Apodanthera glaziovii (Cucurbitaceae) Shows Strong Anti-Inflammatory Activity in Murine Models of Acute Inflammation
Abstract
1. Introduction
2. Material and Methods
2.1. Plant Material and Obtention from A. glaziovii Hidroetanolic Extract
2.2. Qualitative Phytochemical Characterization
2.3. Determination of Total Phenolics
2.4. Determination of Total Flavonoids
2.5. Determination of Total Tannins
2.6. 1H and 13C Nuclear Magnetic Resonance (NMR) and High-Performance Liquid Chromatography (HPLC) Profiles
2.7. Assay of Hemolytic Activity
2.8. Animals
2.9. Acute Oral Toxicity
2.10. Mammalian Erythrocyte Micronucleus Test
2.11. Carrageenan-Induced Paw Edema Model of Acute Inflammation
2.12. Peritonitis Model of Acute Inflammation
2.13. Carrageenan-Induced Air Pouch Model of Acute Inflammation
2.14. Statistical Analysis
3. Results
3.1. Phytochemical Profile of SHE-Ag
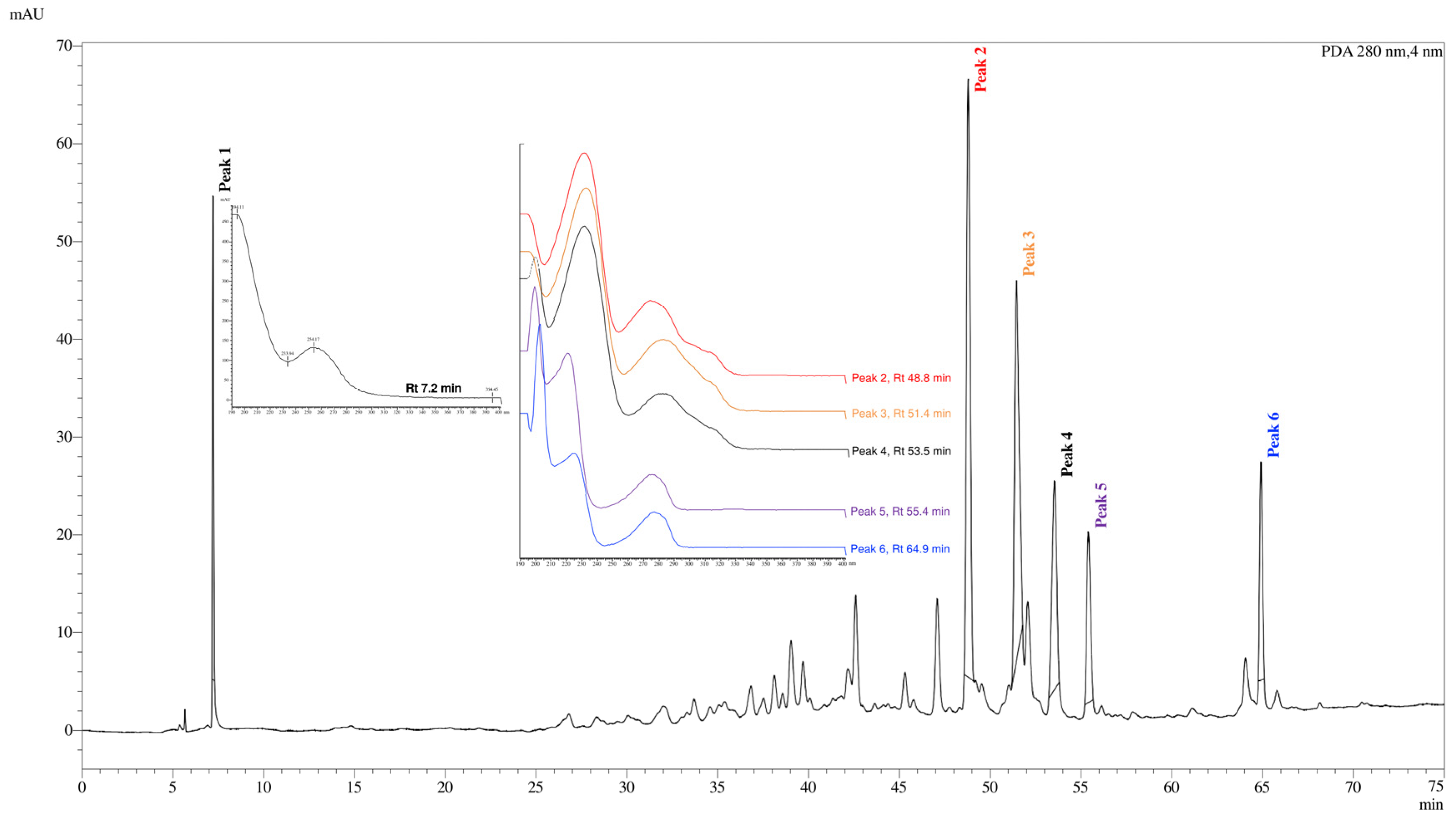
3.2. 1H and 13C Nuclear Magnetic Resonance (NMR) and High-Performance Liquid Chromatography (HPLC) Profile
3.3. Hemolytic Activity
3.4. Acute Oral Toxicity Test
3.5. DNA Damage Results by Micronucleus Test
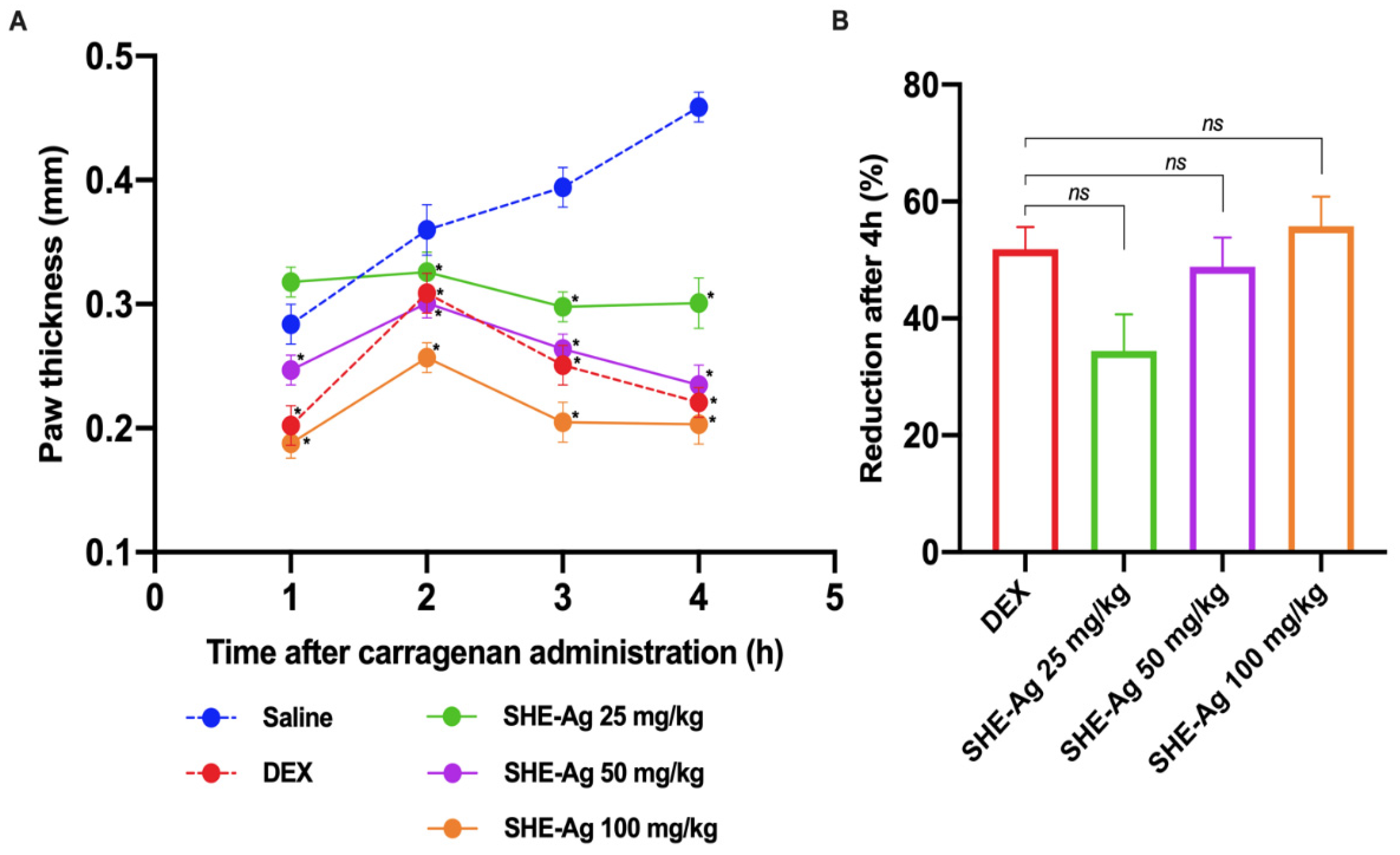
3.6. Carrageenan-Induced Paw Edema
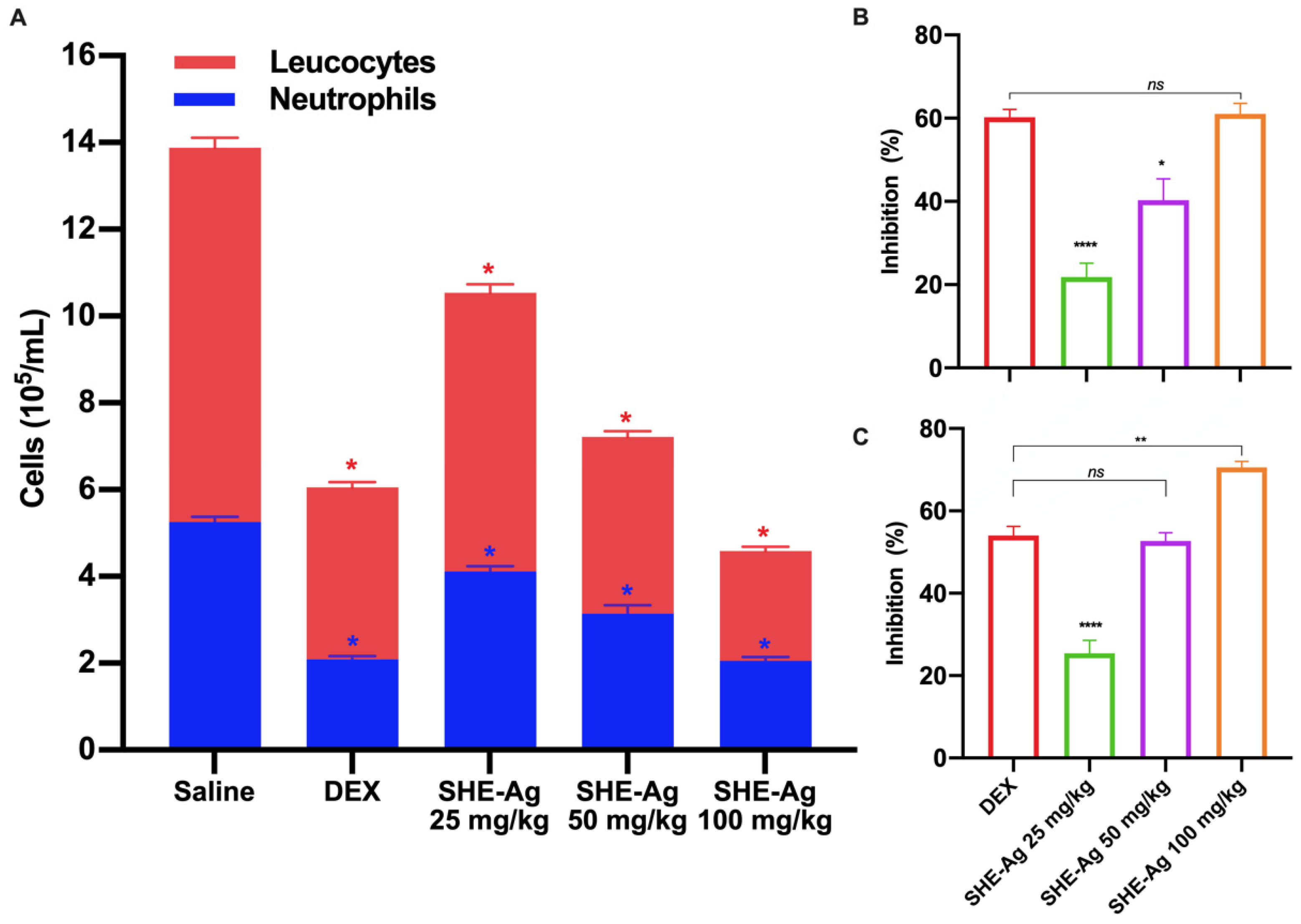
3.7. Peritonitis Test
3.8. Carrageenan-Induced Acute Inflammation in Air Pouch Model
4. Discussion
5. Conclusion Remarks
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Noronha, J.I.; Giardini, I.J.M.; Pasotti, D.V.; Teixeira, C.M.P.P. Análise Da Prevalência Da Automedicação Com Anti-Inflamatórios Não Esteroidais Em Drogaria De Espírito Santo Do Pinhal-SP. Rev. Fac. Saber 2021, 6, 814–822. [Google Scholar]
- Mehmood, K.T.; Al-Baldawi, S.; Salazar, G.Z.; Zúñiga, D.; Balasubramanian, S. Antipyretic Use in Noncritically Ill Patients With Fever: A Review. Cureus 2024, 16, e51943. [Google Scholar] [CrossRef] [PubMed]
- Sohail, R.; Mathew, M.; Patel, K.K.; Reddy, S.A.; Haider, Z.; Naria, M.; Habib, A.; Abdin, Z.U.; Chaudhry, W.R.; Akbar, A. Effects of Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) and Gastroprotective NSAIDs on the Gastrointestinal Tract: A Narrative Review. Cureus 2023, 15, e37080. [Google Scholar] [CrossRef] [PubMed]
