Aspherical, Nano-Structured Drug Delivery System with Tunable Release and Clearance for Pulmonary Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. Particle Preparation
2.4. Particle Characterization
2.5. Disintegration Tests
2.6. Aerodynamic Particle Size Analysis
2.7. Cell Viability
2.8. Cellular Uptake
2.9. Statistical Evaluation
3. Results and Discussion
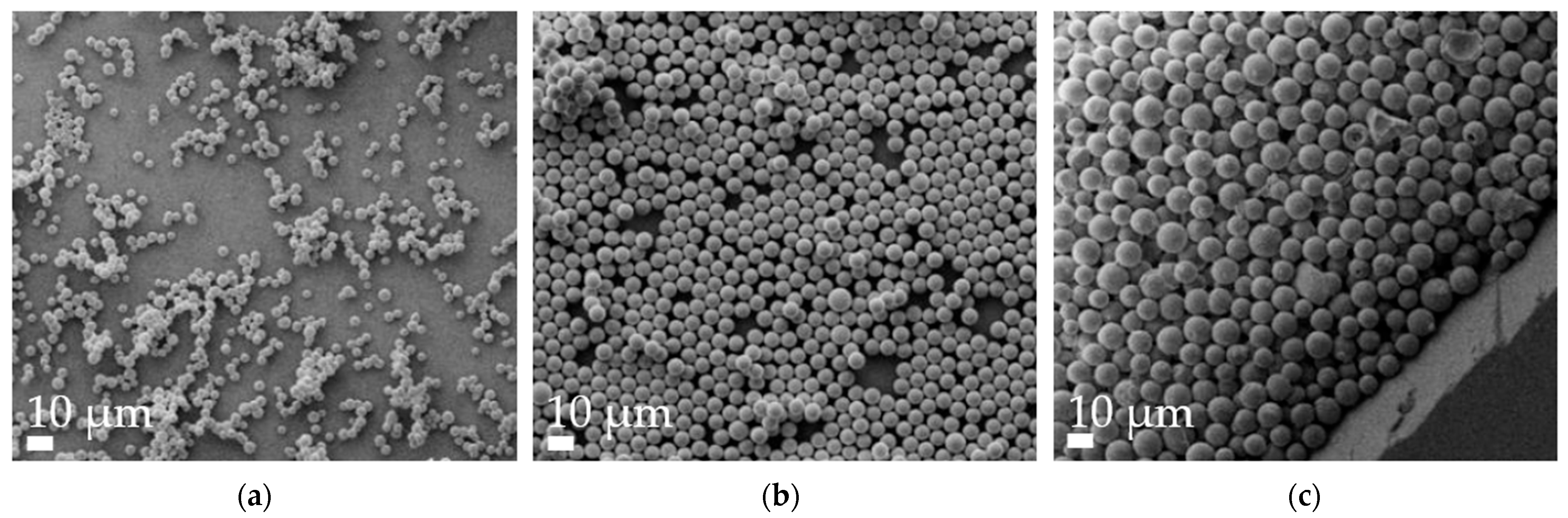
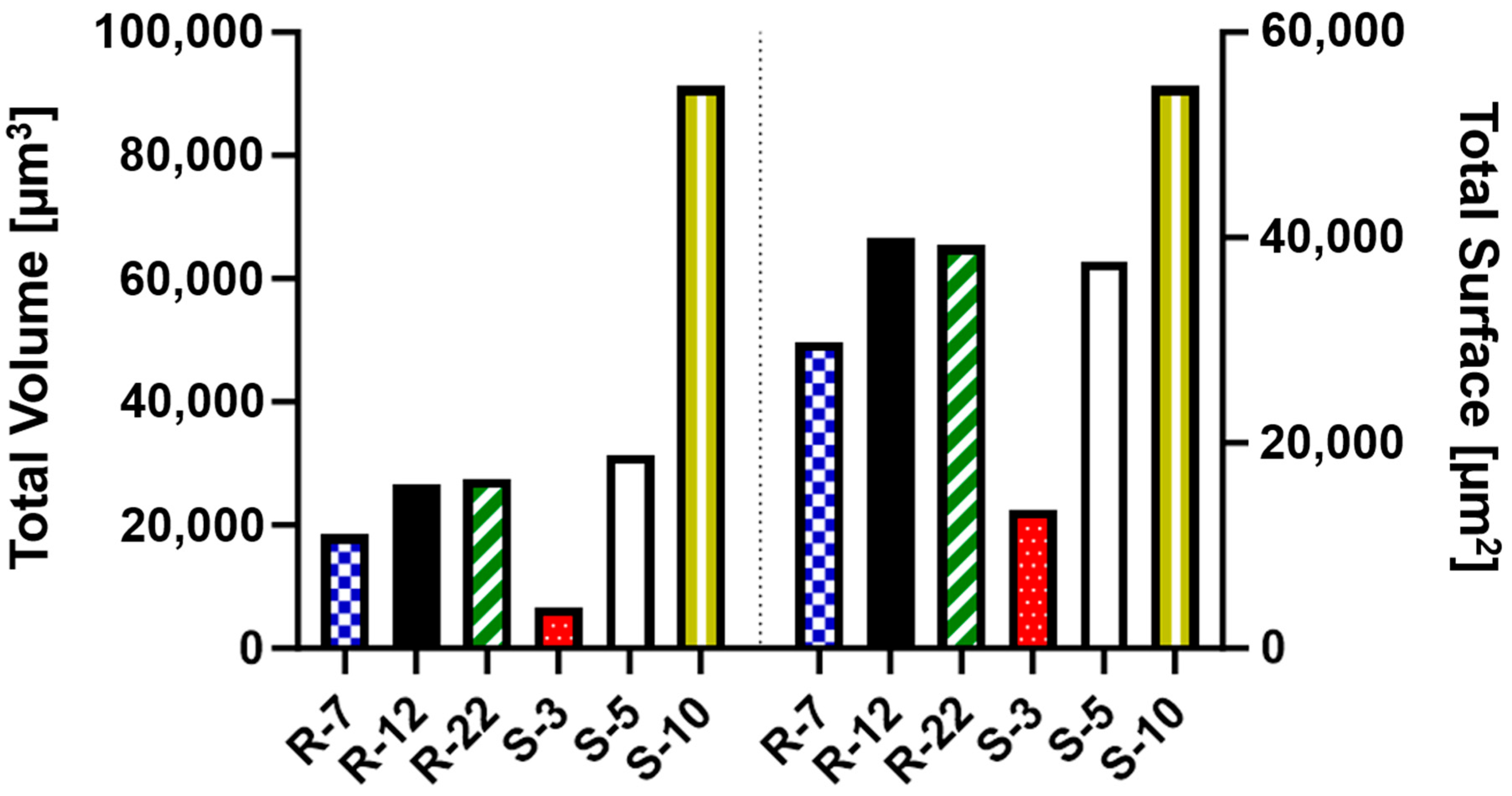
3.1. Particle Characterization
3.2. Disintegration Tests
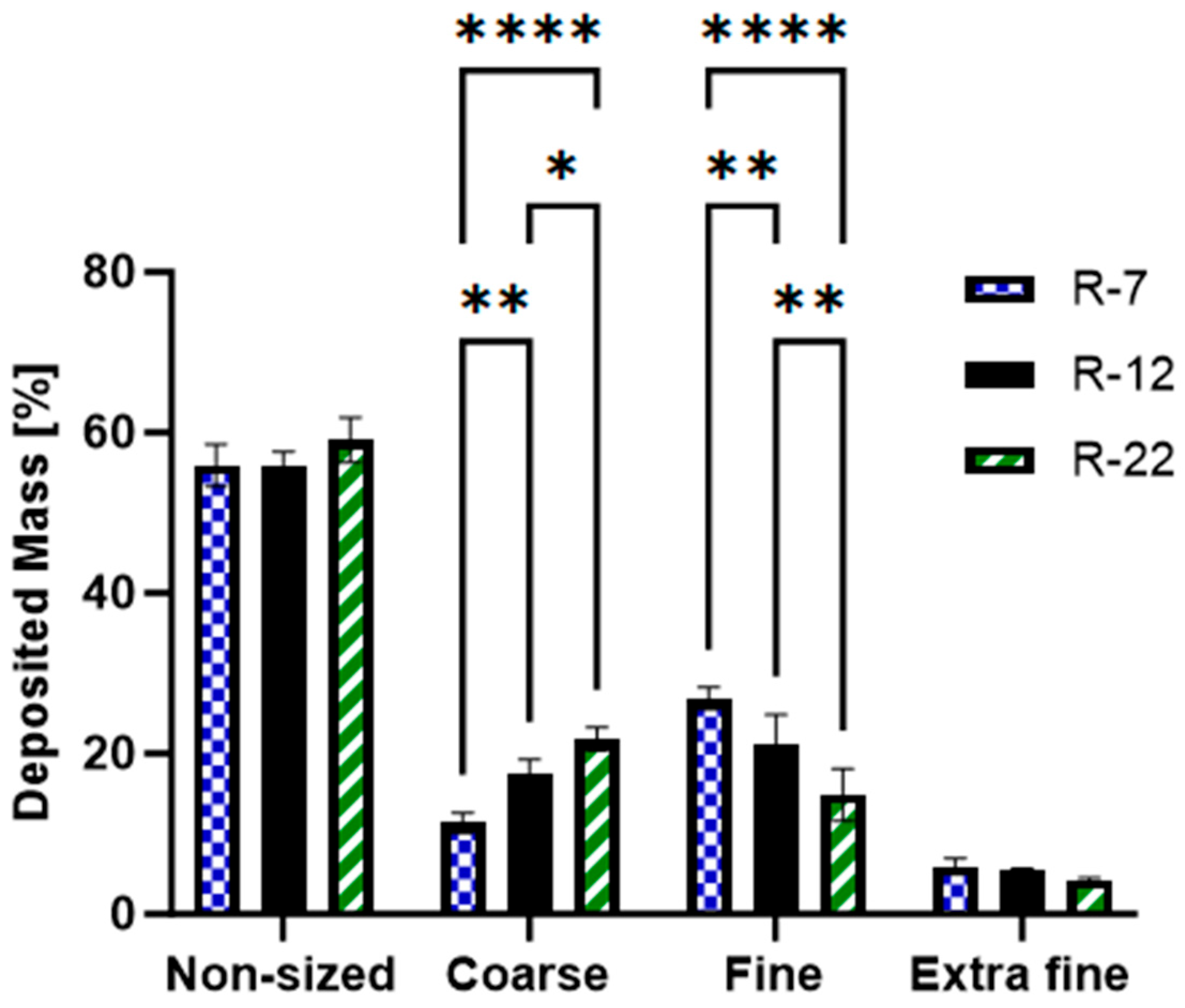
3.3. Aerodynamic Particle Size Analysis
- Non-sized: big particles with extra-thoracic deposition (capsule, IP and pre-seperator).
- Coarse: pertaining to the upper respiratory tract, particle sizes between 4.46 and 12.80 µm (stages 1 and 2).
- Fine: associated with the bronchial region, covering particles between 0.94 and 4.46 µm (stages 3, 4 and 5).
- Extra Fine: relates to the alveoli, including particles smaller than 0.94 µm (stages 6, 7 and 8) [88].
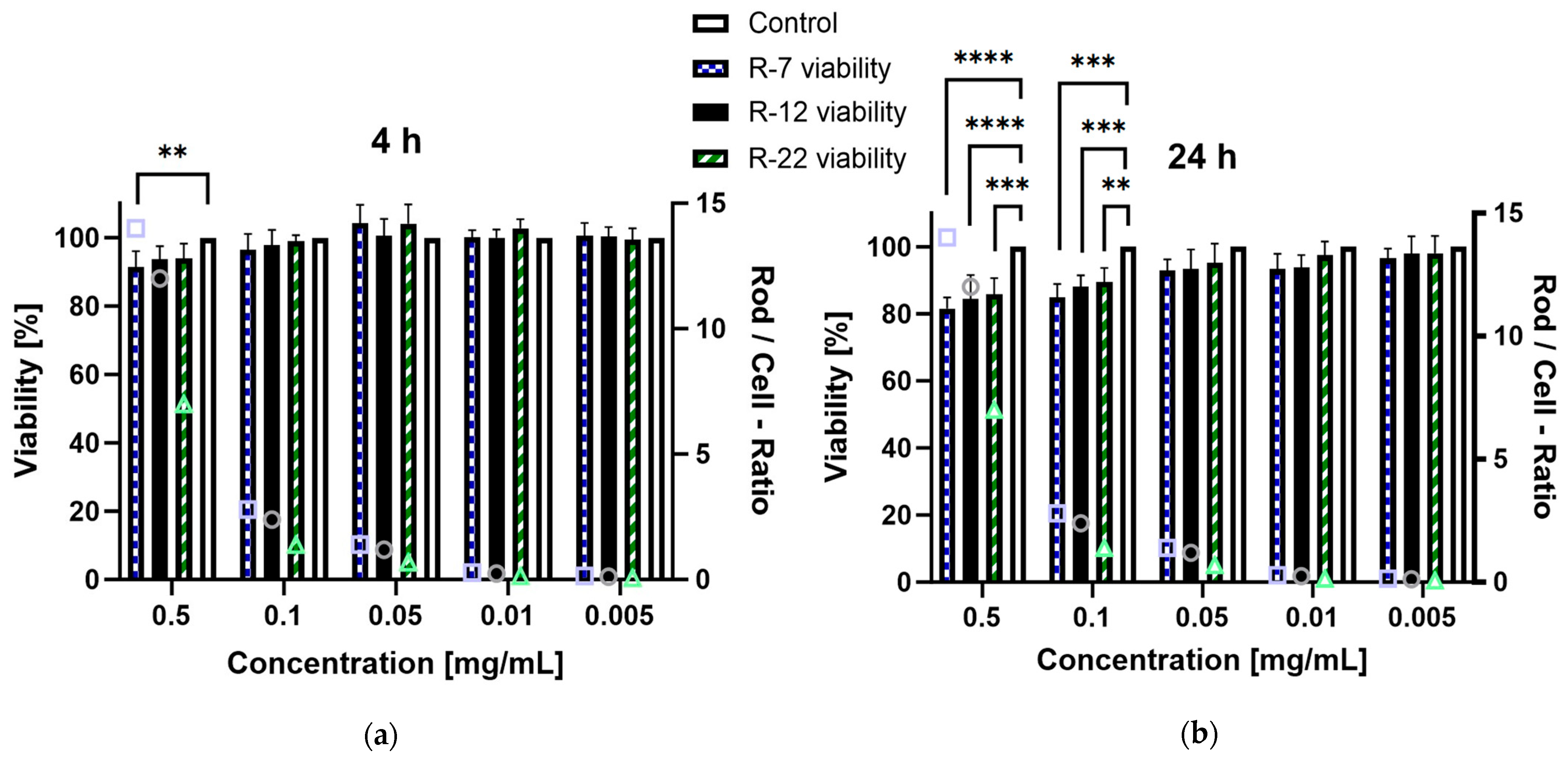
3.4. Cell Viability
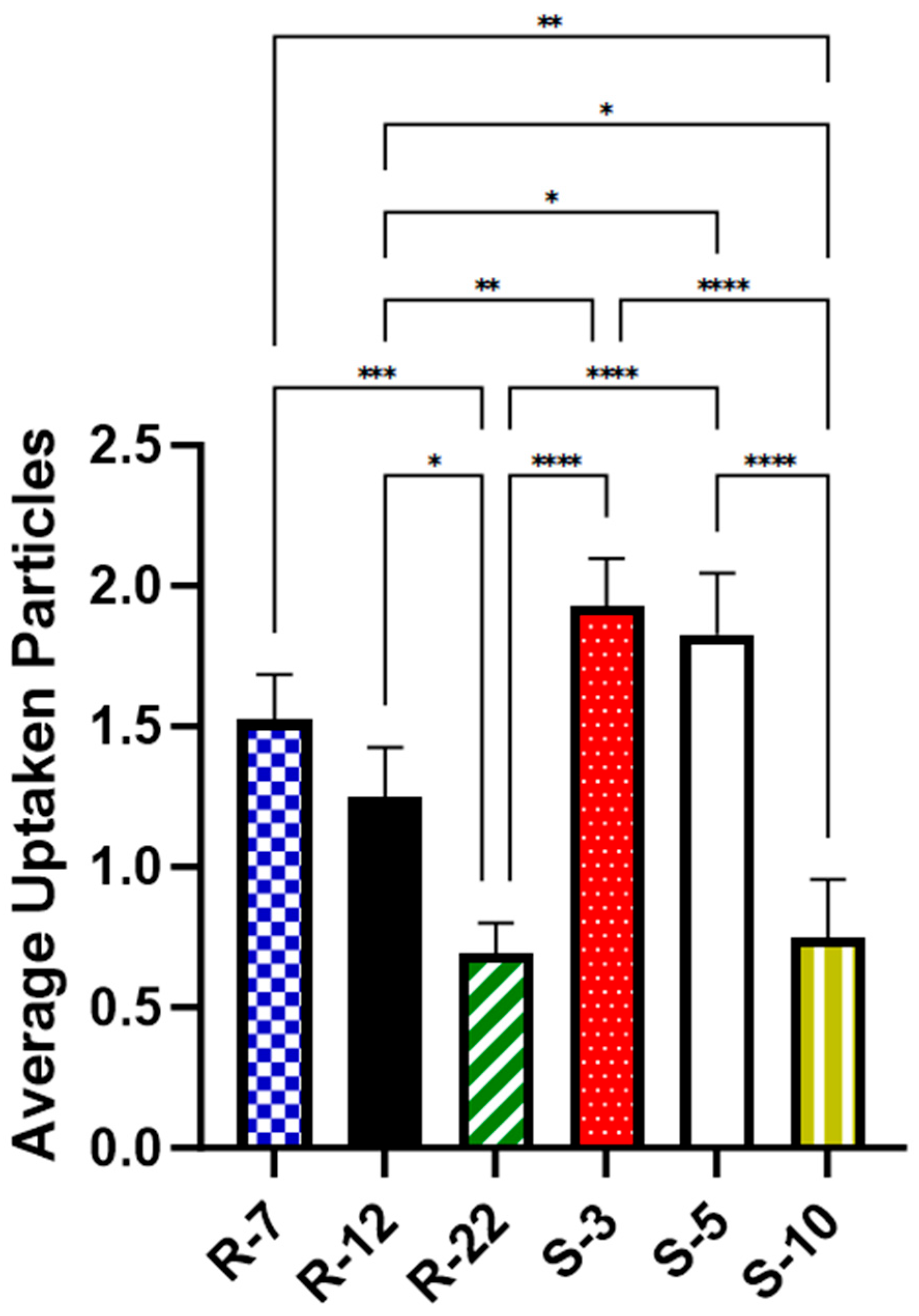
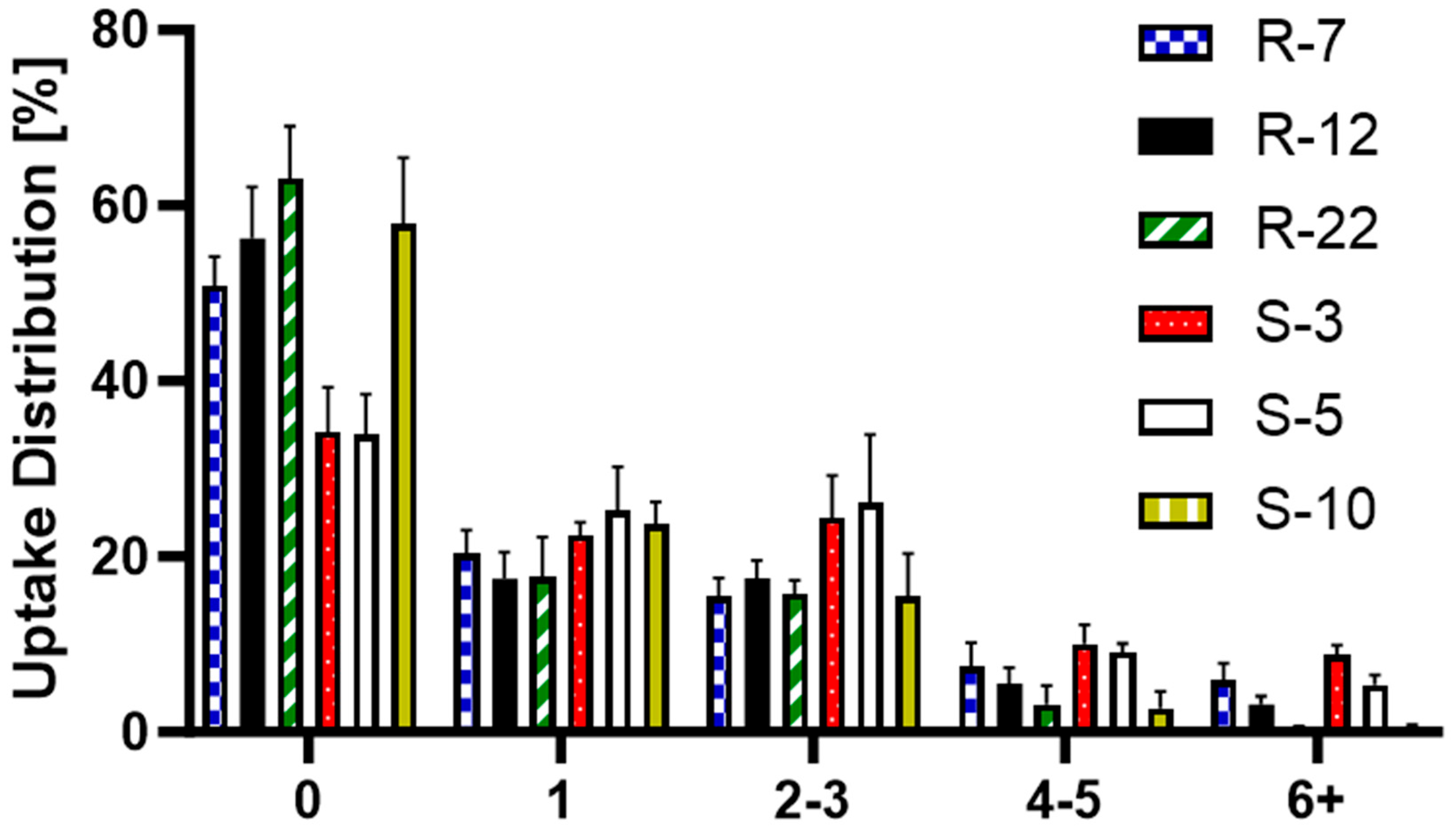
3.5. Cellular Uptake
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Soriano, J.B.; Kendrick, P.J.; Paulson, K.R.; Gupta, V.; Abrams, E.M.; Adedoyin, R.A.; Adhikari, T.B.; Advani, S.M.; Agrawal, A.; Ahmadian, E.; et al. Prevalence and Attributable Health Burden of Chronic Respiratory Diseases, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet Respir. Med. 2020, 8, 585–596. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Y.; Chen, X.; Feng, Y.; Ren, X.; Yang, M.; Ding, T. Targeted Delivery of Inhalable Drug Particles in a Patient-Specific Tracheobronchial Tree with Moderate COVID-19: A Numerical Study. Powder Technol. 2022, 405, 117520. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.S.; Lau, R.W.M. Effect of Particle Shape on Dry Particle Inhalation: Study of Flowability, Aerosolization, and Deposition Properties. AAPS PharmSciTech 2009, 10, 1252. [Google Scholar] [CrossRef] [PubMed]
- Ruge, C.A.; Kirch, J.; Lehr, C.-M. Pulmonary Drug Delivery: From Generating Aerosols to Overcoming Biological Barriers—Therapeutic Possibilities and Technological Challenges. Lancet Respir. Med. 2013, 1, 402–413. [Google Scholar] [CrossRef] [PubMed]
- Racanelli, A.C.; Kikkers, S.A.; Choi, A.M.K.; Cloonan, S.M. Autophagy and Inflammation in Chronic Respiratory Disease. Autophagy 2018, 14, 221–232. [Google Scholar] [CrossRef]
- Ganguly, K.; Carlander, U.; Garessus, E.D.; Fridén, M.; Eriksson, U.G.; Tehler, U.; Johanson, G. Computational Modeling of Lung Deposition of Inhaled Particles in Chronic Obstructive Pulmonary Disease (COPD) Patients: Identification of Gaps in Knowledge and Data. Crit. Rev. Toxicol. 2019, 49, 160–173. [Google Scholar] [CrossRef]
- Canan, C.H.; Gokhale, N.S.; Carruthers, B.; Lafuse, W.P.; Schlesinger, L.S.; Torrelles, J.B.; Turner, J. Characterization of Lung Inflammation and Its Impact on Macrophage Function in Aging. J. Leukoc. Biol. 2014, 96, 473–480. [Google Scholar] [CrossRef]
- Mohning, M.P.; Thomas, S.M.; Barthel, L.; Mould, K.J.; McCubbrey, A.L.; Frasch, S.C.; Bratton, D.L.; Henson, P.M.; Janssen, W.J. Phagocytosis of Microparticles by Alveolar Macrophages during Acute Lung Injury Requires MerTK. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2018, 314, L69–L82. [Google Scholar] [CrossRef]
