1. Introduction
Reactive oxygen species (ROS), such as superoxide, hydroxyl radicals, and hydrogen peroxide, are powerful oxidants produced in the body to maintain the homeostasis of biological systems [
1]. For example, mitochondrial ROS are involved in the electron transport chain for obtaining biological energy. Intracellular ROS are used as signaling molecules to maintain redox balance. In general, glutathione (GSH) and endogenous antioxidant proteins (e.g., catalase, superoxide dismutase, and glutathione peroxidase) are used to control the intracellular redox balance [
2,
3,
4,
5,
6]. When large amounts of ROS are produced, the endogenous antioxidant system cannot scavenge excessively generated ROS, and the intracellular redox balance cannot be maintained. An excess amount of ROS which cannot be appropriately reduced by the endogenous antioxidant system is harmful because these species oxidize intracellular components such as DNA, proteins, and lipids [
7]. The accumulation of this oxidative damage is called oxidative stress. In the case of cancer, for example, many immune cells such as macrophages invade tumor tissues and produce a large number of ROS, which act as bullets to combat uncontrollable cancer cells. However, it has also been reported that ROS activate several important signals, such as nuclear factor kappa B (NF-κB), which conversely activates the progression and metastasis of tumors [
8,
9,
10,
11,
12]. Therefore, the generation of ROS in the tumor environment is a strategy for directly killing cancer cells, whereas scavenging ROS in the tumor environment is also a strategy for anticancer therapy. Many antioxidants have been developed to scavenge overproduced ROS. However, none of these effectively function as anticancer drugs. For example, it was previously reported that
N-acetyl cysteine (NAC) inhibited cell growth in cancer [
8,
13,
14]. However, a recently published meta-analysis did not confirm the efficacy of antioxidants, including NAC, for cancer treatment [
15,
16,
17]. This is because small-molecule drugs diffuse into the entire body immediately after administration and are rapidly metabolized. Therefore, large or frequent doses are required to maintain efficacy; however, this causes severe adverse effects because small-molecule antioxidants are internalized in normal cells, causing dysfunction of the intracellular redox homeostasis. Thus, the conventional antioxidants developed to date have a narrow or no therapeutic window and show almost no effect for anticancer therapy.
Although the physical encapsulation of small-molecule drugs, including antioxidants, into nanoparticles such as liposomes and polymer micelles has been developed to reduce their adverse effects, the therapeutic efficacy is poor because the small-molecule drugs leak from the nanoparticles during circulation throughout the whole body [
18,
19,
20,
21]. Therefore, to reduce the immediate diffusion and metabolism of drugs, we are developing self-assembling nanoparticle drugs composed of hydrophilic poly(ethylene glycol) (PEG) and hydrophobic drug-based polymers bearing antioxidants and amino acids with covalent bonds [
22,
23,
24,
25,
26]. In particular, amino acid-based self-assembling drugs release their corresponding amino acids through biodegradation by endogenous enzymes, and their therapeutic effects are enhanced by the release of amino acids [
23,
24,
25,
26]. For example, we previously designed poly(cysteine)-based self-assembling nanoparticle drugs (Nano
Cys(SS)) whose thiol groups were protected by tertiary butylthio (S
tBu) groups and showed antitumor effects in a xenograft mouse model [
26]. The results indicated that the
tert-butyldithiol group was effectively cleaved in the tumor environment and induced an antioxidant effect there. Under the pathological conditions of acute inflammatory diseases, combined with cytokine storms, such as sepsis, ROS levels have been reported to rapidly increase simultaneously [
27,
28,
29,
30,
31]. Therefore, the reactivity of disulfide bonds may not be sufficient for a rapid response to the treatment of such acute inflammatory diseases because ROS levels immediately increase with the overproduction of inflammatory cytokines throughout the body.
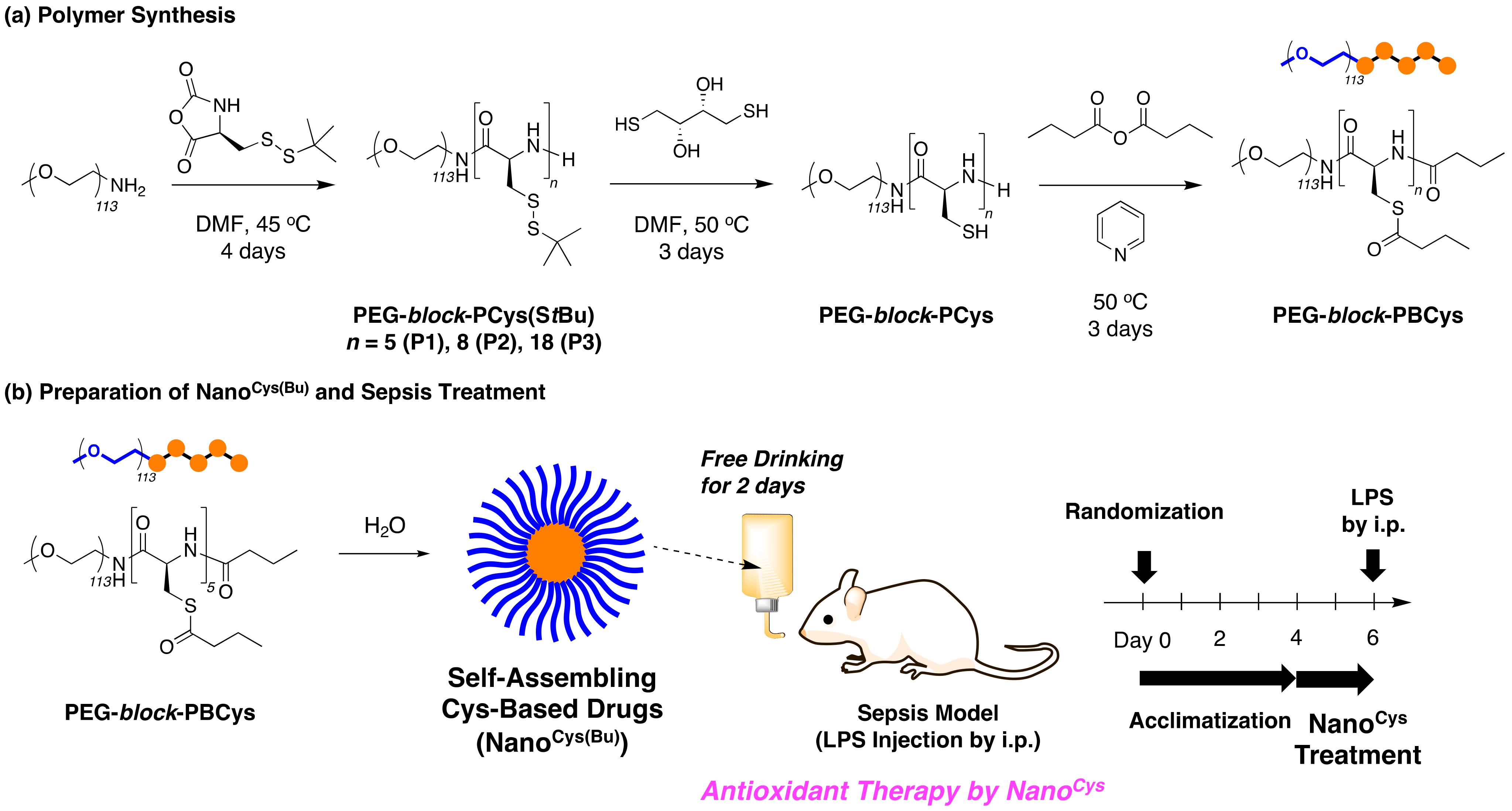
To this end, we designed new PEG- and poly(cysteine) (PCys)-based block copolymers whose thiol groups are protected by thioester bonds to enhance the reactivity of Nano
