Cataleptogenic Effect of Haloperidol Formulated in Water-Soluble Calixarene-Based Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Methods
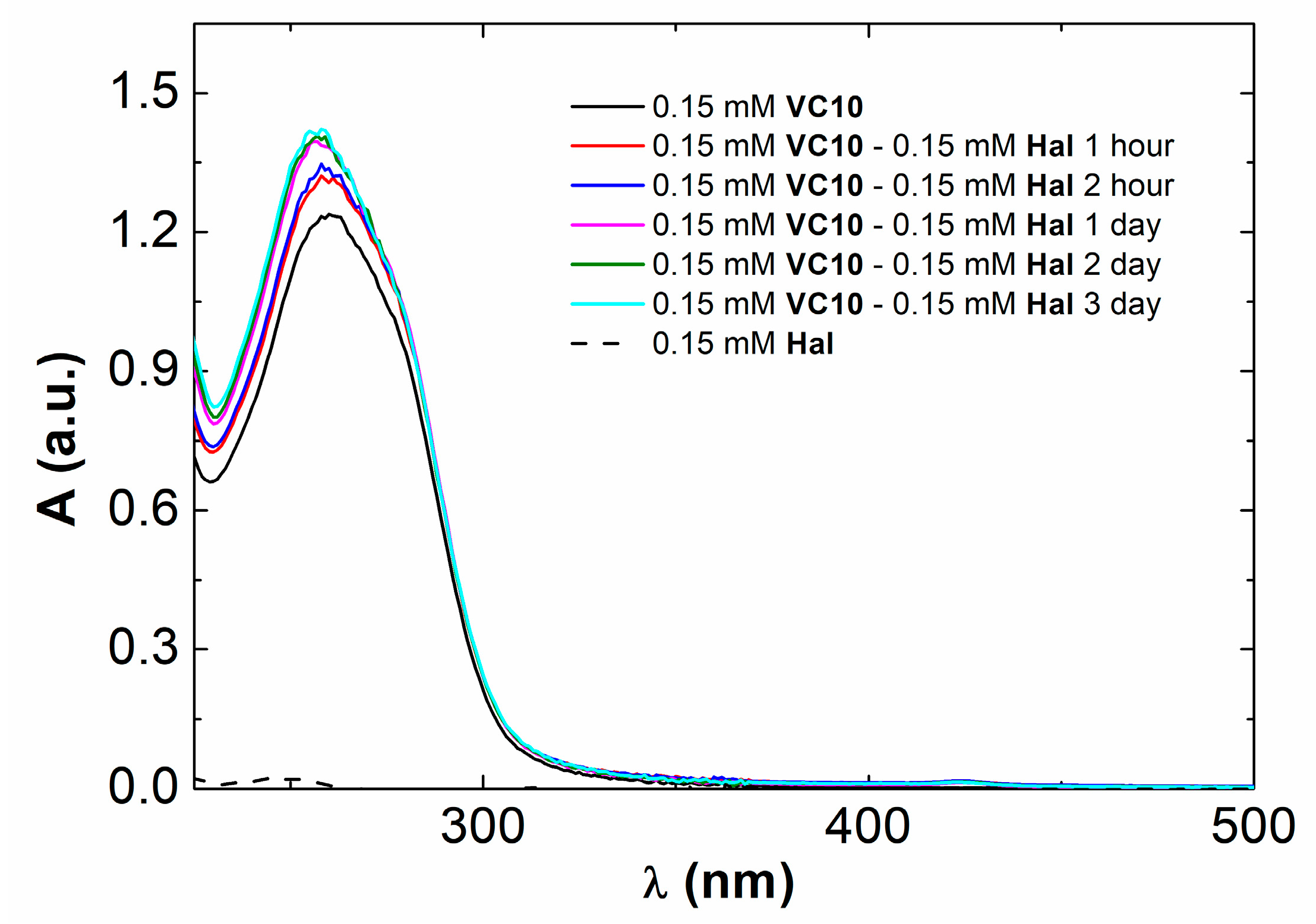
2.2.1. UV-Vis Spectroscopy
2.2.2. NMR Spectroscopy
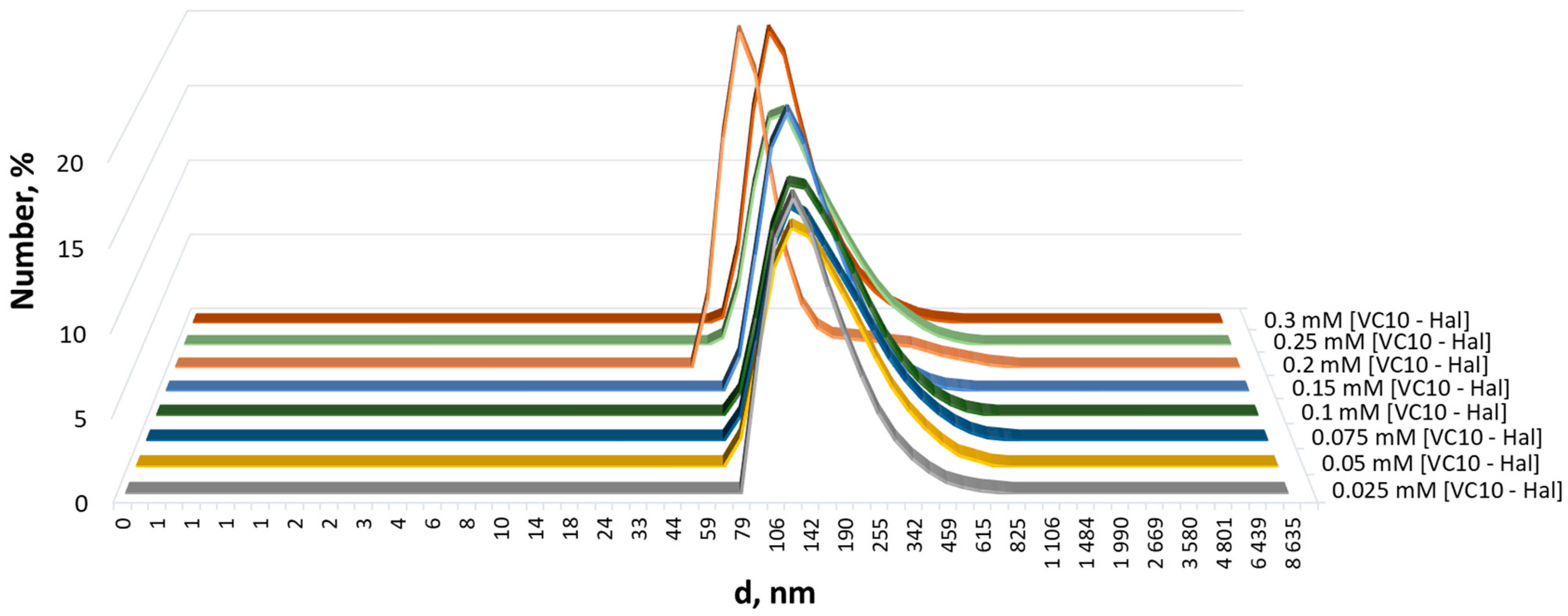
2.2.3. Dynamic Light Scattering
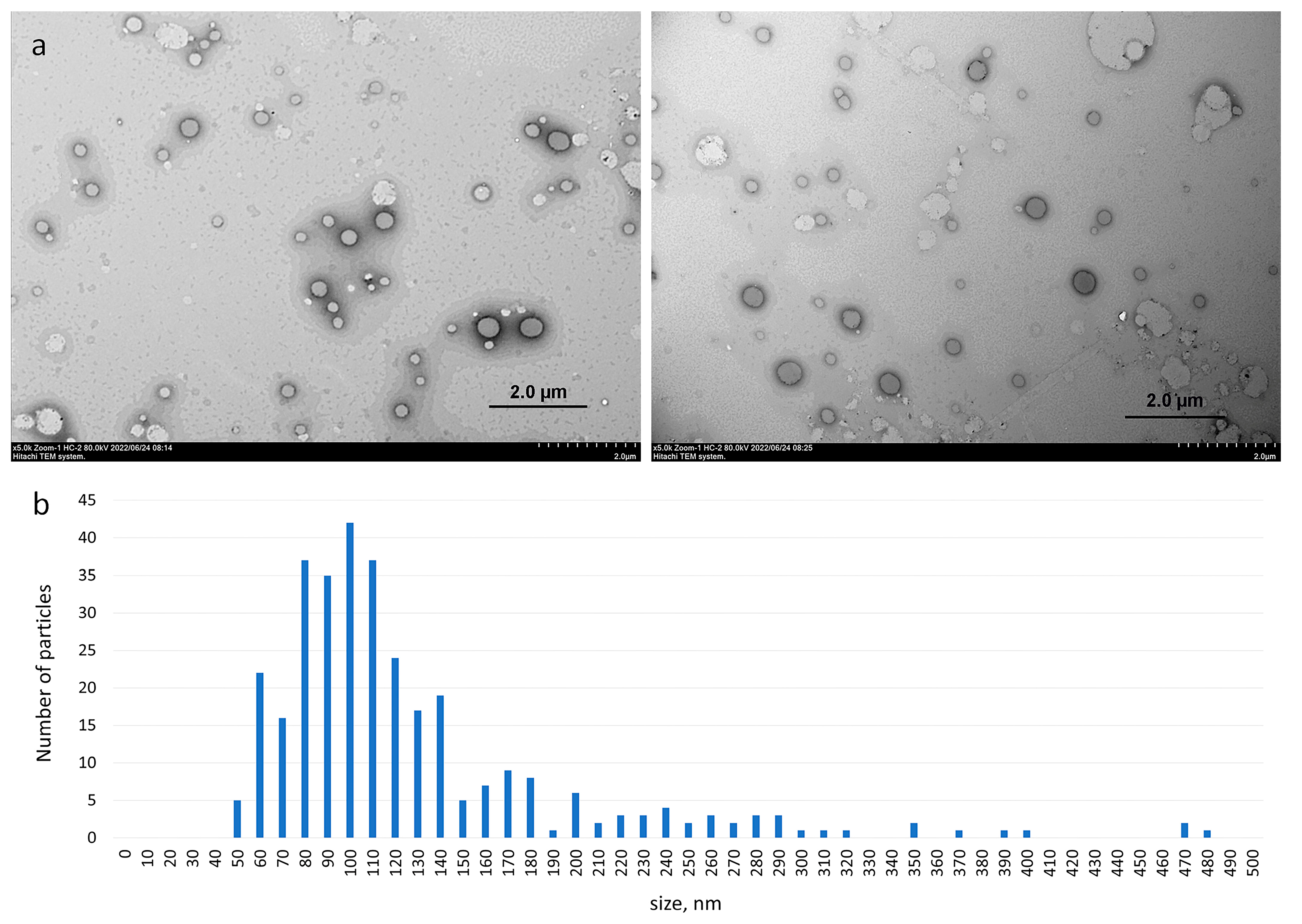
2.2.4. Transmission Electron Microscopy
2.2.5. Fluorescence Spectroscopy
2.2.6. Circular Dichroism Spectroscopy
2.2.7. In Vivo Experiments
Toxicity Assay
“Open Field” Test
Catalepsy Test
Statistical Analysis
3. Results and Discussions
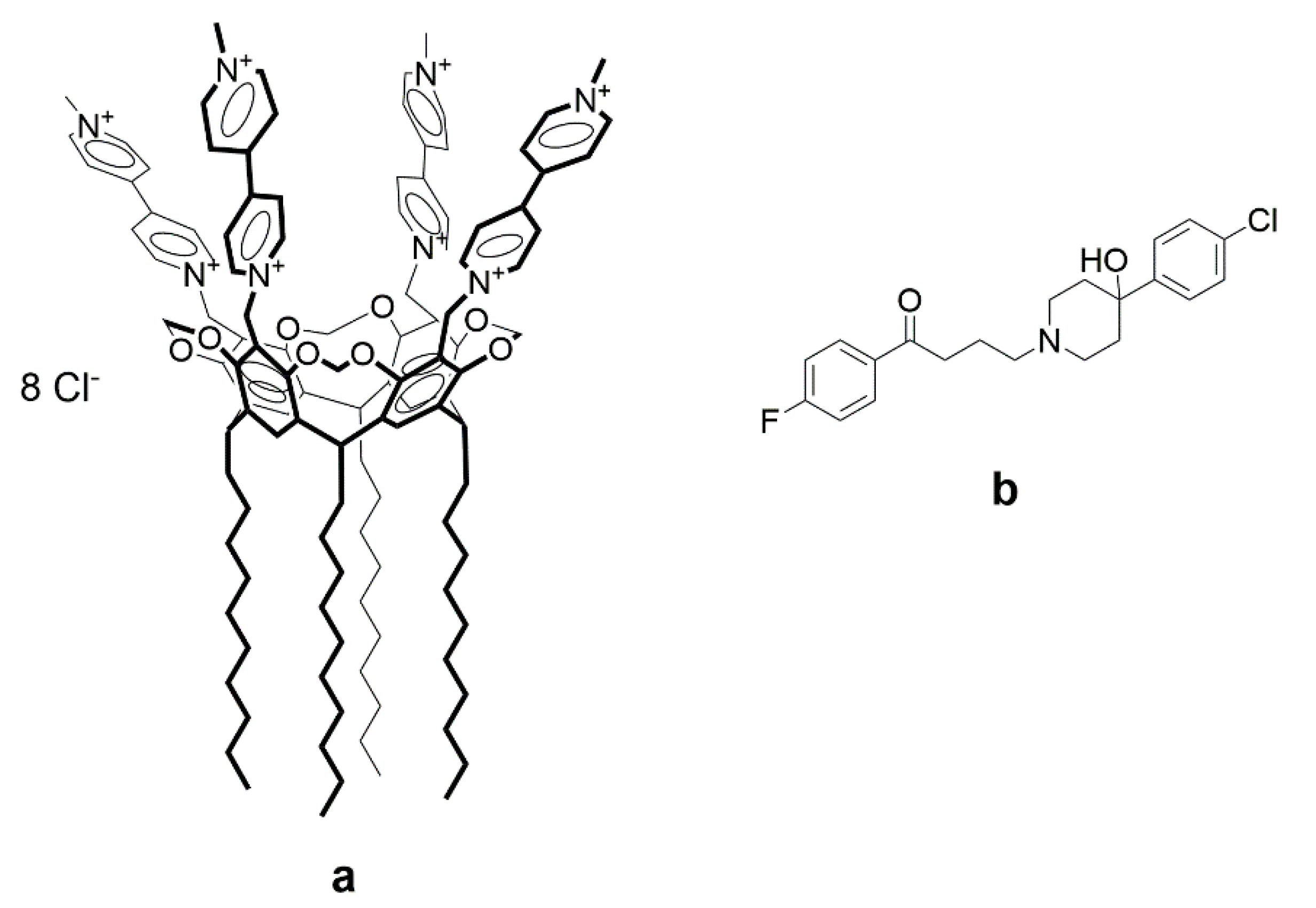
3.1. Coaggregation of Haloperidol and Viologen Decyl Calix[4]resorcinol
3.2. Morphology of Aggregates Based on Haloperidol and Viologen Decyl Calix[4]resorcinol
3.3. Mucoadhesive Properties of Calix[4]resorcinol–Haloperidol Nanoparticles
3.4. Toxicity Studies
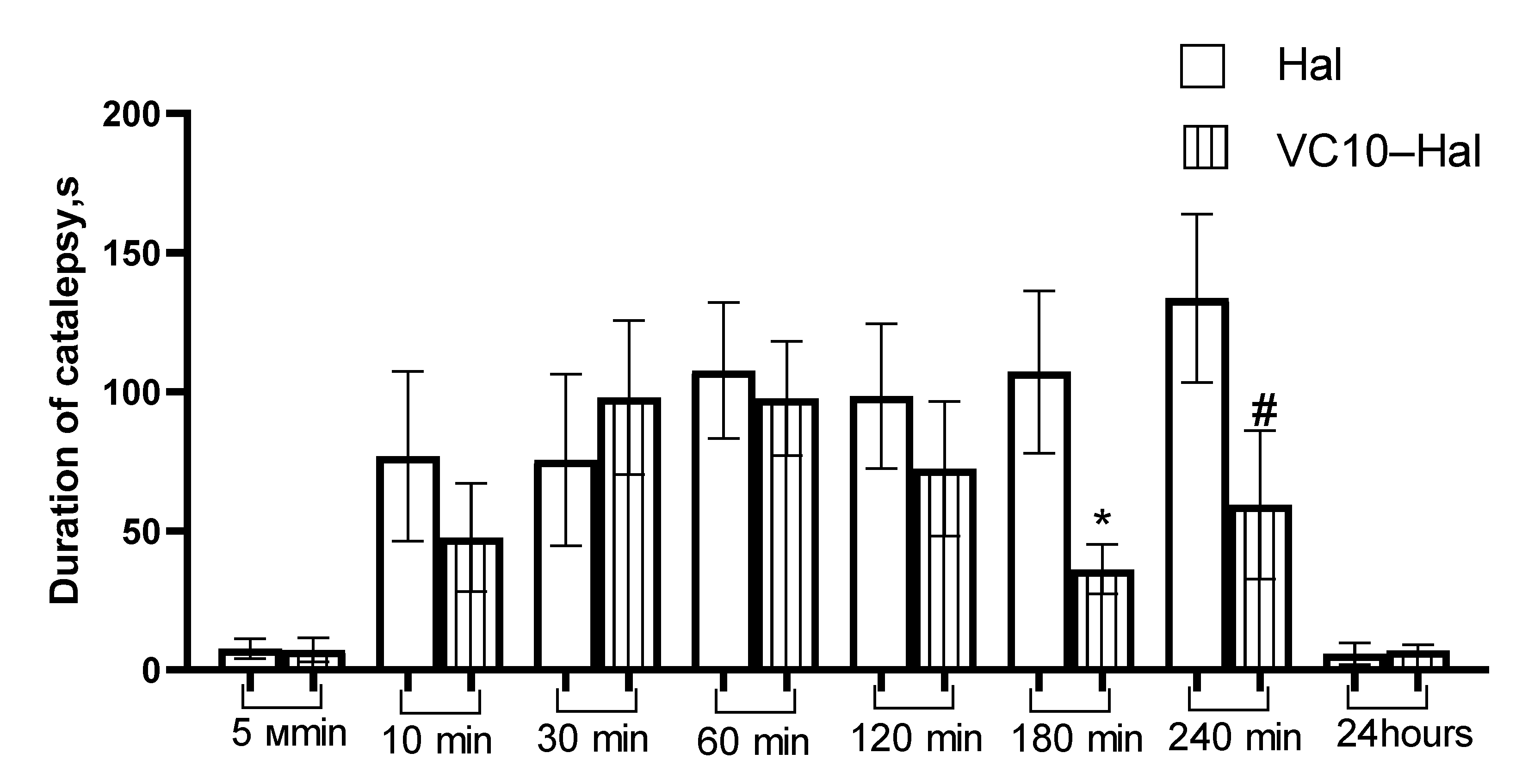
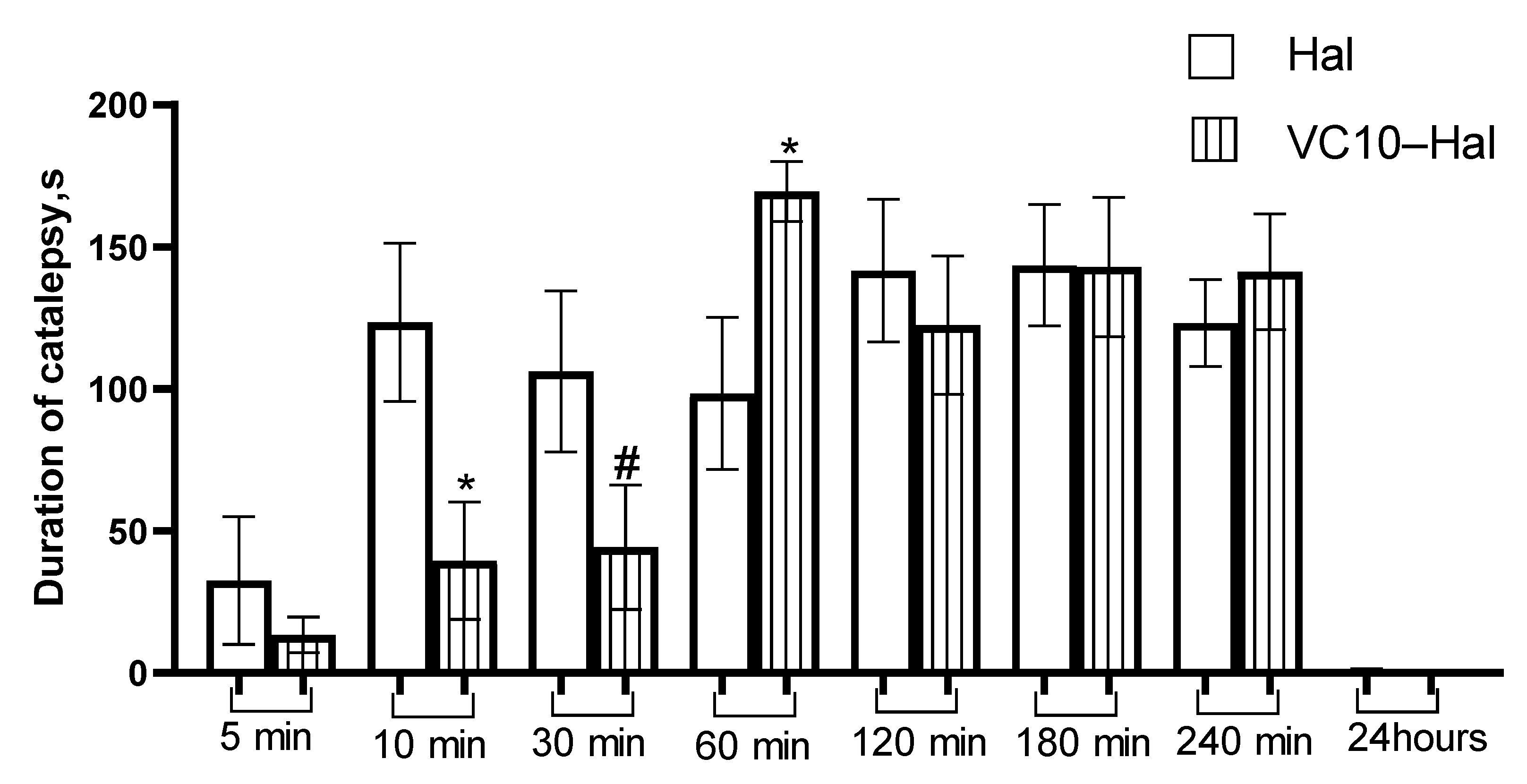
3.5. In Vivo Catalepsy Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gozalbes, R.; Jacewicz, M.; Annand, R.; Tsaioun, K.; Pineda-Lucena, A. QSAR-based permeability model for drug-like compounds. Bioorganic Med. Chem. 2011, 19, 2615–2624. [Google Scholar] [CrossRef]
- Šoltýsová, I.; Toropilová, D.; de Vringer, T. Lipid Based Formulations of Biopharmaceutics Classification System (BCS) Class II Drugs: Strategy, Formulations, Methods and Saturation. Folia Vet. 2016, 60, 63–69. [Google Scholar] [CrossRef]
- Shan, X.; Williams, A.C.; Khutoryanskiy, V.V. Polymer structure and property effects on solid dispersions with haloperidol: Poly(N-vinyl pyrrolidone) and poly(2-oxazolines) studies. Int. J. Pharm. 2020, 590, 119884. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xing, H.; Zhao, Y.; Ma, Z. Pharmaceutical dispersion techniques for dissolution and bioavailability enhancement of poorly water-soluble drugs. Pharmaceutics 2018, 10, 74. