Enhancing Acute Migraine Treatment: Exploring Solid Lipid Nanoparticles and Nanostructured Lipid Carriers for the Nose-to-Brain Route
Abstract
1. Introduction
2. Acute Migraine
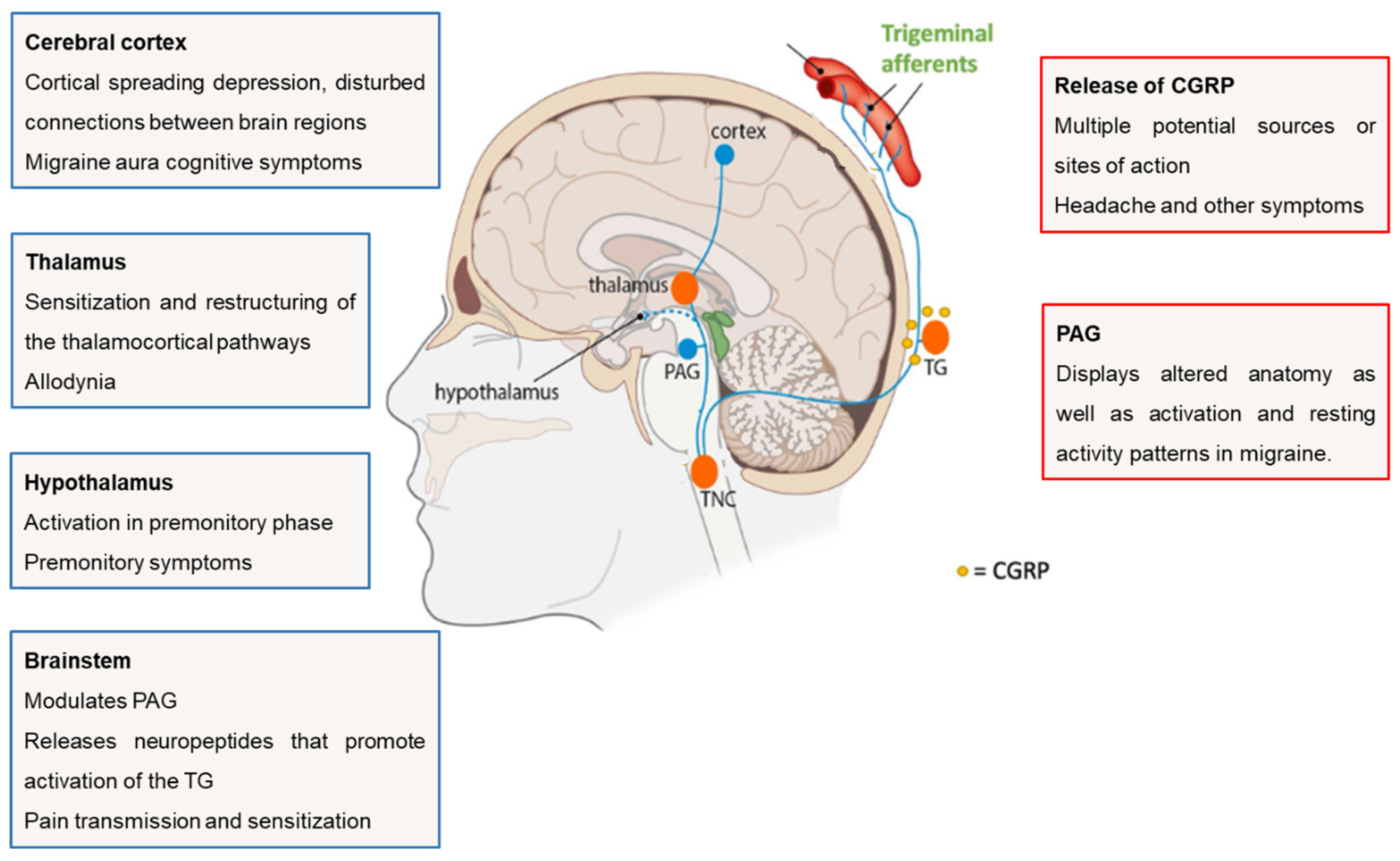
2.1. Functional Anatomy and Pathophysiology
2.2. Pharmacological Treatment of Migraine
2.2.1. Acute Treatment
2.2.2. Preventive Treatment
2.2.3. Non-Invasive Strategies to Overcome Treatment Limitations
2.3. Nasal Products Approved for the Treatment of Acute Migraine
3. Nose-to-Brain Route—An Overview
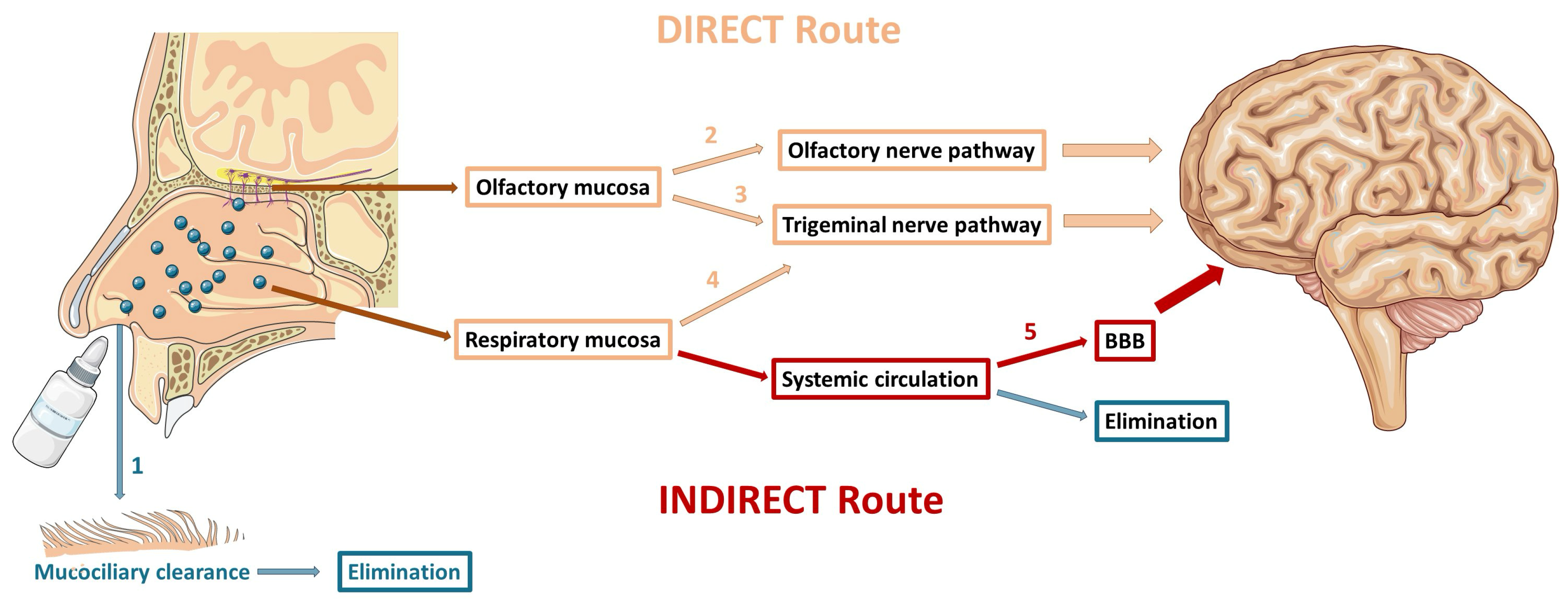
3.1. Mechanisms of Drug Delivery to the Brain
3.1.1. Olfactory Nerve Pathway
3.1.2. Trigeminal Nerve Pathway
3.1.3. Indirect Transport
3.2. Challenges of Nose-to-Brain Drug Delivery
4. Main Features of SLN and NLC
4.1. Specificities of SLN and NLC for Nose-to-Brain Drug Delivery
4.2. Recent In Vivo Studies with SLN and NLC to Improve the Treatment of Acute Migraine via the Nose-to-Brain Route
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Feigin, V.L.; Vos, T.; Nichols, E.; Owolabi, M.O.; Carroll, W.M.; Dichgans, M.; Deuschl, G.; Parmar, P.; Brainin, M.; Murray, C. The global burden of neurological disorders: Translating evidence into policy. Lancet Neurol. 2020, 19, 255–265. [Google Scholar] [CrossRef]
- Puledda, F.; Silva, E.M.; Suwanlaong, K.; Goadsby, P.J. Migraine: From pathophysiology to treatment. J. Neurol. 2023, 270, 3654–3666. [Google Scholar] [CrossRef] [PubMed]
- de Boer, I.; Verhagen, I.E.; Souza, M.N.P.; Ashina, M. Place of next generation acute migraine specific treatments among triptans, non-responders and contraindications to triptans and possible combination therapies. Cephalalgia 2023, 43, 3331024221143773. [Google Scholar] [CrossRef] [PubMed]
- Mungoven, T.J.; Henderson, L.A.; Meylakh, N. Chronic Migraine Pathophysiology and Treatment: A Review of Current Perspectives. Front. Pain Res. 2021, 2, 705276. [Google Scholar] [CrossRef] [PubMed]
- Eigenbrodt, A.K.; Ashina, H.; Khan, S.; Diener, H.C.; Mitsikostas, D.D.; Sinclair, A.J.; Pozo-Rosich, P.; Martelletti, P.; Ducros, A.; Lanteri-Minet, M.; et al. Diagnosis and management of migraine in ten steps. Nat. Rev. Neurol. 2021, 17, 501–514. [Google Scholar] [CrossRef]
- Cooper, W.; Ray, S.; Aurora, S.K.; Shrewsbury, S.B.; Fuller, C.; Davies, G.; Hoekman, J. Delivery of Dihydroergotamine Mesylate to the Upper Nasal Space for the Acute Treatment of Migraine: Technology in Action. J. Aerosol. Med. Pulm. Drug Deliv. 2022, 35, 321–332. [Google Scholar] [CrossRef]
- Martin, V.; Hoekman, J.; Aurora, S.K.; Shrewsbury, S.B. Nasal Delivery of Acute Medications for Migraine: The Upper Versus Lower Nasal Space. J. Clin. Med. 2021, 10, 2468. [Google Scholar] [CrossRef]
- Hoekman, J.; Ray, S.; Aurora, S.K.; Shrewsbury, S.B. The Upper Nasal Space—A Novel Delivery Route Ideal for Central Nervous System Drugs. US Neurol. 2020, 16, 25–31. [Google Scholar] [CrossRef]
- Tanna, V.; Sawarkar, S.P.; Ravikumar, P. Exploring Nose to Brain Nano Delivery for Effective Management of Migraine. Curr. Drug Deliv. 2022, 20, 144–157. [Google Scholar] [CrossRef]
- Kataria, I.; Shende, P. Nose-to-brain lipid nanocarriers: An active transportation across BBB in migraine management. Chem. Phys. Lipids 2022, 243, 105177. [Google Scholar] [CrossRef]
- Assadpour, S.; Shiran, M.R.; Asadi, P.; Akhtari, J.; Sahebkar, A. Harnessing Intranasal Delivery Systems of Sumatriptan for the Treatment of Migraine. Biomed. Res. Int. 2022, 2022, 3692065. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.R.; Liu, M.; Khan, M.W.; Zhai, G. Progress in brain targeting drug delivery system by nasal route. J. Control. Release 2017, 268, 364–389. [Google Scholar] [CrossRef]
- Erdő, F.; Bors, L.A.; Farkas, D.; Bajza, Á.; Gizurarson, S. Evaluation of intranasal delivery route of drug administration for brain targeting. Brain Res. Bull. 2018, 143, 155–170. [Google Scholar] [CrossRef]
- Formica, M.L.; Real, D.A.; Picchio, M.L.; Catlin, E.; Donnelly, R.F.; Paredes, A.J. On a highway to the brain: A review on nose-to-brain drug delivery using nanoparticles. Appl. Mater. Today 2022, 29, 101631. [Google Scholar] [CrossRef]
- Battaglia, L.; Panciani, P.P.; Muntoni, E.; Capucchio, M.T.; Biasibetti, E.; De Bonis, P.; Mioletti, S.; Fontanella, M.; Swaminathan, S. Lipid nanoparticles for intranasal administration: Application to nose-to-brain delivery. Expert Opin. Drug Deliv. 2018, 15, 369–378. [Google Scholar] [CrossRef]
- Nguyen, T.-T.-L.; Maeng, H.-J. Pharmacokinetics and Pharmacodynamics of Intranasal Solid Lipid Nanoparticles and Nanostructured Lipid Carriers for Nose-to-Brain Delivery. Pharmaceutics 2022, 14, 572. [Google Scholar] [CrossRef]
- Pescador Ruschel, M.A.; De Jesus, O. Migraine Headache. In StatPearls; Copyright © 2023; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2023. [Google Scholar]
- Belopasova, A.V.; Dobrynina, L.A.; Gubanova, M.V.; Suslina, A.D. Achievements of Recent Decades in the Diagnosis and Study of Migraine Pathogenesis. Human Physiol. 2021, 46, 870–879. [Google Scholar] [CrossRef]
- Fan, L.; Wu, Y.; Wei, J.; Xia, F.; Cai, Y.; Zhang, S.; Miao, J.; Zhou, Y.; Liu, C.; Yan, W.; et al. Global, regional, and national time trends in incidence for migraine, from 1990 to 2019: An age-period-cohort analysis for the GBD 2019. J. Headache Pain 2023, 24, 79. [Google Scholar] [CrossRef]