Cys against ROS, since thioester bonds exhibit high hydrolytic reactivity. These block copolymers were amphiphilic in order to form the nanoparticles with several nanometers in the aqueous condition. The nanoparticle structures enable us to avoid the cellular uptake by normal cells, preventing the disruption of the intracellular redox homeostasis. In addition, the extended bioavailability would suppress the oxidative damage caused by the severe systemic inflammation. To synthesize the targeted polymers, PEG-based block copolymers were synthesized through ring-opening polymerization of the
α-amino acid
N-carboxyanhydride of
S-
tert-butylmercapto-
L-Cys (NCA-Cys(S
tBu)), with a PEG macroinitiator carrying an amino group end (
Scheme 1a). The disulfide bonds were deprotected using a reducing agent, and the free thiol groups were protected using butyric anhydride to produce a new Cys-based block copolymer. The block copolymers formed the new nanoparticles (Nano
Cys(Bu)) in water, and Nano
Cys(Bu) was stable under a variety of pH conditions. The obtained Nano
Cys(Bu) was applied to sepsis mice to investigate the potential of Nano
Cys(Bu). Nano
Cys(Bu) was supplied to mice by free drinking and was confirmed to prolong the half-survival life of the sepsis mouse models prepared by the intraperitoneal injection of lipopolysaccharides (LPS) compared to the non-treatment and cysteine administration groups (
Scheme 1b). In this study, we have confirmed the enhanced pharmacokinetic characteristics of our nanoparticle-type antioxidants based on poly(cysteine). These antioxidants effectively suppress the adverse effects of cysteine, leading to improved therapeutic outcomes in sepsis treatments.
2. Materials and Methods
2.1. Materials
S-tert-butylmercapto-L-cysteine (Cys(StBu); Sigma-Aldrich, Saint Louis, MO, USA), triphosgene (TCI, purity > 98.0%), (1S)-(-)-α-pinene (TCI, purity > 97.0%), PEG-OH (Sigma-Aldrich, Saint Louis, MO, USA, Mn~5000), methanesulfonyl chloride (MsCl; TCI, purity > 99.0%), triethylamine (Et3N; TCI, purity > 99.0%), chloroform (Wako, Tokyo, Japan, purity~99.0%), isopropanol (IPA; Wako, purity~98%), hexane (Wako, purity~95%), ammonia solution (Wako, ~28 wt%), 1,2,3,4-tetrahydronaphtalene (tetralin; TCI, purity > 98.0%), DL-dithiothreitol (DTT; TCI, purity > 98.0%), pyridine (Wako, purity~99.0%), butyric anhydride (TCI, purity > 98.0%), and trifluoroacetic acid (TFA; TCI, purity~98.0%) were used as received. Super-dehydrated tetrahydrofuran (THF; Kanto Chemicals, Tokyo, Japan, purity > 99.5%) and super-dehydrated N,N-dimethylformamide (DMF; Kanto Chemicals, purity > 99.5%) were further purified using a purification column (solvent dispensing system; glass contour; HANSEN & CO., Ltd., London, UK). Water for supplying the solution to the mice was prepared through the process of distillation and deionization using Milli-Q reference (Merck, Rahway, NJ, USA). Lipopolysaccharide (LPS from Escherichia coli O111:B4; Aldrich) was used as received.
2.2. Characterization
The number-averaged and weight-averaged molecular weights (Mn, and Mw, respectively) and the molecular weight distribution (Đ) of the polymers were measured using gel permeation chromatography (GPC) in DMF at 40 °C (flow rate: 0.40 mL/min) on two polystyrene gel columns (TOSHO TSKgel GMHHR-M; exclusion limit: molecular weight (MW) = 4.0 × 106; particle size: 5 μm; pore size: N/A; 7.8 cm i.d. × 30 cm) connected to a pump (JASCO, PU-4180), a refractive index (RI) detector (JASCO, RI-2031), and a UV/Vis detector (JASCO, UV-4075). The columns were calibrated using 18 standard poly(ethylene oxide) (PEO) and poly(ethylene glycol) (PEG) samples (Merck; Mp = 238–1,180,000). The 1H nuclear magnetic resonance (NMR) spectra were recorded in CDCl3 or DMSO-d6 at room temperature (22–23 °C) using an AVANCE-600 NMR spectrometer (Bruker) operating at 600 MHz (1H). Dynamic light scattering (DLS) measurements were performed using a Zetasizer Nano ZSP (Malvern) equipped with a He–Ne laser (λ = 633 nm) at 37 °C ([polymer] = 1.0 mg/mL). The measurement angle was 173°, and the data were analyzed using the non-negative least squares (NNLS) method. Transmission electron microscopy (TEM) was performed using a JEM-1400 (JEOL) instrument at an accelerating voltage of 80 kV. The samples were prepared by drop-casting an aqueous solution of the micelles (10 mg/mL) onto carbon-coated grids (OKENSHOJI; ELS-C10 STEM Cu100P) and staining with a 1 wt% phosphotungstic acid solution (10 μL, pH = 7.4).
