Cell Therapy for Retinal Degenerative Diseases: Progress and Prospects
Abstract
1. Introduction
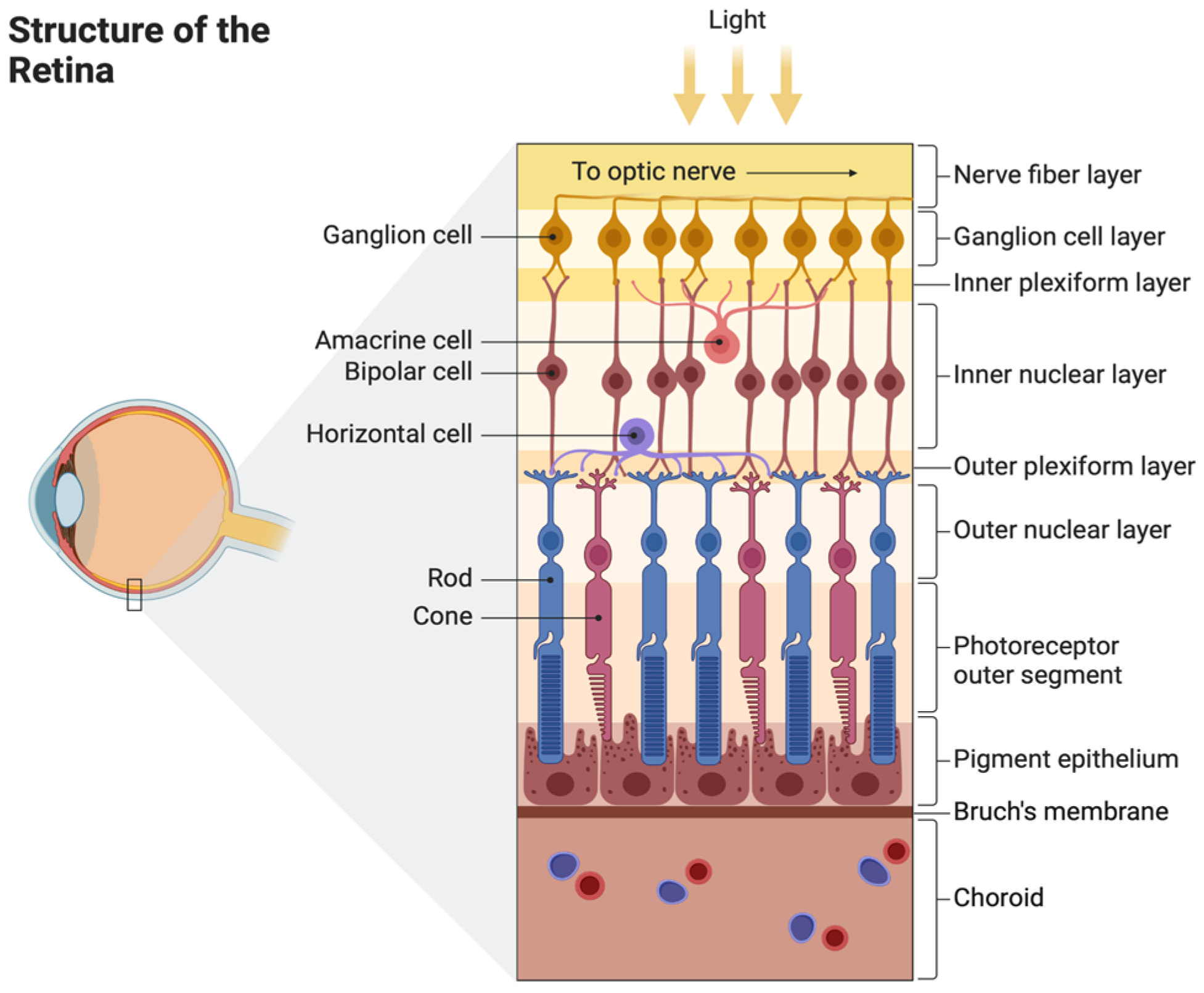
2. Anatomy and Physiology of the Retina
2.1. Structure of the Retina and Choroid
2.2. Retinal Blood Supply
3. Retinal Degenerative Diseases
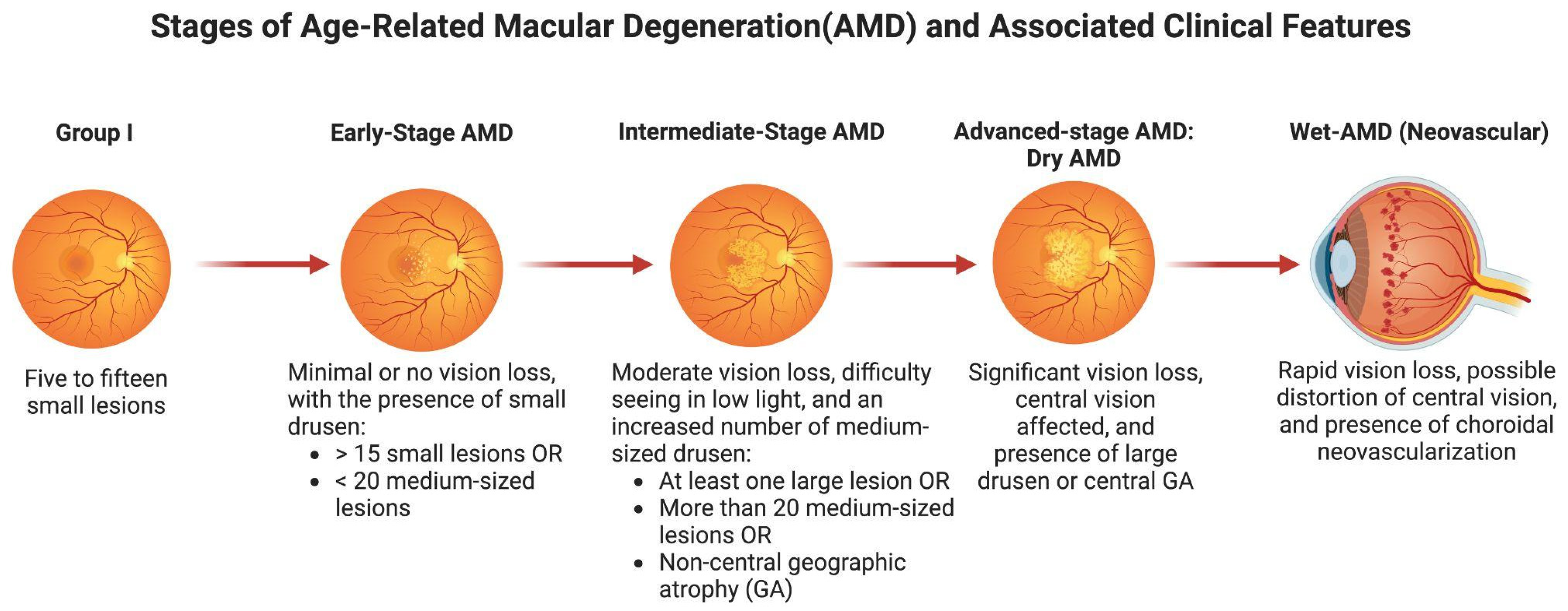
3.1. Age-Related Macular Degeneration (AMD)
3.2. Retinitis Pigmentosa (RP) (Disease)
3.3. Glaucoma
3.4. Stargardt Disease (SD)
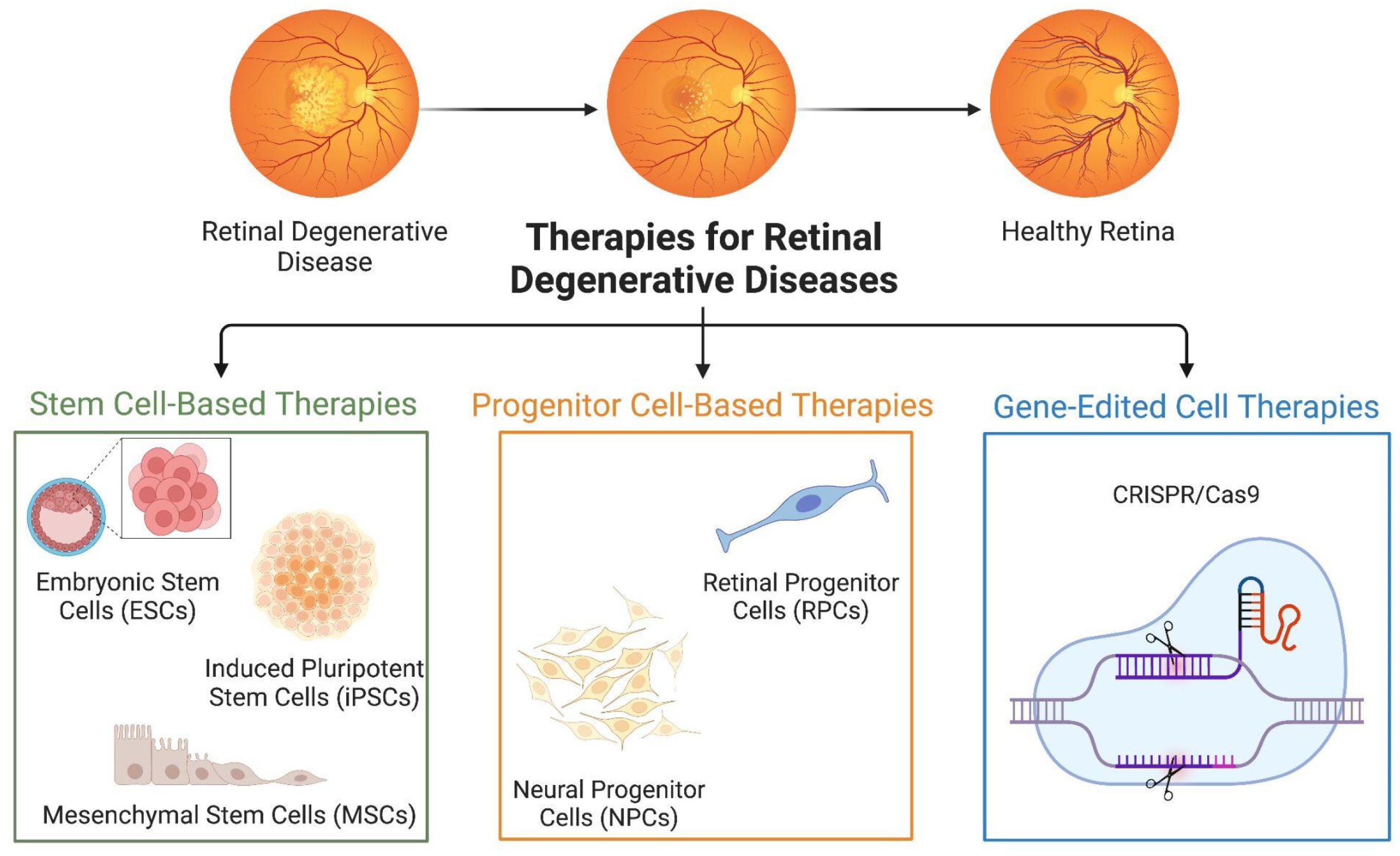
4. Types of Cell Therapies
4.1. Retinitis Pigmentosa (RP)
4.1.1. Embryonic Stem Cells
4.1.2. Induced Pluripotent Stem Cells
4.1.3. Mesenchymal Stem Cells
4.2. Progenitor Cell-Based Therapies
4.2.1. Retinal Progenitor Cells
4.2.2. Neural Progenitor Cells
4.3. Gene-Edited Cell Therapies
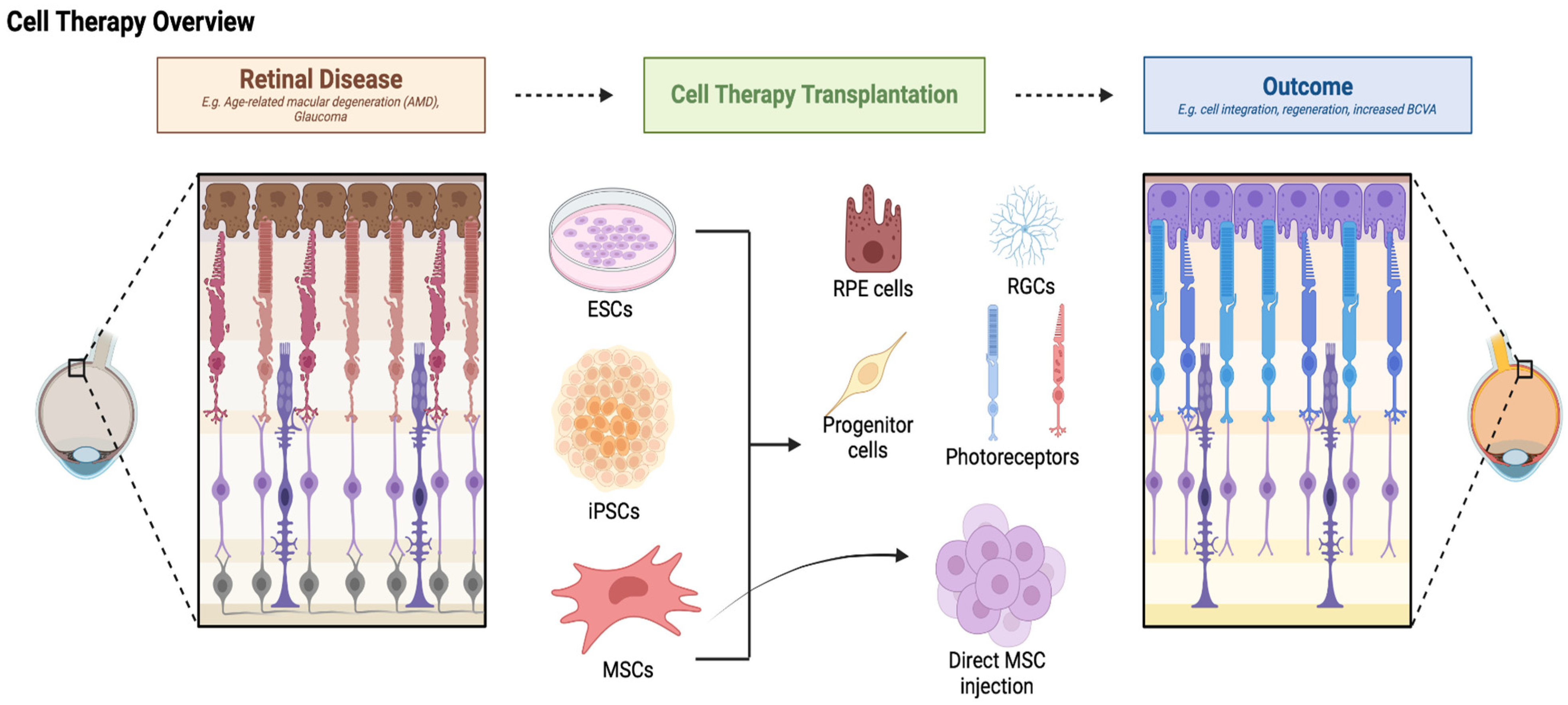
5. Mechanisms of Action
5.1. Cell Replacement
5.2. Neuroprotection and Paracrine Effects
6. Cell Therapy for Retinal Degenerative Diseases
6.1. Preclinical Studies
6.1.1. Preclinical Studies Using ESCs
6.1.2. Preclinical Studies Using iPSCs
6.1.3. Preclinical Studies Using MSCs
6.1.4. Preclinical Studies Using Progenitor Cells
6.2. Clinical Trials
6.2.1. Clinical Trials Using hESCs
6.2.2. Clinical Trials Using hiPSCs
6.2.3. Clinical Trials Using MSCs
6.2.4. Clinical Trials Using Progenitor Cells
7. Future Directions
7.1. Advances in Cell Therapy Techniques
7.2. Combination Therapies
7.3. Barrier to Clinical Translation
7.4. Ethical Issues
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mahabadi, N.; Al Khalili, Y. Neuroanatomy, Retina. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Delaey, C.; van de Voorde, J. Regulatory Mechanisms in the Retinal and Choroidal Circulation. Ophthalmic Res. 2000, 32, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Vinores, S.A. Breakdown of the Blood–Retinal Barrier. Encycl. Eye 2010, 5, 216–222. [Google Scholar] [CrossRef]
- Forrester, J.V.; McMenamin, P.G.; Dando, S.J. CNS Infection and Immune Privilege. Nat. Rev. Neurosci. 2018, 19, 655–671. [Google Scholar] [CrossRef] [PubMed]
- Ellis, R.R. Age-Related Macular Degeneration (AMD): An Overview. Available online: https://www.webmd.com/eye-health/macular-degeneration/age-related-macular-degeneration-overview (accessed on 16 August 2024).