- Tai, F.W.D.; McAlindon, M.E. Non-Steroidal Anti-Inflammatory Drugs and the Gastrointestinal Tract. Clin. Med. J. R. Coll. Physicians Lond. 2021, 21, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Minhas, D.; Nidhaan, A.; Husni, M.E. Recommendations for the Use of Nonsteroidal Anti-Inflammatory Drugs and Cardiovascular Disease Risk: Decades Later, Any New Lessons Learned? Rheum. Dis. Clin. North. Am. 2023, 49, 179–191. [Google Scholar] [CrossRef]
- Parreira, N.S.M.; Silva, P.V.; Rodrigues, V.R. Automedicação Prolongada De Corticoides: Riscos E Motivações. Rev. Cient. ITPAC Porto 2021, 1, 1–11. [Google Scholar]
- Moudgil, K.D.; Venkatesha, S.H. The Anti-Inflammatory and Immunomodulatory Activities of Natural Products to Control Autoimmune Inflammation. Int. J. Mol. Sci. 2023, 24, 95. [Google Scholar] [CrossRef]
- Gono, C.M.P.; Ahmadi, P.; Hertiani, T.; Septiana, E.; Putra, M.Y.; Chianese, G. A Comprehensive Update on the Bioactive Compounds from Seagrasses. Mar. Drugs 2022, 20, 406. [Google Scholar] [CrossRef]
- Santos, M.O.; De Almeida, B.V.; Ribeiro, D.A.; De Macêdo, D.G.; Macêdo, M.J.F.; Macedo, J.G.F.; De Sousa, F.F.S.; De Oliveira, L.G.S.; Saraiva, M.E.; Araújo, T.M.S.; et al. The Conservation of Native Priority Medicinal Plants in a Caatinga Area in Ceará, Northeastern Brazil. Acad. Bras. Cienc. 2017, 89, 2675–2685. [Google Scholar] [CrossRef]
- Silvestre, G.F.G.; Lucena, R.P.; Silva, H.A. Cucurbitacins and the Immune System: Update in Research on Anti- Inflammatory, Antioxidant, and Immunomodulatory Mechanisms. Curr. Med. Chem. 2022, 29, 3774–3789. [Google Scholar] [CrossRef]
- Hafeez, N. View of Phytochemical and Biological Studies of Cucurbitaceae A Mini-Review. Phytopharm. Res. J. 2024, 3, 13–23. [Google Scholar]
- Rolnik, A.; Olas, B. Vegetables from the Cucurbitaceae Family and Their Products: Positive Effect on Human Health. Nutrition 2020, 78, 110788. [Google Scholar] [CrossRef] [PubMed]
- Silvestre, G.F.G.; Lucena, R.P.; Oliveira, G.D.; Pereira, H.N.; Dias, J.A.B.; Souza, I.A.; Alves, H.S. Anti-Tumor and Anti-Inflammatory Activity In Vivo of Apodanthera congestiflora Cogn. (Cucurbitaceae). Pharmaceutics 2021, 13, 743. [Google Scholar] [CrossRef] [PubMed]