- Allais, G.; Chiarle, G.; Sinigaglia, S.; Airola, G.; Schiapparelli, P.; Benedetto, C. Gender-related differences in migraine. Neurol. Sci. 2020, 41 (Suppl. S2), 429–436. [Google Scholar] [CrossRef]
- Ong, J.J.Y.; De Felice, M. Migraine Treatment: Current Acute Medications and Their Potential Mechanisms of Action. Neurotherapeutics 2018, 15, 274–290. [Google Scholar] [CrossRef]
- Dodick, D.W. A Phase-by-Phase Review of Migraine Pathophysiology. Headache 2018, 58 (Suppl. S1), 4–16. [Google Scholar] [CrossRef] [PubMed]
- Muehlberger, T. What Is Migraine? Migraine Surg. 2018, 7–30. [Google Scholar] [CrossRef]
- Gawde, P.; Shah, H.; Patel, H.; Bharathi, K.S.; Patel, N.; Sethi, Y.; Kaka, N. Revisiting Migraine: The Evolving Pathophysiology and the Expanding Management Armamentarium. Cureus 2023, 15, e34553. [Google Scholar] [CrossRef] [PubMed]
- Pescador, M.A.; Ruschel, O.D.J. Migraine Headache. 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK560787/ (accessed on 2 September 2023).
- Haanes, K.A.; Edvinsson, L. Pathophysiological Mechanisms in Migraine and the Identification of New Therapeutic Targets. CNS Drugs 2019, 33, 525–537. [Google Scholar] [CrossRef]
- Cohen, C.F.; Roh, J.; Lee, S.H.; Park, C.K.; Berta, T. Targeting Nociceptive Neurons and Transient Receptor Potential Channels for the Treatment of Migraine. Int. J. Mol. Sci. 2023, 24, 7897. [Google Scholar] [CrossRef]
- van Welie, F.C.; Kreft, L.A.; Huisman, J.M.A.; Terwindt, G.M. Sex-specific metabolic profiling to explain the increased CVD risk in women with migraine: A narrative review. J. Headache Pain 2023, 24, 64. [Google Scholar] [CrossRef]
- Yam, M.F.; Loh, Y.C.; Tan, C.S.; Khadijah Adam, S.; Abdul Manan, N.; Basir, R. General Pathways of Pain Sensation and the Major Neurotransmitters Involved in Pain Regulation. Int. J. Mol. Sci. 2018, 19, 2164. [Google Scholar] [CrossRef]
- Parada, C.A.; Tambeli, C.H.; Green, P.G.; Cairns, B.E. Primary afferent nociceptor as a target for the relief of pain. Pain Res. Treat. 2012, 2012, 348043. [Google Scholar] [CrossRef][Green Version]
- Guo, S.; Jansen-Olesen, I.; Olesen, J.; Christensen, S.L. Role of PACAP in migraine: An alternative to CGRP? Neurobiol. Dis. 2023, 176, 105946. [Google Scholar] [CrossRef]
- Zagami, A.S.; Edvinsson, L.; Goadsby, P.J. Pituitary adenylate cyclase activating polypeptide and migraine. Ann. Clin. Transl. Neurol. 2014, 1, 1036–1040. [Google Scholar] [CrossRef]
- Vilas, D.; Rubio, S.; Gea, M.; Rios, J.; Ispierto, L.; Hernandez-Perez, M.; Pare, M.; Millan, M.; Dorado, L. Periaqueductal gray matter echogenicity as a marker of migraine chronification: A case control study. J. Headache Pain 2023, 24, 41. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, A. How triggers trigger acute migraine attacks: A hypothesis. Med. Hypotheses 2010, 74, 750–753. [Google Scholar] [CrossRef] [PubMed]
- Todd, A.J. An Historical Perspective: The Second Order Neuron in the Pain Pathway. Front. Pain Res. 2022, 3, 845211. [Google Scholar] [CrossRef] [PubMed]
- Knight, Y.E.; Goadsby, P.J. The periaqueductal grey matter modulates trigeminovascular input: A role in migraine? Neuroscience 2021, 106, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Hamel, E.; Currents, H. Serotonin and Migraine: Biology and Clinical Implications. Cephalalgia 2007, 27, 1293–1300. [Google Scholar] [CrossRef]
- Burstein, R.; Noseda, R.; Borsook, D. Migraine: Multiple processes, complex pathophysiology. J. Neurosci. 2015, 35, 6619–6629. [Google Scholar] [CrossRef]
- Sudershan, A.; Mahajan, K.; Singh, K.; Dhar, M.K.; Kumar, P. The complexities of migraine: A debate among migraine researchers: A review. Clin. Neurol. Neurosurg. 2022, 214, 107136. [Google Scholar] [CrossRef]
- Puledda, F.; Sacco, S.; Diener, H.C.; Ashina, M.; Al-Khazali, H.M.; Ashina, S.; Burstein, R.; Liebler, E.; Cipriani, A.; Chu, M.K.; et al. International Headache Society global practice recommendations for the acute pharmacological treatment of migraine. Cephalalgia 2024, 44, 3331024241252666. [Google Scholar] [CrossRef]
- Pellesi, L.; Do, T.P.; Hougaard, A. Pharmacological management of migraine: Current strategies and future dir. Expert Opin. Pharmacother. 2024, 25, 673–683. [Google Scholar] [CrossRef]
- Pehlivanlar, E.; Carradori, S.; Simsek, R. Migraine and Its Treatment from the Medicinal Chemistry Perspective. ACS Pharmacol. Transl. Sci. 2024, 7, 951–966. [Google Scholar] [CrossRef]
- Nicolas, S.; Nicolas, D. Triptans. In StatPearls; Copyright © 2023; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2023. [Google Scholar]
- Karlsson, W.K.; Ostinelli, E.G.; Zhuang, Z.A.; Kokoti, L.; Christensen, R.H.; Al-Khazali, H.M.; Deligianni, C.I.; Tomlinson, A.; Ashina, H.; Ruiz de la Torre, E.; et al. Comparative effects of drug interventions for the acute management of migraine episodes in adults: Systematic review and network meta-analysis. BMJ 2024, 386, e080107. [Google Scholar] [CrossRef] [PubMed]
- Danesh, A.; Gottschalk, P.C.H. Beta-Blockers for Migraine Prevention: A Review Article. Curr. Treat. Options Neurol. 2019, 21, 20. [Google Scholar] [CrossRef] [PubMed]
- Naegel, S.; Obermann, M. Topiramate in the prevention and treatment of migraine: Efficacy, safety and patient preference. Neuropsychiatr. Dis. Treat. 2009, 6, 17–28. [Google Scholar]
- Pavelic, A.R.; Wober, C.; Riederer, F.; Zebenholzer, K. Monoclonal Antibodies against Calcitonin Gene-Related Peptide for Migraine Prophylaxis: A Systematic Review of Real-World Data. Cells 2022, 12, 143. [Google Scholar] [CrossRef]
