Drug Repurposing of Metformin for the Treatment of Haloperidol-Related Behavior Disorders and Oxidative Stress: A Preliminary Study
Abstract
1. Introduction
2. Materials and Methods
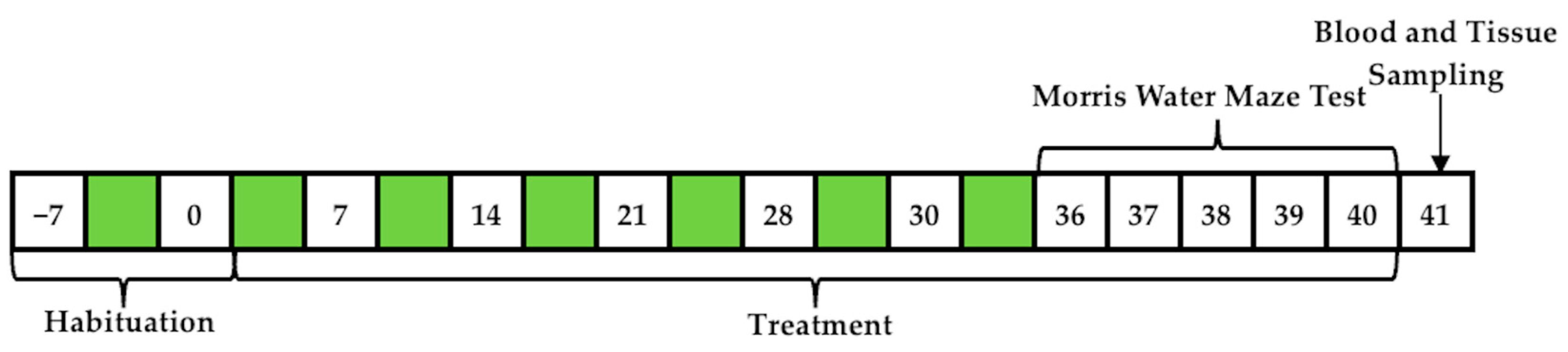
2.1. Animals and Treatment
2.2. Chemicals and Reagents
2.3. Behavioral Assessment
Morris Water Maze
2.4. Experimental Procedures for Collecting Samples
2.4.1. Determination of MDA
2.4.2. Determination of GSH/GSSG Ratio
2.4.3. Immunoassay for BDNF
2.5. Statistical Analysis
3. Results
3.1. Morris Water Maze
3.1.1. Escape Latency
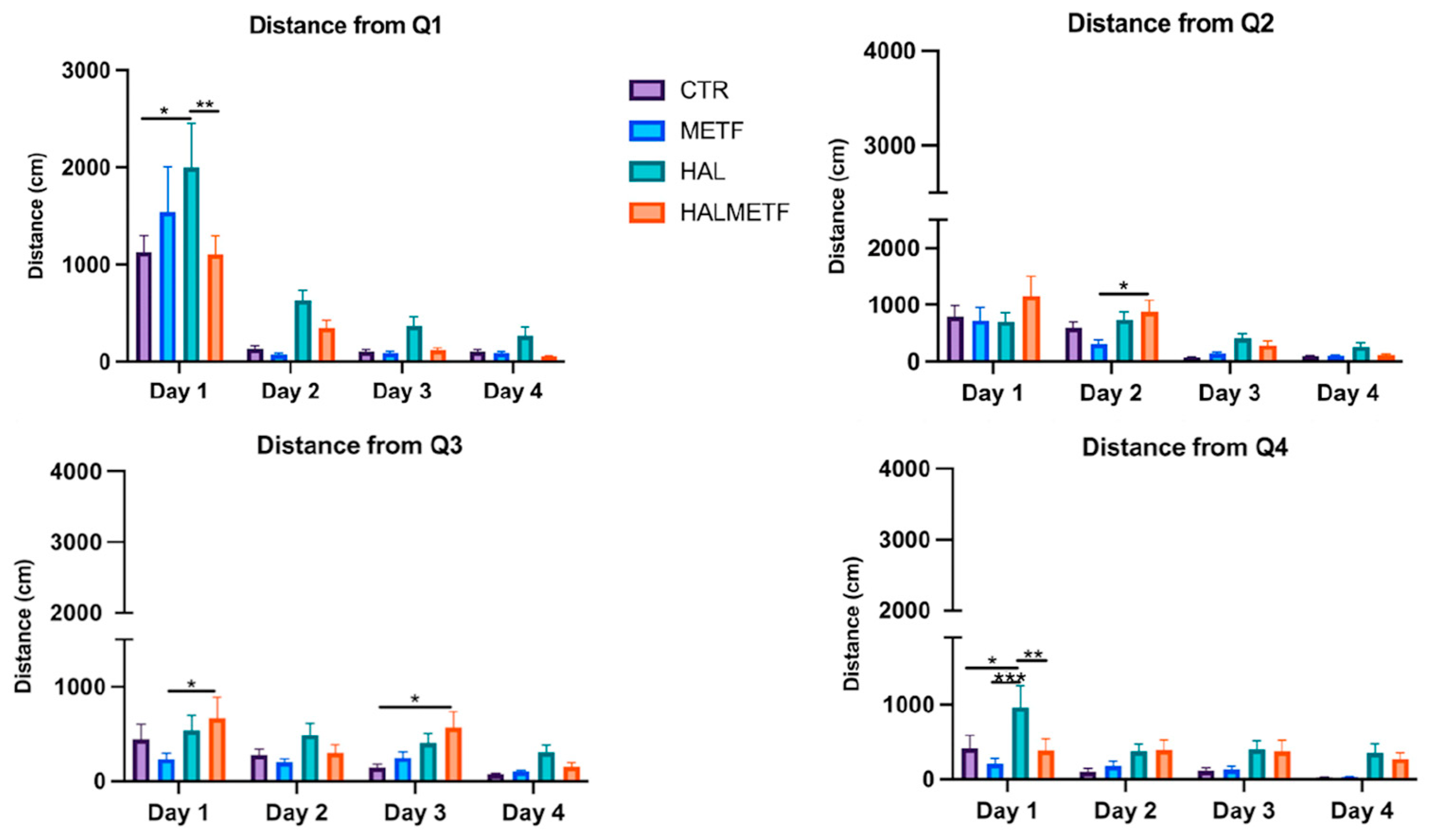
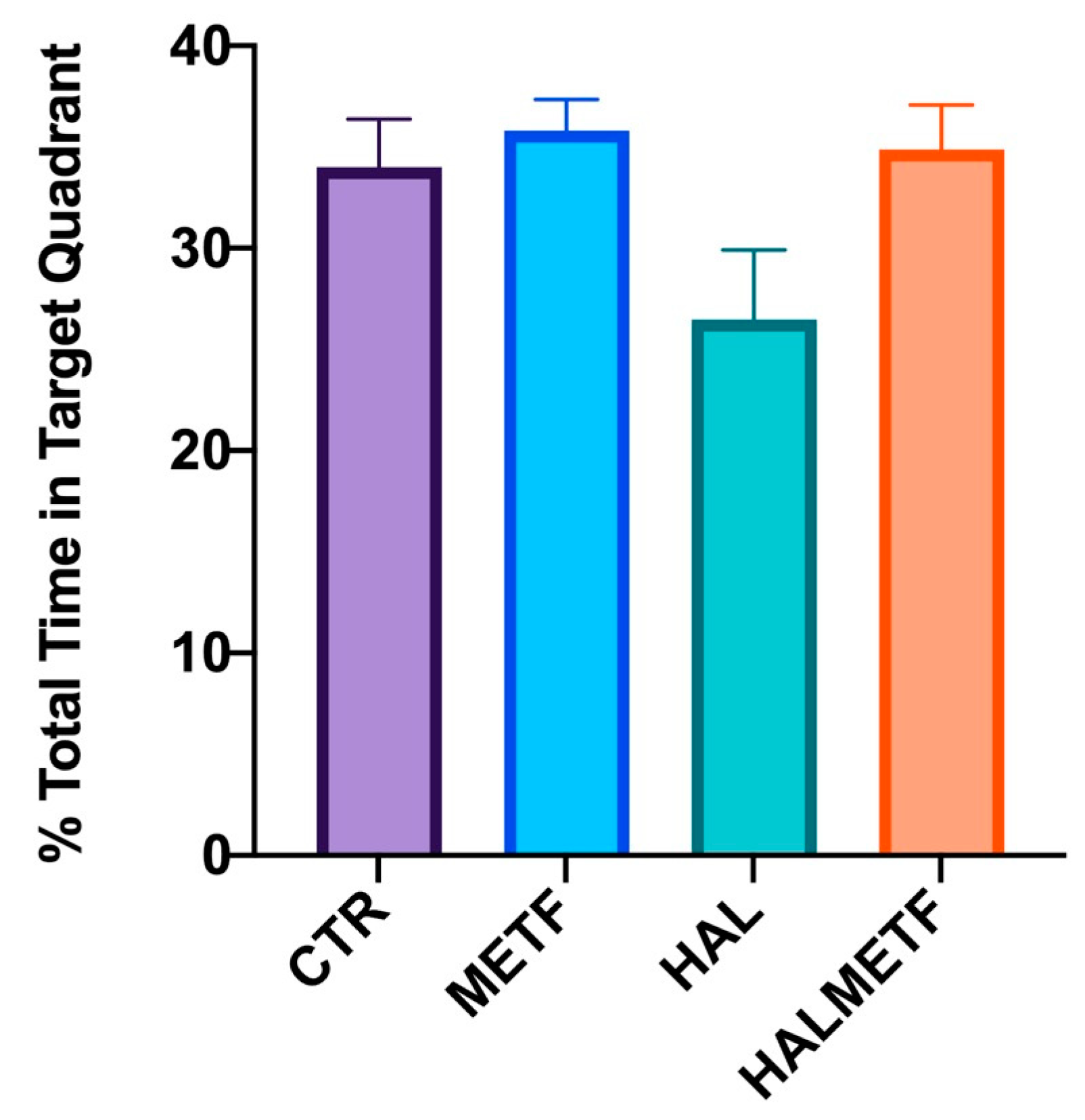
3.1.2. Distance in Quadrant
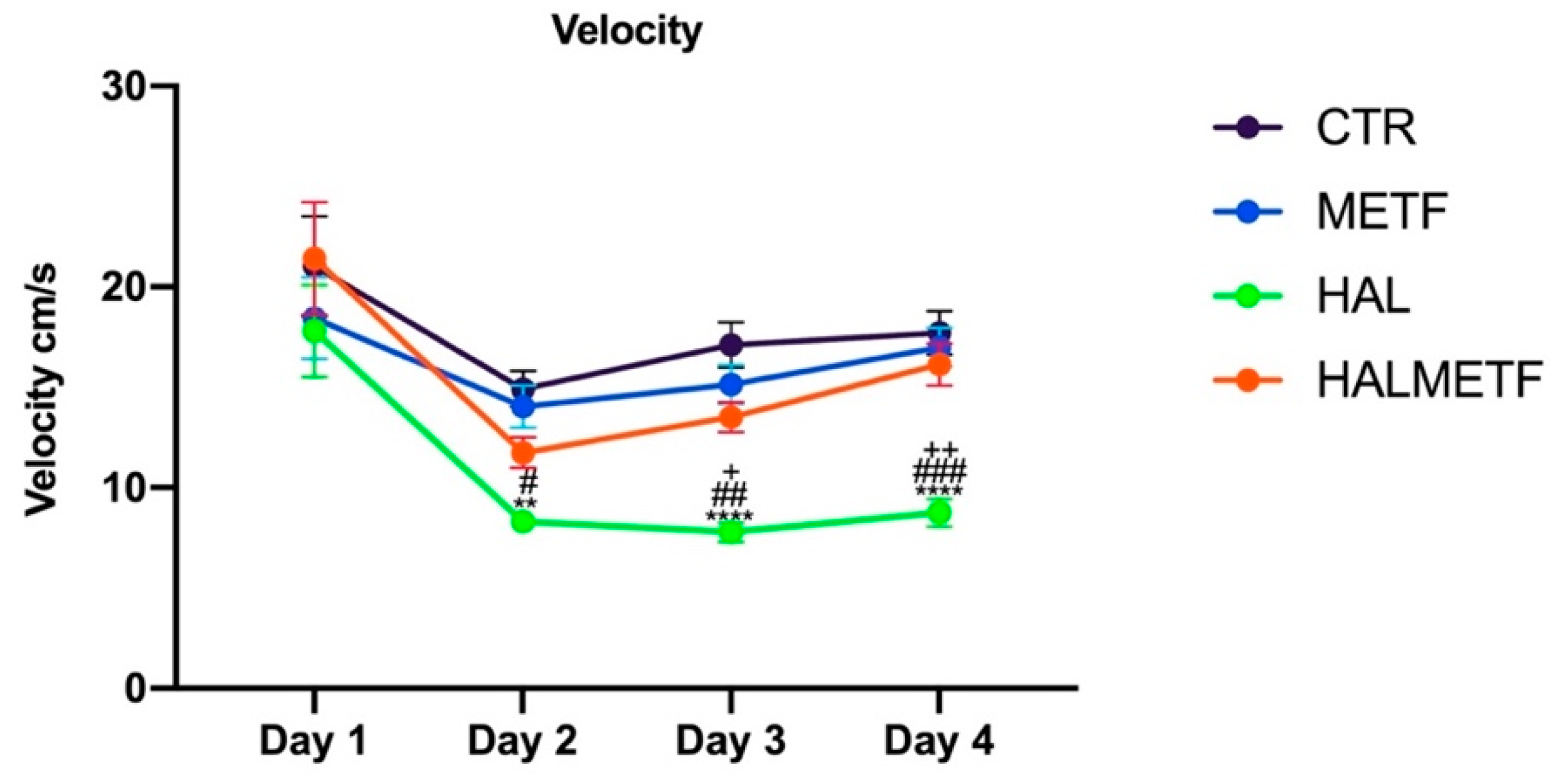
3.1.3. Swim Speed
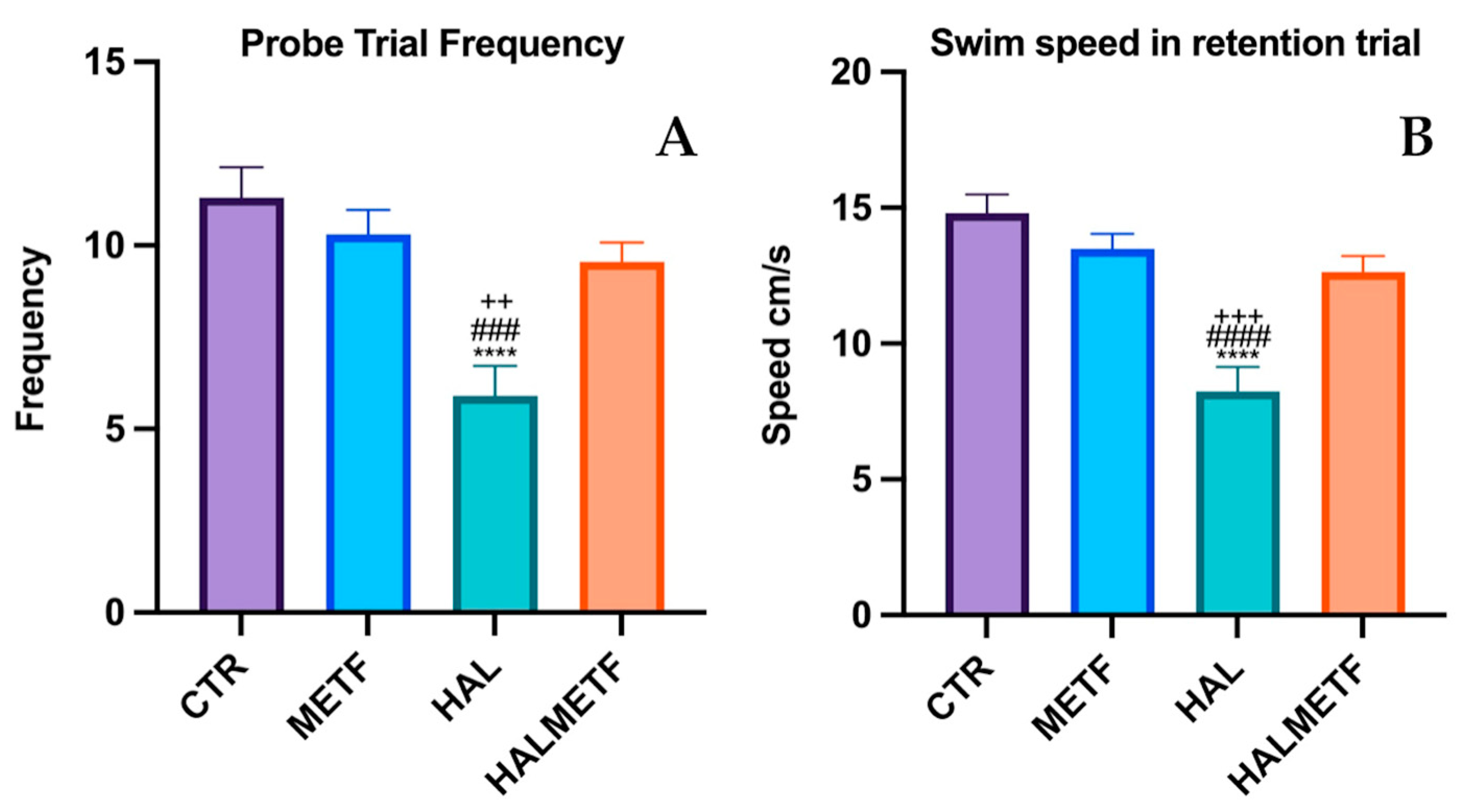
3.1.4. Probe Trial
3.2. Biochemical Parameters
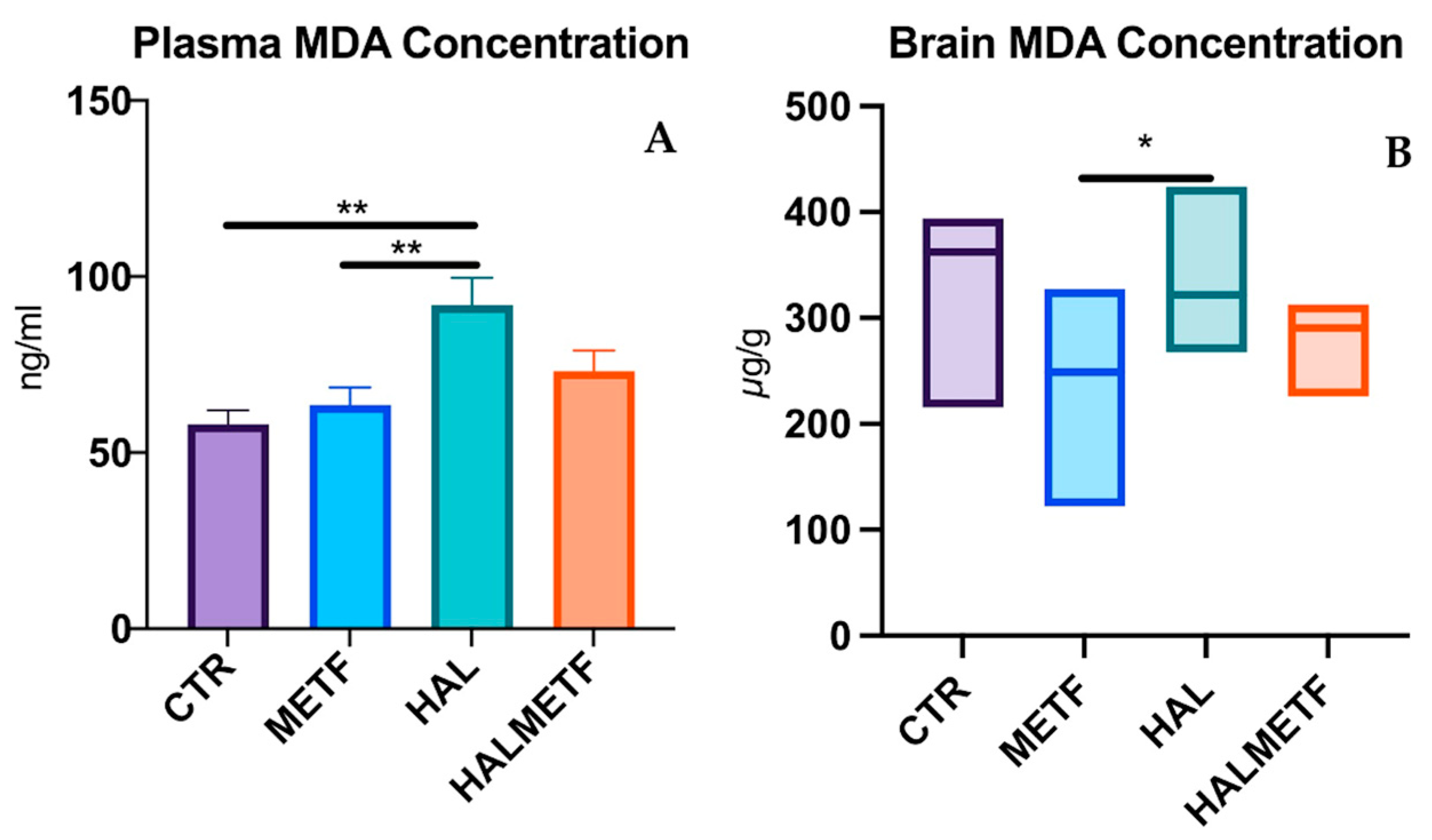
3.2.1. Plasma and Brain Level of MDA
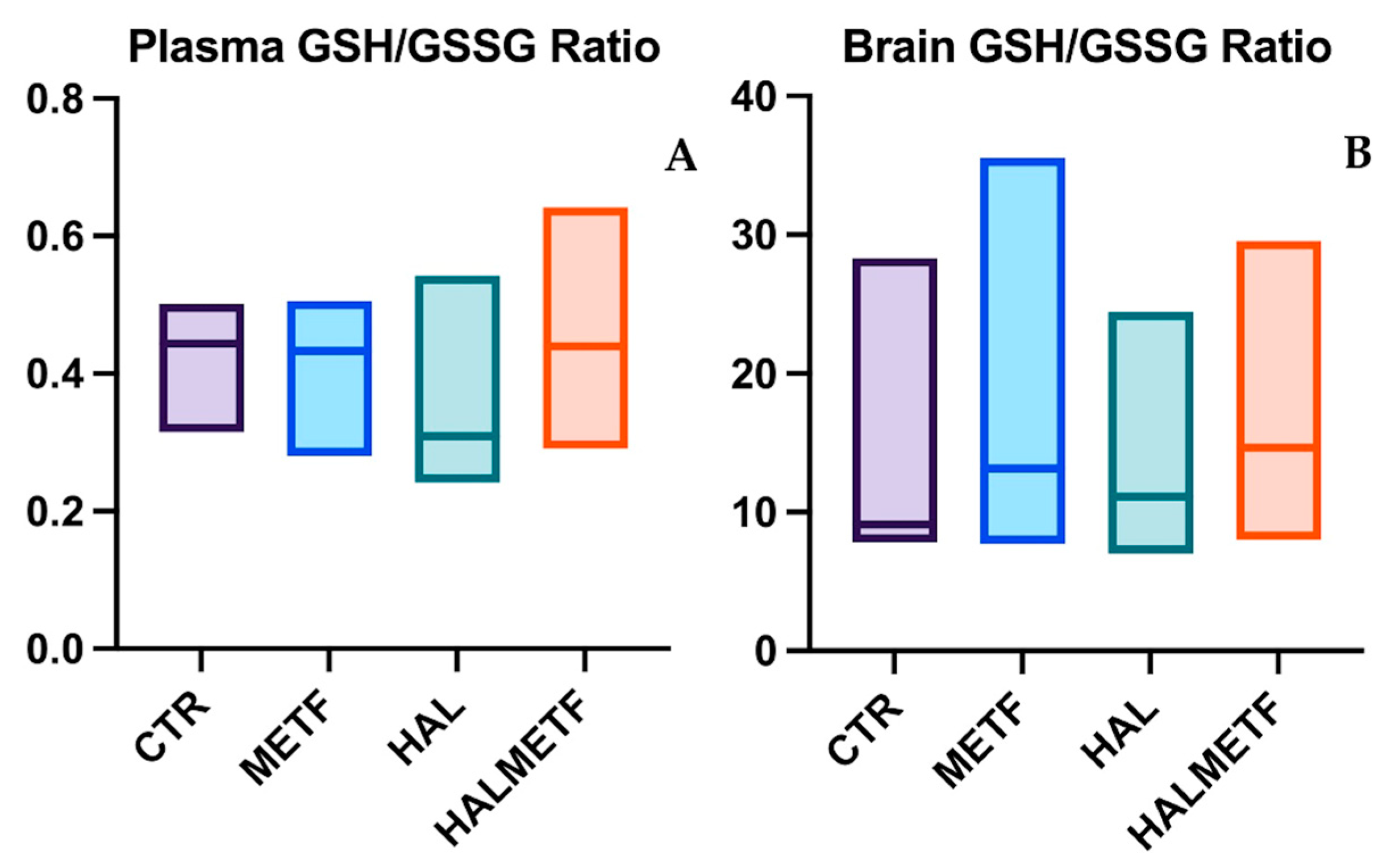
3.2.2. Plasma and Brain GSH/GSSG Ratio
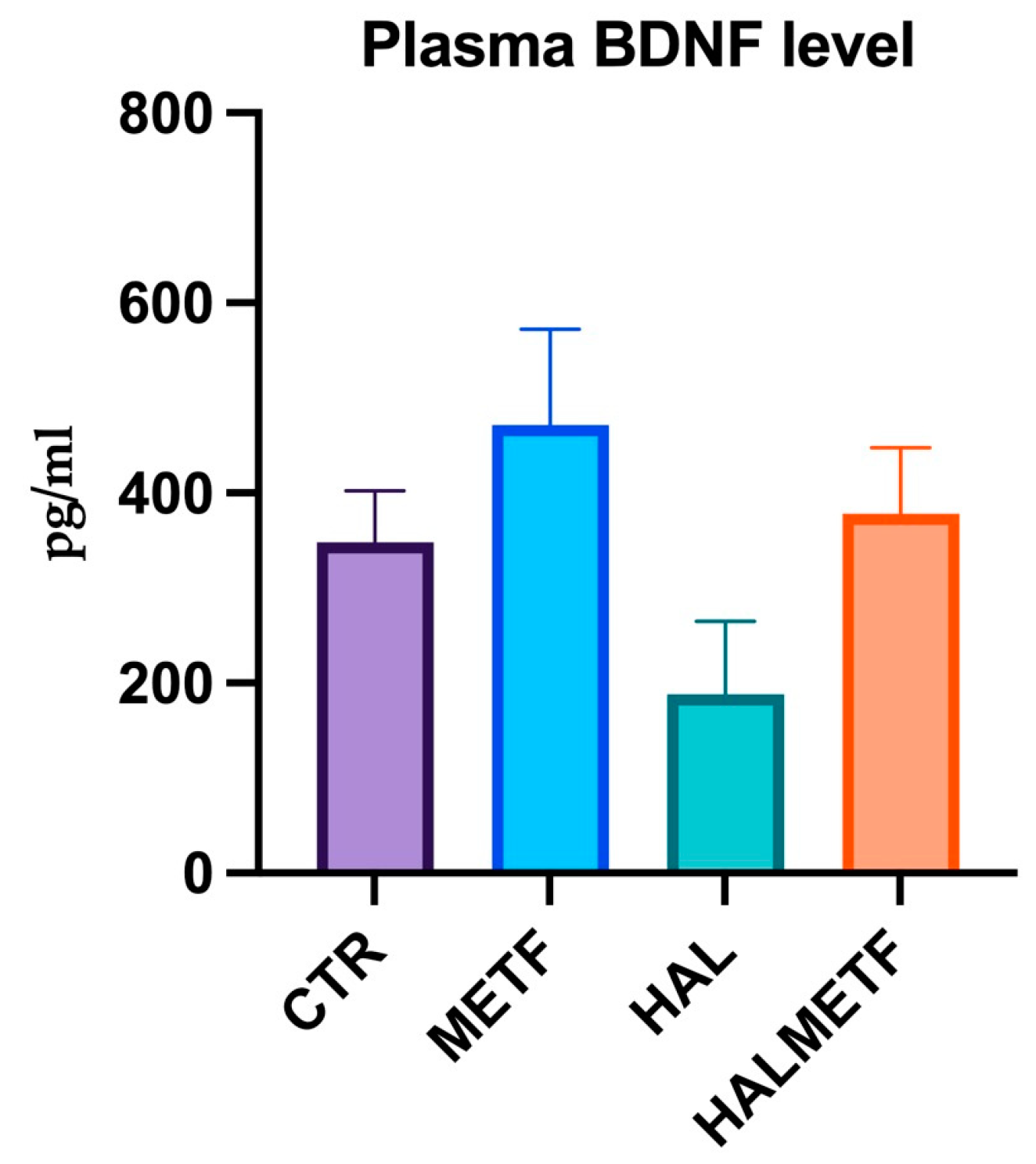
3.2.3. Plasma Level of BDNF
4. Discussion
5. Limitations of the Study
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sportelli, C.; Urso, D.; Jenner, P.; Chaudhuri, K.R. Metformin as a Potential Neuroprotective Agent in Prodromal Parkinson’s Disease-Viewpoint. Fron. Neurol. 2020, 11, 556. [Google Scholar] [CrossRef]
- Sluggett, J.K.; Koponen, M.; Bell, J.S.; Taipale, H.; Tanskanen, A.; Tiihonen, J.; Uusitupa, M.; Tolppanen, A.M.; Hartikainen, S. Metformin and Risk of Alzheimer’s Disease among Community-Dwelling People with Diabetes: A National Case-Control Study. J. Clin. Endocrinol. Metab. 2020, 105, dgz234. [Google Scholar] [CrossRef]
- Ha, J.; Choi, D.W.; Kim, K.J.; Cho, S.Y.; Kim, H.; Kim, K.Y.; Koh, Y.; Nam, C.M.; Kim, E. Association of metformin use with Alzheimer’s disease in patients with newly diagnosed type 2 diabetes: A population-based nested case-control study. Sci. Rep. 2021, 11, 24069. [Google Scholar] [CrossRef]
- Liao, W.; Xu, J.; Li, B.; Ruan, Y.; Li, T.; Liu, J. Deciphering the Roles of Metformin in Alzheimer’s Disease: A Snapshot. Front. Pharmacol. 2022, 12, 728315. [Google Scholar] [CrossRef] [PubMed]
- Isop, L.M.; Neculau, A.E.; Necula, R.D.; Kakucs, C.; Moga, M.A.; Dima, L. Metformin: The Winding Path from Understanding Its Molecular Mechanisms to Proving Therapeutic Benefits in Neurodegenerative Disorders. Pharmaceuticals 2023, 16, 1714. [Google Scholar] [CrossRef] [PubMed]
- Horvath, G.; Kis, G.; Kekesi, G.; Büki, A.; Adlan, L.G.; Szűcs, E.; Heni, H.E.; Benyhe, S. Interaction of clozapine with metformin in a schizophrenia rat model. Sci. Rep. 2021, 11, 16862. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.P.; Lorenzo, C.; Habib, S.L.; Jo, B.; Espinoza, S.E. Differential effects of metformin on age related comorbidities in older men with type 2 diabetes. J. Diabetes Complicat. 2017, 31, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Wang, X.; Ye, X.; Ares, I.; Lopez-Torres, B.; Martínez, M.; Martínez-Larrañaga, M.R.; Wang, X.; Anadón, A.; Martínez, M.A. Mitochondria as an important target of metformin: The mechanism of action, toxic and side effects, and new therapeutic applications. Pharmacol. Res. 2022, 177, 106114. [Google Scholar] [CrossRef] [PubMed]