- Vilar, C.J.; Carvalho, M.C.; Furtado, C.M. Effects of the Aqueous Extracts of Plants of the Genera Apodanthera (Cucurbitaceae) And Jatropha (Euphorbiaceae) on the Lethality of the Venom of Bothrops Jararaca (Serpentes, Viperidae). Biol. Geral Exper. 2007, 7, 32–39. [Google Scholar]
- Matos, F.J.A. Introdução a Fitoquímica Experimental, 3rd ed.; Imprensa Universitária: Fortaleza, Brazil, 2009. [Google Scholar]
- Barbosa, W.L.R. Manual Para Análise Fitoquímica e Cromatográfica de Extratos Vegetais; Universidade Federal do Pará (UFPA): Belém, Brazil, 2001. [Google Scholar]
- Chandra, S.; Mejia, E.G. Polyphenolic Compounds, Antioxidant Capacity, and Quinone Reductase Activity of an Aqueous Extract of Ardisia Compressa in Comparison to Mate (Llex Paraguariensis) and Green (Camellia Sinensis) Teas. J. Agric. Food Chem. 2004, 52, 3583–3589. [Google Scholar] [CrossRef] [PubMed]
- Meda, A.; Lamien, C.E.; Romito, M.; Millogo, J.; Nacoulma, O.G. Determination of the Total Phenolic, Flavonoid and Proline Contents in Burkina Fasan Honey, as Well as Their Radical Scavenging Activity. Food Chem. 2005, 91, 571–577. [Google Scholar] [CrossRef]
- Makkar, H.P.S.; Becker, K. Vanillin-HCl Method for Condensed Tannins: Effect of Organic Solvents Used for Extraction of Tannins. J. Chem. Ecol. 1993, 19, 613–621. [Google Scholar] [CrossRef]
- Pinto, D.S.; Duarte, F.M.; Costa, J.I.V.; De Almeida Filho, G.G.; Alves, H.S.; Célia, M.; Chaves, O.; De Luna, H.; Pessôa, F. Antibacterial and Hemolytic Activities from Piper Montealegreanum Yuncker (Piperaceae). Antiinfect. Agents 2012, 10, 1–5. [Google Scholar] [CrossRef]
- OECD/OCDE. 423Guideline for Testing of Chemicals Acute Oral Toxicity-Acute Toxic Class Method; OECD: Paris, France, 2001. [Google Scholar]
- Oliveira, A.M.; Freitas, A.F.S.; Paiva, P.M.G.; Napoleão, T.H. Genotoxicity Assessment of Saline Extract from Pilosocereus Gounellei (Cactaceae) and Its Chemopreventive Effect against Cyclophosphamide-Induced DNA Damage. Heliyon 2020, 6, e03811. [Google Scholar] [CrossRef]
- OECD. Test No. 474: Mammalian Erythrocyte Micronucleus Test; OECD: Paris, France, 2014. [Google Scholar]
