Rectal Bioavailability of Amoxicillin from Hollow-Type Suppositories: Effect of Chemical Form of Amoxicillin
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
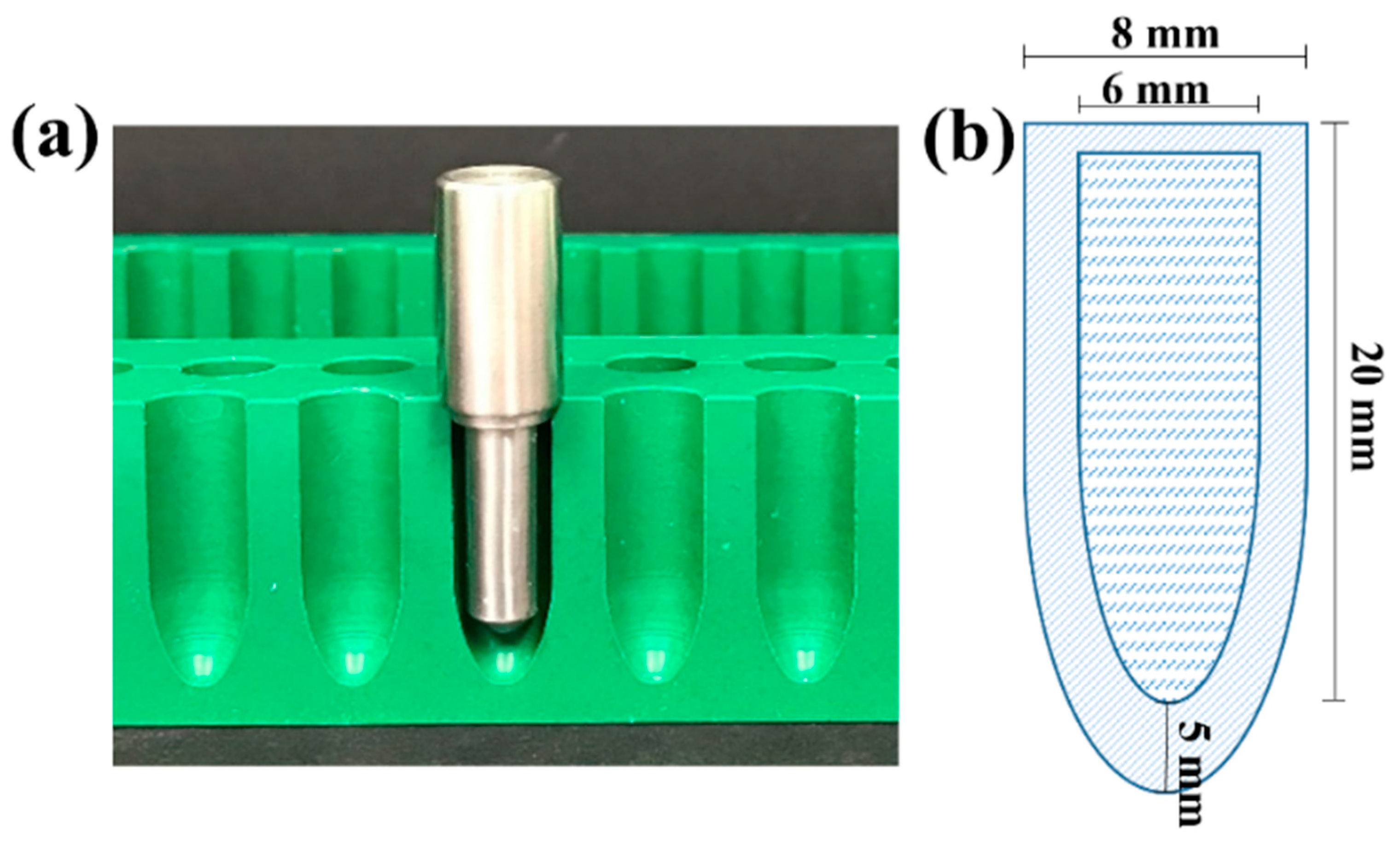
2.2. Preparation of Amoxicillin HTS
2.3. Characterisation of Amoxicillin HT Suppositories
2.3.1. Uniformity Tests
2.3.2. Hardness of HT Suppositories
2.4. Characterisation of Drug–Base Interactions
2.4.1. Characterisation of the Core Using Powder X-ray Diffraction (XRD)