- Pilcer, G.; Amighi, K. Formulation Strategy and Use of Excipients in Pulmonary Drug Delivery. Int. J. Pharm. 2010, 392, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Patton, J.S. The Lungs as a Portal of Entry for Systemic Drug Delivery. Proc. Am. Thorac. Soc. 2004, 1, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-B.; Xu, D.; Bai, L.; Zhou, Y.-M.; Zhang, H.; Cui, Y.-L. A Review of Non-Invasive Drug Delivery through Respiratory Routes. Pharmaceutics 2022, 14, 1974. [Google Scholar] [CrossRef] [PubMed]
- Labaki, W.W.; Han, M.K. Chronic Respiratory Diseases: A Global View. Lancet Respir. Med. 2020, 8, 531–533. [Google Scholar] [CrossRef] [PubMed]
- Agustí, A.; Celli, B.R.; Criner, G.J.; Halpin, D.; Anzueto, A.; Barnes, P.; Bourbeau, J.; Han, M.K.; Martinez, F.J.; de Oca, M.M.; et al. Global Initiative for Chronic Obstructive Lung Disease 2023 Report: GOLD Executive Summary. Eur. Respir. J. 2023, 61, 2300239. [Google Scholar] [CrossRef] [PubMed]
- Reddel, H.K.; Bacharier, L.B.; Bateman, E.D.; Brightling, C.E.; Brusselle, G.G.; Buhl, R.; Cruz, A.A.; Duijts, L.; Drazen, J.M.; FitzGerald, J.M.; et al. Global Initiative for Asthma Strategy 2021: Executive Summary and Rationale for Key Changes. Eur. Respir. J. 2022, 59, 2102730. [Google Scholar] [CrossRef] [PubMed]
- Hadinoto, K.; Cheow, W.S. Nano-Antibiotics in Chronic Lung Infection Therapy against Pseudomonas Aeruginosa. Colloids Surf. B Biointerfaces 2014, 116, 772–785. [Google Scholar] [CrossRef]
- Sazali, N.B.; Chan, L.W.; Wong, T.W. Nano-Enabled Agglomerates and Compact: Design Aspects of Challenges. Asian J. Pharm. Sci. 2023, 18, 100794. [Google Scholar] [CrossRef]
- Tsuda, A.; Henry, F.S.; Butler, J.P. Particle Transport and Deposition: Basic Physics of Particle Kinetics. Compr. Physiol. 2013, 3, 1437–1471. [Google Scholar]
- Heijerman, H.; Westerman, E.; Conway, S.; Touw, D. Inhaled Medication and Inhalation Devices for Lung Disease in Patients with Cystic Fibrosis: A European Consensus. J. Cyst. Fibros. 2009, 8, 295–315. [Google Scholar] [CrossRef] [PubMed]
- Tsapis, N.; Bennett, D.; Jackson, B.; Weitz, D.A.; Edwards, D.A. Trojan Particles: Large Porous Carriers of Nanoparticles for Drug Delivery. Proc. Natl. Acad. Sci. USA 2002, 99, 12001–12005. [Google Scholar] [CrossRef]
- Torge, A.; Pavone, G.; Jurisic, M.; Lima-Engelmann, K.; Schneider, M. A Comparison of Spherical and Cylindrical Microparticles Composed of Nanoparticles for Pulmonary Application. Aerosol Sci. Technol. 2019, 53, 53–62. [Google Scholar] [CrossRef]
- Fischer, T.; Winter, I.; Drumm, R.; Schneider, M. Cylindrical Microparticles Composed of Mesoporous Silica Nanoparticles for the Targeted Delivery of a Small Molecule and a Macromolecular Drug to the Lungs: Exemplified with Curcumin and siRNA. Pharmaceutics 2021, 13, 844. [Google Scholar] [CrossRef] [PubMed]
- Fischer, T.; Tschernig, T.; Drews, F.; Brix, K.; Meier, C.; Simon, M.; Kautenburger, R.; Schneider, M. siRNA Delivery to Macrophages Using Aspherical, Nanostructured Microparticles as Delivery System for Pulmonary Administration. Eur. J. Pharm. Biopharm. 2021, 158, 284–293. [Google Scholar] [CrossRef]