- Winter, C.A.; Risley, E.A.; Nuss, G.W. Carrageenin-Induced Edema in Hind Paw of the ’Rat as an Assay for Antiinflammatory Dkugp. Merck Znstitute Ther. Res. 1972, 111, 544–547. [Google Scholar]
- Cavalcante, G.S.; Macário, A.O.; Soares, A.F.F.; Paiva, P.M.G.; Napoleão, T.H. Antinociceptive and Anti-Inflammatory Effects of Saline Extract and Lectin-Rich Fraction from Microgramma vacciniifolia Rhizome in Mice. Chem. Biodivers. 2021, 18, e2100125. [Google Scholar] [CrossRef] [PubMed]
- Lapa, A.J.; Souccar, C.; Lima-Landman, T.R.; Castro, M.A.S.; Lima, T.C.M. Plantas Medicinais: Métodos de Avaliação Da Atividade Farmacológica; Sociedade Brasileira de Plantas Medicinais: Sao Paulo, Brazil, 2008; Volume 144. [Google Scholar]
- Sedgwick, A.D.; Lees, P. Studies of Eicosanoid Production in the Air Pouch Model of Synovial Inflammation. Agents Actions 1986, 18, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Barros, C.D.; Amato, A.A.; Oliveira, T.B.; Iannini, K.B.R.; Silva, A.L.; Silva, T.G.; Leite, E.S.; Hernandes, M.Z.; Lima, M.C.A.; Galdino, S.L.; et al. Synthesis and Anti-Inflammatory Activity of New Arylidene-Thiazolidine-2,4-Diones as PPARγ Ligands. Bioorg Med. Chem. 2010, 18, 3805–3811. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Jeong, J.H.; Shin, J.Y.; Kim, D.G.; Yu, K.W. Immunomodulatory and Anti-Inflammatory Activity of Mulberry (Morus alba) Leaves Fermented with Hericium Erinaceum Mycelium by Solid-State Culture. J. Korean Soc. Food Sci. Nutr. 2011, 40, 1333–1339. [Google Scholar] [CrossRef]
- Himeno, E.; Nagao, T.; Honda, J.; Okabe, H.; Irino, N.; Nakasumi, T. Studies on the Constituents of the Root of Cayaponia tayuya (Vell.) Cogn. III. Structures of Cayaponosides, 29-nor-1,2,3,4,5,10-Exadehydrocucurbit-6-Ene Glucosides. Chem. Pharm. Bull. 1994, 42, 2370–2372. [Google Scholar] [CrossRef][Green Version]
- Achenbach, H.; Waibel, R.; Hefter-Bübl, U.; Constenla, M.A. Constituents of Fevillea cordifolia: New Norcucurbitacin and Cucurbitacin Glycosides. J. Nat. Prod. 1993, 56, 1506–1519. [Google Scholar] [CrossRef]
- Nakano, K.; Kanai, Y.; Murakami, K.; Takaishi, Y.; Tomimatsu, T. Nor-Cucurbitacin Glucosides From Caputo nigri. Phytochemistry 1994, 37, 817–820. [Google Scholar] [CrossRef]