- Hageman, G.S.; Gehrs, K.; Johnson, L.V.; Anderson, D. Age-Related Macular Degeneration (AMD). In Webvision: The Organization of the Retina and Visual System; Kolb, H., Fernandez, E., Nelson, R., Eds.; University of Utah Health Sciences Center: Salt Lake City, UT, USA, 1995. [Google Scholar]
- Fernandes, A.R.; Zielińska, A.; Sanchez-Lopez, E.; dos Santos, T.; Garcia, M.L.; Silva, A.M.; Karczewski, J.; Souto, E.B. Exudative versus Nonexudative Age-Related Macular Degeneration: Physiopathology and Treatment Options. Int. J. Mol. Sci. 2022, 23, 2592. [Google Scholar] [CrossRef] [PubMed]
- Somasundaran, S.; Constable, I.J.; Mellough, C.B.; Carvalho, L.S. Retinal Pigment Epithelium and Age-Related Macular Degeneration: A Review of Major Disease Mechanisms. Clin. Exp. Ophthalmol. 2020, 48, 1043–1056. [Google Scholar] [CrossRef]
- Ruan, Y.; Jiang, S.; Gericke, A. Age-Related Macular Degeneration: Role of Oxidative Stress and Blood Vessels. Int. J. Mol. Sci. 2021, 22, 1296. [Google Scholar] [CrossRef]
- Pugazhendhi, A.; Hubbell, M.; Jairam, P.; Ambati, B. Neovascular Macular Degeneration: A Review of Etiology, Risk Factors, and Recent Advances in Research and Therapy. Int. J. Mol. Sci. 2021, 22, 1170. [Google Scholar] [CrossRef]
- Dry Macular Degeneration—Symptoms and Causes—Mayo Clinic. Available online: https://www.mayoclinic.org/diseases-conditions/dry-macular-degeneration/symptoms-causes/syc-20350375 (accessed on 16 August 2024).
- Ruia, S.; Kaufman, E.J. Macular Degeneration. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Cunningham ET Jr, Adamis AP, Altaweel M, Aiello LP, Bressler NM, D’Amico DJ, Goldbaum M, Guyer DR, Katz B, Patel M, Schwartz SD; Macugen Diabetic Retinopathy Study Group. A Phase II Randomized Double-Masked Trial of Pegaptanib, an Anti–Vascular Endothelial Growth Factor Aptamer, for Diabetic Macular Edema. Ophthalmology 2005, 112, 1747–1757. [Google Scholar] [CrossRef]
- O’Neal, T.B.; Luther, E.E. Retinitis Pigmentosa. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Glaucoma|National Eye Institute. Available online: https://www.nei.nih.gov/learn-about-eye-health/eye-conditions-and-diseases/glaucoma (accessed on 28 September 2024).
- Types of Glaucoma|National Eye Institute. Available online: https://www.nei.nih.gov/learn-about-eye-health/eye-conditions-and-diseases/glaucoma/types-glaucoma (accessed on 28 September 2024).
- Carreon, T.; van der Merwe, E.; Fellman, R.L.; Johnstone, M.; Bhattacharya, S.K. Aqueous Outflow—A Continuum from Trabecular Meshwork to Episcleral Veins. Prog. Retin. Eye Res. 2017, 57, 108–133. [Google Scholar] [CrossRef]
- Glaucoma and Eye Pressure|National Eye Institute. Available online: https://www.nei.nih.gov/learn-about-eye-health/eye-conditions-and-diseases/glaucoma/glaucoma-and-eye-pressure (accessed on 28 September 2024).
- Vernazza, S.; Tirendi, S.; Bassi, A.M.; Traverso, C.E.; Saccà, S.C. Neuroinflammation in Primary Open-Angle Glaucoma. J. Clin. Med. 2020, 9, 3172. [Google Scholar] [CrossRef]
- Glaucoma Medicines|National Eye Institute. Available online: https://www.nei.nih.gov/Glaucoma/glaucoma-medicines (accessed on 28 September 2024).
- Laser Treatment for Glaucoma|National Eye Institute. Available online: https://www.nei.nih.gov/learn-about-eye-health/eye-conditions-and-diseases/glaucoma/treatment (accessed on 28 September 2024).
- Hu, B.-Y.; Xin, M.; Chen, M.; Yu, P.; Zeng, L.-Z. Mesenchymal Stem Cells for Repairing Glaucomatous Optic Nerve. Int. J. Ophthalmol. 2024, 17, 748–760. [Google Scholar] [CrossRef] [PubMed]
- Stargardt Disease|National Eye Institute. Available online: https://www.nei.nih.gov/learn-about-eye-health/eye-conditions-and-diseases/stargardt-disease (accessed on 28 September 2024).
- Ghenciu, L.A.; Hațegan, O.A.; Stoicescu, E.R.; Iacob, R.; Șișu, A.M. Emerging Therapeutic Approaches and Genetic Insights in Stargardt Disease: A Comprehensive Review. Int. J. Mol. Sci. 2024, 25, 8859. [Google Scholar] [CrossRef] [PubMed]
- Auricchio, A.; Trapani, I.; Allikmets, R. Gene Therapy of ABCA4-Associated Diseases. Cold Spring Harb. Perspect. Med. 2015, 5, a017301. [Google Scholar] [CrossRef] [PubMed]
- Yalla, G.R.; Kuriyan, A.E. Cell Therapy for Retinal Disease. Curr. Opin. Ophthalmol. 2024, 35, 178–184. [Google Scholar] [CrossRef]
- Radu, M.; Brănișteanu, D.C.; Pirvulescu, R.A.; Dumitrescu, O.M.; Ionescu, M.A.; Zemba, M. Exploring Stem-Cell-Based Therapies for Retinal Regeneration. Life 2024, 14, 668. [Google Scholar] [CrossRef]
- Cerneckis, J.; Cai, H.; Shi, Y. Induced Pluripotent Stem Cells (iPSCs): Molecular Mechanisms of Induction and Applications. Signal Transduct. Target. Ther. 2024, 9, 112. [Google Scholar] [CrossRef]
- Voisin, A.; Pénaguin, A.; Gaillard, A.; Leveziel, N. Stem Cell Therapy in Retinal Diseases. Neural Regen. Res. 2022, 18, 1478–1485. [Google Scholar] [CrossRef]
- Adak, S.; Magdalene, D.; Deshmukh, S.; Das, D.; Jaganathan, B.G. A Review on Mesenchymal Stem Cells for Treatment of Retinal Diseases. Stem Cell Rev. Rep. 2021, 17, 1154–1173. [Google Scholar] [CrossRef]
- Sharma, A.; Jaganathan, B.G. Stem Cell Therapy for Retinal Degeneration: The Evidence to Date. BTT 2021, 15, 299–306. [Google Scholar] [CrossRef]
- German, O.L.; Vallese-Maurizi, H.; Soto, T.B.; Rotstein, N.P.; Politi, L.E. Retina Stem Cells, Hopes and Obstacles. World J. Stem Cells 2021, 13, 1446–1479. [Google Scholar] [CrossRef]
- Shahin, S.; Tan, P.; Chetsawang, J.; Lu, B.; Svendsen, S.; Ramirez, S.; Conniff, T.; Alfaro, J.S.; Fernandez, M.; Fulton, A.; et al. Human Neural Progenitors Expressing GDNF Enhance Retinal Protection in a Rodent Model of Retinal Degeneration. Stem Cells Transl. Med. 2023, 12, 727–744. [Google Scholar] [CrossRef] [PubMed]
- Nair, D.S.R.; Thomas, B.B. Stem Cell-Based Treatment Strategies for Degenerative Diseases of the Retina. Curr. Stem Cell Res. Ther. 2022, 17, 214–225. [Google Scholar] [CrossRef] [PubMed]
- Drag, S.; Dotiwala, F.; Upadhyay, A.K. Gene Therapy for Retinal Degenerative Diseases: Progress, Challenges, and Future Directions. Investig. Ophthalmol. Vis. Sci. 2023, 64, 39. [Google Scholar] [CrossRef] [PubMed]
- Surendran, H.; Soundararajan, L.; Reddy, V.B.K.; Subramani, J.; Stoddard, J.; Reynaga, R.; Tschetter, W.; Ryals, R.C.; Pal, R. An Improved Protocol for Generation and Characterization of Human-Induced Pluripotent Stem Cell-Derived Retinal Pigment Epithelium Cells. STAR Protoc. 2022, 3, 101803. [Google Scholar] [CrossRef]
- Petrus-Reurer, S.; Winblad, N.; Kumar, P.; Gorchs, L.; Chrobok, M.; Wagner, A.K.; Bartuma, H.; Lardner, E.; Aronsson, M.; Plaza Reyes, Á.; et al. Generation of Retinal Pigment Epithelial Cells Derived from Human Embryonic Stem Cells Lacking Human Leukocyte Antigen Class I and II. Stem Cell Rep. 2020, 14, 648–662. [Google Scholar] [CrossRef]
- Shrestha, R.; Wen, Y.-T.; Tsai, R.-K. Induced Pluripotent Stem Cells and Derivative Photoreceptor Precursors as Therapeutic Cells for Retinal Degenerations. Tzu Chi Med. J. 2019, 32, 101–112. [Google Scholar] [CrossRef]
- Wu, K.Y.; Kulbay, M.; Toameh, D.; Xu, A.Q.; Kalevar, A.; Tran, S.D. Retinitis Pigmentosa: Novel Therapeutic Targets and Drug Development. Pharmaceutics 2023, 15, 685. [Google Scholar] [CrossRef]
- Li, L.; Turner, J.E. Inherited Retinal Dystrophy in the RCS Rat: Prevention of Photoreceptor Degeneration by Pigment Epithelial Cell Transplantation. Exp. Eye Res. 1988, 47, 911–917. [Google Scholar] [CrossRef]
