Influenza Virus Inactivated by Heavy Ion Beam Irradiation Stimulates Antigen-Specific Immune Responses
Abstract
1. Introduction
2. Material and Methods
2.1. Mice
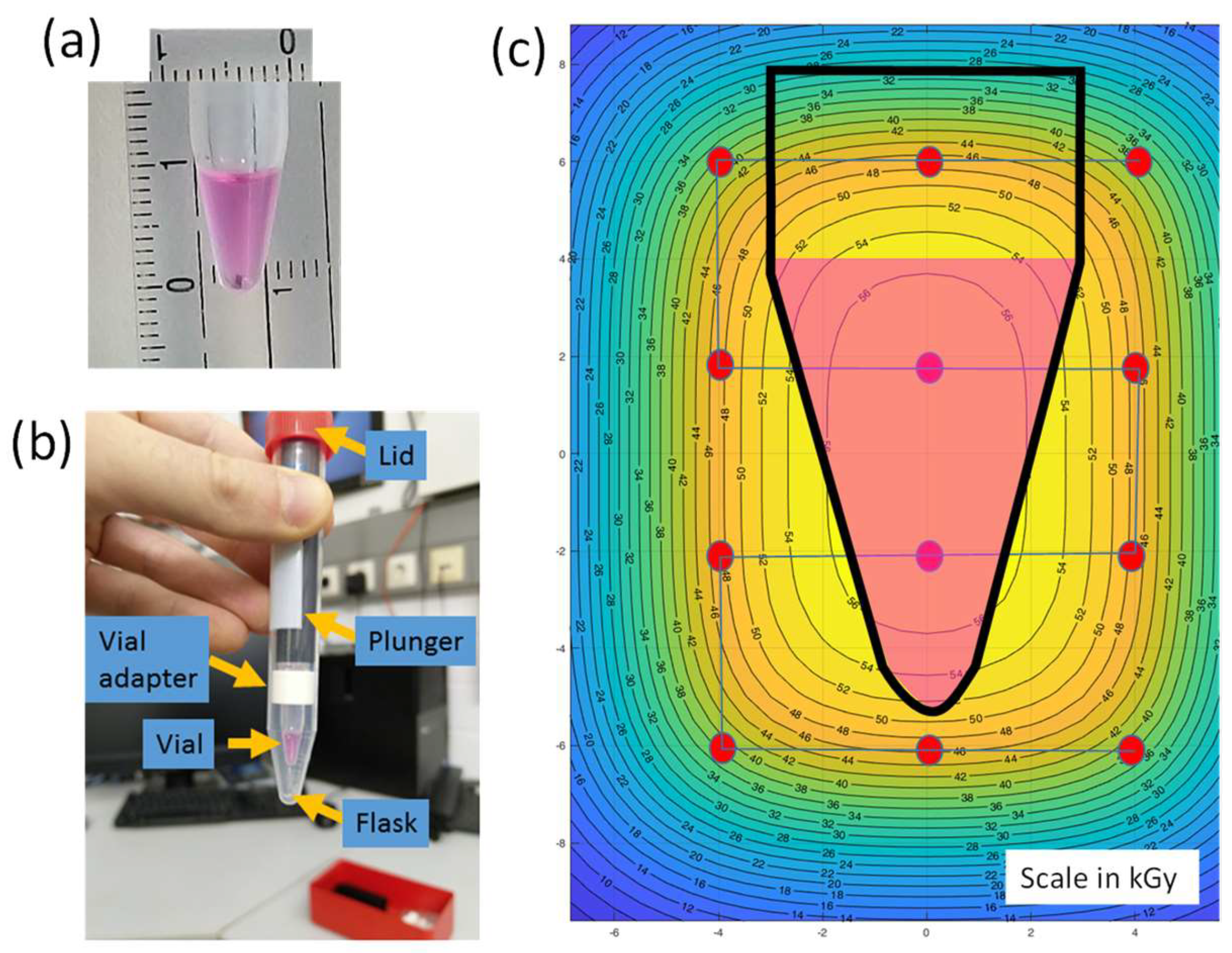
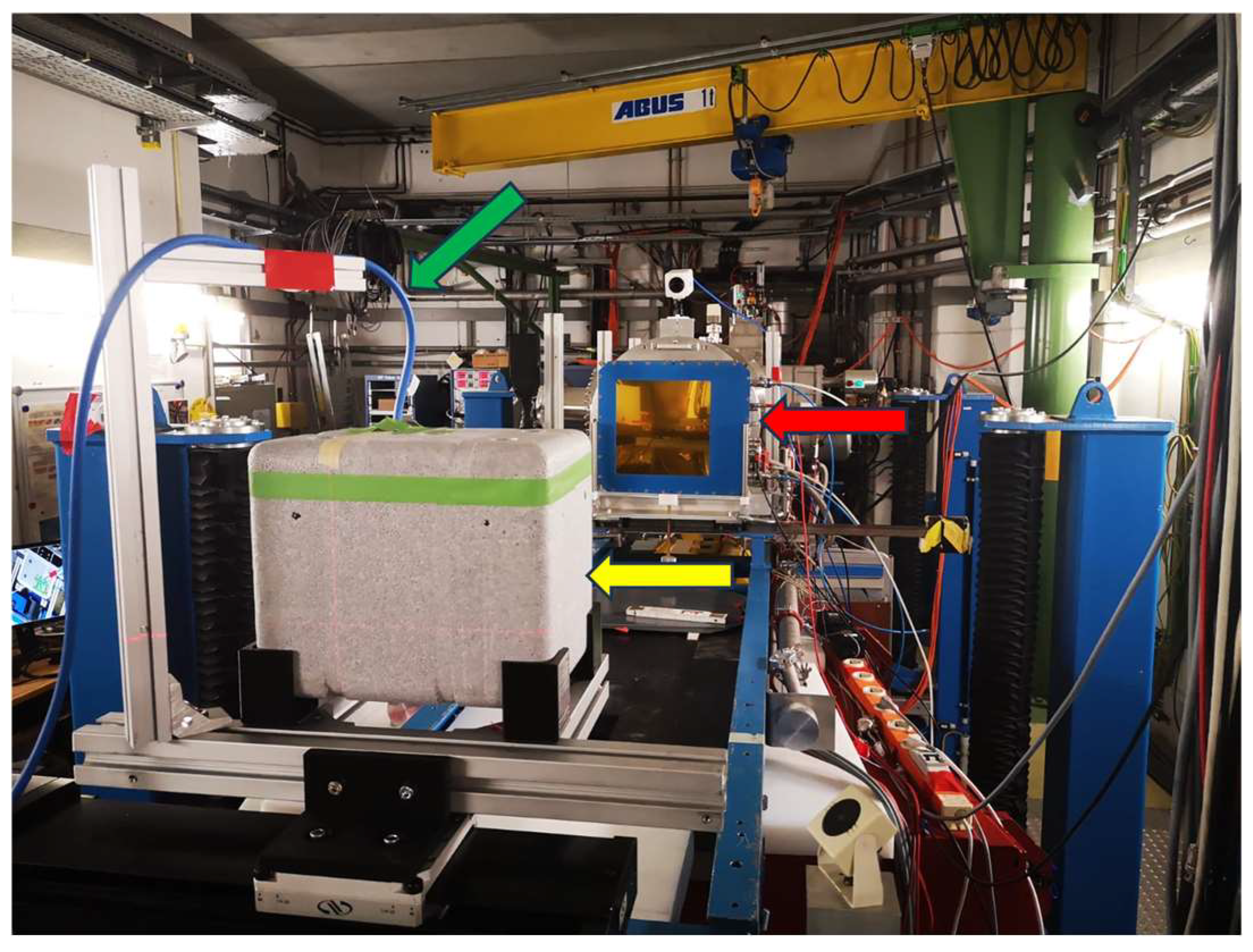
2.2. Virus Inactivation Using Heavy-Ion Beams
2.3. Validation of Irradiation Efficacy
2.4. Validation of Virus Integrity
2.5. Immunization Protocols
2.6. Sample Collection
2.7. Detection of Antigen-Specific Antibodies
2.8. Evaluation of Antigen-Specific Cellular Responses
2.9. Statistical Analysis
3. Results
