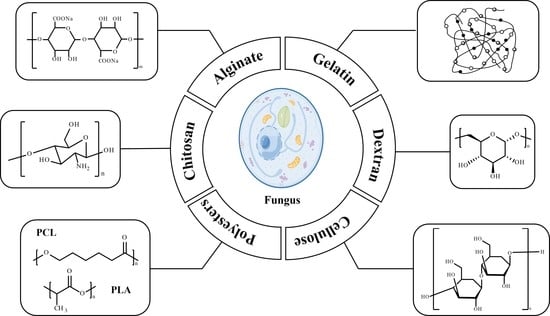
Emerging Polymer-Based Nanosystem Strategies in the Delivery of Antifungal Drugs
Abstract
1. Introduction
2. Polymers for Antifungal Drug Delivery Nanosystems
2.1. Chitosan
2.2. Alginate
| Loaded Drugs | Role of Chitosan | Other Components | Fungal | Zeta Potential (mV) | Diameters (nm) | Loading Content (LC) | Encapsulation Efficiency (EE) | Drug Release | PDI | Antifungal Efficacy In Vitro | Administration Route/In Vivo Study | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| O. syriacum essential oil (OSEO) and imidazolium-Zn(II)Salen | matrix | / | Candida albicans | +58.39 | 120.15 ± 62.65 | 22.41% | 35.17% | 80% (50 h) | 0.31–0.39 | ZOI: 29.48 ± 1.26 mm; MIC; 3.25–45.25 μg/mL | N/N | [47] |
| Iron oxide nanoparticles/chlorhexidine (CHX) | matrix | / | Candida albicans/Aspergillus flavus | +18.10 ± 0.82 | 33.6 ± 10.7 | / | / | / | 1.25 ± 0.06 | MIC: 400 μg/mL | topical administration/N | [49] |
| Cinnamic acid grafted CS | matrix | cinnamic acid grafted chitosan | M. canis | −69.74 | 263.0 ± 81.4 | / | 84.93% | / | / | inhibition: 53.96%, MIC: 200 μg/mL | vaginal administration/N | [45] |
| Metronidazol | coating layer | / | C. albicans | +10.6 ± 1.3 | 188.7 | / | 12 μg/mg | 63% (8 h) | / | MIC: 18 to 36 μg/mL | N/N | [61] |
| Fluconazole (Flu) | matrix | / | C. albicans | +3.36 | 82 | 60.2% | 78.7% | 8.12% (94 h) | / | MIC: 1.25 mg/mL, ZOI: 22.3 ± 1.6 mm | N/N | [46] |
| Ceftriaxone | matrix | / | / | +32 ± 2.4 | 56 | 54.37% | 79.43% | 8.12% (94 h) | / | ZOI: 19.5 ± 0.6 mm | N/N | [62] |
| Carvacrol | matrix | / | C. albicans, C. glabrata, C. krusei, C. tropicalis | / | 281.6 ± 2 | 25.5% | 56% | 50% (72 h) | 0.235 ± 0.03 | MIC: 24 μg/mL | N/N | [63] |
| AmB | matrix | / | C. albicans | +15.84 ± 1.41 | 174.47 ± 5.12 | 3.05 ± 0.13% | / | 80.6% (25 h) | 0.17 | MIC: 1 μg/mL | N/N | [64] |
2.3. Gelatin
2.4. Dextran
2.5. Cellulose
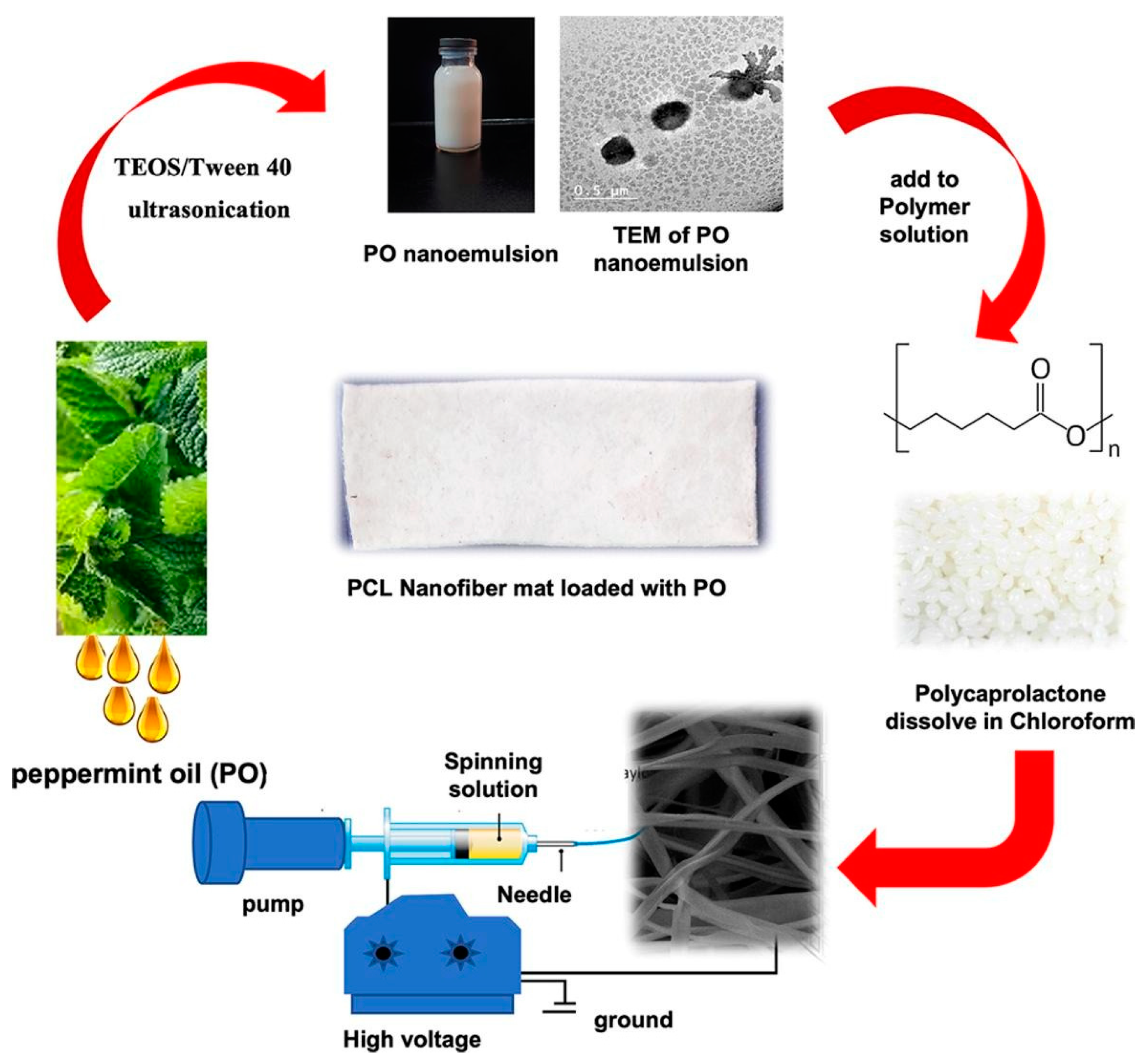
2.6. Polyesters
| Polymers/Role | Other Components | Loaded Drugs | Fungal | Zeta Potential (mV) | Diameters (nm) | Loading Content (LC) | Encapsulation Efficiency (EE) | Drug Release | PDI | Antifungal Efficacy In Vitro | Administration Route/In Vivo Study | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PLA/matrix | / | Bovine Lactoferrin | Aspergillus nidulans | / | 495 ± 127 | 20 wt% | / | 17.7 ± 4.4% (7 weeks) | / | Significantly inhibit mycelium growth | Antifungal dressings/N | [139] |
| PLA/coating layer | Mesoporous silica nanoparticles | Levofloxacin | Candida albicans | / | 5.4 | 33.3 wt% | 98.32% | 92% (280 min) | / | ZOI: 43 mm at 72 h | N/N | [141] |
| PLA/core | Polyacrylonitrile/cellulose | Chitin | Aspergillus niger | −10.5 ± 1.3 | 350–400 | 15 wt% | / | / | / | >99% for fungal spores (>2 µm) | N/N | [140] |
| PLA/matrix | Cellulose nanofibrils | Silver nanoparticles | Fusarium/Aspergillus/Curvularia | / | 1.44 ± 0.32 μm | <0.1 wt% | / | / | / | inhibition > 95% | Antifungal dressings/N | [137] |
| PLGA/matrix | / | Amp B | Candida albicans | −10.9 ± 1.9 | 343.17 ± 24.74 | 5.7% | 85% | 45.6% (48 h) | / | inhibition: 99.65% | Topical administration/Y | [133] |
| PLGA/matrix | / | Amp B | Candida albicans | −10.9 ± 1.9 | 287.8 ± 8.64 | 5.7 ± 0.12% | 85 ± 2.4% | / | 85 ± 2.4 | diffusion distance: 1.55 ± 0.11 μm | Topical administration/Y | [142] |
| PLA/matrix | / | Carvacrol | Candida albicans | 1.54 ± 1.07 μm | 28 wt% | / | 90% (150 h) | / | inhibition: 92–96% | Antifungal dressings/N | [136] | |
| PLA/matrix | PEG | Amp B | Candida albicans | / | 25.3 ± 2.7 | 40 mg/batch | 56.5 ± 3.9% | 59.4 ± 5.7% (24 h) | / | inhibition: 90.8% | Oral administration/Y | [143] |
| PLGA/core | / | Butenafine | Candida albicans, Aspergillus niger | −20.3 | 267.21 ± 3.54 | 1% | 72.43 ± 3.11% | 42.76 ± 2.87% (48 h) | 0.227 | ZOI: 20.54 ± 1.8 mm at 48 h | Topical administration/N | [144] |
| PLA/matrix | Cashew gum | Amp B | Candida albicans | −24.3 ± 2.3 | 1025 ± 143 | 9.1% | 89.7% | 52.2 ± 3.9% (168 h) | 0.307 | MIC: 0.25 μg/mL | Oral administration/N | [145] |
| PLA/matrix | / | Hypocrellin A | Candida auris | / | 699 | 2% | / | / | inhibition: 99.9% | Topical administration/Y | [138] | |
| PCL/matrix | Squalene | Squalene | Candida albicans | −48 ± 2.00 | 254 ± 6.81 | 30.98 ± 2.20% | 86.09 ± 0.28% | 85% (4 h) | 0.23 ± 3.03 | inhibition: 92.47% | Topical administration/Y | [146] |
| PCL/coating layer | / | Peppermint oil | Candida albicans/Aspergillus niger | / | / | / | / | / | / | ZOI: 20.6 mm at 48 h | Antifungal dressings/N | [147] |
| PCL/coating layer | / | Essential oils | Candida albicans | −11 ± 1 | 200 | 52 ± 3% | 84 ± 6% | / | 0.09 ± 0.02 | inhibition: 89% | N/N | [148] |
| PCL/matrix | / | 4-Nerolidylcatechol | Microsporum canis | −9.30 ± 0.17 | 143.5 ± 1.36 | / | 100% | / | 0.232 ± 0.00 | MIC: 0.625 μg/mL. MFC: 0.625 μg/mL. | Cutaneous administration/Y | [149] |
| PCL/coating layer | / | Miconazole nitrate | Candida albicans | –31.22 ± 2.1 | 89 ± 3.63 | 24.1 ± 0.65% | 98 ± 5.21% | 90% (48 h) | 0.35 | MIC: 0.75 μg/mL | Cutaneous administration/N | [150] |
| PCL/matrix | / | Diphenyl diselenide | Candida albicans | −10.1 ± 2.21 | 240 ± 52 | 5.07 ± 0.14 mg/g | 98% | / | 0.17 ± 0.08 | MIC: 0.5 μg/mL | Cutaneous administration/Y | [151] |
| PCL/matrix | / | Amp B | / | 0 | 183 | 5 mg/mL | 86% | 78% (48 h) | 0.211 | / | N/N | [152] |
| PCL/matrix | Pluronic | Chloramphenicol | Candida | −22.4 | 123.5 | / | 98.3% | 88% (96 h) | / | MIC: 2 μg/mL | Antifungal dressings/Y | [153] |
| PCL/coating layer | Polyethyleglicol | Am B | Albicans/Glabrata/Auris | −8.8 ± 0.1 | 226 | 16.40 ± 0.18 wt% | / | 38% (100 h) | 0.25 | MIC: 0.11 μg/mL | N/N | [154] |
3. Summary and Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Köhler, J.R.; Casadevall, A.; Perfect, J. The spectrum of fungi that infects humans. Cold Spring Harb. Perspect. Med. 2014, 5, a019273. [Google Scholar] [CrossRef]
- American Academy of Microbiology. American Academy of Microbiology. American Academy of Microbiology Colloquia Reports. In One Health: Fungal Pathogens of Humans, Animals, and Plants: Report on an American Academy of Microbiology Colloquium held in Washington, DC, USA, on 18 October 2017; American Society for Microbiology: Washington, DC, USA, 2019. [Google Scholar]