- Gouras, P.; Kjeldbye, H.; Sullivan, B.; Reppucci, V.; Britfis, M.; Wapner, F.; Goluboff, E. Transplanted Retinal Pigment Epithelium Modifies the Retinal Degeneration in the RC5 Rat. Investig. Ophthalmol. 1989, 30, 586–588. [Google Scholar]
- Schraermeyer, U.; Thumann, G.; Luther, T.; Kociok, N.; Arnhold, S.; Kruttwig, K.; Andressen, C.; Addicks, K.; Bartz-Schmidt, K.U. Subretinally Transplanted Embryonic Stem Cells Rescue Photoreceptor Cells from Degeneration in the RCS Rats. Cell Transplant. 2001, 10, 673–680. [Google Scholar] [CrossRef]
- Kicic, A.; Shen, W.-Y.; Wilson, A.S.; Constable, I.J.; Robertson, T.; Rakoczy, P.E. Differentiation of Marrow Stromal Cells into Photoreceptors in the Rat Eye. J. Neurosci. 2003, 23, 7742–7749. [Google Scholar] [CrossRef]
- Haruta, M.; Sasai, Y.; Kawasaki, H.; Amemiya, K.; Ooto, S.; Kitada, M.; Suemori, H.; Nakatsuji, N.; Ide, C.; Honda, Y.; et al. In Vitro and In Vivo Characterization of Pigment Epithelial Cells Differentiated from Primate Embryonic Stem Cells. Investig. Ophthalmol. Vis. Sci. 2004, 45, 1020–1025. [Google Scholar] [CrossRef]
- Klassen, H.J.; Ng, T.F.; Kurimoto, Y.; Kirov, I.; Shatos, M.; Coffey, P.; Young, M.J. Multipotent Retinal Progenitors Express Developmental Markers, Differentiate into Retinal Neurons, and Preserve Light-Mediated Behavior. Investig. Ophthalmol. Vis. Sci. 2004, 45, 4167–4173. [Google Scholar] [CrossRef] [PubMed]
- Lund, R.D.; Wang, S.; Klimanskaya, I.; Holmes, T.; Ramos-Kelsey, R.; Lu, B.; Girman, S.; Bischoff, N.; Sauvé, Y.; Lanza, R. Human Embryonic Stem Cell–Derived Cells Rescue Visual Function in Dystrophic RCS Rats. Cloning Stem Cells 2006, 8, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Vugler, A.; Carr, A.-J.; Lawrence, J.; Chen, L.L.; Burrell, K.; Wright, A.; Lundh, P.; Semo, M.; Ahmado, A.; Gias, C.; et al. Elucidating the Phenomenon of HESC-Derived RPE: Anatomy of Cell Genesis, Expansion and Retinal Transplantation. Exp. Neurol. 2008, 214, 347–361. [Google Scholar] [CrossRef]
- Castanheira, P.; Torquetti, L.; Nehemy, M.B.; Goes, A.M. Retinal Incorporation and Differentiation of Mesenchymal Stem Cells Intravitreally Injected in the Injured Retina of Rats. Arq. Bras. Oftalmol. 2008, 71, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Idelson, M.; Alper, R.; Obolensky, A.; Ben-Shushan, E.; Hemo, I.; Yachimovich-Cohen, N.; Khaner, H.; Smith, Y.; Wiser, O.; Gropp, M.; et al. Directed Differentiation of Human Embryonic Stem Cells into Functional Retinal Pigment Epithelium Cells. Cell Stem Cell 2009, 5, 396–408. [Google Scholar] [CrossRef] [PubMed]
- Lamba, D.A.; Gust, J.; Reh, T.A. Transplantation of Human Embryonic Stem Cell-Derived Photoreceptors Restores Some Visual Function in Crx-Deficient Mice. Cell Stem Cell 2009, 4, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Jagatha, B.; Divya, M.S.; Sanalkumar, R.; Indulekha, C.L.; Vidyanand, S.; Divya, T.S.; Das, A.V.; James, J. In Vitro Differentiation of Retinal Ganglion-like Cells from Embryonic Stem Cell Derived Neural Progenitors. Biochem. Biophys. Res. Commun. 2009, 380, 230–235. [Google Scholar] [CrossRef]
- Osakada, F.; Jin, Z.-B.; Hirami, Y.; Ikeda, H.; Danjyo, T.; Watanabe, K.; Sasai, Y.; Takahashi, M. In Vitro Differentiation of Retinal Cells from Human Pluripotent Stem Cells by Small-Molecule Induction. J. Cell Sci. 2009, 122, 3169–3179. [Google Scholar] [CrossRef]
- Meyer, J.S.; Shearer, R.L.; Capowski, E.E.; Wright, L.S.; Wallace, K.A.; McMillan, E.L.; Zhang, S.-C.; Gamm, D.M. Modeling Early Retinal Development with Human Embryonic and Induced Pluripotent Stem Cells. Proc. Natl. Acad. Sci. USA 2009, 106, 16698–16703. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Chen, Q.; Sun, X.; Shen, W.; Liu, B.; Zhong, X.; Leng, Y.; Li, C.; Zhang, W.; Chai, F.; et al. Generation of Retinal Ganglion–like Cells from Reprogrammed Mouse Fibroblasts. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5970–5978. [Google Scholar] [CrossRef] [PubMed]
- Lamba, D.A.; McUsic, A.; Hirata, R.K.; Wang, P.-R.; Russell, D.; Reh, T.A. Generation, Purification and Transplantation of Photoreceptors Derived from Human Induced Pluripotent Stem Cells. PLoS ONE 2010, 5, e8763. [Google Scholar] [CrossRef] [PubMed]
- Tucker, B.A.; Park, I.-H.; Qi, S.D.; Klassen, H.J.; Jiang, C.; Yao, J.; Redenti, S.; Daley, G.Q.; Young, M.J. Transplantation of Adult Mouse iPS Cell-Derived Photoreceptor Precursors Restores Retinal Structure and Function in Degenerative Mice. PLoS ONE 2011, 6, e18992. [Google Scholar] [CrossRef] [PubMed]
- Shirai, H.; Mandai, M.; Matsushita, K.; Kuwahara, A.; Yonemura, S.; Nakano, T.; Assawachananont, J.; Kimura, T.; Saito, K.; Terasaki, H.; et al. Transplantation of Human Embryonic Stem Cell-Derived Retinal Tissue in Two Primate Models of Retinal Degeneration. Proc. Natl. Acad. Sci. USA 2016, 113, E81–E90. [Google Scholar] [CrossRef]
- Santos-Ferreira, T.; Völkner, M.; Borsch, O.; Haas, J.; Cimalla, P.; Vasudevan, P.; Carmeliet, P.; Corbeil, D.; Michalakis, S.; Koch, E.; et al. Stem Cell–Derived Photoreceptor Transplants Differentially Integrate Into Mouse Models of Cone-Rod Dystrophy. Investig. Ophthalmol. Vis. Sci. 2016, 57, 3509–3520. [Google Scholar] [CrossRef]
- Venugopalan, P.; Wang, Y.; Nguyen, T.; Huang, A.; Muller, K.J.; Goldberg, J.L. Transplanted Neurons Integrate into Adult Retinas and Respond to Light. Nat. Commun. 2016, 7, 10472. [Google Scholar] [CrossRef]
- Holan, V.; Hermankova, B.; Kossl, J. Perspectives of Stem Cell–Based Therapy for Age-Related Retinal Degenerative Diseases. Cell Transplant. 2017, 26, 1538–1541. [Google Scholar] [CrossRef]
- Wu, H.; Li, J.; Mao, X.; Li, G.; Xie, L.; You, Z. Transplantation of Rat Embryonic Stem Cell-Derived Retinal Cells Restores Visual Function in the Royal College of Surgeons Rats. Doc. Ophthalmol. 2018, 137, 71–78. [Google Scholar] [CrossRef]
- Sharma, R.; Khristov, V.; Rising, A.; Jha, B.S.; Dejene, R.; Hotaling, N.; Li, Y.; Stoddard, J.; Stankewicz, C.; Wan, Q.; et al. Clinical-Grade Stem Cell-Derived Retinal Pigment Epithelium Patch Rescues Retinal Degeneration in Rodents and Pigs. Sci. Transl. Med. 2019, 11, eaat5580. [Google Scholar] [CrossRef]
- Tu, H.-Y.; Watanabe, T.; Shirai, H.; Yamasaki, S.; Kinoshita, M.; Matsushita, K.; Hashiguchi, T.; Onoe, H.; Matsuyama, T.; Kuwahara, A.; et al. Medium- to Long-Term Survival and Functional Examination of Human iPSC-Derived Retinas in Rat and Primate Models of Retinal Degeneration. EBioMedicine 2019, 39, 562–574. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Gao, F.; Zhang, M.; Zheng, Y.; Zhang, F.; Xu, L.; Cao, L.; He, W. Intravitreal Injection of Human Retinal Progenitor Cells for Treatment of Retinal Degeneration. Med. Sci. Monit. 2020, 26, e921184-1–e921184-10. [Google Scholar] [CrossRef] [PubMed]
- Salas, A.; Duarri, A.; Fontrodona, L.; Ramírez, D.M.; Badia, A.; Isla-Magrané, H.; Ferreira-de-Souza, B.; Zapata, M.Á.; Raya, Á.; Veiga, A.; et al. Cell Therapy with hiPSC-Derived RPE Cells and RPCs Prevents Visual Function Loss in a Rat Model of Retinal Degeneration. Mol. Ther. Methods Clin. Dev. 2021, 20, 688–702. [Google Scholar] [CrossRef] [PubMed]
- Surendran, H.; Nandakumar, S.; Reddy, V.B.K.; Stoddard, J.; Mohan, K.V.; Upadhyay, P.K.; McGill, T.J.; Pal, R. Transplantation of Retinal Pigment Epithelium and Photoreceptors Generated Concomitantly via Small Molecule-Mediated Differentiation Rescues Visual Function in Rodent Models of Retinal Degeneration. Stem Cell Res. Ther. 2021, 12, 70. [Google Scholar] [CrossRef] [PubMed]