2.4.2. Differential Scanning Calorimetry (DSC)
2.4.3. Drug-Excipient Interactions Using FTIR
2.5. In Vitro Drug Release and Kinetics
2.6. Stability of Amoxicillin in HTS
2.7. Bioavailability of Amoxicillin in a Rabbit Model
2.7.1. Study Design and Sample Analysis
2.7.2. Pharmacokinetics and Data Analysis
2.8. Rectal Tissue Compatibility
3. Results and Discussion
3.1. Characterisation of Amoxicillin HTS
3.1.1. Uniformity Tests
3.1.2. Hardness Test of HTS
3.2. Characterisation of Drug–Base Interactions
3.2.1. Characterisation of the Drug-Loaded SNA Core Using XRD
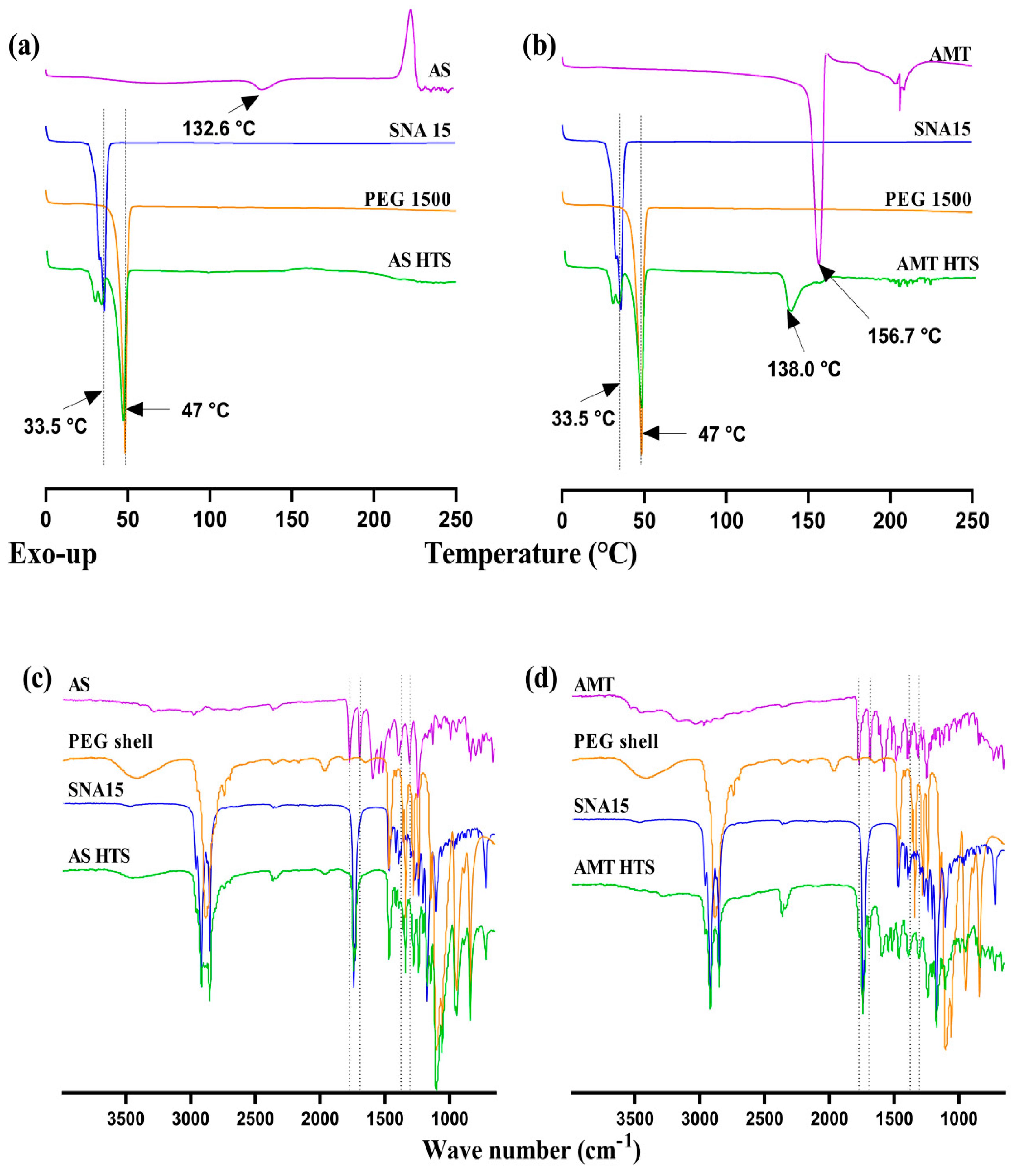
3.2.2. Differential Scanning Calorimetry (DSC)
3.2.3. Fourier Transform Infrared Spectroscopy (FTIR)
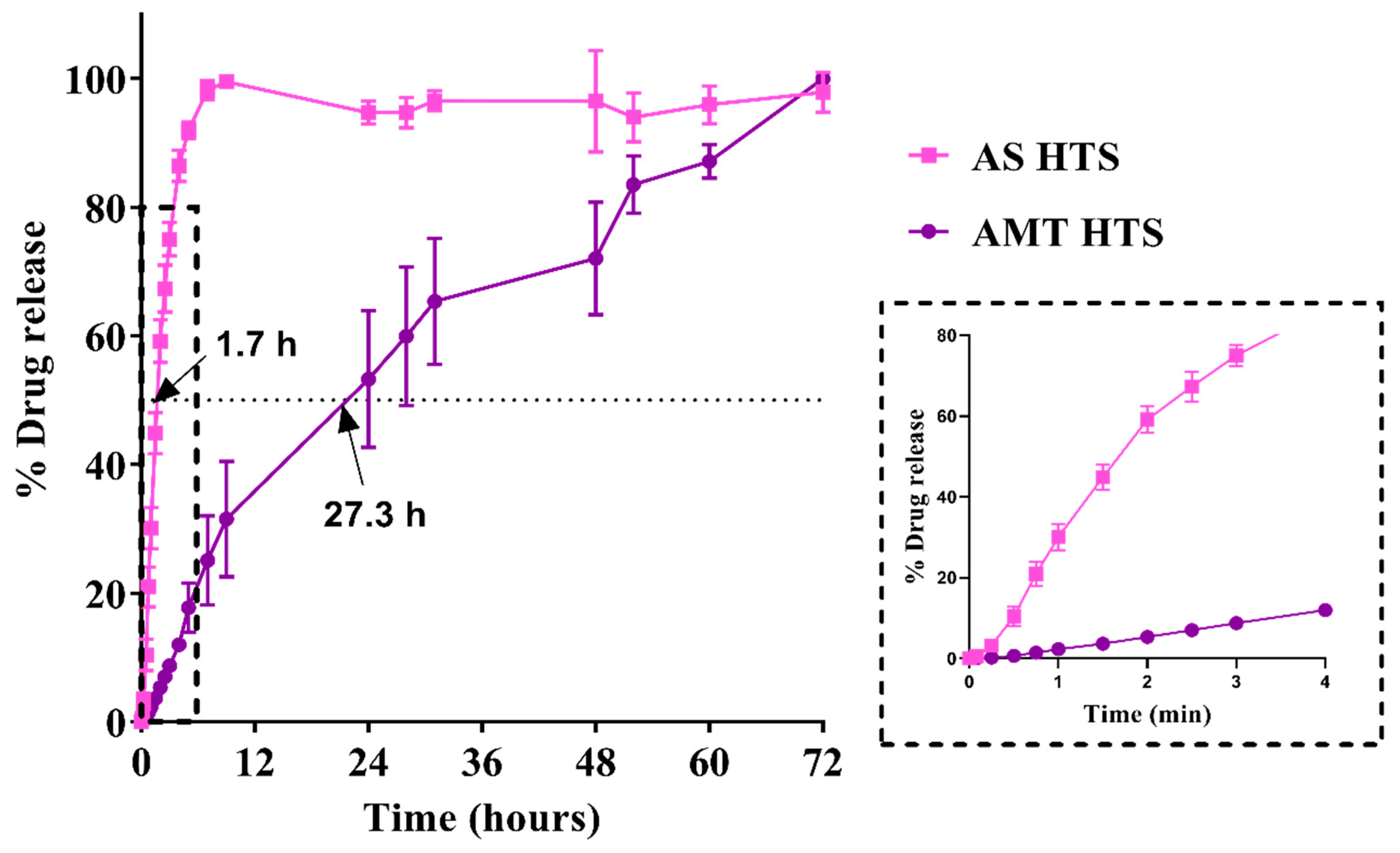
3.3. In Vitro Drug Release
3.4. Stability of HTS
3.4.1. Change in Hardness: Effect of Amoxicillin Form
3.4.2. Content of Amoxicillin over Time
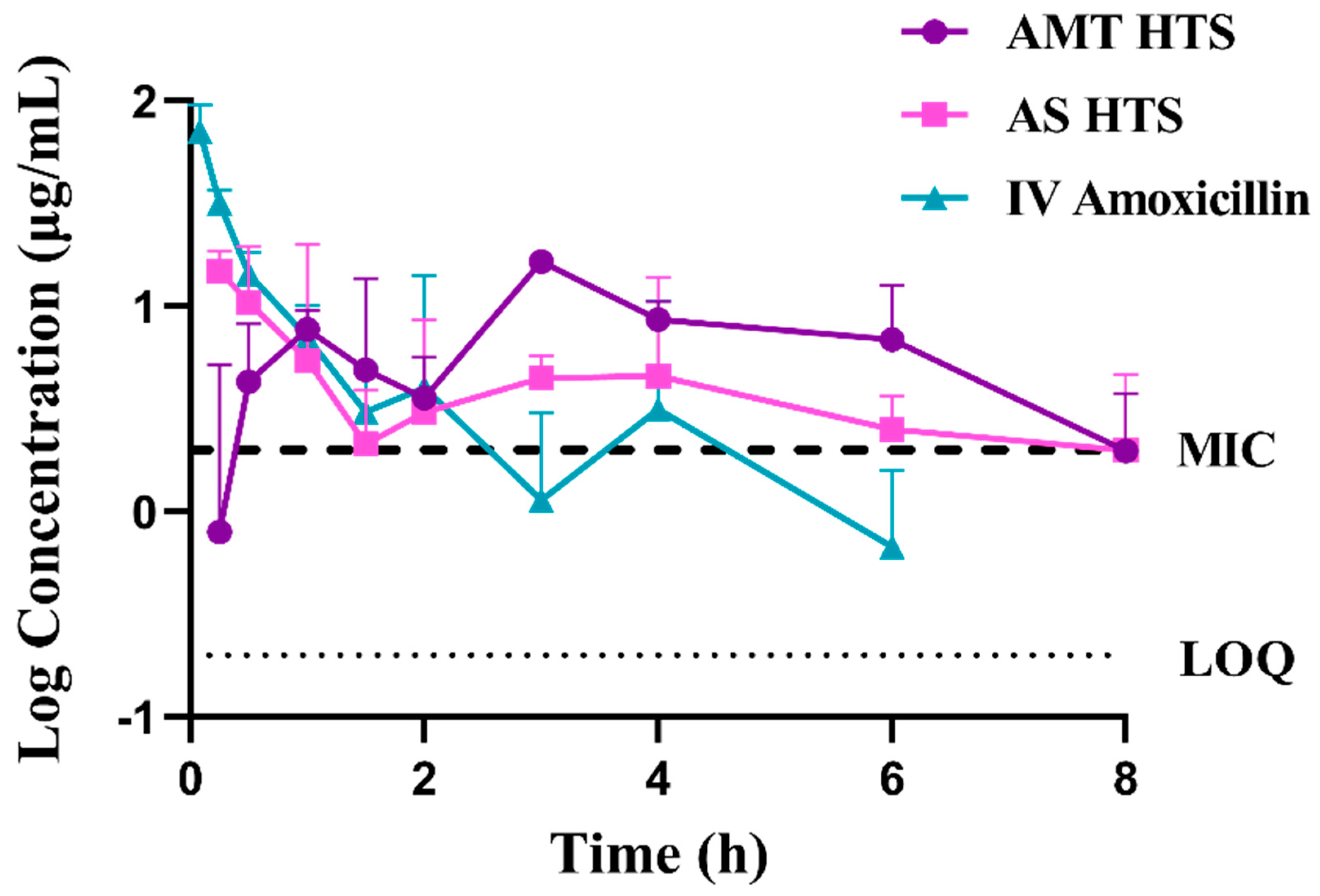
3.5. Absolute Bioavailability in Rabbits
3.6. Rectal Tissue Compatibility
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Purohit, T.J.; Hanning, S.M.; Wu, Z. Advances in rectal drug delivery systems. Pharm. Dev. Technol. 2018, 23, 942–952. [Google Scholar] [CrossRef] [PubMed]
- Amoxicillin Dispersible Tablets: Market and Supply Update. Available online: https://www.unicef.org/supply/media/511/file/amoxicillin-dispersible-tablets-market-and-supply-update.pdf (accessed on 6 April 2017).