- Rena, G.; Hardie, D.G.; Pearson, E.R. The mechanisms of action of metformin. Diabetologia 2017, 60, 1577–1585. [Google Scholar] [CrossRef] [PubMed]
- Alhowail, A.H.; Chigurupati, S.; Sajid, S.; Mani, V. Ameliorative effect of metformin on cyclophosphamide-induced memory impairment in mice. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 9660–9666. [Google Scholar] [CrossRef]
- Lu, M.; Chen, H.; Nie, F.; Wei, X.; Tao, Z.; Ma, J. The potential role of metformin in the treatment of Parkinson’s disease. J. Bio-X. 2020, 3, 27–35. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, K.; Huang, K.; Gu, Y.; Hu, Y.; Pan, S.; Ji, Z. Metformin Improves Neurologic Outcome Via AMP-Activated Protein Kinase-Mediated Autophagy Activation in a Rat Model of Cardiac Arrest and Resuscitation. J. Am. Heart Assoc. 2018, 7, e008389. [Google Scholar] [CrossRef] [PubMed]
- Takata, F.; Dohgu, S.; Matsumoto, J.; Machida, T.; Kaneshima, S.; Matsuo, M.; Sakaguchi, S.; Takeshige, Y.; Yamauchi, A.; Kataoka, Y. Metformin induces up-regulation of blood-brain barrier functions by activating AMP-activated protein kinase in rat brain microvascular endothelial cells. Biochem. Biophys. Res. Commun. 2013, 433, 586–590. [Google Scholar] [CrossRef] [PubMed]
- Pintana, H.; Apaijai, N.; Pratchayasakul, W.; Chattipakorn, N.; Chattipakorn, S.C. Effects of metformin on learning and memory behaviors and brain mitochondrial functions in high fat diet induced insulin resistant rats. Life Sci. 2012, 91, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Aksoz, E.; Gocmez, S.S.; Sahin, T.D.; Aksit, D.; Aksit, H.; Utkan, T. The protective effect of metformin in scopolamine-induced learning and memory impairment in rats. Pharmacol. Rep. 2019, 71, 818–825. [Google Scholar] [CrossRef]
- Asadbegi, M.; Yaghmaei, P.; Salehi, I.; Ebrahim-Habibi, A.; Komaki, A. Neuroprotective effects of metformin against Aβ-mediated inhibition of long-term potentiation in rats fed a high-fat diet. Brain Res. Bull. 2016, 121, 178–185. [Google Scholar] [CrossRef]
- Keshavarzi, S.; Kermanshahi, S.; Karami, L.; Motaghinejad, M.; Motevalian, M.; Sadr, S. Protective role of metformin against methamphetamine induced anxiety, depression, cognition impairment and neurodegeneration in rat: The role of CREB/BDNF and Akt/GSK3 signaling pathways. Neurotoxicology 2019, 72, 74–84. [Google Scholar] [CrossRef]
- Ghada, F.S.; Ghada, H.; Monica Gamal, F.; Walaa, I. Neuroprotective Effects of Metformin Versus Selegiline on Parkinson’s Disease Model By Reserpine through the Interrelation of α Synuclein and Antioxidants on Behavioral Changes in Rats. Egypt. J. Basic Clin. Pharmacol. 2019, 9, 101450. [Google Scholar] [CrossRef]