- Denning, D.W.; Kneale, M.; Sobel, J.D.; Rautemaa-Richardson, R. Global burden of recurrent vulvovaginal candidiasis: A systematic review. Lancet Infect. Dis. 2018, 18, e339–e347. [Google Scholar] [CrossRef]
- Clancy, C.J.; Nguyen, M.H. Emergence of Candida auris: An International Call to Arms. Clin. Infect. Dis. 2016, 64, 141–143. [Google Scholar] [CrossRef]
- Chan, J.F.; Lau, S.K.; Yuen, K.Y.; Woo, P.C. Talaromyces (Penicillium) marneffei infection in non-HIV-infected patients. Emerg. Microbes Infect. 2016, 5, e19. [Google Scholar] [CrossRef]
- Latgé, J.P.; Chamilos, G. Aspergillus fumigatus and Aspergillosis in 2019. Clin. Microbiol. Rev. 2019, 33, e00140-18. [Google Scholar] [CrossRef] [PubMed]
- McCarty, T.P.; Pappas, P.G. Invasive Candidiasis. Infect. Dis. Clin. North Am. 2016, 30, 103–124. [Google Scholar] [CrossRef] [PubMed]
- Binder, U.; Maurer, E.; Lass-Flörl, C. Mucormycosis—From the pathogens to the disease. Clin. Microbiol. Infect. 2014, 20, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Rokas, A. Evolution of the human pathogenic lifestyle in fungi. Nat. Microbiol. 2022, 7, 607–619. [Google Scholar] [CrossRef]
- Du, W.; Gao, Y.; Liu, L.; Sai, S.; Ding, C. Striking Back against Fungal Infections: The Utilization of Nanosystems for Antifungal Strategies. Int. J. Mol. Sci. 2021, 22, 10104. [Google Scholar] [CrossRef]
- Dellenbach, P.; Thomas, J.L.; Guerin, V.; Ochsenbein, E.; Contet-Audonneau, N. Topical treatment of vaginal candidosis with sertaconazole and econazole sustained-release suppositories. Int. J. Gynecol. Obstet. 2000, 71, 47–52. [Google Scholar] [CrossRef]
- Gayam, V.; Khalid, M.; Dahal, S.; Garlapati, P.; Gill, A. Hyperacute liver injury following intravenous fluconazole: A rare case of dose-independent hepatotoxicity. J. Fam. Med. Prim. Care 2018, 7, 451. [Google Scholar] [CrossRef] [PubMed]
- Young, G.A.R.; Bosly, A.; Gibbs, D.L.; Durrant, S. A double-blind comparison of fluconazole and nystatin in the prevention of candidiasis in patients with leukaemia. Eur. J. Cancer 1999, 35, 1208–1213. [Google Scholar] [CrossRef] [PubMed]
- Torre, P.d.l.; Meyer, D.K.; Reboli, A.C. Anidulafungin: A novel echinocandin for candida infections. Future Microbiol. 2008, 3, 593–601. [Google Scholar] [CrossRef]
- Singal, A. Butenafine and superficial mycoses: Current status. Expert Opin. Drug Metab. Toxicol. 2008, 4, 999–1005. [Google Scholar] [CrossRef]
- Gupta, A.K.; Stec, N.; Summerbell, R.C.; Shear, N.H.; Piguet, V.; Tosti, A.; Piraccini, B.M. Onychomycosis: A review. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 1972–1990. [Google Scholar] [CrossRef]
- Ryder, N.S. Squalene epoxidase as a target for the allylamines. Biochem. Soc. Trans. 1991, 19, 774–777. [Google Scholar] [CrossRef]
- Crawford, F.; Hollis, S. Topical treatments for fungal infections of the skin and nails of the foot. Cochrane Database Syst. Rev. 2007, 3, CD001434. [Google Scholar] [CrossRef]
- Mohd-Assaad, N.; McDonald, B.A.; Croll, D. Multilocus resistance evolution to azole fungicides in fungal plant pathogen populations. Mol. Ecol. 2016, 25, 6124–6142. [Google Scholar] [CrossRef]
- Mohr, J.; Johnson, M.; Cooper, T.; Lewis, J.S.; Ostrosky-Zeichner, L. Current Options in Antifungal Pharmacotherapy. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2008, 28, 614–645. [Google Scholar] [CrossRef] [PubMed]
- Roemer, T.; Krysan, D.J. Antifungal drug development: Challenges, unmet clinical needs, and new approaches. Cold Spring Harb. Perspect. Med. 2014, 4, a019703. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Xie, H. Nanoparticles in Daily Life: Applications, Toxicity and Regulations. J. Environ. Pathol. Toxicol. Oncol. 2018, 37, 209–230. [Google Scholar] [CrossRef] [PubMed]
- Mu, L.; Feng, S.S. A novel controlled release formulation for the anticancer drug paclitaxel (Taxol®): PLGA nanoparticles containing vitamin E TPGS. J. Control. Release 2003, 86, 33–48. [Google Scholar] [CrossRef] [PubMed]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007, 2, 751–760. [Google Scholar] [CrossRef]
- Choi, H.; Lee, D.G. Lycopene induces apoptosis in Candida albicans through reactive oxygen species production and mitochondrial dysfunction. Biochimie 2015, 115, 108–115. [Google Scholar] [CrossRef]
- Erdoğar, N.; Akkın, S.; Bilensoy, E. Nanocapsules for Drug Delivery: An Updated Review of the Last Decade. Recent Pat. Drug Deliv. Formul. 2018, 12, 252–266. [Google Scholar] [CrossRef]
- Panahi, Y.; Farshbaf, M.; Mohammadhosseini, M.; Mirahadi, M.; Khalilov, R.; Saghfi, S.; Akbarzadeh, A. Recent advances on liposomal nanoparticles: Synthesis, characterization and biomedical applications. Artif. Cells Nanomed. Biotechnol. 2017, 45, 788–799. [Google Scholar] [CrossRef]
- Farrag, Y.; Ide, W.; Montero, B.; Rico, M.; Rodríguez-Llamazares, S.; Barral, L.; Bouza, R. Preparation of starch nanoparticles loaded with quercetin using nanoprecipitation technique. Int. J. Biol. Macromol. 2018, 114, 426–433. [Google Scholar] [CrossRef]
- Asefa, T.; Tao, Z. Biocompatibility of Mesoporous Silica Nanoparticles. Chem. Res. Toxicol. 2012, 25, 2265–2284. [Google Scholar] [CrossRef] [PubMed]
- Frank, L.A.; Contri, R.V.; Beck, R.C.; Pohlmann, A.R.; Guterres, S.S. Improving drug biological effects by encapsulation into polymeric nanocapsules. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnology 2015, 7, 623–639. [Google Scholar] [CrossRef] [PubMed]
- Negi, P.; Singh, A.; Pundir, S.; Parashar, A.; Upadhyay, N.; Agarwal, S.; Chauhan, R.; Tambuwala, M.M. Essential oil and nanocarrier-based formulations approaches for vaginal candidiasis. Ther. Deliv. 2023, 14, 207–225. [Google Scholar] [CrossRef]
- Araujo, V.H.S.; Duarte, J.L.; Carvalho, G.C.; Silvestre, A.L.P.; Fonseca-Santos, B.; Marena, G.D.; Ribeiro, T.d.C.; dos Santos Ramos, M.A.; Bauab, T.M.; Chorilli, M. Nanosystems against candidiasis: A review of studies performed over the last two decades. Crit. Rev. Microbiol. 2020, 46, 508–547. [Google Scholar] [CrossRef]
- Bangia, R.; Sharma, G.; Dogra, S.; Katare, O.P. Nanotechnological interventions in dermatophytosis: From oral to topical, a fresh perspective. Expert Opin. Drug Deliv. 2019, 16, 377–396. [Google Scholar] [CrossRef] [PubMed]
- Nami, S.; Aghebati-Maleki, A.; Aghebati-Maleki, L. Current applications and prospects of nanoparticles for antifungal drug delivery. EXCLI J. 2021, 20, 562–584. [Google Scholar] [CrossRef] [PubMed]
