Innovative Strategies for Drug Delivery to the Ocular Posterior Segment
Abstract
1. Introduction
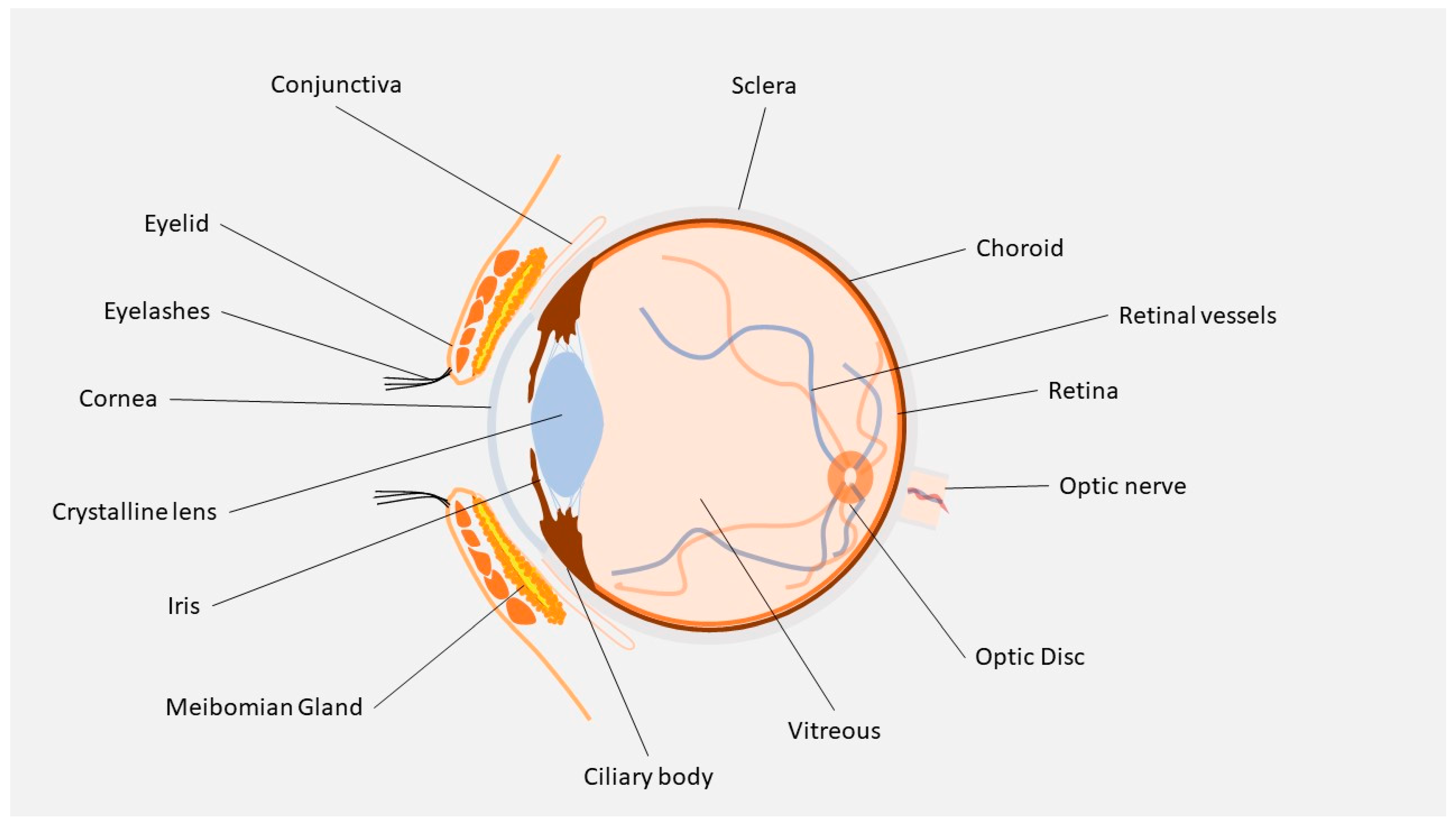
2. Ocular Barriers
2.1. Lacrimal Wash-Out and Mucosal Capillary Circulation
2.2. Cornea
2.3. Conjunctiva
2.4. Sclera
2.5. BAB and BRB
3. Methods
4. Innovative Drug Delivery Systems
4.1. Nanomedicine
4.1.1. Liposomes
4.1.2. Nanomicelles
4.1.3. Nanospheres and Solid Lipids
4.1.4. Dendrimers
4.1.5. Organic Nanopolymers
4.1.6. Chitosan and Chitosan-Based Nanotechnologies
Chitosan Nanoparticles
Chitosan Micelles
Chitosan Lipid Nanoparticles and Liposomes
Chitosan in Combined Drug Delivery Systems
4.1.7. Metallic and Other Inorganic Nanomaterials
4.1.8. Encapsulated Cell Technology
4.2. Other Topical Absorption Enhancers
4.2.1. Cell-Penetrating Peptides (CPPs)
4.2.2. Cyclodextrins
4.2.3. BAC
4.2.4. Iontophoresis
4.3. Sustained Drug-Release Systems
4.3.1. Ocular Inserts
4.3.2. Ocular Implants
4.3.3. Hydrogels
4.3.4. Contact Lens
4.3.5. Microneedles
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Edelhauser, H.F.; Rowe-Rendleman, C.L.; Robinson, M.R.; Dawson, D.G.; Chader, G.J.; Grossniklaus, H.E.; Rittenhouse, K.D.; Wilson, C.G.; Weber, D.A.; Kuppermann, B.D.; et al. Ophthalmic Drug Delivery Systems for the Treatment of Retinal Diseases: Basic Research to Clinical Applications. Investig. Opthalmology Vis. Sci. 2010, 51, 5403–5420. [Google Scholar] [CrossRef] [PubMed]
- Thrimawithana, T.R.; Young, S.; Bunt, C.R.; Green, C.; Alany, R.G. Drug delivery to the posterior segment of the eye. Drug Discov. Today 2011, 16, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Bansal, P.; Garg, S.; Sharma, Y.; Venkatesh, P. Posterior Segment Drug Delivery Devices: Current and Novel Therapies in Development. J. Ocul. Pharmacol. Ther. 2016, 32, 135–144. [Google Scholar] [CrossRef]
- Awwad, S.; Ahmed, A.H.M.; Sharma, G.; Heng, J.S.; Khaw, P.T.; Brocchini, S.; Lockwood, A. Principles of pharmacology in the eye. Br. J. Pharmacol. 2017, 174, 4205–4223. [Google Scholar] [CrossRef]
- Gholizadeh, S.; Wang, Z.; Chen, X.; Dana, R.; Annabi, N. Advanced nanodelivery platforms for topical ophthalmic drug delivery. Drug Discov. Today 2021, 26, 1437–1449. [Google Scholar] [CrossRef] [PubMed]
- Löscher, M.; Seiz, C.; Hurst, J.; Schnichels, S. Topical Drug Delivery to the Posterior Segment of the Eye. Pharmaceutics 2022, 14, 134. [Google Scholar] [CrossRef] [PubMed]
- Kang-Mieler, J.J.; Rudeen, K.M.; Liu, W.; Mieler, W.F. Advances in ocular drug delivery systems. Eye 2020, 34, 1371–1379. [Google Scholar] [CrossRef]
- Yadav, D.; Varma, L.T.; Yadav, K. Drug delivery to posterior segment of the eye: Conventional delivery strategies their barriers and restrictions. In Drug Delivery for the Retina and Posterior Segment Disease; Patel, J.K., Sutariya, V., Kanwar, J.R., Pathak, Y.V., Eds.; Springer: Cham, Switzerland, 2018; pp. 51–67. [Google Scholar] [CrossRef]
- Akhter, M.H.; Ahmad, I.; Alshahrani, M.Y.; Al-Harbi, A.I.; Khalilullah, H.; Afzal, O.; Altamimi, A.S.A.; Najib Ullah, S.N.M.; Ojha, A.; Karim, S. Drug Delivery Challenges and Current Progress in Nanocarrier-Based Ocular Therapeutic System. Gels 2022, 8, 82. [Google Scholar] [CrossRef]
- Wang, R.; Gao, Y.; Liu, A.; Zhai, G. A review of nanocarrier-mediated drug delivery systems for posterior segment eye disease: Challenges analysis and recent advances. J. Drug Target. 2021, 29, 687–702. [Google Scholar] [CrossRef]
- Joseph, R.R.; Venkatraman, S.S. Drug delivery to the eye: What benefits do nanocarriers offer? Nanomedicine 2017, 12, 683–702. [Google Scholar] [CrossRef]
- Urtti, A.; Salminen, L. Minimizing systemic absorption of topically administered ophthalmic drugs. Surv. Ophthalmol. 1993, 37, 435–456. [Google Scholar] [CrossRef] [PubMed]
- Urtti, A. Challenges and obstacles of ocular pharmacokinetics and drug delivery. Adv. Drug Deliv. Rev. 2006, 58, 1131–1135. [Google Scholar] [CrossRef] [PubMed]
- Maurice, D.M.; Mishima, S. Ocular pharmacokinetics. In Handbook of Experimental Pharmacology; Sears, M.L., Ed.; Springer: Berlin/Heidelberg, Germany, 1984; Volume 69, pp. 16–119. [Google Scholar]
- Hornof, M.; Toropainen, E.; Urtti, A. Cell culture models of the ocular barriers. Eur. J. Pharm. Biopharm. 2005, 60, 207–225. [Google Scholar] [CrossRef]
- Prausnitz, M.R.; Noonan, J.S. Permeability of cornea, sclera, and conjunctiva: A literature analysis for drug delivery to the eye. J. Pharm. Sci. 1998, 87, 1479–1488. [Google Scholar] [CrossRef]
- Hämäläinen, K.M.; Kontturi, K.; Murtomäki, L.; Auriola, S.; Urtti, A. Estimation of pore size and porosity of biomembranes from permeability measurements of polyethylene glycols using an effusion-like approach. J. Control. Release 1997, 49, 97–104. [Google Scholar] [CrossRef]
- Geroski, D.H.; Edelhauser, H.F. Transscleral drug delivery for posterior segment disease. Adv. Drug Deliv. Rev. 2001, 52, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Hämäläinen, K.M.; Kananen, K.; Auriola, S.; Kontturi, K.; Urtti, A. Characterization of paracellular aqueous penetration routes in cornea conjunctiva sclera. Investig. Ophthalmol. Vis. Sci. 1997, 38, 627–634. [Google Scholar]
- Raghava, S.; Hammond, M.; Kompella, U.B. Periocular routes for retinal drug delivery. Exp. Opin. Drug Deliv. 2004, 1, 99–114. [Google Scholar] [CrossRef]
- Loftsson, T.; Sigurdsson, H.H.; Konrádsdóttir, F.; Gísladóttir, S.; Jansook, P.; Stefánsson, E. Topical drug delivery to the posterior segment of the eye: Anatomical and physiological considerations. Pharmazie 2008, 63, 171–179. [Google Scholar]
- Tawfik, M.; Chen, F.; Goldberg, J.L.; Sabel, B.A. Nanomedicine and drug delivery to the retina: Current status and implications for gene therapy. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2022, 395, 1477–1507. [Google Scholar] [CrossRef]
- Cunha-Vaz, J.G. The blood-retinal barriers. Doc. Ophthalmol. 1976, 41, 287–327. [Google Scholar] [CrossRef]
- Das, S.; Suresh, P.K. Drug delivery to the eye: Special reference to nanoparticle. Int. J. Drug Deliv. 2010, 2, 12–21. [Google Scholar] [CrossRef]
- Zamboulis, A.; Nanaki, S.; Michailidou, G.; Koumentakou, I.; Lazaridou, M.; Ainali, N.M.; Xanthopoulou, E.; Bikiaris, D.N. Chitosan and its Derivatives for Ocular Delivery Formulations: Recent Advances and Developments. Polymers 2020, 12, 1519. [Google Scholar] [CrossRef] [PubMed]
- Almeida, H.; Amaral, M.H.; Lobão, P.; Silva, A.C.; Lobo, J.M.S. Applications of polymeric and lipid nanoparticles in ophthalmic pharmaceutical formulations: Present and future considerations. J. Pharm. Sci. 2014, 17, 278–293. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.K.; O’Hanlon, D.E.; Harrold, S.; Man, S.T.; Wang, Y.Y.; Cone, R.; Hanes, J. Rapid transport of large polymeric nanoparticles in fresh undiluted human mucus. Proc. Natl. Acad. Sci. USA 2007, 104, 1482–1487. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.K.; Wang, Y.Y.; Hida, K.; Cone, R.; Hanes, J. Nanoparticles reveal that human cervicovaginal mucus is riddled with pores larger than viruses. Proc. Natl. Acad. Sci. USA 2010, 107, 598–603. [Google Scholar] [CrossRef]
- Kompella, U.B.; Amrite, A.C.; Pacha Ravi, R.; Durazo, S.A. Nanomedicines for back of the eye drug delivery, gene delivery, and imaging. Prog. Retin. Eye Res. 2013, 36, 172–198. [Google Scholar] [CrossRef]
- Kaur, I.P.; Kakkar, S. Nanotherapy for posterior eye diseases. J. Control. Release 2014, 193, 100–112. [Google Scholar] [CrossRef]
- Liu, H.-A.; Liu, Y.-L.; Ma, Z.-Z.; Wang, J.-C.; Zhang, Q. A Lipid Nanoparticle System Improves siRNA Efficacy in RPE Cells and a Laser-Induced Murine CNV Model. Investig. Opthalmol. Vis. Sci. 2011, 52, 4789–4794. [Google Scholar] [CrossRef]
- Gross, N.; Ranjbar, M.; Evers, C.; Hua, J.; Schulze, B.; Michaelis, U.; Hansen, L.L.; Agostini, H.T. Choroidal neovascularization reduced by targeted drug delivery with cationic liposome-encapsulated paclitaxel or targeted photodynamic therapy with verteporfin encapsulated in cationic liposomes. Mol. Vis. 2013, 19, 54. [Google Scholar]