- Markowicz-Piasecka, M.; Sikora, J.; Szydłowska, A.; Skupień, A.; Mikiciuk-Olasik, E.; Huttunen, K.M. Metformin—A Future Therapy for Neurodegenerative Diseases. Pharm. Res. 2017, 34, 2614–2627. [Google Scholar] [CrossRef]
- Shohamy, D.; Adcock, R.A. Dopamine and adaptive memory. Trends Cogn. Sci. 2010, 14, 464–472. [Google Scholar] [CrossRef]
- Reilly, J.L.; Harris, M.S.; Khine, T.T.; Keshavan, M.S.; Sweeney, J.A. Antipsychotic drugs exacerbate impairment on a working memory task in first-episode schizophrenia. Biol. Psychiatry. 2007, 62, 818–821. [Google Scholar] [CrossRef] [PubMed]
- Grober, E.; Hall, C.B.; Lipton, R.B.; Zonderman, A.B.; Resnick, S.M.; Kawas, C. Memory impairment, executive dysfunction, and intellectual decline in preclinical Alzheimer’s disease. J. Int. Neuropsychol. Soc. 2008, 14, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Nakahata, N.; Nakamura, T.; Kawarabayashi, T.; Seino, Y.; Ichii, S.; Ikeda, Y.; Amari, M.; Takatama, M.; Murashita, K.; Ihara, K.; et al. Age-Related Cognitive Decline and Prevalence of Mild Cognitive Impairment in the Iwaki Health Promotion Project. J. Alzheimers Dis. 2021, 84, 1233–1245. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.M.; Leem, Y.H. Chronic stress-induced memory deficits are reversed by regular exercise via AMPK-mediated BDNF induction. Neuroscience 2016, 324, 271–285. [Google Scholar] [CrossRef]
- MacKenzie, N.E.; Kowalchuk, C.; Agarwal, S.M.; Costa-Dookhan, K.A.; Caravaggio, F.; Gerretsen, P.; Chintoh, A.; Remington, G.J.; Taylor, V.H.; Müeller, D.J.; et al. Antipsychotics, Metabolic Adverse Effects, and Cognitive Function in Schizophrenia. Front. Psychiatry. 2018, 9, 622. [Google Scholar] [CrossRef]
- Adem, A.; Madjid, N.; Stiedl, O.; Bonito-Oliva, A.; Konradsson-Geuken, Å.; Holst, S.; Fisone, G.; Ögren, S.O. Atypical but not typical antipsychotic drugs ameliorate phencyclidine-induced emotional memory impairments in mice. Eur. Neuropsychopharmacol. 2019, 29, 616–628. [Google Scholar] [CrossRef]
- Trask, S.; Dulka, B.N.; Helmstetter, F.J. Age-Related Memory Impairment Is Associated with Increased zif268 Protein Accumulation and Decreased Rpt6 Phosphorylation. Int. J. Mol. Sci. 2020, 21, 5352. [Google Scholar] [CrossRef]
- Lisica, D.; Koso-Drljević, M.; Stürmer, B.; Džubur, A.; Valt, C. Working memory impairment in relation to the severity of anxiety symptoms. Cogn. Emot. 2022, 36, 1093–1108. [Google Scholar] [CrossRef]
- Marwari, S.; Dawe, G.S. Effects of haloperidol on cognitive function and behavioural flexibility in the IntelliCage social home cage environment. Behav. Brain Res. 2019, 371, 111976. [Google Scholar] [CrossRef]
- Sykes, D.A.; Moore, H.; Stott, L.; Holliday, N.; Javitcs, J.A.; Lane, J.R.; Charlton, S.J. Extrapyramidal side effects of antipsychotics are linked to their association kinetics at dopamine D2 receptors. Nat. Commun. 2017, 8, 763. [Google Scholar] [CrossRef] [PubMed]
