Ectoine, from a Natural Bacteria Protectant to a New Treatment of Dry Eye Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal
2.2. Mouse Model of Experimental Dry Eye Disease
2.3. Ectoine Topical Treatment
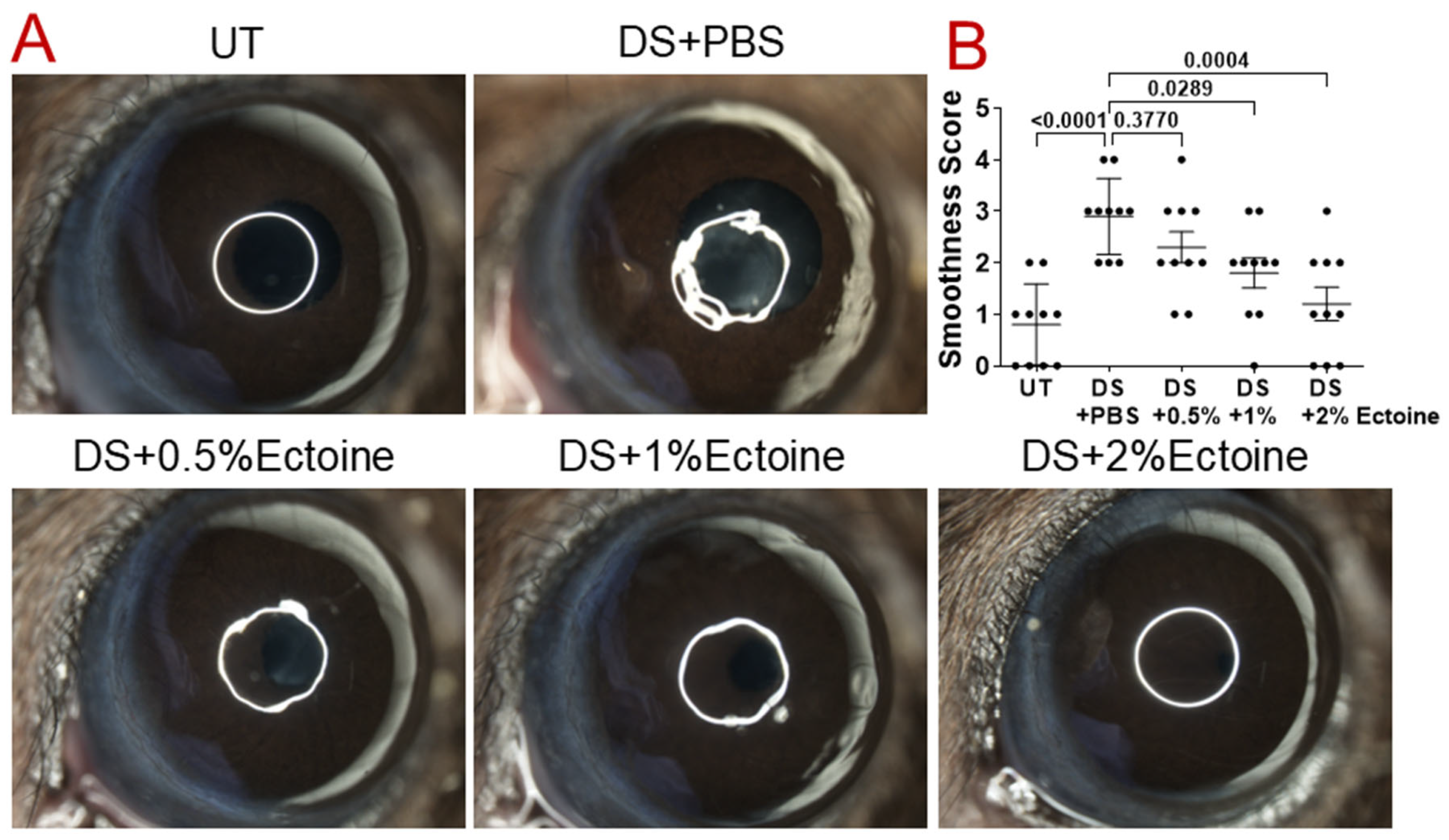
2.4. Evaluation of Corneal Smoothness
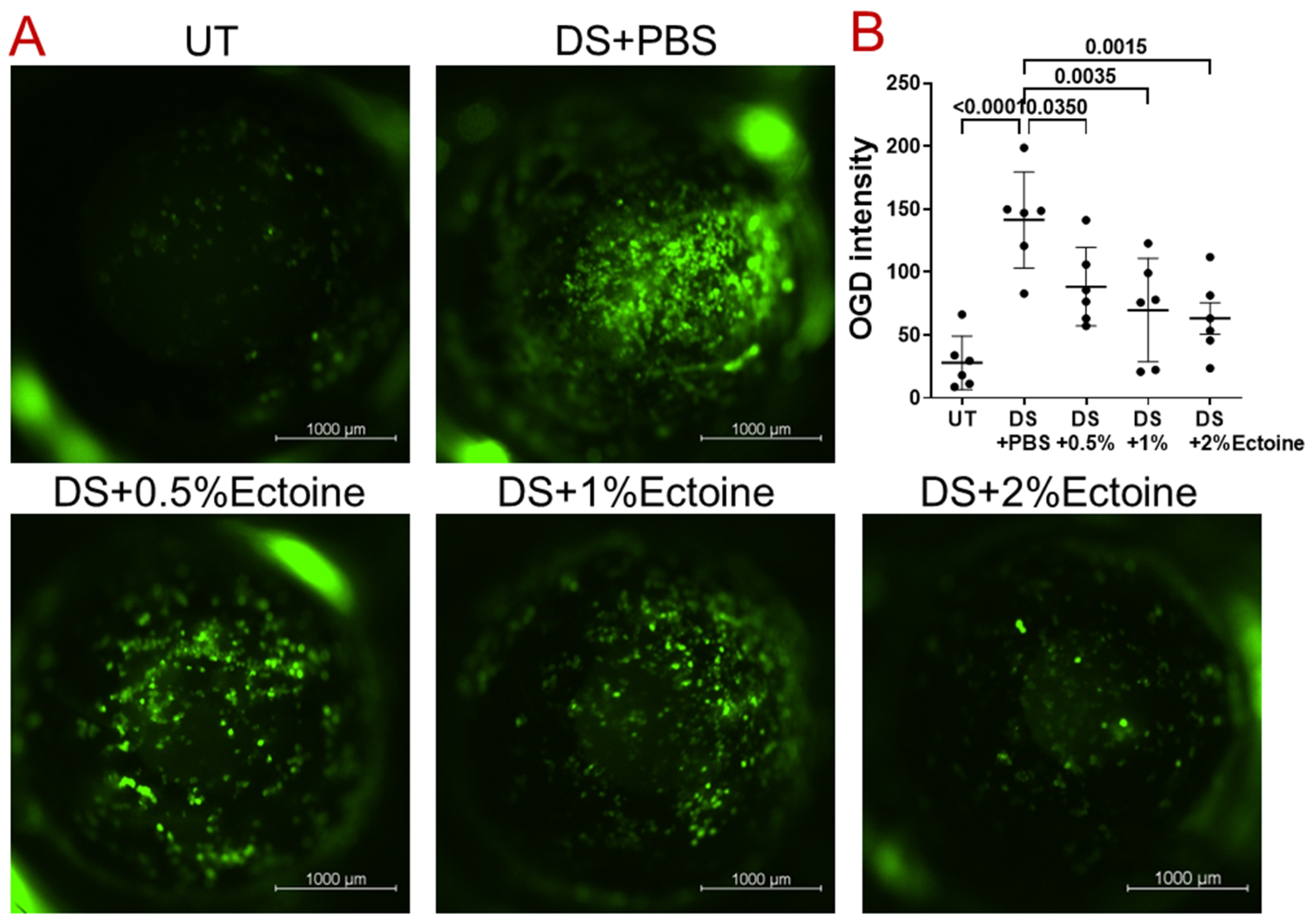
2.5. Evaluation of Corneal Defect via Oregon Green Dextran (OGD) Fluorescent Staining
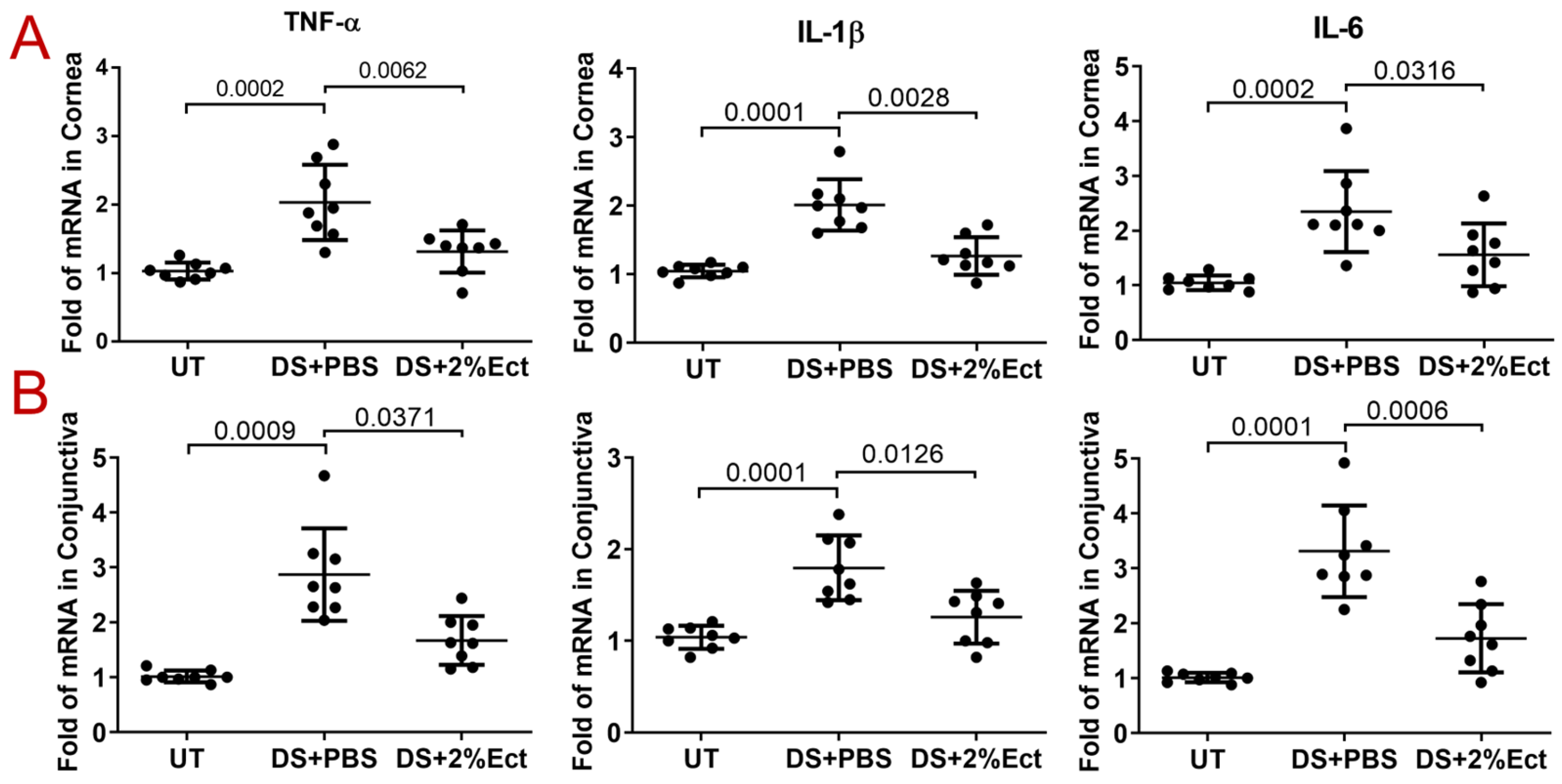
2.6. Evaluation of Ocular Surface Inflammation
2.7. RNA Isolation, Reverse Transcription (RT), and Quantitative Real-Time PCR (qPCR)
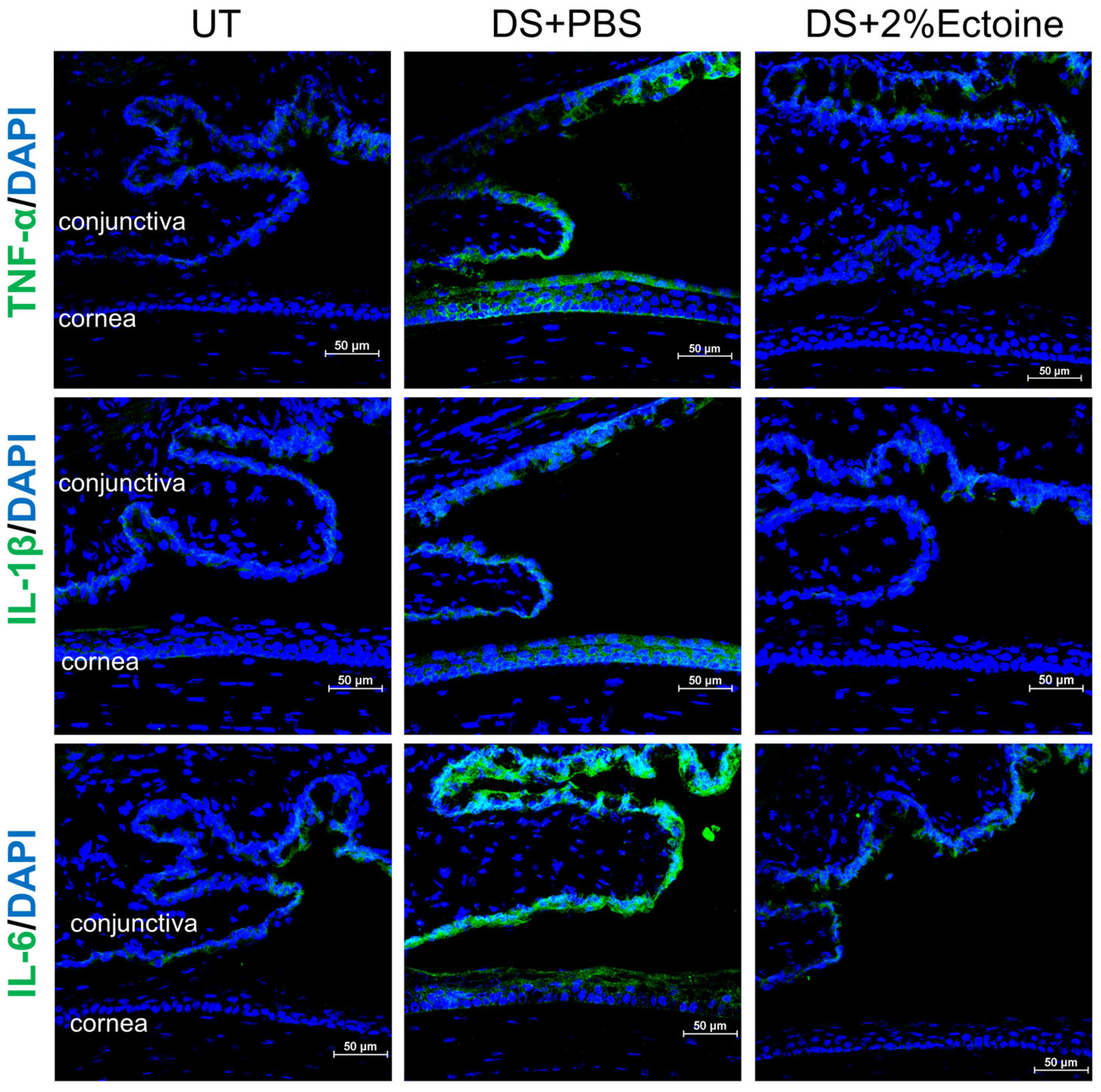
2.8. Immunofluorescent Staining and Laser Scanning Confocal Microscopy
2.9. Statistical Analysis
3. Results
3.1. Efficacy of Ectoine at Protecting Corneal Epithelium in the Murine Experimental Dry Eye Model
3.1.1. Corneal Smoothness
3.1.2. OGD Fluorescent Staining
3.2. Effect of Ectoine on Suppressing Pro-Inflammatory Cytokines in the Murine Experimental Dry Eye Model
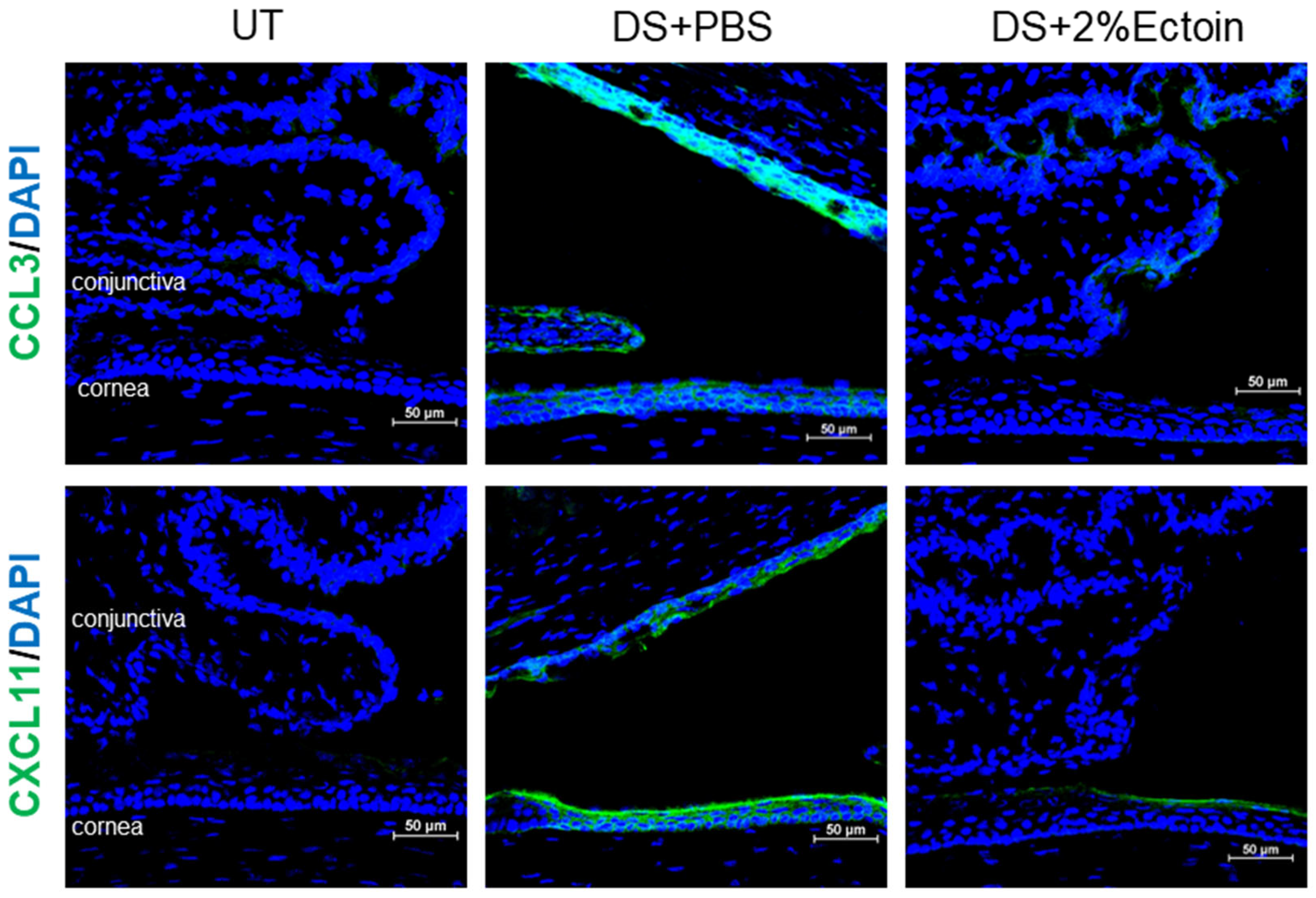