- Crommelin, D.J.; van Hoogevest, P.; Storm, G. The role of liposomes in clinical nanomedicine development. What now? Now what? J. Control. Release 2020, 318, 256–263. [Google Scholar] [CrossRef]
- Gu, Y.; Xu, C.; Wang, Y.; Zhou, X.; Fang, L.; Cao, F. Multifunctional nanocomposites based on liposomes layered double hydroxides conjugated with glycylsarcosine for efficient topical drug delivery to the posterior segment of the eye. Mol. Pharm. 2019, 16, 2845–2857. [Google Scholar] [CrossRef] [PubMed]
- Kamaleddin, M.A. Nano-ophthalmology: Applications and considerations. Nanomedicine 2017, 13, 1459–1472. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Zhang, S.; Wang, B.; Cui, S.; Yan, J. Toxicity of cationic lipids and cationic polymers in gene delivery. J. Control. Release 2006, 114, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Bochot, A.; Fattal, E. Liposomes for intravitreal drug delivery: A state of the art. J. Control. Release 2012, 161, 628–634. [Google Scholar] [CrossRef]
- Bachu, R.D.; Chowdhury, P.; Al-Saedi, Z.H.; Karla, P.K.; Boddu, S.H.S. Ocular drug delivery barriers—Role of nanocarriers in the treatment of anterior segment ocular diseases. Pharmaceutics 2018, 10, 28. [Google Scholar] [CrossRef]
- Cholkar, K.; Patel, A.; Dutt Vadlapudi, A.K.; Mitra, A. Novel nanomicellar formulation approaches for anterior posterior segment ocular drug delivery. Recent Pat. Nanomed. 2012, 2, 82–95. [Google Scholar] [CrossRef]
- Patel, A.; Cholkar, K.; Agrahari, V.; Mitra, A.K. Ocular drug delivery systems: An overview. World J. Pharmacol. 2013, 2, 47–64. [Google Scholar] [CrossRef]
- Mandal, A.; Cholkar, K.; Khurana, V.; Shah, A.; Agrahari, V.; Bishit, R.; Pal, D.; Mitra, A.K. Topical formulation of self-assembled antiviral prodrug nanomicelles for targeted retinal delivery. Mol. Pharm. 2017, 14, 2056–2069. [Google Scholar] [CrossRef]
- Cholkar, K.; Gunda, S.; Earla, R.; Pal, D.; Mitra, A.K. Nanomicellar Topical Aqueous Drop Formulation of Rapamycin for Back-of-the-Eye Delivery. AAPS PharmSciTech 2015, 16, 610–622. [Google Scholar] [CrossRef]
- Velagaleti, P.R.; Anglade, E.; Khan, I.J.; Gilger, B.C. Topical delivery of hydrophobic drugs using a novel mixed nanomicellar technology to treat diseases of the anterior and posterior segments of the eye. Drug Deliv. Technol. 2010, 10, 42–47. [Google Scholar]
- Alshamrani, M.; Sikder, S.; Coulibaly, F.; Mandal, A.; Pal, D.; Mitra, A.K. Self-assembling topical nanomicellar formulation to improve curcumin absorption across ocular tissues. AAPS PharmSciTech 2019, 20, 254. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.; Gote, V.; Pal, D.; Ogundele, A.; Mitra, A.K. Ocular pharmacokinetics of a topical ophthalmic nanomicellar solution of cyclosporine Cequa® for dry eye disease. Pharm. Res. 2019, 36, 36. [Google Scholar] [CrossRef]
- Vadlapudi, A.D.; Mitra, A.K. Nanomicelles: An emerging platform for drug delivery to the eye. Ther. Deliv. 2013, 4, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Vadlapudi, A.D.; Cholkar, K.; Vadlapatla, R.K.; Mitra, A.K. Aqueous nanomicellar formulation for topical delivery of biotinylated lipid prodrug of acyclovir: Formulation development and ocular biocompatibility. J. Ocul. Pharmacol. Ther. 2014, 30, 49–58. [Google Scholar] [CrossRef]
- Trivedi, R.; Kompella, U.B. Nanomicellar formulations for sustained drug delivery: Strategies and underlying principles. Nanomedicine 2010, 5, 485–505. [Google Scholar] [CrossRef] [PubMed]
- Ideta, R.; Yanagi, Y.; Tamaki, Y.; Tasaka, F.; Harada, A.; Kataoka, K. Effective accumulation of polyion complex micelle to experimental choroidal neovascularization in rats. FEBS Lett. 2004, 557, 21–25. [Google Scholar] [CrossRef]
- Panda, J.J.; Yandrapu, S.; Kadam, R.S.; Chauhan, V.S.; Kompella, U.B. Self-assembled phenylalanine-α β-dehydrophenylalanine nanotubes for sustained intravitreal delivery of a multi-targeted tyrosine kinase inhibitor. J. Control. Release 2013, 172, 1151. [Google Scholar] [CrossRef]
- Honda, M.; Asai, T.; Oku, N.; Araki, Y.; Tanaka, M.; Ebihara, N. Liposomes and nanotechnology in drug development: Focus on ocular targets. Int. J. Nanomed. 2013, 8, 495–504. [Google Scholar] [CrossRef]
- Apaolaza, P.S.; Del Pozo-Rodriguez, A.; Solinis, M.A.; Rodriguez, J.M.; Friedrich, U.; Torrecilla, J.; Weber, B.H.F.; Rodríguez-Gascón, A. Structural recovery of the retina in a retinoschisin-deficient mouse after gene replacement therapy by solid lipid nanoparticles. Biomaterials 2016, 90, 40–49. [Google Scholar] [CrossRef]
- Kalomiraki, M.; Thermos, K.; Chaniotakis, N.A. Dendrimers as tunable vectors of drug delivery systems and biomedical and ocular applications. Int. J. Nanomed. 2016, 11, 1. [Google Scholar]
- Kokaz, S.F.; Deb, P.K.; Borah, P.; Bania, R.; Venugopala, K.N.; Nair, A.B.; Singh, V.; Al-Shar’I, N.A.; Hourani, W.; Tekade, R.K. Dendrimers: Properties and applications in biomedical field. Nanoeng. Biomater. 2022, 2, 215–243. [Google Scholar]
- Yavuz, B.; Pehlivan, S.B.; Vural, I.; Ünlü, N. In Vitro/In Vivo Evaluation of Dexamethasone—PAMAM Dendrimer Complexes for Retinal Drug Delivery. J. Pharm. Sci. 2015, 104, 3814–3823. [Google Scholar] [CrossRef] [PubMed]
- Whitcup, S.M.; Nussenblatt, R.B.; Lightman, S.L.; Hollander, D.A. Inflammation in Retinal Disease. Int. J. Inflamm. 2013, 2013, 724648. [Google Scholar] [CrossRef] [PubMed]
- Iezzi, R.; Guru, B.R.; Glybina, I.V.; Mishra, M.K.; Kennedy, A.; Kannan, R.M. Dendrimer-based targeted intravitreal therapy for sustained attenuation of neuroinflammation in retinal degeneration. Biomaterials 2012, 33, 979–988. [Google Scholar] [CrossRef]
- Kambhampati, S.P.; Clunies-Ross, A.J.M.; Bhutto, I.; Mishra, M.; Edwarda, M.; McLeod, D.S.; Kannan, R.M.; Lutty, G. Systemic and Intravitreal Delivery of Dendrimers to Activated Microglia/Macrophage in Ischemia/Reperfusion Mouse Retina. Investig. Opthalmol. Vis. Sci. 2015, 56, 4413–4424. [Google Scholar] [CrossRef]
- Lancina, M.G., III; Singh, S.; Kompella, U.B.; Husain, S.; Yang, H. Fast dissolving dendrimer nanofiber mats as alternative to eye drops for more efficient antiglaucoma drug delivery. ACS Biomater. Sci. Eng. 2017, 3, 1861–1868. [Google Scholar] [CrossRef]
- Mishra, V.; Jain, N.K. Acetazolamide encapsulated dendritic nano-architectures for effective glaucoma management in rabbits. Int. J. Pharm. 2014, 461, 380–390. [Google Scholar] [CrossRef]
- Kang, S.J.; Durairaj, C.; Kompella, U.B.; O’Brien, J.M.; Grossniklaus, H.E. Subconjunctival nanoparticle carboplatin in the treatment of murine retinoblastoma. Arch. Ophthalmol. 2009, 127, 1043–1047. [Google Scholar] [CrossRef]
- Albertazzi, L.; Gherardini, L.; Brondi, M.; Sato, S.S.; Bifone, A.; Pizzorusso, T.; Ratto, G.M.; Bardi, G. In vivo distribution and toxicity of PAMAM dendrimers in the central nervous system depend on their surface chemistry. Mol. Pharm. 2013, 10, 249–260. [Google Scholar] [CrossRef]
- Robinson, B.V.; Sullivan, F.M.; Borzelleca, J.F.; Schwartz, S.L. PVP: A Critical Review of the Kinetics and Toxicology of Polyvinylpyrrolidone Povidone; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Bruining, M.J.; Edelbroek-Hoogendoorn, P.S.; Blaauwgeers, H.G.; Mooyh, C.M.; Hendrikse, F.H.; Koole, L.H. New biodegradable networks of poly(N-vinylpyrrolidinone) designed for controlled nonburst degradation in the vitreous body. J. Biomed. Mater. Res. 1999, 47, 189–197. [Google Scholar] [CrossRef]
- Hong, Y.; Chirila, T.V.; Vijayasekaran, S.; Dalton, P.D.; Tahija, S.G.; Cuypers, M.J.; Constable, I.J. Crosslinked poly-1-vinyl-2-pyrrolidinone as a vitreous substitute. J. Biomed. Mater. Res. 1996, 30, 441–448. [Google Scholar] [CrossRef]
- Gupta, S.V. Physicochemical requirements for polymers and poly-mer-based nanomaterial for ophthalmic drug delivery. In Nano-Biomaterials for Ophthalmic Drug Delivery; Pathak, Y., Sutariya, V., Hirani, A.A., Eds.; Springer: Cham, Switzerland, 2016; pp. 131–146. [Google Scholar]
- Tawfik, M.; Zhang, X.; Grigartzik, L.; Heiduschka, P.; Hintz, W.; Heinrich-Noack, P.; van Wachem, B.; Bernhard, A.S. Gene therapy with caspase-3 small interfering RNA-nanoparticles is neuroprotective after optic nerve damage. Neural Regen. Res. 2021, 16, 2534. [Google Scholar] [PubMed]