- van Hoogstraten, W.S.; MaassenVanDenBrink, A. The need for new acutely acting antimigraine drugs: Moving safely outside acute medication overuse. J. Headache Pain 2019, 20, 54. [Google Scholar] [CrossRef]
- Ha, H.; Gonzalez, A. Migraine Headache Prophylaxis. Am. Fam. Physician 2019, 99, 17–24. [Google Scholar]
- Diener, H.C.; Charles, A.; Goadsby, P.J.; Holle, D. New therapeutic approaches for the prevention and treatment of migraine. Lancet Neurol. 2015, 14, 1010–1022. [Google Scholar] [CrossRef]
- Ashina, M.; Lanteri-Minet, M.; Pozo-Rosich, P.; Ettrup, A.; Christoffersen, C.L.; Josiassen, M.K.; Phul, R.; Sperling, B. Safety and efficacy of eptinezumab for migraine prevention in patients with two-to-four previous preventive treatment failures (DELIVER): A multi-arm, randomised, double-blind, placebo-controlled, phase 3b trial. Lancet Neurol. 2022, 21, 597–607. [Google Scholar] [CrossRef]
- Haghdoost, F.; Puledda, F.; Garcia-Azorin, D.; Huessler, E.M.; Messina, R.; Pozo-Rosich, P. Evaluating the efficacy of CGRP mAbs and gepants for the preventive treatment of migraine: A systematic review and network meta-analysis of phase 3 randomised controlled trials. Cephalalgia 2023, 43, 3331024231159366. [Google Scholar] [CrossRef]
- Messina, R.; Huessler, E.M.; Puledda, F.; Haghdoost, F.; Lebedeva, E.R.; Diener, H.C. Safety and tolerability of monoclonal antibodies targeting the CGRP pathway and gepants in migraine prevention: A systematic review and network meta-analysis. Cephalalgia 2023, 43, 3331024231152169. [Google Scholar] [CrossRef]
- Vernieri, F.; Brunelli, N.; Messina, R.; Costa, C.M.; Colombo, B.; Torelli, P.; Quintana, S.; Cevoli, S.; Favoni, V.; d’Onofrio, F.; et al. Discontinuing monoclonal antibodies targeting CGRP pathway after one-year treatment: An observational longitudinal cohort study. J. Headache Pain 2021, 22, 154. [Google Scholar] [CrossRef] [PubMed]
- Steiner, T.J.; Jensen, R.; Katsarava, Z.; Linde, M.; MacGregor, E.A.; Osipova, V.; Paemeleire, K.; Olesen, J.; Peters, M.; Martelletti, P. Aids to management of headache disorders in primary care (2nd edition): On behalf of the European Headache Federation and Lifting The Burden: The Global Campaign against Headache. J. Headache Pain 2019, 20, 57. [Google Scholar] [CrossRef] [PubMed]
- Herman, T.F.; Santos, C. First Pass Effect. In StatPearls; Copyright © 2023; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2023. [Google Scholar]
- Costa, C.P.; Moreira, J.N.; Sousa Lobo, J.M.; Silva, A.C. Intranasal delivery of nanostructured lipid carriers, solid lipid nanoparticles and nanoemulsions: A current overview of in vivo studies. Acta Pharm. Sin. B 2021, 11, 925–940. [Google Scholar] [CrossRef]
- Jeong, S.H.; Jang, J.H.; Lee, Y.B. Drug delivery to the brain via the nasal route of administration: Exploration of key targets and major consideration factors. J. Pharm. Investig. 2023, 53, 119–152. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Das, S.; Sarma, K.N. Nose-to-Brain Drug Delivery: An Update to the Alternative Path to Successful Targeted Anti-Migraine Drugs. Int. J. Appl. Pharm. 2021, 13, 67–75. [Google Scholar] [CrossRef]
- Correia, A.C.; Monteiro, A.R.; Silva, R.; Moreira, J.N.; Sousa Lobo, J.M.; Silva, A.C. Lipid nanoparticles strategies to modify pharmacokinetics of central nervous system targeting drugs: Crossing or circumventing the blood-brain barrier (BBB) to manage neurological disorders. Adv. Drug Deliv. Rev. 2022, 189, 114485. [Google Scholar] [CrossRef]
- Pandit, R.; Chen, L.; Gotz, J. The blood-brain barrier: Physiology and strategies for drug delivery. Adv. Drug Deliv. Rev. 2020, 165–166, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.P.; Barreiro, S.; Moreira, J.N.; Silva, R.; Almeida, H.; Sousa Lobo, J.M.; Silva, A.C. In Vitro Studies on Nasal Formulations of Nanostructured Lipid Carriers (NLC) and Solid Lipid Nanoparticles (SLN). Pharmaceuticals 2021, 14, 711. [Google Scholar] [CrossRef]
- Aurora, S.K.; Shrewsbury, S.B.; Ray, S.; Hindiyeh, N.; Nguyen, L. A link between gastrointestinal disorders and migraine: Insights into the gut-brain connection. Headache 2021, 61, 576–589. [Google Scholar] [CrossRef]
- Crowe, T.P.; Hsu, W.H. Evaluation of Recent Intranasal Drug Delivery Systems to the Central Nervous System. Pharmaceutics 2022, 14, 629. [Google Scholar] [CrossRef]
- Duquesnoy, C.; Mamet, J.P.; Sumner, D.; Fuseau, E. Comparative clinical pharmacokinetics of single doses of sumatriptan following subcutaneous, oral, rectal and intranasal administration. Eur. J. Pharm. Sci. 1998, 6, 99–104. [Google Scholar] [CrossRef] [PubMed]
- IMITREX (Sumatriptan) Nasal Spray. Available online: https://gskpro.com/content/dam/global/hcpportal/en_US/Prescribing_Information/Imitrex_Nasal_Spray/pdf/IMITREX-NASAL-SPRAY-PI-PIL.PDF (accessed on 10 September 2023).
- Dihydroergotamine Mesylate Nasal Spray. Available online: https://pi.bauschhealth.com/globalassets/BHC/PI/Migranal-PI.pdf (accessed on 10 September 2023).
- Gallagher, R.M. Acute treatment of migraine with dihydroergotamine nasal spray. Dihydroergotamine Working Group. Arch. Neurol. 1996, 53, 1285–1291. [Google Scholar] [CrossRef] [PubMed]
- ZOMIG (Zolmitriptan) Nasal Spray. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/021450s007lbl.pdf (accessed on 10 September 2023).