2.3. Synthesis of Cys-Based Block Copolymers
α-Amino acid
N-carboxyanhydride of
S-
tert-butylmercapto-
L-Cys (NCA-Cys(S
tBu)), a PEG macroinitiator carrying an amino end group (PEG-NH
2), and PEG-
block-PCys(StBu) were prepared using the method reported previously [
26]. To prepare PEG-
block-PCys(StBu) (
P1(SS)), for example, PEG-NH
2 (44.1 g, 8.82 mmol) was placed into a 1000 mL round-bottom flask, equipped with a three-way stopcock, and purged by N
2 after drying under the reduced pressure. DMF (111 mL) and tetralin (0.5 mL) were added by a syringe technique under an N
2 atmosphere. After the complete solubilization of PEG-NH
2, the solution of NCA-Cys(StBu) in DMF (1000 mM, 52 mL, 52 mmol) was added to the flask, and the mixture solution was kept at 45 °C for 4 d. The polymerization was quenched by liq. N
2 and purified by dialysis of the solution against methanol/water (=9/1,
v/
v) in a regenerated cellulose membrane (Spectra/Por 3
®; molecular weight cut-off (MWCO) 1000).
2.3.1. Deprotection of S-tert-Butylmercapto (StBu) Groups on PEG-block-PCys(StBu)
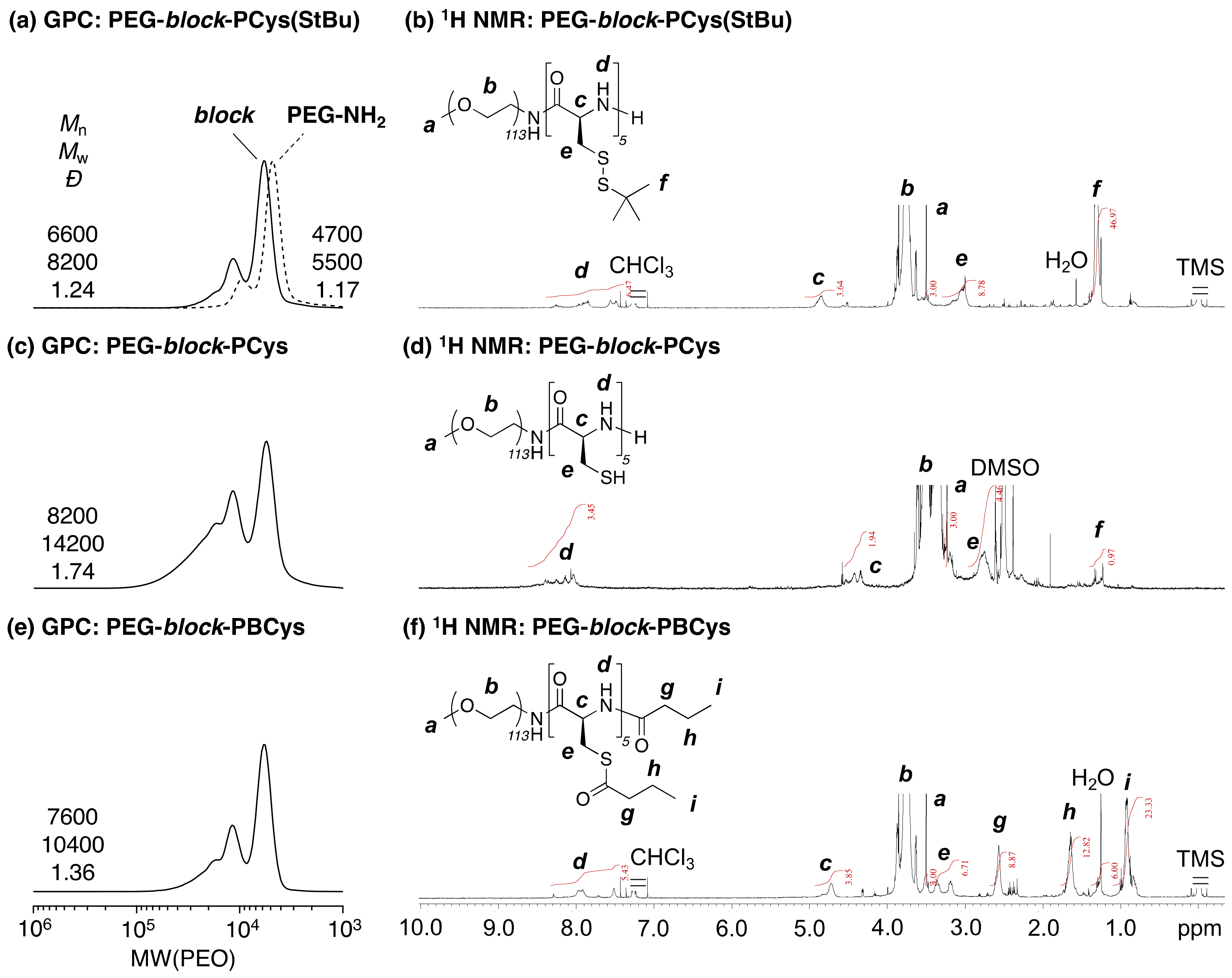
PEG-block-PCys(StBu) (P1(SS)) (90.1 g, 44 mmol of SS bonds; Mn(GPC) = 6600, Đ(GPC) = 1.24, DP(Cys)NMR = 5, Mn(NMR) = 6000) was obtained by evaporation after dialysis against methanol (Spectra/Por® 3; molecular weight cut-off (MWCO) = 1000). DMF (300 mL) was added, and the polymer was completely solubilized. DTT (27.2 g, 176 mmol; DTT/SS bonds = 4/1 mol/mol) was added, and the solution was purged with an N2 flow for 15 min. The solution was stirred at 50 °C for 3 d. The solution was then dialyzed against methanol (MWCO = 1000). The inner solution was evaporated, and PEG-block-PCys (P1(SH)) was obtained. GPC (DMF, PEO standard): Mn(GPC) = 8200, Đ(GPC) = 1.74. 1H NMR (600 MHz, DMSO, r.t., δ = 2.50 ppm (DMSO)): δ 8.43–7.94 (brs, –COCH(CH2SH)NH–), 4.56–4.27 (brs, –COCH(CH2SH)NH–), 3.59–3.44 (brs, –OCH2CH2–), 3.23 (3H, s, –OCH3), 2.91–2.64 (brs, –CH2SH).