- Colthurst, M.J.; Williams, R.L.; Hiscott, P.S.; Grierson, I. Biomaterials used in the posterior segment of the eye. Biomaterials 2000, 21, 649–665. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y.; Zhang, C.; Wang, Y.; Song, C. Pharmacokinetics and tolerance study of intravitreal injection of dexamethasone-loaded nanoparticles in rabbits. Int. J. Nanomed. 2009, 4, 175. [Google Scholar] [CrossRef]
- Bisht, R.; Jaiswal, J.K.; Rupenthal, I.D. Nanoparticle-loaded biodegradable light-responsive in situ forming injectable implants for effective peptide delivery to the posterior segment of the eye. Med. Hypotheses 2017, 103, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Chen, Y.; Hu, Y.; Maypo, A.S.; Kompella, U.B.; Longera, R.; Ma, J. Nanoparticle-mediated expression of an angiogenic inhibitor ameliorates ischemia-induced retinal neovascularization and diabetes-induced retinal vascular leakage. Diabetes 2009, 58, 1902–1913. [Google Scholar] [CrossRef]
- Singh, S.R.; Grossniklaus, H.E.; Kang, S.J.; Edelhauser, H.F.; Ambati, B.K.; Kompella, U.B. Intravenous transferrin RGD peptide dual-targeted nanoparticles enhance anti-VEGF intraceptor gene delivery to laser-induced CNV. Gene Ther. 2009, 16, 645–659. [Google Scholar] [CrossRef]
- Salama, H.A.; Ghorab, M.; Mahmoud, A.A.; Hady, M.A. PLGA nanoparticles as subconjunctival injection for management of glaucoma. AAPS PharmSciTech 2017, 18, 2517–2528. [Google Scholar] [CrossRef]
- Bourges, J.L.; Gautier, S.E.; Delie, F.; Bejjani, R.A.; Jeanny, J.; Gurny, R.; Benezra, D.; Behar-Cohen, F.F. Ocular drug delivery targeting the retinal pigment epithelium using polylactide nanoparticles. Investig. Ophthalmol. Vis. Sci. 2003, 44, 3562–3569. [Google Scholar] [CrossRef] [PubMed]
- Sakai, T.; Kohno, H.; Ishihara, T.; Higaki, M.; Saito, S.; Matsushima, M.; Kitahara, K. Treatment of experimental autoimmune uveoretinitis with poly lactic acid nanoparticles encapsulating betamethasone phosphate. Exp. Eye Res. 2006, 82, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Fialho, S.L.; Behar-Cohen, F.; Silva-Cunha, A. Dexamethasone-loaded poly ε-caprolactone intravitreal implants: A pilot study. Eur. J. Pharm. Biopharm. 2008, 68, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Yenice, İ.; Mocan, M.C.; Palaska, E.; Bochot, A.; Bilensoy, E.; Vural, I.; Irkeç, M.; Hincal, A.A. Hyaluronic acid coated poly-ɛ-caprolactone nanospheres deliver high concentrations of cyclosporine A into the cornea. Exp. Eye Res. 2008, 87, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Gong, C.; Shi, S.; Liu, X.; Wei, Y.; Qian, Z. Toxicity evaluation of biodegradable thermosensitive PEG-PCL-PEG hydrogel as a potential in situ sustained ophthalmic drug delivery system. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 92, 129–137. [Google Scholar] [CrossRef]
- Li, V.H.; Wood, R.W.; Kreuter, J.; Harmia, T.; Robinson, J.R. Ocular drug delivery of progesterone using nanoparticles. J. Microencapsul. 1986, 3, 213–218. [Google Scholar] [CrossRef]
- Vote, B.J.; Elder, M.J. Cyanoacrylate glue for corneal perforations: A description of a surgical technique a review of the literature. Clin. Exp. Ophthalmol. 2000, 28, 437–442. [Google Scholar] [CrossRef]
- Ramge, P.; Unger, R.E.; Oltrogge, J.B.; Zenker, D.; Begley, D.; Kreuter, J.; Von Briesen, H. Polysorbate-80 coating enhances uptake of polybutylcyanoacrylate PBCA -nanoparticles by human and bovine primary brain capillary endothelial cells. Eur. J. Neurosci. 2000, 12, 1931–1940. [Google Scholar] [CrossRef]
- Leggat, P.A.; Smith, D.R.; Kedjarune, U. Surgical applications of cyanoacrylate adhesives: A review of toxicity. ANZ J. Surg. 2007, 77, 209–213. [Google Scholar] [CrossRef]
- Wilson, B. Brain targeting PBCA nanoparticles and the blood–brain barrier. Nanomedicine 2009, 4, 499–502. [Google Scholar] [CrossRef]
- Voigt, N.; Henrich-Noack, P.; Kockentiedt, S.; Hintz, W.; Tomas, J.; Sabel, B.A. Surfactants not size or zeta-potential influence blood–brain barrier passage of polymeric nanoparticles. Eur. J. Pharm. Biopharm. 2014, 87, 19–29. [Google Scholar] [CrossRef]
- Voigt, N.; Henrich-Noack, P.; Kockentiedt, S.; Hintz, W.; Tomas, J.; Sabel, B.A. Toxicity of polymeric nanoparticles in vivo and in vitro. J. Nanopart. Res. 2014, 16, 2379. [Google Scholar] [CrossRef] [PubMed]
- Sabel, B.A.; Engelmann, R.; Humphrey, M.F. In vivo confocal neuroimaging ICON of CNS neurons. Nat. Med. 1997, 3, 244–247. [Google Scholar] [CrossRef] [PubMed]
- Prilloff, S.; Fan, J.; Henrich-Noack, P.; Sabel, B.A. In vivo confocal neuroimaging ICON: Non-invasive functional imaging of the mammalian CNS with cellular resolution. Eur. J. Neurosci. 2010, 31, 521–528. [Google Scholar] [CrossRef]
- Henrich-Noack, P.; Prilloff, S.; Voigt, N.; Jin, J.; Hintz, W.; Tomas, J.; Sabel, B.A. In vivo visualisation of nanoparticle entry into central nervous system tissue. Arch. Toxicol. 2012, 86, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- You, Q.; Hopf, T.; Hintz, W.; Rannabauer, S.; Voigt, N.; van Wachem, B.; Henrich-Noack, P.; Sabel, B.A. Major effects on blood-retinabarrier passage by minor alterations in design of polybutylcyanoacrylate nanoparticles. J. Drug Target. 2019, 27, 338–346. [Google Scholar] [CrossRef] [PubMed]
- You, Q.; Sokolov, M.; Grigartzik, L.; Hintz, W.; van Wachem, B.; Henrich-Noack, P.; Sabel, B.A. How nanoparticle physicochemical parameters affect drug delivery to cells in the retina via systemic interactions. Mol. Pharm. 2019, 16, 5068–5075. [Google Scholar] [CrossRef]
- Caramella, C.; Ferrari, F.; Bonferoni, M.C.; Rossi, S.; Sandri, G. Chitosan and Its Derivatives as Drug Penetration Enhancers. J. Drug Deliv. Sci. Technol. 2010, 20, 5–13. [Google Scholar] [CrossRef]
- De Campos, A.M.; Sánchez, A.; Alonso, M.J. Chitosan nanoparticles: A new vehicle for the improvement of the delivery of drugs to the ocular surface. Application to cyclosporin A. Int. J. Pharm. 2001, 224, 159–168. [Google Scholar] [CrossRef]
- Felt, O.; Furrer, P.; Mayer, J.M.; Plazonnet, B.; Buri, P.; Gurny, R. Topical use of chitosan in ophthalmology:Tolerance assessment and evaluation of precorneal retention. Int. J. Pharm. 1999, 180, 185–193. [Google Scholar] [CrossRef]
- Taghe, S.; Mirzaeei, S. Preparation and characterization of novel, mucoadhesive of ofloxacin nanoparticles for ocular drug delivery. Braz. J. Pharm. Sci. 2019, 55, 1–12. [Google Scholar] [CrossRef]
- Badiee, P.; Varshochian, R.; Rafiee-Tehrani, M.; Abedin Dorkoosh, F.; Khoshayand, M.R.; Dinarvand, R. Ocular implant containing bevacizumab-loaded chitosan nanoparticles intended for choroidal neovascularization treatment. J. Biomed. Mater. Res. Part A 2018, 106, 2261–2271. [Google Scholar] [CrossRef] [PubMed]
- Elsaid, N.; Jackson, T.L.; Elsaid, Z.; Alqathama, A.; Somavarapu, S. PLGA microparticles entrapping chitosan-based nanoparticles for the ocular delivery of ranibizumab. Mol. Pharm. 2016, 13, 2923–2940. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.C.; Silva, S.; Sarmento, B.; Pintado, M. Chitosan nanoparticles for daptomycin delivery in oculartreatment of bacterial endophthalmitis. Drug. Deliv. 2015, 22, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, L.; Fang, L.; Cao, F. Multifunctional Carboxymethyl Chitosan Derivatives-Layered Double Hydroxide Hybrid Nanocomposites for Efficient Drug Delivery to the Posterior Segment of the Eye. Acta Biomater. 2020, 104, 104–114. [Google Scholar] [CrossRef]
- Savin, C.L.; Popa, M.; Delaite, C.; Costuleanu, M.; Costin, D.; Peptu, C.A. Chitosan grafted-poly(ethyleneglycol) methacrylate nanoparticles as carrier for controlled release of bevacizumab. Mater. Sci. Eng. C 2019, 98, 843–860. [Google Scholar] [CrossRef]
- Xu, X.; Sun, L.; Zhou, L.; Cheng, Y.; Cao, F. Functional chitosan oligosaccharide nanomicelles for topical ocular drug delivery of dexamethasone. Carbohydr. Polym. 2020, 227, 115356. [Google Scholar] [CrossRef]
- Li, J.; Cheng, T.; Tian, Q.; Cheng, Y.; Zhao, L.; Zhang, X.; Qu, Y. A More Efficient Ocular Delivery System of Triamcinolone Acetonide as Eye Drop to the Posterior Segment of the Eye. Drug Deliv. 2019, 26, 188–198. [Google Scholar] [CrossRef]