- Voltan, A.; Quindós, G.; Alarcón, K.; Fusco-Almeida, A.M.; Mendes-Giannini, M.J.; Chorilli, M. Fungal diseases: Could nanostructured drug delivery systems be a novel paradigm for therapy? Int. J. Nanomed. 2016, 11, 3715–3730. [Google Scholar] [CrossRef] [PubMed]
- Perinelli, D.R.; Fagioli, L.; Campana, R.; Lam, J.K.W.; Baffone, W.; Palmieri, G.F.; Casettari, L.; Bonacucina, G. Chitosan-based nanosystems and their exploited antimicrobial activity. Eur. J. Pharm. Sci. 2018, 117, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Calvo, N.L.; Sreekumar, S.; Svetaz, L.A.; Lamas, M.C.; Moerschbacher, B.M.; Leonardi, D. Design and Characterization of Chitosan Nanoformulations for the Delivery of Antifungal Agents. Int. J. Mol. Sci. 2019, 20, 3686. [Google Scholar] [CrossRef] [PubMed]
- Almawash, S. Solid lipid nanoparticles, an effective carrier for classical antifungal drugs. Saudi Pharm. J. 2023, 31, 1167–1180. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, N.C.; de Sá, L.L.F.; de Carvalho, A.L.M. Nanostructured Lipid Carriers as a Novel Strategy for Topical Antifungal Therapy. AAPS PharmSciTech 2021, 23, 32. [Google Scholar] [CrossRef]
- Abbas, H.S.; Krishnan, A. Magnetic Nanosystems as a Therapeutic Tool to Combat Pathogenic Fungi. Adv. Pharm. Bull. 2020, 10, 512–523. [Google Scholar] [CrossRef]
- Liu, R.; Zhao, J.; Han, Q.; Hu, X.; Wang, D.; Zhang, X.; Yang, P. One-Step Assembly of a Biomimetic Biopolymer Coating for Particle Surface Engineering. Adv. Mater. 2018, 30, 1802851. [Google Scholar] [CrossRef]
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H.; Chan, W.Y. Chitosan: An Update on Potential Biomedical and Pharmaceutical Applications. Mar. Drugs 2015, 13, 5156–5186. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wu, X.; Quan, L.; Ao, Q. Characteristics of Marine Biomaterials and Their Applications in Biomedicine. Mar. Drugs 2022, 20, 372. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.C.; Cox, B.G. Kinetics of Amide Formation through Carbodiimide/N-Hydroxybenzotriazole (HOBt) Couplings. J. Org. Chem. 2007, 72, 8863–8869. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, S.Y.B.; Almeida, R.R.; Pinto, N.A.R.; de Mayrinck, C.; Vieira, S.S.; Haddad, J.F.; Leitão, A.A.; Guimarães, L.G.d.L. Encapsulation of essential oils using cinnamic acid grafted chitosan nanogel: Preparation, characterization and antifungal activity. Int. J. Biol. Macromol. 2021, 166, 902–912. [Google Scholar] [CrossRef]
- El Rabey, H.A.; Almutairi, F.M.; Alalawy, A.I.; Al-Duais, M.A.; Sakran, M.I.; Zidan, N.S.; Tayel, A.A. Augmented control of drug-resistant Candida spp. via fluconazole loading into fungal chitosan nanoparticles. Int. J. Biol. Macromol. 2019, 141, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Hassan, Y.A.; Khedr, A.I.M.; Alkabli, J.; Elshaarawy, R.F.M.; Nasr, A.M. Co-delivery of imidazolium Zn(II)salen and Origanum Syriacum essential oil by shrimp chitosan nanoparticles for antimicrobial applications. Carbohydr. Polym. 2021, 260, 117834. [Google Scholar] [CrossRef]
- Liu, Q.; Gao, L.; Qin, Y.; Ji, N.; Dai, L.; Xiong, L.; Sun, Q. Incorporation of oxidized debranched starch/chitosan nanoparticles for enhanced hydrophobicity of corn starch films. Food Packag. Shelf Life 2023, 35, 101032. [Google Scholar] [CrossRef]
- Ma, S.; Moser, D.; Han, F.; Leonhard, M.; Schneider-Stickler, B.; Tan, Y. Preparation and antibiofilm studies of curcumin loaded chitosan nanoparticles against polymicrobial biofilms of Candida albicans and Staphylococcus aureus. Carbohydrate Polymers 2020, 241, 116254. [Google Scholar] [CrossRef]
- Biao, L.; Tan, S.; Wang, Y.; Guo, X.; Fu, Y.; Xu, F.; Zu, Y.; Liu, Z. Synthesis, characterization and antibacterial study on the chitosan-functionalized Ag nanoparticles. Mater. Sci. Eng. C 2017, 76, 73–80. [Google Scholar] [CrossRef]
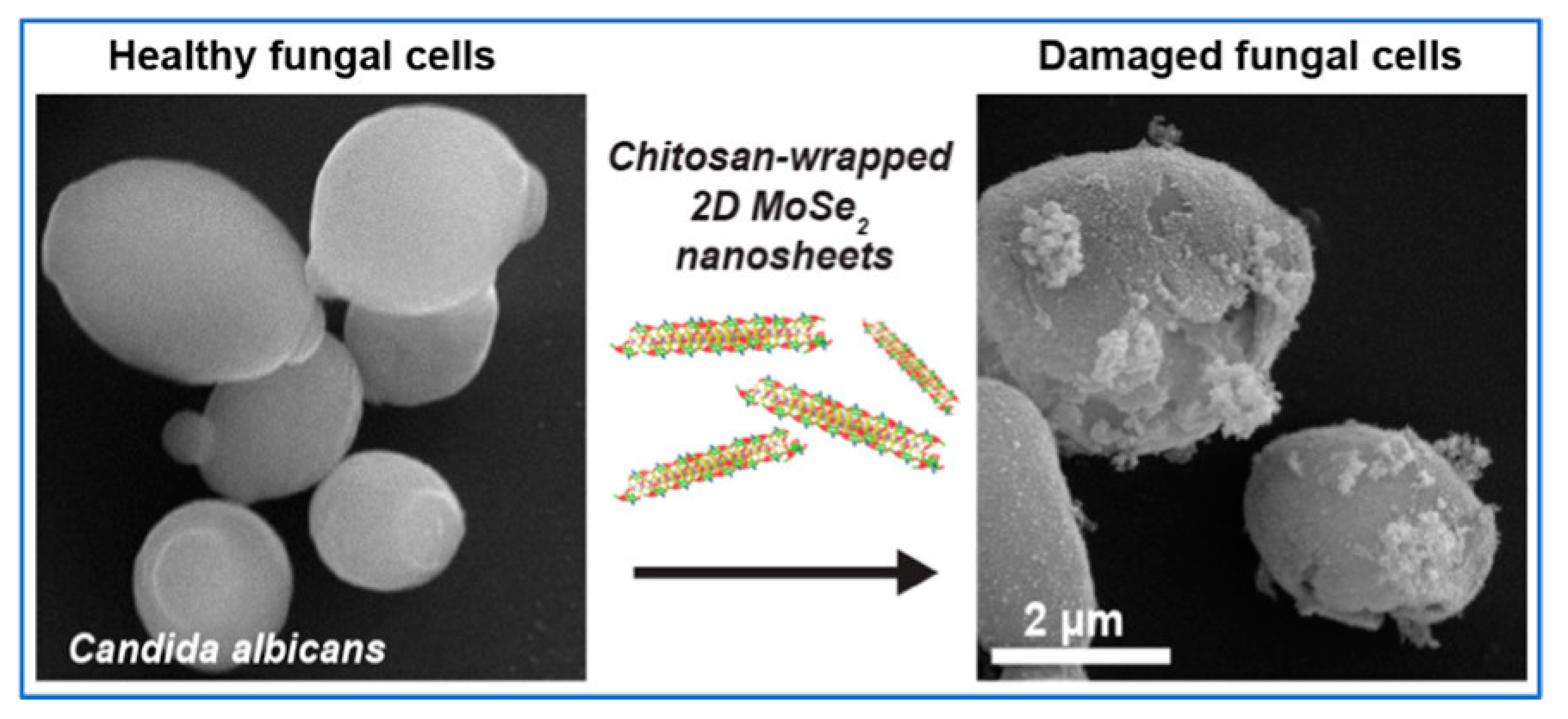
- Saha, S.; Gilliam, M.S.; Wang, Q.H.; Green, A.A. Eradication of Fungi Using MoSe2/Chitosan Nanosheets. ACS Appl. Nano Mater. 2022, 5, 133–148. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Hosseini, H.; Mohammadifar, M.A.; Mortazavian, A.M.; Mohammadi, A.; Khosravi-Darani, K.; Shojaee-Aliabadi, S.; Dehghan, S.; Khaksar, R. Incorporation of essential oil in alginate microparticles by multiple emulsion/ionic gelation process. Int. J. Biol. Macromol. 2013, 62, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Paques, J.P.; van der Linden, E.; van Rijn, C.J.M.; Sagis, L.M.C. Preparation methods of alginate nanoparticles. Adv. Colloid Interface Sci. 2014, 209, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Cheng, J.; Ao, Q. Preparation of Alginate-Based Biomaterials and Their Applications in Biomedicine. Mar. Drugs 2021, 19, 264. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Zhou, J.; An, Y.; Li, M.; Zhang, J.; Yang, S. Modification, 3D printing process and application of sodium alginate based hydrogels in soft tissue engineering: A review. Int. J. Biol. Macromol. 2023, 232, 123450. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Y.; He, J.; Sun, C.; Lu, W.; Zhang, Y.; Fang, Y. Doubling growth of egg-box structure during Calcium-mediated molecular assembly of alginate. J. Colloid Interface Sci. 2023, 634, 747–756. [Google Scholar] [CrossRef]
- Zheng, J.; Han, Y.; Wei, L.; Li, M.; Zhu, L. Sludge-derived biopolymers for in-situ synthesis of magnetic ALE-Fe-Zr composites for phosphate removal. Chem. Eng. J. 2023, 456, 140842. [Google Scholar] [CrossRef]
- Augustine, R.; Rajarathinam, K. Synthesis and characterization of silver nanoparticles and its immobilization on alginate coated sutures for the prevention of surgical wound infections and the in vitro release studies. Int. J. Nanodimensions 2012, 2, 205–212. [Google Scholar]
- Maestrelli, F.; Jug, M.; Cirri, M.; Kosalec, I.; Mura, P. Characterization and microbiological evaluation of chitosan-alginate microspheres for cefixime vaginal administration. Carbohydr. Polym. 2018, 192, 176–183. [Google Scholar] [CrossRef]
- Abid, S.; Uzair, B.; Niazi, M.B.K.; Fasim, F.; Bano, S.A.; Jamil, N.; Batool, R.; Sajjad, S. Bursting the Virulence Traits of MDR Strain of Candida albicans Using Sodium Alginate-based Microspheres Containing Nystatin-loaded MgO/CuO Nanocomposites. Int. J. Nanomed. 2021, 16, 1157–1174. [Google Scholar] [CrossRef]
