3D Printing of Biodegradable Polymeric Microneedles for Transdermal Drug Delivery Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Procedures
2.2. Design
2.3. Material
2.4. Drug-Loading Preparation
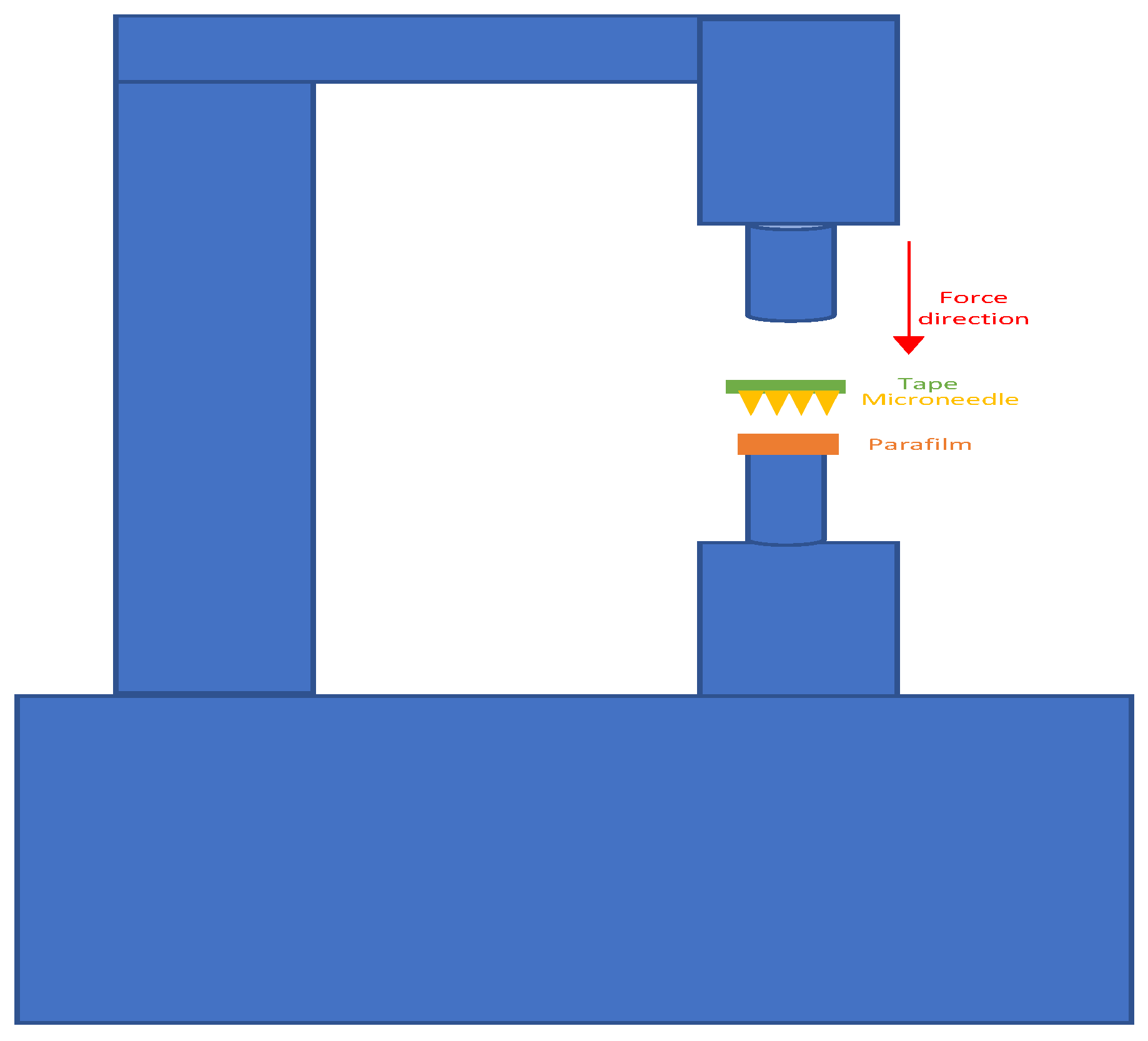
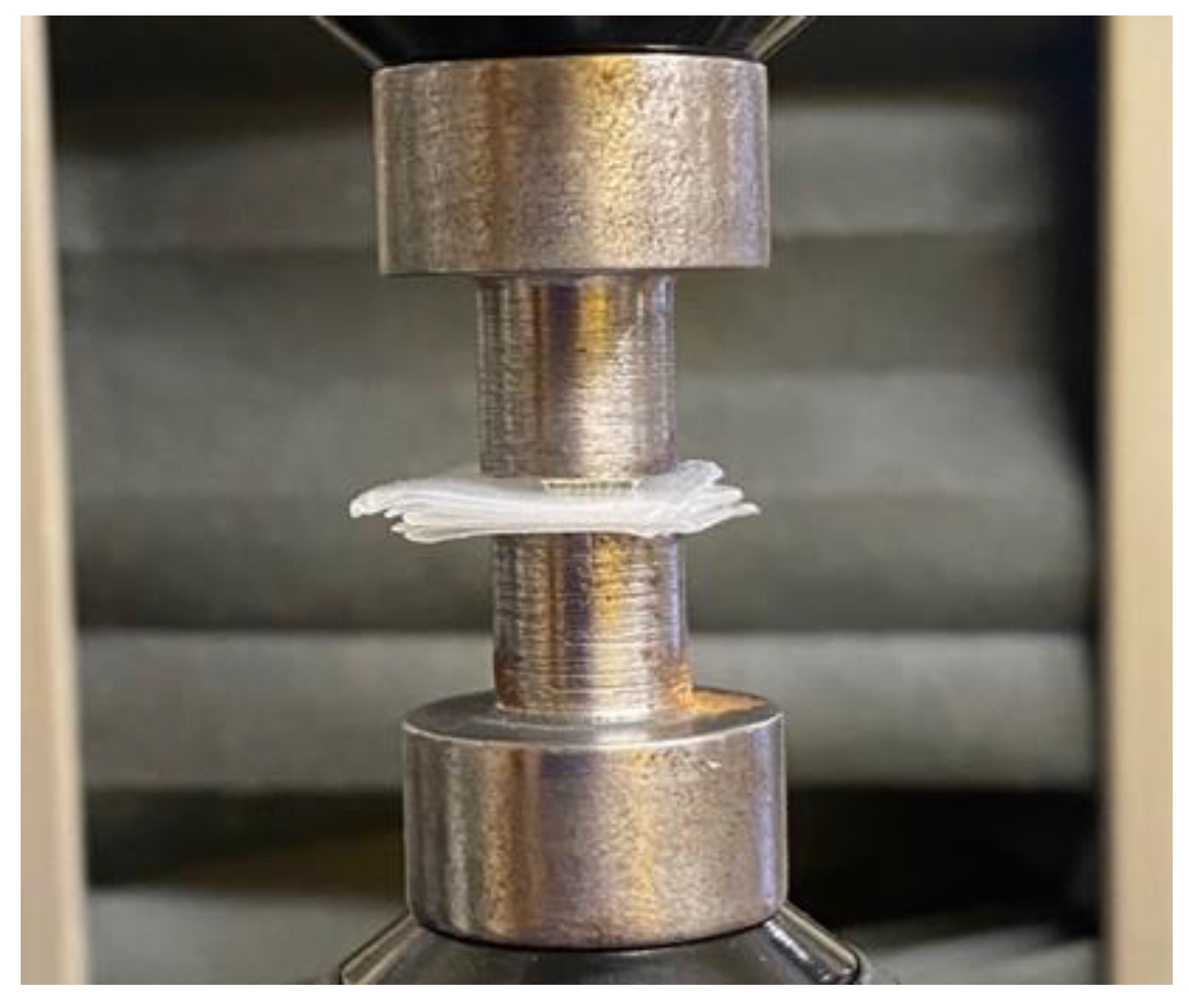
2.5. Mechanical Testing
2.6. Insertion Test
3. Results
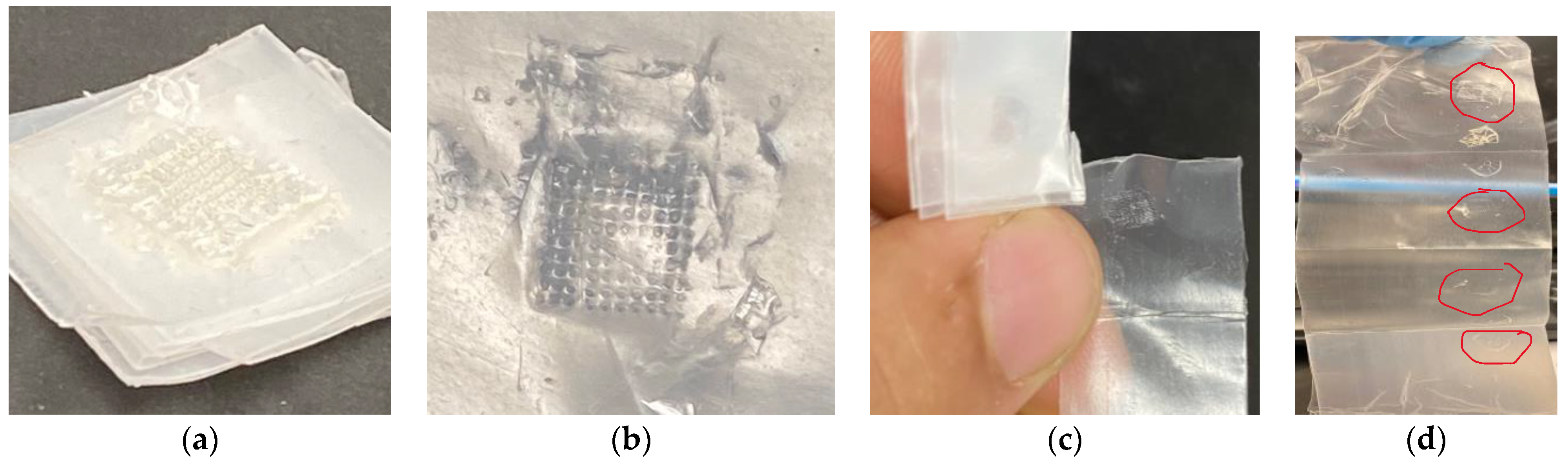
3.1. Printing Accuracy of 3D-Printed and Biodegradable Polymer MN Array
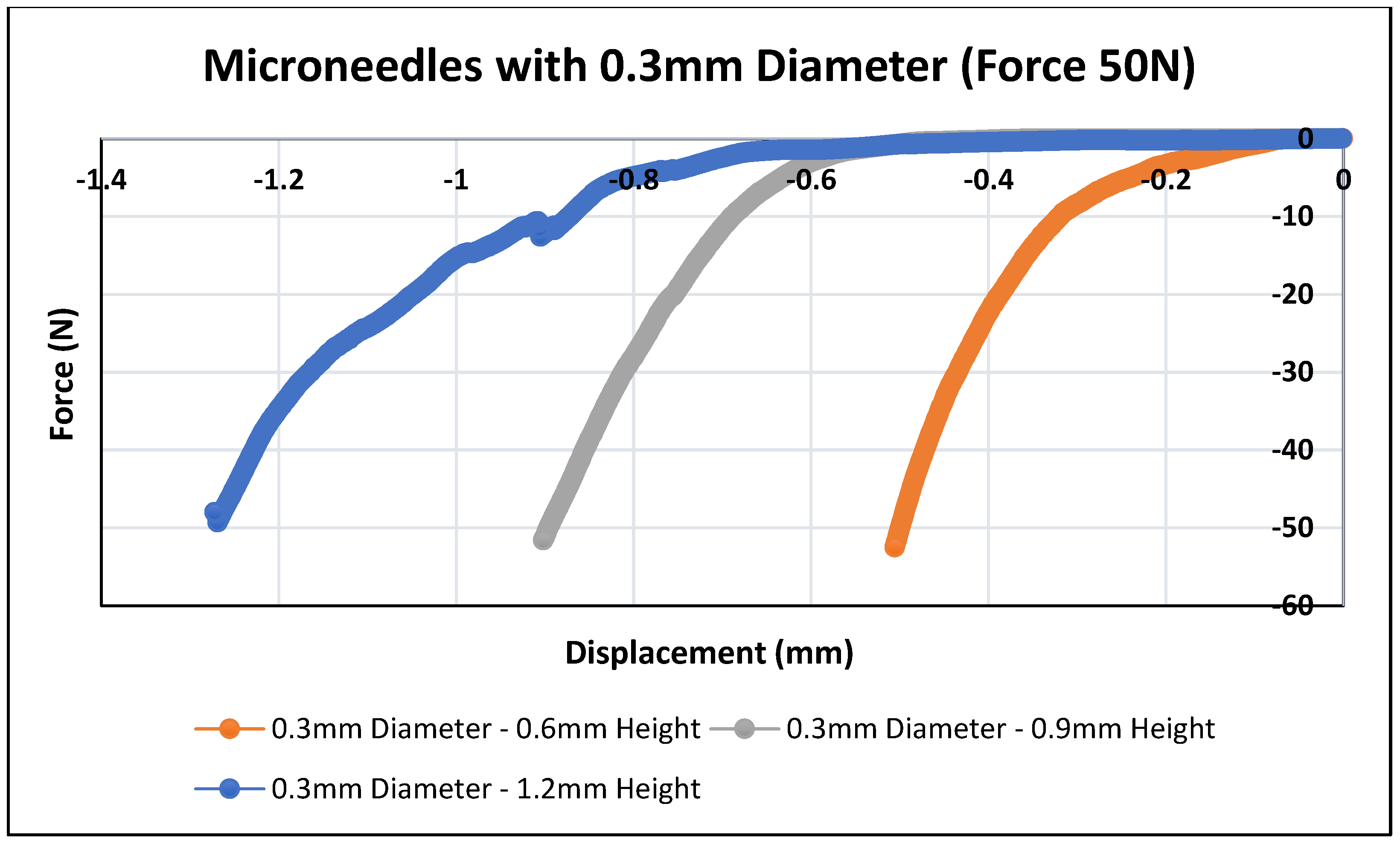
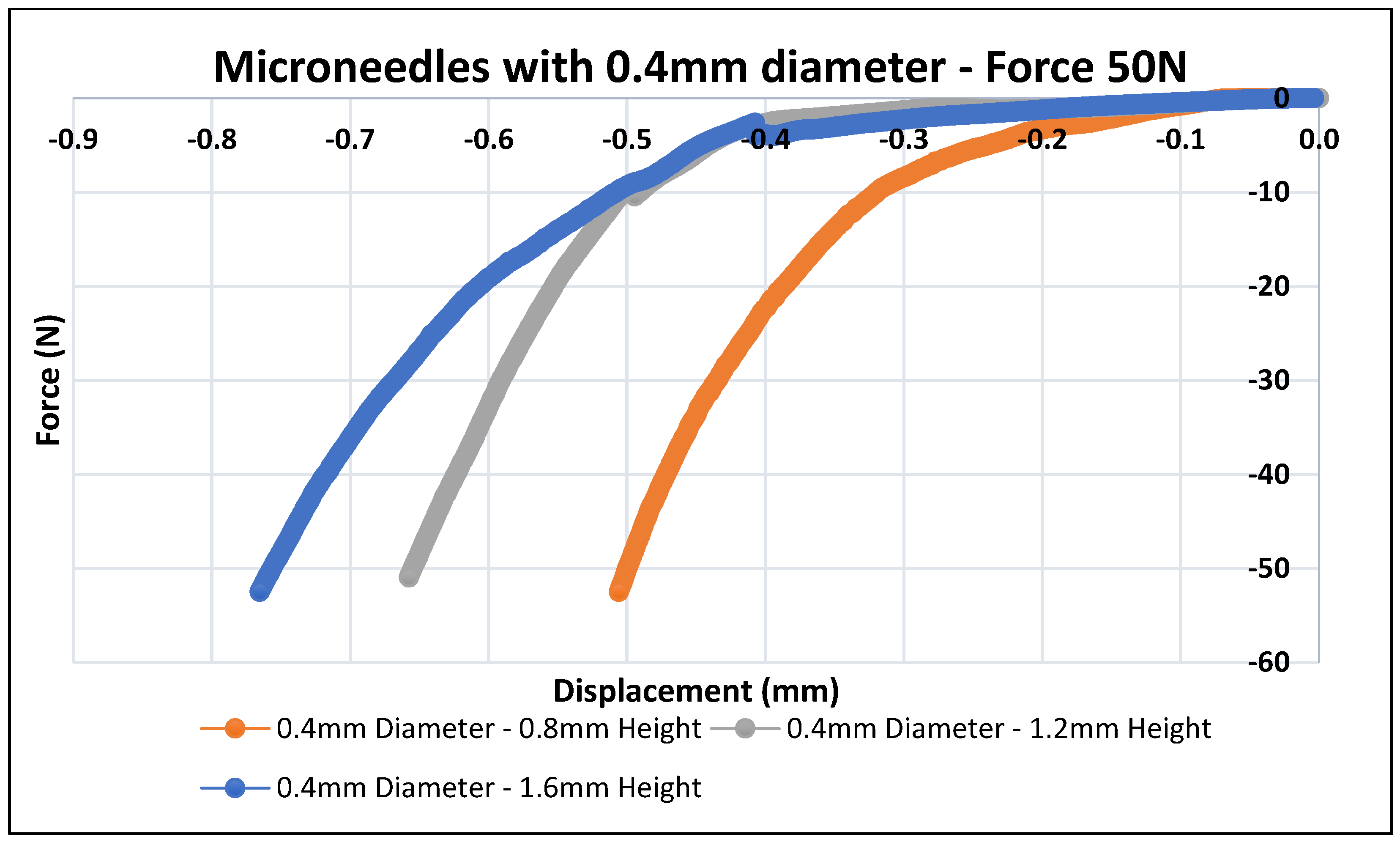
3.2. Mechanical Testing of Biodegradable Polymeric MN
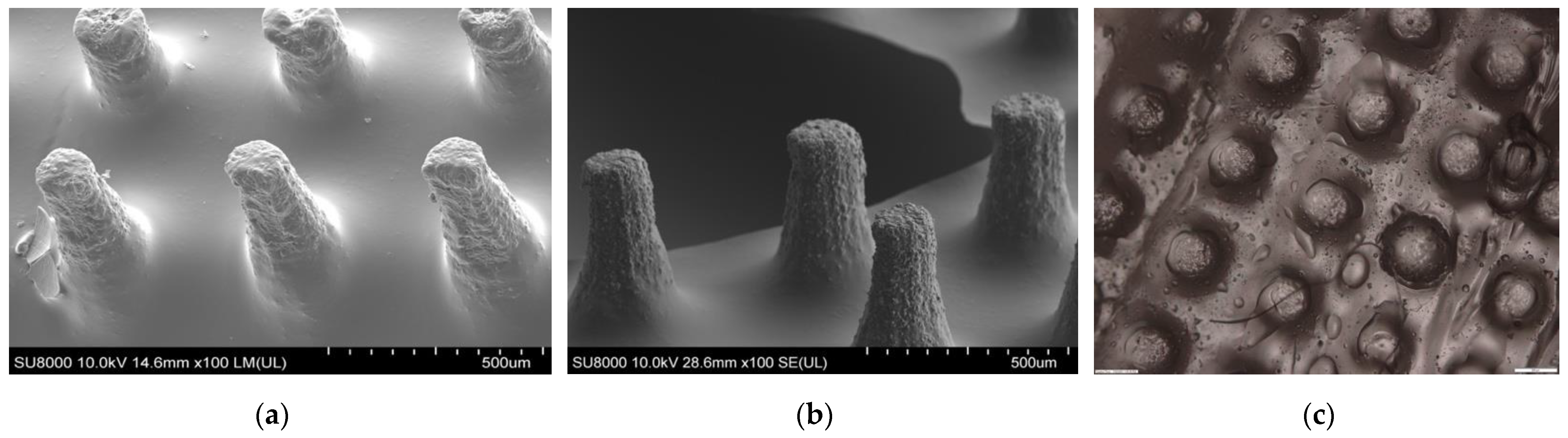
3.3. Insertion Test of Polymeric MN
3.4. Comparative Analysis between AM and Laser Ablation Method
4. Limitations and Future Work
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, T.R.R.; Mcmillan, H.; Mooney, K.; Alkilani, A.Z.; Donnelly, R.F. 6—Microneedles for drug delivery and monitoring. In Microfluidic Devices for Biomedical Applications; Li, X., Zhou, Y., Eds.; Woodhead Publishing Series in Biomaterials; Woodhead Publishing: Sawston, UK, 2013; pp. 185–230. [Google Scholar] [CrossRef]
- Prausnitz, M.R. Microneedles for transdermal drug delivery. In Advanced Drug Delivery Reviews; Elsevier: Amsterdam, The Netherlands, 2004; Volume 56, pp. 581–587. [Google Scholar] [CrossRef]
- Donnelly, R.F.; Prausnitz, M.R. The promise of microneedle technologies for drug delivery. Drug Deliv. and Transl. Res. 2024, 14, 573–580. [Google Scholar] [CrossRef]
- Glover, K.; Mishra, D.; Gade, S.; Lalitkumar, K.; Wu, Y.; Paredes, A.J.; Donnelly, R.F.; Singh, T.R.R. Microneedles for advanced ocular drug delivery. Adv. Drug Deliv. Rev. 2023, 201, 115082. [Google Scholar] [CrossRef]