- Cheng, T.; Li, J.; Cheng, Y.; Zhang, X.; Qu, Y. Triamcinolone acetonide-chitosan coated liposomes efficiently treated retinal edema as eye drops. Exp. Eye Res. 2019, 188, 107805. [Google Scholar] [CrossRef]
- Khalil, M.; Hasmi, U.; Riaz, R.; Rukh Abbas, S. Chitosan coated liposomes (CCL) containing triamcinolone acetonide for sustained delivery: A potential topical treatment for posterior segment diseases. Int. J. Biol. Macromol. 2020, 143, 483–491. [Google Scholar] [CrossRef]
- Jiang, F.; Tang, Z.; Zhang, Y.; Ju, Y.; Gao, H.; Sun, N.; Liu, F.; Gu, P.; Zhang, W. Enhanced proliferation and differentiation of retinal progenitor cells through a self-healing injectable hydrogel. Biomater. Sci. 2019, 7, 2335–2347. [Google Scholar] [CrossRef]
- Moreno, M.; Pow, P.Y.; Tabitha, T.S.T.; Nirmal, S.; Larsson, A.; Radhakrishnan, K.; Nirmal, J.; Quah, S.T.; Geifman Shochat, S.; Agrawal, R.; et al. Modulating release of ranibizumab and aflibercept from thiolated chitosan-based hydrogels for potential treatment of ocular neovascularization. Expert Opin. Drug Deliv. 2017, 14, 913–925. [Google Scholar] [CrossRef]
- Nagai, N.; Saijo, S.; Song, Y.; Kaji, H.; Abe, T. A drug refillable device for transscleral sustained drug delivery to the retina. Eur. J. Pharm. Biopharm. 2019, 136, 184–191. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, J.H.; Kim, K.W.; Kim, K.; Kim, M.H.; Yu, Y.S. Intravenously administered gold nanoparticles pass through the blood–retinal barrier depending on the particle size, and induce no retinal toxicity. Nanotechnology 2009, 20, 505101. [Google Scholar] [CrossRef] [PubMed]
- Kalishwaralal, K.; Barathmanikanth, S.; Pandian, S.R.K.; Deepak, V.; Gurunathan, S. Silver nano—A trove for retinal therapies. J. Control. Release 2010, 145, 76–90. [Google Scholar] [CrossRef] [PubMed]
- Sheikpranbabu, S.; Kalishwaralal, K.; Lee, K.J.; Vaidyanathan, R.; Eom, S.H.; Gurunathan, S. The inhibition of advanced glycation end-products-induced retinal vascular permeability by silver nanoparticles. Biomaterials 2010, 31, 2260–2271. [Google Scholar] [CrossRef] [PubMed]
- Masse, F.; Ouellette, M.; Lamoureux, G.; Boisselier, E. Gold nanoparticles in ophthalmology. Med. Res. Rev. 2018, 39, 302–327. [Google Scholar] [CrossRef]
- Giannaccini, M.; Pedicini, L.; De Matienzo, G.; Chiellini, F.; Dente, L.; Raffa, V. Magnetic nanoparticles: A strategy to target the choroidal layer in the posterior segment of the eye. Sci. Rep. 2017, 7, 43092. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Cai, X.; Zhou, X.; Wong, L.; Karakoti, A.S.; Seal, S.; McGinnis, J.F. Nanoceria extend photoreceptor cell lifespan in tubby mice by modulation of apoptosis/survival signaling pathways. Neurobiol. Dis. 2011, 42, 514–523. [Google Scholar] [CrossRef]
- Kyosseva, S.V.; McGinnis, J.F. Cerium oxide nanoparticles as promising ophthalmic therapeutics for the treatment of retinal diseases. World J. Ophthalmol. 2015, 5, 23–30. [Google Scholar] [CrossRef]
- Maccarone, R.; Tisi, A.; Passacantando, M.; Ciancaglini, M. Ophthalmic Applications of Cerium Oxide Nanoparticles. J. Ocul. Pharmacol. Ther. 2020, 36, 376–383. [Google Scholar] [CrossRef]
- Wong, L.L.; Pye, Q.N.; Chen, L.; Seal, S.; McGinnis, J.F. Defining the Catalytic Activity of Nanoceria in the P23H-1 Rat, a Photoreceptor Degeneration Model. PLoS ONE 2015, 10, e0121977. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Seal, S.; McGinnis, J.F. Non-toxic retention of nanoceria in murine eyes. Mol. Vis. 2016, 22, 1176–1187. [Google Scholar] [PubMed]
- Jo, D.H.; Kim, J.H.; Yu, Y.S.; Lee, T.G.; Kim, J.H. Antiangiogenic effect of silicate nanoparticle on retinal neovascularization induced by vascular endothelial growth factor. Nanomedicine 2012, 8, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Troll, J.; Jeong, H.H.; Wei, Q.; Stang, M.; Ziemssen, F.; Wang, Z.; Mingdong, D.; Schnichels, S.; Qui, T.; et al. A swarm of slippery micropropellers penetrates the vitreous body of the eye. Sci. Adv. 2018, 4, eaat4388. [Google Scholar] [CrossRef]
- Jo, D.H.; Lee, T.G.; Kim, J.H. Nanotechnology and nanotoxicology in retinopathy. Int. J. Mol. Sci. 2011, 12, 8288–8301. [Google Scholar] [CrossRef]
- Soderstjerna, E.; Bauer, P.; Cedervall, T.; Abdshill, H.; Johansson, F.; Englund Johansson, U. Silver and gold nanoparticles exposure to in vitro cultured retina—Studies on nanoparticle internalization, apoptosis, oxidative stress, glial- and microglial activity. PLoS ONE 2014, 9, e105359. [Google Scholar] [CrossRef]
- Chan, Y.J.; Liao, P.L.; Tsai, C.H.; Cheng, Y.; Lin, F.; Ho, J.; Chen, C.; Li, C. Titanium dioxide nanoparticles impair the inner blood-retinal barrier and retinal electrophysiology through rapid ADAM17 activation claudin-5 degradation. Part. Fibre Toxicol. 2021, 18, 4. [Google Scholar] [CrossRef]
- Zhang, K.; Hopkins, J.J.; Heier, J.S.; Birch, D.G.; Halperin, L.S.; Albini, T.A.; Brown, D.M.; Jaffe, G.J.; Tao, W.; Williams, G.A. Ciliary neurotrophic factor delivered by encapsulated cell intraocular implants for treatment of geographic atrophy in age-related macular degeneration. Proc. Natl. Acad. Sci. USA 2011, 108, 6241–6245. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, L.; Weinreb, R.N. Ophthalmic drug discovery: Novel targets mechanisms for retinal diseases and glaucoma. Nat. Rev. Drug Discov. 2012, 11, 541–559. [Google Scholar] [CrossRef]
- Wong, F.S.; Tsang, K.K.; Lo, A.C. Delivery of therapeutics to posterior eye segment: Cell-encapsulating systems. Neural Regen. Res. 2017, 12, 576–577. [Google Scholar]
- Moiseev, R.V.; Morrison, P.W.J.; Steele, F.; Khutoryanskiy, V.V. Penetration Enhancers in Ocular Drug Delivery. Pharmaceutics 2019, 11, 321. [Google Scholar] [CrossRef] [PubMed]
- Kaur, I.P.; Smitha, R. Penetration Enhancers and Ocular Bioadhesives: Two New Avenues for Ophthalmic Drug Delivery. Drug Dev. Ind. Pharm. 2002, 28, 353–369. [Google Scholar] [CrossRef] [PubMed]
- Zambito, Y.; Di Colo, G. Chitosan and Its Derivatives as Intraocular Penetration Enhancers. J. Drug Deliv. Sci. Technol. 2010, 20, 45–52. [Google Scholar] [CrossRef]
- Bechara, C.; Sagan, S. Cell-Penetrating Peptides: 20 Years Later, Where Do We Stand? FEBS Lett. 2013, 587, 1693–1702. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lin, H.; Lin, S.; Qu, J.; Xiao, J.; Huang, Y.; Xiao, Y.; Fu, X.; Yang, Y.; Li, X. Cell-Penetrating Peptide TAT-Mediated Delivery of Acidic FGF to Retina and Protection against Ischemia-Reperfusion Injury in Rats. J. Cell. Mol. Med. 2010, 14, 1998–2005. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, Y.; Cheng, Y.; Tan, H.; Li, Z.; Qu, Y.; Mu, G.; Wang, F. Tat PTD-Endostatin: A Novel Anti-Angiogenesis Protein with Ocular Barrier Permeability via Eye-Drops. Biochim. Biophys. Acta Gen. Subj. 2015, 1850, 1140–1149. [Google Scholar] [CrossRef]
- Li, Y.; Li, L.; Li, Z.; Sheng, J.; Zhang, X.; Feng, D.; Zhang, X.; Yin, F.; Wang, A.; Wang, F. Tat PTD-Endostatin-RGD: A Novel Protein with Anti-Angiogenesis Effect in Retina via Eye Drops. Biochim. Biophys. Acta Gen. Subj. 2016, 1860, 2137–2147. [Google Scholar] [CrossRef]
- Chu, Y.; Chen, N.; Yu, H.; Mu, H.; He, B.; Hua, H.; Wang, A.; Sun, K. Topical Ocular Delivery to Laser-Induced Choroidal Neovascularization by Dual Internalizing RGD and TAT Peptide-Modified Nanoparticles. Int. J. Nanomed. 2017, 12, 1353–1368. [Google Scholar] [CrossRef]
- Atlasz, T.; Werling, D.; Song, S.; Szabo, E.; Vaczy, A.; Kovari, P.; Tamas, A.; Reglodi, D.; Yu, R. Retinoprotective Effects of TAT-Bound Vasoactive Intestinal Peptide and Pituitary Adenylate Cyclase Activating Polypeptide. J. Mol. Neurosci. 2019, 68, 397–407. [Google Scholar] [CrossRef]
- Liu, C.; Tai, L.; Zhang, W.; Wei, G.; Pan, W.; Lu, W. Penetratin, a Potentially Powerful Absorption Enhancer for Noninvasive Intraocular Drug Delivery. Mol. Pharm. 2014, 11, 1218–1227. [Google Scholar] [CrossRef]
- Liu, C.; Jiang, K.; Tai, L.; Liu, Y.; Wei, G.; Lu, W.; Pan, W. Facile Noninvasive Retinal Gene Delivery Enabled by Penetratin. ACS Appl. Mater. Interfaces 2016, 8, 19256–19267. [Google Scholar] [CrossRef]