- McAlister, E.; Dutton, B.; Vora, L.K.; Zhao, L.; Ripolin, A.; Zahari, D.; Quinn, H.L.; Tekko, I.A.; Courtenay, A.J.; Kelly, S.A.; et al. Directly compressed tablets: A novel drug-containing reservoir combined with hydrogel-forming microneedle arrays for transdermal drug delivery. Adv. Healthc. Mater. 2021, 10, e2001256. [Google Scholar] [CrossRef] [PubMed]
- Hanning, S.M.; Matiz, S.; Krasser, K.; Orlu, M.; Dodoo, C.; Gaisford, S.; Tuleu, C. Characterisation of rectal amoxicillin (RAMOX) for the treatment of pneumonia in children. Drug Deliv. Transl. Res. 2020, 11, 944–955. [Google Scholar] [CrossRef] [PubMed]
- Purohit, T.J.; Hanning, S.M.; Amirapu, S.; Wu, Z. Rectal bioavailability of amoxicillin sodium in rabbits: Effects of suppository base and drug dose. J. Control Release 2021, 338, 858–869. [Google Scholar] [CrossRef]
- Webster, J.A.; Dowse, R.; Walker, R.B. In vitro release of amoxycillin from lipophilic suppositories. Drug Dev. Ind. Pharm. 1998, 24, 395–399. [Google Scholar] [CrossRef]
- Binson, G.; Grignon, C.; Le Moal, G.; Lazaro, P.; Lelong, J.; Roblot, F.; Venisse, N.; Dupuis, A. Overcoming stability challenges during continuous intravenous administration of high-dose amoxicillin using portable elastomeric pumps. PLoS ONE 2019, 14, e0221391. [Google Scholar] [CrossRef]
- Purohit, T.J.; Wu, Z.; Hanning, S.M. Simple and reliable extraction and a validated high performance liquid chromatographic assay for quantification of amoxicillin from plasma. J. Chromatogr. A 2020, 1611, 460611. [Google Scholar] [CrossRef]
- Suppocire® NA 15. Available online: https://www.gattefosse.com/pharmaceuticals-products/suppocire-na-15 (accessed on 17 October 2017).
- Innovative Delivery Systems for Paediatric Medicines: Technology Landscape. Available online: https://unitaid.org/assets/Innovative-delivery-systems-for-paediatric-medicines-technology-landscape.pdf (accessed on 15 November 2020).
- Ilomuanya, M.O.; Salako, B.B.; Ologunagba, M.O.; Shonekan, O.O.; Owodeha-Ashaka, K.; Osahon, E.S.; Amenaghawon, A.N. Formulation and Optimization of Metronidazole and Lactobacillus spp. Layered Suppositories via a Three-Variable, Five-Level Central Composite Design for the Management of Bacterial Vaginosis. Pharmaceutics 2022, 14, 2337. [Google Scholar] [CrossRef]
- Shiohira, H.; Fujii, M.; Koizumi, N.; Kondoh, M.; Watanabe, Y. Novel chronotherapeutic rectal aminophylline delivery system for therapy of asthma. Int. J. Pharm. 2009, 379, 119–124. [Google Scholar] [CrossRef]
- Promoting the quality of medicines (PQM). Product Information Report: Amoxicillin; U.S. Pharmacopoeial Convention: Rockville, Maryland, 2017. [Google Scholar]
- Brayfield, A. Martindale: The Complete Drug Reference, 38th ed.; Pharmaceutical Press: London, UK, 2014; Volume A, p. 2057. [Google Scholar]
- Purohit, T.J. Development of Amoxicillin Suppositories and In Vitro/In Vivo Evaluations. Ph.D. Thesis, University of Auckland, Auckland, New Zealand, 2021. [Google Scholar]
- British Pharmacopoeia Commission. British Pharmacopoeia, Volume 3: Rectal Preparations; The Stationery Office: London, UK, 2020. [Google Scholar]
- Zhang, Y.; Huo, M.; Zhou, J.; Zou, A.; Li, W.; Yao, C.; Xie, S. DDSolver: An add-in program for modeling and comparison of drug dissolution profiles. AAPS J. 2010, 12, 263–271. [Google Scholar] [CrossRef]
- Shah, V.P.; Tsong, Y.; Sathe, P.; Liu, J.P. In vitro dissolution profile comparison—statistics and analysis of the similarity factor, f2. Pharm. Res. 1998, 15, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Bruschi, M.L. (Ed.) Mathematical models of drug release. In Strategies to Modify the Drug Release from Pharmaceutical Systems; Woodhead Publishing: Soston, UK, 2015; pp. 63–86. [Google Scholar]
- Wu, Z.; Tucker, I.G.; Razzak, M.; Yang, L.; McSporran, K.; Medlicott, N.J. Absorption and tissue tolerance of ricobendazole in the presence of hydroxypropyl-beta-cyclodextrin following subcutaneous injection in sheep. Int. J. Pharm. 2010, 397, 96–102. [Google Scholar] [CrossRef] [PubMed]
- USP30-NF25. Chapter 9: Quality Control of Suppositories. Available online: https://pdf4pro.com/view/quality-control-of-suppositories-pharmaceutical-press-39f578.html (accessed on 2 May 2017).