P2(SH) and P3(SH) with different molecular weights were synthesized in the same manner.
2.3.2. Protection of Thiol Groups on PEG-block-PCys and Preparation of Micelle Solution
The obtained PEG-block-PCys (P1(SH)) was solubilized in pyridine (100 mL) and butyric anhydride (150 mL), and the solution was purged with N2 for 15 min. The solution was stirred at 50 °C for 3 d. The acylation reaction solution was directly dialyzed against water for preparing NanoCys(Bu)(P1) (MWCO = 1000), followed by concentrating the obtained solution using a centrifugal evaporator (EYELA, CVE-3100). The concentration of the resulting solution was determined by weighing the obtained polymer after lyophilizing 100 μL of the solution. Finally, the solution was diluted with MilliQ water to achieve the target concentration. GPC (DMF, PEO standard): Mn(GPC) = 7600, Đ(GPC) = 1.36. 1H NMR (600 MHz, CDCl3/TFA = 15/1 (v/v), r.t., δ = 7.26 ppm (CHCl3)): δ 8.32–7.45 (brs, –COCH(CH2SCO(CH2)2CH3)NH–), 4.89–4.60 (brs, –COCH(CH2SCO(CH2)2CH3)NH–), 3.96–3.58 (brs, –OCH2CH2–), 3.5 (3H, s, –OCH3), 3.44–3.10 (brs, –CH2 SCO(CH2)2CH3), 2.68–2.46 (brs, –SCOCH2CH2CH3), 1.80–1.53 (brs, –SCOCH2CH2CH3), 1.10–0.74 (brs, –SCOCH2CH2CH3).
NanoCys(Bu)(P2) and NanoCys(Bu)(P3) were prepared in the same manner, after the protection reactions of P2(SH) and P3(SH).
2.4. Animal Experiments
This study was conducted in strict accordance with the University of Tsukuba Guidelines for Animal Care and Laboratory Use, Japan (experimental plan approval #22-195). BALB/cA mice (seven weeks old, male) were purchased from Charles River Japan, Inc. (Yokohama, Japan). The mice were held under a light/dark cycle of 14 h/10 h at a temperature of 23.5 ± 2.5 °C and a humidity of 52.5% ± 12.5%.
2.4.1. Preparation and Treatment of Sepsis Mouse Model
BALB/cA mice (body weight = 27–31 g) were randomized into four groups (N = 8) after seven days of acclimation. After randomization, the mice were further acclimatized to free drinking of Milli-Q water for four days. The aqueous solutions of Nano
Cys and Cys or MilliQ water were supplied to mice by free drinking for two days (
Scheme 1b; [Cys] = 4.4 mM; [polymer] = 5.0 mg/mL). All mice received an intraperitoneal injection of LPS (LPS = 5.0 mg/kg-BW). The mice were then closely monitored for survival on an hourly basis.
2.4.2. Statistical Analysis
Statistical analysis was conducted using GraphPad Prism 9 software (GraphPad Software Inc., Boston, MA, USA, 2020), and the survival curves were compared using the log-rank (Mantel–Cox) test.
4. Discussion
Several diseases are associated with acute or chronic inflammation. In most cases, ROS are overproduced at the inflammatory sites. For example, after influenza infection in mice, the number of viruses increases rapidly, followed by an increase in the severity score and ROS level for several days up to one week [
33,
34,
35]. Therefore, it is crucial to maintain the efficiency of antioxidant therapy for an extended period, without harmful effects. As explained in the Introduction, the problems associated with conventional antioxidants are their rapid diffusion and excretion, owing to their small size. In addition, small antioxidants induce dysfunction in the intracellular redox balance after internalization in normal cells. Therefore, conventional antioxidants cannot be used to treat most oxidative-stress-related diseases. Cysteine is a naturally occurring amino acid with a reduction potential. One of the well-known synthetic derivatives,
N-acetylcysteine (NAC), has strong antioxidant properties, but it induces powerful adverse effects. To solve the problems of low molecular weight antioxidants, especially cysteine, we designed new Cys-based polymers which self-assemble in aqueous media to form core–shell-type nanoparticles covered with biocompatible PEG chains to develop antioxidant chemotherapy. Our idea was to extend the bioavailability of antioxidants by increasing the size of the self-assembled nanoparticles. It is also anticipated that a decrease in cellular uptake will prevent the dysfunction of intracellular redox homeostasis [
36]. The disulfide protective group was converted to a thioester group to increase the deprotection speed, anticipating an increase in the antioxidant capacity. The thioester group is known to be much more active in hydrolysis than is the conventional ester group; therefore, we confirmed its self-assembling character, as well as its stability against increased pH and concentration. Nano
Cys(Bu), thus prepared, was applied for sepsis treatment due to its significant association with oxidative stress, its rapid deterioration in pathology, and its induction of systemic inflammation.