- Jiang, K.; Gao, X.; Shen, Q.; Zhan, C.; Zhang, Y.; Xie, C.; Wei, G.; Lu, W. Discerning the Composition of Penetratin for Safe Penetration from Cornea to Retina. Acta Biomater. 2017, 63, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, L.; Li, L.; Han, M.; Tang, S.; Wang, T.; Han, J.; He, X.; He, X.; Wang, A.; et al. A Novel Dendrimer-Based Complex Co-Modified with Cyclic RGD Hexapeptide and Penetratin for Noninvasive Targeting and Penetration of the Ocular Posterior Segment. Drug Deliv. 2019, 26, 989–1001. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.N.; Cashman, S.M.; Kumar-Singh, R. Cell-Penetrating Peptide for Enhanced Delivery of Nucleic Acids and Drugs to Ocular Tissues Including Retina and Cornea. Mol. Ther. 2008, 16, 107–114. [Google Scholar] [CrossRef] [PubMed]
- De Cogan, F.; Hill, L.J.; Lynch, A.; Morgan-Warren, P.J.; Lechner, J.; Berwick, M.R.; Peacock, A.F.A.; Chen, M.; Scott, R.A.H.; Xu, H.; et al. Topical Delivery of Anti-VEGF Drugs to the Ocular Posterior Segment Using Cell-Penetrating Peptides. Investig. Ophthalmol. Vis. Sci. 2017, 58, 2578–2590. [Google Scholar] [CrossRef] [PubMed]
- Sigurdsson, H.H.; Konradsdottir, F.; Loftsson, T.; Stefansson, E. Topical and systemic absorption in delivery of dexamethasone to the anterior and posterior segments of the eye. Acta Ophthalmol. Scand. 2007, 85, 598–602. [Google Scholar] [CrossRef]
- Loftsson, T.; Hreinsdottir, D.; Stefansson, E. Cyclodextrin microparticles for drug delivery to the posterior segment of the eye: Aqueous dexamethasone eye drops. J. Pharm. Pharmacol. 2007, 59, 629–635. [Google Scholar] [CrossRef]
- Tanito, M.; Hara, K.; Takai, Y.; Matsuoka, Y.; Nishimura, N.; Jansook, P.; Loftsson, T.; Stéfansson, E.; Ohira, A. Topical Dexamethasone-Cyclodextrin Microparticle Eye Drops for Diabetic Macular Edema. Investig. Opthalmol. Vis. Sci. 2011, 52, 7944–7948. [Google Scholar]
- Ohira, A.; Hara, K.; Jóhannesson, G.; Tanito, M.; Ásgrímsdóttir, G.M.; Lund, S.H.; Loftsson, T.; Stéfansson, E. Topical dexamethasone γ-cyclodextrin nanoparticle eye drops increase visual acuity and decrease macular thickness in diabetic macular oedema. Acta Ophthalmol. 2015, 93, 610–615. [Google Scholar] [CrossRef]
- Shulman, S.; Jóhannesson, G.; Stefánsson, E.; Loewenstein, A.; Rosenblatt, A.; Habot-Wilner, Z. Topical dexamethasone-cyclodextrin nanoparticle eye drops for non-infectious uveitic macular oedema and vitritis—A pilot study. Acta Ophthalmol. 2015, 93, 411–415. [Google Scholar] [CrossRef]
- Krag, S.; Hessellund, A. Topical dexamethasone-cyclodextrin microparticle eye drops for uveitic macular oedema. Acta Ophthalmol. 2014, 92, e689–e690. [Google Scholar] [CrossRef]
- Mahaling, B.; Katti, D.S. Understanding the Influence of Surface Properties of Nanoparticles and Penetration Enhancers for Improving Bioavailability in Eye Tissues In Vivo. Int. J. Pharm. 2016, 501, 1–9. [Google Scholar] [CrossRef]
- Behar-Cohen, F.F.; El Aouni, A.; Gautier, S.; David, G.; Davis, J.; Chapon, P.; Parel, J.M. Transscleral Coulomb-controlled Iontophoresis of Methylprednisolone into the Rabbit Eye: Influence of Duration of Treatment, Current Intensity and Drug Concentration on Ocular Tissue and Fluid Levels. Exp. Eye Res. 2002, 74, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Güngör, S.; Delgado-Charro, M.B.; Ruiz-Perez, B.; Schubert, W.; Isom, P.; Moslemy, P.; Patane, M.A.; Guy, R.H. Trans-scleral iontophoretic delivery of low molecular weight therapeutics. J. Control. Release 2010, 147, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.E.; Assang, C.; Patane, M.A.; From, S.; Korenfeld, M.; Avion Study Investigators. Evaluation of dexamethasone phosphate delivered by ocular iontophoresis for treating noninfectious anterior uveitis. Ophthalmology 2012, 119, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Chopra, P.; Hao, J.; Li, S.K. Sustained release micellar carrier systems for iontophoretic transport of dexamethasone across human sclera. J. Control. Release 2012, 160, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Molokhia, S.A.; Jeong, E.K.; Higuchi, W.I.; Li, S.K. Examination of penetration routes distribution of ionic permeants during after transscleral iontophoresis with magnetic resonance imaging. Int. J. Pharm. 2007, 335, 46–53. [Google Scholar] [CrossRef]
- Souza, J.G.; Dias, K.; Pereira, T.A.; Spuri Bernardi, D.; Lopez, R.F.V. Topical delivery of ocular therapeutics: Carrier systems physical methods. J. Pharm. Pharmacol. 2014, 66, 507–530. [Google Scholar] [CrossRef]
- Dabral, K.; Uniyal, Y. Ocular inserts: Novel approach for drug delivery into eyes. GSC Biol. Pharm. Sci. 2019, 7, 001–007. [Google Scholar] [CrossRef]
- Kumari, A.; Sharma, P.K.; Garg, V.K.; Garg, G. Ocular inserts—Advancement in therapy of eye diseases. J. Adv. Pharm. Technol. Res. 2010, 1, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Thakur, R.; Swami, G. Promising implication of ocuserts in ocular disease. J. Drug Deliv. Ther. 2012, 2, 2. [Google Scholar] [CrossRef]
- Kearns, V.R.; Williams, R.L. Drug delivery systems for the eye. Expert Rev. Med. Devices 2009, 6, 277–290. [Google Scholar] [CrossRef]
- Lee, S.S.; Hughes, P.; Ross, A.D.; Robinson, M.R. Biodegradable Implants for Sustained Drug Release in the Eye. Pharm. Res. 2010, 27, 2043–2053. [Google Scholar] [CrossRef]
- Pelusi, L.; Mandatori, D.; Mastropasqua, L.; Agnifili, L.; Allegretti, M.; Nubile, M.; Pandolfi, A. Innovation in the Development of Synthetic and Natural Ocular Drug Delivery Systems for Eye Diseases Treatment: Focusing on Drug-Loaded Ocular Inserts, Contacts, and Intraocular Lenses. Pharmaceutics 2023, 15, 625. [Google Scholar] [CrossRef] [PubMed]
- Brandt, J.D.; Sall, K.; DuBiner, H.; Benza, R.; Alster, Y.; Walker, G.; Semba, C.P. Six-month intraocular pressure reduction with a topical bimatoprost ocular insert: Results of a phase II randomized controlled study. Ophthalmology 2016, 123, 1685–1694. [Google Scholar] [CrossRef]
- Manickavasagam, D.; Wehrung, D.; Chamsaz, E.A.; Sanders, M.; Bouhenni, R.; Crish, S.D.; Joy, A.; Oyewumi, M.O. Assessment of alkoxylphenacyl-based polycarbonates as a potential platform for controlled delivery of a model anti-glaucoma drug. Eur. J. Pharm. Biopharm. 2016, 107, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Franca, J.R.; Foureaux, G.; Fuscaldi, L.L.; Ribeiro, T.G.; Castilho, R.O.; Yoshida, I.M.; Cardoso, V.N.; Fernandes, S.O.A.; Cronemberg, S.; Nogueira, J.C.; et al. Chitosan/hydroxyethyl cellulose inserts for sustained-release of dorzolamide for glaucoma treatment: In vitro and in vivo evaluation. Int. J. Pharm. 2019, 570, 118662. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jiang, Y.-Y.; Lin, N. Promise of latanoprost and timolol loaded combinatorial nanosheet for therapeutic applications in glaucoma. J. King Saud Univ. Sci. 2020, 32, 1042–1047. [Google Scholar] [CrossRef]
- Dubald, M.; Bourgeois, S.; Andrieu, V.; Fessi, H. Ophthalmic Drug Delivery Systems for Antibiotherapy—A Review. Pharmaceutics 2018, 10, 10. [Google Scholar] [CrossRef]
- Terreni, E.; Burgalassi, S.; Chetoni, P.; Tampucci, S.; Zucchetti, E.; Fais, R.; Ghelardi, E.; Lupetti, A.; Monti, D. Development and Characterization of a Novel Peptide-Loaded Antimicrobial Ocular Insert. Biomolecules 2020, 10, 664. [Google Scholar] [CrossRef]
- Sadeghi, A.M.; Farjadian, F.; Alipour, S. Sustained release of linezolid in ocular insert based on lipophilic modified structure of sodium alginate. Iran. J. Basic Med. Sci. 2021, 24, 331–340. [Google Scholar]
- Grimaudo, M.A.; Nicoli, A.; Santi, P.; Concheiro, A.; Alvarez-Lorenzo, C. Cyclosporine-loaded cross-linked inserts of sodium hyaluronan and hydroxypropyl-beta-cyclodextrin for ocular administration. Carbohydr. Polym. 2018, 201, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Grimaudo, M.A.; Concheiro, A.; Alvarez-Lorenzo, C. Crosslinked Hyaluronan Electrospun Nanofibers for Ferulic Acid Ocular Delivery. Pharmaceutics 2020, 12, 274. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, G.; Mirzaeei, S.; Taghe, S.; Mohammadi, P. Preparation and Evaluation of EudragitI L100 Nanoparticles Loaded Impregnated with KT Tromethamine Loaded PVA -HEC Insertions for Ophthalmic Drug Delivery. Adv. Pharm. Bull. 2019, 9, 593–600. [Google Scholar] [CrossRef]