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Kim, D.S.; Mustapha, O.; Yousaf, A.M.; Kim, J.S.; Kim, D.W.; Yong, C.S.; Youn, Y.S.; Oh, K.T.; Lim, S.J.; et al. Comparison of a revaprazan-loaded solid dispersion, solid SNEDDS and inclusion compound: Physicochemical characterisation and pharmacokinetics. Colloids Surf. B Biointerfaces 2018, 162, 420–426. [Google Scholar] [CrossRef]
- Zhang, Y.; Ren, T.; Gou, J.; Zhang, L.; Tao, X.; Tian, B.; Tian, P.; Yu, D.; Song, J.; Liu, X.; et al. Strategies for improving the payload of small molecular drugs in polymeric micelles. J. Control. Release 2017, 261, 352–366. [Google Scholar] [CrossRef]
- Ibatullina, M.R.; Zhil’tsova, E.P.; Lukashenko, S.S.; Zakharova, L.Y. Supramolecular Systems of Metal Complexes of 1-Cetyl-4-aza-1-azoniabicyclo[2,2,2]octane Bromide for Increasing Griseofulvin Solubility. Colloid. J. 2020, 82, 8–15. [Google Scholar] [CrossRef]
- Zhou, S.; Shang, Q.; Wang, N.; Li, Q.; Song, A.; Luan, Y. Rational design of a minimalist nanoplatform to maximize immunotherapeutic efficacy: Four birds with one stone. J. Control Release 2020, 328, 617–630. [Google Scholar] [CrossRef]
- Zhang, M.; Qin, X.; Zhao, Z.; Du, Q.; Li, Q.; Jiang, Y.; Luan, Y. A self-amplifying nanodrug to manipulate the Janus-faced nature of ferroptosis for tumor therapy. Nanoscale Horiz. 2022, 7, 198–210. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, C.; Xiao, M.; Ganesan, K.; Gao, F.; Liu, Q.; Ye, Z.; Sui, Y.; Zhang, F.; Wei, K.; et al. A tumor-targeted delivery of oral isoliquiritigenin through encapsulated zein phosphatidylcholine hybrid nanoparticles prevents triple-negative breast cancer. J. Drug Deliv. Sci. Technol. 2023, 79, 103922. [Google Scholar] [CrossRef]