- Charlesworth, B.R.; Dowson, A.J.; Purdy, A.; Becker, W.J.; Boes-Hansen, S.; Färkkilä, M. Speed of onset and efficacy of zolmitriptan nasal spray in the acute treatment of migraine: A randomised, double-blind, placebo-controlled, dose-ranging study versus zolmitriptan tablet. CNS Drugs 2003, 17, 653–667. [Google Scholar] [CrossRef] [PubMed]
- Winner, P.; Farkas, V.; Štillová, H.; Woodruff, B.; Liss, C.; Lillieborg, S.; Raines, S. Efficacy and tolerability of zolmitriptan nasal spray for the treatment of acute migraine in adolescents: Results of a randomized, double-blind, multi-center, parallel-group study (TEENZ). Headache 2016, 56, 1107–1119. [Google Scholar] [CrossRef] [PubMed]
- ONZETRA™ XsailTM (Sumatriptan Nasal Powder). Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/206099s000lbl.pdf (accessed on 11 September 2023).
- Obaidi, M.; Offman, E.; Messina, J.; Carothers, J.; Djupesland, P.G.; Mahmoud, R.A. Improved pharmacokinetics of sumatriptan with Breath Powered nasal delivery of sumatriptan powder. Headache 2013, 53, 1323–1333. [Google Scholar] [CrossRef]
- Cady, R. A novel intranasal breath-powered delivery system for sumatriptan: A review of technology and clinical application of the investigational product AVP-825 in the treatment of migraine. Expert Opin. Drug Deliv. 2015, 12, 1565–1577. [Google Scholar] [CrossRef]
- TOSYMRA™ (Sumatriptan) Nasal Spray. Available online: https://www.upsher-smith.com/wp-content/uploads/TOS-MI.pdf (accessed on 12 September 2023).
- Munjal, S.; Brand-Schieber, E.; Allenby, K.; Spierings, E.L.H.; Cady, R.K.; Rapoport, A.M. A multicenter, open-label, long-term safety and tolerability study of DFN-02, an intranasal spray of sumatriptan 10 mg plus permeation enhancer DDM, for the acute treatment of episodic migraine. J. Headache Pain 2017, 18, 31. [Google Scholar] [CrossRef]
- Munjal, S.; Gautam, A.; Offman, E.; Brand-Schieber, E.; Allenby, K.; Fisher, D.M. A Randomized Trial Comparing the Pharmacokinetics, Safety, and Tolerability of DFN-02, an Intranasal Sumatriptan Spray Containing a Permeation Enhancer, With Intranasal and Subcutaneous Sumatriptan in Healthy Adults. Headache 2016, 56, 1455–1465. [Google Scholar] [CrossRef]
- TRUDHESATM (Dihydroergotamine Mesylate) Nasal Spray. Available online: https://www.trudhesa.com/trudhesa-prescribing-information.pdf (accessed on 11 September 2023).
- Dhillon, S. Zavegepant: First Approval. Drugs 2023, 83, 825–831. [Google Scholar] [CrossRef]
- Freeman, S.C.; Karp, D.A.; Kahwaji, C.I. Physiology, Nasal. 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK526086/ (accessed on 2 September 2023).
- Lofts, A.; Abu-Hijleh, F.; Rigg, N.; Mishra, R.K.; Hoare, T. Using the Intranasal Route to Administer Drugs to Treat Neurological and Psychiatric Illnesses: Rationale, Successes, and Future Needs. CNS Drugs 2022, 36, 739–770. [Google Scholar] [CrossRef]
- Torres, J.; Costa, I.; Peixoto, A.F.; Silva, R.; Sousa Lobo, J.M.; Silva, A.C. Intranasal Lipid Nanoparticles Containing Bioactive Compounds Obtained from Marine Sources to Manage Neurodegenerative Diseases. Pharmaceuticals 2023, 16. [Google Scholar] [CrossRef] [PubMed]
- Keller, L.A.; Merkel, O.; Popp, A. Intranasal drug delivery: Opportunities and toxicologic challenges during drug development. Drug Deliv. Transl. Res. 2022, 12, 735–757. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Minko, T. Nanotherapeutics for Nose-to-Brain Drug Delivery: An Approach to Bypass the Blood Brain Barrier. Pharmaceutics 2021, 13, 2049. [Google Scholar] [CrossRef] [PubMed]
- Bahadur, S.; Pardhi, D.M.; Rautio, J.; Rosenholm, J.M.; Pathak, K. Intranasal Nanoemulsions for Direct Nose-to-Brain Delivery of Actives for CNS Disorders. Pharmaceutics 2020, 12, 1230. [Google Scholar] [CrossRef]
- Ying, C.; Zhang, J.; Zhang, H.; Gao, S.; Guo, X.; Lin, J.; Wu, H.; Hong, Y. Stem cells in central nervous system diseases: Promising therapeutic strategies. Exp. Neurol. 2023, 369, 114543. [Google Scholar] [CrossRef]
- Yu, P.; Chen, W.; Jiang, L.; Jia, Y.; Xu, X.; Shen, W.; Jin, N.; Du, H. Olfactory dysfunction and the role of stem cells in the regeneration of olfactory neurons. Heliyon 2024, 10, e29948. [Google Scholar] [CrossRef]
- Moradi, F.; Dashti, N. Targeting neuroinflammation by intranasal delivery of nanoparticles in neurological diseases: A comprehensive review. Naunyn Schmiedebergs Arch. Pharmacol. 2022, 395, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, V.N.; Tzabazis, A.Z.; Klukinov, M.; Manering, N.A.; Yeomans, D.C. Intranasal Administration for Pain: Oxytocin and Other Polypeptides. Pharmaceutics 2021, 13, 1088. [Google Scholar] [CrossRef]
- Costa, C.; Moreira, J.N.; Amaral, M.H.; Sousa Lobo, J.M.; Silva, A.C. Nose-to-brain delivery of lipid-based nanosystems for epileptic seizures and anxiety crisis. J. Control. Release 2019, 295, 187–200. [Google Scholar] [CrossRef]
- Yang, R.; Wei, T.; Goldberg, H.; Wang, W.; Cullion, K.; Kohane, D.S. Getting Drugs Across Biological Barriers. Adv. Mater. 2017, 29, 1606596. [Google Scholar] [CrossRef]
- Sigurdsson, H.H.; Kirch, J.; Lehr, C.M. Mucus as a barrier to lipophilic drugs. Int. J. Pharm. 2013, 453, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Martins, P.P.; Smyth, H.D.C.; Cui, Z. Strategies to facilitate or block nose-to-brain drug delivery. Int. J. Pharm. 2019, 570, 118635. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.S.; Oh, K.T.; Choi, H.G.; Lim, S.J. Liposomal Formulations for Nose-to-Brain Delivery: Recent Advances and Future Perspectives. Pharmaceutics 2019, 11, 540. [Google Scholar] [CrossRef] [PubMed]
- Velloso, M.I.; Landoni, M.F. Penetration Enhancers for the Development of Intranasal Formulations for Use in Equines. Int. J. Equine Sci. 2022, 1, 16–32. [Google Scholar]
- Du, L.; Chen, L.; Liu, F.; Wang, W.; Huang, H. Chapter Eight—Nose-to-brain drug delivery for the treatment of CNS disease: New development and strategies. Int. Rev. Neurobiol. 2023, 171, 255–297. [Google Scholar]
- Patharapankal, E.J.; Ajiboye, A.L.; Mattern, C.; Trivedi, V. Nose-to-Brain (N2B) Delivery: An Alternative Route for the Delivery of Biologics in the Management and Treatment of Central Nervous System Disorders. Pharmaceutics 2023, 16, 66. [Google Scholar] [CrossRef]
- Alexander, A.; Saraf, S. Nose-to-brain drug delivery approach: A key to easily accessing the brain for the treatment of Alzheimer’s disease. Neural Regen. Res. 2018, 13, 2102–2104. [Google Scholar] [CrossRef]
- Pires, P.C.; Rodrigues, M.; Alves, G.; Santos, A.O. Strategies to Improve Drug Strength in Nasal Preparations for Brain Delivery of Low Aqueous Solubility Drugs. Pharmaceutics 2022, 14, 588. [Google Scholar] [CrossRef]
- Sorokowski, P.; Karwowski, M.; Misiak, M.; Marczak, M.K.; Dziekan, M.; Hummel, T.; Sorokowska, A. Sex Differences in Human Olfaction: A Meta-Analysis. Front. Psychol. 2019, 10, 242. [Google Scholar] [CrossRef]