- Duarri, A.; Rodríguez-Bocanegra, E.; Martínez-Navarrete, G.; Biarnés, M.; García, M.; Ferraro, L.L.; Kuebler, B.; Aran, B.; Izquierdo, E.; Aguilera-Xiol, E.; et al. Transplantation of Human Induced Pluripotent Stem Cell-Derived Retinal Pigment Epithelium in a Swine Model of Geographic Atrophy. Int. J. Mol. Sci. 2021, 22, 10497. [Google Scholar] [CrossRef]
- He, X.-Y.; Zhao, C.-J.; Xu, H.; Chen, K.; Bian, B.-S.-J.; Gong, Y.; Weng, C.-H.; Zeng, Y.-X.; Fu, Y.; Liu, Y.; et al. Synaptic Repair and Vision Restoration in Advanced Degenerating Eyes by Transplantation of Retinal Progenitor Cells. Stem Cell Rep. 2021, 16, 1805–1817. [Google Scholar] [CrossRef]
- Liang, Q.; Li, Q.; Ren, B.; Yin, Z.Q. Intravenous Infusion of Small Umbilical Cord Mesenchymal Stem Cells Could Enhance Safety and Delay Retinal Degeneration in RCS Rats. BMC Ophthalmol. 2022, 22, 67. [Google Scholar] [CrossRef]
- Zhang, J.; Li, P.; Zhao, G.; He, S.; Xu, D.; Jiang, W.; Peng, Q.; Li, Z.; Xie, Z.; Zhang, H.; et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles Protect Retina in a Mouse Model of Retinitis Pigmentosa by Anti-Inflammation through miR-146a-Nr4a3 Axis. Stem Cell Res. Ther. 2022, 13, 394. [Google Scholar] [CrossRef]
- Brown, C.; Agosta, P.; McKee, C.; Walker, K.; Mazzella, M.; Alamri, A.; Svinarich, D.; Chaudhry, G.R. Human Primitive Mesenchymal Stem Cell-Derived Retinal Progenitor Cells Improved Neuroprotection, Neurogenesis, and Vision in Rd12 Mouse Model of Retinitis Pigmentosa. Stem Cell Res. Ther. 2022, 13, 148. [Google Scholar] [CrossRef]
- Di Pierdomenico, J.; Gallego-Ortega, A.; Martínez-Vacas, A.; García-Bernal, D.; Vidal-Sanz, M.; Villegas-Pérez, M.P.; García-Ayuso, D. Intravitreal and Subretinal Syngeneic Bone Marrow Mononuclear Stem Cell Transplantation Improves Photoreceptor Survival but Does Not Ameliorate Retinal Function in Two Rat Models of Retinal Degeneration. Acta Ophthalmol. 2022, 100, e1313–e1331. [Google Scholar] [CrossRef]
- Dezfuly, A.R.; Safaee, A.; Amirpour, N.; Kazemi, M.; Ramezani, A.; Jafarinia, M.; Dehghani, A.; Salehi, H. Therapeutic Effects of Human Adipose Mesenchymal Stem Cells and Their Paracrine Agents on Sodium Iodate Induced Retinal Degeneration in Rats. Life Sci. 2022, 300, 120570. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Liu, J.; Guo, M.; Sung, T.-C.; Wang, T.; Yu, T.; Tian, Z.; Fan, G.; Wu, W.; Higuchi, A. Comparison of Retinal Degeneration Treatment with Four Types of Different Mesenchymal Stem Cells, Human Induced Pluripotent Stem Cells and RPE Cells in a Rat Retinal Degeneration Model. J. Transl. Med. 2023, 21, 910. [Google Scholar] [CrossRef] [PubMed]
- Sheedlo, H.J.; Li, L.; Gaur, V.P.; Young, R.W.; Seaton, A.D.; Stovall, S.V.; Jaynes, C.D.; Turner, J.E. Photoreceptor Rescue in the Dystrophic Retina by Transplantation of Retinal Pigment Epithelium. In International Review of Cytology; Jeon, K.W., Friedlander, M., Eds.; Academic Press: Cambridge, MA, USA, 1992; Volume 138, pp. 1–49. [Google Scholar]
- Schwartz, S.D.; Hubschman, J.-P.; Heilwell, G.; Franco-Cardenas, V.; Pan, C.K.; Ostrick, R.M.; Mickunas, E.; Gay, R.; Klimanskaya, I.; Lanza, R. Embryonic Stem Cell Trials for Macular Degeneration: A Preliminary Report. Lancet 2012, 379, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, S.D.; Regillo, C.D.; Lam, B.L.; Eliott, D.; Rosenfeld, P.J.; Gregori, N.Z.; Hubschman, J.-P.; Davis, J.L.; Heilwell, G.; Spirn, M.; et al. Human Embryonic Stem Cell-Derived Retinal Pigment Epithelium in Patients with Age-Related Macular Degeneration and Stargardt’s Macular Dystrophy: Follow-up of Two Open-Label Phase 1/2 Studies. Lancet 2015, 385, 509–516. [Google Scholar] [CrossRef]
- Song, W.K.; Park, K.-M.; Kim, H.-J.; Lee, J.H.; Choi, J.; Chong, S.Y.; Shim, S.H.; Del Priore, L.V.; Lanza, R. Treatment of Macular Degeneration Using Embryonic Stem Cell-Derived Retinal Pigment Epithelium: Preliminary Results in Asian Patients. Stem Cell Rep. 2015, 4, 860–872. [Google Scholar] [CrossRef]
- Mehat, M.S.; Sundaram, V.; Ripamonti, C.; Robson, A.G.; Smith, A.J.; Borooah, S.; Robinson, M.; Rosenthal, A.N.; Innes, W.; Weleber, R.G.; et al. Transplantation of Human Embryonic Stem Cell-Derived Retinal Pigment Epithelial Cells in Macular Degeneration. Ophthalmology 2018, 125, 1765–1775. [Google Scholar] [CrossRef]
- Sung, Y.; Lee, M.J.; Choi, J.; Jung, S.Y.; Chong, S.Y.; Sung, J.H.; Shim, S.H.; Song, W.K. Long-Term Safety and Tolerability of Subretinal Transplantation of Embryonic Stem Cell-Derived Retinal Pigment Epithelium in Asian Stargardt Disease Patients. Br. J. Ophthalmol. 2021, 105, 829–837. [Google Scholar] [CrossRef]
- Li, S.; Liu, Y.; Wang, L.; Wang, F.; Zhao, T.; Li, Q.; Xu, H.; Meng, X.; Hao, J.; Zhou, Q.; et al. A Phase I Clinical Trial of Human Embryonic Stem Cell-derived Retinal Pigment Epithelial Cells for Early-stage Stargardt Macular Degeneration: 5-years’ Follow-up. Cell Prolif. 2021, 54, e13100. [Google Scholar] [CrossRef]
- da Cruz, L.; Fynes, K.; Georgiadis, O.; Kerby, J.; Luo, Y.H.; Ahmado, A.; Vernon, A.; Daniels, J.T.; Nommiste, B.; Hasan, S.M.; et al. Phase 1 Clinical Study of an Embryonic Stem Cell–Derived Retinal Pigment Epithelium Patch in Age-Related Macular Degeneration. Nat. Biotechnol. 2018, 36, 328–337. [Google Scholar] [CrossRef]
- Monville, C.; Bertin, S.; Devisme, C.; Brazhnikova, E.; Jaillard, C.; Walter, H.; Plancheron, A.; Jarraya, M.; Bejanariu, A.; Abbas, S.; et al. Phase I/II Open-Label Study of Implantation into One Eye of hESC-Derived RPE in Patients with Retinitis Pigmentosa Due to Monogenic Mutation: First Safety Results. Investig. Ophthalmol. Vis. Sci. 2023, 64, 3829. [Google Scholar]
- Humayun, M.S.; Clegg, D.O.; Dayan, M.S.; Kashani, A.H.; Rahhal, F.M.; Avery, R.L.; Salehi-Had, H.; Chen, S.; Chan, C.; Palejwala, N.; et al. Long-Term Follow-up of a Phase 1/2a Clinical Trial of a Stem Cell-Derived Bioengineered Retinal Pigment Epithelium Implant for Geographic Atrophy. Ophthalmology 2024, 131, 682–691. [Google Scholar] [CrossRef] [PubMed]
- Kashani, A.H.; Lebkowski, J.S.; Rahhal, F.M.; Avery, R.L.; Salehi-Had, H.; Chen, S.; Chan, C.; Palejwala, N.; Ingram, A.; Dang, W.; et al. One-Year Follow-Up in a Phase 1/2a Clinical Trial of an Allogeneic RPE Cell Bioengineered Implant for Advanced Dry Age-Related Macular Degeneration. Trans. Vis. Sci. Technol. 2021, 10, 13. [Google Scholar] [CrossRef]
- Telander, D. OpRegen® Retinal Pigment Epithelium (RPE) Cell Therapy for Patients with Geographic Atrophy (GA): Month 24 Results from the Phase 1/2a Trial. 2024. [Google Scholar]
- Mandai, M.; Watanabe, A.; Kurimoto, Y.; Hirami, Y.; Morinaga, C.; Daimon, T.; Fujihara, M.; Akimaru, H.; Sakai, N.; Shibata, Y.; et al. Autologous Induced Stem-Cell–Derived Retinal Cells for Macular Degeneration. N. Engl. J. Med. 2017, 376, 1038–1046. [Google Scholar] [CrossRef] [PubMed]
- Takagi, S.; Mandai, M.; Gocho, K.; Hirami, Y.; Yamamoto, M.; Fujihara, M.; Sugita, S.; Kurimoto, Y.; Takahashi, M. Evaluation of Transplanted Autologous Induced Pluripotent Stem Cell-Derived Retinal Pigment Epithelium in Exudative Age-Related Macular Degeneration. Ophthalmol. Retin. 2019, 3, 850–859. [Google Scholar] [CrossRef] [PubMed]