- Kicuntod, J.; Khuntawee, W.; Wolschann, P. Inclusion complexation of pinostrobin with various cyclodextrin derivatives. J. Mol. Graph. Model. 2016, 63, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Ezawa, T.; Inoue, Y.; Murata, I.; Takao, K.; Sugita, Y.; Kanamoto, I. Characterization of the Dissolution Behavior of Piperine/Cyclodextrins Inclusion Complexes. AAPS PharmSciTech 2018, 19, 923–933. [Google Scholar] [CrossRef] [PubMed]
- Morina, D.; Sessevmez, M.; Sinani, G.; Mülazımoğlu, L.; Cevher, E. Oral tablet formulations containing cyclodextrin complexes of poorly water soluble cefdinir to enhance its bioavailability. J. Drug Deliv. Sci. Technol. 2020, 57, 101742. [Google Scholar] [CrossRef]
- Sbârcea, L.; Tănase, I.-M.; Ledeți, A.; Cîrcioban, D.; Vlase, G.; Barvinschi, P.; Miclău, M.; Văruţ, R.-M.; Trandafirescu, C.; Ledeți, I. Encapsulation of Risperidone by Methylated β-Cyclodextrins: Physicochemical and Molecular Modeling Studies. Molecules 2020, 25, 5694. [Google Scholar] [CrossRef]
- Aloisio, C.; Antimisiaris, S.G.; Longhi, M.R. Liposomes containing cyclodextrins or meglumine to solubilize and improve the bioavailability of poorly soluble drugs. J. Mol. Liq. 2017, 229, 106–113. [Google Scholar] [CrossRef]
- Qian, X.; Wang, G.; Li, J.; Zhang, X.; Zhang, M.; Yang, Q.; Zhang, Z.; Li, Y. Improving oral bioavailability of water-insoluble idebenone with bioadhesive liposomes. J. Drug Deliv. Sci. Technol. 2022, 75, 103640. [Google Scholar] [CrossRef]
- Yang, W.; de Villiers, M.M. Aqueous solubilization of furosemide by supramolecular complexation with 4-sulphonic calix[n]arenes. J. Pharm. Pharmacol. 2004, 56, 703–708. [Google Scholar] [CrossRef]
- Yang, W.; Otto, D.; Liebenberg, W.; de Villiers, M. Effect of para-Sulfonato-Calix[n]arenes on the Solubility, Chemical Stability, and Bioavailability of a Water Insoluble Drug Nifedipine. Curr. Drug Discov. Technol. 2008, 5, 129–139. [Google Scholar] [CrossRef]