- Andersen, T.; Mishchenko, E.; Flaten, G.E.; Sollid, J.U.E.; Mattsson, S.; Tho, I.; Škalko-Basnet, N. Chitosan-Based Nanomedicine to Fight Genital Candida Infections: Chitosomes. Mar. Drugs 2017, 15, 64. [Google Scholar] [CrossRef]
- Alshubaily, F.A.; Al-Zahrani, M.H. Appliance of fungal chitosan/ceftriaxone nano-composite to strengthen and sustain their antimicrobial potentiality against drug resistant bacteria. Int. J. Biol. Macromol. 2019, 135, 1246–1251. [Google Scholar] [CrossRef]
- Vitali, A.; Stringaro, A.; Colone, M.; Muntiu, A.; Angiolella, L. Antifungal Carvacrol Loaded Chitosan Nanoparticles. Antibiotics 2022, 11, 11. [Google Scholar] [CrossRef]
- Tan, Y.; Ma, S.; Ding, T.; Ludwig, R.; Lee, J.; Xu, J. Enhancing the Antibiofilm Activity of β-1,3-Glucanase-Functionalized Nanoparticles Loaded with Amphotericin B Against Candida albicans Biofilm. Front. Microbiol. 2022, 13, 815091. [Google Scholar] [CrossRef]
- Martín, M.J.; Calpena, A.C.; Fernández, F.; Mallandrich, M.; Gálvez, P.; Clares, B. Development of alginate microspheres as nystatin carriers for oral mucosa drug delivery. Carbohydr. Polym. 2015, 117, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Sarmento, B.; Ribeiro, A.; Veiga, F.; Sampaio, P.; Neufeld, R.; Ferreira, D. Alginate/chitosan nanoparticles are effective for oral insulin delivery. Pharm. Res. 2007, 24, 2198–2206. [Google Scholar] [CrossRef] [PubMed]
- Bhosale, V.A.; Srivastava, V.; Valamla, B.; Yadav, R.; Singh, S.B.; Mehra, N.K. Preparation and Evaluation of Modified Chitosan Nanoparticles Using Anionic Sodium Alginate Polymer for Treatment of Ocular Disease. Pharmaceutics 2022, 14, 2802. [Google Scholar] [CrossRef] [PubMed]
- Spadari, C.d.C.; de Bastiani, F.W.M.d.S.; Lopes, L.B.; Ishida, K. Alginate nanoparticles as non-toxic delivery system for miltefosine in the treatment of candidiasis and cryptococcosis. Int. J. Nanomed. 2019, 14, 5187–5199. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, S.; Yang, Y.; Tong, Z.; Shen, T.; Yu, Z.; Xie, J.; Yao, Y.; Gao, B.; Li, Y.C.; et al. Development of multifunctional copper alginate and bio-polyurethane bilayer coated fertilizer: Controlled-release, selenium supply and antifungal. Int. J. Biol. Macromol. 2023, 224, 256–265. [Google Scholar] [CrossRef]
- Ahmed, T.A.; Alzahrani, M.M.; Sirwi, A.; Alhakamy, N.A. Study the Antifungal and Ocular Permeation of Ketoconazole from Ophthalmic Formulations Containing Trans-Ethosomes Nanoparticles. Pharmaceutics 2021, 13, 151. [Google Scholar] [CrossRef]
- Abdelghany, S.; Alkhawaldeh, M.; AlKhatib, H.S. Carrageenan-stabilized chitosan alginate nanoparticles loaded with ethionamide for the treatment of tuberculosis. J. Drug Deliv. Sci. Technol. 2017, 39, 442–449. [Google Scholar] [CrossRef]
- Begines, B.; Ortiz, T.; Pérez-Aranda, M.; Martínez, G.; Merinero, M.; Argüelles-Arias, F.; Alcudia, A. Polymeric Nanoparticles for Drug Delivery: Recent Developments and Future Prospects. Nanomaterials 2020, 10, 1403. [Google Scholar] [CrossRef]
- Germovsek, E.; Barker, C.I.; Sharland, M. What do I need to know about aminoglycoside antibiotics? Arch. Dis. Child.-Educ. Pract. 2017, 102, 89. [Google Scholar] [CrossRef]
- Zavascki, A.P.; Goldani, L.Z.; Li, J.; Nation, R.L. Polymyxin B for the treatment of multidrug-resistant pathogens: A critical review. J. Antimicrob. Chemother. 2007, 60, 1206–1215. [Google Scholar] [CrossRef]
- Dhand, C.; Venkatesh, M.; Barathi, V.A.; Harini, S.; Bairagi, S.; Goh Tze Leng, E.; Muruganandham, N.; Low, K.Z.W.; Fazil, M.H.U.T.; Loh, X.J.; et al. Bio-inspired crosslinking and matrix-drug interactions for advanced wound dressings with long-term antimicrobial activity. Biomaterials 2017, 138, 153–168. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, H.M.; Taha, G.M.; El-Alfy, E.A.; El-Bisi, M.K. Enhancing antibacterial action of gauze by adding gelatin nanoparticles loaded with spectinomycin and chloramphenicol. Cellulose 2022, 29, 5677–5688. [Google Scholar] [CrossRef]
- Aparna, V.; Melge, A.R.; Rajan, V.K.; Biswas, R.; Jayakumar, R.; Gopi Mohan, C. Carboxymethylated ɩ-carrageenan conjugated amphotericin B loaded gelatin nanoparticles for treating intracellular Candida glabrata infections. Int. J. Biol. Macromol. 2018, 110, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Ilhan, E.; Cesur, S.; Sulutas, R.B.; Pilavci, E.; Dalbayrak, B.; Kaya, E.; Arisan, E.D.; Tinaz, G.B.; Sengor, M.; Kijeńska-Gawrońska, E.; et al. The Role of Multilayer Electrospun Poly(Vinyl Alcohol)/Gelatin nanofibers loaded with Fluconazole and Cinnamaldehyde in the Potential Treatment of Fungal Keratitis. Eur. Polym. J. 2022, 176, 111390. [Google Scholar] [CrossRef]
- Asgari, Q.; Alishahi, M.; Davani, F.; Caravan, D.; Khorram, M.; Enjavi, Y.; Barzegar, S.; Esfandiari, F.; Zomorodian, K. Fabrication of amphotericin B-loaded electrospun core–shell nanofibers as a novel dressing for superficial mycoses and cutaneous leishmaniasis. Int. J. Pharm. 2021, 606, 120911. [Google Scholar] [CrossRef]
- Ambrosio, J.A.R.; Pinto, B.C.d.S.; Godoy, D.d.S.; Carvalho, J.A.; Abreu, A.d.S.; da Silva, B.G.M.; Leonel, L.d.C.; Costa, M.S.; Beltrame Junior, M.; Simioni, A.R. Gelatin nanoparticles loaded methylene blue as a candidate for photodynamic antimicrobial chemotherapy applications in Candida albicans growth. J. Biomater. Sci. Polym. Ed. 2019, 30, 1356–1373. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Fontana, F.; Shahbazi, M.A.; Santos, H.A. Acetalated dextran based nano-and microparticles: Synthesis, fabrication, and therapeutic applications. Chem. Commun. 2021, 57, 4212–4229. [Google Scholar] [CrossRef]
- Hernández-Rivas, M.; Guzmán, E.; Fernández-Peña, L.; Akanno, A.; Greaves, A.; Léonforte, F.; Ortega, F.; Rubio, R.G.; Luengo, G.S. Deposition of Synthetic and Bio-Based Polycations onto Negatively Charged Solid Surfaces: Effect of the Polymer Cationicity, Ionic Strength, and the Addition of an Anionic Surfactant. Colloids Interfaces 2020, 4, 33. [Google Scholar] [CrossRef]
- Sagitha, P.; Reshmi, C.R.; Sundaran, S.P.; Binoy, A.; Mishra, N.; Sujith, A. In-vitro evaluation on drug release kinetics and antibacterial activity of dextran modified polyurethane fibrous membrane. Int. J. Biol. Macromol. 2019, 126, 717–730. [Google Scholar] [CrossRef]
