; Azoulay, E.; Warris, A. Liposomal Amphotericin B—The Present. J. Antimicrob. Chemother. 2022, 77, ii11–ii20. [Google Scholar] [CrossRef]
- Nydert, P.; El-Edelbi, R.; Obaya, S.; Nilsson, K.M.; Lindemalm, S. 5PSQ-140 Reconstution Practice by a Paediatric and Neonatal Ward-Based Pharmacist. Eur. J. Hosp. Pharm. 2018, 215, A228–A229. [Google Scholar]

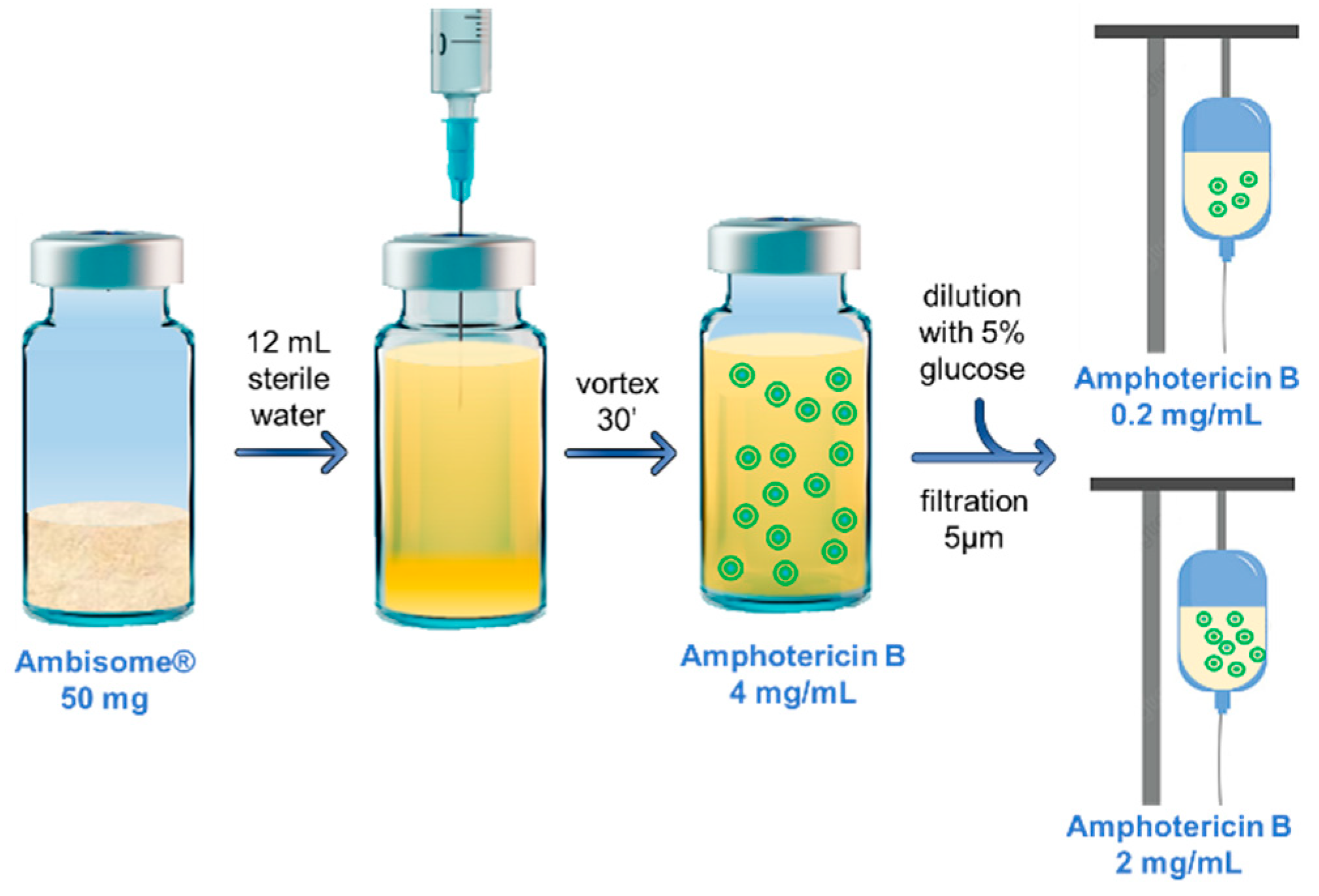
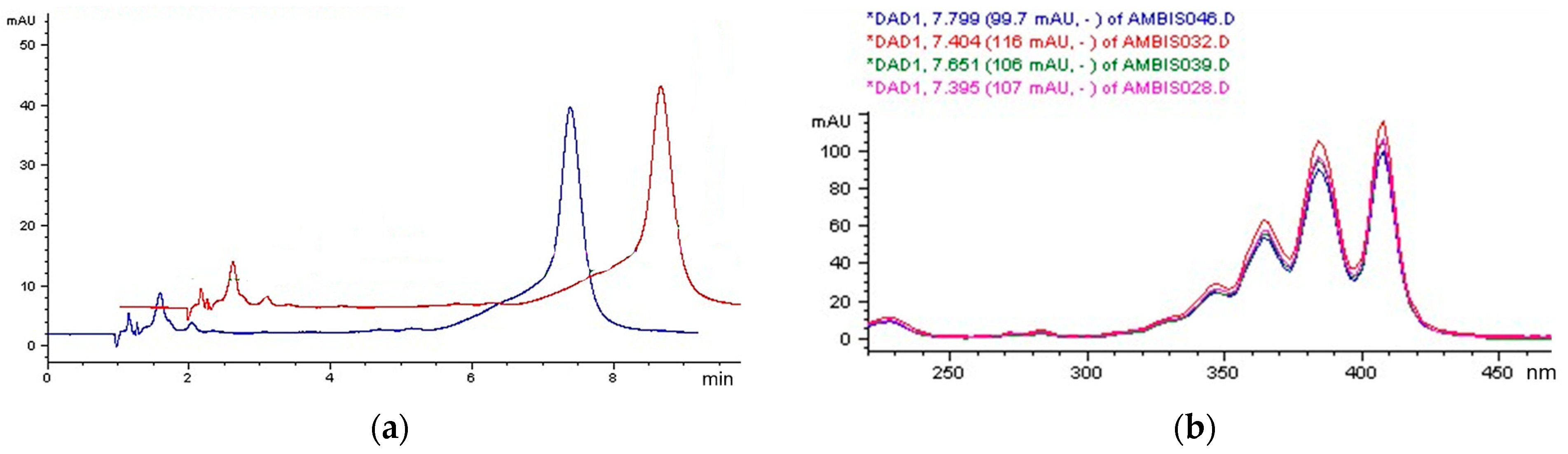
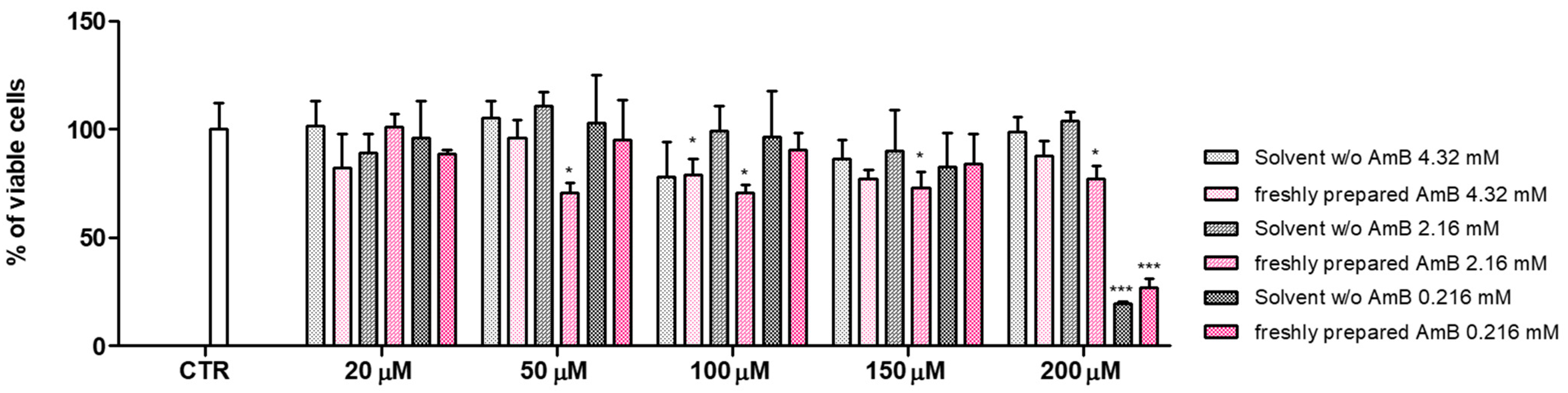
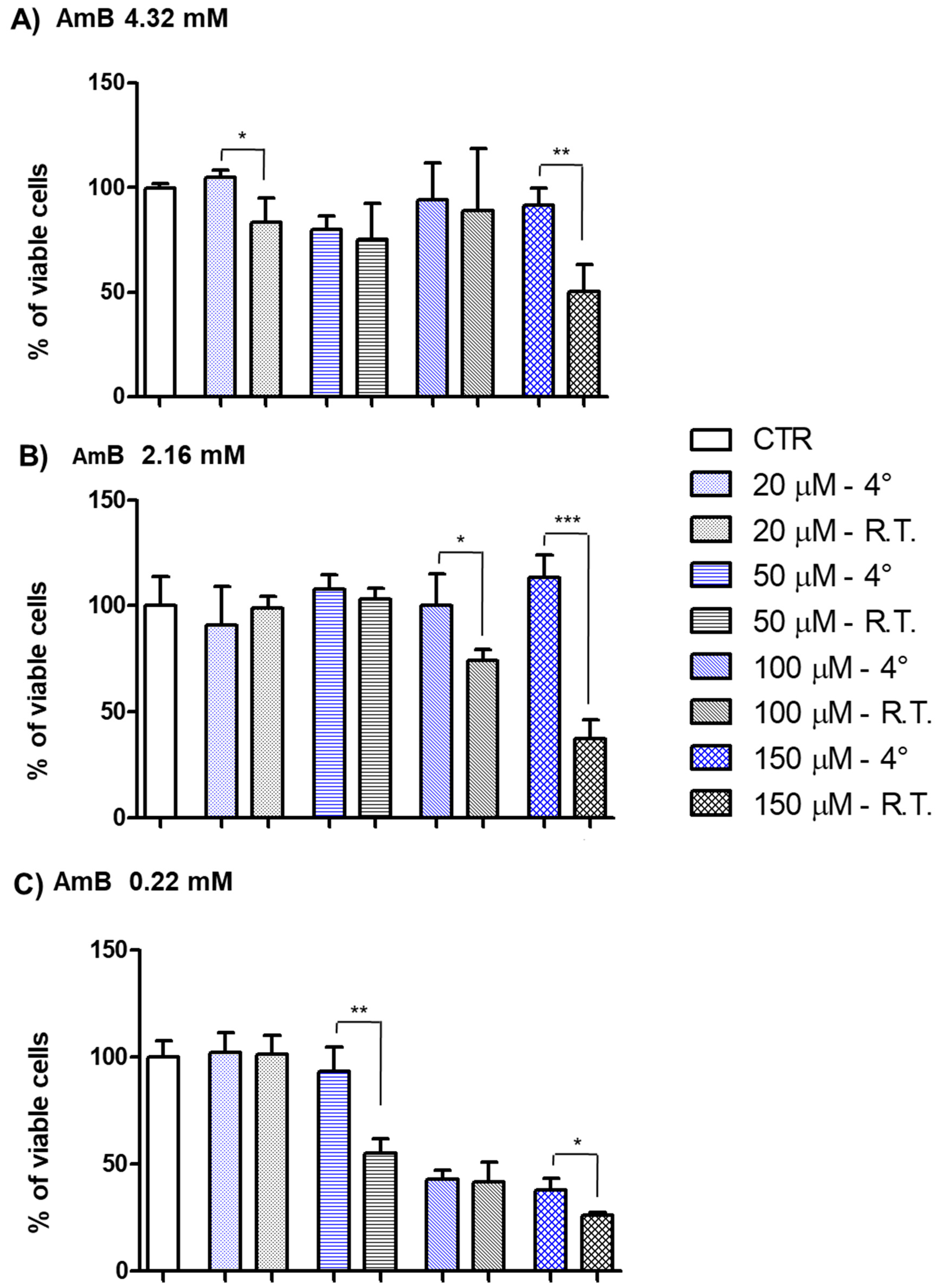
| Diluent | Concentration of AmB (mg/mL) | Maximum Duration of Storage at 4 °C | Maximum Duration of Storage at 25 °C | ||
|---|---|---|---|---|---|
| Manufacturer | Hospital | Manufacturer | Hospital | ||
| Water for injection | 4.0 | 7 days | 7 days | 24 h | N 1 |
| 5% glucose | 2.0 | 7 days | 24 h | 48 h | N |
| 5% glucose | 0.2 | 4 days | 4 days | 24 h | N |
| Sample | NTA System | Dynamic Light Scattering | Storage at 4 °C | |||||