- Hill, S.K.; Bishop, J.R.; Palumbo, D.; Sweeney, J.A. Effect of second-generation antipsychotics on cognition: Current issues and future challenges. Expert. Rev. Neurother. 2010, 10, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Terry, A.; Hill, W.; Parikh, V.; Evans, D.; Waller, J.; Mahadik, S. Differential effects of chronic haloperidol and olanzapine exposure on brain cholinergic markers and spatial learning in rats. Psychopharmacology 2002, 164, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Mihaylova, A.S.; Kostadinov, I.D.; Doncheva, N.D.; Zlatanova, H.I.; Delev, D.P. Effects of Pramipexole on Learning and Memory Processes in Naïve and Haloperidol-challenged Rats in Active Avoidance Test. Folia Med. 2019, 61, 258–265. [Google Scholar] [CrossRef]
- Saeedi, H.; Remington, G.; Christensen, B.K. Impact of haloperidol, a dopamine D2 antagonist, on cognition and mood. Schizophr. Res. 2006, 85, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Stuchlik, A.; Rehakova, L.; Telensky, P.; Vales, K. Morris water maze learning in Long-Evans rats is differentially affected by blockade of D1-like and D2-like dopamine receptors. Neurosci. Lett. 2007, 422, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Kemppainen, N.; Laine, M.; Laakso, M.P.; Kaasinen, V.; Någren, K.; Vahlberg, T.; Kurki, T.; Rinne, J.O. Hippocampal dopamine D2 receptors correlate with memory functions in Alzheimer’s disease. Eur. J. Neurosci. 2003, 18, 149–154. [Google Scholar] [CrossRef]
- Joy, C.B.; Adams, C.E.; Lawrie, S.M. Haloperidol versus placebo for schizophrenia. Cochrane Database Syst. Rev. 2006, 4, CD003082. [Google Scholar] [CrossRef]
- Raudenska, M.; Gumulec, J.; Babula, P.; Stracina, T.; Sztalmachova, M.; Polanska, H.; Adam, V.; Kizek, R.; Novakova, M.; Masarik, M. Haloperidol cytotoxicity and its relation to oxidative stress. Mini Rev. Med. Chem. 2013, 13, 1993–1998. [Google Scholar] [CrossRef]
- Sally, A.; El-Awdan, G.A.; Abdel, J.; Dalia, O.S. Alleviation of haloperidol induced oxidative stress in rats: Effects of sucrose vs grape seed extract. Bull. Fac. Pharm. Cairo Univ. 2015, 53, 29–35. [Google Scholar] [CrossRef]
- Alzoubi, K.H.; Mayyas, F.A.; Mahafzah, R.; Khabour, O.F. Melatonin prevents memory impairment induced by high-fat diet: Role of oxidative stress. Behav. Brain Res. 2018, 336, 93–98. [Google Scholar] [CrossRef]
- Tseng, H.C.; Wang, M.H.; Fang, C.H.; Lin, Y.W.; Soung, H.S. Involvement of Antioxidant and Prevention of Mitochondrial Dysfunction, Anti-Neuroinflammatory Effect and Anti-Apoptotic Effect: Betaine Ameliorates Haloperidol-Induced Orofacial Dyskinesia in Rats. Brain Sci. 2023, 13, 1064. [Google Scholar] [CrossRef]
- Samad, N.; Haleem, D.J. Antioxidant effects of rice bran oil mitigate repeated haloperidol-induced tardive dyskinesia in male rats. Metab. Brain Dis. 2017, 32, 1099–1107. [Google Scholar] [CrossRef]