- De Marco Castro, E.; Calder, P.C.; Roche, H.M. β-1,3/1,6-Glucans and Immunity: State of the Art and Future Directions. Mol. Nutr. Food Res. 2021, 65, 1901071. [Google Scholar] [CrossRef] [PubMed]
- Abid, M.; Naveed, M.; Azeem, I.; Faisal, A.; Faizan Nazar, M.; Yameen, B. Colon specific enzyme responsive oligoester crosslinked dextran nanoparticles for controlled release of 5-fluorouracil. Int. J. Pharm. 2020, 586, 119605. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Liang, Y.; Shi, M.; Guo, B.; Gao, Y.; Yin, Z. Biocompatible conductive hydrogels based on dextran and aniline trimer as electro-responsive drug delivery system for localized drug release. Int. J. Biol. Macromol. 2019, 140, 255–264. [Google Scholar] [CrossRef]
- Delvart, A.; Moreau, C.; D’Orlando, A.; Falourd, X.; Cathala, B. Dextran-based polyelectrolyte multilayers: Effect of charge density on film build-up and morphology. Colloids Surf. B Biointerfaces 2022, 210, 112258. [Google Scholar] [CrossRef]
- Vercauteren, R.; Bruneel, D.; Schacht, E.; Duncan, R. Effect of the Chemical Modification of Dextran on the Degradation by Dextranase. J. Bioact. Compat. Polym. 1990, 5, 4–15. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, J.; Wang, Y.; Li, J.; Bao, J.; Xu, X.; Zhang, C.; Li, Y.; Wu, H.; Gu, Z. Reduced polydopamine nanoparticles incorporated oxidized dextran/chitosan hybrid hydrogels with enhanced antioxidative and antibacterial properties for accelerated wound healing. Carbohydr. Polym. 2021, 257, 117598. [Google Scholar] [CrossRef]
- Prasher, P.; Sharma, M.; Singh, S.K.; Haghi, M.; MacLoughlin, R.; Chellappan, D.K.; Gupta, G.; Paudel, K.R.; Hansbro, P.M.; George Oliver, B.G.; et al. Advances and applications of dextran-based nanomaterials targeting inflammatory respiratory diseases. J. Drug Deliv. Sci. Technol. 2022, 74, 103598. [Google Scholar] [CrossRef]
- Chen, H.; Wang, H.; Wei, Y.; Hu, M.; Dong, B.; Fang, H.; Chen, Q. Super-resolution imaging reveals the subcellular distribution of dextran at the nanoscale in living cells. Chin. Chem. Lett. 2022, 33, 1865–1869. [Google Scholar] [CrossRef]
- Chircov, C.; Ștefan, R.-E.; Dolete, G.; Andrei, A.; Holban, A.M.; Oprea, O.-C.; Vasile, B.S.; Neacșu, I.A.; Tihăuan, B. Dextran-Coated Iron Oxide Nanoparticles Loaded with Curcumin for Antimicrobial Therapies. Pharmaceutics 2022, 14, 1057. [Google Scholar] [CrossRef]
- Cakić, M.; Glišić, S.; Nikolić, G.; Nikolić, G.M.; Cakić, K.; Cvetinov, M. Synthesis, characterization and antimicrobial activity of dextran sulphate stabilized silver nanoparticles. J. Mol. Struct. 2016, 1110, 156–161. [Google Scholar] [CrossRef]
- Anusuya, S.; Sathiyabama, M. Preparation of β-d-glucan nanoparticles and its antifungal activity. Int. J. Biol. Macromol. 2014, 70, 440–443. [Google Scholar] [CrossRef] [PubMed]
- Tuchilus, C.G.; Nichifor, M.; Mocanu, G.; Stanciu, M.C. Antimicrobial activity of chemically modified dextran derivatives. Carbohydr. Polym. 2017, 161, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Finbloom, J.A.; Raghavan, P.; Kwon, M.; Kharbikar, B.N.; Yu, M.A.; Desai, T.A. Codelivery of synergistic antimicrobials with polyelectrolyte nanocomplexes to treat bacterial biofilms and lung infections. Sci. Adv. 2023, 9, eade8039. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.-T.; Hadinoto, K. Ternary nanoparticle complex of antibiotic, polyelectrolyte, and mucolytic enzyme as a potential antibiotic delivery system in bronchiectasis therapy. Colloids Surf. B Biointerfaces 2020, 193, 111095. [Google Scholar] [CrossRef]
- Sakima, V.T.; Barbugli, P.A.; Cerri, P.S.; Chorilli, M.; Carmello, J.C.; Pavarina, A.C.; Mima, E.G. Antimicrobial Photodynamic Therapy Mediated by Curcumin-Loaded Polymeric Nanoparticles in a Murine Model of Oral Candidiasis. Molecules 2018, 23, 2075. [Google Scholar] [CrossRef]
- Tiyaboonchai, W.; Limpeanchob, N. Formulation and characterization of amphotericin B–chitosan–dextran sulfate nanoparticles. Int. J. Pharm. 2007, 329, 142–149. [Google Scholar] [CrossRef]
- Cheow, W.S.; Kiew, T.Y.; Yang, Y.; Hadinoto, K. Amorphization Strategy Affects the Stability and Supersaturation Profile of Amorphous Drug Nanoparticles. Mol. Pharm. 2014, 11, 1611–1620. [Google Scholar] [CrossRef]
- Tian, W.; Gao, X.; Zhang, J.; Yu, J.; Zhang, J. Cellulose nanosphere: Preparation and applications of the novel nanocellulose. Carbohydr. Polym. 2022, 277, 118863. [Google Scholar] [CrossRef]
- Chen, S.; Xia, Y.; Zhang, B.; Chen, H.; Chen, G.; Tang, S. Disassembly of lignocellulose into cellulose, hemicellulose, and lignin for preparation of porous carbon materials with enhanced performances. J. Hazard. Mater. 2021, 408, 124956. [Google Scholar] [CrossRef]
- Lehrhofer, A.F.; Goto, T.; Kawada, T.; Rosenau, T.; Hettegger, H. The in vitro synthesis of cellulose—A mini-review. Carbohydr. Polym. 2022, 285, 119222. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, H. Celluloses as support materials for antibacterial agents: A review. Cellulose 2021, 28, 2715–2761. [Google Scholar] [CrossRef]
- Esmaeili, A.; Haseli, M. Electrospinning of thermoplastic carboxymethyl cellulose/poly(ethylene oxide) nanofibers for use in drug-release systems. Mater. Sci. Eng. C 2017, 77, 1117–1127. [Google Scholar] [CrossRef] [PubMed]
- Henschen, J.; Li, D.; Ek, M. Preparation of cellulose nanomaterials via cellulose oxalates. Carbohydr. Polym. 2019, 213, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Arora, J.K.; Sinha, T.J.M.; Srivastava, S. Functionalization of nanocrystalline cellulose for decontamination of Cr(III) and Cr(VI) from aqueous system: Computational modeling approach. Clean Technol. Environ. Policy 2014, 16, 1179–1191. [Google Scholar] [CrossRef]
- Yuan, Z.; Cheng, J.; Lan, G.; Lu, F. A cellulose/Konjac glucomannan–based macroporous antibacterial wound dressing with synergistic and complementary effects for accelerated wound healing. Cellulose 2021, 28, 5591–5609. [Google Scholar] [CrossRef]
- Forero-Doria, O.; Polo, E.; Marican, A.; Guzmán, L.; Venegas, B.; Vijayakumar, S.; Wehinger, S.; Guerrero, M.; Gallego, J.; Durán-Lara, E.F. Supramolecular hydrogels based on cellulose for sustained release of therapeutic substances with antimicrobial and wound healing properties. Carbohydr. Polym. 2020, 242, 116383. [Google Scholar] [CrossRef]