- Himeno, E.; Nagao, T.; Honda, J.; Okabe, H.; Irino, N.; Nakasumi, T. Structures of Cayaponosides A, B, C and D, Glucosides of New nor-Cucurbitacins in the Roots of Cayaponia tayuya. Chem. Pharm. Bull. 1992, 40, 2885–2887. [Google Scholar] [CrossRef][Green Version]
- Hayashi, M. The Micronucleus Test-Most Widely Used in Vivo Genotoxicity Test. Genes. Environ. 2016, 38, 18. [Google Scholar] [CrossRef]
- Tice, R.R.; Agurell, E.; Anderson, D.; Burlinson, B.; Hartmann, A.; Kobayashi, H.; Miyamae, Y.; Rojas, E.; Ryu, J.C.; Sasaki, Y.F. Single Cell Gel/Comet Assay: Guidelines for in Vitro and in Vivo Genetic Toxicology Testing. Environ. Mol. Mutagen. 2000, 35, 206–221. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Singha, S.; Kar, A.; Chanda, J.; Banerjee, S.; Dasgupta, B.; Haldar, P.K.; Sharma, N. Therapeutic Importance of Cucurbitaceae: A Medicinally Important Family. J. Ethnopharmacol. 2022, 282, 114599. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.L.; Santos, C.A.C.; Oliveira, G.; Silva, M.T.; Silva, B.B.; Cunha, J.E.B.L.; Ruhoff, A.; Santos, C.A.G. Remote Sensing-Based Assessment of Land Degradation and Drought Impacts over Terrestrial Ecosystems in Northeastern Brazil. Sci. Total Environ. 2022, 835, 155490. [Google Scholar] [CrossRef] [PubMed]
- Lima, L.F.P. Estudos Taxonômicos e Morfopolínicos Em Cucurbitaceae Brasileiras. Ph.D. Thesis, Universidade Federal do Rio Grande do Sul, Rio Grande do Sul, Brazil, 2010. [Google Scholar]
- Lobo, R.A.A.M.; Lobo, A.C.B.N.M.; Oliveira, A.F.M.; Andrade, L.H.C. Ethnomedicinal Plants for Veterinary Use in Gypsy Communities of the northeast of Brazil. Bol. Latinoam. Caribe Plantas Med. Aromat. 2020, 19, 179–187. [Google Scholar] [CrossRef]
- Roque, A.A.; Rocha, R.M.; Loiola, M.I.B. Uso e Diversidade de Plantas Medicinais Da Caatinga Na Comunidade Rural de Laginhas, Município de Caicó, Rio Grande Do Norte (Nordeste Do Brasil). Rev. Bras. Pl. Med. 2010, 12, 31–42. [Google Scholar] [CrossRef]
- Shyaula, S.L.; Manandhar, M.D. Manandhar/Comprehensive Insights in Vegetables of Nepal 235; Nepal Academy of Science and Technology (NAST): Khumaltar, Nepal, 2021; ISBN 978-1405125093.