- Girgis, G.N.S. Formulation and Evaluation of Atorvastatin Calcium-Poly-epsilon-Caprolactone Nanoparticles Loaded Ocular Inserts for Sustained Release and Antiinflammatory Efficacy. Curr. Pharm. Biotechnol. 2020, 21, 1688–1698. [Google Scholar] [CrossRef] [PubMed]
- Bertens, C.J.; Martino, C.; van Osch, M.C.; Lataster, A.; Dias, A.J.; Biggelaar, F.J.V.D.; Tuinier, R.; Nuijts, R.M.; Gijs, M. Design of the ocular coil, a new device for non-invasive drug delivery. Eur. J. Pharm. Biopharm. 2020, 150, 120–130. [Google Scholar] [CrossRef]
- Bertens, C.J.F.; Gijs, M.; Dias, A.A.J.; Biggelaar, F.J.H.M.V.D.; Ghosh, A.; Sethu, S.; Nuijts, R.M.M.A. Pharmacokinetics and efficacy of a ketorolac-loaded ocular coil in New Zealand white rabbits. Drug Deliv. 2021, 28, 400–407. [Google Scholar] [CrossRef]
- Thakkar, S.; Misra, M. Electrospun polymeric nanofibers: New horizons in drug delivery. Eur. J. Pharm. Sci. 2017, 107, 148–167. [Google Scholar] [CrossRef]
- Singla, J.; Bajaj, T.; Goyal, A.K.; Rath, G. Development of Nanofibrous Ocular Insert for Retinal Delivery of Fluocinolone Acetonide. Curr. Eye Res. 2019, 44, 541–550. [Google Scholar] [CrossRef]
- Balguri, S.P.; Adelli, G.R.; Tatke, A.; Janga, K.Y.; Bhagav, P.; Majumdar, S. Melt-Cast Noninvasive Ocular Inserts for Posterior Segment Drug Delivery. J. Pharm. Sci. 2017, 106, 3515–3523. [Google Scholar] [CrossRef]
- Alambiaga-Caravaca, A.M.; Domenech-Monsell, I.M.; Sebastián-Morelló, M.; Calatayud-Pascual, M.A.; Merino, V.; Rodilla, V.; López-Castellano, A. Development, characterization, and ex vivo evaluation of an insert for the ocular administration of progesterone. Int. J. Pharm. 2021, 606, 120921. [Google Scholar] [CrossRef]
- Shastri, D.H.; Silva, A.C.; Almeida, H. Ocular Delivery of Therapeutic Proteins: A Review. Pharmaceutics 2023, 15, 205. [Google Scholar] [CrossRef] [PubMed]
- Ben-Arzi, A.; Ehrlich, R.; Neumann, R. Retinal Diseases: The Next Frontier in Pharmacodelivery. Pharmaceutics 2022, 14, 904. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.F.; Parks, D.J.; Mellow, S.D.; Ferris, F.L.; Walton, R.C.; Remaley, N.A.; Chew, E.Y.; Ashton, P.; Davis, M.D.; Nussenblatt, R.B. Treatment of cytomegalovirus retinitis with an intraocular sustained-release ganciclovir implant. A randomized controlled clinical trial. Arch. Ophthalmol. 1994, 112, 1531–1539. [Google Scholar] [CrossRef]
- Jaffe, G.J.; Martin, D.; Callanan, D.; Pearson, P.A.; Levy, B.; Comstock, T. Fluocinolone Acetonide Implant (Retisert) for Noninfectious Posterior Uveitis: Thirty-Four–Week Results of a Multicenter Randomized Clinical Study. Ophthalmology 2006, 113, 1020–1027. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.; Stinnett, S.S.; Jaffe, G.J. Prospective study of a fluocinolone acetonide implant for chronic macular edema from central retinal vein occlusion: Thirty-six-month results. Ophthalmology 2012, 119, 132–137. [Google Scholar] [CrossRef]
- Pearson, P.A.; Comstock, T.L.; Ip, M.; Callanan, D.; Morse, L.S.; Ashton, P.; Levy, B.; Mann, E.S.; Eliott, D. Fluocinolone Acetonide Intravitreal Implant for Diabetic Macular Edema: A 3-Year Multicenter, Randomized, Controlled Clinical Trial. Ophthalmology 2011, 118, 1580–1587. [Google Scholar] [CrossRef]
- Campochiaro, P.A.; Brown, D.M.; Pearson, A.; Chen, S.; Boyer, D.; Ruiz-Moreno, J.; Garretson, B.; Gupta, A.; Hariprasad, S.M.; Bailey, C.; et al. Sustained Delivery Fluocinolone Acetonide Vitreous Inserts Provide Benefit for at Least 3 Years in Patients with Diabetic Macular Edema. Ophthalmology 2012, 119, 2125–2132. [Google Scholar] [CrossRef]
- Pearce, W.; Hsu, J.; Yeh, S. Advances in drug delivery to the posterior segment. Curr. Opin. Ophthalmol. 2015, 26, 233–239. [Google Scholar] [CrossRef]
- Testi, I.; Pavesio, C. Preliminary evaluation of YUTIQ™ (fluocinolone acetonide intravitreal implant 0.18 mg) in posterior uveitis. Ther. Deliv. 2019, 10, 621–625. [Google Scholar] [CrossRef]
- Christoforidis, J.B.; Chang, S.; Jiang, A.; Wang, J.; Cebulla, C.M. Intravitreal devices for the treatment of vitreous inflammation. Mediat. Inflamm. 2012, 2012, 126463. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, A.; Joshi, M.; Christoforidis, J. Drug delivery implants in the treatment of vitreous inflammation. Mediat. Inflamm. 2013, 2013, 780634. [Google Scholar] [CrossRef] [PubMed]
- Ranade, S.V.; Wieland, M.R.; Tam, T.; Rea, J.C.; Horvath, J.; Hieb, A.R.; Jia, W.; Grace, L.; Barteselli, G.; Stewart, J.M. The Port Delivery System with ranibizumab: A new paradigm for long-acting retinal drug delivery. Drug Deliv. 2022, 29, 1326–1334. [Google Scholar] [CrossRef] [PubMed]
- Iovino, C.; Mastropasqua, R.; Lupidi, M.; Bacherini, D.; Pellegrini, M.; Bernabei, F.; Borrelli, E.; Sacconi, R.; Carnevali, A.; D’aloisio, R.; et al. Intravitreal Dexamethasone Implant as a Sustained Release Drug Delivery Device for the Treatment of Ocular Diseases: A Comprehensive Review of the Literature. Pharmaceutics 2020, 12, 703. [Google Scholar] [CrossRef] [PubMed]
- Mohan, S.; Ratra, D. Intravitreal Implants. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Gholamali, I.; Yadollahi, M. Doxorubicin-loaded carboxymethyl cellulose/Starch/ZnO nanocomposite hydrogel beads as an anticancer drug carrier agent. Int. J. Biol. Macromol. 2020, 160, 724–7355. [Google Scholar] [CrossRef]
- Sadasivam, R.; Packirisamy, G.; Goswami, M. Biocompatible soft hydrogel lens as topical implants for diabetic retinopathy. Mater. Lett. 2023, 318, 132174. [Google Scholar] [CrossRef]
- Tan, C.S.; Ngo, W.K.; Chay, I.W.; Ting, D.S.; Sadda, S.R. Neovascular Age-Related Macular Degeneration (nAMD): A Review of Emerging Treatment Options. Clin. Ophthalmol. 2022, 16, 917–933. [Google Scholar] [CrossRef]
- Zheng, C.; Xi, H.; Wen, D.; Ke, Y.; Zhang, X.; Ren, X.; Li, X. Biocompatibility and Efficacy of a Linearly Cross-Linked Sodium Hyaluronic Acid Hydrogel as a Retinal Patch in Rhegmatogenous Retinal Detachment Repairment. Front. Bioeng. Biotechnol. 2022, 10, 914675. [Google Scholar] [CrossRef]
- Vermonden, T.; Censi, R.; Hennink, W.E. Hydrogels for protein delivery. Chem. Rev. 2012, 112, 2853–2888. [Google Scholar] [CrossRef]
- Yu, Y.; Lau, L.C.; Lo, A.C.; Chau, Y. Injectable chemically crosslinked hydrogel for the controlled release of bevacizumab in vitreous: A 6-month in vivo study. Transl. Vis. Sci. Technol. 2015, 4, 5. [Google Scholar] [CrossRef]
- Buwalda, S.J.; Vermonden, T.; Hennink, W.E. Hydrogels for therapeutic delivery: Current developments and future directions. Biomacromolecules 2017, 18, 316–330. [Google Scholar] [CrossRef]
- Buwalda, S.J.; Bethry, A.; Hunger, S.; Kandoussi, S.; Coudane, J.; Nottelet, B. Ultrafast in situ forming poly(ethylene glycol)- poly(amido amine) hydrogels with tunable drug release properties via controllable degradation rates. Eur. J. Pharm. Biopharm. 2019, 139, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Bae, K.H.; Wang, L.S.; Kurisawa, M. Injectable biodegradable hydrogels: Progress and challenges. J. Mater. Chem. B 2013, 1, 5371. [Google Scholar] [CrossRef] [PubMed]
- Franssen, O.; Vandervennet, L.; Roders, P.; Hennink, W.E. Degradable dextran hydrogels: Controlled release of a model protein from cylinders and microspheres. J. Control. Release 1999, 60, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Ran, R.; Ma, Y.; Zhang, M. Polymeric hydrogel as a vitreous substitute: Current research, challenges, and future directions. Biomed. Mater. 2021, 16, 042012. [Google Scholar] [CrossRef]
- Censi, R.; Vermonden, T.; Van, M.J. Photopolymerized thermosensitive hydrogels for tailorable diffusion-controlled protein delivery. J. Control. Release 2009, 140, 230–236. [Google Scholar] [CrossRef]