- Le, Z.; Yu, J.; Quek, Y.J.; Bai, B.; Li, X.; Shou, Y.; Myint, B.; Xu, C.; Tay, A. Design principles of microneedles for drug delivery and sampling applications. Mater. Today 2023, 63, 137–169. [Google Scholar] [CrossRef]
- Liao, Z.; Zhou, Q.; Gao, B. Electrochemical Microneedles: Innovative Instruments in Health Care. Biosensors 2022, 12, 801. [Google Scholar] [CrossRef]
- Ozyilmaz, E.D.; Turan, A.; Comoglu, T. An overview on the advantages and limitations of 3D printing of microneedles. Pharm. Dev. Technol. 2021, 26, 923–933. [Google Scholar] [CrossRef]
- Li, X.; Shan, W.; Yang, Y.; Joralmon, D.; Zhu, Y.; Chen, Y.; Yuan, Y.; Xu, H.; Rong, J.; Dai, R.; et al. Limpet Tooth-Inspired Painless Microneedles Fabricated by Magnetic Field-Assisted 3D Printing. Adv. Funct. Mater. 2021, 31, 2003725. [Google Scholar] [CrossRef]
- Aldawood, F.K.; Andar, A.; Desai, S. A Comprehensive Review of Microneedles: Types, Materials, Processes, Characterizations and Applications. Polymers 2021, 13, 2815. [Google Scholar] [CrossRef] [PubMed]
- Filho, D.; Guerrero, M.; Pariguana, M.; Marican, A.; Durán-Lara, E.F. Hydrogel-Based Microneedle as a Drug Delivery System. Pharmaceutics 2023, 15, 2444. [Google Scholar] [CrossRef] [PubMed]
- Bauleth-Ramos, T.; El-Sayed, N.; Fontana, F.; Lobita, M.; Shahbazi, M.-A.; Santos, H.A. Recent approaches for enhancing the performance of dissolving microneedles in drug delivery applications. Mater. Today 2023, 63, 239–287. [Google Scholar] [CrossRef]
- Chakraborty, R.; Afrose, N.; Kuotsu, K. Novel synergistic approaches of protein delivery through physical enhancement for transdermal microneedle drug delivery: A review. J. Drug Deliv. Sci. Technol. 2023, 84, 104467. [Google Scholar] [CrossRef]