- Park, S.S.; Bauer, G.; Abedi, M.; Pontow, S.; Panorgias, A.; Jonnal, R.; Zawadzki, R.J.; Werner, J.S.; Nolta, J. Intravitreal Autologous Bone Marrow CD34+ Cell Therapy for Ischemic and Degenerative Retinal Disorders: Preliminary Phase 1 Clinical Trial Findings. Investig. Ophthalmol. Vis. Sci. 2015, 56, 81–89. [Google Scholar] [CrossRef]
- Park, S.S.; Bauer, G.; Fury, B.; Abedi, M.; Perotti, N.; Coleal-Gergum, D.; Nolta, J.A. Phase I Study of Intravitreal Injection of Autologous CD34+ Stem Cells from Bone Marrow in Eyes with Vision Loss from Retinitis Pigmentosa (AAO Meeting Paper). Ophthalmol. Sci. 2024, 5, 100589. [Google Scholar] [CrossRef]
- Siqueira, R.C.; Messias, A.; Voltarelli, J.C.; Scott, I.U.; Jorge, R. Intravitreal Injection of Autologous Bone Marrow–Derived Mononuclear Cells for Hereditary Retinal Dystrophy: A Phase I Trial. Retina 2011, 31, 1207–1214. [Google Scholar] [CrossRef]
- Siqueira, R.C.; Messias, A.; Messias, K.; Arcieri, R.S.; Ruiz, M.A.; Souza, N.F.; Martins, L.C.; Jorge, R. Quality of Life in Patients with Retinitis Pigmentosa Submitted to Intravitreal Use of Bone Marrow-Derived Stem Cells (Reticell-Clinical Trial). Stem Cell Res. Ther. 2015, 6, 29. [Google Scholar] [CrossRef]
- Tuekprakhon, A.; Sangkitporn, S.; Trinavarat, A.; Pawestri, A.R.; Vamvanij, V.; Ruangchainikom, M.; Luksanapruksa, P.; Pongpaksupasin, P.; Khorchai, A.; Dambua, A.; et al. Intravitreal Autologous Mesenchymal Stem Cell Transplantation: A Non-Randomized Phase I Clinical Trial in Patients with Retinitis Pigmentosa. Stem Cell Res. Ther. 2021, 12, 52. [Google Scholar] [CrossRef]
- Siqueira, R.C.; Costa Cotrim, C.; Messias, A.; Sousa, M.V.d.; Toscano, L.; Jorge, R. Intravitreal Autologous Bone Marrow Derived Stem Cells in Dry Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2016, 57, 3704. [Google Scholar]
- Weiss, J.N.; Levy, S. Stem Cell Ophthalmology Treatment Study (SCOTS): Bone Marrow-Derived Stem Cells in the Treatment of Stargardt Disease. Medicines 2021, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.N.; Levy, S. Stem Cell Ophthalmology Treatment Study (SCOTS): Bone Marrow-Derived Stem Cells in the Treatment of Age-Related Macular Degeneration. Medicines 2020, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.N.; Levy, S. Stem Cell Ophthalmology Treatment Study: Bone Marrow Derived Stem Cells in the Treatment of Retinitis Pigmentosa. Stem Cell Investig. 2018, 5, 18. [Google Scholar] [CrossRef] [PubMed]
- Özmert, E.; Arslan, U. Management of Retinitis Pigmentosa by Wharton’s Jelly Derived Mesenchymal Stem Cells: Preliminary Clinical Results. Stem Cell Res. Ther. 2020, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Tang, L.; Zhou, Y. Subretinal Injection: A Review on the Novel Route of Therapeutic Delivery for Vitreoretinal Diseases. Ophthalmic Res. 2017, 58, 217–226. [Google Scholar] [CrossRef]
- Chiang, B.; Jung, J.H.; Prausnitz, M.R. The Suprachoroidal Space as a Route of Administration to the Posterior Segment of the Eye. Adv. Drug Deliv. Rev. 2018, 126, 58–66. [Google Scholar] [CrossRef]
- Lotfi, M.; Morshedi Rad, D.; Mashhadi, S.S.; Ashouri, A.; Mojarrad, M.; Mozaffari-Jovin, S.; Farrokhi, S.; Hashemi, M.; Lotfi, M.; Ebrahimi Warkiani, M.; et al. Recent Advances in CRISPR/Cas9 Delivery Approaches for Therapeutic Gene Editing of Stem Cells. Stem Cell Rev. Rep. 2023, 19, 2576–2596. [Google Scholar] [CrossRef]
- Siles, L.; Ruiz-Nogales, S.; Navinés-Ferrer, A.; Méndez-Vendrell, P.; Pomares, E. Efficient Correction of ABCA4 Variants by CRISPR-Cas9 in hiPSCs Derived from Stargardt Disease Patients. Mol. Ther. Nucleic Acids 2023, 32, 64–79. [Google Scholar] [CrossRef]
- Klymenko, V.; González Martínez, O.G.; Zarbin, M. Recent Progress in Retinal Pigment Epithelium Cell-Based Therapy for Retinal Disease. Stem Cells Transl. Med. 2024, 13, 317–331. [Google Scholar] [CrossRef]
- Ballios, B.G.; Cooke, M.J.; van der Kooy, D.; Shoichet, M.S. A Hydrogel-Based Stem Cell Delivery System to Treat Retinal Degenerative Diseases. Biomaterials 2010, 31, 2555–2564. [Google Scholar] [CrossRef]
- Silverman, M.S.; Hughes, S.E. Transplantation of Photoreceptors to Light-Damaged Retina. Investig. Ophthalmol. Vis. Sci. 1989, 30, 1684–1690. [Google Scholar]
- Song, M.J.; Quinn, R.; Nguyen, E.; Hampton, C.; Sharma, R.; Park, T.S.; Koster, C.; Voss, T.; Tristan, C.; Weber, C.; et al. Bioprinted 3D Outer Retina Barrier Uncovers RPE-Dependent Choroidal Phenotype in Advanced Macular Degeneration. Nat. Methods 2023, 20, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.-B.; Okamoto, S.; Osakada, F.; Homma, K.; Assawachananont, J.; Hirami, Y.; Iwata, T.; Takahashi, M. Modeling Retinal Degeneration Using Patient-Specific Induced Pluripotent Stem Cells. PLoS ONE 2011, 6, e17084. [Google Scholar] [CrossRef] [PubMed]
- Rohowetz, L.J.; Koulen, P. Stem Cell-Derived Retinal Pigment Epithelium Cell Therapy: Past and Future Directions. Front. Cell Dev. Biol. 2023, 11, 1098406. [Google Scholar] [CrossRef]
- Zheng, Y.L. Some Ethical Concerns About Human Induced Pluripotent Stem Cells. Sci. Eng. Ethics 2016, 22, 1277–1284. [Google Scholar] [CrossRef]
| Disease Model | Animal Model | Protocol Description | Observed Effect | Reference |
|---|---|---|---|---|
| Retinal degeneration | Royal College of Surgeon (RCS) rats | Subretinal transplantation of donor RPE in host eye | RPE cells can be successfully transplanted into normal neonatal and adult rat eyes. | [40] |
| Retinal degeneration | Royal College of Surgeon (RCS) rats | Transplantation of donor RPE into subretinal space of dystrophic rat retina | Transplantation of RPE cells can prevent photoreceptor degeneration for at least 4 months. | [41] |
| Retinal degeneration | Royal College of Surgeon (RCS) rats | Subretinal transplantation of embryonic stem cells | Transplantation appeared to delay photoreceptor degeneration. | [42] |
| Retinal degeneration | Royal College of Surgeon (RCS) rats | Adult CD90 marrow stromal cells induced into cells with photoreceptor markers in vitro and then transplanted into RCS rats | MSC differentiated with autologous transplantation and integrated into the host retina with no teratoma formation. | [43] |
| AMD | Royal College of Surgeon (RCS) rats | Transplantation of RPE derived from primate ESC into subretinal space | Recovery of retinal function post-transplantation. | [44] |
| RP | C57BL/6 rho−/− mice at 4 week of age or C3H rd mice at 4 weeks of age | Isolated retinal progenitor cells from day 1 eGFP transgenic CH7Bl/6 mice and expanded them; then, transplanted into mice with retinal degeneration | Donor cells integrated into retina and mice who received the transplant showed improved light-mediated behavior. | [45] |
| AMD | Royal College of Surgeon (RCS) rats | RPE derived from human ESC and transplanted into subretinal space of RCS rats | Cell survived in host, photoreceptors were restored, and vision improved. | [46] |
| AMD and RP | Royal College of Surgeon (RCS) rats | hESC-derived RPE was transplanted in the subretinal space of RCS rats | Cell survived in host, photoreceptors were restored, and vision improved. | [47] |
| Retinal injury and damage | 10–12-week-old Wistar rats | Adult rat retinas underwent retinal damage via laser and then received bone marrow mesenchymal stem cell transplants | Bone marrow MSC survived in the retina and was incorporated into the outer nuclear layer, inner nuclear layer, and ganglion cell layer. Cells expressed rhodopsin and parvalbumin. | [48] |