To prepare the PCys segment in the block copolymer, NCA-Cys(S
tBu) was directly polymerized with PEG-NH
2. The alkyldithio group was used as a protecting group for the free thiol group in cysteine because acyl-protection is not sufficiently stable in NCA ring-opening polymerization [
37]. Disulfide bonds on the PCys segments were cleaved by DTT in DMF at 50 °C after the polymerization of NCA-Cys(S
tBu), and PEG-
block-PCys (
P1(SH)), which possess a free thiol group as a side chain, was obtained. In this deprotection reaction, the disulfide bonds were almost completely cleaved because of the excess amount of DTT (disulfide bonds/DTT = 1/4 mol/mol), as confirmed by
1H NMR spectra before and after the reaction (
Figure 1b,d). The GPC curve of PEG-
block-PCys (
P1(SH)) became broader than that before deprotection (PEG-
block-PCys(StBu) (
P1(SS)), probably because of the aggregation of the polymer chains (
Figure 1a,c). The GPC curve of PEG-
block-PBCys (
P1(Bu)) after the protection reaction with butyric anhydride was narrower than that of PEG-
block-PCys (
P1(SH)) (
Figure 1c,e). Therefore, the broadening of the GPC profile of
P1(SH) was not due to the crosslinking of free thiol groups in the side chain of the polymer, but rather due to aggregation of the polymer through hydrogen bonding of the thiol groups.
The composition of the obtained block copolymers, PEG-
block-PCys(S
tBu), could be easily controlled by the initial concentration ratio of [Cys-NCA] versus [PEG-NH
2] and the three different prepared samples (
P1(SS),
P2(SS),
P3(SS)); the degree of polymerization (DP) was confirmed by these
1H NMR spectra (
Figure 1,
Figure S1,
Table S1;
DP(Cys)
NMR = 5 (
P1(SS)), 8 (
P2(SS)), and 18(
P3(SS))). PEG-
block-PBCys copolymers (
P1(Bu)–
P3(Bu)) were successfully synthesized using butyric anhydride in pyridine. These molecular weights, determined by GPC, did not increase significantly (
Figure 2a,c), probably because of the absorption of the polymers on the substrates of the GPC columns used. The longer PCys segments would interact with the GPC columns more than would the other copolymers. The
1H NMR spectrum of
P1(Bu) suggested that the number of acyl-protecting groups in
P1(Bu) was 6, determined by peaks
g,
h, and
i (
Figure 1f). Thus, one polymer chain of
P1(Bu) had five acyl-protecting groups on the side chains and another on the terminal end chain. Therefore, all thiol and amino end groups were quantitatively modified, and the main chain of thet PCys segments was not affected through the deprotection and protection reactions at all. The
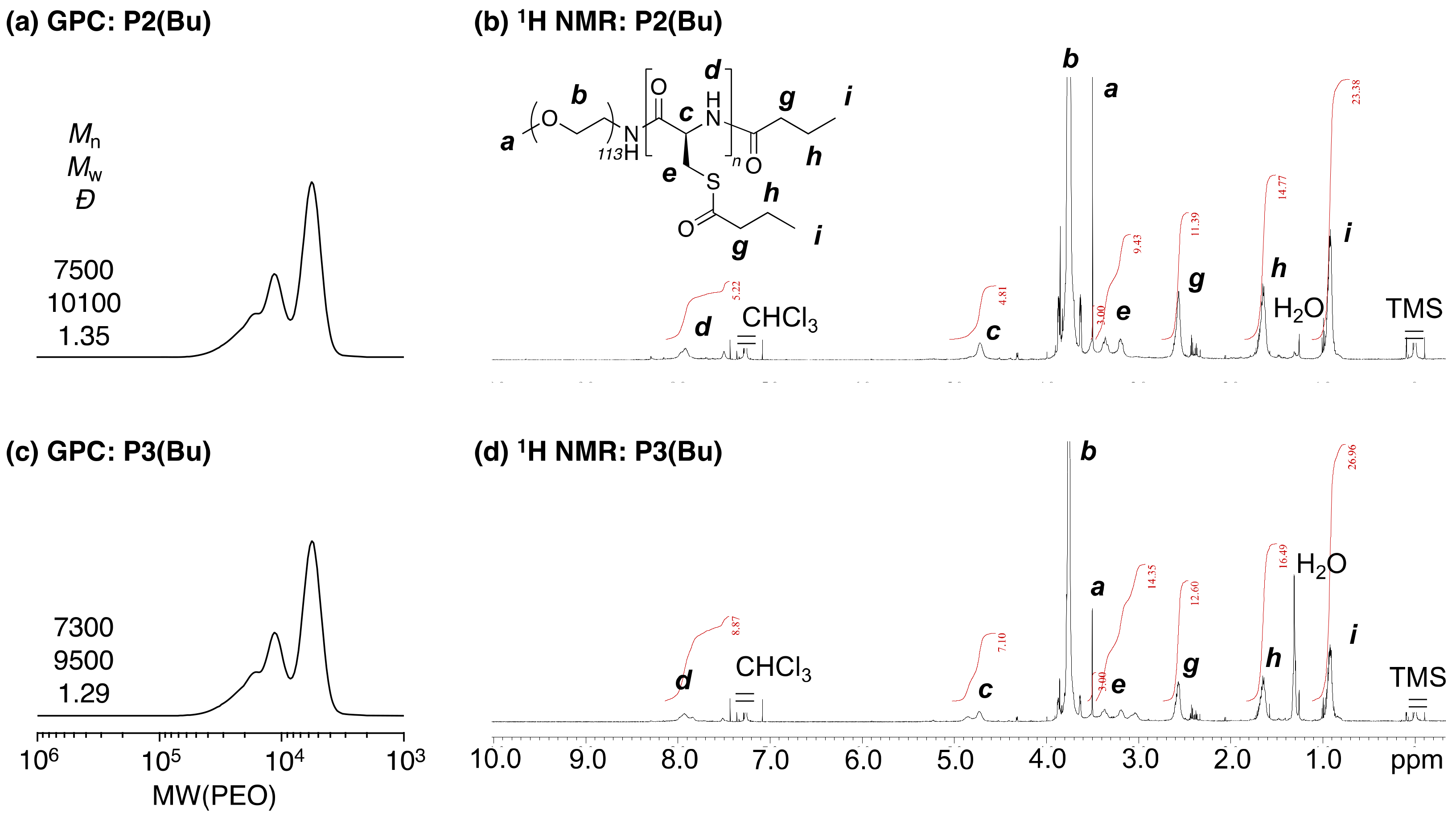
1H NMR spectra of
P2(Bu) and
P3(Bu) indicated that these polymers had 7 and 8 acyl-protecting groups, respectively (
Figure 2b,d). The smaller than expected values suggested that these polymers showed much stronger molecular interactions than did
P1(Bu), and these molecular dynamics decreased. According to the results using the
P1(Bu)-based copolymers,
P2(Bu) and
P3(Bu) were also successfully synthesized, but the extent of the modification efficiency decreased due to the decreased molecular interaction.