- Joshi, N.; Machekposhti, S.A.; Narayan, R.J. Evolution of Transdermal Drug Delivery Devices and Novel Microneedle Technologies: A Historical Perspective and Review. JID Innov. 2023, 3, 100225. [Google Scholar] [CrossRef]
- Khalid, R.; Mahmood, S.; Sofian, Z.M.; Hilles, A.R.; Hashim, N.M.; Ge, Y. Microneedles and Their Application in Transdermal Delivery of Antihypertensive Drugs—A Review. Pharmaceutics 2023, 15, 2029. [Google Scholar] [CrossRef]
- Nguyen, H.X.; Nguyen, C.N. Microneedle-Mediated Transdermal Delivery of Biopharmaceuticals. Pharmaceutics 2023, 15, 277. [Google Scholar] [CrossRef]
- Donnelly, R.F.; Morrow, D.I.; McCarron, P.A.; David Woolfson, A.; Morrissey, A.; Juzenas, P.; Juzeniene, A.; Iani, V.; McCarthy, H.O.; Moan, J. Microneedle Arrays Permit Enhanced Intradermal Delivery of a Preformed Photosensitizer. Photochem. Photobiol. 2009, 85, 195–204. [Google Scholar] [CrossRef]
- Dangol, M.; Kim, S.; Li, C.G.; Lahiji, S.F.; Jang, M.; Ma, Y.; Huh, I.; Jung, H. Anti-obesity effect of a novel caffeine-loaded dissolving microneedle patch in high-fat diet-induced obese C57BL/6J mice. J. Control. Release 2017, 265, 41–47. [Google Scholar] [CrossRef]
- Perez Cuevas, M.B.; Kodani, M.; Choi, Y.; Joyce, J.; O’Connor, S.M.; Kamili, S.; Prausnitz, M.R. Hepatitis B vaccination using a dissolvable microneedle patch is immunogenic in mice and rhesus macaques. Bioeng. Transl. Med. 2018, 3, 186–196. [Google Scholar] [CrossRef]
- Poirier, D.; Renaud, F.; Dewar, V.; Strodiot, L.; Wauters, F.; Janimak, J.; Shimada, T.; Nomura, T.; Kabata, K.; Kuruma, K.; et al. Hepatitis B surface antigen incorporated in dissolvable microneedle array patch is antigenic and thermostable. Biomaterials 2017, 145, 256–265. [Google Scholar] [CrossRef]