| AMD and RP | Royal College of Surgeon (RCS) rats | RPE derived from human ESC and transplanted into subretinal space of RCS rats | Functional rescue in transplanted eyes compared to controls. | [49] |
| RP | Crx−/− mice (model of Leber’s Congenital Amaurosis) | Retinal cells derived from human ESC were injected into mice retina using intraocular injection | Human ESC expressed markers for rod and cone photoreceptor cells once in subretinal space of mice and restored light response. | [50] |
| Glaucoma | ES cell culture from mouse D3-ES cells | Embryonic stem cells were differentiated in vitro and also transplanted in vivo | Embryonic cells can be used to treat degenerative diseases as they generate RGC-like cells in vitro and also differentiate into RGC cells in vivo after transplantation. | [51] |
| Retinal degeneration | hESC and iPSC | Provide a defined method of inducing hESC and iPSC into retinal progenitors, RPE, and photoreceptors | Induced retinal progenitor cells expressed RX, MITF, PAX6, and CHX10. Hexagonal pigmented cells expressed RPE65 and CRALBP. Photoreceptors expressed recoverin, rhodopsin, and phototransduction genes. | [52] |
| Retinal degeneration | hESC and iPSC | Determine whether hESC and iPSC model retinal development upon differentiation | Demonstrated that retinal cell specification from hESC and iPSC follows a sequence and time course similar to normal retinal development. | [53] |
| Glaucoma | BALB/c mice | Trying to see if induced pluripotent stem cells can express retinal progenitor cell genes and differentiate into retinal ganglion cells. Injected iPS-derived retinal ganglion-like cells into the retina | iPS cells express Pax6, Rx, Otx2, Lhx2, and Nestin genes inherently and over expression of Math5 and DN differentiate iPS into RG-like cells. Inhibiting Hes1 increases RGC genes. iPS-derived RG-like cells survive in retina but cannot integrate post-transplant. | [54] |
| N/a | Normal retina, adult wild-type mice | Generate iPSC with OCT4, SOX2, NANOG, and LIN28 to derive photoreceptors for use in cell therapy for retinal transplantation | FACS-purified iPSC-derived photoreceptors can integrate into normal mouse retina and express photoreceptor markers. | [55] |
| RP | 4–6-week-old dsRed-positive C57B1G mice were fibroblast donors and 4–6 weeks rhodopsin-null mice were transplant recipients | Adult dsRed mouse dermal fibroblast-derived iPSCs were transplanted in degenerative hosts | Cells formed teratomas. At 33 days, post-differentiation cells had markers for photoreceptors. CRX, recoverin, and rhodopsin. Increased retinol function in hosts with degenerative retina post-transplant. | [56] |
| RP | Monkey models | Determine ability of hESC-retina graft to transplant in rats and then conduct a pilot transplant in newly developed monkey models of retinal degeneration | Developed monkey models for study of retinal transplantation. Demonstrated hESC-retina graft to be effective in transplantation. | [57] |
| Retinal degeneration | Mouse models with mild degeneration (prom 1−/−) or severe degeneration (Cpfl1/Rho−/−) | Derived photoreceptors from organoids and subretinal transplantation in wild-type hosts | Retinal organoids had high photoreceptors and survived in the subretinal space of all mice. In mild degeneration cells integrated and had mature morphology. In the severe degeneration model, transplants remained in subretinal space and had rod-specific markers but no mature morphology. | [58] |
| Glaucoma | 1–3-month Sprague Dawley rats | Transplanted GFP-labeled retinal ganglion cells into normal rat retinas by intravitreal injection | Cells integrated into the retina of adult rats (1–3 months) and made synapses post-transplantation. | [59] |
| Retinal degeneration | Female mice of inbred strain BALB/c age 7–9 weeks | Cultured MSCs to see growth factor expression, anti-inflammatory effects, and differentiation | Mesenchymal cells can differentiate into cells that show retinal markers, produce neuroprotective factors for retinal regeneration, and inhibit production of pro-inflammatory cytokines. | [60] |
| Retinal degeneration | 4-week albino Royal College of Surgeon (RCS) rats | Isolated rat embryonic stem cells and induced them into retinal progenitor cells in vitro; transplanted into RCS rat retina | Visual function was restored in RCS rats. Potential clinical application of ESC cell therapy. | [61] |
| Retinal degeneration | Mice and pigs | Oncogene mutation-free iPSC was taken from AMD patients and differentiated into iPSC retinal pigment epithelium patches | Protocol was robust and efficient in generating RPE cells and rescuing degenerating retina in mice and pigs. | [62] |
| Retinal degeneration | Rhodopsin mutant SD-Foxn1 Tg (S334ter)3LacRrrc nude rats and 2 monkeys | Transplanted human iPSC retinas into animal models | Mature photoreceptors survived in the host retina for 5 months (rat) and 2 years (monkey). Some light responses detected in grafted areas in rats (4 of 7) and monkeys. | [63] |
| Retinal degeneration | BALB/c-mu mice | Transplanted human retinal progenitor cells via intravitreal injection into BALB/c-mu mice | Differentiated hRPCs had high retinal markers, no teratoma was formed, and retinal function improved. Slowed retinal degeneration. However, hRPCs were no longer effective 12 weeks post-transplant. | [64] |
| Retinal degeneration | Royal College of Surgeon (RCS) rats | Compared combined hiPSC-derived RPE and retinal precursor cell (RPCs) transplantation to either alone; in vivo monitoring conducted | Combined transplantation of hiPSC-derived RPE and RPC may be better than either transplant alone in retinal degeneration. Better visual response and conservation of outer nuclear layer. | [65] |
| RP | Royal College of Surgeon (RCS) rats | hiPSC-derived retinal cells and photoreceptor progenitor (PRP) cells transplanted in vivo via trans-scleral subretinal injection | Strong efficacy and safety for hiPSC-derived RPE and PRP cells in animals. No animal had teratoma formation and there was graft survival and integration. RPE transplant rescued vision function and there was functional photoreceptor activity. | [66] |
| AMD—geographic atrophy | Swine | Subretinal transplantation of hiPSC-derived RPE into healthy and degenerative retina areas | In vitro analysis showed the hiPSC-RPE cells to be differentiated, have typical epithelial morphology, and RPE-related gene expression. In the healthy retina, they engrafted and formed mature epithelium, but were patchy in atrophic areas. | [67] |
| Retinal degeneration | Royal College of Surgeon (RCS) rats | Transplanted retinal progenitor cells derived from mouse ESC-derived retinal organoids into RCS rats | The transplanted cells migrated to the inner retina and differentiated into photoreceptors, interneurons, and ganglion cells. The grafted cells elicited robust responses to light stimuli and integrated with the host retina. | [68] |
| RP | Royal College of Surgeon (RCS) rats | Derived umbilical cord mesenchymal stem cells (UCMSC) and then intravenously infused into RCS rats | Small UCMSC became stuck in lungs less and left quicker than UCMSC. Inflammation was inhibited and neurotrophic factors upregulated in retina and serum after transplantation. May be a potential therapeutic approach and delay degeneration in rats. | [69] |