- Panchal, J.G.; Patel, R.V.; Menon, S.K. Preparation and physicochemical characterization of carbamazepine (CBMZ): Para-sulfonated calix[n]arene inclusion complexes. J. Incl. Phenom. Macrocycl. Chem. 2010, 67, 201–208. [Google Scholar] [CrossRef]
- Patel, M.B.; Valand, N.N.; Modi, N.R.; Joshi, K.V.; Harikrishnan, U.; Kumar, S.P.; Jasrai, Y.T.; Menon, S.K. Effect of p-sulfonatocalix[4]resorcinarene (PSC[4]R) on the solubility and bioavailability of a poorly water soluble drug lamotrigine (LMN) and computational investigation. RSC Adv. 2013, 3, 15971–15981. [Google Scholar] [CrossRef]
- Yasir, M.; Sara, U.V.S. Solid lipid nanoparticles for nose to brain delivery of haloperidol: In vitro drug release and pharmacokinetics evaluation. Acta Pharm. Sin. B 2014, 4, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Porfiryeva, N.N.; Semina, I.I.; Salakhov, I.A.; Moustafine, R.I.; Khutoryanskiy, V.V. Mucoadhesive and mucus-penetrating interpolyelectrolyte complexes for nose-to-brain drug delivery. Nanomed. Nanotechnol. Biol. Med. 2021, 37, 102432. [Google Scholar] [CrossRef] [PubMed]
- Filippov, S.K.; Khusnutdinov, R.R.; Inham, W.; Liu, C.; Nikitin, D.O.; Semina, I.I.; Garvey, C.J.; Nasibullin, S.F.; Khutoryanskiy, V.V.; Zhang, H.; et al. Hybrid nanoparticles for haloperidol encapsulation: Quid est optimum? Polymers 2021, 13, 4189. [Google Scholar] [CrossRef]
- Kashapov, R.R.; Kashapova, N.E.; Ziganshina, A.Y.; Syakaev, V.V.; Khutoryanskiy, V.V.; Zakharova, L.Y. Interaction of mucin with viologen and acetate derivatives of calix[4]resorcinols. Colloids Surf. B Biointerfaces 2021, 208, 112089. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Zhang, Z.; Yu, X.; Bai, B.; Qi, S. Hydrophilic Tetraphenylethene-Based Tetracationic Cyclophanes: NADPH Recognition and Cell Imaging with Fluorescent Switch. Front. Chem. 