- Möhwald, M.; Pinnapireddy, S.R.; Wonnenberg, B.; Pourasghar, M.; Jurisic, M.; Jung, A.; Fink-Straube, C.; Tschernig, T.; Bakowsky, U.; Schneider, M. Aspherical, Nanostructured Microparticles for Targeted Gene Delivery to Alveolar Macrophages. Adv. Healthc. Mater. 2017, 6, 1700478. [Google Scholar] [CrossRef]
- He, W.; Kapate, N.; Shields, C.W.; Mitragotri, S. Drug Delivery to Macrophages: A Review of Targeting Drugs and Drug Carriers to Macrophages for Inflammatory Diseases. Adv. Drug Deliv. Rev. 2020, 165–166, 15–40. [Google Scholar] [CrossRef] [PubMed]
- Daum, N.; Tscheka, C.; Neumeyer, A.; Schneider, M. Novel Approaches for Drug Delivery Systems in Nanomedicine: Effects of Particle Design and Shape. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2012, 4, 52–65. [Google Scholar] [CrossRef]
- Fish, B.R. Aerosol Physics of Asbestos Fibers: Interception; U.S. Department of Energy Office of Scientific and Technical Information: Oak Ridge, TN, USA, 1974; ORNL-TM-4051, 4265252. [Google Scholar]
- Hunstad, D.A.; Justice, S.S. Intracellular Lifestyles and Immune Evasion Strategies of Uropathogenic Escherichia coli. Annu. Rev. Microbiol. 2010, 64, 203–221. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.C.; Keller, A.; Odell, J.A.; Ottewill, R.H. Preparation of Monodisperse Ellipsoidal Polystyrene Particles. Colloid Polym. Sci. 1993, 271, 469–479. [Google Scholar] [CrossRef]
- Champion, J.A.; Katare, Y.K.; Mitragotri, S. Making Polymeric Micro- and Nanoparticles of Complex Shapes. Proc. Natl. Acad. Sci. USA 2007, 104, 11901–11904. [Google Scholar] [CrossRef]
- Gratton, S.E.A.; Pohlhaus, P.D.; Lee, J.; Guo, J.; Cho, M.J.; DeSimone, J.M. Nanofabricated Particles for Engineered Drug Therapies: A Preliminary Biodistribution Study of PRINTTM Nanoparticles. J. Control. Release 2007, 121, 10–18. [Google Scholar] [CrossRef]
- Xu, J.; Wong, D.H.C.; Byrne, J.D.; Chen, K.; Bowerman, C.; DeSimone, J.M. Future of the Particle Replication in Nonwetting Templates (PRINT) Technology. Angew. Chem. Int. Ed. Engl. 2013, 52, 6580–6589. [Google Scholar] [CrossRef]
- Sadeghi, I.; Lu, X.; Sarmadi, M.; Langer, R.; Jaklenec, A. Micromolding of Thermoplastic Polymers for Direct Fabrication of Discrete, Multilayered Microparticles. Small Methods 2022, 6, 2200232. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Su, W.-C.; Cheng, Y.S. Fiber Deposition in the Tracheobronchial Region: Deposition Equations. Inhal. Toxicol. 2008, 20, 1191–1198. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Kleinstreuer, C. Analysis of Non-Spherical Particle Transport in Complex Internal Shear Flows. Phys. Fluids 2013, 25, 091904. [Google Scholar] [CrossRef]
- Kaialy, W.; Alhalaweh, A.; Velaga, S.P.; Nokhodchi, A. Effect of Carrier Particle Shape on Dry Powder Inhaler Performance. Int. J. Pharm. 2011, 421, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Larhrib, H.; Martin, G.P.; Marriott, C.; Prime, D. The Influence of Carrier and Drug Morphology on Drug Delivery from Dry Powder Formulations. Int. J. Pharm. 2003, 257, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.K.; Sarode, A.; Kanabar, D.D.; Muth, A.; Kunda, N.K.; Mitragotri, S.; Gupta, V. Bioinspired Particle Engineering for Non-Invasive Inhaled Drug Delivery to the Lungs. Mater. Sci. Eng. C 2021, 128, 112324. [Google Scholar] [CrossRef] [PubMed]