|---|---|---|---|---|---|---|---|---|
| Concentration (Particles/mL) | Mean Diameter (nm) | Mode (nm) | Percentile Values | Z-Average (nm) | PDI | Zeta Potential (mV) | Time (Days) | |
| Liposomal AmB 4 mg/mL in sterile water | 3.94 × 1013 ± 1.43 × 1012 | 87.0 ± 1.0 | 68.3 ± 0.7 | D10 = 58.8 ± 0.2 | 91.9 ± 7.2 | 0.220 | −32.6 ± 7.3 | 0 |
| D50 = 81.4 ± 1.1 | ||||||||
| D90 = 122.4 ± 0.8 | ||||||||
| 1.33 × 1014 ± 6.24 × 1012 | 92.6 ± 1.1 * | 75.8 ± 2.0 | D10 = 64.2 ± 0.5 | 93.9 ± 5.2 | 0.189 | −33.9 ± 6.7 | 2 | |
| D50 = 84.0 ± 1.1 | ||||||||
| D90 = 135.2 ± 2.0 | ||||||||
| 1.28 × 1014 ± 6.81 × 1012 | 104.0 ± 3.5 ** | 81.5 ± 2.5 | D10 = 62.2 ± 2.2 | 92.9 ± 4.7 | 0.210 | −32.4 ± 7.2 | 7 | |
| D50 = 96.9 ± 3.3 | ||||||||
| D90 = 154.1 ± 3.7 | ||||||||
| Liposomal AmB 2 mg/mL in 5% glucose | 8.47 × 1013 ± 1.80 × 1012 | 89.7 ± 1.4 | 75.4 ± 0.8 | D10 = 63.6 ± 1.9 | 97.5 ± 8.4 | 0.181 | −41.4 ± 10.9 | 0 |
| D50 = 82.5 ± 0.8 | ||||||||
| D90 = 128.7 ± 1.7 | ||||||||