2021, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ziganshina, A.Y.; Kharlamov, S.V.; Korshin, D.E.; Mukhitova, R.K.; Kazakova, E.K.; Latypov, S.K.; Yanilkin, V.V.; Konovalov, A.I. Electrochemical behaviour of a molecular capsule based on methylviologen-resorcinarene and sulfonatomethylene-resorcinarene. Tetrahedron Lett. 2008, 49, 5312–5315. [Google Scholar] [CrossRef]
- Mironov, A.N.; Bunyatyan, N.D.; Vasiliev, A.N.; Verstakova, O.L.; Zhuravleva, M.V.; Lepakhin, V.K.; Korobov, N.V.; Merkulov, V.A.; Orekhov, S.N.; Sakaeva, I.V.; et al. Guidelines for Conducting Preclinical Studies of Drugs; Grif and K: Tula, Russia, 2012; 944p, ISBN 9785812514663. [Google Scholar]
- Sestakova, N.; Puzserova, A.; Kluknavsky, M.; Bernatova, I. Determination of motor activity and anxiety-related behaviour in rodents: Methodological aspects and role of nitric oxide. Interdiscip. Toxicol. 2013, 6, 126–135. [Google Scholar] [CrossRef]
- Henry, B.L.; Minassian, A.; Young, J.W.; Paulus, M.P.; Geyer, M.A.; Perry, W. Cross-species assessments of motor and exploratory behavior related to bipolar disorder. Neurosci. Biobehav. Rev. 2010, 34, 1296–1306. [Google Scholar] [CrossRef]
- Miyamoto, S.; Duncan, G.E.; Marx, C.E.; Lieberman, J.A. Treatments for schizophrenia: A critical review of pharmacology and mechanisms of action of antipsychotic drugs. Mol. Psychiatry 2005, 10, 79–104. [Google Scholar] [CrossRef]
- Vogel, H.G. Psychotropic and Neurotropic Activity. In Drug Discovery and Evaluation; Springer: Berlin/Heidelberg, Germany, 2007; pp. 565–876. ISBN 978-3-540-71420-0. [Google Scholar]
- Natfji, A.A.; Nikitin, D.O.; Semina, I.I.; Moustafine, R.I.; Khutoryanskiy, V.V.; Lin, H.; Stephens, G.J.; Watson, K.A.; Osborn, H.M.I.; Greco, F. Conjugation of haloperidol to PEG allows peripheral localisation of haloperidol and eliminates CNS extrapyramidal effects. J. Control. Release 2020, 322, 227–235. [Google Scholar] [CrossRef]