3.3. Effect of Ectoine on Suppressing Chemokines That Attract the Infiltration of Immune Cells to the Ocular Surface
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stapleton, F.; Alves, M.; Bunya, V.Y.; Jalbert, I.; Lekhanont, K.; Malet, F.; Na, K.S.; Schaumberg, D.; Uchino, M.; Vehof, J.; et al. TFOS DEWS II Epidemiology Report. Ocul. Surf. 2017, 15, 334–365. [Google Scholar] [CrossRef] [PubMed]
- Bradley, J.L.; Özer Stillman, I.; Pivneva, I.; Guerin, A.; Evans, A.M.; Dana, R. Dry eye disease ranking among common reasons for seeking eye care in a large US claims database. Clin. Ophthalmol. 2019, 13, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, A.J.; Cruickshanks, K.J.; Fischer, M.E.; Huang, G.H.; Klein, B.E.; Klein, R.; Dalton, D.S. Dry eye in the beaver dam offspring study: Prevalence, risk factors, and health-related quality of life. Am. J. Ophthalmol. 2014, 157, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Scifo, C.; Barabino, S.; De Pasquale, G.; Blanco, A.R.; Mazzone, M.G.; Rolando, M. Effects of a new lipid tear substitute in a mouse model of dry eye. Cornea 2010, 29, 802–806. [Google Scholar] [CrossRef] [PubMed]