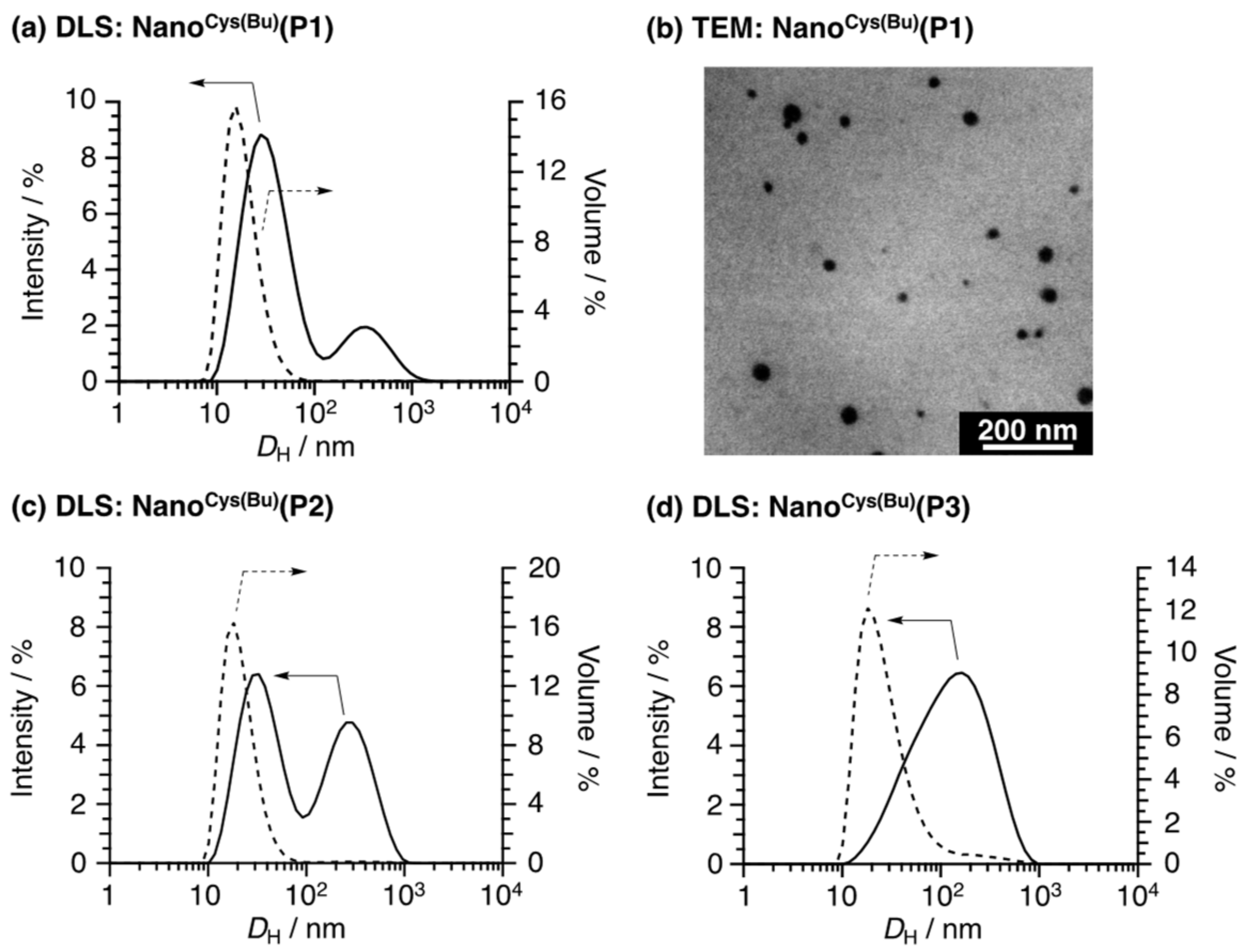
Cysteine-based antioxidant polymer nanoparticles (Nano
Cys(Bu)) were prepared by direct dialysis of the acylation reaction mixture against water. After the dialysis, Nano
Cys(Bu) was characterized by DLS and TEM. The hydrodynamic diameter (
DH,intensity) of Nano
Cys(Bu)(
P1) according to scattering intensity showed the bimodal distributions, the size of which was 37 and 376 nm (
Figure 3a,
Table S1). Here, the large aggregates should be nearly negligible, as the light scattering intensity is emphasized because it is proportional to ten to the power of six of the size [
38]. Actually, the volume-averaged hydrodynamic diameter,
DH,volume, of Nano
Cys(Bu)(
P1) in water was 19 nm, and no large aggregate was observed. Therefore, the large nanoparticles scarcely existed in the solution. This was also confirmed by the TEM image, showing the particles with tens of nanometer (
Figure 3b). Nano
Cys(Bu)(
P2) and Nano
Cys(Bu)(
P3) showed slightly larger sizes compared to Nano
Cys(Bu)(
P1) (
Table S1;
DH,volume = 20 (
P1), 26 (
P2), 39 (
P3) nm). However, this small difference is unlikely to have a significant effect on in vivo toxicity.