- Edens, C.; Dybdahl-Sissoko, N.C.; Weldon, W.C.; Oberste, M.S.; Prausnitz, M.R. Inactivated polio vaccination using a microneedle patch is immunogenic in the rhesus macaque. Vaccine 2015, 33, 4683–4690. [Google Scholar] [CrossRef]
- Jang, M.; Baek, S.; Kang, G.; Yang, H.; Kim, S.; Jung, H. Dissolving microneedle with high molecular weight hyaluronic acid to improve skin wrinkles, dermal density and elasticity. Int. J. Cosmet. Sci. 2020, 42, 302–309. [Google Scholar] [CrossRef]
- Sakuraba, K.; Kojima, Y.; Terahara, T.; Kuma, H.; Tokudome, Y. Non-invasive Microneedle Application Increases Ceramide and Natural Moisturizing Factors in a Reconstructed Human Skin Model. Biol. Pharm. Bull. 2023, 46, 1310–1315. [Google Scholar] [CrossRef]
- Chang, H.; Zheng, M.; Yu, X.; Than, A.; Seeni, R.Z.; Kang, R.; Tian, J.; Khanh, D.P.; Liu, L.; Chen, P.; et al. A Swellable Microneedle Patch to Rapidly Extract Skin Interstitial Fluid for Timely Metabolic Analysis. Adv. Mater. 2017, 29, 1702243. [Google Scholar] [CrossRef]
- Grogan, S.P.; Dorthé, E.W.; Glembotski, N.E.; Gaul, F.; D’Lima, D.D. Cartilage tissue engineering combining microspheroid building blocks and microneedle arrays. Connect. Tissue Res. 2020, 61, 229–243. [Google Scholar] [CrossRef]
- Rong, X.; Mehwish, N.; Niu, X.; Zhu, N.; Lee, B.H. Human Albumin-Based Hydrogels for Their Potential Xeno-Free Microneedle Applications. Macromol. Biosci. 2023, 23, 2200463. [Google Scholar] [CrossRef]
- Global Medical Devices Market Report and Forecast 2023–2031. Available online: https://www.researchandmarkets.com/reports/5786892/global-medical-devices-market-report-forecast (accessed on 20 November 2023).