- Božič, M.; Liu, P.; Mathew, A.P.; Kokol, V. Enzymatic phosphorylation of cellulose nanofibers to new highly-ions adsorbing, flame-retardant and hydroxyapatite-growth induced natural nanoparticles. Cellulose 2014, 21, 2713–2726. [Google Scholar] [CrossRef]
- Zainal, S.H.; Mohd, N.H.; Suhaili, N.; Anuar, F.H.; Lazim, A.M.; Othaman, R. Preparation of cellulose-based hydrogel: A review. J. Mater. Res. Technol. 2021, 10, 935–952. [Google Scholar] [CrossRef]
- Thakur, V.K.; Thakur, M.K.; Gupta, R.K. Graft copolymers from cellulose: Synthesis, characterization and evaluation. Carbohydr. Polym. 2013, 97, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tian, Y.; Zheng, W.; Feng, Y.; Huang, R.; Shao, J.; Tang, R.; Wang, P.; Jia, Y.; Zhang, J.; et al. Composites of Bacterial Cellulose and Small Molecule-Decorated Gold Nanoparticles for Treating Gram-Negative Bacteria-Infected Wounds. Small 2017, 13, 1700130. [Google Scholar] [CrossRef]
- Li, J.; Cha, R.; Mou, K.; Zhao, X.; Long, K.; Luo, H.; Zhou, F.; Jiang, X. Nanocellulose-Based Antibacterial Materials. Adv. Healthc. Mater. 2018, 7, 1800334. [Google Scholar] [CrossRef]
- Vilela, C.; Oliveira, H.; Almeida, A.; Silvestre, A.J.D.; Freire, C.S.R. Nanocellulose-based antifungal nanocomposites against the polymorphic fungus Candida albicans. Carbohydr. Polym. 2019, 217, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Rimpy; Ahuja, M. Fluconazole-loaded TEOS-modified nanocellulose 3D scaffolds—Fabrication, characterization and its application as vaginal drug delivery system. J. Drug Deliv. Sci. Technol. 2022, 75, 103646. [Google Scholar] [CrossRef]
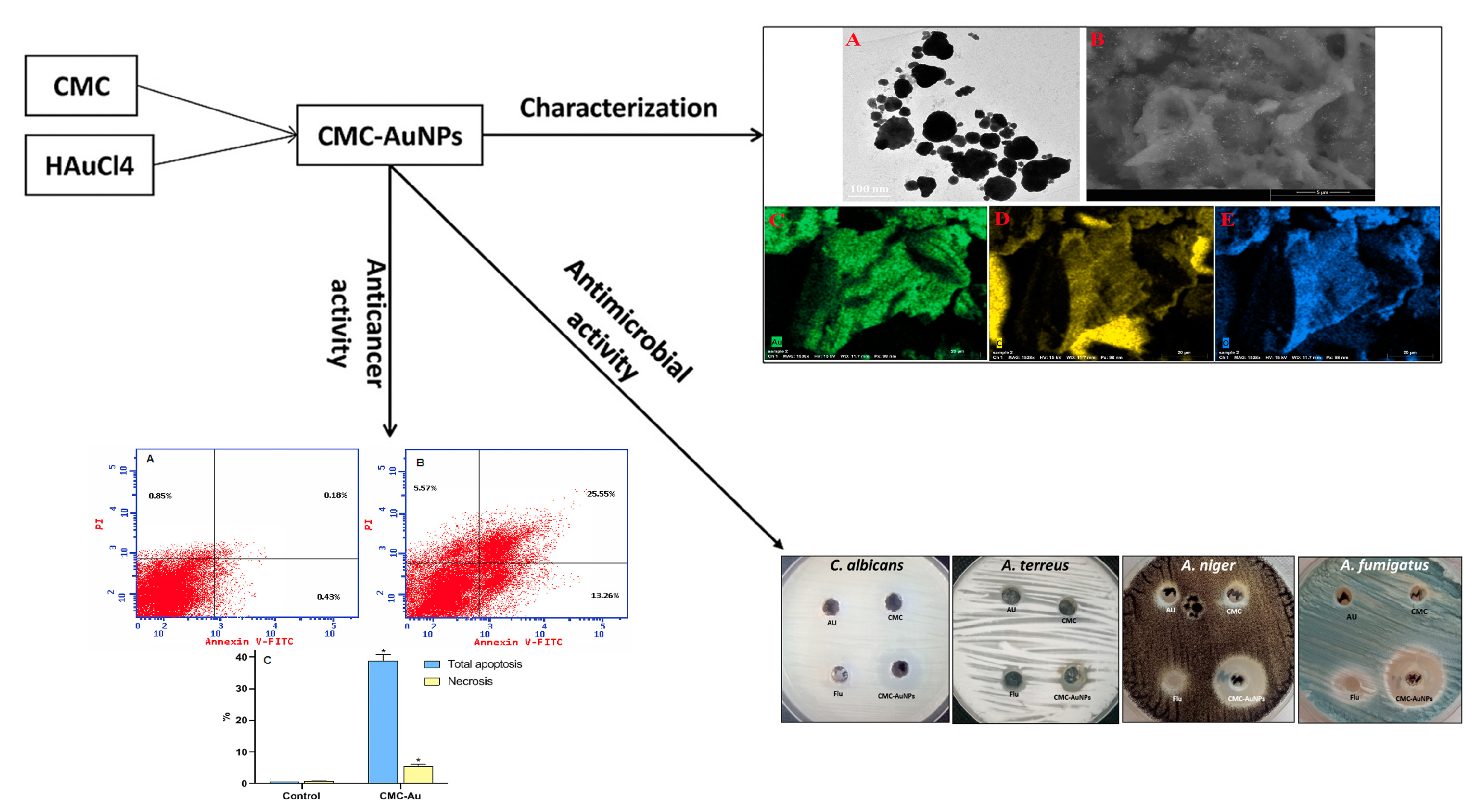
- Doghish, A.S.; Hashem, A.H.; Shehabeldine, A.M.; Sallam, A.-A.M.; El-Sayyad, G.S.; Salem, S.S. Nanocomposite based on gold nanoparticles and carboxymethyl cellulose: Synthesis, characterization, antimicrobial, and anticancer activities. J. Drug Deliv. Sci. Technol. 2022, 77, 103874. [Google Scholar] [CrossRef]
- Thakkar, M.; Islam, M.S.; Railkar, A.; Mitra, S. Antisolvent precipitative immobilization of micro and nanostructured griseofulvin on laboratory cultured diatom frustules for enhanced aqueous dissolution. Colloids Surf. B Biointerfaces 2020, 196, 111308. [Google Scholar] [CrossRef]
- Bellmann, T.; Luber, R.; Kischio, L.; Karl, B.; Pötzinger, Y.; Beekmann, U.; Kralisch, D.; Wiegand, C.; Fischer, D. Bacterial nanocellulose patches as a carrier for hydrating formulations to improve the topical treatment of nail diseases. Int. J. Pharm. 2022, 628, 122267. [Google Scholar] [CrossRef]
- Azzaoui, K.; Mejdoubi, E.; Lamhamdi, A.; Jodeh, S.; Hamed, O.; Berrabah, M.; Jerdioui, S.; Salghi, R.; Akartasse, N.; Errich, A.; et al. Preparation and characterization of biodegradable nanocomposites derived from carboxymethyl cellulose and hydroxyapatite. Carbohydr. Polym. 2017, 167, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Kaur, K.; Kumar, P.; Kush, P. Amphotericin B loaded ethyl cellulose nanoparticles with magnified oral bioavailability for safe and effective treatment of fungal infection. Biomed. Pharmacother. 2020, 128, 110297. [Google Scholar] [CrossRef]
- Kapileshwari, G.R.; Barve, A.R.; Kumar, L.; Bhide, P.J.; Joshi, M.; Shirodkar, R.K. Novel drug delivery system of luliconazole—Formulation and characterisation. J. Drug Deliv. Sci. Technol. 2020, 55, 101302. [Google Scholar] [CrossRef]
- Robles, E.; Salaberria, A.M.; Herrera, R.; Fernandes, S.C.M.; Labidi, J. Self-bonded composite films based on cellulose nanofibers and chitin nanocrystals as antifungal materials. Carbohydr. Polym. 2016, 144, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Schmücker, C.; Stevens, G.W.; Mumford, K.A. Liquid marble formation and solvent vapor treatment of the biodegradable polymers polylactic acid and polycaprolactone. J. Colloid Interface Sci. 2018, 514, 349–356. [Google Scholar] [CrossRef]
- Gupta, P.K.; Gahtori, R.; Govarthanan, K.; Sharma, V.; Pappuru, S.; Pandit, S.; Mathuriya, A.S.; Dholpuria, S.; Bishi, D.K. Recent trends in biodegradable polyester nanomaterials for cancer therapy. Mater. Sci. Eng. C 2021, 127, 112198. [Google Scholar] [CrossRef]
- Elsawy, M.A.; Kim, K.-H.; Park, J.-W.; Deep, A. Hydrolytic degradation of polylactic acid (PLA) and its composites. Renew. Sustain. Energy Rev. 2017, 79, 1346–1352. [Google Scholar] [CrossRef]
- Ajioka, M.; Enomoto, K.; Suzuki, K.; Yamaguchi, A. The basic properties of poly(lactic acid) produced by the direct condensation polymerization of lactic acid. J. Environ. Polym. Degrad. 1995, 3, 225–234. [Google Scholar] [CrossRef]
- Darie-Niță, R.N.; Râpă, M.; Frąckowiak, S. Special Features of Polyester-Based Materials for Medical Applications. Polymers 2022, 14, 951. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-Y.; Zhang, L.-M. Preparation of a polysaccharide–polyester diblock copolymer and its micellar characteristics. Carbohydr. Polym. 2007, 69, 196–201. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, Z.; Gu, J.G.; Zhou, W.; Liang, X.; Zhou, G.; Han, C.C.; Xu, S.; Liu, Y. Mechanism of a long-term controlled drug release system based on simple blended electrospun fibers. J. Control Release 2020, 320, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Duygulu, N.E.; Ciftci, F.; Ustundag, C.B. Electrospun drug blended poly(lactic acid) (PLA) nanofibers and their antimicrobial activities. J. Polym. Res. 2020, 27, 232. [Google Scholar] [CrossRef]
- Castro-Aguirre, E.; Iñiguez-Franco, F.; Samsudin, H.; Fang, X.; Auras, R. Poly(lactic acid)—Mass production, processing, industrial applications, and end of life. Adv. Drug Deliv. Rev. 2016, 107, 333–366. [Google Scholar] [CrossRef]
- Yang, M.; Xie, S.; Adhikari, V.P.; Dong, Y.; Du, Y.; Li, D. The synergistic fungicidal effect of low-frequency and low-intensity ultrasound with amphotericin B-loaded nanoparticles on C. albicans in vitro. Int. J. Pharm. 2018, 542, 232–241. [Google Scholar] [CrossRef]
- Li, Z.; Liu, L.; Chen, B.; Zhao, T.; Ran, L.; Yuan, X.; Cao, Z.; Wu, T. Structure and antimicrobial properties of long-chain branched poly (lactic acid). J. Biomed. Mater. Res. Part A 2019, 107, 2458–2467. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Xu, L.; Ahmed, A. Batch Preparation and Characterization of Electrospun Porous Polylactic Acid-Based Nanofiber Membranes for Antibacterial Wound Dressing. Adv. Fiber Mater. 2022, 4, 832–844. [Google Scholar] [CrossRef]