- Tsubota, K.; Kawashima, M.; Inaba, T.; Dogru, M.; Matsumoto, Y.; Ishida, R.; Kaido, M.; Kojima, T.; Uchino, M.; Uchino, Y.; et al. The antiaging approach for the treatment of dry eye. Cornea 2012, 31, S3–S8. [Google Scholar] [CrossRef] [PubMed]
- Craig, J.P.; Nichols, K.K.; Akpek, E.K.; Caffery, B.; Dua, H.S.; Joo, C.K.; Liu, Z.; Nelson, J.D.; Nichols, J.J.; Tsubota, K.; et al. TFOS DEWS II Definition and Classification Report. Ocul. Surf. 2017, 15, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Bron, A.J.; de Paiva, C.S.; Chauhan, S.K.; Bonini, S.; Gabison, E.E.; Jain, S.; Knop, E.; Markoulli, M.; Ogawa, Y.; Perez, V.; et al. TFOS DEWS II pathophysiology report. Ocul. Surf. 2017, 15, 438–510. [Google Scholar] [CrossRef]
- Baudouin, C.; Aragona, P.; Messmer, E.M.; Tomlinson, A.; Calonge, M.; Boboridis, K.G.; Akova, Y.A.; Geerling, G.; Labetoulle, M.; Rolando, M. Role of hyperosmolarity in the pathogenesis and management of dry eye disease: Proceedings of the OCEAN group meeting. Ocul. Surf. 2013, 11, 246–258. [Google Scholar] [CrossRef]