- Baker-Sediako, R.D.; Richter, B.; Blaicher, M.; Thiel, M.; Hermatschweiler, M. Industrial perspectives for personalized microneedles. Beilstein J. Nanotechnol. 2023, 14, 857–864. [Google Scholar] [CrossRef]
- Ma, X.; Zhou, Q.; Gao, B. Recent advances of biosensors on microneedles. Anal. Methods 2023, 15, 5711–5730. [Google Scholar] [CrossRef]
- Li, J.; Ge, R.; Lin, K.; Wang, J.; He, Y.; Lu, H.; Dong, H. Advances in the Application of Microneedles in the Treatment of Local Organ Diseases. Small 2023, 2306222. [Google Scholar] [CrossRef]
- Global Microneedle Drug Delivery Systems Market Size and Forecast to 2030. Available online: https://www.skyquestt.com/report/microneedle-drug-delivery-systems-market (accessed on 20 November 2023).
- Microneedle Drug Delivery Systems Market Report, Trends Forecast By 2032. January 2023. Available online: https://www.thebusinessresearchcompany.com/report/microneedle-drug-delivery-systems-global-market-report (accessed on 20 November 2023).
- Henry, S.; McAllister, D.V.; Allen, M.G.; Prausnitz, M.R. Microfabricated microneedles: A novel approach to transdermal drug delivery. J. Pharm. Sci. 1998, 87, 922–925. [Google Scholar] [CrossRef]
- Shriky, B.; Babenko, M.; Whiteside, B.R. Dissolving and Swelling Hydrogel-Based Microneedles: An Overview of Their Materials, Fabrication, Characterization Methods, and Challenges. Gels 2023, 9, 806. [Google Scholar] [CrossRef]
- Faraji Rad, Z. Microneedle Technologies for Food and Crop Health: Recent Advances and Future Perspectives. Adv. Eng. Mater. 2023, 25, 2201194. [Google Scholar] [CrossRef]
- Aldawood, F.K.; Desai, S. Laser Fabrication of Polymeric Microneedles for Transdermal Drug Delivery. In Proceedings of the 2021 IISE Annual Conference, Online, 22–25 May 2021. [Google Scholar]
- Aldawood, F.K.; Desai, S.; Parupelli, S.K.; Andar, A. Polymeric Microneedles Using Additive Manufacturing for Therapeutic Applications. In Proceedings of the IIE Annual Conference. Proceedings, New Orleans, LA, USA, 21–23 May 2023. [Google Scholar]
- Perkins, J.; Xu, Z.; Smith, C.; Roy, A.; Kumta, P.N.; Waterman, J.; Conklin, D.; Desai, S. Direct writing of polymeric coatings on magnesium alloy for tracheal stent applications. Ann. Biomed. Eng. 2015, 43, 1158–1165. [Google Scholar] [CrossRef]
- Perkins, J.; Hong, Y.; Ye, S.-H.; Wagner, W.R.; Desai, S. Direct writing of bio-functional coatings for cardiovascular applications. J. Biomed. Mater. Res. A 2014, 102, 4290–4300. [Google Scholar] [CrossRef]
- Tettey, F.; Saudi, S.; Davies, D.; Shrestha, S.; Johnson, K.; Fialkova, S.; Subedi, K.; Bastakoti, B.P.; Sankar, J.; Desai, S.; et al. Fabrication and Characterization of Zn Particle Incorporated Fibrous Scaffolds for Potential Application in Tissue Healing and Regeneration. ACS Appl. Mater. Interfaces 2023, 15, 48913–48929. [Google Scholar] [CrossRef]
- Desai, S.; Perkins, J.; Harrison, B.S.; Sankar, J. Understanding release kinetics of biopolymer drug delivery microcapsules for biomedical applications. Mater. Sci. Eng. B 2010, 168, 127–131. [Google Scholar] [CrossRef]
- Desai, S. Methods and Apparatus of Manufacturing Micro and Nano-Scale Features. U.S. Patent US8573757B2, 5 November 2013. [Google Scholar]
- Ako, H.; O’Mahony, J.; Hughes, H.; McLoughlin, P.; O’Reilly, N.J. A novel approach to the manufacture of dissolving microneedles arrays using aerosol jet printing. Appl. Mater. Today 2023, 35, 101958. [Google Scholar] [CrossRef]
- Tamez-Tamez, J.I.; Vázquez-Lepe, E.; Rodriguez, C.A.; Martínez-López, J.I.; García-López, E. Assessment of geometrical dimensions and puncture feasibility of microneedles manufactured by micromilling. Int. J. Adv. Manuf. Technol. 2023, 126, 4983–4996. [Google Scholar] [CrossRef]
- Lammerding, L.C.; Breitkreutz, J. Technical evaluation of precisely manufacturing customized microneedle array patches via inkjet drug printing. Int. J. Pharm. 2023, 642, 123173. [Google Scholar] [CrossRef]
- Dong, C.-W.; Jeon, J.-Y.; Kang, H.-M.; Park, W.-T. Tip fabrication methods of hollow metal microneedles. J. Mech. Sci. Technol. 2023, 37, 261–269. [Google Scholar] [CrossRef]
- Anjani, Q.K.; Cárcamo-Martínez, Á.; Wardoyo, L.A.H.; Moreno-Castellanos, N.; Sabri, A.H.B.; Larrañeta, E.; Donnelly, R.F. MAP-box: A novel, low-cost and easy-to-fabricate 3D-printed box for the storage and transportation of dissolving microneedle array patches. Drug Deliv. Transl. Res. 2024, 14, 208–222. [Google Scholar] [CrossRef]