- Scaffaro, R.; Lopresti, F.; D’Arrigo, M.; Marino, A.; Nostro, A. Efficacy of poly(lactic acid)/carvacrol electrospun membranes against Staphylococcus aureus and Candida albicans in single and mixed cultures. Appl. Microbiol. Biotechnol. 2018, 102, 4171–4181. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Yao, Q.; Yu, F.; Chen, L.; Zhang, S.; Sun, H.; Lin, J.; Fu, Y. Surface modified electrospun poly(lactic acid) fibrous scaffold with cellulose nanofibrils and Ag nanoparticles for ocular cell proliferation and antimicrobial application. Mater. Sci. Eng. C 2020, 111, 110767. [Google Scholar] [CrossRef]
- Liu, X.; Guo, C.; Zhuang, K.; Chen, W.; Zhang, M.; Dai, Y.; Tan, L.; Ran, Y. A recyclable and light-triggered nanofibrous membrane against the emerging fungal pathogen Candida auris. PLoS Pathog. 2022, 18, e1010534. [Google Scholar] [CrossRef] [PubMed]
- Machado, R.; da Costa, A.; Silva, D.M.; Gomes, A.C.; Casal, M.; Sencadas, V. Antibacterial and Antifungal Activity of Poly(Lactic Acid)–Bovine Lactoferrin Nanofiber Membranes. Macromol. Biosci. 2018, 18, 1700324. [Google Scholar] [CrossRef]
- Jalvo, B.; Mathew, A.P.; Rosal, R. Coaxial poly(lactic acid) electrospun composite membranes incorporating cellulose and chitin nanocrystals. J. Membr. Sci. 2017, 544, 261–271. [Google Scholar] [CrossRef]
- Abdelbar, M.F.; Shams, R.S.; Morsy, O.M.; Hady, M.A.; Shoueir, K.; Abdelmonem, R. Highly ordered functionalized mesoporous silicate nanoparticles reinforced poly (lactic acid) gatekeeper surface for infection treatment. Int. J. Biol. Macromol. 2020, 156, 858–868. [Google Scholar] [CrossRef]
- Yang, M.; Du, K.; Hou, Y.; Xie, S.; Dong, Y.; Li, D.; Du, Y. Synergistic Antifungal Effect of Amphotericin B-Loaded Poly(Lactic-Co-Glycolic Acid) Nanoparticles and Ultrasound against Candida albicans Biofilms. Antimicrob. Agents Chemother. 2019, 63, e02022-02018. [Google Scholar] [CrossRef] [PubMed]
- Radwan, M.A.; AlQuadeib, B.T.; Šiller, L.; Wright, M.C.; Horrocks, B. Oral administration of amphotericin B nanoparticles: Antifungal activity, bioavailability and toxicity in rats. Drug Deliv. 2017, 24, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Alshehri, S.; Imam, S.S. Formulation and evaluation of butenafine loaded PLGA-nanoparticulate laden chitosan nano gel. Drug Deliv. 2021, 28, 2348–2360. [Google Scholar] [CrossRef]
- Richter, A.R.; Carneiro, M.J.; de Sousa, N.A.; Pinto, V.P.T.; Freire, R.S.; de Sousa, J.S.; Mendes, J.F.S.; Fontenelle, R.O.S.; Feitosa, J.P.A.; Paula, H.C.B.; et al. Self-assembling cashew gum-graft-polylactide copolymer nanoparticles as a potential amphotericin B delivery matrix. Int. J. Biol. Macromol. 2020, 152, 492–502. [Google Scholar] [CrossRef]
- AbouSamra, M.M.; Basha, M.; Awad, G.E.A.; Mansy, S.S. A promising nystatin nanocapsular hydrogel as an antifungal polymeric carrier for the treatment of topical candidiasis. J. Drug Deliv. Sci. Technol. 2019, 49, 365–374. [Google Scholar] [CrossRef]
- El-Naggar, M.E.; Abdelgawad, A.M.; Abdel-Sattar, R.; Gibriel, A.A.; Hemdan, B.A. Potential antimicrobial and antibiofilm efficacy of essential oil nanoemulsion loaded polycaprolactone nanofibrous dermal patches. Eur. Polym. J. 2023, 184, 111782. [Google Scholar] [CrossRef]
- Kapustová, M.; Puškárová, A.; Bučková, M.; Granata, G.; Napoli, E.; Annušová, A.; Mesárošová, M.; Kozics, K.; Pangallo, D.; Geraci, C. Biofilm inhibition by biocompatible poly(ε-caprolactone) nanocapsules loaded with essential oils and their cyto/genotoxicity to human keratinocyte cell line. Int. J. Pharm. 2021, 606, 120846. [Google Scholar] [CrossRef]
- Greatti, V.R.; Oda, F.; Sorrechia, R.; Kapp, B.R.; Seraphim, C.M.; Weckwerth, A.C.V.B.; Chorilli, M.; Silva, P.B.D.; Eloy, J.O.; Kogan, M.J.; et al. Poly-ε-caprolactone Nanoparticles Loaded with 4-Nerolidylcatechol (4-NC) for Growth Inhibition of Microsporum canis. Antibiotics 2020, 9, 894. [Google Scholar] [CrossRef]
- Abdel-Rashid, R.S.; Helal, D.A.; Alaa-Eldin, A.A.; Abdel-Monem, R. Polymeric versus lipid nanocapsules for miconazole nitrate enhanced topical delivery: In vitro and ex vivo evaluation. Drug Deliv. 2022, 29, 294–304. [Google Scholar] [CrossRef]
- Zimmermann, E.S.; Ferreira, L.M.; Denardi, L.B.; Sari, M.H.M.; Cervi, V.F.; Nogueira, C.W.; Alves, S.H.; Cruz, L. Mucoadhesive gellan gum hydrogel containing diphenyl diselenide-loaded nanocapsules presents improved anti-candida action in a mouse model of vulvovaginal candidiasis. Eur. J. Pharm. Sci. 2021, 167, 106011. [Google Scholar] [CrossRef]
- Saqib, M.; Ali Bhatti, A.S.; Ahmad, N.M.; Ahmed, N.; Shahnaz, G.; Lebaz, N.; Elaissari, A. Amphotericin B Loaded Polymeric Nanoparticles for Treatment of Leishmania Infections. Nanomaterials 2020, 10, 1152. [Google Scholar] [CrossRef] [PubMed]
- Kalita, S.; Kandimalla, R.; Devi, B.; Kalita, B.; Kalita, K.; Deka, M.; Kataki, A.; Sharma, A.; Kotoky, J. Dual delivery of chloramphenicol and essential oil by poly-ε-caprolactone–Pluronic nanocapsules to treat MRSA-Candida co-infected chronic burn wounds. RSC Adv. 2017, 3, 1749–1758. [Google Scholar] [CrossRef]
- Arias, E.R.; Angarita-Villamizar, V.; Baena, Y.; Parra-Giraldo, C.; Perez, L.D. Phospholipid-Conjugated PEG-b-PCL Copolymers as Precursors of Micellar Vehicles for Amphotericin B. Polymers 2021, 13, 1747. [Google Scholar] [CrossRef] [PubMed]
- Hauser, M.; Nowack, B. Probabilistic modelling of nanobiomaterial release from medical applications into the environment. Environ. Int. 2021, 146, 106184. [Google Scholar] [CrossRef]



| Loaded Drugs | Role of Alginate | Other Components | Fungal | Zeta Potential (mV) | Diameters (nm) | Loading Content (LC) | Encapsulation Efficiency (EE) | Drug Release | PDI | Antifungal Efficacy In Vitro | Administration Route/In Vivo Study | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nystatin (Nys) | internal phase | / | C. albicans | −37.42 ± 1.07 (pH 7.5); −35.22 ± 1.40 (pH 5.5) | 24,410 | Surface 7.63 ± 1.81% | / | About 62% (18 h) | / | exhibited a marked fungicidal activity | oral mucosa administration/Y | [65] |
| Inside 17.45 ± 2.34% | ||||||||||||