- Kajero, J.A.; Seedat, S.; Ohaeri, J.U.; Akindele, A.; Aina, O. The effects of cannabidiol on behavioural and oxidative stress parameters induced by prolonged haloperidol administration. Acta Neuropsychiatr. 2022, 1–11. [Google Scholar] [CrossRef]
- Park, S.W.; Lee, C.H.; Lee, J.G.; Lee, S.J.; Kim, N.R.; Choi, S.M.; Kim, Y.H. Differential effects of ziprasidone and haloperidol on immobilization stress-induced mRNA BDNF expression in the hippocampus and neocortex of rats. J. Psychiatr. Res. 2009, 43, 274–281. [Google Scholar] [CrossRef]
- Parikh, V.; Khan, M.M.; Mahadik, S.P. Olanzapine counteracts reduction of brain-derived neurotrophic factor and TrkB receptors in rat hippocampus produced by haloperidol. Neurosci. Lett. 2004, 356, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Cunha, C.; Brambilla, R.; Thomas, K.L. A simple role for BDNF in learning and memory? Front. Mol. Neurosci. 2010, 3, 865. [Google Scholar] [CrossRef]
- Bekinschtein, P.; Cammarota, M.; Medina, J.H. BDNF and memory processing. Neuropharmacology 2014, 76, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Parikh, V.; Khan, M.M.; Mahadik, S.P. Differential effects of antipsychotics on expression of antioxidant enzymes and membrane lipid peroxidation in rat brain. J. Psychiatr. Res. 2003, 37, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Yu, H.; Liu, J.; Chen, Y.; Wang, Q.; Xiang, L. Metformin attenuates hyperalgesia and allodynia in rats with painful diabetic neuropathy induced by streptozotocin. Eur. J. Pharmacol. 2015, 764, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Diniz Vilela, D.; Gomes Peixoto, L.; Teixeira, R.R.; Belele Baptista, N.; Carvalho Caixeta, D.; Vieira de Souza, A.; Machado, H.L.; Pereira, M.N.; Sabino-Silva, R.; Espindola, F.S. The Role of Metformin in Controlling Oxidative Stress in Muscle of Diabetic Rats. Oxid. Med. Cell Longev. 2016, 2016, 6978625. [Google Scholar] [CrossRef] [PubMed]
- Gamad, N.; Malik, S.; Suchal, K.; Vasisht, S.; Tomar, A.; Arava, S.; Arya, D.S.; Bhatia, J. Metformin alleviates bleomycin-induced pulmonary fibrosis in rats: Pharmacological effects and molecular mechanisms. Biomed. Pharmacother. 2018, 97, 1544–1553. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, H.; Jahanbazi, F.; Sadeghi, H.; Omidifar, N.; Alipoor, B.; Kokhdan, E.P.; Mousavipoor, S.M.; Mousavi-Fard, S.H.; Doustimotlagh, A.H. Metformin attenuates oxidative stress and liver damage after bile duct ligation in rats. Res. Pharm. Sci. 2019, 14, 122–129. [Google Scholar] [CrossRef]
- Zaremba, M.; Rosiak, M.; Cudna, A.; Kaplon-Cieslicka, A.; Opolski, G.; Filipiak, K.J.; Malek, L.; Postula, M. Serum Brain-Derived Neurotrophic Factor is Related to Platelet Reactivity and Metformin Treatment in Adult Patients with Type 2 Diabetes Mellitus. Can. J. Diabetes 2019, 43, 19–26. [Google Scholar] [CrossRef]