- Gowda, B.H.J.; Ahmed, M.G.; Hani, U.; Kesharwani, P.; Wahab, S.; Paul, K. Microneedles as a momentous platform for psoriasis therapy and diagnosis: A state-of-the-art review. Int. J. Pharm. 2023, 632, 122591. [Google Scholar] [CrossRef]
- Sawaira, T.; Jamil, A.; Aziz, S.; Mujahid, A.; Hussain, T.; Afzal, A. Low-cost, disposable sensors based on polystyrene waste and V–TiO2 microneedle composites for diagnosis of renal malfunction and muscle dystrophy. Compos. Commun. 2023, 37, 101398. [Google Scholar] [CrossRef]
- Adarkwa, E.; Roy, A.; Ohodnicki, J.; Lee, B.; Kumta, P.N.; Desai, S. 3D printing of drug-eluting bioactive multifunctional coatings for orthopedic applications. Int. J. Bioprint. 2023, 9, 661. [Google Scholar] [CrossRef]
- Adarkwa, E.; Kotoka, R.; Desai, S. 3D printing of polymeric Coatings on AZ31 Mg alloy Substrate for Corrosion Protection of biomedical implants. Med. Devices Sens. 2021, 4, e10167. [Google Scholar] [CrossRef]
- Parupelli, S.K.; Desai, S. A Comprehensive Review of Additive Manufacturing (3D Printing): Processes, Applications and Future Potential. AJAS 2019, 16, 244–272. [Google Scholar] [CrossRef]
- Parupelli, S.K.; Saudi, S.; Bhattarai, N.; Desai, S. 3D printing of PCL-ceramic composite scaffolds for bone tissue engineering applications. Int. J. Bioprint. 2023, 9, 0196. [Google Scholar] [CrossRef]
- Chen, Z.; Lin, Y.; Lee, W.; Ren, L.; Liu, B.; Liang, L.; Wang, Z.; Jiang, L. Additive Manufacturing of Honeybee-Inspired Microneedle for Easy Skin Insertion and Difficult Removal. ACS Appl. Mater. Interfaces 2018, 10, 29338–29346. [Google Scholar] [CrossRef]
- Johnson, R.; Procopio, A.T. Low cost additive manufacturing of microneedle masters. 3D Print. Med. 2019, 5, 2. [Google Scholar] [CrossRef]
- Li, R.; Zhang, L.; Jiang, X.; Li, L.; Wu, S.; Yuan, X.; Cheng, H.; Jiang, X.; Gou, M. 3D-printed microneedle arrays for drug delivery. J. Control. Release 2022, 350, 933–948. [Google Scholar] [CrossRef]
- Anbazhagan, G.; Suseela, S.B.; Sankararajan, R. Design, analysis and fabrication of solid polymer microneedle patch using CO2 laser and polymer molding. Drug Deliv. Transl. Res. 2023, 13, 1813–1827. [Google Scholar] [CrossRef]
- Sil, D.; Bhowmik, S.; Patel, P.; Kurmi, B.D. Promising role of microneedles in therapeutic and biomedical applications. J. Drug Deliv. Sci. Technol. 2024, 91, 105273. [Google Scholar] [CrossRef]
- Vora, L.K.; Sabri, A.H.; Naser, Y.; Himawan, A.; Hutton, A.R.; Anjani, Q.K.; Volpe-Zanutto, F.; Mishra, D.; Li, M.; Rodgers, A.M.; et al. Long-acting microneedle formulations. Adv. Drug Deliv. Rev. 2023, 201, 115055. [Google Scholar] [CrossRef]
- Luo, X.; Yang, L.; Cui, Y. Microneedles: Materials, fabrication, and biomedical applications. Biomed. Microdevices 2023, 25, 20. [Google Scholar] [CrossRef] [PubMed]
- Umeyor, C.E.; Shelke, V.; Pol, A.; Kolekar, P.; Jadhav, S.; Tiwari, N.; Anure, A.; Nayak, A.; Bairagi, G.; Agale, A.; et al. Biomimetic microneedles: Exploring the recent advances on a microfabricated system for precision delivery of drugs, peptides, and proteins. Future J. Pharm. Sci. 2023, 9, 103. [Google Scholar] [CrossRef]
- Detamornrat, U.; McAlister, E.; Hutton, A.R.J.; Larrañeta, E.; Donnelly, R.F. The Role of 3D Printing Technology in Microengineering of Microneedles. Small 2022, 18, 2106392. [Google Scholar] [CrossRef]
- Caudill, C.L.; Perry, J.L.; Tian, S.; Luft, J.C.; DeSimone, J.M. Spatially controlled coating of continuous liquid interface production microneedles for transdermal protein delivery. J. Control. Release 2018, 284, 122–132. [Google Scholar] [CrossRef]
- Mathew, E.; Pitzanti, G.; Santos, A.L.G.D.; Lamprou, D.A. Optimization of Printing Parameters for Digital Light Processing 3D Printing of Hollow Microneedle Arrays. Pharmaceutics 2021, 13, 1837. [Google Scholar] [CrossRef]
- Antonara, L.; Dallas, P.P.; Rekkas, D.M. A novel 3D printing enabled method for fast and reliable construction of polymeric microneedles using experimental design. J. Drug Deliv. Sci. Technol. 2022, 68, 102888. [Google Scholar] [CrossRef]
- Caudill, C.; Perry, J.L.; Iliadis, K.; Tessema, A.T.; Lee, B.J.; Mecham, B.S.; Tian, S.; DeSimone, J.M. Transdermal vaccination via 3D-printed microneedles induces potent humoral and cellular immunity. Proc. Natl. Acad. Sci. USA 2021, 118, e2102595118. [Google Scholar] [CrossRef]