- Champion, J.A.; Katare, Y.K.; Mitragotri, S. Particle Shape: A New Design Parameter for Micro- and Nanoscale Drug Delivery Carriers. J. Control. Release 2007, 121, 3–9. [Google Scholar] [CrossRef]
- Mathaes, R.; Winter, G.; Besheer, A.; Engert, J. Influence of Particle Geometry and PEGylation on Phagocytosis of Particulate Carriers. Int. J. Pharm. 2014, 465, 159–164. [Google Scholar] [CrossRef]
- Tschernig, T.; Fischer, T.; Grissmer, A.; Beckmann, A.; Meier, C.; Lipp, P.; Schneider, M. Silica Nanoparticles of Microrods Enter Lung Epithelial Cells. Biomed. Rep. 2018, 9, 156–160. [Google Scholar] [CrossRef]
- Kohler, D.; Schneider, M.; Krüger, M.; Lehr, C.-M.; Möhwald, H.; Wang, D. Template-Assisted Polyelectrolyte Encapsulation of Nanoparticles into Dispersible, Hierarchically Nanostructured Microfibers. Adv. Mater. 2011, 23, 1376–1379. [Google Scholar] [CrossRef]
- Koenneke, A.; Pourasghar, M.; Schneider, M. Nano-Structured Microparticles for Inhalation. In Delivery of Drugs; Volume 2: Expectations and Realities of Multifunctional Drug Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2020; pp. 119–160. ISBN 978-0-12-817776-1. [Google Scholar]
- Zimmermann, U.; Klöck, G.; Federlin, K.; Hannig, K.; Kowalski, M.; Bretzel, R.G.; Horcher, A.; Entenmann, H.; Sieber, U.; Zekorn, T. Production of Mitogen-Contamination Free Alginates with Variable Ratios of Mannuronic Acid to Guluronic Acid by Free Flow Electrophoresis. Electrophoresis 1992, 13, 269–274. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and Biomedical Applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Browne, E.; Kavanagh, S.; Devery, S. The Cytotoxicity of Phorbol 12- Myristate 13-Acetate and Lipopolysaccharide on THP-1 Cells and an Optimized Differentiation Protocol. In Proceedings of the BiTaP, Online, 8–9 September 2022; p. 5. [Google Scholar]
- Zhou, J.; Zhu, P.; Jiang, J.; Zhang, Q.; Wu, Z.; Yao, X.; Tang, H.; Lu, N.; Yang, Y.; Chen, Z. Involvement of CD147 in Overexpression of MMP-2 and MMP-9 and Enhancement of Invasive Potential of PMA-Differentiated THP-1. BMC Cell Biol. 2005, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Tscheka, C.; Hittinger, M.; Lehr, C.-M.; Schneider-Daum, N.; Schneider, M. Macrophage Uptake of Cylindrical Microparticles Investigated with Correlative Microscopy. Eur. J. Pharm. Biopharm. 2015, 95, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Najafabadi, A.R.; Gilani, K.; Barghi, M.; Rafiee-Tehrani, M. The Effect of Vehicle on Physical Properties and Aerosolisation Behaviour of Disodium Cromoglycate Microparticles Spray Dried Alone or with L-Leucine. Int. J. Pharm. 2004, 285, 97–108. [Google Scholar] [CrossRef]
- Aquino, R.P.; Prota, L.; Auriemma, G.; Santoro, A.; Mencherini, T.; Colombo, G.; Russo, P. Dry Powder Inhalers of Gentamicin and Leucine: Formulation Parameters, Aerosol Performance and in Vitro Toxicity on CuFi1 Cells. Int. J. Pharm. 2012, 426, 100–107. [Google Scholar] [CrossRef]
- Mathaes, R.; Manning, M.C.; Winter, G.; Engert, J.; Wilson, G.A. Shape Characterization of Subvisible Particles Using Dynamic Imaging Analysis. J. Pharm. Sci. 2020, 109, 375–379. [Google Scholar] [CrossRef]