- Ilochonwu, B.C.; Mihajlovic, M.; Maas-Bakker, R.F.; Rousou, C.; Tang, M.; Chen, M.; Hennink, W.E.; Vermonden, T. Hyaluronic Acid-PEG-Based Diels–Alder In Situ Forming Hydrogels for Sustained Intraocular Delivery of Bevacizumab. Biomacromolecules 2022, 23, 2914–2929. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Chen, M.; Liu, Y.; Zhang, D.; Shen, J.; Ni, N.; Tang, Z.; Ju, Y.; Dai, X.; Zhuang, A.; et al. Injectable Anti-Inflammatory Supramolecular Nanofiber Hydrogel to Promote Anti-VEGF Therapy in Age-Related Macular Degeneration Treatment. Adv. Mater. 2023, 35, e2204994. [Google Scholar] [CrossRef]
- Janet, T.; Stuart, W.; Kevin, H.; Gary, O.; Gabe, F.; Nicole, M.; Tomas, N.; Benjamin, M.; Benjamin, Y. In-vitro release of Bevacizumab from hydrogel-based drug delivery systems. Investig. Ophthalmol. Vis. Sci. 2015, 56, 222. [Google Scholar]
- Ilochonwu, B.C.; Urtti, A.; Hennink, W.E.; Vermonden, T. Intravitreal hydrogels for sustained release of therapeutic proteins. J. Control. Release 2020, 326, 419–441. [Google Scholar] [CrossRef]
- Wei, Y.; Alexandre, U.; Ma, X. Hydrogels to Support Transplantation of Human Embryonic Stem Cell-Derived Retinal Pigment Epithelial Cells. Brain Sci. 2022, 12, 1620. [Google Scholar] [CrossRef]
- Zheng, C.; Wen, D.; Xu, K.; Zhang, X.; Ren, X.; Li, X. Advances in biomaterials as a retinal patch for the repair of rhegmatogenous retinal detachment. Front. Bioeng. Biotechnol. 2022, 10, 997243. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Banworth, M.J.; Makkia, R.; Conley, S.M.; Al-Ubaidi, M.R.; Cooper, M.J.; Naash, M.I. Genomic DNA nanoparticles rescue rhodopsin-associated retinitis pigmentosa phenotype. FASEB J. 2015, 29, 2535–2544. [Google Scholar] [PubMed]
- Zheng, M.; Mitra, R.N.; Filonov, N.A.; Han, Z. Nanoparticle-mediated rhodopsin cDNA but not intron-containing DNA delivery causes transgene silencing in a rhodopsin knockout model. FASEB J. 2016, 30, 1076–1086. [Google Scholar] [CrossRef] [PubMed]
- Ottonelli, I.; Bighinati, A.; Adani, E.; Loll, F.; Caraffi, R.; Vandelli, M.A.; Boury, F.; Tosi, G.; Duskey, J.T.; Marigo, V.; et al. Optimization of an Injectable Hydrogel Depot System for the Controlled Release of Retinal-Targeted Hybrid Nanoparticles. Pharmaceutics 2023, 15, 25. [Google Scholar] [CrossRef] [PubMed]
- Osswald, C.R.; Kang-Mieler, J.J. Controlled and extended in vitro release of bioactive anti-vascular endothelial growth factors from a microsphere-hydrogel drug delivery system. Curr. Eye Res. 2016, 41, 1216–1222. [Google Scholar] [CrossRef]
- Kim, S.; Kang-Mieler, J.J.; Liu, W.; Wang, Z.; Yiu, G.; Teixeira, L.B.C.; Mieler, W.F.; Thomasy, S.M. Safety and Biocompatibility of Aflibercept-Loaded Microsphere Thermo-Responsive Hydrogel Drug Delivery System in a Nonhuman Primate Model. Transl. Vis. Sci. Technol. 2020, 9, 30. [Google Scholar] [CrossRef]
- Rudeen, K.M.; Liu, W.; Mieler, W.F.; Kang-Mieler, J.J. Simultaneous Release of Aflibercept and Dexamethasone from an Ocular Drug Delivery System. Curr. Eye Res. 2022, 47, 1034–1042. [Google Scholar] [CrossRef]
- Holgado, M.A.; Anguiano-Domínguez, A.; Martín-Banderas, L. Contact lenses as drug-delivery systems: A promising therapeutic tool. Lentes de contacto para vehiculizar principios activos: Una prometedora herramienta terapéutica. Arch. Soc. Española Oftalmol. (Engl. Ed.) 2020, 95, 24–33. [Google Scholar] [CrossRef]
- Peral, A.; Martinez-Aguila, A.; Pastrana, C.; Huete-Toral, F.; Carpena-Torres, C.; Carracedo, G. Contact lenses as a drug delivery system for glaucoma: A Review. Appl. Sci. 2020, 10, 151. [Google Scholar] [CrossRef]
- Stiler-Wyszyńska, S.; Golba, S.; Jurek-Suliga, J.; Kuczkowski, S. Review of the latest solutions in the use of contact lenses as controlled release systems for ophthalmic drugs. Polym Med. 2023; ahead of print. [Google Scholar] [CrossRef]
- Franco, P.; De Marco, I. Contact Lenses as Ophthalmic Drug Delivery Systems: A Review. Polymers 2021, 13, 1102. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, I.M.; Marques, C.S.; Oliveira, R.S.; Coelho, P.B.; Costa, P.C.; Ferreira, D.C. Sustained drug release by contact lenses for glaucoma treatment—A review. J. Control. Release 2015, 202, 76–82. [Google Scholar] [CrossRef] [PubMed]
- González-Chomón, C.; Silva, M.; Concheiro, A.; Alvarez-Lorenzo, C. Biomimetic contact lenses eluting olopatadine for allergic conjunctivitis. Acta Biomater. 2016, 41, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Malakooti, N.; Alexander, C.; Alvarez-Lorenzo, C. Imprinted contact lenses for sustained release of polymyxin B and related antimicrobial peptides. J. Pharm. Sci. 2015, 104, 3386–3394. [Google Scholar] [CrossRef] [PubMed]
- Varela-Garcia, A.; Gomez-Amoza, J.L.; Concheiro, A.; Alvarez-Lorenzo, C. Imprinted Contact Lenses for Ocular Administration of Antiviral Drugs. Polymers 2020, 12, 2026. [Google Scholar] [CrossRef]
- Phan, C.M.; Subbaraman, L.; Jones, L. Contact lenses for antifungal ocular drug delivery: A review. Expert Opin. Drug Deliv. 2014, 11, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Dixon, P.; Ghosh, T.; Mondal, K.; Konar, A.; Chauhan, A.; Hazra, S. Controlled delivery of pirfenidone through vitamin E-loaded contact lens ameliorates corneal inflammation. Drug Deliv. Transl. Res. 2018, 8, 1114–1126. [Google Scholar] [CrossRef]
- Torres-Luna, C.; Hu, N.; Tammareddy, T.; Domszy, R.; Yang, J.; Wang, N.S.; Yang, A. Extended delivery of non-steroidal anti-inflammatory drugs through contact lenses loaded with Vitamin E and cationic surfactants. Contact Lens Anterior Eye 2019, 42, 546–552. [Google Scholar] [CrossRef]
- Gade, S.K.; Nirmal, J.; Garg, P.; Venuganti, V.V.K. Corneal delivery of moxifloxacin and dexamethasone combination using drug-eluting mucoadhesive contact lens to treat ocular infections. Int. J. Pharm. 2020, 591, 120023. [Google Scholar] [CrossRef]
- De Guzman, L.M.C.; De Guzman, G.Q.; Borromeo, E.C. Brinzolamide-loaded soft contact lens for ophthalmic delivery. Ther. Deliv. 2022, 13, 233–247. [Google Scholar] [CrossRef]
- Dang, H.; Dong, C.; Zhang, L. Sustained latanoprost release from PEGylated solid lipid nanoparticle-laden soft contact lens to treat glaucoma. Pharm. Dev. Technol. 2022, 27, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Rivera, F.; Fernández-Villanueva, D.; Concheiro, A.; Alvarez-Lorenzo, C. α-Lipoic Acid in Soluplus(®) Polymeric Nanomicelles for Ocular Treatment of Diabetes-Associated Corneal Diseases. J. Pharm. Sci. 2016, 105, 2855–2863. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Rivera, F.; Concheiro, A.; Alvarez-Lorenzo, C. Epalrestat-loaded silicone hydrogels as contact lenses to address diabetic eye complications. Eur. J. Pharm. Biopharm. 2018, 122, 126–136. [Google Scholar] [CrossRef]
- Zaidun, N.H.; Thent, Z.C.; Latiff, A.A. Combating oxidative stress disorders with citrus flavonoid: Naringenin. Life Sci. 2018, 208, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.; Dowling, J.; Ryan, R.; McLoughlin, P.; Fitzhenry, L. Controlled release of naringenin from soft hydrogel contact lens: An investigation into lens critical properties and in vitro release. Int. J. Pharm. 2022, 621, 121793. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.E.; Bengani, L.C.; Tulsan, R.; Maidana, D.E.; Salvador-Culla, B.; Kobashi, H.; Kolovou, P.E.; Zhai, H.; Taghizadeh, K.; Kuang, L.; et al. Topical sustained drug delivery to the retina with a drug-eluting contact lens. Biomaterials 2019, 217, 119285. [Google Scholar] [CrossRef]
- Keith, C.; Anuj, C. Contact Lens Based Drug Delivery to the Posterior Segment via Iontophoresis in Cadaver Rabbit Eyes. Pharm. Res. 2019, 36, 87. [Google Scholar]
- Moffatt, K.; Wang, Y.; Singh, T.R.R.; Donnelly, R.F. Microneedles for enhanced transdermal and intraocular drug delivery. Curr. Opin. Pharmacol. 2017, 36, 14–21. [Google Scholar] [CrossRef]
- Gadziński, P.; Froelich, A.; Wojtyłko, M.; Białek, A.; Krysztofiak, J.; Osmałek, T. Microneedle-based ocular drug delivery systems—Recent advances and challenges. Beilstein J. Nanotechnol. 2022, 13, 1167–1184. [Google Scholar] [CrossRef]
- [Press Release] Bausch + Lomb and Clearside Biomedical Announce FDA Approval of XIPERE™ (Triamcinolone Acetonide Injectable Suspension) for Suprachoroidal Use for the Treatment of Macular Edema Associated with Uveitis. 2021. Available online: https://ir.clearsidebio.com/news-relea (accessed on 12 March 2023).