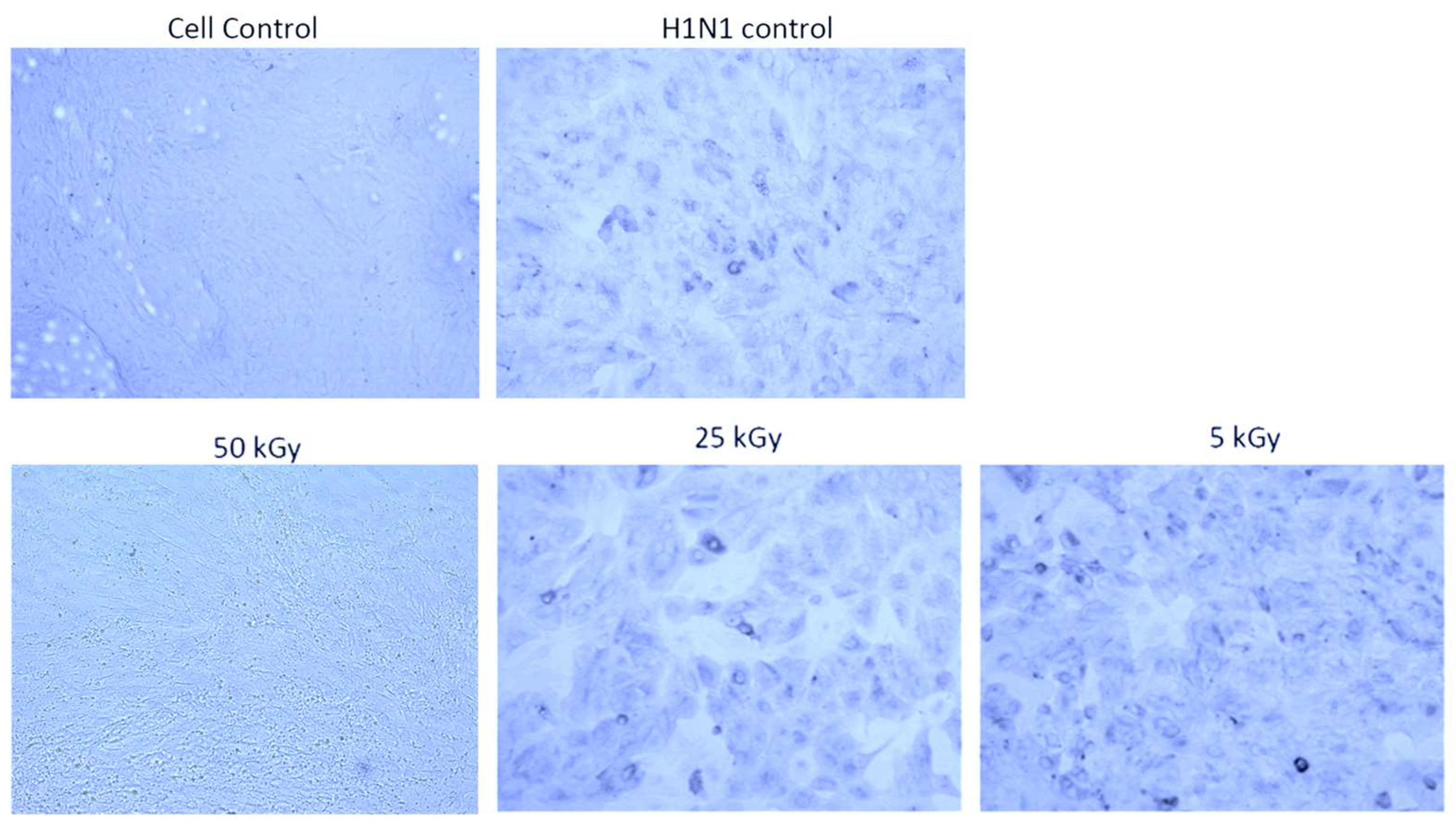
3.1. Irradiation Using a Heavy Ion Beam Efficiently Inactivates Influenza Virus
3.2. Irradiation Using a Heavy Ion Beam Maintains Structural Integrity of the Influenza Virus
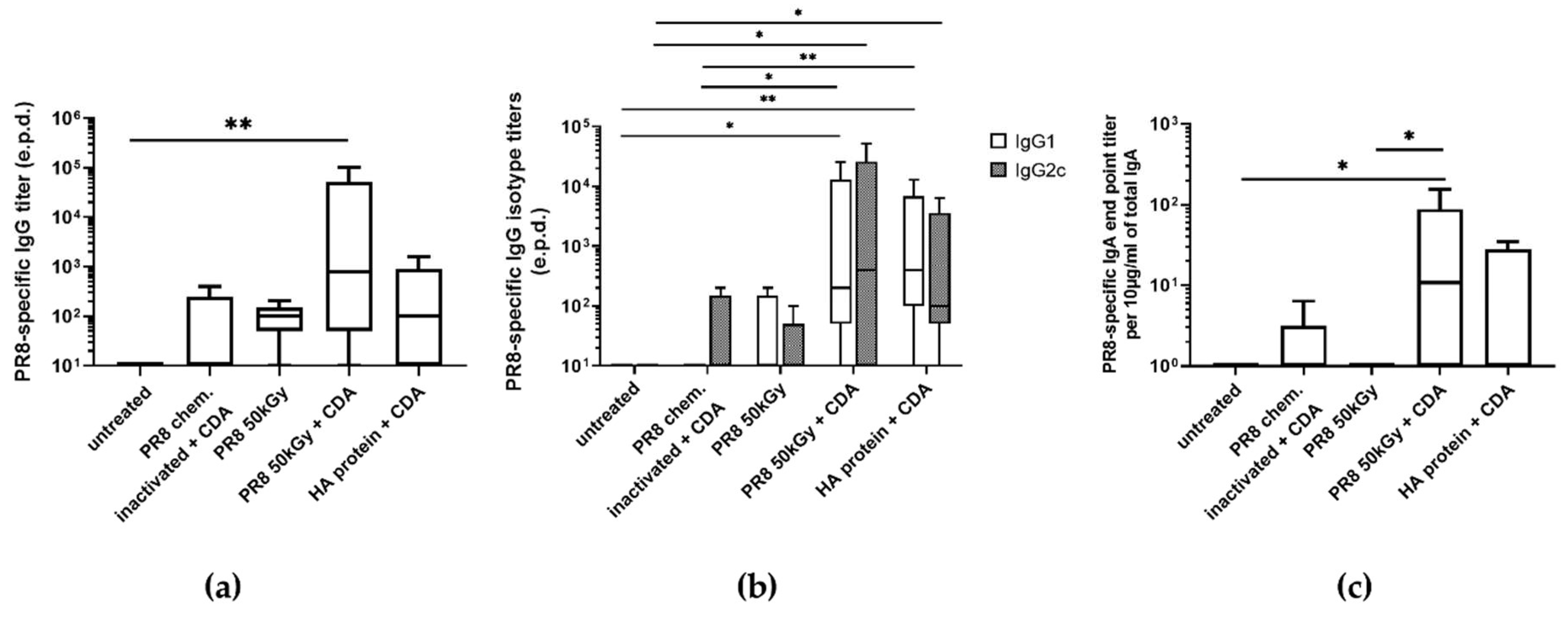
3.3. Immunization of Mice Using Heavy Ion Beam-Inactivated Influenza Virus Stimulated Humoral Immune Responses
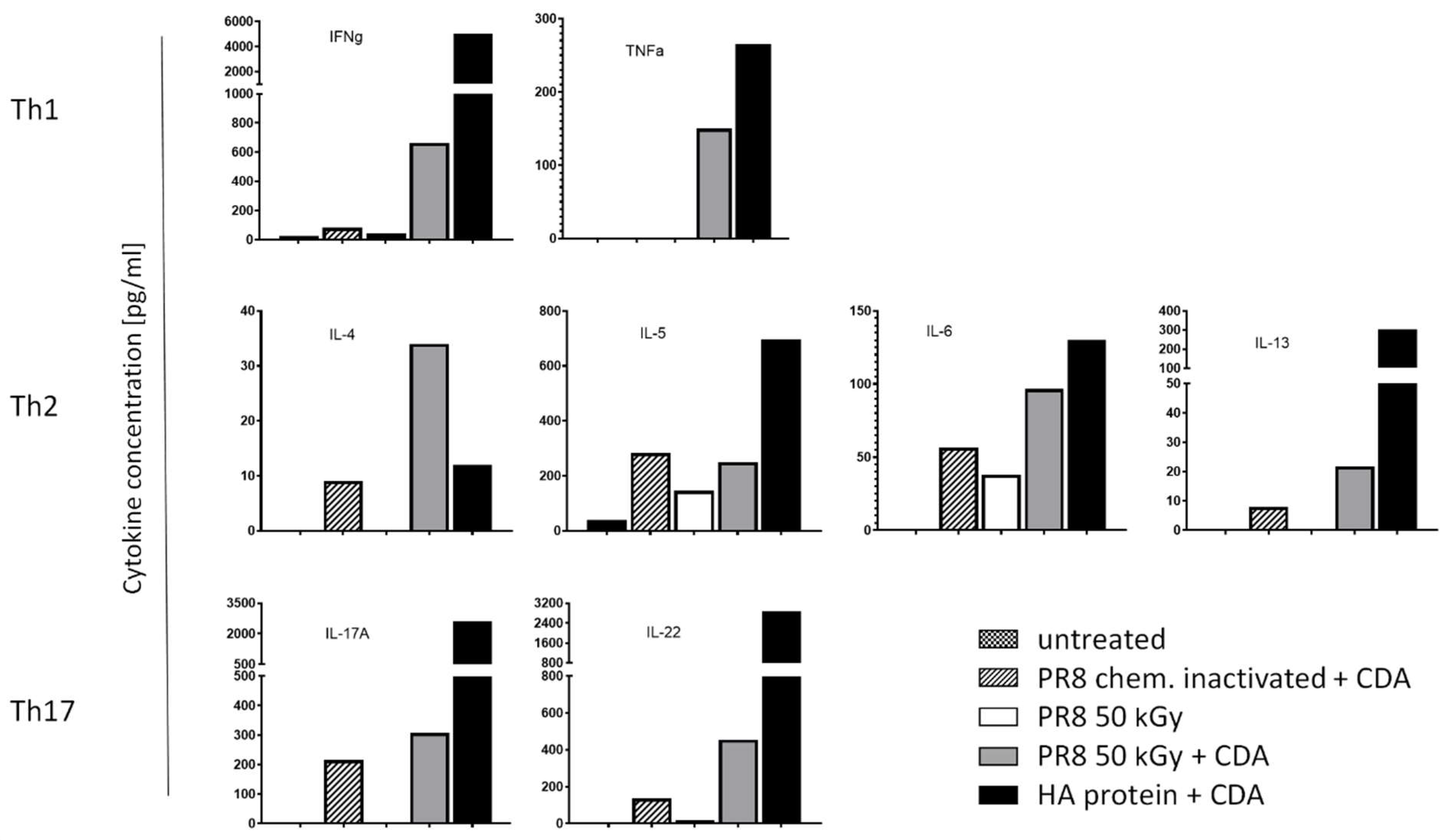
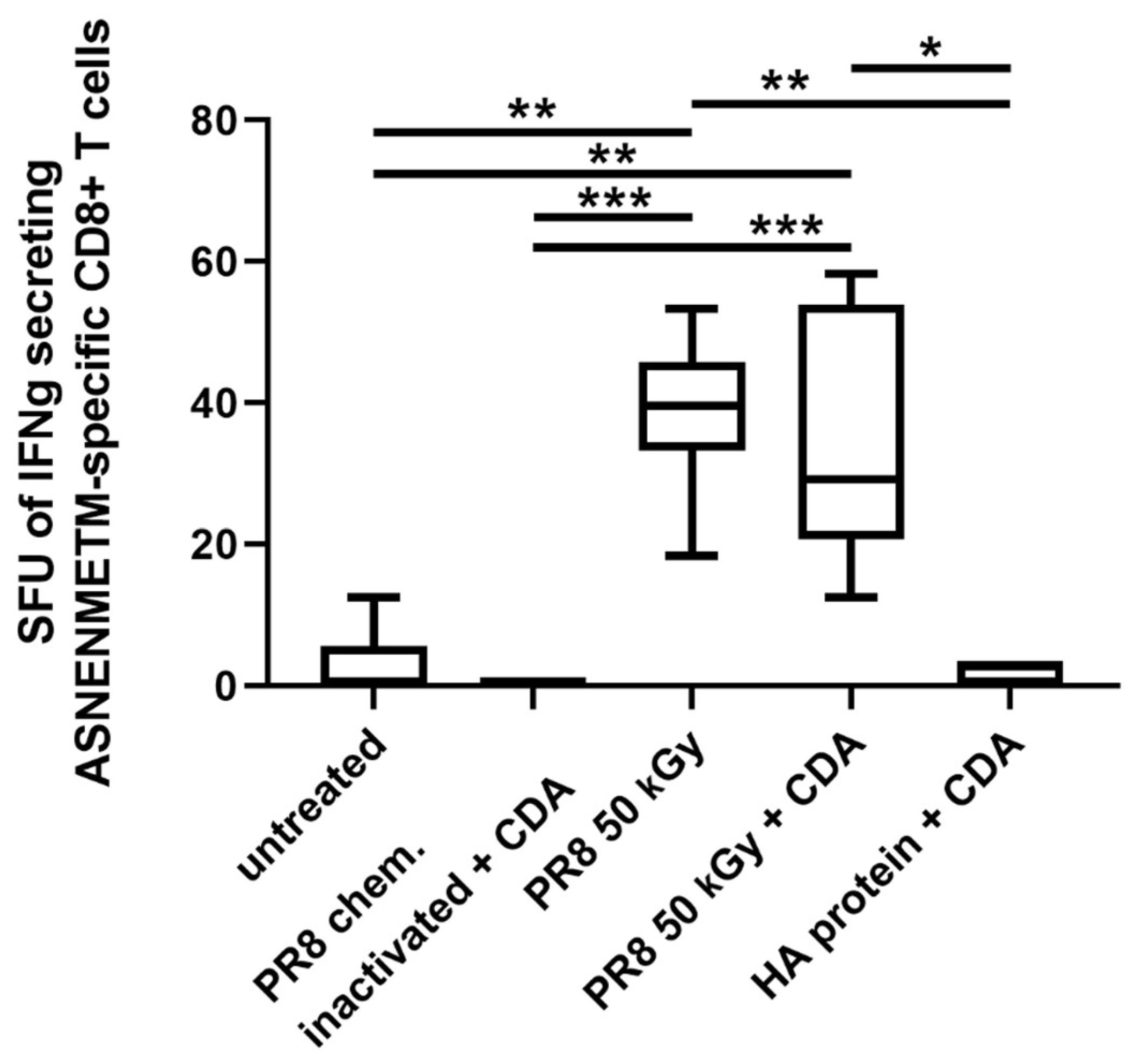
3.4. Immunization of Mice with Influenza Virus Inactivated Using Heavy Ion Beam-Stimulated Antigen-Specific Cellular Immune Responses