- Inostroza, M.; Cid, E.; Brotons-Mas, J.; Gal, B.; Aivar, P.; Uzcategui, Y.G.; Sandi, C.; de la Prida, L.M. Hippocampal-Dependent Spatial Memory in the Water Maze is Preserved in an Experimental Model of Temporal Lobe Epilepsy in Rats. PLoS ONE 2011, 6, e22372. [Google Scholar] [CrossRef] [PubMed]
- Fogarasi, E.; Croitoru, M.D.; Fülöp, I.; Nemes-Nagy, E.; Tripon, R.G.; Simon-Szabo, Z.; Muntean, D.L. Malondialdehyde levels can be measured in serum and saliva by using a fast HPLC method with visible detection/Determinarea printr-o metodă HPLC-VIS rapidă a concentraţiilor serice şi salivare ale malondialdehidei. Rev. Romana Medicina Lab. 2016, 24, 319–326. [Google Scholar] [CrossRef]
- Jîtcă, G.; Fogarasi, E.; Ősz, B.-E.; Vari, C.E.; Tero-Vescan, A.; Miklos, A.; Bătrînu, M.-G.; Rusz, C.M.; Croitoru, M.D.; Dogaru, M.T. A Simple HPLC/DAD Method Validation for the Quantification of Malondialdehyde in Rodent’s Brain. Molecules 2021, 26, 5066. [Google Scholar] [CrossRef]
- Jîtcă, G.; Fogarasi, E.; Ősz, B.-E.; Vari, C.E.; Fülöp, I.; Croitoru, M.D.; Rusz, C.M.; Dogaru, M.T. Profiling the Concentration of Reduced and Oxidized Glutathione in Rat Brain Using HPLC/DAD Chromatographic System. Molecules 2021, 26, 6590. [Google Scholar] [CrossRef]
- Ouslimani, N.; Peynet, J.; Bonnefont-Rousselot, D.; Thérond, P.; Legrand, A.; Beaudeux, J.L. Metformin decreases intracellular production of reactive oxygen species in aortic endothelial cells. Metabolism 2005, 54, 829–834. [Google Scholar] [CrossRef]
- Lu, X.Y.; Huang, S.; Chen, Q.B.; Zhang, D.; Li, W.; Ao, R.; Leung, F.C.; Zhang, Z.; Huang, J.; Tang, Y.; et al. Metformin Ameliorates Aβ Pathology by Insulin-Degrading Enzyme in a Transgenic Mouse Model of Alzheimer’s Disease. Oxid. Med. Cell Longev. 2020, 2020, 2315106. [Google Scholar] [CrossRef]
- Nataya, S.; Kornrawee, S.; Salinee, N.; Pornthip, C.; Anusara, A.; Apiwat, S.; Wanassanan, P.; Peter, W.; Jariya, U.W. Effect of metformin treatment on memory and hippocampal neurogenesis decline correlated with oxidative stress induced by methotrexate in rats. Biomed. Pharmacother. 2021, 144, 112280. [Google Scholar] [CrossRef]
- Perera, J.; Tan, J.H.; Jeevathayaparan, S.; Srikumar, C.; Nagaraja, H. Neuroprotective Effects of Alpha Lipoic Acid on Haloperidol-Induced Oxidative Stress in the Rat Brain. Cell Biosci. 2011, 1, 12. [Google Scholar] [CrossRef]
- Valvassori, S.S.; Cararo, J.H.; Menegas, S.; Possamai-Della, T.; Aguiar-Geraldo, J.M.; Araujo, S.L.; Mastella, G.A.; Quevedo, J.; Zugno, A.I. Haloperidol elicits oxidative damage in the brain of rats submitted to the ketamine-induced model of schizophrenia. Brain Res. Bull. 2021, 170, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Chokhawala, K.; Stevens, L. Antipsychotic Medications. [Updated 2023 Feb 26]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK519503/ (accessed on 2 March 2024).