- Ciulla, T.; Yeh, S. Microinjection via the suprachoroidal space: A review of a novel mode of administration. Am. J. Manag. Care 2022, 28 (Suppl. 13), S243–S252. [Google Scholar] [CrossRef]
- Kansara, V.S.; Hancock, S.E.; Muya, L.W.; Ciulla, T.A. Suprachoroidal delivery enables targeting, localization and durability of small molecule suspensions. J. Control. Release 2022, 349, 1045–1051. [Google Scholar] [CrossRef] [PubMed]
- Roy, G.; Garg, P.; Venuganti, V.V.K. Microneedle scleral patch for minimally invasive delivery of triamcinolone to the posterior segment of eye. Int. J. Pharm. 2022, 612, 121305. [Google Scholar] [CrossRef] [PubMed]
- Amer, M.; Chen, R.K. Hydrogel-Forming Microneedle Arrays for Sustained and Controlled Ocular Drug Delivery. ASME J. Med. Diagn. 2020, 3, 041003. [Google Scholar] [CrossRef]
- Wu, Y.; Vora, L.K.; Wang, Y.; Adrianto, M.F.; Tekko, I.A.; Waite, D.; Donnelly, R.F.; Thakur, R.R.S. Long-acting nanoparticle-loaded bilayer microneedles for protein delivery to the posterior segment of the eye. Eur. J. Pharm. Biopharm. 2021, 165, 306–318. [Google Scholar] [CrossRef]
- Singh, R.R.T.; Tekko, I.; McAvoy, K.; McMillan, H.; Jones, D.; Donnelly, R.F. Minimally invasive microneedles for ocular drug delivery. Expert Opin. Drug Deliv. 2017, 14, 525–537. [Google Scholar] [CrossRef]
- Jones, A.T.; Sayers, E.J. Cell entry of cell penetrating peptides: Tales of tails wagging dogs. J. Control. Release 2012, 161, 582–591. [Google Scholar] [CrossRef]
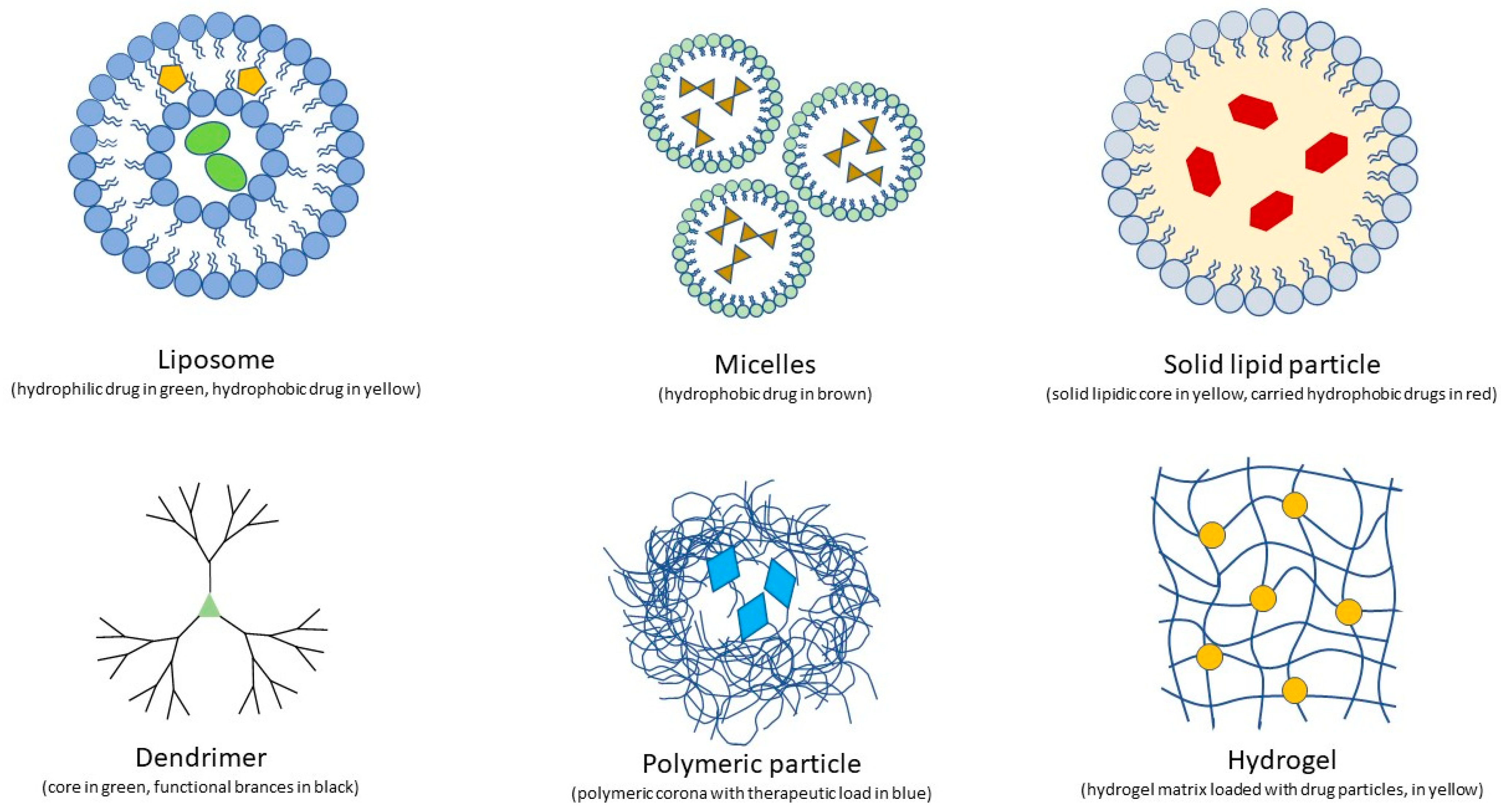
| Brief Description | Developmental Stage | Advantages | Disadvantages | ||
|---|---|---|---|---|---|
| Nanomedicine | Liposomes | Membrane-like vesicular structures carrying drugs across ocular barriers, e.g., Visudyne (Bausch&Lomb) to deliver verteporfin to the retina in nAMD Dexamethasone and tacrolimus also experimented with using liposomes to treat vitreoretinal inflammation and macular edema | Approved (Visudyne) |
|
|
| Nanomicelles | Amphiphilic monolayered vesicles carrying drugs. Topical preparations able to vehicle drugs to the retina via the conjunctival-scleral route in a safe and non-invasive way. Intravitreal administration is more invasive but may be useful to obtain sustained drug release (e.g., intravitreal pazopanib-loaded nanotubes) | Pre-clinical |
|
| |
| Nanospheres and nanocapsules | Self-assembling polymeric nanospheres and nanocapsules | Pre-clinical |
|
| |
| Solid lipids | Solid lipid core stabilized by surfactants. Stable, extremely resistant (autoclave), and economic | Pre-clinical |
|
| |
| Dendrimers | Ramified polymers consisting in a core with functional groups on its branches Intravitreal steroids, subconjunctival antiblastic, and topical antiglaucomatous molecules have been tested on animal models | Pre-clinical |
|
| |
| Organic nanopolymers | Chemical compounds acting as carrier for retinal drugs including dexamethasone and anti-VEGF, enhancing their bioavailability and targeting and prolonging their release | Pre-clinical |
|
| |
| Chitosan | Highly diffuse polysaccharide used for decades in pharmaceutics. Versatile penetration enhancer employed as a coating or component of different drug-loaded particles and drug delivery systems. | Approved products containing chitosan |
| ||
| Metals and other inorganic materials | Metallic nanoparticles with intrinsic therapeutic properties. Magnetism can be used to direct ab-externo metallic nanocarriers to target tissues (e.g., helical vectors containing iron and nickel) | Pre-clinical |
|
| |
| Encapsulated cells | Implantable polymeric scaffold containing human RPE cells secreting growth factors | Pre-clinical |
|
| |
| Topical drugs penetration enhancers | Cell-penetrating peptides | Short chains of peptides trespassing membranes. Studied on animal models of choroidal neovascularization, retinal neovascularization and oxygen-induced retinopathy | Pre-clinical |
| |
| Cyclodextrins | Oligosaccharides with a truncated cone-shape, more lipophilic centrally and a more hydrophilic on the outer surface, which can be used to aggregate with and augment the topical absorption of hydrophobic drugs | P2 |
|
| |
| Benzalkonium chloride | Enhancing penetration by breaking down tight junction of corneal epithelia. | Pre-clinical as enhancer for posterior segment disease treatment |
|
| |
| Iontophoresis | Application of low-amplitude electrical current to vehicle drugs across the conjunctival-scleral barrier | P1 (Visulex) Launched (EyeGate II) |
|
| |
| Sustained Drug-release Systems | Ocular inserts | Sterile, non-implantable, thin, multi-layered routes of administration with solid or semisolid consistency | Pre-clinical |
|
|
| Non-biodegradable Ocular Implants | Scleral and intrascleral (disc) implants and intravitreal implants (encapsulated cells) which do not experience change in structure and are made up of ethylene vinyl acetate, polyvinyl alcohol, polysulfone capillary fiber | Approved |
|
| |
| Biodegradable Ocular Implants | Injectable microparticles, intravitreal implant (injectable rod), intra-scleral and epi-scleral implant (disc, plug) which degrade and disintegrate over time. They are made up of polylactic acid, polyglycolic acid, PLGA, or polycaprolactones | Approved | |||
| Hydrogels | Three-dimensional network structures of crosslinked hydrophilic monomers which can deliver drugs via multiple administration routes such as topical administration, intracameral injection and intravitreal injection | Pre-clinical |
|
| |
| Contact lens | Contact lenses act as a drug-reservoir, incorporating functional monomers or nanoparticles in a polymeric matrix | Pre-clinical or approved |
|
| |
| Microneedles | Drug-loaded arrays of microneedles, inserted in sclera/suprachoroidal space | Pre-clinical or approved |
|
| |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gabai, A.; Zeppieri, M.; Finocchio, L.; Salati, C. Innovative Strategies for Drug Delivery to the Ocular Posterior Segment. Pharmaceutics 2023, 15, 1862. https://doi.org/10.3390/pharmaceutics15071862
Gabai A, Zeppieri M, Finocchio L, Salati C. Innovative Strategies for Drug Delivery to the Ocular Posterior Segment. Pharmaceutics. 2023; 15(7):1862. https://doi.org/10.3390/pharmaceutics15071862
Chicago/Turabian StyleGabai, Andrea, Marco Zeppieri, Lucia Finocchio, and Carlo Salati. 2023. "Innovative Strategies for Drug Delivery to the Ocular Posterior Segment" Pharmaceutics 15, no. 7: 1862. https://doi.org/10.3390/pharmaceutics15071862
APA StyleGabai, A., Zeppieri, M., Finocchio, L., & Salati, C. (2023). Innovative Strategies for Drug Delivery to the Ocular Posterior Segment. Pharmaceutics, 15(7), 1862. https://doi.org/10.3390/pharmaceutics15071862