- Rassu, G.; Ferraro, L.; Pavan, B.; Giunchedi, P.; Gavini, E.; Dalpiaz, A. The Role of Combined Penetration Enhancers in Nasal Microspheres on In Vivo Drug Bioavailability. Pharmaceutics 2018, 10, 206. [Google Scholar] [CrossRef]
- Giunchedi, P.; Gavini, E.; Bonferoni, M.C. Nose-to-Brain Delivery. Pharmaceutics 2020, 12, 138. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Fukakusa, S.; Torikai, Y.; Suzuki, C.; Sonohata, I.; Kawahata, T.; Magata, Y.; Kawai, K.; Haruta, S. Effective nose-to-brain drug delivery using a combination system targeting the olfactory region in monkeys. J. Control. Release 2023, 359, 384–399. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.; Peters, J.M.; Detyniecki, K.; Tatum, W.; Rabinowicz, A.L.; Carrazana, E. The nose has it: Opportunities and challenges for intranasal drug administration for neurologic conditions including seizure clusters. Epilepsy Behav. Rep. 2023, 21, 100581. [Google Scholar] [CrossRef] [PubMed]
- Alabsi, W.; Eedara, B.B.; Encinas-Basurto, D.; Polt, R.; Mansour, H.M. Nose-to-Brain Delivery of Therapeutic Peptides as Nasal Aerosols. Pharmaceutics 2022, 14, 1870. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xiong, G.; Tsang, W.C.; Schatzlein, A.G.; Uchegbu, I.F. Nose-to-Brain Delivery. J. Pharmacol. Exp. Ther. 2019, 370, 593–601. [Google Scholar] [CrossRef]
- Gänger, S.; Schindowski, K. Tailoring Formulations for Intranasal Nose-to-Brain Delivery: A Review on Architecture, Physico-Chemical Characteristics and Mucociliary Clearance of the Nasal Olfactory Mucosa. Pharmaceutics 2018, 10, 116. [Google Scholar] [CrossRef]
- Tekade, A.R.; Mittha, P.S.; Pisal, C.S. Nanostructured Lipid Carriers for Nose to Brain Delivery Targeting CNS: Diversified Role of Liquid Lipids for Synergistic Action. Adv. Pharm. Bull. 2022, 12, 763–771. [Google Scholar] [CrossRef]
- Musielak, E.; Feliczak-Guzik, A.; Nowak, I. Synthesis and Potential Applications of Lipid Nanoparticles in Medicine. Materials 2022, 15, 682. [Google Scholar] [CrossRef]
- Xu, L.; Wang, X.; Liu, Y.; Yang, G.; Falconer, R.J.; Zhao, C.-X. Lipid Nanoparticles for Drug Delivery. Adv. NanoBiomed Res. 2021, 2, 109. [Google Scholar] [CrossRef]
- Viegas, C.; Patricio, A.B.; Prata, J.M.; Nadhman, A.; Chintamaneni, P.K.; Fonte, P. Solid Lipid Nanoparticles vs. Nanostructured Lipid Carriers: A Comparative Review. Pharmaceutics 2023, 15, 1593. [Google Scholar] [CrossRef]
- Shan, X.; Gong, X.; Li, J.; Wen, J.; Li, Y.; Zhang, Z. Current approaches of nanomedicines in the market and various stage of clinical translation. Acta Pharm. Sin. B 2022, 12, 3028–3048. [Google Scholar] [CrossRef] [PubMed]
- Pasarin, D.; Ghizdareanu, A.I.; Enascuta, C.E.; Matei, C.B.; Bilbie, C.; Paraschiv-Palada, L.; Veres, P.A. Coating Materials to Increase the Stability of Liposomes. Polymers 2023, 15, 782. [Google Scholar] [CrossRef]
- Alshaer, W.; Nsairat, H.; Lafi, Z.; Hourani, O.M.; Al-Kadash, A.; Esawi, E.; Alkilany, A.M. Quality by Design Approach in Liposomal Formulations: Robust Product Development. Molecules 2022, 28, 10. [Google Scholar] [CrossRef] [PubMed]
- Scioli Montoto, S.; Muraca, G.; Ruiz, M.E. Solid Lipid Nanoparticles for Drug Delivery: Pharmacological and Biopharmaceutical Aspects. Front. Mol. Biosci. 2020, 7, 587997. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.H.; Mäder, K.; Gohla, S. Solid lipid nanoparticles (SLN) for controlled drug delivery—A review of the state of the art. Eur. J. Pharm. Biopharm. 2000, 50, 161–177. [Google Scholar] [CrossRef]
- Mehnert, W.; Mäder, K. Solid lipid nanoparticles: Production, characterization and applications. Adv. Drug Deliv. Rev. 2001, 47, 165–196. [Google Scholar] [CrossRef]
- Shidhaye, S.S.; Vaidya, R.; Sutar, S.; Patwardhan, A.; Kadam, V.J. Solid lipid nanoparticles and nanostructured lipid carriers—Innovative generations of solid lipid carriers. Curr. Drug Deliv. 2008, 5, 324–331. [Google Scholar] [CrossRef]
- Müller, R.H.; Radtke, M.; Wissing, S.A. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv. Drug Deliv. Rev. 2002, 54 (Suppl. S1), S131–S155. [Google Scholar] [CrossRef]
- Hald Albertsen, C.; Kulkarni, J.A.; Witzigmann, D.; Lind, M.; Petersson, K.; Simonsen, J.B. The role of lipid components in lipid nanoparticles for vaccines and gene therapy. Adv. Drug Deliv. Rev. 2022, 188, 114416. [Google Scholar] [CrossRef]
- Oehlke, K.; Behsnilian, D.; Mayer-Miebach, E.; Weidler, P.G.; Greiner, R. Edible solid lipid nanoparticles (SLN) as carrier system for antioxidants of different lipophilicity. PLoS ONE 2017, 12, e0171662. [Google Scholar] [CrossRef]
- Fonseca-Santos, B.; Silva, P.B.; Rigon, R.B.; Sato, M.R.; Chorilli, M. Formulating SLN and NLC as Innovative Drug Delivery Systems for Non-Invasive Routes of Drug Administration. Curr. Med. Chem. 2020, 27, 3623–3656. [Google Scholar] [CrossRef] [PubMed]
- Gugleva, V.; Andonova, V. Drug delivery to the brain–lipid nanoparticles-based approach. Pharmacia 2023, 70, 113–120. [Google Scholar] [CrossRef]
- Shankar, R.; Joshi, M.; Pathak, K. Lipid Nanoparticles: A Novel Approach for Brain Targeting. Pharm. Nanotechnol. 2018, 6, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Cunha, S.; Swedrowska, M.; Bellahnid, Y.; Xu, Z.; Sousa Lobo, J.M.; Forbes, B.; Silva, A.C. Thermosensitive in situ hydrogels of rivastigmine-loaded lipid-based nanosystems for nose-to-brain delivery: Characterisation, biocompatibility, and drug deposition studies. Int. J. Pharm. 2022, 620, 121720. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, N.; Awasthi, R.; Sharma, B.; Kharkwal, H.; Kulkarni, G.T. Lipid Nanoparticles as Carriers for Bioactive Delivery. Front. Chem. 2021, 9, 580118. [Google Scholar] [CrossRef]
- Correia, A.C.; Moreira, J.N.; Sousa Lobo, J.M.; Silva, A.C. Design of experiment (DoE) as a quality by design (QbD) tool to optimize formulations of lipid nanoparticles for nose-to-brain drug delivery. Expert Opin. Drug Deliv. 2023, 20, 1731–1748. [Google Scholar] [CrossRef]
- Cunha, S.; Forbes, B.; Sousa Lobo, J.M.; Silva, A.C. Improving Drug Delivery for Alzheimer’s Disease Through Nose-to-Brain Delivery Using Nanoemulsions, Nanostructured Lipid Carriers (NLC) and in situ Hydrogels. Int. J. Nanomed. 2021, 16, 4373–4390. [Google Scholar] [CrossRef]
- Committee for Medicinal Products for Human Use. Guideline on the Pharmaceutical Quality of Inhalation and Nasal Products; European Medicines Agency: London, UK, 2006. [Google Scholar]
- Patel, D.; Patel, B.; Wairkar, S. Intranasal delivery of biotechnology-based therapeutics. Drug Discov. Today 2022, 27, 103371. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, R. Steps to Success in Nose-to-Brain Drug Delivery. 2022. Available online: https://www.ondrugdelivery.com/steps-to-success-in-nose-to-brain-drug-delivery/ (accessed on 17 September 2023).