- Kamyar, M.; Razavi, B.M.; Hasani, F.V.; Mehri, S.; Foroutanfar, A.; Hosseinzadeh, H. Crocin prevents haloperidol-induced orofacial dyskinesia: Possible an antioxidant mechanism. Iran. J. Basic. Med. Sci. 2016, 19, 1070–1079. [Google Scholar]
- de Araújo, D.P.; Camboim, T.G.M.; Silva, A.P.M.; Silva, C.D.F.; de Sousa, R.C.; Barbosa, M.D.A.; Oliveira, L.C.; Cavalcanti, J.R.L.P.; Lucena, E.E.S.; Guzen, F.P. Behavioral and neurochemical effects of alpha lipoic acid associated with omega-3 in tardive dyskinesia induced by chronic haloperidol in rats. Can. J. Physiol. Pharmacol. 2017, 95, 837–843. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, D.; Goswami, S.; Gahalain, N. Protective effect of hesperetin against haloperidol-induced orofacial dyskinesia and catalepsy in rats. Nutr. Neurosci. 2018, 21, 667–675. [Google Scholar] [CrossRef]
- Pillai, A.; Parikh, V.; Terry, A.V., Jr.; Mahadik, S.P. Long-term antipsychotic treatments and crossover studies in rats: Differential effects of typical and atypical agents on the expression of antioxidant enzymes and membrane lipid peroxidation in rat brain. J. Psychiatr. Res. 2007, 41, 372–386. [Google Scholar] [CrossRef]
- Vairetti, M.; Ferrigno, A.; Canonico, P.L.; Battaglia, A.; Bertè, F.; Richelmi, P. Nicergoline reverts haloperidol-induced loss of detoxifying-enzyme activity. Eur. J. Pharmacol. 2004, 505, 121–125. [Google Scholar] [CrossRef]
- Krzysztof, Ł.; Dariusz, S.; Bożena, G.; Anna, B.; Sebastian, L.; Bogusław, O. Quantification of metformin by the HPLC method in brain regions, cerebrospinal fluid and plasma of rats treated with lipopolysaccharide. Pharmacol. Rep. 2010, 62, 956–965. [Google Scholar] [CrossRef]
- Ou, Z.; Kong, X.; Sun, X.; He, X.; Zhang, L.; Gong, Z.; Huang, J.; Xu, B.; Long, D.; Li, J.; et al. Metformin treatment prevents amyloid plaque deposition and memory impairment in APP/PS1 mice. Brain Behav. Immun. 2018, 69, 351–363. [Google Scholar] [CrossRef]
- Bonnefont-Rousselot, D.; Raji, B.; Walrand, S.; Gardès-Albert, M.; Jore, D.; Legrand, A.; Peynet, J.; Vasson, M.P. An intracellular modulation of free radical production could contribute to the beneficial effects of metformin towards oxidative stress. Metabolism 2003, 52, 586–589. [Google Scholar] [CrossRef]
- Sritawan, N.; Prajit, R.; Chaisawang, P.; Sirichoat, A.; Pannangrong, W.; Wigmore, P.; Welbat, J.U. Metformin alleviates memory and hippocampal neurogenesis decline induced by methotrexate chemotherapy in a rat model. Biomed. Pharmacother. 2020, 131, 110651. [Google Scholar] [CrossRef] [PubMed]
- Abílio, V.C.; Araujo, C.C.; Bergamo, M.; Calvente, P.R.; D’Almeida, V.; Ribeiro Rde, A.; Frussa-Filho, R. Vitamin E attenuates reserpine-induced oral dyskinesia and striatal oxidized glutathione/reduced glutathione ratio (GSSG/GSH) enhancement in rats. Prog. Neuropsychopharmacol. Biol. Psychiatry 2003, 27, 109–114. [Google Scholar] [CrossRef]
- Swarnkar, S.; Singh, S.; Sharma, S.; Mathur, R.; Patro, I.K.; Nath, C. Rotenone induced neurotoxicity in rat brain areas: A histopathological study. Neurosci. Lett. 2011, 501, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Courtes, A.A.; Arantes, L.P.; Barcelos, R.P.; da Silva, I.K.; Boligon, A.A.; Athayde, M.L.; Puntel, R.L.; Soares, F.A. Protective Effects of Aqueous Extract of Luehea divaricata against Behavioral and Oxidative Changes Induced by 3-Nitropropionic Acid in Rats. Evid. Based Complement. Alternat Med. 2015, 2015, 723431. [Google Scholar] [CrossRef] [PubMed]
- Alzoubi, K.H.; Malkawi, B.S.; Khabour, O.F.; El-Elimat, T.; Alali, F.Q. Arbutus andrachne L. Reverses Sleep Deprivation-Induced Memory Impairments in Rats. Mol. Neurobiol. 2018, 55, 1150–1156. [Google Scholar] [CrossRef]
- Fuchs, M.; Viel, C.; Lehto, A.; Lau, H.; Klein, J. Oxidative stress in rat brain during experimental status epilepticus: Effect of antioxidants. Front. Pharmacol. 2023, 14, 1233184. [Google Scholar] [CrossRef]
- Harish, G.; Venkateshappa, C.; Mahadevan, A.; Pruthi, N.; Srinivas Bharath, M.M.; Shankar, S.K. Glutathione metabolism is modulated by postmortem interval, gender difference and agonal state in postmortem human brains. Neurochem. Int. 2011, 59, 1029–1042. [Google Scholar] [CrossRef]
- Mischley, L.K.; Standish, L.J.; Weiss, N.S.; Padowski, J.M.; Kavanagh, T.J.; White, C.C.; Rosenfeld, M.E. Glutathione as a Biomarker in Parkinson’s Disease: Associations with Aging and Disease Severity. Oxid. Med. Cell Longev. 2016, 2016, 9409363. [Google Scholar] [CrossRef]
- Nitti, M.; Marengo, B.; Furfaro, A.L.; Pronzato, M.A.; Marinari, U.M.; Domenicotti, C.; Traverso, N. Hormesis and Oxidative Distress: Pathophysiology of Reactive Oxygen Species and the Open Question of Antioxidant Modulation and Supplementation. Antioxidants 2022, 11, 1613. [Google Scholar] [CrossRef]
- Tristan, C.; Shahani, N.; Sedlak, T.W.; Sawa, A. The diverse functions of GAPDH: Views from different subcellular compartments. Cell Signal. 2011, 23, 317–323. [Google Scholar] [CrossRef]
- Peralta, D.; Bronowska, A.K.; Morgan, B.; Dóka, É.; Van Laer, K.; Nagy, P.; Gräter, F.; Dick, T.P. A proton relay enhances H2O2 sensitivity of GAPDH to facilitate metabolic adaptation. Nat. Chem. Biol. 2015, 11, 156–163. [Google Scholar] [CrossRef]
- Hildebrandt, T.; Knuesting, J.; Berndt, C.; Morgan, B.; Scheibe, R. Cytosolic thiol switches regulating basic cellular functions: GAPDH as an information hub? Biol. Chem. 2015, 396, 523–537. [Google Scholar] [CrossRef] [PubMed]
- Kuehne, A.; Emmert, H.; Soehle, J.; Winnefeld, M.; Fischer, F.; Wenck, H.; Gallinat, S.; Terstegen, L.; Lucius, R.; Hildebrand, J.; et al. Acute Activation of Oxidative Pentose Phosphate Pathway as First-Line Response to Oxidative Stress in Human Skin Cells. Mol. Cell. 2015, 59, 359–371. [Google Scholar] [CrossRef]
- Terry, A.; Hill, W.; Parikh, V.; Waller, J.L.; Evans, D.R.; Mahadik, S.P. Differential Effects of Haloperidol, Risperidone, and Clozapine Exposure on Cholinergic Markers and Spatial Learning Performance in Rats. Neuropsychopharmacology 2003, 28, 300–309. [Google Scholar] [CrossRef]
- Xu, H.; Yang, H.J.; Rose, G.M. Chronic haloperidol-induced spatial memory deficits accompany the upregulation of D(1) and D(2) receptors in the caudate putamen of C57BL/6 mouse. Life Sci. 2012, 91, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Ning, H.; Cao, D.; Wang, H.; Kang, B.; Xie, S.; Meng, Y. Effects of haloperidol, olanzapine, ziprasidone, and PHA-543613 on spatial learning and memory in the Morris water maze test in naïve and MK-801-treated mice. Brain Behav. 2017, 7, e00764. [Google Scholar] [CrossRef]
- Osacka, J.; Kiss, A.; Bacova, Z.; Tillinger, A. Effect of Haloperidol and Olanzapine on Hippocampal Cells’ Proliferation in Animal Model of Schizophrenia. Int. J. Mol. Sci. 2022, 23, 7711. [Google Scholar] [CrossRef]
- Scharfman, H.; Goodman, J.; Macleod, A.; Phani, S.; Antonelli, C.; Croll, S. Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Exp. Neurol. 2005, 192, 348–356. [Google Scholar] [CrossRef]
- Rossi, C.; Angelucci, A.; Costantin, L.; Braschi, C.; Mazzantini, M.; Babbini, F.; Fabbri, M.E.; Tessarollo, L.; Maffei, L.; Berardi, N.; et al. Brain-derived neurotrophic factor (BDNF) is required for the enhancement of hippocampal neurogenesis following environmental enrichment. Eur. J. Neurosci. 2006, 24, 1850–1856. [Google Scholar] [CrossRef] [PubMed]