- Marques, M.R.C.; Loebenberg, R.; Almukainzi, M. Simulated Biological Fluids with Possible Application in Dissolution Testing. Dissolution Technol. 2011, 18, 15–28. [Google Scholar] [CrossRef]
- Marple, V.A.; Roberts, D.L.; Romay, F.J.; Miller, N.C.; Truman, K.G.; Van Oort, M.; Olsson, B.; Holroyd, M.J.; Mitchell, J.P.; Hochrainer, D. Next Generation Pharmaceutical Impactor (A New Impactor for Pharmaceutical Inhaler Testing). Part I: Design. J. Aerosol Med. 2003, 16, 283–299. [Google Scholar] [CrossRef] [PubMed]
- Newman, S.P.; Morén, F.; Trofast, E.; Talaee, N.; Clarke, S.W. Terbutaline Sulphate Turbuhaler: Effect of Inhaled Flow Rate on Drug Deposition and Efficacy. Int. J. Pharm. 1991, 74, 209–213. [Google Scholar] [CrossRef]
- Borgström, L. On the Use of Dry Powder Inhalers in Situations Perceived as Constrained. J. Aerosol Med. 2001, 14, 281–287. [Google Scholar] [CrossRef]
- Abdelrahim, M.E.; Chrystyn, H. Aerodynamic Characteristics of Nebulized Terbutaline Sulphate Using the Next Generation Impactor (NGI) and CEN Method. J. Aerosol Med. Pulm. Drug Deliv. 2009, 22, 19–28. [Google Scholar] [CrossRef]
- Van Meerloo, J.; Kaspers, G.J.L.; Cloos, J. Cell Sensitivity Assays: The MTT Assay. In Cancer Cell Culture; Cree, I.A., Ed.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2011; Volume 731, pp. 237–245. ISBN 978-1-61779-079-9. [Google Scholar]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Ashraf, S.; Hassan Said, A.; Hartmann, R.; Assmann, M.; Feliu, N.; Lenz, P.; Parak, W.J. Quantitative Particle Uptake by Cells as Analyzed by Different Methods. Angew. Chem. Int. Ed. 2020, 59, 5438–5453. [Google Scholar] [CrossRef]
- Paul, D.; Achouri, S.; Yoon, Y.-Z.; Herre, J.; Bryant, C.E.; Cicuta, P. Phagocytosis Dynamics Depends on Target Shape. Biophys. J. 2013, 105, 1143–1150. [Google Scholar] [CrossRef]
- Johnstone, S.A.; Masin, D.; Mayer, L.; Bally, M.B. Surface-Associated Serum Proteins Inhibit the Uptake of Phosphatidylserine and Poly(Ethylene Glycol) Liposomes by Mouse Macrophages. Biochim. Biophys. Acta-Biomembr. 2001, 1513, 25–37. [Google Scholar] [CrossRef]
- Al-Fityan, S.; Diesel, B.; Fischer, T.; Ampofo, E.; Schomisch, A.; Mashayekhi, V.; Schneider, M.; Kiemer, A.K. Nanostructured Microparticles Repolarize Macrophages and Induce Cell Death in an In Vitro Model of Tumour-Associated Macrophages. Pharmaceutics 2023, 15, 1895. [Google Scholar] [CrossRef] [PubMed]
- Nishiumi, H.; Deiringer, N.; Krause, N.; Yoneda, S.; Torisu, T.; Menzen, T.; Friess, W.; Uchiyama, S. Utility of Three Flow Imaging Microscopy Instruments for Image Analysis in Evaluating Four Types of Subvisible Particle in Biopharmaceuticals. J. Pharm. Sci. 2022, 111, 3017–3028. [Google Scholar] [CrossRef]
- Kikuchi, A.; Kawabuchi, M.; Watanabe, A.; Sugihara, M.; Sakurai, Y.; Okano, T. Effect of Ca2+-Alginate Gel Dissolution on Release of Dextran with Different Molecular Weights. J. Control. Release 1999, 58, 21–28. [Google Scholar] [CrossRef]
- Grant, G.T.; Mon, E.R.; Rees, S.D.A. Biological Interactions between Polysacchartdes and Divalent Cations: The Egg-Box Model. FEBS Lett. 1973, 32, 195–198. [Google Scholar] [CrossRef]
- Kikuchi, A.; Kawabuchi, M.; Sugihara, M.; Sakurai, Y.; Okano, T. Pulsed Dextran Release from Calcium-Alginate Gel Beads. J. Control. Release 1997, 47, 21–29. [Google Scholar] [CrossRef]