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gote, V.; Bolla, P.K.; Kommineni, N.; Butreddy, A.; Nukala, P.K.; Palakurthi, S.S.; Khan, W. A Comprehensive Review of mRNA Vaccines. Int. J. Mol. Sci. 2023, 24, 2700. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Mendonca, L.; Yang, Y.; Gao, Y.; Shen, C.; Liu, J.; Ni, T.; Ju, B.; Liu, C.; Tang, X.; et al. The Architecture of Inactivated SARS-CoV-2 with Postfusion Spikes Revealed by Cryo-EM and Cryo-ET. Structure 2020, 28, 1218–1224.e1214. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Parthasarathy, H.; Sah, V.; Tandel, D.; Vedagiri, D.; Reddy, S.; Harshan, K.H. Inactivation of SARS-CoV-2 by beta-propiolactone causes aggregation of viral particles and loss of antigenic potential. Virus Res. 2021, 305, 198555. [Google Scholar] [CrossRef] [PubMed]
- Bonnafous, P.; Nicolai, M.C.; Taveau, J.C.; Chevalier, M.; Barriere, F.; Medina, J.; Le Bihan, O.; Adam, O.; Ronzon, F.; Lambert, O. Treatment of influenza virus with beta-propiolactone alters viral membrane fusion. Biochim. Biophys. Acta 2014, 1838, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Elveborg, S.; Monteil, V.M.; Mirazimi, A. Methods of Inactivation of Highly Pathogenic Viruses for Molecular, Serology or Vaccine Development Purposes. Pathogens 2022, 11, 271. [Google Scholar] [CrossRef] [PubMed]
- Furuya, Y. Return of inactivated whole-virus vaccine for superior efficacy. Immunol. Cell Biol. 2012, 90, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Morehouse, K.M.; Komolprasert, V. Overview of Irradiation of Food and Packaging. Available online: https://www.fda.gov/food/irradiation-food-packaging/overview-irradiation-food-and-packaging (accessed on 4 March 2024).