- Videres, L.C.C.A. Apodanthera congestiflora e Myracrodruon urundeuva: Investigação Das Biológicas Em Preparações Brutas e Produtos Isolados; Universidade Federal De Pernambuco Centro De Biociências Programa De Pós-Graduação Em Ciências Biológicas: Recife, Brazil, 2017. [Google Scholar]
- Pant, P.; Pandey, S.; Dall’Acqua, S. The Influence of Environmental Conditions on Secondary Metabolites in Medicinal Plants: A Literature Review. Chem. Biodivers. 2021, 18, e2100345. [Google Scholar] [CrossRef]
- Santos, C.H.C. Metabólitos Secundários e Avaliação de Atividades Biológicas de Siolmatra brasiliensis (Cogn.) Baill. (Cucurbitaceae) e Espécies Do Gênero Ziziphus mill. (Rhamnaceae). Ph.D. Thesis, Universidade Federal Rural Do Rio De Janeiro Instituto De Química Programa De Pós-Graduação Em Química, Seropédica, Brazil, 2012. [Google Scholar]
- Krepsky, P.B. Contribuição Para O Desenvolvimento De Método Para Análise De Cucurbitacinas Em Ebracteata Cogn. E Luffa operculata (L.) Cogn., Empregando E Espectrofotometria No UV. Master’s Thesis, Universidade Federal De Santa Catarina Centro De Ciências Da Saúde Programa De Pós-Graduação Em Farmácia, Trindade, Brazil, 2003. [Google Scholar]
- Ezeani, C.; Ezenyi, I.; Erhunse, N.; Sahal, D.; Akunne, T.; Okoli, C. Assessment of Antimalarial Medicinal Plants Used in Nigerian Ethnomedicine Reveals Antimalarial Potential of Cucurbita pepo Leaf Extract. Heliyon 2022, 8, e09916. [Google Scholar] [CrossRef]
- Espitia-Baena, J.E.; Robledo-Restrepo, S.M.; Cuadrado-Cano, B.S.; Rosario, H.D.; Gómez-Estradai, H.A. Neglected Tropical Diseases: Multi-Target-Directed Ligands in the Search for Novel Lead Candidates against Trypanosoma and Leishmania. J. Med. Chem. 2009, 52, 7339–7359. [Google Scholar] [CrossRef]
- Oda, K.; Matsuda, H.; Murakami, T.; Katayama, S.; Ohgitani, T.; Yoshikawa, M. Adjuvant and Haemolytic Activities of 47 Saponins Derived from Medicinal and Food Plants. Biol. Chem. 2000, 381, 67–74. [Google Scholar] [CrossRef]
- Chwalek, M.; Lalun, N.; Bobichon, H.; Plé, K.; Voutquenne-Nazabadioko, L. Structure–Activity Relationships of Some Hederagenin Diglycosides: Haemolysis, Cytotoxicity and Apoptosis Induction. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2006, 1760, 1418–1427. [Google Scholar] [CrossRef]
- Chen, Z.; Duan, H.; Tong, X.; Hsu, P.; Han, L.; Morris-Natschke, S.L.; Yang, S.; Liu, W.; Lee, K.-H. Cytotoxicity, Hemolytic Toxicity, and Mechanism of Action of Pulsatilla Saponin D and Its Synthetic Derivatives. J. Nat. Prod. 2018, 81, 465–474. [Google Scholar] [CrossRef]
- Ahmad, M.H.; Zezi, A.U.; Anafi, S.B.; Alshargi, O.Y.; Mohammed, M.; Mustapha, S.; Bala, A.A.; Muhammad, S.; Julde, S.M.; Wada, A.S.; et al. Sub-Acute Toxicity Study on Hydromethanolic Leaves Extract of Combretum hypopilinum (Combretaceae) Diels in Wistar Rats. Toxicol. Res. 2022, 38, 487–502. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.E.; Hall, M.E. Tratado de Fisiologia Médica, 14th ed.; Júnior, M.A.C., Ed.; Elsevier: Rio de Janeiro, Brazil, 2021; ISBN 978-0-323-59712-8. [Google Scholar]