Based on these results, we have confirmed the preparation of cysteine-based nanoparticles with several tens of nanometers, possessing acyl-protective groups as a side chain. Before we applied the obtained Nano
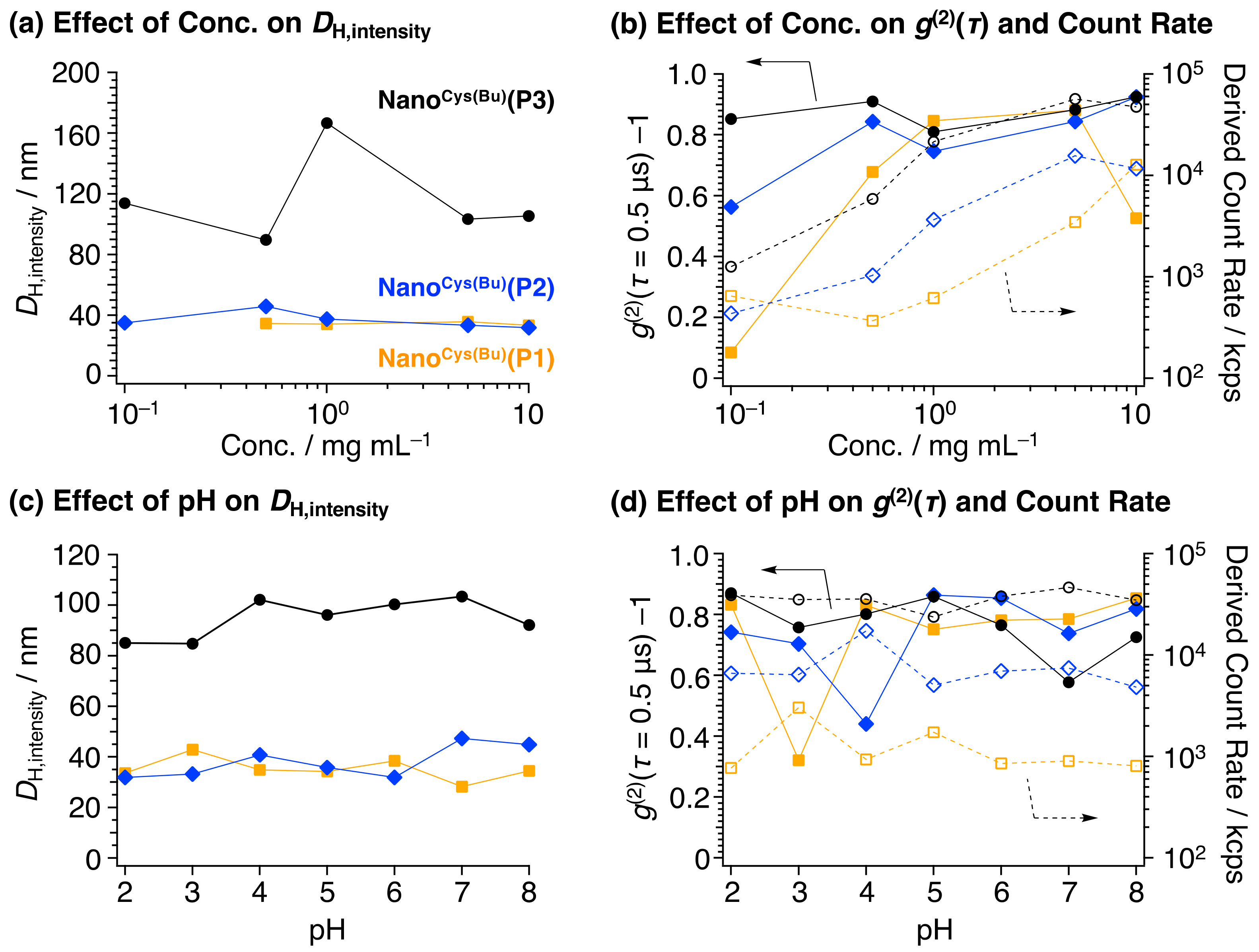
Cys(Bu) to sepsis model mice, their stability was investigated in terms of pH and concentration. The effect of the polymer concentration on the nanoparticle formation was investigated by changing the concentration from 0.1 to 10 mg/mL. The initial value of the ACF (
g(2)(
τ = 0.5 μs)–1) at 0.5 μs is the important physical parameter for determining the micelle formation. When polymer micelles are formed, the initial value of the ACF is expected to be greater than 0.5 [
38]. In the case of Nano
Cys(Bu)(
P1)), at a concentration of 0.1 mg/mL, the value was found to be less than 0.5, suggesting the absence of self-assembling nanoparticles, possibly due to the weaker coagulation force of the hydrophobic PCys segment (
Figure 4b and
Figure S4a). However, as the hydrophobic PCys segment increased, the ACF values exceeded 0.5, indicating the formation of a self-assembling structure. The size of the self-assembled structures of Nano
Cys(Bu) showed nearly the same hydrodynamic diameters (
Figure 4a and
Figure S4b). Since the pH in the digestive tract varies widely from 2 to 8, the effect of pH on the nanoparticle formation was also investigated. As a result, none of the Nano
Cys(Bu) samples significantly changed their size or ACF (
Figure 4d and
Figure S5), indicating that Nano
Cys(Bu) would be stable traveling from the stomach to the intestinal area, although the newly designed Nano
Cys(Bu)s possess thioester groups in the side chain of the PBCys segment. Due to the fact that the amino-end group of the PCys chain was converted to an amide group by acylation, these polymers are not susceptible to pH variations. Further investigation on the physicochemical characteristics will be conducted, and the results will be published elsewhere.