- Schwartz, L.; Guais, A.; Pooya, M.; Abolhassani, M. Is inflammation a consequence of extracellular hyperosmolarity? J. Inflamm. 2009, 6, 21. [Google Scholar] [CrossRef]
- Montani, G. Intrasubject tear osmolarity changes with two different types of eyedrops. Optom. Vis. Sci. 2013, 90, 372–377. [Google Scholar] [CrossRef]
- Yancey, P.H. Organic osmolytes as compatible, metabolic and counteracting cytoprotectants in high osmolarity and other stresses. J. Exp. Biol. 2005, 208, 2819–2830. [Google Scholar] [CrossRef]
- Weinisch, L.; Kirchner, I.; Grimm, M.; Kuhner, S.; Pierik, A.J.; Rossello-Mora, R.; Filker, S. Glycine Betaine and Ectoine Are the Major Compatible Solutes Used by Four Different Halophilic Heterotrophic Ciliates. Microb. Ecol. 2019, 77, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Ongagna-Yhombi, S.Y.; Boyd, E.F. Biosynthesis of the osmoprotectant ectoine, but not glycine betaine, is critical for survival of osmotically stressed Vibrio parahaemolyticus cells. Appl. Environ. Microbiol. 2013, 79, 5038–5049. [Google Scholar] [CrossRef] [PubMed]