- Del Gaudio, P.; Colombo, P.; Colombo, G.; Russo, P.; Sonvico, F. Mechanisms of Formation and Disintegration of Alginate Beads Obtained by Prilling. Int. J. Pharm. 2005, 302, 1–9. [Google Scholar] [CrossRef]
- Moya, M.L.; Morley, M.; Khanna, O.; Opara, E.C.; Brey, E.M. Stability of Alginate Microbead Properties in Vitro. J. Mater. Sci Mater. Med. 2012, 23, 903–912. [Google Scholar] [CrossRef] [PubMed]
- Vreeker, R.; Li, L.; Fang, Y.; Appelqvist, I.; Mendes, E. Drying and Rehydration of Calcium Alginate Gels. Curr. Biol. 1997, 7, R126. [Google Scholar] [CrossRef]
- Mitchell, J.P.; Nagel, M.W. Cascade Impactors for the Size Characterization of Aerosols from Medical Inhalers: Their Uses and Limitations. J. Aerosol Med. 2003, 16, 341–377. [Google Scholar] [CrossRef] [PubMed]
- Kleinstreuer, C. Drug-Targeting Methodologies with Applications: A Review. WJCC 2014, 2, 742. [Google Scholar] [CrossRef] [PubMed]
- Raula, J.; Seppälä, J.; Malm, J.; Karppinen, M.; Kauppinen, E.I. Structure and Dissolution of L-Leucine-Coated Salbutamol Sulphate Aerosol Particles. AAPS PharmSciTech 2012, 13, 707–712. [Google Scholar] [CrossRef][Green Version]
- Moghaddam, P.H.; Ramezani, V.; Esfandi, E.; Vatanara, A.; Nabi-Meibodi, M.; Darabi, M.; Gilani, K.; Najafabadi, A.R. Development of a Nano–Micro Carrier System for Sustained Pulmonary Delivery of Clarithromycin. Powder Technol. 2013, 239, 478–483. [Google Scholar] [CrossRef]
- Hinds, W.C. Aerosol Technology: Properties, Behavior, and Measurement of Airborne Particles, 2nd ed.; Wiley: New York, NY, USA, 1999; ISBN 978-0-471-19410-1. [Google Scholar]
- Kasper, G. Dynamics and Measurement of Smokes. I Size Characterization of Nonspherical Particles. Aerosol Sci. Technol. 1982, 1, 187–199. [Google Scholar] [CrossRef]
- De Boer, A.H.; Gjaltema, D.; Hagedoorn, P.; Frijlink, H.W. Characterization of Inhalation Aerosols: A Critical Evaluation of Cascade Impactor Analysis and Laser Diffraction Technique. Int. J. Pharm. 2002, 249, 219–231. [Google Scholar] [CrossRef]
- Cheng, Y.-S.; Yeh, H.-C.; Allen, M.D. Dynamic Shape Factor of a Plate-Like Particle. Aerosol Sci. Technol. 1988, 8, 109–123. [Google Scholar] [CrossRef]
- Newman, S.P. Fine Particle Fraction: The Good and the Bad. J. Aerosol Med. Pulm. Drug Deliv. 2022, 35, 2–10. [Google Scholar] [CrossRef]
- Cunningham, E.; Larmor, J. On the Velocity of Steady Fall of Spherical Particles through Fluid Medium. Proc. R. Soc. Lond. Ser. A Contain. Pap. A Math. Phys. Character 1997, 83, 357–365. [Google Scholar] [CrossRef]
- Allen, M.D.; Raabe, O.G. Slip Correction Measurements of Spherical Solid Aerosol Particles in an Improved Millikan Apparatus. Aerosol Sci. Technol. 1985, 4, 269–286. [Google Scholar] [CrossRef]
- Graf, C. Silica, Amorphous. In Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2018; pp. 1–43. ISBN 978-0-471-23896-6. [Google Scholar]
- Hales, T.C. An Overview of the Kepler Conjecture. arXiv 2002, arXiv:math/9811071. [Google Scholar]
- Lau, R.; Chuah, H.K.L. Dynamic Shape Factor for Particles of Various Shapes in the Intermediate Settling Regime. Adv. Powder Technol. 2013, 24, 306–310. [Google Scholar] [CrossRef]
- Human Respiratory Tract Model for Radiological Protection. A Report of a Task Group of the International Commission on Radiological Protection. Ann. ICRP 1994, 24, 1–482.