- Kashapov, R.; Razuvayeva, Y.; Ziganshina, A.; Sapunova, A.; Lyubina, A.; Amerhanova, S.; Kulik, N.; Voloshina, A.; Nizameev, I.; Salnikov, V.; et al. Effect of preorganization and amphiphilicity of calix[4]arene platform on functional properties of viologen derivatives. J. Mol. Liq. 2022, 345, 117801. [Google Scholar] [CrossRef]
- Kashapov, R.R.; Kharlamov, S.V.; Sultanova, E.D.; Mukhitova, R.K.; Kudryashova, Y.R.; Zakharova, L.Y.; Ziganshina, A.Y.; Konovalov, A.I. Controlling the size and morphology of supramolecular assemblies of viologen-resorcin[4]arene cavitands. Chem.-A Eur. J. 2014, 20, 14018–14025. [Google Scholar] [CrossRef] [PubMed]
- Gadelle, F.; Koros, W.J.; Schechter, R.S. Solubilization of Aromatic Solutes in Block Copolymers. Macromolecules 1995, 28, 4883–4892. [Google Scholar] [CrossRef]
- Shah, V.; Bharatiya, B.; Patel, V.; Mishra, M.K.; Shukla, A.D.; Shah, D.O. Interaction of salicylic acid analogues with Pluronic® micelles: Investigations on micellar growth and morphological transition. J. Mol. Liq. 2019, 277, 563–570. [Google Scholar] [CrossRef]
- Hanio, S.; Schlauersbach, J.; Lenz, B.; Spiegel, F.; Böckmann, R.A.; Schweins, R.; Nischang, I.; Schubert, U.S.; Endres, S.; Pöppler, A.C.; et al. Drug-Induced Dynamics of Bile Colloids. Langmuir 2021, 37, 2543–2551. [Google Scholar] [CrossRef] [PubMed]
- Albarkah, Y.A.; Green, R.J.; Khutoryanskiy, V.V. Probing the Mucoadhesive Interactions between Porcine Gastric Mucin and Some Water-Soluble Polymers. Macromol. Biosci. 2015, 15, 1546–1553. [Google Scholar] [CrossRef]
- Fraiji, L.K.; Hayes, D.M.; Werner, T.C. Static and dynamic fluorescence quenching experiments for the physical chemistry laboratory. J. Chem. Educ. 1992, 69, 424–428. [Google Scholar] [CrossRef]