- Arshad, M.S.; Zafar, S.; Zahra, A.T.; Zaman, M.H.; Akhtar, A.; Kucuk, I.; Farhan, M.; Chang, M.W.; Ahmad, Z. Fabrication and characterisation of self-applicating heparin sodium microneedle patches. J. Drug Target. 2021, 29, 60–68. [Google Scholar] [CrossRef]
- Larrañeta, E.; Moore, J.; Vicente-Pérez, E.M.; González-Vázquez, P.; Lutton, R.; Woolfson, A.D.; Donnelly, R.F. A proposed model membrane and test method for microneedle insertion studies. Int. J. Pharm. 2014, 472, 65–73. [Google Scholar] [CrossRef]
- Liu, R.X.; He, Y.T.; Liang, L.; Hu, L.F.; Liu, Y.; Yu, R.X.; Chen, B.Z.; Cui, Y.; Guo, X.D. Mechanical evaluation of polymer microneedles for transdermal drug delivery: In vitro and in vivo. J. Ind. Eng. Chem. 2022, 114, 181–189. [Google Scholar] [CrossRef]
- Larrañeta, E.; Stewart, S.; Fallows, S.J.; Birkhäuer, L.L.; McCrudden, M.T.; Woolfson, A.D.; Donnelly, R.F. A facile system to evaluate in vitro drug release from dissolving microneedle arrays. Int. J. Pharm. 2016, 497, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Lalehpour, A.; Janeteas, C.; Barari, A. Surface roughness of FDM parts after post-processing with acetone vapor bath smoothing process. Int. J. Adv. Manuf. Technol. 2018, 95, 1505–1520. [Google Scholar] [CrossRef]
- Krieger, K.J.; Bertollo, N.; Dangol, M.; Sheridan, J.T.; Lowery, M.M.; O’Cearbhaill, E.D. Simple and customizable method for fabrication of high-aspect ratio microneedle molds using low-cost 3D printing. Microsyst. Nanoeng. 2019, 5, 42. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.R.; Caudill, C.L.; Tumbleston, J.R.; Bloomquist, C.J.; Moga, K.A.; Ermoshkin, A.; Shirvanyants, D.; Mecham, S.J.; Luft, J.C.; DeSimone, J.M. Single-Step Fabrication of Computationally Designed Microneedles by Continuous Liquid Interface Production. PLoS ONE 2016, 11, e0162518. [Google Scholar] [CrossRef] [PubMed]
- Kundu, A.; Arnett, P.; Bagde, A.; Azim, N.; Kouagou, E.; Singh, M.; Rajaraman, S. DLP 3D Printed ‘Intelligent’ Microneedle Array (iμNA) for Stimuli Responsive Release of Drugs and Its In Vitro and Ex Vivo Characterization. J. Microelectromechan. Syst. 2020, 29, 685–691. [Google Scholar] [CrossRef]
- Shah, S.A.; Oakes, R.S.; Kapnick, S.M.; Jewell, C.M. Mapping the Mechanical and Immunological Profiles of Polymeric Microneedles to Enable Vaccine and Immunotherapy Applications. Front. Immunol. 2022, 13, 843355. [Google Scholar] [CrossRef]
- Ning, X.; Wiraja, C.; Lio, D.C.S.; Xu, C. A Double-Layered Microneedle Platform Fabricated through Frozen Spray-Coating. Adv. Healthc. Mater. 2020, 9, 2000147. [Google Scholar] [CrossRef]
- Chi, Y.; Huang, Y.; Kang, Y.; Dai, G.; Liu, Z.; Xu, K.; Zhong, W. The effects of molecular weight of hyaluronic acid on transdermal delivery efficiencies of dissolving microneedles. Eur. J. Pharm. Sci. 2022, 168, 106075. [Google Scholar] [CrossRef]
- Römgens, M.; Bader, D.L.; Bouwstra, J.A.; Baaijens, F.P.T.; Oomens, C.W.J. Monitoring the penetration process of single microneedles with varying tip diameters. J. Mech. Behav. Biomed. Mater. 2014, 40, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Potts, M.R.; Evans, S.L.; Pullin, R.; Coulman, S.A.; Birchall, J.C.; Wyatt, H. An analysis of the relationship between microneedle spacing, needle force and skin strain during the indentation phase prior to skin penetration. Comput. Methods Biomech. Biomed. Eng. 2023, 26, 1719–1731. [Google Scholar] [CrossRef] [PubMed]
- Jeggy, C. Micro-Injection Moulding: From Process to Modelling; Presses Universitaires de Louvain: Louvain-la-Neuve, Belgium, 2004. [Google Scholar]
- Olowe, M.; Parupelli, S.K.; Desai, S. A Review of 3D-Printing of Microneedles. Pharmaceutics 2022, 14, 2693. [Google Scholar] [CrossRef] [PubMed]
- Lutton, R.E.M.; Larrañeta, E.; Kearney, M.-C.; Boyd, P.; Woolfson, A.D.; Donnelly, R.F. A novel scalable manufacturing process for the production of hydrogel-forming microneedle arrays. Int. J. Pharm. 2015, 494, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Rad, Z.F.; Prewett, P.D.; Davies, G.J. High-resolution two-photon polymerization: The most versatile technique for the fabrication of microneedle arrays. Microsyst. Nanoeng. 2021, 7, 71. [Google Scholar] [CrossRef]