- Ammari, W.G.; Mohammad, M.K.; Tayyem, R.F. The Impact of Patients’ Real-Life Environmental Temperature and Humidity Use Conditions of Tiotropium Dry Powder Inhaler on Its Aerosol Emission Characteristics. Eur. J. Pharm. Sci. 2019, 133, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Zhou, X.; Dai, W.; Yu, J.; Zhou, Z.; Tong, Z.; Yu, A. In Vitro and In Silico Investigations on Drug Delivery in the Mouth-Throat Models with Handihaler®. Pharm. Res. 2022, 39, 3005–3019. [Google Scholar] [CrossRef]
- Roberts, D.L.; Mitchell, J.P. Measurement of Aerodynamic Particle Size Distribution of Orally Inhaled Products by Cascade Impactor: How to Let the Product Specification Drive the Quality Requirements of the Cascade Impactor. AAPS PharmSciTech 2019, 20, 57. [Google Scholar] [CrossRef]
- Roberts, D.L.; Mitchell, J.P. Quality Requirements for Cascade Impactors Assigned to Batch Release Testing of a Specific Drug Product; Part II: The Concept of “Sufficient” as Applied to Impactor Quality Specifications; CSC Publishing: Singapore, 2019. [Google Scholar]
- Klinger-Strobel, M.; Lautenschläger, C.; Fischer, D.; Mainz, J.G.; Bruns, T.; Tuchscherr, L.; Pletz, M.W.; Makarewicz, O. Aspects of Pulmonary Drug Delivery Strategies for Infections in Cystic Fibrosis—Where Do We Stand? Expert Opin. Drug Deliv. 2015, 12, 1351–1374. [Google Scholar] [CrossRef] [PubMed]
- De Boer, A.H.; Hagedoorn, P.; Hoppentocht, M.; Buttini, F.; Grasmeijer, F.; Frijlink, H.W. Dry Powder Inhalation: Past, Present and Future. Expert Opin. Drug Deliv. 2017, 14, 499–512. [Google Scholar] [CrossRef]
- DeHaan, W.H.; Finlay, W.H. Predicting Extrathoracic Deposition from Dry Powder Inhalers. J. Aerosol Sci. 2004, 35, 309–331. [Google Scholar] [CrossRef]
- Heyder, J. Deposition of Inhaled Particles in the Human Respiratory Tract and Consequences for Regional Targeting in Respiratory Drug Delivery. Proc. Am. Thorac. Soc. 2004, 1, 315–320. [Google Scholar] [CrossRef]
- Darquenne, C. Deposition Mechanisms. J. Aerosol Med. Pulm. Drug Deliv. 2020, 33, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Rostami, A.A. Computational Modeling of Aerosol Deposition in Respiratory Tract: A Review. Inhal. Toxicol. 2009, 21, 262–290. [Google Scholar] [CrossRef]
- Forrester, M.A.; Wassall, H.J.; Hall, L.S.; Cao, H.; Wilson, H.M.; Barker, R.N.; Vickers, M.A. Similarities and Differences in Surface Receptor Expression by THP-1 Monocytes and Differentiated Macrophages Polarized Using Seven Different Conditioning Regimens. Cell. Immunol. 2018, 332, 58–76. [Google Scholar] [CrossRef]
- López-García, J.; Lehocký, M.; Humpolíček, P.; Sáha, P. HaCaT Keratinocytes Response on Antimicrobial Atelocollagen Substrates: Extent of Cytotoxicity, Cell Viability and Proliferation. J. Funct. Biomater. 2014, 5, 43–57. [Google Scholar] [CrossRef]
- Tomatis, M.; Turci, F.; Ceschino, R.; Riganti, C.; Gazzano, E.; Martra, G.; Ghigo, D.; Fubini, B. High Aspect Ratio Materials: Role of Surface Chemistry vs. Length in the Historical “Long and Short Amosite Asbestos Fibers”. Inhal. Toxicol. 2010, 22, 984–998. [Google Scholar] [CrossRef]
- Aderem, A.; Underhill, D.M. Mechanisms of Phagocytosis in Macrophages. Annu. Rev. Immunol. 1999, 17, 593–623. [Google Scholar] [CrossRef]
- Geiser, M. Morphological Aspects of Particle Uptake by Lung Phagocytes. Microsc. Res. Tech. 2002, 57, 512–522. [Google Scholar] [CrossRef]
- Samuelson, D.R.; Welsh, D.A.; Shellito, J.E. Regulation of Lung Immunity and Host Defense by the Intestinal Microbiota. Front. Microbiol. 2015, 6, 1085. [Google Scholar] [CrossRef]
- Pluen, A.; Netti, P.A.; Jain, R.K.; Berk, D.A. Diffusion of Macromolecules in Agarose Gels: Comparison of Linear and Globular Configurations. Biophys. J. 1999, 77, 542–552. [Google Scholar] [CrossRef]
- Pacheco, P.; White, D.; Sulchek, T. Effects of Microparticle Size and Fc Density on Macrophage Phagocytosis. PLoS ONE 2013, 8, e60989. [Google Scholar] [CrossRef]