- Prajapati, J.B.; Patel, G.C. Nose to brain delivery of Rotigotine loaded solid lipid nanoparticles: Quality by design based optimization and characterization. J. Drug Deliv. Sci. Technol. 2021, 63, 102377. [Google Scholar] [CrossRef]
- Yasir, M.; Chauhan, I.; Zafar, A.; Verma, M.; Alruwaili, N.K.; Noorulla, K.M.; Singh, A.P.; Tura, A.J. Glyceryl behenate-based solid lipid nanoparticles as a carrier of haloperidol for nose to brain delivery: Formulation development, in-vitro, and in-vivo evaluation. Braz. J. Pharm. Sci. 2022, 58, e20254. [Google Scholar] [CrossRef]
- Ramalingam, P.; Ganesan, P.; Prabakaran, D.S.; Gupta, P.K.; Jonnalagadda, S.; Govindarajan, K.; Vishnu, R.; Sivalingam, K.; Sodha, S.; Choi, D.K.; et al. Lipid Nanoparticles Improve the Uptake of α-Asarone Into the Brain Parenchyma: Formulation, Characterization, In Vivo Pharmacokinetics, and Brain Delivery. AAPS PharmSciTech 2020, 21, 299. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.U.; Shehzad, A.; Ahmed, M.B.; Lee, Y.S. Intranasal Delivery of Nanoformulations: A Potential Way of Treatment for Neurological Disorders. Molecules 2020, 25, 1929. [Google Scholar] [CrossRef] [PubMed]
- Boyuklieva, R.; Pilicheva, B. Micro- and Nanosized Carriers for Nose-to-Brain Drug Delivery in Neurodegenerative Disorders. Biomedicines 2022, 10, 1706. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, F.; Hanieh, P.N.; Chan, L.K.N.; Angeloni, L.; Passeri, D.; Rossi, M.; Wang, J.T.; Imbriano, A.; Carafa, M.; Marianecci, C. Chitosan Glutamate-Coated Niosomes: A Proposal for Nose-to-Brain Delivery. Pharmaceutics 2018, 10, 38. [Google Scholar] [CrossRef]
- Yermak, I.M.; Davydova, V.N.; Volod’ko, A.V. Mucoadhesive Marine Polysaccharides. Mar. Drugs 2022, 20, 522. [Google Scholar] [CrossRef]
- Pandey, V.; Gadeval, A.; Asati, S.; Jain, P.; Jain, N.; Roy, R.K.; Tekade, M.; Soni, V.; Tekade, R.K. Formulation strategies for nose-to-brain delivery of therapeutic molecules. In Drug Delivery Systems; Academic Press: Cambridge, MA, USA, 2020; pp. 291–332. [Google Scholar]
- Doub, W.H.; Suman, J.M.; Copley, M.; Goodey, A.P.; Hosseini, S.; Mitchell, J.P. Laboratory Performance Testing of Aqueous Nasal Inhalation Products for Droplet/Particle Size Distribution: An Assessment from the International Pharmaceutical Aerosol Consortium on Regulation and Science (IPAC-RS). AAPS PharmSciTech 2023, 24, 208. [Google Scholar] [CrossRef]
- Bioavailability and Bioequivalence Studies for Nasal Aerosols and Nasal Sprays for Local Action. Guidance for Industry 2003. Available online: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM070111.pdf (accessed on 27 September 2023).
- Sijs, R.; Kooij, S.; Holterman, H.J.; van de Zande, J.; Bonn, D. Drop size measurement techniques for sprays: Comparison of image analysis, phase Doppler particle analysis, and laser diffraction. AIP Adv. 2021, 11, 015315. [Google Scholar] [CrossRef]
- Thomas, B.J.; Absar, M.; Delvadia, R.; Conti, D.S.; Witzmann, K.; Guo, C. Analytical method development for characterizing ingredient-specific particle size distributions of nasal spray suspension products. J. Pharm. Sci. 2021, 110, 2778–2788. [Google Scholar] [CrossRef] [PubMed]
- Le Guellec, S.; Ehrmann, S.; Vecellio, L. In vitro–in vivo correlation of intranasal drug deposition. Adv. Drug Deliv. Rev. 2021, 170, 340–352. [Google Scholar] [CrossRef]
- Liu, Q.; Absar, M.; Saluja, B.; Guo, C.; Chowdhury, B.; Lionberger, R.; Conner, D.P.; Li, B.V. Scientific Considerations for the Review and Approval of First Generic Mometasone Furoate Nasal Suspension Spray in the United States from the Bioequivalence Perspective. AAPS J. 2019, 21, 14. [Google Scholar] [CrossRef]
- Hallworth, G.W.; Padfield, J.M. A comparison of the regional deposition in a model nose of a drug discharged from metered aerosol and metered-pump nasal delivery systems. J. Allergy Clin. Immunol. 1986, 77, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Chari, S.; Sridhar, K.; Kleinstreuer, C. Effects of subject-variability on nasally inhaled drug deposition, uptake, and clearance. J. Aerosol Sci. 2022, 165, 106021. [Google Scholar] [CrossRef]
- Williams, G.; Suman, J.D. In Vitro Anatomical Models for Nasal Drug Delivery. Pharmaceutics 2022, 14, 1353. [Google Scholar] [CrossRef]
- Xi, J.; Wang, Z.; Nevorski, D.; White, T.; Zhou, Y. Nasal and Olfactory Deposition with Normal and Bidirectional Intranasal Delivery Techniques: In Vitro Tests and Numerical Simulations. J. Aerosol Med. Pulm. Drug Deliv. 2017, 30, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Walenga, R.L.; Newman, B.; Babiskin, A.; Zhao, L. In Silico and Experimental Methods to Support Generic Nasal Drug Product (NDP) Development. Respir. Drug Deliv. 2021, 2021, 141–150. [Google Scholar]
- Chen, J.Z.; Finlay, W.H.; Martin, A. In Vitro Regional Deposition of Nasal Sprays in an Idealized Nasal Inlet: Comparison with In Vivo Gamma Scintigraphy. Pharm. Res. 2022, 39, 3021–3028. [Google Scholar] [CrossRef]
- Chen, J.Z.; Kiaee, M.; Martin, A.R.; Finlay, W.H. In vitro assessment of an idealized nose for nasal spray testing: Comparison with regional deposition in realistic nasal replicas. Int. J. Pharm. 2020, 582, 119341. [Google Scholar] [CrossRef]
- Pina Costa, C.; Nizic Nodilo, L.; Silva, R.; Martins, E.; Zadravec, D.; Kalogjera, L.; Nuno Moreira, J.; Manuel Sousa Lobo, J.; Hafner, A.; Catarina Silva, A. In situ hydrogel containing diazepam-loaded nanostructured lipid carriers (DZP-NLC) for nose-to-brain delivery: Development, characterization and deposition studies in a 3D-printed human nasal cavity model. Int. J. Pharm. 2023, 644, 123345. [Google Scholar] [CrossRef]
- Pardeshi, C.V.; Belgamwar, V.S. Improved brain pharmacokinetics following intranasal administration of N,N,N-trimethyl chitosan tailored mucoadhesive NLCs. Mater. Technol. 2019, 35, 249–266. [Google Scholar] [CrossRef]
- Som Chaudhury, S.; Sinha, K.; Das Mukhopadhyay, C. Intranasal route: The green corridor for Alzheimer’s disease therapeutics. J. Drug Deliv. Sci. Technol. 2021, 66, 102791. [Google Scholar] [CrossRef]
- Pires, P.C.; Santos, A.O. Nanosystems in nose-to-brain drug delivery: A review of non-clinical brain targeting studies. J. Control. Release 2018, 270, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Youssef, N.; Kassem, A.A.; Farid, R.M.; Ismail, F.A.; El-Massik, M.A.E.; Boraie, N.A. A novel nasal almotriptan loaded solid lipid nanoparticles in mucoadhesive in situ gel formulation for brain targeting: Preparation, characterization and in vivo evaluation. Int. J. Pharm. 2018, 548, 609–624. [Google Scholar] [CrossRef] [PubMed]
- Salem, L.H.; El-Feky, G.S.; Fahmy, R.H.; El Gazayerly, O.N.; Abdelbary, A. Coated Lipidic Nanoparticles as a New Strategy for Enhancing Nose-to-Brain Delivery of a Hydrophilic Drug Molecule. J. Pharm. Sci. 2020, 109, 2237–2251. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, D.; Sonar, P.K.; Parashar, P.; Chaudhary, S.K.; Upadhyay, S.; Saraf, S.K. Augmented Brain Delivery of Cinnarizine Through Nanostructured Lipid Carriers Loaded in situ Gel: In vitro and Pharmacokinetic Evaluation. BioNanoScience 2021, 11, 159–171. [Google Scholar] [CrossRef]
- Bakshi, V.; Amarachinta, P.R.; Chettupalli, A.K. Design, Development and Optimization of Solid Lipid Nanoparticles of Rizatriptan for Intranasal delivery: Invitro & Invivo assessment. Mater. Today Proc. 2022, 66, 2342–2357. [Google Scholar] [CrossRef]
- Masjedi, M.; Azadi, A.; Heidari, R.; Mohammadi-Samani, S. Nose-to-brain delivery of sumatriptan-loaded nanostructured lipid carriers: Preparation, optimization, characterization and pharmacokinetic evaluation. J. Pharm. Pharmacol. 2020, 72, 1341–1351. [Google Scholar] [CrossRef]
| Phases of Migraine | Premonitory (Few Hours to Days) | Aura (5–60 min) | Headache (4–72 h) | Postdrome (24–48 h) |
|---|---|---|---|---|
| Associated symptoms | Impaired concentration | Numbness of face | Giddiness | Cognitive difficulties |