- Bird, A.E. Amoxicillin. In Analytical Profiles of Drug Substances and Excipients; Brittain, H.G., Ed.; Academic Press: Cambridge, MA, USA, 1994; Volume 23, pp. 1–52. [Google Scholar]
- Fogazzi, G.B.; Cantu, M.; Saglimbeni, L.; Daudon, M. Amoxycillin, a rare but possible cause of crystalluria. Nephrol. Dial. Transpl. 2003, 18, 212–214. [Google Scholar] [CrossRef] [PubMed]
- Brophy, M.R.; Deasy, P.B. Application of the Higuchi model for drug release from dispersed matrices to particles of general shape. Int. J. Pharm. 1987, 37, 41–47. [Google Scholar] [CrossRef]
- Jurczak, E.; Mazurek, A.H.; Szeleszczuk, Ł.; Pisklak, D.M.; Zielińska-Pisklak, M. Pharmaceutical hydrates analysis-Overview of methods and recent advances. Pharmaceutics 2020, 12, 959. [Google Scholar] [CrossRef]
- Sood, J.; Sapra, B.; Bhandari, S.; Jindal, M.; Tiwary, A.K. Understanding pharmaceutical polymorphic transformations I: Influence of process variables and storage conditions. Ther. Deliv. 2014, 5, 1123–1142. [Google Scholar] [CrossRef]
- Hamad, F.A.; Egelle, E.; Cummings, K.; Russell, P. Investigation of the melting process of polyethylene glycol 1500 (PEG 1500) in a rectagular enclosure. Int. J. Heat. Mass. Tran. 2017, 114, 1234–1247. [Google Scholar] [CrossRef]
- Falqi, F.H.; Bin-Dahman, O.A.; Hussain, M.; Al-Harthi, M.A. Preparation of miscible PVA/PEG blends and effect of graphene concentration on thermal, crystallization, morphological, and mechanical properties of PVA/PEG (10 wt%) blend. Int. J. Polym. Sci. 2018, 2018, 8527693. [Google Scholar] [CrossRef]
- Han, S.; Kim, C.; Kwon, D. Thermal degradation of poly(ethyleneglycol). Polym. Degrad. Stab. 1995, 47, 203–208. [Google Scholar] [CrossRef]
- Paberit, R.; Rilby, E.; Gohl, J.; Swenson, J.; Refaa, Z.; Johansson, P.; Jansson, H. Cycling stability of poly(ethylene glycol) of six molecular weights: Influence of thermal conditions for energy applications. Acs Appl. Energ. Mater. 2020, 3, 10578–10589. [Google Scholar] [CrossRef]
- Brittain, H.G. Polymorphism and solvatomorphism 2008. J. Pharm. Sci. 2010, 99, 3648–3664. [Google Scholar] [CrossRef] [PubMed]
- Giron, D.; Goldbronn, C.; Mutz, M.; Pfeffer, S.; Piechon, P.; Schwab, P. Solid state characterizations of pharmaceutical hydrates. J. Therm. Anal. Calorim. 2002, 68, 453–465. [Google Scholar] [CrossRef]
- United States Pharmacopeial Convention. The United States Pharmacopoeia 41. The National Formulary 36; United States Pharmacopeial Convention: Rockville, MD, USA, 2018; Available online: https://www.usp.org (accessed on 8 April 2020).