| Voriconazole | coating layer | Chitosan | / | −24 ± 0.9 | 185 ± 1 | 10.38 ± 0.87% | 91.31 ± 1.05% | About 68% (50 h) | / | / | corneal administration/N | [67] |
| Miltefosine | matrix | / | C. albicans | −39.7 ± 5.2 | 279.1 ± 56.7 | / | 81.70 ± 6.64% | 55.24% (181 h) | / | MIC: 0.03 to 2 µg/mL | mucosal and oral administration/Y | [68] |
| /C. gattii. | ||||||||||||
| Sodium selenate | coating layer | / | Fusarium oxysporum Schltdl | −7.25 | 80 | / | / | About 60% (40 h) | / | / | N/N | [69] |
| Miltefosine | matrix | / | Galleria mellonella caterpillars | −39.7 ± 5.2 | 279.1 ± 56.7 | About 80% | 81.70% ± 6.64 | 55.24% (181 h) | / | MIC: 0.03 µg/mL | mucosal and oral administration/Y | [68] |
| Ketoconazole | matrix | poloxamer 407, carbopol 940 | Candida albicans | +82.2 ± 64.94 | 34.8 ± 73.34 | / | 97.5 ± 41.95% | 43.75 ± 5.38% (6 h) | / | / | ocular administration/Y | [70] |
| Ethionamide | matrix | Chitosan | Mycobacterial | −24 ± 9 | 324 ± 62 | 59% | About 100% (80 h) | 0.35 ± 0.09 | MIC: 0.43 µg/mL | inhalation and intravenous administration/N | [71] |
| Loaded Drugs | Role of Gelatin | Other Components | Fungal | Zeta Potential (mV) | Diameters (nm) | Loading Content (LC) | Encapsulation Efficiency (EE) | Drug Release | PDI | Antifungal Efficacy In Vitro | Administration Route/In Vivo Study | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Spectinomycin | matrix | / | / | / | 250.9 | 0.1–0.5 g/100 mL | / | / | / | ZOI: 22 mm | oral administration/N | [76] |
| Fluconazole/Cinnamaldehyde | matrix | Poly(Vinyl Alcohol) | Candida albicans | / | 334 ± 56 | 0.2 + 2.6 wt% | 73.84% (CA) and 68.58% (FLU) | CA87% (8 h)/FLU61% (12 h) | / | ZOI: 36 ± 1 mm | corneal administration/N | [78] |
| Amp B | matrix | Carboxymethyl ι-carrageenan | Candida glabrata | −25 ± 5.3 | 343 ± 12 | 2 wt% | 78 ± 0.68% | 99% (40 days) | <0.3 | No viable C. glabrata was detected in Macrophage cells. | N/N | [77] |
| Amp B | shell-forming components | polyethylene oxide | Candida tropicalis/Candida krusei/Candida parapsilosis/Candida glabrata/Candida dubliniensis/Aspergillus flavus | / | 351 ± 73 | 0–9% | / | 78% (11 h) | / | ZOI: 19 ± 0.5 mm | Topical administration/N | [79] |
| Mmethylene blue | matrix | / | Candida albicans | 30.8 | 100 | 3.13% to 6.75% | 84.0 ± 1.3% | 48% (180 h) | 0.107 | / | N/N | [80] |
| Daptomycin/Polymyxin B/Tobramycin/Vancomycin/Caspofungin/Amp B | matrix | polydopamine | Candida albicans | / | 998 ± 250 | 0.5% | / | 80% (24 h) | / | ZOI: 31 mm | wound dressings/Y | [75] |
| Loaded Drugs | Role of Dextran | Other Components | Fungal | Zeta Potential (mV) | Diameters (nm) | Loading Content (LC) | Encapsulation Efficiency (EE) | Drug Release | PDI | Antifungal Efficacy In Vitro | Administration Route/In Vivo Study | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tobramycin/AgNPs | matrix | / | Pseudomonas aeruginosa (PA) | −39.2 ± 1.5 | 167.2 ± 3.56 | >75% | Ag > 95%/Tob78 ± 2.5% | / | 0.241 ± 0.008 | MIC: 2 μg/mL | intratracheal instillation/Y | [96] |
| Ciprofloxacin (CIP)/mucolytic enzyme papain (PAP) | matrix | / | PA | −51.0 ± 1.9 | 223 ± 99 | / | 88% | 100% (40 min) | 0.51 ± 0.05 | / | N/N | [97] |
| Curcumin (CUR) | matrix | poly-lactic acid | / | +35 (±7.23) | 248 (±86.39) | / | 73.81% | 50% (16 h) | 0.21 ± 0.09 | / | oral mucosa administration/Y | [98] |
| Amp B | coating layer | poly-lactic acid | / | 37 | 644 ± 52 | / | 56% | 100% (5 min) | 0.27 | / | intravenous administration/Y | [99] |
| Itraconazole (ITZ) | matrix | / | / | −47 ± 0.8 | 400 ± 120 | 65 ± 6% | 93 ± 2% | / | / | / | N/N | [100] |
| Loaded Drugs | Role of Cellulose | Other Components | Fungal | Zeta Potential (mV) | Diameters (nm) | Loading Content (LC) | Encapsulation Efficiency (EE) | Drug Release | PDI | Antifungal Efficacy In Vitro | Administration Route/In Vivo Study | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [2-(methacryloyloxy)ethyl] trimethylammonium chloride solution | matrix | / | Candida albicans | / | / | 40% | / | / | / | inhibition > 99.9% | Antifungal dressings/N | [115] |
| Fluconazole | matrix | tetraethyl orthosilicate | Candida albicans | RNF-25.4 ± 1.13/WNF-24.4 ± 1.15 | RNF for 441.7/WNF for 407.7 | 1% w/v | / | 30% (24 h) | 0.735 for RNF/0.655 for WNF | ZOI: 39 mm | Vaginal administration/Y | [116] |
| ciclopirox olamine and Boswellia serrata | matrix | / | Candida albicans, Candida parapsilosis | / | / | 10.1 ± 3.1% | 10.0 ± 2.2% | 79.1 ± 17.7% (48 h) | / | ZOI: 20 mm | Topical administration/N | [119] |
| gold nanoparticles | matrix | / | C. albicans, A. terreus, A. niger, and A. fumigatus | −3.16 | 54.49 | / | / | pH 5.5, >45%. pH 7 < 5% pH 9 <1%. | / | MIC: 20 μg/mL | N/N | [117] |
| hydroxyapatite | matrix | lysine | Candida albicans | / | 600 | 50–70% | / | / | / | ZOI: 28 mm | N/N | [120] |
| Griseofulvin | stabilizer | diatom | / | −13 ± 2 | 2–3 ± 0.5 μm | / | / | / | 0.675 | / | N/N | [118] |
| Amp | matrix | / | Candida albicans | −16.10 ± 2.6 | 150 ± 9.23 | 5 μg/mL | 60 ± 2% | 18 ± 2.1% (12 h) | 0.258 ± 0.005 | MIC: 0.145 ± 0.01 µg/mL | Oral administration/Y | [121] |
| Lliconazole | matrix | Polyvinyl alcohol | Candida albicans, Aspergillus niger | −14.6–32.3 | 300–600 | 1% | 70–80% | up to 8 h | 0.108~0.497 | strong antifungal activity | Topical administration/N | [122] |
| Citin’ nanocrystals | matrix | / | Aspergillus | / | 60 | 0–10 % | / | / | / | inhibition: 98.87% | Antifungal dressings/N | [123] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xin, Y.; Quan, L.; Zhang, H.; Ao, Q. Emerging Polymer-Based Nanosystem Strategies in the Delivery of Antifungal Drugs. Pharmaceutics 2023, 15, 1866. https://doi.org/10.3390/pharmaceutics15071866
Xin Y, Quan L, Zhang H, Ao Q. Emerging Polymer-Based Nanosystem Strategies in the Delivery of Antifungal Drugs. Pharmaceutics. 2023; 15(7):1866. https://doi.org/10.3390/pharmaceutics15071866
Chicago/Turabian StyleXin, Yuan, Liang Quan, Hengtong Zhang, and Qiang Ao. 2023. "Emerging Polymer-Based Nanosystem Strategies in the Delivery of Antifungal Drugs" Pharmaceutics 15, no. 7: 1866. https://doi.org/10.3390/pharmaceutics15071866
APA StyleXin, Y., Quan, L., Zhang, H., & Ao, Q. (2023). Emerging Polymer-Based Nanosystem Strategies in the Delivery of Antifungal Drugs. Pharmaceutics, 15(7), 1866. https://doi.org/10.3390/pharmaceutics15071866