| RP | rd10 mice | Intravitreal injection of MSCs into mouse retina | Increase in survival rate of photoreceptors and visual function enhancement was observed through optomotor and electroretinogram responses. | [70] |
| RP | rd12 mouse models with retinal degeneration | Intravitreal injection of adult MSC-derived RPCs into mouse retina | Transplanted RPCs led to improved vision and function. Observed anti-inflammation, retinal protection, and increased expression of genes involved in neurogenesis. | [71] |
| RP | Two animal models: RCS and P23H-1 rats | Utilized either intravitreal or subretinal injections of bone marrow mononuclear stem cell transplantations | Both forms of injections increased cell survival, as seen through mitigation of photoreceptor degeneration. No enhanced retinal function observed. | [72] |
| RP and AMD | Sodium iodate-induced retinal injury rat model | Transplantation of human adipose-derived MSCs | Transplantation facilitated photoreceptor regeneration and restoration of retinal function. | [73] |
| Retinal degeneration | 3-week-old RCS rats | Compared subretinal transplant of stem cells, human adipose-derived stem cells, amniotic fluid stem cells, bone marrow stem cells, dental pulp stem cells, induced pluripotent stem cells, and hiPSC-derived RPE | Rats transplanted with any stem cell other than hiPSC had better visual function 4 weeks post-injection. Rats with hiPSC maintained visual function 8 weeks post-injection. | [74] |
| Trial Stage | Type of Cell Used | Disease | Sample Size | Approach | Country | Identifier |
|---|---|---|---|---|---|---|
| Phase I/II | hESC-derived RPE (MA09-hRPE) | SMD | 13 | Subretinal injection of 50,000–200,000 cells | USA | NCT01345006 |
| Phase I and II—completed | hESC-derived RPE (MA09-hRPE) | Dry AMD | 13 | Subretinal injection of 50,000–150,000 cells in 5 cohorts | USA | NCT01344993 |
| Terminated | hESC-derived RPE (MA09-hRPE) | Advanced Dry AMD | 10 | Transplantation of MA09-hRPE | Republic of Korea | NCT01674829 |
| Phase I/II—completed | hESC-derived RPE (MA09-hRPE) | SMD | 12 | 5 cohorts with 50,000–200,000 cell injections | UK | NCT01469832 |
| Phase I—completed | hESC-derived RPE (MA09-hRPE) | Stargardt Macular Dystrophy | 3 | Subretinal transplantation of MA09-hRPE cells | Republic of Korea | NCT01625559 |
| Phase I/II—unknown status | hESC-derived RPE | AMD and Stargardt | 15 | Subretinal transplantation | China | NCT02749734 |
| Phase I/II—enrolling | hESC-RPE | AMD | 36 | Evaluating occurrence of late-onset adverse effects after hESC-RPE subretinal transplant | UK, USA | NCT03167203 |
| Phase 1 | hESC-derived RPE | RP | 10 | Transplant into subretinal space | China | NCT03944239 |
| Phase 1—recruiting | PF-05206388—hESC-derived RPE | Wet AMD | 10 | Implantation of PF-05206388 | UK | NCT01691261 |
| Phase I and II—active | hESC-derived RPE | RP | 12 | Implantation of monolayer therapeutical patch into eye with worse acuity | France | NCT03963154 |
| Phase I/II—completed | hESC-derived RPE | Dry AMD, wet AMD, and Stargardt disease | 15 | Compare the safety of surgical implantation of hESC-RPE monolayer on s polymeric scaffold versus hESC-RPE injections into subretinal space | Brazil | NCT02903576 |
| Phase I/II—unknown status | hESC-derived RPE on parylene membrane (CPCB-RPE1) | Advanced dry AMD patients with geographic atrophy and central fovea involvement | 16 | Subretinal implantation of 100,000 differentiated RPE cells attached to a small parylene membrane | USA | NCT02590692 |
| Phase I/IIa—active, not recruiting | OpRegen hESC-derived RPE | Dry AMD | 24 | Subretinal transplantation of 50,000–200,000 cells; see how cells engraft, survive, and moderate disease progression | Israel | NCT02286089 |
| Phase I/II—enrolling | Retinal stem and progenitor cells | AMD | 20 | Cultured retinal stem and progenitor cells are injected subretinally | Belarus | NCT05187104 |
| Phase I/II—unknown status | hESC-derived RPE | Dry AMD | 10 | Transplant into subretinal space | China | NCT03046407 |
| Phase I/II—recruiting | RPESC-RPE-4W (allogeneic RPE stem-cell-derived RPE cells isolated from human cadaver) | Dry AMD | 18 | Patients will receive 50,000, 150,000, or 250,000 RPESC-RPE-4W cells in the macula of the eye. | USA | NCT04627428 |
| Phase 1 | Autologous iPSC-derived RPE | AMD | 6 | Determine safety of transplanting iPSC-derived RPE sheets | Japan | UMIN000011929 |
| Phases I/IIa—recruiting | Autologous iPSC-derived RPE | Dry AMD | 20 | Subretinal transplantation of autologous iPSC-derived RPE in one eye | USA | NCT04339764 |
| Phase I/IIa—recruiting | hiPSC-derived Eyecyte-RPE | Geographic atrophy secondary to dry AMD | 54 | Single-dose subretinal injection at varying doses: 100,000, 200,000, and 300,000 | India | NCT06394232 |
| Phase I—recruiting | Induced pluripotent stem cell (iPSC) | AMD | 10 | Autologous transplantation of iPSC-derived retinal pigment epithelium (RPE) into subretinal pace | Beijing | NCT05445063 |
| Phase I | CD34+ stem cells from bone marrow | Irreversibly blind patients due to various retinal conditions | 15 | CD34+ bone marrow stem cells intravitreal | USA | NCT01736059 (pilot) |
| Phase I—completed | Autologous CD34+ stem cells harvested from bone marrow | RP | 4 | Intravitreal injection into 1 eye and followed for 6 months | USA | NCT04925687 |
| Phase 1—completed | Autologous bone marrow stem cells | RP | 5 | Single intravitreal injection | Brazil | NCT01068561 |
| Phase II—completed | Autologous bone marrow stem cells | RP | 50 | Single intravitreal injection | Brazil | NCT01560715 |
| Phase I | Adult human bone-marrow-derived MSC | RP | 14 | Intravitreal injection | Thailand | NCT01531348 |
| Phase I/II—completed | Autologous bone marrow stem cell | AMD or Stargardt with best-corrected ETDRS visual acuity <20/200 | 20 | Intravitreal injection | Brazil | NCT01518127 |
| Not noted | Autologous bone-marrow-derived stem cells | AMD, RP, Stargardt | 500 | Injection of autologous bone-marrow-derived stem cells | USA, United Arab Emirates | NCT03011541 |
| Phase 3—completed | Umbilical cord Wahrton’s jelly-derived mesenchymal stem cells | RP | 32 | Cells implanted in sub-tenon space | Turkey | NCT04224207 |
| Phase I—recruiting | Allogeneic adult umbilical cord-derived mesenchymal stem cells (UC-MSCs) | RP | 20 | Intravenous and sub-tenon delivery of 100 million UC-MSCs | Antigua and Barbuda, Argentia, Mexico | NCT05147701 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, K.Y.; Dhaliwal, J.K.; Sasitharan, A.; Kalevar, A. Cell Therapy for Retinal Degenerative Diseases: Progress and Prospects. Pharmaceutics 2024, 16, 1299. https://doi.org/10.3390/pharmaceutics16101299
Wu KY, Dhaliwal JK, Sasitharan A, Kalevar A. Cell Therapy for Retinal Degenerative Diseases: Progress and Prospects. Pharmaceutics. 2024; 16(10):1299. https://doi.org/10.3390/pharmaceutics16101299
Chicago/Turabian StyleWu, Kevin Y., Jaskarn K. Dhaliwal, Akash Sasitharan, and Ananda Kalevar. 2024. "Cell Therapy for Retinal Degenerative Diseases: Progress and Prospects" Pharmaceutics 16, no. 10: 1299. https://doi.org/10.3390/pharmaceutics16101299
APA StyleWu, K. Y., Dhaliwal, J. K., Sasitharan, A., & Kalevar, A. (2024). Cell Therapy for Retinal Degenerative Diseases: Progress and Prospects. Pharmaceutics, 16(10), 1299. https://doi.org/10.3390/pharmaceutics16101299