Finally, the effect of the newly designed Nano
Cys(Bu) on a sepsis shock model in mice was investigated. Sepsis is a severe systemic inflammatory response syndrome (SIRS) that causes multiorgan dysfunction and blood clots, and the mortality rate is high, especially in the case of elderly and infant patients. The mechanism has not been completely elucidated; however, the cytokine storm caused by the overproduction of inflammatory cytokines, such as interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-
α), is considered as a key target [
27,
28,
29,
30,
31]. Recently, inhibitors of these cytokines have been developed [
30]; however, it remains difficult to immediately suppress the overproduction of cytokines. Furthermore, accompanied by the production of cytokines, a large amount of ROS is rapidly generated simultaneously, an event which is called a radical storm. The overproduction of ROS causes damage to cells and tissues throughout the body [
27,
28,
33,
34,
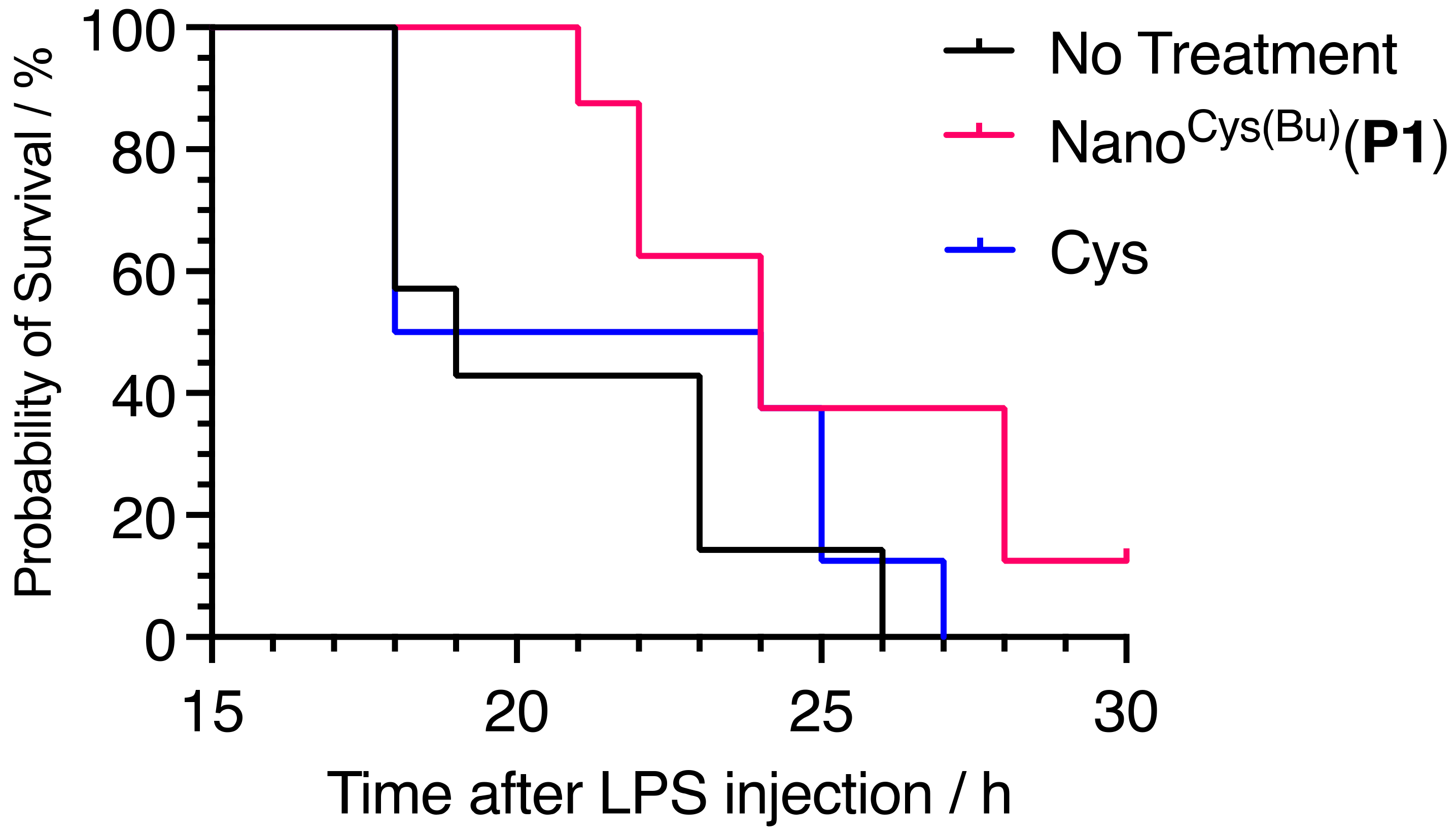
35]. Thus, antioxidants have a great potential for application in the treatment of sepsis. However, low molecular weight antioxidants are ineffective against sepsis. Several methods can be used to induce sepsis in mice, including the intraperitoneal (i.p.) injection of lipopolysaccharides (LPS), cecal ligation and puncture, and the intravenous injection of bacteria. For the present study, we chose the i.p. LPS model due to its well-established capacity to induce significant ROS generation and the development of sepsis syndrome. In this study, Nano
Cys(Bu) and Cys were supplied to BALB/cA mice by free drinking for two days, and the sepsis shock model was induced by the intraperitoneal injection of LPS (LPS = 5.0 mg/kg-BW). We used Nano
Cys(Bu)(
P1) here, as it is stable at high concentrations, but tends to disintegrate at low concentrations. We anticipated that it would form stable spherical structures in the GI tract. However, it tends to disintegrate after migrating into the intestinal mucosa, which can be anticipated to increase the speed of enzymatic hydrolysis. In fact, we have previously confirmed a more pronounced impact of shorter PCys segments on inhibiting tumor growth [
26]. Oral administration of Cys itself did not show any therapeutic effect; indeed, the half-survival time was almost the same as that in the no-treatment group. In contrast, Nano
Cys(Bu)(
P1) prolonged the half-survival time compared with that of the control group. Therefore, our self-assembling poly(cysteine)-based nanoparticles, Nano
Cys(Bu), have great potential for improving sepsis treatment. A detailed investigation into the effects of Nano
Cys(Bu) on sepsis treatment will be performed, and the results will be published in the future.