| Mental slowing | Expressive language dysfunction | Insomnia | Lack of comprehension | |
| Speech dysfunction | Scintillating scotoma | Depressed mood | Depressed mood | |
| Drowsiness | Flashes of lights | Anxiety | Euphoric mood | |
| Yawning | Scotoma | Nasal congestion | Somnolence | |
| Fatigue | Paraesthesia/numbness | Neck pain/stiffness | Asthenia | |
| Food cravings | Motor dysfunction | HEADACHE Unilateral Severe disability Worsens with activity Throbbing | Tiredness | |
| Neck pain and stiffness | ASSOCIATED SYMPTOMS Nausea and vomiting Photophobia/phonophobia | Diuresis | ||
| Photophobia | ||||
| Nausea | ||||
| Anorexia | ||||
| Diarrhea |
| Treatment | Drug Class | Drug | AEs | Contraindications | References |
|---|---|---|---|---|---|
| Acute | First-line medication | ||||
| Non-steroidal anti-inflammatory drugs (NSAIDs) | Acetylsalicylic acid | Gastric effects | Patients with inflammatory bowel disease, renal dysfunction and who have had gastric bypass surgery. | [5,21] | |
| Ibuprofen | |||||
| Naproxen | |||||
| Diclofenac potassium | |||||
| Other simple analgesics | Paracetamol | Gastrointestinal effects | Patients with hepatic disease and renal failure. | ||
| Antiemetic drugs | Metoclopramide | Drowsiness, weight gain, blurred vision, cardiac arrhythmias, urinary retention, extrapyramidal symptoms, and infertility | Patients with gastrointestinal bleeding, epilepsy, renal failure, cardiac arrhythmia, and Parkinson’s disease. | ||
| Chlorpromazine | |||||
| Prochlorperazine | |||||
| Second-line medication | |||||
| Triptans | Sumatriptan | Nausea, dizziness, coronary vasoconstriction, chest pressure and tingling in the limbs | Patients with cardio- or cerebrovascular disease, uncontrolled hypertension, ischemic bowel, pregnant patients, or those who have used another triptan in the last 24 h. | [3,42,43,44] | |
| Zolmitriptan | |||||
| Rizatriptan | |||||
| Naratriptan | |||||
| Almotriptan | |||||
| Frovatriptan | |||||
| Third-line medication | |||||
| Ditans | Lasmiditan | Dizziness, nausea and somnolence | Pregnant women and patients using drugs that are P-glycoprotein substrates. | [2,3,5,42] | |
| Gepants | Ubrogepant | Fatigue and nausea | Patients with hypersensitivity and hepatic impairment. | ||
| Rimegepant | |||||
| Preventive | First-line medication | ||||
| Beta blockers | Metoprolol | Dizziness, cold hands or feet and difficulties in sleeping | Patients with asthma, cardiac failure, Raynaud disease, atrioventricular block and diabetes mellitus. | [5,41,45,46] | |
| Propranolol | |||||
| Anticonvulsant | Topiramate | Fatigue, cognitive disturbance, weight loss and paresthesia | Pregnant and lactating patients; and patients with nephrolithiasis and glaucoma. | ||
| Second-line medication | |||||
| Antidepressant | Amitriptyline | Dry mouth, fatigue, dizziness and sweating | Patients with age ˂ 6 years, glaucoma, prostatic adenoma hyperplasia and heart insufficiency. | [5,46] | |
| Calcium channel blocker | Flunarizine | Fatigue, weight gain, depression, hyperkinesia, tremor, parkinsonism and gastrointestinal side effects | Patients with familial parkinsonism, focal dystonia and depression. | ||
| Anticonvulsant | Valproic acid | Fatigue dizziness, tremorand elevation of liver enzymes/disturbance in liver function | Patients with liver failure, pregnancy, alcoholism and polycystic ovaries. | ||
| Third-line medication | |||||
| Calcitonin gene-related peptide monoclonal antibodies | Erenumab | Constipation, gastric pain, and chest pain | Patients with inflammatory bowel disease, coronary heart disease, chronic obstructive pulmonary disease and subarachnoid hemorrhage. | [5,47] | |
| Fremanezumab | |||||
| Galcanezumab | |||||
| Drug | Product Details | Brand Name | Key Results | References |
|---|---|---|---|---|
| Sumatriptan | Dose: 5, 10, or 20 mg Liquid formulation delivered via traditional nasal spray | IMITREX® | Pharmacokinetic studies demonstrated a Cmax blood of 69.5 ng/mL and 12.9 ng/mL following subcutaneous and nasal administration of sumatriptan, respectively. Pharmacokinetic studies demonstrated that the mean bioavailability following nasal administration is 15.8%, compared with the subcutaneous route. Greater percentage of patients had headache relief 2 h after treatment with 10 or 20 mg of IMITREX® vs. placebo. Frequent AEs include nasal cavity/sinuses discomfort, burning, dizziness, nausea, vomiting, unusual taste and throat discomfort. | [65,66] |
| Dihydroergotamine mesylate | Dose: 2 mg Liquid formulation delivered to the respiratory region | MIGRANAL® | Pharmacokinetic studies demonstrated that the mean bioavailability following nasal administration is 32%, compared with the intravenous administration. Greater percentage of patients had headache relief 4 h after treatment with 2 mg of MIGRANAL® vs. placebo. Frequent AEs include rhinitis, nausea, unusual taste, application site reactions and dizziness. | [67,68] |
| Zolmitriptan | Dose: 2.5 or 5 mg Liquid formulation delivered to the nasopharynx and lower nasal space | ZOMIG® | Pharmacokinetic studies demonstrated that the mean bioavailability following nasal administration is 102%, compared with the oral tablet. Greater percentage of patients had headache relief 2 h after treatment with 2.5 or 5 mg of ZOMIG® vs. placebo. One multi-attack trial for adults showed that the headache response with ZOMIG® was consistently maintained during the 2 h. Frequent AE include unusual taste (adolescents), paresthesia, hyperesthesia and somnolence. | [69,70,71] |
| Sumatriptan | Dose: 22 mg Nasal powder delivered via breath to the upper nasal space | ONZETRATM XsailTM | Pharmacokinetic studies demonstrated that administration of sumatriptan nasal powder (ONZETRATM XsailTM) resulted in 27% higher Cmax (20.8 vs. 16.4 ng/mL) and 75% higher early exposure (AUC0–15min, 2.1 vs. 1.2 ng*h/mL) comparative to the sumatriptan nasal spray (IMITREX®). Pharmacokinetic studies demonstrated that the mean bioavailability following nasal administration is 19%, compared with the subcutaneous route. Greater percentage of patients had headache relief 2 h after treatment with 22 mg ONZETRATM XsailTM vs. placebo. Frequent AEs include unusual taste, nasal discomfort and rhinorrhea. | [72,73,74] |
| Sumatriptan | Dose: 10 mg Liquid formulation containing a permeation-enhancing excipient (0.2% 1-O-n-Dodecyl-β-D-maltopyranoside) | TOSYMRA™ | Pharmacokinetic studies comparing a single dose of 10 mg TOSYMRATM to 20 mg IMITREX® demonstrated that TOSYMRATM was more rapidly absorbed, with Cmax values of 63.9 and 21.4 ng/mL and AUC0–2h values of 48.4 and 24.7 ng*h/mL for TOSYMRATM and IMITREX®, respectively. Pharmacokinetic studies demonstrated that the mean bioavailability following nasal administration is 58%, compared with the subcutaneous route. Greater percentage of patients had headache relief 2 h after treatment with 10 mg TOSYMRATM vs. placebo. Frequent AEs include application site pain and reaction, unusual taste, upper respiratory infection, sinusitis and nasopharyngitis. | [75,76,77] |
| Dihydroergotamine mesylate | Dose: 1.45 mg Liquid formulation delivered to the upper nasal space | TrudhesaTM | Greater percentage of patients had headache relief 4 h after treatment with 2 mg TrudhesaTM vs. placebo. In patients with migraine-associated nausea, photophobia, and phonophobia at baseline there was a lower incidence of these symptoms at 2- and 4-h following administration of TrudhesaTM nasal spray vs. placebo. Frequent AEs include application site reaction, rhinitis, nausea, vomiting, somnolence, pharyngitis and diarrhea. | [58,78] |
| Zavegepant | Dose: 10 mg Liquid formulation delivered via nasal spray | Zavzpret™ | Greater percentage of patients had headache relief 2 h after treatment with 10 mg ZavzpretTM vs. placebo. Frequent AEs include unusual taste, nausea, nasal discomfort, and vomiting. | [79] |
| Limitations | Strategies | Description | References |