| 7.06 × 1013 ± 1.43 × 1012 | 91.9 ± 0.3 | 78.9 ± 3.2 | D10 = 64.0 ± 0.9 | 102.2 ± 4.6 | 0.150 | −42.0 ± 11.5 | 1 | |
| D50 = 87.3 ± 0.5 | ||||||||
| D90 = 125.3 ± 0.8 | ||||||||
| 8.18 × 1012 ± 2.11 × 1011 | 90.9 ± 1.2 | 79.3 ± 0.8 | D10 = 66.2 ± 2.1 | 99.3 ± 5.2 | 0.146 | −42.2 ± 10.9 | 2 | |
| D50 = 84.6 ± 0.7 | ||||||||
| D90 = 127.1 ± 3.2 | ||||||||
| 8.80 × 1013 ± 1.68 × 1012 | 85.1 ± 0.6 * | 73.6 ± 0.3 | D10 = 62.2 ± 0.6 | 99.3 ± 6.5 | 0.160 | −40.9 ± 10.7 | 4 | |
| D50 = 79.5 ± 0.6 | ||||||||
| D90 = 117.0 ± 2.6 | ||||||||
| Liposomal AmB 0.2 mg/mL in 5% glucose | 9.48 × 1012 ± 3.17 × 1011 | 91.1 ± 1.1 | 78.8 ± 2.5 | D10 = 63.5 ± 1.1 | 108.9 ± 7.1 | 0.137 | −44.4 ± 15.9 | 0 |
| D50 = 87.0 ± 0.8 | ||||||||
| D90 = 130.0 ± 2.6 | ||||||||
| 9.19 × 1012 ± 1.92 × 1011 | 87.6 ± 0.3 | 77.3 ± 2.1 | D10 = 63.9 ± 0.4 | 112.4 ± 5.6 | 0.136 | −45.9 ± 14.5 | 1 | |
| D50 = 82.7 ± 0.6 | ||||||||
| D90 = 118.1 ± 1.9 | ||||||||