- Lone, M.S.; Afzal, S.; Chat, O.A.; Aswal, V.K.; Dar, A.A. Temperature- and Composition-Induced Multiarchitectural Transitions in the Catanionic System of a Conventional Surfactant and a Surface-Active Ionic Liquid. ACS Omega 2021, 6, 11974–11987. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, L.; Huang, X.; Tan, X.; Luo, T.; Li, W. Temperature-induced vesicle to micelle transition in cationic/cationic mixed surfactant systems. Soft Matter 2015, 11, 8848–8855. [Google Scholar] [CrossRef]
- Nikogeorgos, N.; Efler, P.; Kayitmazer, A.B.; Lee, S. “Bio-glues” to enhance slipperiness of mucins: Improved lubricity and wear resistance of porcine gastric mucin (PGM) layers assisted by mucoadhesion with chitosan. Soft Matter 2015, 11, 489–498. [Google Scholar] [CrossRef]
- Shrivastava, H.Y.; Nair, B.U. Structural modification and aggregation of mucin by chromium(III) complexes. J. Biomol. Struct. Dyn. 2003, 20, 575–587. [Google Scholar] [CrossRef] [PubMed]
- Kandsi, F.; Lafdil, F.Z.; Elbouzidi, A.; Bouknana, S.; Miry, A.; Addi, M.; Conte, R.; Hano, C.; Gseyra, N. Evaluation of Acute and Subacute Toxicity and LC-MS/MS Compositional Alkaloid Determination of the Hydroethanolic Extract of Dysphania ambrosioides (L.) Mosyakin and Clemants Flowers. Toxins 2022, 14, 475. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, D.C.; Donovan, H. Catalepsy as a rodent model for detecting antipsychotic drugs with extrapyramidal side effect liability. Psychopharmacology 1995, 120, 128–133. [Google Scholar] [CrossRef]
- Pandey, M.; Jain, N.; Kanoujia, J.; Hussain, Z.; Gorain, B. Advances and Challenges in Intranasal Delivery of Antipsychotic Agents Targeting the Central Nervous System. Front. Pharmacol. 2022, 13, 865590. [Google Scholar] [CrossRef] [PubMed]



| Ksv 103 (M−1) | |||
|---|---|---|---|
| 298 K | 304 K | 310 K | |
| PGM–VC10 | 1762 ± 28 | 1635 ± 42 | 1516 ± 42 |
| PGM–[VC10–Hal] | 1702 ± 39 | 1563 ± 39 | 1405 ± 25 |
| PGM–VC10 | PGM–[VC10–Hal] | |||||
|---|---|---|---|---|---|---|