- Heldt, S.A.; Stanek, L.; Chhatwal, J.P.; Ressler, K.J. Hippocampus-specific deletion of BDNF in adult mice impairs spatial memory and extinction of aversive memories. Mol. Psychiatry. 2007, 12, 656–670. [Google Scholar] [CrossRef]
- Huang, T.T.; Leu, D.; Zou, Y. Oxidative stress and redox regulation on hippocampal-dependent cognitive functions. Arch. Biochem. Biophys. 2015, 576, 2–7. [Google Scholar] [CrossRef]
- Radecki, D.T.; Brown, L.M.; Martinez, J.; Teyler, T.J. BDNF protects against stress-induced impairments in spatial learning and memory and LTP. Hippocampus 2005, 15, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Won, D.H.; Kim, B.S.; Chang, H.; Kim, Y.I.; Jo, S.A.; Leem, Y.H. Exercise ameliorates cognition impairment due to restraint stress-induced oxidative insult and reduced BDNF level. Biochem. Biophys. Res. Commun. 2013, 434, 245–251. [Google Scholar] [CrossRef]
- Novkovic, T.; Mittmann, T.; Manahan-Vaughan, D. BDNF contributes to the facilitation of hippocampal synaptic plasticity and learning enabled by environmental enrichment. Hippocampus 2015, 25, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Anirudhan, A.; Ahmad, S.F.; Emran, T.B.; Angulo-Bejarano, P.I.; Sharma, A.; Ahmed, S.S.S.J. Comparative Efficacy of Metformin and Glimepiride in Modulating Pharmacological Network to Increase BDNF Levels and Benefit Type 2 Diabetes-Related Cognitive Impairment. Biomedicines 2023, 11, 2939. [Google Scholar] [CrossRef]
- Radahmadi, M.; Hosseini, N.; Alaei, H.; Sharifi, M.R. The Effect of Preventive, Therapeutic and Protective Exercises on Hippocampal Memory Mediators in Stressed Rats. Malays. J. Med. Sci. 2016, 23, 29–37. [Google Scholar] [CrossRef]
- Vásquez, C.E.; Riener, R.; Reynolds, E.; Britton, G.B. NMDA receptor dysregulation in chronic state: A possible mechanism underlying depression with BDNF downregulation. Neurochem. Int. 2014, 79, 88–97. [Google Scholar] [CrossRef]
- Polacchini, A.; Metelli, G.; Francavilla, R.; Baj, G.; Florean, M.; Mascaretti, L.G.; Tongiorgi, E. A method for reproducible measurements of serum BDNF: Comparison of the performance of six commercial assays. Sci. Rep. 2015, 5, 17989. [Google Scholar] [CrossRef]
- Li, M.; Du, W.; Shao, F.; Wang, W. Cognitive dysfunction and epigenetic alterations of the BDNF gene are induced by social isolation during early adolescence. Behav. Brain Res. 2016, 313, 177–183. [Google Scholar] [CrossRef]
- Hacioglu, G.; Senturk, A.; Ince, I.; Alver, A. Assessment of oxidative stress parameters of brain-derived neurotrophic factor heterozygous mice in acute stress model. Iran. J. Basic. Med. Sci. 2016, 19, 388–393. [Google Scholar]
- González-Rodríguez, P.; Ugidos, I.F.; Pérez-Rodríguez, D.; Anuncibay-Soto, B.; Santos-Galdiano, M.; Font-Belmonte, E.; Gonzalo-Orden, J.M.; Fernández-López, A. Brain-derived neurotrophic factor alleviates the oxidative stress induced by oxygen and glucose deprivation in an ex vivo brain slice model. J. Cell Physiol. 2019, 234, 9592–9604. [Google Scholar] [CrossRef]
- Wu, A.; Ying, Z.; Gomez-Pinilla, F. The interplay between oxidative stress and brain-derived neurotrophic factor modulates the outcome of a saturated fat diet on synaptic plasticity and cognition. Eur. J. Neurosci. 2004, 19, 1699–1707. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Sun, Y.; Chen, N.; Chen, S.; Xiu, M.; Zhang, X. Interaction of oxidative stress and BDNF on executive dysfunction in patients with chronic schizophrenia. Psychoneuroendocrinology 2020, 111, 104473. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Li, H.; Du, X.; Zhou, J.; Yuan, L.; Ren, H.; Yang, X.; Zhang, G.; Chen, X. Circulating Brain-Derived Neurotrophic Factor, Antioxidant Enzymes Activities, and Mitochondrial DNA in Bipolar Disorder: An Exploratory Report. Front. Psychiatry 2020, 11, 514658. [Google Scholar] [CrossRef]
- Simpson, J.; Bree, D.; Kelly, J.P. Effect of early life housing manipulation on baseline and drug-induced behavioural responses on neurochemistry in the male rat. Prog. Neuropsychopharmacol. Biol. Psychiatry 2012, 37, 252–263. [Google Scholar] [CrossRef]
- Evans, J.; Sun, Y.; McGregor, A.; Connor, B. Allopregnanolone regulates neurogenesis and depressive/anxiety-like behaviour in a social isolation rodent model of chronic stress. Neuropharmacology 2012, 63, 1315–1326. [Google Scholar] [CrossRef]
- Demaré, S.; Kothari, A.; Calcutt, N.A.; Fernyhough, P. Metformin as a potential therapeutic for neurological disease: Mobilizing AMPK to repair the nervous system. Expert. Rev. Neurother. 2021, 21, 45–63. [Google Scholar] [CrossRef] [PubMed]
- Howell, J.J.; Hellberg, K.; Turner, M.; Talbott, G.; Kolar, M.J.; Ross, D.S.; Hoxhaj, G.; Saghatelian, A.; Shaw, R.J.; Manning, B.D. Metformin Inhibits Hepatic mTORC1 Signaling via Dose-Dependent Mechanisms Involving AMPK and the TSC Complex. Cell Metab. 2017, 25, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.H.; Jackson, S.; Seaman, M.; Brown, K.; Kempkes, B.; Hibshoosh, H.; Levine, B. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature 1999, 402, 672–676. [Google Scholar] [CrossRef]
- Okerlund, N.D.; Schneider, K.; Leal-Ortiz, S.; Montenegro-Venegas, C.; Kim, S.A.; Garner, L.C.; Waites, C.L.; Gundelfinger, E.D.; Reimer, R.J.; Garner, C.C. Bassoon Controls Presynaptic Autophagy through Atg5. Neuron 2017, 93, 897–913.e7. [Google Scholar] [CrossRef]
- Glatigny, M.; Moriceau, S.; Rivagorda, M.; Ramos-Brossier, M.; Nascimbeni, A.C.; Lante, F.; Shanley, M.R.; Boudarene, N.; Rousseaud, A.; Friedman, A.K.; et al. Autophagy Is Required for Memory Formation and Reverses Age-Related Memory Decline. Curr. Biol. 2019, 29, 435–448.e8. [Google Scholar] [CrossRef] [PubMed]
- Khoury, R.; Saad, J.; Jabre, V.; Ghayad, L.M.; Khalifeh, M.; Houbeika, R.; El Ahmad, P.; Mezher, A.; El Masri, D.; Haddad, Z.; et al. Autophagy regulates the release of exercise factors and their beneficial effects on spatial memory recall. Heliyon 2023, 9, e14705. [Google Scholar] [CrossRef] [PubMed]


| Day 1 | Q1 | Q2 | Q3 | Q4 |
| Day 2 | Q2 | Q3 | Q4 | Q1 |
| Day 3 | Q3 | Q4 | Q1 | Q2 |
| Day 4 | Q4 | Q1 | Q2 | Q3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jîtcă, G.; Gáll, Z.; Jîtcă, C.-M.; Buț, M.-G.; Májai, E. Drug Repurposing of Metformin for the Treatment of Haloperidol-Related Behavior Disorders and Oxidative Stress: A Preliminary Study. Pharmaceutics 2024, 16, 403. https://doi.org/10.3390/pharmaceutics16030403
Jîtcă G, Gáll Z, Jîtcă C-M, Buț M-G, Májai E. Drug Repurposing of Metformin for the Treatment of Haloperidol-Related Behavior Disorders and Oxidative Stress: A Preliminary Study. Pharmaceutics. 2024; 16(3):403. https://doi.org/10.3390/pharmaceutics16030403
Chicago/Turabian StyleJîtcă, George, Zsolt Gáll, Carmen-Maria Jîtcă, Mădălina-Georgiana Buț, and Erzsébet Májai. 2024. "Drug Repurposing of Metformin for the Treatment of Haloperidol-Related Behavior Disorders and Oxidative Stress: A Preliminary Study" Pharmaceutics 16, no. 3: 403. https://doi.org/10.3390/pharmaceutics16030403
APA StyleJîtcă, G., Gáll, Z., Jîtcă, C.-M., Buț, M.-G., & Májai, E. (2024). Drug Repurposing of Metformin for the Treatment of Haloperidol-Related Behavior Disorders and Oxidative Stress: A Preliminary Study. Pharmaceutics, 16(3), 403. https://doi.org/10.3390/pharmaceutics16030403