- Arama, C.; Troye-Blomberg, M. The path of malaria vaccine development: Challenges and perspectives. J. Intern. Med. 2014, 275, 456–466. [Google Scholar] [CrossRef] [PubMed]
- Pica, N.; Palese, P. Toward a universal influenza virus vaccine: Prospects and challenges. Annu. Rev. Med. 2013, 64, 189–202. [Google Scholar] [CrossRef] [PubMed]
- De Remigis, A.; de Gruijl, T.D.; Uram, J.N.; Tzou, S.C.; Iwama, S.; Talor, M.V.; Armstrong, T.D.; Santegoets, S.J.; Slovin, S.F.; Zheng, L.; et al. Development of thyroglobulin antibodies after GVAX immunotherapy is associated with prolonged survival. Int. J. Cancer 2015, 136, 127–137. [Google Scholar] [CrossRef]
- Le, D.T.; Wang-Gillam, A.; Picozzi, V.; Greten, T.F.; Crocenzi, T.; Springett, G.; Morse, M.; Zeh, H.; Cohen, D.; Fine, R.L.; et al. Safety and survival with GVAX pancreas prime and Listeria Monocytogenes-expressing mesothelin (CRS-207) boost vaccines for metastatic pancreatic cancer. J. Clin. Oncol. 2015, 33, 1325–1333. [Google Scholar] [CrossRef]
- Qin, L.; Smith, B.D.; Tsai, H.L.; Yaghi, N.K.; Neela, P.H.; Moake, M.; Fu, J.; Kasamon, Y.L.; Prince, G.T.; Goswami, M.; et al. Induction of high-titer IgG antibodies against multiple leukemia-associated antigens in CML patients with clinical responses to K562/GVAX immunotherapy. Blood Cancer J. 2013, 3, e145. [Google Scholar] [CrossRef] [PubMed]
- Francis, Z.; Incerti, S.; Zein, S.A.; Lampe, N.; Guzman, C.A.; Durante, M. Monte Carlo Simulation of SARS-CoV-2 Radiation-Induced Inactivation for Vaccine Development. Radiat. Res. 2021, 195, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Zeitlin, C.; Guetersloh, S.B.; Heilbronn, L.H.; Miller, J. Measurements of materials shielding properties with 1GeV/nuc 56Fe. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2006, 252, 308–318. [Google Scholar] [CrossRef]
- Luoni, F.; Weber, U.; Boscolo, D.; Durante, M.; Reidel, C.-A.; Schuy, C.; Zink, K.; Horst, F. Beam Monitor Calibration for Radiobiological Experiments With Scanned High Energy Heavy Ion Beams at FAIR. Front. Phys. 2020, 8, 568145. [Google Scholar] [CrossRef]
- Ebensen, T.; Debarry, J.; Pedersen, G.K.; Blazejewska, P.; Weissmann, S.; Schulze, K.; McCullough, K.C.; Cox, R.J.; Guzman, C.A. Mucosal Administration of Cycle-Di-Nucleotide-Adjuvanted Virosomes Efficiently Induces Protection against Influenza H5N1 in Mice. Front. Immunol. 2017, 8, 1223. [Google Scholar] [CrossRef] [PubMed]
- Schulze, K.; Ebensen, T.; Chandrudu, S.; Skwarczynski, M.; Toth, I.; Olive, C.; Guzman, C.A. Bivalent mucosal peptide vaccines administered using the LCP carrier system stimulate protective immune responses against Streptococcus pyogenes infection. Nanomedicine 2017, 13, 2463–2474. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.; Schulze, K.; Ebensen, T.; Weissmann, S.; Hansen, S.; Guzman, C.A.; Lehr, C.M. Inverse micellar sugar glass (IMSG) nanoparticles for transfollicular vaccination. J. Control. Release 2015, 206, 140–152. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Bao, L.; Mao, H.; Wang, L.; Xu, K.; Yang, M.; Li, Y.; Zhu, L.; Wang, N.; Lv, Z.; et al. Development of an inactivated vaccine candidate for SARS-CoV-2. Science 2020, 369, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, M.O.R. The Efficacy of Three Doses of Live Attenuated, Oral Rotavirus Vaccine 116E; NCT05958771; University of Chile: Santiago, Chile, 2023. [Google Scholar]
- Grant, P. The Effects of Attenuated Versus Inactivated Flu Vaccine in Twin Sets (FLU-TW); Stanford University: Stanford, CA, USA, 2023. [Google Scholar]
- Delrue, I.; Verzele, D.; Madder, A.; Nauwynck, H.J. Inactivated virus vaccines from chemistry to prophylaxis: Merits, risks and challenges. Expert Rev. Vaccines 2012, 11, 695–719. [Google Scholar] [CrossRef]
- Paul-Ehrlich-Institut (PEI). Vaccines for Humans. Available online: https://www.pei.de/EN/medicinal-products/vaccines-human/vaccines-human-node.html (accessed on 5 March 2024).