| 8.20 × 1012 ± 6.82 × 1010 | 90.7 ± 0.5 | 77.0 ± 1.8 | D10 = 64.9 ± 0.9 | 109.2 ± 8.2 | 0.133 | −46.5 ± 13.9 | 2 | |
| D50 = 83.2 ± 0.5 | ||||||||
| D90 = 131.2 ± 1.2 | ||||||||
| 7.47 × 1012 ± 2.62 × 1011 | 88.6 ± 0.6 | 75.9 ± 1.4 | D10 = 64.5 ± 0.7 | 109.0 ± 6.5 | 0.131 | −45.7 ± 16.0 | 4 | |
| D50 = 82.8 ± 0.9 | ||||||||
| D90 = 124.0 ± 2.7 | ||||||||
| Time Period | Vials of AmBisome® Used | Number of Therapies | Vials per Therapy | Cost per Therapy (€) | |
|---|---|---|---|---|---|
| Before | January 2022 | 418 | 72 | 5.55 ± 0.35 | 814.74 |
| February 2022 | 306 | 58 | |||
| After | June 2022 | 152 | 83 | 2.29 ± 0.38 | 336.17 |
| July 2022 | 201 | 91 | |||
| August 2022 | 251 | 99 | |||
| September 2022 | 280 | 106 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuccari, G.; Villa, C.; Iurilli, V.; Barabino, P.; Zorzoli, A.; Marimpietri, D.; Caviglia, D.; Russo, E. AmBisome® Formulations for Pediatrics: Stability, Cytotoxicity, and Cost-Effectiveness Studies. Pharmaceutics 2024, 16, 466. https://doi.org/10.3390/pharmaceutics16040466
Zuccari G, Villa C, Iurilli V, Barabino P, Zorzoli A, Marimpietri D, Caviglia D, Russo E. AmBisome® Formulations for Pediatrics: Stability, Cytotoxicity, and Cost-Effectiveness Studies. Pharmaceutics. 2024; 16(4):466. https://doi.org/10.3390/pharmaceutics16040466
Chicago/Turabian StyleZuccari, Guendalina, Carla Villa, Valentina Iurilli, Paola Barabino, Alessia Zorzoli, Danilo Marimpietri, Debora Caviglia, and Eleonora Russo. 2024. "AmBisome® Formulations for Pediatrics: Stability, Cytotoxicity, and Cost-Effectiveness Studies" Pharmaceutics 16, no. 4: 466. https://doi.org/10.3390/pharmaceutics16040466
APA StyleZuccari, G., Villa, C., Iurilli, V., Barabino, P., Zorzoli, A., Marimpietri, D., Caviglia, D., & Russo, E. (2024). AmBisome® Formulations for Pediatrics: Stability, Cytotoxicity, and Cost-Effectiveness Studies. Pharmaceutics, 16(4), 466. https://doi.org/10.3390/pharmaceutics16040466