| n | logKa | Kd 10−8 (M) | n | logKa | Kd 10−8 (M) | |
| 298 K | 1.21 ± 0.01 | 7.47 ± 0.06 | 3.40 | 1.30 ± 0.01 | 7.96 ± 0.04 | 1.10 |
| 304 K | 1.17 ± 0.03 | 7.18 ± 0.17 | 6.28 | 1.12 ± 0.02 | 6.87 ± 0.11 | 13.25 |
| 310 K | 1.13 ± 0.04 | 6.90 ± 0.29 | 11.06 | 1.05 ± 0.04 | 6.45 ± 0.22 | 32.71 |
| ΔG° (kJ·mol−1) | |||
|---|---|---|---|
| 298 K | 304 K | 310 K | |
| PGM–VC10 | −42.62 | −41.79 | −40.95 |
| PGM–[VC10–Hal] | −45.41 | −39.98 | −38.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kashapova, N.E.; Kashapov, R.R.; Ziganshina, A.Y.; Nikitin, D.O.; Semina, I.I.; Salnikov, V.V.; Khutoryanskiy, V.V.; Moustafine, R.I.; Zakharova, L.Y. Cataleptogenic Effect of Haloperidol Formulated in Water-Soluble Calixarene-Based Nanoparticles. Pharmaceutics 2023, 15, 921. https://doi.org/10.3390/pharmaceutics15030921
Kashapova NE, Kashapov RR, Ziganshina AY, Nikitin DO, Semina II, Salnikov VV, Khutoryanskiy VV, Moustafine RI, Zakharova LY. Cataleptogenic Effect of Haloperidol Formulated in Water-Soluble Calixarene-Based Nanoparticles. Pharmaceutics. 2023; 15(3):921. https://doi.org/10.3390/pharmaceutics15030921
Chicago/Turabian StyleKashapova, Nadezda E., Ruslan R. Kashapov, Albina Y. Ziganshina, Dmitry O. Nikitin, Irina I. Semina, Vadim V. Salnikov, Vitaliy V. Khutoryanskiy, Rouslan I. Moustafine, and Lucia Y. Zakharova. 2023. "Cataleptogenic Effect of Haloperidol Formulated in Water-Soluble Calixarene-Based Nanoparticles" Pharmaceutics 15, no. 3: 921. https://doi.org/10.3390/pharmaceutics15030921
APA StyleKashapova, N. E., Kashapov, R. R., Ziganshina, A. Y., Nikitin, D. O., Semina, I. I., Salnikov, V. V., Khutoryanskiy, V. V., Moustafine, R. I., & Zakharova, L. Y. (2023). Cataleptogenic Effect of Haloperidol Formulated in Water-Soluble Calixarene-Based Nanoparticles. Pharmaceutics, 15(3), 921. https://doi.org/10.3390/pharmaceutics15030921