- Hua, S. Comparison of in vitro dialysis release methods of loperamide-encapsulated liposomal gel for topical drug delivery. Int. J. Nanomed. 2014, 9, 735–744. [Google Scholar] [CrossRef] [PubMed]
- Ozguney, I.; Kardhiqi, A.; Yildiz, G.; Ertan, G. In vitro-in vivo evaluation of in situ gelling and thermosensitive ketoprofen liquid suppositories. Eur. J. Drug Metab. Pharm. 2014, 39, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, K.K.; Agarwal, A.K.; Singh, P.J.; Sharma, S.N. Comparative study of ano-rectal region in mammals. J. Anat. Soc. India 2002, 51, 220–224. [Google Scholar]
- Karimi, R. Biomedical & pharmaceutical sciences with patient care correlations. In Biomedical and Pharmaceutical Sciences with Patient Care Correlations; Jones & Bartlett Learning: Burlington, MA, USA, 2015; pp. 498–499. [Google Scholar]



| Model | AMT HT | AS HT | ||||
|---|---|---|---|---|---|---|
| R2 | Slope | Intercept | R2 | Slope | Intercept | |
| First-order | 0.6869 | −0.0255 | 2.0870 | 0.9930 ** | −0.2533 | 2.0890 |
| Hixson Crowell | 0.9336 | 0.0458 | 0.0145 | 0.9748 | 0.4629 | 0.1072 |
| Higuchi | 0.9804 ** | 12.4800 | 8.5010 | 0.9405 | 41.5000 | 7.7850 |
| Korsmeyer Peppas # | 0.9519 | 1.0200 | 0.2977 | 0.6049 | 1.0610 | 1.2080 |
| PK Parameter | IV Amoxicillin (n = 3) | AMT HTS (n = 3) | AS HTS (n = 3) |
|---|---|---|---|
| AUC0–8h (μg/mL·h) | 33.95 ± 11.34 | 56.67 ± 7.45 | 39.97 ± 17.92 |
| AUC0–∞ (μg/mL·h2) | 36.09 ± 13.35 | 63.43 ± 6.09 | 213.96 ± 99.88 |
| Tmax1 (h) | - | 1.33 ± 0.29 | 0.50 ± 0.43 |
| Tmax2 (h) | - | 3.00 ± 0.00 | 3.33 ± 1.15 |
| Cmax1 (μg/mL) | 73.40 ± 20.48 | 9.24 ± 1.60 | 16.89 ± 6.23 |
| Cmax2 (μg/mL) | - | 16.57 ± 1.54 | 9.22 ± 2.64 |
| t1/2 (h) | 1.41 ± 0.49 | 1.86 ± 0.44 | 3.92 ± 3.02 |
| MRT (h) | 1.05 ± 0.83 | 6.83 ± 0.40 | 5.36 ± 0.88 |
| MAT (h) | - | 6.19 ± 0.40 | 4.72 ± 0.88 |
| F (%) | 100 | 68.25 ± 6.55 | 72.75 ± 32.19 |
| Vd (L) | 5.82 ± 1.46 | 10.64 ± 2.43 | 2.22 ± 29.72 |
| Cl (L/h) | 3.07 ± 1.24 | 3.97 ± 0.40 | 4.82 ± 2.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Purohit, T.J.; Amirapu, S.; Wu, Z.; Hanning, S.M. Rectal Bioavailability of Amoxicillin from Hollow-Type Suppositories: Effect of Chemical Form of Amoxicillin. Pharmaceutics 2023, 15, 1865. https://doi.org/10.3390/pharmaceutics15071865
Purohit TJ, Amirapu S, Wu Z, Hanning SM. Rectal Bioavailability of Amoxicillin from Hollow-Type Suppositories: Effect of Chemical Form of Amoxicillin. Pharmaceutics. 2023; 15(7):1865. https://doi.org/10.3390/pharmaceutics15071865
Chicago/Turabian StylePurohit, Trusha J., Satya Amirapu, Zimei Wu, and Sara M. Hanning. 2023. "Rectal Bioavailability of Amoxicillin from Hollow-Type Suppositories: Effect of Chemical Form of Amoxicillin" Pharmaceutics 15, no. 7: 1865. https://doi.org/10.3390/pharmaceutics15071865
APA StylePurohit, T. J., Amirapu, S., Wu, Z., & Hanning, S. M. (2023). Rectal Bioavailability of Amoxicillin from Hollow-Type Suppositories: Effect of Chemical Form of Amoxicillin. Pharmaceutics, 15(7), 1865. https://doi.org/10.3390/pharmaceutics15071865