- Hirota, K.; Hasegawa, T.; Hinata, H.; Ito, F.; Inagawa, H.; Kochi, C.; Soma, G.-I.; Makino, K.; Terada, H. Optimum Conditions for Efficient Phagocytosis of Rifampicin-Loaded PLGA Microspheres by Alveolar Macrophages. J. Control. Release 2007, 119, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Rosales, C.; Uribe-Querol, E. Phagocytosis: A Fundamental Process in Immunity. Biomed. Res. Int. 2017, 2017, 9042851. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S. Phagocytosis: An Immunobiologic Process. Immunity 2016, 44, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Champion, J.A.; Walker, A.; Mitragotri, S. Role of Particle Size in Phagocytosis of Polymeric Microspheres. Pharm. Res. 2008, 25, 1815–1821. [Google Scholar] [CrossRef]
- Doshi, N.; Mitragotri, S. Macrophages Recognize Size and Shape of Their Targets. PLoS ONE 2010, 5, e10051. [Google Scholar] [CrossRef]
- Möller, J.; Luehmann, T.; Hall, H.; Vogel, V. The Race to the Pole: How High-Aspect Ratio Shape and Heterogeneous Environments Limit Phagocytosis of Filamentous Escherichia coli Bacteria by Macrophages. Nano Lett. 2012, 12, 2901–2905. [Google Scholar] [CrossRef]
- Gog, J.R.; Murcia, A.; Osterman, N.; Restif, O.; McKinley, T.J.; Sheppard, M.; Achouri, S.; Wei, B.; Mastroeni, P.; Wood, J.L.N.; et al. Dynamics of Salmonella Infection of Macrophages at the Single Cell Level. J. R. Soc. Interface 2012, 9, 2696–2707. [Google Scholar] [CrossRef]
- Padmore, T.; Stark, C.; Turkevich, L.A.; Champion, J.A. Quantitative Analysis of the Role of Fiber Length on Phagocytosis and Inflammatory Response by Alveolar Macrophages. Biochim. Biophys. Acta-Gen. Subj. 2017, 1861, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Champion, J.A.; Mitragotri, S. Role of Target Geometry in Phagocytosis. Proc. Natl. Acad. Sci. USA 2006, 103, 4930–4934. [Google Scholar] [CrossRef] [PubMed]
- Spano, A.; Barni, S.; Sciola, L. PMA Withdrawal in PMA-treated Monocytic THP-1 Cells and Subsequent Retinoic Acid Stimulation, Modulate Induction of Apoptosis and Appearance of Dendritic Cells. Cell Prolif. 2013, 46, 328–347. [Google Scholar] [CrossRef]
- Ombid, R.J.L.; Oyong, G.G.; Cabrera, E.C.; Espulgar, W.V.; Saito, M.; Tamiya, E.; Pobre, R.F. In-Vitro Study of Monocytic THP-1 Leukemia Cell Membrane Elasticity with a Single-Cell Microfluidic-Assisted Optical Trapping System. Biomed. Opt. Express 2020, 11, 6027–6037. [Google Scholar] [CrossRef] [PubMed]
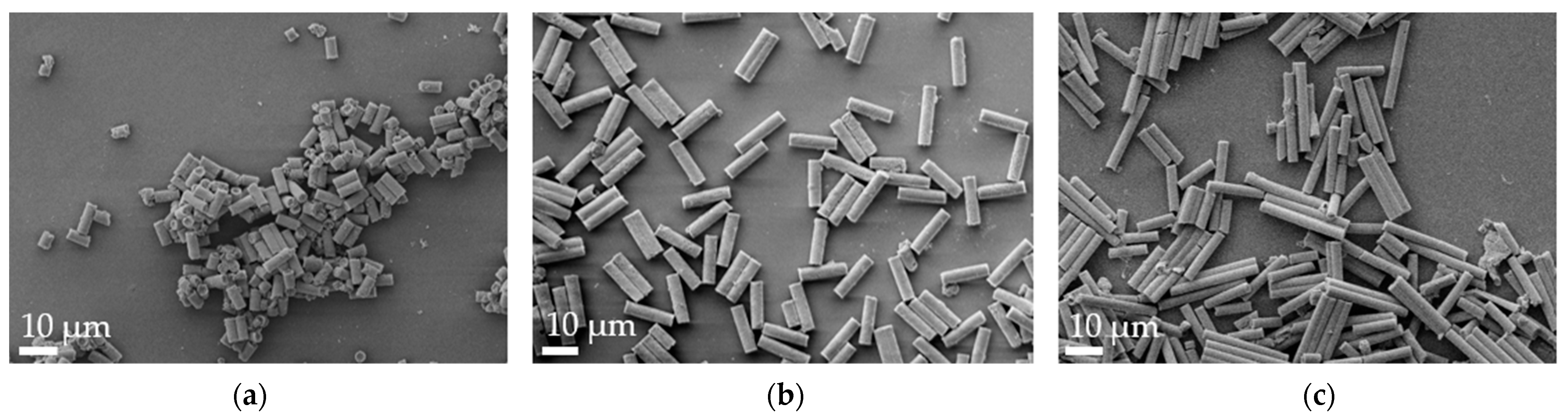

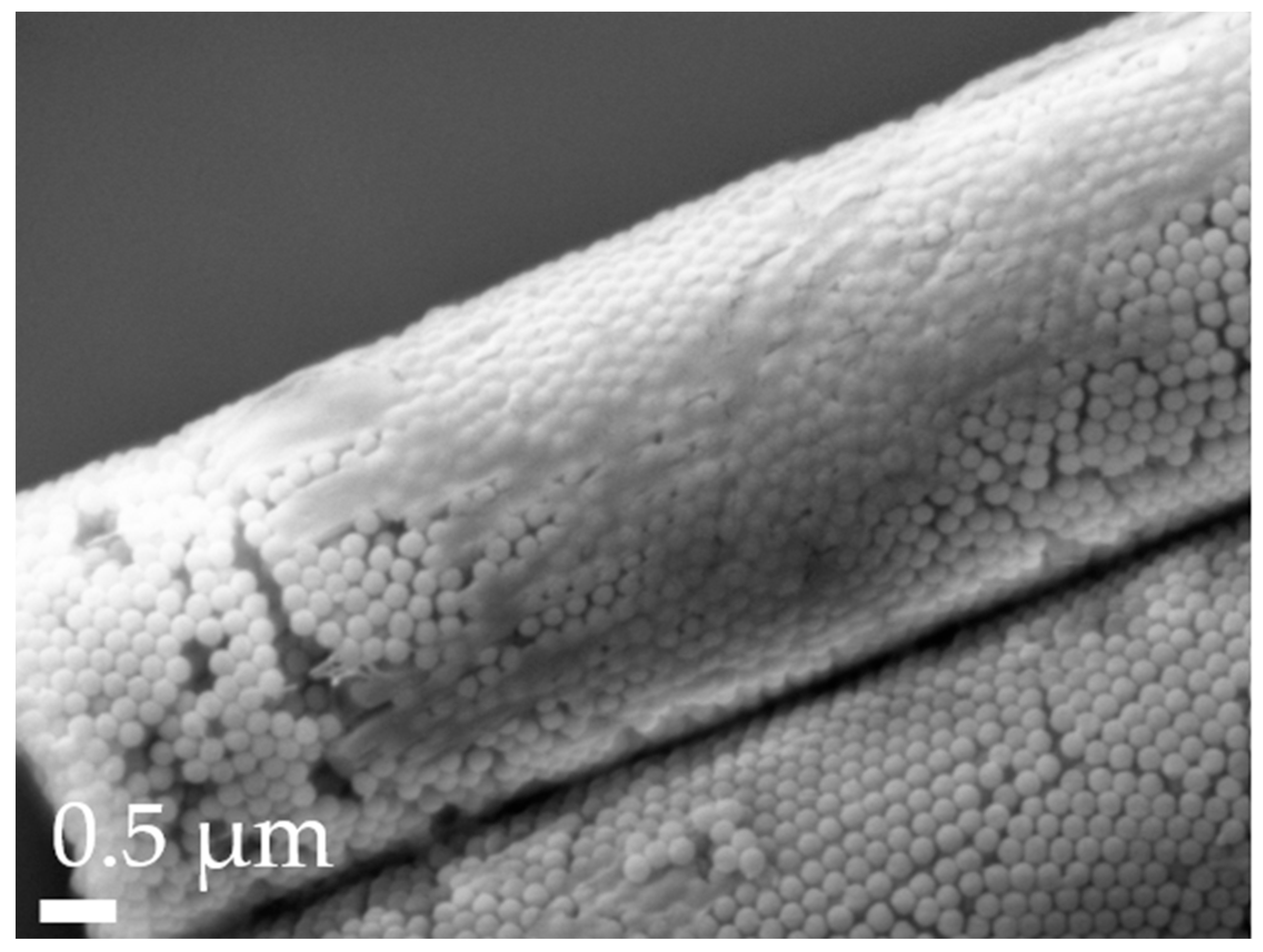

| Formulation | Length [µm] | Width [µm] |
|---|---|---|
| R-7 | 7.84 ± 0.59 | 3.46 ± 0.38 |
| R-12 | 12.65 ± 1.16 | 3.43 ± 0.77 |
| R-22 | 22.44 ± 0.62 | 3.70 ± 0.86 |
| Formulation | t90 [h] | t50 [h] | t10 [h] |
|---|---|---|---|
| Standard (1%, 1×) | 0.49 | 2.20 | 9.77 |
| 2× | 0.65 | 3.04 | 14.07 |
| 4× | 0.88 | 3.75 | 15.94 |
| 0.25% | 0.41 | 1.33 | 4.29 |
| 4% | 0.79 | 3.89 | 21.89 |
| Formulation | MMAD [µm] | GSD | FPF [%] |
|---|---|---|---|
| R-7 | 2.88 ± 0.23 | 2.49 ± 0.16 | 34.27 ± 2.95 |
| R-12 | 3.62 ± 0.35 | 2.27 ± 0.11 | 30.95 ± 2.25 |
| R-22 | 4.72 ± 0.48 | 2.26 ± 0.13 | 23.31 ± 2.86 **** |
| S-3 | 4.72 ± 0.18 | 2.32 ± 0.17 | 24.19 ± 2.65 *** |
| S-5 | 7.11 ± 0.02 | 2.10 ± 0.03 | 16.92 ± 4.51 **** |
| S-10 | 10.95 ± 0.85 **** | 2.66 ± 0.10 | 14.34 ± 1.90 **** |
| Formulation | (Calculated) | (Literature, [82]) | da [µm] |
|---|---|---|---|
| R-7 | 3.49 ± 0.54 | 1.75 | 4.18 |
| R-12 | 3.26 ± 0.60 | 2.25 | 4.47 |
| R-22 | 2.78 ± 0.56 | 3.0 | 4.65 |
| S-3 | / | 1 | 4.49 |
| S-5 | / | 1 | 7.42 |
| S-10 | / | 1 | 14.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pioch, T.; Fischer, T.; Schneider, M. Aspherical, Nano-Structured Drug Delivery System with Tunable Release and Clearance for Pulmonary Applications. Pharmaceutics 2024, 16, 232. https://doi.org/10.3390/pharmaceutics16020232
Pioch T, Fischer T, Schneider M. Aspherical, Nano-Structured Drug Delivery System with Tunable Release and Clearance for Pulmonary Applications. Pharmaceutics. 2024; 16(2):232. https://doi.org/10.3390/pharmaceutics16020232
Chicago/Turabian StylePioch, Tomas, Thorben Fischer, and Marc Schneider. 2024. "Aspherical, Nano-Structured Drug Delivery System with Tunable Release and Clearance for Pulmonary Applications" Pharmaceutics 16, no. 2: 232. https://doi.org/10.3390/pharmaceutics16020232
APA StylePioch, T., Fischer, T., & Schneider, M. (2024). Aspherical, Nano-Structured Drug Delivery System with Tunable Release and Clearance for Pulmonary Applications. Pharmaceutics, 16(2), 232. https://doi.org/10.3390/pharmaceutics16020232