|---|---|---|---|
| Mucociliary clearance mechanism | Increased contact time of the formulation with the nasal mucosa for improved absorption of the drug | Absorption enhancers: cyclodextrins, sodium hyaluronate, Cremophor RH40, chitosan and cyclopentyladenosin | [58,59,64] |
| Mucoadhesive agents: chitosan, and carboxymethylcellulose | [12,58] | ||
| Viscosity enhancers: pectin, Pluronic®, Carbopol®, cellulose derivatives and chitosan | [12] | ||
| Mucoadhesive systems: nanoparticulate drug delivery systems | [12,14,59,60,64] | ||
| Enzymatic and P-glycoprotein activity | Disturb the normal function of enzymes in the nasal epithelium | Enzyme modulators: P-glycoprotein inhibitors and CYP450 inhibitors | [59,60,64] |
| Protection of drugs against enzymatic degradation and efflux transport mechanisms | Nanoparticulate drug delivery systems | [60] | |
| Systemic absorption | Prevent deposition of the formulation in the respiratory region | Delivery devices designed to deposit the formulation in the olfactory region: ViaNase™, SipNose, OptiMist™, Precision Olfactory Device (POD®), VersiDoser®, VRx2TM, DARTTM and MAD NasalTM | [59,60,64] |
| Tight junctions | Transiently decrease nasal epithelial tight junctions’ tightness | Compounds that modulate the permeability of tight junctions: chitosan, 12-O-tetradecanotlophorbol-13-acetate (TPA), papaverine, poly-L-arginine and bisindolylmaleimide | [64] |
| Chelating agents: disodium ethylenediaminetetraacetate (EDTA) | [81] | ||
| Absorption enhancers: polysorbate 80, propylene glycol, and polyethylene glycol 400 | [81] | ||
| Physicochemical characteristics of drug molecules | Increase the nasal permeability of hydrophilic drugs | Nanoparticulate systems | [58,59,101] |
| Absorption enhancers: cyclodextrins and chitosan | |||
| Increase the nasal permeability of lipophilic drugs | Nanoparticulate drug delivery systems | [14] | |
| Prodrugs | [12] | ||
| Damage to the nasal mucosa | Appropriately select the excipients of the formulation | Excipients generally recognized as safe (GRAS) | [102] |
| Keep nasal mucosa moist | Humectants: glycerin, sorbitol, and mannitol | [12] | |
| Formulations with pH similar to the nasal cavity (5.5–6.5) | pH adjustment and buffers: citric acid, sodium chloride, sodium hydroxide, hydrochloric acid, and potassium phosphate | [81] | |
| Isotonic formulations | Isotonizing excipients: glycerin, sodium chloride, glucose or dextrose | [90] | |
| Insufficient in vivo studies in humans | Use non-human primates with anatomical and physiological resemblance to humans | Preclinical investigations with cynomolgus monkey (Macaca fascicularis) | [103] |
| Drug | Formulations Tested | Constituents of SLN and NLC | AUC0-t brain ± SD AUC0-t blood ± SD (µg*h/mL) | Tmax Brain (h) | Cmax brain± SD Cmax blood ± SD (µg/mL) | DTE (%) | DTP (%) | Relevant Results | References |
|---|---|---|---|---|---|---|---|---|---|
| Almotriptan malate (ALM) | IN ALM-loaded SLN in-situ gel | Solid lipid: Precirol® ATO 5 Emulsifier(s): Polyvinyl alcohol (PVA) and Poloxamer 188 | 7.87 ± 0.09 8.77 ± 0.08 | 0.17 | 2.41 ± 0.04 2.69 ± 0.02 | 335.7 | 70.21 | Higher Cmax brain of IN ALM-loaded SLN in-situ gel (1.7-fold vs. IN free ALM in-situ gel and 2-fold vs. IV ALM solution); Faster onset of IN ALM-loaded SLN in-situ gel (Tmax brain = 0.17 h); The toxicological results indicated the higher safety profile of IN ALM-loaded SLN in situ gel for nasal administration. | [157] |
| IN free ALM in-situ gel | 6.25 ± 0.03 9.15 ± 0.07 | 2 | 1.43 ± 0.02 3.09 ± 0.05 | 255.1 | 60.80 | ||||
| IV ALM solution | 3.32 ± 0.04 12.43 ± 0.09 | 0.5 | 1.23 ± 0.02 3.20 ± 0.06 | - | - | ||||
| Almotriptan malate (ALM) | IN ALM-loaded NLC | Solid lipid: Compritol® ATO 888 Liquid lipid: Labrafil® M 2125 CS Emulsifier(s): Tween® 80 and Lauroglycol | 27,291.00 ± 0.02 15,348.60 ± 0.03 | 0.17 | 3.44 ± 0.03 1.54 ± 0.02 | - | - | Higher Cmax brain of IN ALM-loaded NLC (7.2-fold vs. IN ALM solution and 6.6-fold vs. oral marketed formulation); Faster onset of IN ALM-loaded NLC (Tmax brain = 0.17 h); The toxicological results indicated the IN ALM-loaded NLC as safe for nasal administration. | [158] |
| IN ALM solution | 3387.00 ± 0.05 2541.60 ± 0.05 | 0.33 | 0.48 ± 0.04 0.25 ± 0.03 | - | - | ||||
| Oral marketed ALM formulation (tablet) | 5982.60 ± 0.03 7579.20 ± 0.04 | 1 | 0.52 ± 0.05 0.58 ± 0.03 | - | - | ||||
| Cinnarizine (CIN) | IN CIN-loaded NLC in situ gel | Solid lipid: Cetyl palmitate Liquid lipid: Oleic acid Emulsifier(s): Poloxamer 188 and Soya lecithin | 108,000 ± 111.5 41,076 ± 57.46 | 1 | 786.65 ± 7.4 345.29 ± 11.2 | - | - | Higher Cmax brain of IN CIN-loaded NLC in situ gel (2.1-fold vs. IN CIN solution). | [159] |
| IN CIN solution | 48,432 ± 55.81 54,210 ± 81.9 | 1 | 380.73 ± 2.41 471.31 ± 7.5 | - | - | ||||
| Rizatriptan (RZT) | IN RZT-loaded SLN | Solid lipid: Compritol® ATO 888 Emulsifier(s): Tween® 80 | 1824.82 1894.80 | 1 | 583.20 955.18 | 50.52 * | −97.88 * | Higher Cmax brain of IN RZT-loaded SLN (1.7-fold vs. IV RZT-loaded SLN and 7.3-fold vs. oral marketed formulation); Faster onset of IN RZT-loaded SLN (Tmax brain = 1 h); DTE value of IN RZT-loaded SLN not indicated a more effective drug brain targeting after IN administration vs. IV administration. | [160] |
| IV RZT-loaded SLN | 2375.10 1246.06 | 2 | 351.29 175.12 | - | - | ||||
| Oral marketed Rizatriptan formulation (tablet) | 841.39 −1432.59 | 4 | 79.84 103.12 | - | - | ||||
| Sumatriptan | IN Sumatriptan-loaded NLC | Solid lipid: Stearic acid and Cholesterol Liquid lipid: Triolein Emulsifier(s): Brij® 35 and Brij® 72 | 0.57 0.19 | 2 | 0.18 0.08 | 258.02 | 61.23 | Higher Cmax brain of IN Sumatriptan-loaded NLC (9.4-fold vs. IV Sumatriptan-loaded NLC, 5.6-fold vs. IN Sumatriptan solution and 7.4-fold vs. IV Sumatriptan solution). | [161] |
| IV Sumatriptan-loaded NLC | 0.07 0.06 | 2 | 0.02 0.05 | - | - | ||||
| IN Sumatriptan solution | 0.07 0.10 | 1 | 0.03 0.07 | - | - | ||||
| IV Sumatriptan solution | 0.04 0.36 | 1 | 0.02 0.23 | - | - | ||||
| Zolmitriptan | IN Zolmitriptan-loaded SLN | Solid lipid: Glyceryl monostearate Emulsifier(s): Soya lecithin and Poloxamer 188 | 0.04 ± 2.45 - | 0.5 | 0.04 ± 1.32 - | - | - | Higher Cmax brain of IN Zolmitriptan-loaded SLN (2-fold vs. IN Marketed formulation and 2.3-fold vs. IN Zolmitriptan solution); IN Zolmitriptan-loaded SLN showed a higher Tmax value (0.5 h) due to a slower drug release pattern. | [10] |
| IN marketed Zolmitriptan formulation (Zolmist® nasal spray) | 0.02 ± 1.65 - | 0.17 | 0.03 ± 2.50 - | - | - | ||||
| IN Zolmitriptan solution | 0.02 ± 1.25 - | 0.17 | 0.03 ± 1.56 - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres, J.; Silva, R.; Farias, G.; Sousa Lobo, J.M.; Ferreira, D.C.; Silva, A.C. Enhancing Acute Migraine Treatment: Exploring Solid Lipid Nanoparticles and Nanostructured Lipid Carriers for the Nose-to-Brain Route. Pharmaceutics 2024, 16, 1297. https://doi.org/10.3390/pharmaceutics16101297
Torres J, Silva R, Farias G, Sousa Lobo JM, Ferreira DC, Silva AC. Enhancing Acute Migraine Treatment: Exploring Solid Lipid Nanoparticles and Nanostructured Lipid Carriers for the Nose-to-Brain Route. Pharmaceutics. 2024; 16(10):1297. https://doi.org/10.3390/pharmaceutics16101297
Chicago/Turabian StyleTorres, Joana, Renata Silva, Gonçalo Farias, José Manuel Sousa Lobo, Domingos Carvalho Ferreira, and Ana Catarina Silva. 2024. "Enhancing Acute Migraine Treatment: Exploring Solid Lipid Nanoparticles and Nanostructured Lipid Carriers for the Nose-to-Brain Route" Pharmaceutics 16, no. 10: 1297. https://doi.org/10.3390/pharmaceutics16101297
APA StyleTorres, J., Silva, R., Farias, G., Sousa Lobo, J. M., Ferreira, D. C., & Silva, A. C. (2024). Enhancing Acute Migraine Treatment: Exploring Solid Lipid Nanoparticles and Nanostructured Lipid Carriers for the Nose-to-Brain Route. Pharmaceutics, 16(10), 1297. https://doi.org/10.3390/pharmaceutics16101297