- Bownik, A.; Stepniewska, Z. Ectoine as a promising protective agent in humans and animals. Arh. Hig. Rada Toksikol. 2016, 67, 260–265. [Google Scholar] [CrossRef]
- Casale, M.; Moffa, A.; Carbone, S.; Fraccaroli, F.; Costantino, A.; Sabatino, L.; Lopez, M.A.; Baptista, P.; Cassano, M.; Rinaldi, V. Topical Ectoine: A Promising Molecule in the Upper Airways Inflammation—A Systematic Review. Biomed. Res. Int. 2019, 2019, 7150942. [Google Scholar] [CrossRef] [PubMed]
- Unfried, K.; Kramer, U.; Sydlik, U.; Autengruber, A.; Bilstein, A.; Stolz, S.; Marini, A.; Schikowski, T.; Keymel, S.; Krutmann, J. Reduction of neutrophilic lung inflammation by inhalation of the compatible solute ectoine: A randomized trial with elderly individuals. Int. J. Chron. Obs. Pulmon Dis. 2016, 11, 2573–2583. [Google Scholar] [CrossRef]
- Eichel, A.; Bilstein, A.; Werkhauser, N.; Mosges, R. Meta-analysis of the efficacy of ectoine nasal spray in patients with allergic rhinoconjunctivitis. J. Allergy 2014, 2014, 292545. [Google Scholar] [CrossRef] [PubMed]
- Marini, A.; Reinelt, K.; Krutmann, J.; Bilstein, A. Ectoine-containing cream in the treatment of mild to moderate atopic dermatitis: A randomised, comparator-controlled, intra-individual double-blind, multi-center trial. Skin. Pharmacol. Physiol. 2014, 27, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Salapatek, A.M.; Werkhauser, N.; Ismail, B.; Mosges, R.; Raskopf, E.; Bilstein, A. Effects of ectoine containing nasal spray and eye drops on symptoms of seasonal allergic rhinoconjunctivitis. Clin. Transl. Allergy 2021, 11, e12006. [Google Scholar] [CrossRef]
- Bilstein, A.; Heinrich, A.; Rybachuk, A.; Mosges, R. Ectoine in the Treatment of Irritations and Inflammations of the Eye Surface. Biomed. Res. Int. 2021, 2021, 8885032. [Google Scholar] [CrossRef]
- The National Academies Collection. Guide for the Care and Use of Laboratory Animals, 8th ed.; National Institutes of Health: Washington, DC, USA, 2011.