- Bhatia, S.S.; Pillai, S.D. Ionizing Radiation Technologies for Vaccine Development—A Mini Review. Front. Immunol. 2022, 13, 845514. [Google Scholar] [CrossRef]
- Sanders, B.; Koldijk, M.; Schuitemaker, H. Inactivated Viral Vaccines. Vaccine Analysis: Strategies, Principles, and Control; Springer: Heidelberg, Germany, 2014; Volume 28, pp. 45–80. [Google Scholar] [CrossRef]
- Sir Karakus, G.; Tastan, C.; Dilek Kancagi, D.; Yurtsever, B.; Tumentemur, G.; Demir, S.; Turan, R.D.; Abanuz, S.; Cakirsoy, D.; Seyis, U.; et al. Preclinical efficacy and safety analysis of gamma-irradiated inactivated SARS-CoV-2 vaccine candidates. Sci. Rep. 2021, 11, 5804. [Google Scholar] [CrossRef] [PubMed]
- Astill, J.; Alkie, T.; Yitbarek, A.; Taha-Abdelaziz, K.; Bavananthasivam, J.; Nagy, E.; Petrik, J.J.; Sharif, S. Examination of the effects of virus inactivation methods on the induction of antibody- and cell-mediated immune responses against whole inactivated H9N2 avian influenza virus vaccines in chickens. Vaccine 2018, 36, 3908–3916. [Google Scholar] [CrossRef] [PubMed]
- Furuya, Y.; Regner, M.; Lobigs, M.; Koskinen, A.; Mullbacher, A.; Alsharifi, M. Effect of inactivation method on the cross-protective immunity induced by whole ‘killed’ influenza A viruses and commercial vaccine preparations. J. Gen. Virol. 2010, 91, 1450–1460. [Google Scholar] [CrossRef] [PubMed]
- Sabbaghi, A.; Miri, S.M.; Keshavarz, M.; Zargar, M.; Ghaemi, A. Inactivation methods for whole influenza vaccine production. Rev. Med. Virol. 2019, 29, e2074. [Google Scholar] [CrossRef] [PubMed]
- Mahler, V.; Junker, A.C. Anaphylaxis to additives in vaccines. Allergo J. Int. 2022, 31, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Sadraeian, M.; Zhang, L.; Aavani, F.; Biazar, E.; Jin, D. Viral inactivation by light. eLight 2022, 2, 18. [Google Scholar] [CrossRef] [PubMed]
- Alsharifi, M.; Mullbacher, A. The gamma-irradiated influenza vaccine and the prospect of producing safe vaccines in general. Immunol. Cell Biol. 2010, 88, 103–104. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.Y.; Gao, Y. Killed whole-HIV vaccine; employing a well established strategy for antiviral vaccines. AIDS Res. Ther. 2017, 14, 47. [Google Scholar] [CrossRef] [PubMed]
- Shahrudin, S.; Chen, C.; David, S.C.; Singleton, E.V.; Davies, J.; Kirkwood, C.D.; Hirst, T.R.; Beard, M.; Alsharifi, M. Gamma-irradiated rotavirus: A possible whole virus inactivated vaccine. PLoS ONE 2018, 13, e0198182. [Google Scholar] [CrossRef]
- Tobin, G.J.; Tobin, J.K.; Gaidamakova, E.K.; Wiggins, T.J.; Bushnell, R.V.; Lee, W.M.; Matrosova, V.Y.; Dollery, S.J.; Meeks, H.N.; Kouiavskaia, D.; et al. A novel gamma radiation-inactivated sabin-based polio vaccine. PLoS ONE 2020, 15, e0228006. [Google Scholar] [CrossRef]
- Dollery, S.J.; Zurawski, D.V.; Bushnell, R.V.; Tobin, J.K.; Wiggins, T.J.; MacLeod, D.A.; Tasker, N.; Alamneh, Y.A.; Abu-Taleb, R.; Czintos, C.M.; et al. Whole-cell vaccine candidates induce a protective response against virulent Acinetobacter baumannii. Front. Immunol. 2022, 13, 941010. [Google Scholar] [CrossRef] [PubMed]
- Fertey, J.; Bayer, L.; Grunwald, T.; Pohl, A.; Beckmann, J.; Gotzmann, G.; Casado, J.P.; Schonfelder, J.; Rogner, F.H.; Wetzel, C.; et al. Pathogens Inactivated by Low-Energy-Electron Irradiation Maintain Antigenic Properties and Induce Protective Immune Responses. Viruses 2016, 8, 319. [Google Scholar] [CrossRef] [PubMed]
- Finkensieper, J.; Mayerle, F.; Renteria-Solis, Z.; Fertey, J.; Makert, G.R.; Lange, F.; Besecke, J.; Schopf, S.; Poremba, A.; Konig, U.; et al. Apicomplexan parasites are attenuated by low-energy electron irradiation in an automated microfluidic system and protect against infection with Toxoplasma gondii. Parasitol. Res. 2023, 122, 1819–1832. [Google Scholar] [CrossRef] [PubMed]
- Dima, V.F.; Ionescu, M.D.; Dima, V.S.; Popa, A.; Ionescu, P. Development of an irradiated vaccine that protects against enterotoxigenic Escherichia coli diarrhoea. Roum. Arch. Microbiol. Immunol. 1992, 51, 5–16. [Google Scholar] [PubMed]
- Durante, M.; Schulze, K.; Incerti, S.; Francis, Z.; Zein, S.; Guzmán, C.A. Virus Irradiation and COVID-19 Disease. Front. Phys. 2020, 8, 565861. [Google Scholar] [CrossRef]
- Rafiepour, P.; Sina, S.; Mortazavi, S.M.J. Inactivation of SARS-CoV-2 by charged particles for Future Vaccine Production Applications: A Monte Carlo study. Radiat. Phys. Chem. 2022, 198, 110265. [Google Scholar] [CrossRef] [PubMed]