- Al-Qahtani, A.A.; Alhamlan, F.S.; Al-Qahtani, A.A. Pro-Inflammatory and Anti-Inflammatory Interleukins in Infectious Diseases: A Comprehensive Review. Trop. Med. Infect. Dis. 2024, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- Fehrenbacher, J.C.; McCarson, K.E. Models of Inflammation: Carrageenan Air Pouch. Curr. Protoc. 2021, 1, e183. [Google Scholar] [CrossRef] [PubMed]
- Ribas, C.M.; Meotti, F.C.; Nascimento, F.P.; Jacques, A.V.; Dafre, A.L.; Rodrigues, A.L.S.; Farina, M.; Soldi, C.; Mendes, B.G.; Pizzolatti, M.G.; et al. Antinociceptive Effect of the Polygala Sabulosa Hydroalcoholic Extract in Mice: Evidence for the Involvement of Glutamatergic Receptors and Cytokine Pathways. Basic. Clin. Pharmacol. Toxicol. 2008, 103, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Passos, F.R.S.; Araújo-Filho, H.G.; Monteiro, B.S.; Shanmugam, S.; Araújo, A.A.S.; Almeida, J.R.G.S.; Thangaraj, P.; Júnior, L.J.Q.; Quintans, J.S.S. Anti-Inflammatory and Modulatory Effects of Steroidal Saponins and Sapogenins on Cytokines: A Review of Pre-Clinical Research. Phytomedicine 2022, 96, 153842. [Google Scholar] [CrossRef]
- Wang, L.; Xu, H.; Li, X.; Chen, H.; Zhang, H.; Zhu, X.; Lin, Z.; Guo, S.; Bao, Z.; Rui, H.; et al. Cucurbitacin E Reduces IL-1β-Induced Inflammation and Cartilage Degeneration by Inhibiting the PI3K/Akt Pathway in Osteoarthritic Chondrocytes. J. Transl. Med. 2023, 21, 880. [Google Scholar] [CrossRef]
- Aljohani, O.S. Phytochemical Evaluation of Cucumis prophetarum: Protective Effects against Carrageenan-Induced Prostatitis in Rats. Drug Chem. Toxicol. 2022, 45, 1461–1469. [Google Scholar] [CrossRef]
- Escandell, J.M.; Recio, M.C.; Máñez, S.; Giner, R.M.; Cerdá-Nicolás, M.; Ríos, J.L. Cucurbitacin R Reduces the Inflammation and Bone Damage Associated with Adjuvant Arthritis in Lewis Rats by Suppression of Tumor Necrosis Factor-α in T Lymphocytes and Macrophages. J. Pharmacol. Exp. Ther. 2007, 320, 581–590. [Google Scholar] [CrossRef]
- Peng, Y.; Chen, T.; Luo, L.; Li, L.; Cao, W.; Xu, X.; Zhang, Y.; Yue, P.; Dai, X.; Ji, Z.; et al. Isoforskolin and Cucurbitacin IIa Promote the Expression of Anti-Inflammatory Regulatory Factor SIGIRR in Human Macrophages Stimulated with Borrelia burgdorferi Basic Membrane Protein A. Int. Immunopharmacol. 2020, 88, 106914. [Google Scholar] [CrossRef]
- Kim, M.; Park, S.Y.; Jin, M.L.; Park, G.; Son, H.J. Cucurbitacin B Inhibits Immunomodulatory Function and the Inflammatory Response in Macrophages. Immunopharmacol. Immunotoxicol. 2015, 37, 473–480. [Google Scholar] [CrossRef]
- Duarte, D.B.; Vasko, M.R.; Fehrenbacher, J.C. Models of Inflammation: Carrageenan Air Pouch. Curr. Protoc. Pharmacol. 2012, 8, e183. [Google Scholar] [CrossRef] [PubMed]
- Recio, M.C.; Prieto, M.; Bonucelli, M.; Orsi, C.; Máñez, S.; Giner, R.M.; Cerdá-Nicolás, M.; Ríos, J.L. AnTi-Inflammatory Activity of Two Cucurbitacins Isolated from Cayaponia Tayuya Roots. Planta Med. 2004, 70, 414–420. [Google Scholar] [CrossRef] [PubMed]

| SHE-Ag (mg/kg) | |||
|---|---|---|---|
| Parameter | Saline | 2000 | 500 |
| Water consumed (mL) | 23.09 ± 1.50 | 19.07 ± 1.04 * | 24.03 ± 1.86 |
| Food consumed (g) | 14.11 ± 0.55 | 10.96 ± 0.97 * | 13.47 ± 0.83 |
| Weight gain (g) | 3.51 ± 0.31 | 3.62 ± 0.25 | 3.42 ± 0.33 |
| SHE-Ag (mg/kg) | |||