- Dursun, D.; Wang, M.; Monroy, D.; Li, D.Q.; Lokeshwar, B.L.; Stern, M.; Pflugfelder, S.C. Experimentally induced dry eye produces ocular surface inflammation and epithelial disease. Adv. Exp. Med. Biol. 2002, 506, 647–655. [Google Scholar]
- Krauss, A.H.; Corrales, R.M.; Pelegrino, F.S.; Tukler-Henriksson, J.; Pflugfelder, S.C.; de Paiva, C.S. Improvement of Outcome Measures of Dry Eye by a Novel Integrin Antagonist in the Murine Desiccating Stress Model. Investig. Ophthalmol. Vis. Sci. 2015, 56, 5888–5895. [Google Scholar] [CrossRef]
- De Paiva, C.S.; Corrales, R.M.; Villarreal, A.L.; Farley, W.; Li, D.Q.; Stern, M.E.; Pflugfelder, S.C. Apical corneal barrier disruption in experimental murine dry eye is abrogated by methylprednisolone and doxycycline. Investig. Ophthalmol. Vis. Sci. 2006, 47, 2847–2856. [Google Scholar] [CrossRef] [PubMed]
- Hua, X.; Deng, R.; Li, J.; Chi, W.; Su, Z.; Lin, J.; Pflugfelder, S.C.; Li, D.Q. Protective Effects of L-Carnitine against Oxidative Injury by Hyperosmolarity in Human Corneal Epithelial Cells. Investig. Ophthalmol. Vis. Sci. 2015, 56, 5503–5511. [Google Scholar] [CrossRef] [PubMed]
- de Paiva, C.S.; Chen, Z.; Corrales, R.M.; Pflugfelder, S.C.; Li, D.-Q. ABCG2 transporter identifies a population of clonogenic human limbal epithelial cells. Stem Cells 2005, 23, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Li, Z.; Oh, H.J.; Im, S.K.; Lee, S.H.; Park, S.H.; You, I.C.; Yoon, K.C. Expression of CCR5 and its ligands CCL3, -4, and -5 in the tear film and ocular surface of patients with dry eye disease. Curr. Eye Res. 2012, 37, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.M.; Bremer, E.; Csonka, L.N.; Kraemer, R.; Poolman, B.; van der Heide, T.; Smith, L.T. Osmosensing and osmoregulatory compatible solute accumulation by bacteria. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2001, 130, 437–460. [Google Scholar] [CrossRef] [PubMed]
- Galinski, E.A.; Pfeiffer, H.P.; Truper, H.G. 1,4,5,6-Tetrahydro-2-methyl-4-pyrimidinecarboxylic acid. A novel cyclic amino acid from halophilic phototrophic bacteria of the genus Ectothiorhodospira. Eur. J. Biochem. 1985, 149, 135–139. [Google Scholar] [CrossRef]
- Czech, L.; Hermann, L.; Stoveken, N.; Richter, A.A.; Hoppner, A.; Smits, S.H.J.; Heider, J.; Bremer, E. Role of the Extremolytes Ectoine and Hydroxyectoine as Stress Protectants and Nutrients: Genetics, Phylogenomics, Biochemistry, and Structural Analysis. Genes 2018, 9, 177. [Google Scholar] [CrossRef]
- Graf, R.; Anzali, S.; Buenger, J.; Pfluecker, F.; Driller, H. The multifunctional role of ectoine as a natural cell protectant. Clin. Dermatol. 2008, 26, 326–333. [Google Scholar] [CrossRef]
- Eiberweiser, A.; Nazet, A.; Kruchinin, S.E.; Fedotova, M.V.; Buchner, R. Hydration and Ion Binding of the Osmolyte Ectoine. J. Phys. Chem. B 2015, 119, 15203–15211. [Google Scholar] [CrossRef]
- Veselovskaya, N.N.; Zherebko, I.B. Assessment of Functional Changes Tear Production under the Action of the Eye Drops on the Base of Natural Molecule of Ectoine and Artificial Tears in Patients with Dry Eye Syndrome on the Background of Endocrine Ophthalmopathy. Fiziol. Zhurnal 2016, 62, 118–121. [Google Scholar] [CrossRef] [PubMed]