- Cerami, L.; Mazur, E.; Nolte, S.; Schaffer, C.B. Femtosecond Laser Micromachining. In Ultrafast Nonlinear Optics; Thomson, R., Leburn, C., Reid, D., Eds.; Scottish Graduate Series; Springer International Publishing: Berlin/Heidelberg, Germany, 2013; pp. 287–321. [Google Scholar] [CrossRef]
- Tabriz, A.G.; Viegas, B.; Okereke, M.; Uddin, M.J.; Lopez, E.A.; Zand, N.; Ranatunga, M.; Getti, G.; Douroumis, D. Evaluation of 3D Printability and Biocompatibility of Microfluidic Resin for Fabrication of Solid Microneedles. Micromachines 2022, 13, 1368. [Google Scholar] [CrossRef]
- Lin, L.; Chung, C.-K. PDMS Microfabrication and Design for Microfluidics and Sustainable Energy Application: Review. Micromachines 2021, 12, 1350. [Google Scholar] [CrossRef]
- Chen, Y.; Xian, Y.; Carrier, A.J.; Youden, B.; Servos, M.; Cui, S.; Luan, T.; Lin, S.; Zhang, X. A simple and cost-effective approach to fabricate tunable length polymeric microneedle patches for controllable transdermal drug delivery. RSC Adv. 2020, 10, 15541–15546. [Google Scholar] [CrossRef] [PubMed]



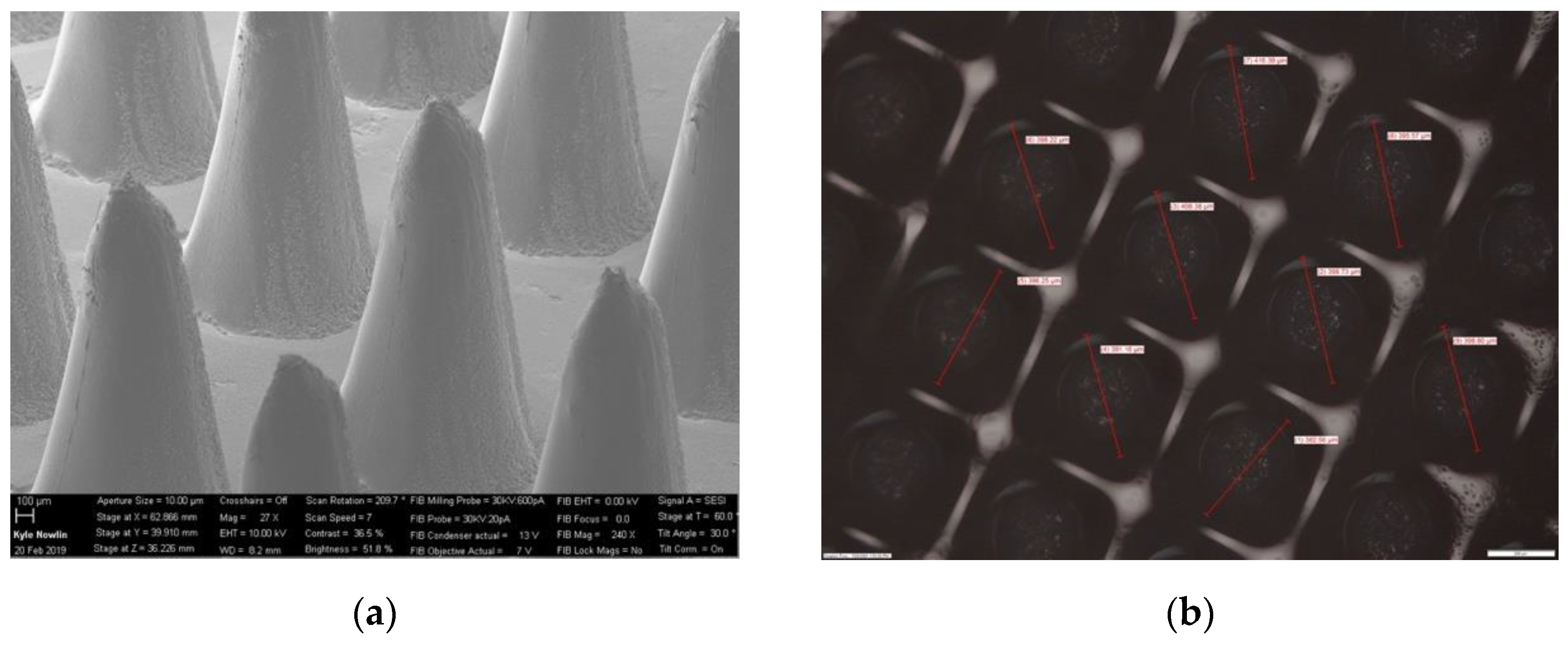
| Diameter = 0.3 mm (n = 16) | ||||
|---|---|---|---|---|
| Aspect Ratio | 2:1 | 3:1 | 4:1 | |
| Design MN | height | 0.60 | 0.90 | 1.20 |
| Diameter of Printed MN (mm) | max | 0.312 | 0.309 | 0.329 |
| min | 0.283 | 0.291 | 0.293 | |
| Printed to Design (Diameter) | % difference from mean | 97.00% | 95.00% | 92.19% |
| Height of Printed MN (mm) | max | 0.58 | 0.85 | 1.16 |
| min | 0.52 | 0.80 | 1.10 | |
| Printed to Design (Height) | % difference from mean | 96.67% | 94.44% | 86.67% |
| Diameter = 0.4 mm (n = 16) | ||||
|---|---|---|---|---|
| Aspect Ratio | 2:1 | 3:1 | 4:1 | |
| Design MN | height (mm) | 0.80 | 1.20 | 1.60 |
| Diameter of Printed MN (mm) | max | 0.439 | 0.416 | 0.397 |
| min | 0.362 | 0.382 | 0.363 | |
| Printed to Design (Diameter) | % difference from mean | 94.25% | 95.50% | 92.75% |
| Height of Printed MN (mm) | max | 0.77 | 1.15 | 1.56 |
| min | 0.70 | 1.10 | 1.51 | |
| Printed to Design (Height) | % difference from mean | 97.50% | 95.83% | 87.50% |
| Design # | Diameter (mm) | Aspect Ratio | Height (mm) | Tip Diameter (µm) | Displacement (mm) at Force = 50 N |
|---|---|---|---|---|---|
| 1 | 0.3 | 2:1 | 0.6 | 36 | 0.51 |
| 2 | 0.3 | 3:1 | 0.9 | 25 | 0.89 |
| 3 | 0.3 | 4:1 | 1.2 | 18 | 1.20 |
| 4 | 0.4 | 2:1 | 0.8 | 45 | 0.50 |
| 5 | 0.4 | 3:1 | 1.2 | 37 | 0.65 |
| 6 | 0.4 | 4:1 | 1.6 | 24 | 0.76 |
| Design # | Diameter (mm) | Aspect Ratio | Height (mm) | Depth of Penetration (mm) | Percentage of Penetration Depth to Needle Height (%) |
|---|---|---|---|---|---|
| 1 | 0.3 | 2:1 | 0.6 | 0.52 | 87 |
| 2 | 0.3 | 3:1 | 0.9 | 0.78 | 87 |
| 3 | 0.3 | 4:1 | 1.2 | 1.17 | 97.5 |
| 4 | 0.4 | 2:1 | 0.8 | 0.65 | 81 |
| 5 | 0.4 | 3:1 | 1.2 | 1.04 | 87 |
| 6 | 0.4 | 4:1 | 1.6 | 1.43 | 89 |
| Aspect to Compare | Laser Ablation | AM | Reference |
|---|---|---|---|
| Fabrication time | Faster | Longer | [79,80] |
| MN design | Not suitable for large-scale production | Complex geometry and customized design | [54,81] |
| MN resolution | Fair accuracy | High accuracy | [82,83] |
| Post-processing | No further steps | Remove support materials and curing | [84] |
| Prepare female MN mold | Double-casting | Single-casting | [85,86] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aldawood, F.K.; Parupelli, S.K.; Andar, A.; Desai, S. 3D Printing of Biodegradable Polymeric Microneedles for Transdermal Drug Delivery Applications. Pharmaceutics 2024, 16, 237. https://doi.org/10.3390/pharmaceutics16020237
Aldawood FK, Parupelli SK, Andar A, Desai S. 3D Printing of Biodegradable Polymeric Microneedles for Transdermal Drug Delivery Applications. Pharmaceutics. 2024; 16(2):237. https://doi.org/10.3390/pharmaceutics16020237
Chicago/Turabian StyleAldawood, Faisal Khaled, Santosh Kumar Parupelli, Abhay Andar, and Salil Desai. 2024. "3D Printing of Biodegradable Polymeric Microneedles for Transdermal Drug Delivery Applications" Pharmaceutics 16, no. 2: 237. https://doi.org/10.3390/pharmaceutics16020237
APA StyleAldawood, F. K., Parupelli, S. K., Andar, A., & Desai, S. (2024). 3D Printing of Biodegradable Polymeric Microneedles for Transdermal Drug Delivery Applications. Pharmaceutics, 16(2), 237. https://doi.org/10.3390/pharmaceutics16020237