|---|---|---|---|
| Parameter | Saline | 2000 | 500 |
| ALB (g/dL) | 20.14 ± 1.62 | 26.45 ± 1.84 * | 20.76 ± 1.54 |
| ALT (U/L) | 59.36 ± 4.25 | 70.04 ± 5.18 * | 60.25 ± 4.34 |
| AST (U/L) | 92.05 ± 7.18 | 115.77 ± 5.39 * | 90.31 ± 4.90 |
| TP (g/dL) | 66.09 ± 5.13 | 68.29 ± 4.23 | 65.50 ± 5.68 |
| ALP (IU/L) | 11.14 ± 0.42 | 10.91 ± 0.31 | 10.77 ± 0.65 |
| GGT (U/L) | 10.26 ± 0.65 | 9.89 ± 0.53 | 9.71 ± 0.82 |
| Urea (mg/dL) | 0.32 ± 0.06 | 0.34 ± 0.05 | 0.36 ± 0.04 |
| CREAT (mg/dL) | 7.44 ± 0.59 | 7.26 ± 0.47 | 7.31 ± 0.40 |
| TC (mg/dL) | 91.03 ± 7.83 | 96.40 ± 8.26 | 90.53 ± 5.54 |
| TG (mg/dL) | 84.97 ± 5.90 | 87.64 ± 5.12 | 83.42 ± 6.65 |
| SHE-Ag (mg/kg) | |||
|---|---|---|---|
| Parameter | Saline | 2000 | 500 |
| Erythrocytes (106/mm3) | 6.21 ± 0.42 | 6.07 ± 0.58 | 6.59 ± 0.41 |
| Hematocrit (%) | 31.18 ± 2.63 | 27.68 ± 2.02 * | 32.12 ± 2.55 |
| Hemoglobin (g/dL) | 13.37 ± 0.40 | 10.64 ± 0.37 * | 13.27 ± 0.45 |
| MCV (fL) | 38.55 ± 2.95 | 40.13 ± 3.25 | 41.51 ± 4.18 |
| MCH (pg) | 15.79 ± 0.62 | 12.88 ± 0.84 * | 15.10 ± 0.98 |
| MCHC (%) | 30.67 ± 2.43 | 31.02 ± 2.72 | 32.13 ± 3.06 |
| Leukocytes (103/mm3) | 7.68 ± 0.56 | 7.33 ± 0.49 | 7.09 ± 0.52 |
| Segmented (%) | 63.46 ± 4.87 | 61.82 ± 3.63 | 59.66 ± 4.19 |
| Lymphocytes (%) | 24.55 ± 2.51 | 25.79 ± 2.49 | 26.12 ± 2.53 |
| Monocytes (%) | 3.42 ± 0.28 | 3.63 ± 0.31 | 3.28 ± 0.37 |
| Treatments | Collection Time | Number of MNPCE per Animal | Mean MPCE | ||||
|---|---|---|---|---|---|---|---|
| M1 | M2 | M3 | M4 | M5 | |||
| Saline | 24 h | 1 | 1 | 0 | 0 | 1 | 0.60 ± 0.04 |
| 48 h | 0 | 1 | 1 | 0 | 1 | 0.60 ± 0.04 | |
| SHE-Ag (2000 mg/kg) | 24 h | 0 | 1 | 0 | 1 | 1 | 0.60 ± 0.04 |
| 48 h | 0 | 1 | 1 | 1 | 0 | 0.60 ± 0.04 | |
| CPA (50 mg/kg) | 24 h | 26 | 28 | 31 | 28 | 26 | 27.50 ± 2.42 * |
| 48 h | 29 | 25 | 27 | 31 | 32 | 28.80 ± 2.30 * | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrade, M.L.d.O.; Marinho, P.A.F.; Oliveira, A.M.d.; Souza, T.A.d.; Cibulski, S.P.; Alves, H.d.S. Apodanthera glaziovii (Cucurbitaceae) Shows Strong Anti-Inflammatory Activity in Murine Models of Acute Inflammation. Pharmaceutics 2024, 16, 1298. https://doi.org/10.3390/pharmaceutics16101298
Andrade MLdO, Marinho PAF, Oliveira AMd, Souza TAd, Cibulski SP, Alves HdS. Apodanthera glaziovii (Cucurbitaceae) Shows Strong Anti-Inflammatory Activity in Murine Models of Acute Inflammation. Pharmaceutics. 2024; 16(10):1298. https://doi.org/10.3390/pharmaceutics16101298
Chicago/Turabian StyleAndrade, Maria Lorena de Oliveira, Pedro Artur Ferreira Marinho, Alisson Macário de Oliveira, Thalisson Amorim de Souza, Samuel Paulo Cibulski, and Harley da Silva Alves. 2024. "Apodanthera glaziovii (Cucurbitaceae) Shows Strong Anti-Inflammatory Activity in Murine Models of Acute Inflammation" Pharmaceutics 16, no. 10: 1298. https://doi.org/10.3390/pharmaceutics16101298
APA StyleAndrade, M. L. d. O., Marinho, P. A. F., Oliveira, A. M. d., Souza, T. A. d., Cibulski, S. P., & Alves, H. d. S. (2024). Apodanthera glaziovii (Cucurbitaceae) Shows Strong Anti-Inflammatory Activity in Murine Models of Acute Inflammation. Pharmaceutics, 16(10), 1298. https://doi.org/10.3390/pharmaceutics16101298