- Pflugfelder, S.C.; Maskin, S.L.; Anderson, B.; Chodosh, J.; Holland, E.J.; de Paiva, C.S.; Bartels, S.P.; Micuda, T.; Proskin, H.M.; Vogel, R. A randomized, double-masked, placebo-controlled, multicenter comparison of loteprednol etabonate ophthalmic suspension, 0.5%, and placebo for treatment of keratoconjunctivitis sicca in patients with delayed tear clearance. Am. J. Ophthalmol. 2004, 138, 444–457. [Google Scholar] [CrossRef] [PubMed]
- Pflugfelder, S.C.; Jones, D.; Ji, Z.; Afonso, A.; Monroy, D. Altered cytokine balance in the tear fluid and conjunctiva of patients with Sjogren’s syndrome keratoconjunctivitis sicca. Curr. Eye Res. 1999, 19, 201–211. [Google Scholar] [CrossRef] [PubMed]
- De Paiva, C.S.; Corrales, R.M.; Villarreal, A.L.; Farley, W.J.; Li, D.Q.; Stern, M.E.; Pflugfelder, S.C. Corticosteroid and doxycycline suppress MMP-9 and inflammatory cytokine expression, MAPK activation in the corneal epithelium in experimental dry eye. Exp. Eye Res. 2006, 83, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, W.; Chauhan, S.K.; Dana, R. Dry eye disease: An immune-mediated ocular surface disorder. Arch. Ophthalmol. 2012, 130, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Enriquez-de-Salamanca, A.; Calonge, M. Cytokines and chemokines in immune-based ocular surface inflammation. Expert. Rev. Clin. Immunol. 2008, 4, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.C.; Park, C.S.; You, I.C.; Choi, H.J.; Lee, K.H.; Im, S.K.; Park, H.Y.; Pflugfelder, S.C. Expression of CXCL9, -10, -11, and CXCR3 in the tear film and ocular surface of patients with dry eye syndrome. Investig. Ophthalmol. Vis. Sci. 2010, 51, 643–650. [Google Scholar] [CrossRef]
- Amparo, F.; Jin, Y.; Hamrah, P.; Schaumberg, D.A.; Dana, R. What is the value of incorporating tear osmolarity measurement in assessing patient response to therapy in dry eye disease? Am. J. Ophthalmol. 2014, 157, 69–77.e62. [Google Scholar] [CrossRef]
- Chen, Y.; Dana, R. Autoimmunity in dry eye disease—An updated review of evidence on effector and memory Th17 cells in disease pathogenicity. Autoimmun. Rev. 2021, 20, 102933. [Google Scholar] [CrossRef]
- El Annan, J.; Chauhan, S.K.; Ecoiffier, T.; Zhang, Q.; Saban, D.R.; Dana, R. Characterization of effector T cells in dry eye disease. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3802–3807. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Lin, N.; Li, J.-M.; Liu, H.; Abu-Romman, A.; Yaman, E.; Bian, F.; de Paiva, C.S.; Pflugfelder, S.C.; Li, D.-Q. Ectoine, from a Natural Bacteria Protectant to a New Treatment of Dry Eye Disease. Pharmaceutics 2024, 16, 236. https://doi.org/10.3390/pharmaceutics16020236
Chen X, Lin N, Li J-M, Liu H, Abu-Romman A, Yaman E, Bian F, de Paiva CS, Pflugfelder SC, Li D-Q. Ectoine, from a Natural Bacteria Protectant to a New Treatment of Dry Eye Disease. Pharmaceutics. 2024; 16(2):236. https://doi.org/10.3390/pharmaceutics16020236
Chicago/Turabian StyleChen, Xin, Na Lin, Jin-Miao Li, Haixia Liu, Anmar Abu-Romman, Ebru Yaman, Fang Bian, Cintia S. de Paiva, Stephen C. Pflugfelder, and De-Quan Li. 2024. "Ectoine, from a Natural Bacteria Protectant to a New Treatment of Dry Eye Disease" Pharmaceutics 16, no. 2: 236. https://doi.org/10.3390/pharmaceutics16020236
APA StyleChen, X., Lin, N., Li, J.-M., Liu, H., Abu-Romman, A., Yaman, E., Bian, F., de Paiva, C. S., Pflugfelder, S. C., & Li, D.-Q. (2024). Ectoine, from a Natural Bacteria Protectant to a New Treatment of Dry Eye Disease. Pharmaceutics, 16(2), 236. https://doi.org/10.3390/pharmaceutics16020236