- Villagomez-Bernabe, B.; Chan, S.W.; Coulter, J.A.; Roseman, A.M.; Currell, F.J. Fast Ion-Beam Inactivation of Viruses, Where Radiation Track Structure Meets RNA Structural Biology. Radiat. Res. 2022, 198, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Bortolami, A.; Mazzetto, E.; Kangethe, R.T.; Wijewardana, V.; Barbato, M.; Porfiri, L.; Maniero, S.; Mazzacan, E.; Budai, J.; Marciano, S.; et al. Protective Efficacy of H9N2 Avian Influenza Vaccines Inactivated by Ionizing Radiation Methods Administered by the Parenteral or Mucosal Routes. Front. Vet. Sci. 2022, 9, 916108. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Seong Seo, H.; Ji, H.J.; Yang, E.; Choi, J.A.; Yang, J.S.; Song, M.; Han, S.H.; Lim, S.; Lim, J.H.; et al. Characterization of humoral and cellular immune features of gamma-irradiated influenza vaccine. Hum. Vaccines Immunother. 2021, 17, 485–496. [Google Scholar] [CrossRef]
- Quan, F.S.; Compans, R.W.; Kang, S.M. Oral vaccination with inactivated influenza vaccine induces cross-protective immunity. Vaccine 2012, 30, 180–188. [Google Scholar] [CrossRef]
- Singleton, E.V.; Gates, C.J.; David, S.C.; Hirst, T.R.; Davies, J.B.; Alsharifi, M. Enhanced Immunogenicity of a Whole-Inactivated Influenza A Virus Vaccine Using Optimised Irradiation Conditions. Front. Immunol. 2021, 12, 761632. [Google Scholar] [CrossRef] [PubMed]
- David, S.C.; Lau, J.; Singleton, E.V.; Babb, R.; Davies, J.; Hirst, T.R.; McColl, S.R.; Paton, J.C.; Alsharifi, M. The effect of gamma-irradiation conditions on the immunogenicity of whole-inactivated Influenza A virus vaccine. Vaccine 2017, 35, 1071–1079. [Google Scholar] [CrossRef] [PubMed]
- Motamedi Sedeh, F.; Khalili, I.; Wijewardana, V.; Unger, H.; Shawrang, P.; Behgar, M.; Moosavi, S.M.; Arbabi, A.; Hosseini, S.M. Improved Whole Gamma Irradiated Avian Influenza Subtype H9N2 Virus Vaccine Using Trehalose and Optimization of Vaccination Regime on Broiler Chicken. Front. Vet. Sci. 2022, 9, 907369. [Google Scholar] [CrossRef] [PubMed]
- Del Campo, J.; Bouley, J.; Chevandier, M.; Rousset, C.; Haller, M.; Indalecio, A.; Guyon-Gellin, D.; Le Vert, A.; Hill, F.; Djebali, S.; et al. OVX836 Heptameric Nucleoprotein Vaccine Generates Lung Tissue-Resident Memory CD8+ T-Cells for Cross-Protection Against Influenza. Front. Immunol. 2021, 12, 678483. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.; Lapuente, D. T Cell Immunity against Influenza: The Long Way from Animal Models Towards a Real-Life Universal Flu Vaccine. Viruses 2021, 13, 199. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.S. Application of radiation technology in vaccines development. Clin. Exp. Vaccine Res. 2015, 4, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Abolaban, F.A.; Djouider, F.M. Gamma irradiation-mediated inactivation of enveloped viruses with conservation of genome integrity: Potential application for SARS-CoV-2 inactivated vaccine development. Open Life Sci. 2021, 16, 558–570. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.P.B.; Linhares, J.H.R.; Dos Santos, T.P.; Pereira, R.C.; Santos, R.T.; da Silva, S.A.; Souza, M.C.O.; da Silva, J.F.A.; Trindade, G.F.; Gomes, V.S.; et al. Inactivated and Immunogenic SARS-CoV-2 for Safe Use in Immunoassays and as an Immunization Control for Non-Clinical Trials. Viruses 2023, 15, 1486. [Google Scholar] [CrossRef] [PubMed]
- Mullbacher, A.; Pardo, J.; Furuya, Y. SARS-CoV-2 Vaccines: Inactivation by Gamma Irradiation for T and B Cell Immunity. Pathogens 2020, 9, 928. [Google Scholar] [CrossRef]
- Turan, R.D.; Tastan, C.; Dilek Kancagi, D.; Yurtsever, B.; Sir Karakus, G.; Ozer, S.; Abanuz, S.; Cakirsoy, D.; Tumentemur, G.; Demir, S.; et al. Gamma-irradiated SARS-CoV-2 vaccine candidate, OZG-38.61.3, confers protection from SARS-CoV-2 challenge in human ACEII-transgenic mice. Sci. Rep. 2021, 11, 15799. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schulze, K.; Weber, U.; Schuy, C.; Durante, M.; Guzmán, C.A. Influenza Virus Inactivated by Heavy Ion Beam Irradiation Stimulates Antigen-Specific Immune Responses. Pharmaceutics 2024, 16, 465. https://doi.org/10.3390/pharmaceutics16040465
Schulze K, Weber U, Schuy C, Durante M, Guzmán CA. Influenza Virus Inactivated by Heavy Ion Beam Irradiation Stimulates Antigen-Specific Immune Responses. Pharmaceutics. 2024; 16(4):465. https://doi.org/10.3390/pharmaceutics16040465
Chicago/Turabian StyleSchulze, Kai, Ulrich Weber, Christoph Schuy, Marco Durante, and Carlos Alberto Guzmán. 2024. "Influenza Virus Inactivated by Heavy Ion Beam Irradiation Stimulates Antigen-Specific Immune Responses" Pharmaceutics 16, no. 4: 465. https://doi.org/10.3390/pharmaceutics16040465
APA StyleSchulze, K., Weber, U., Schuy, C., Durante, M., & Guzmán, C. A. (2024). Influenza Virus Inactivated by Heavy Ion Beam Irradiation Stimulates Antigen-Specific Immune Responses. Pharmaceutics, 16(4), 465. https://doi.org/10.3390/pharmaceutics16040465






