Sustainable Nanomaterials for Biomedical Applications
Abstract
1. Introduction
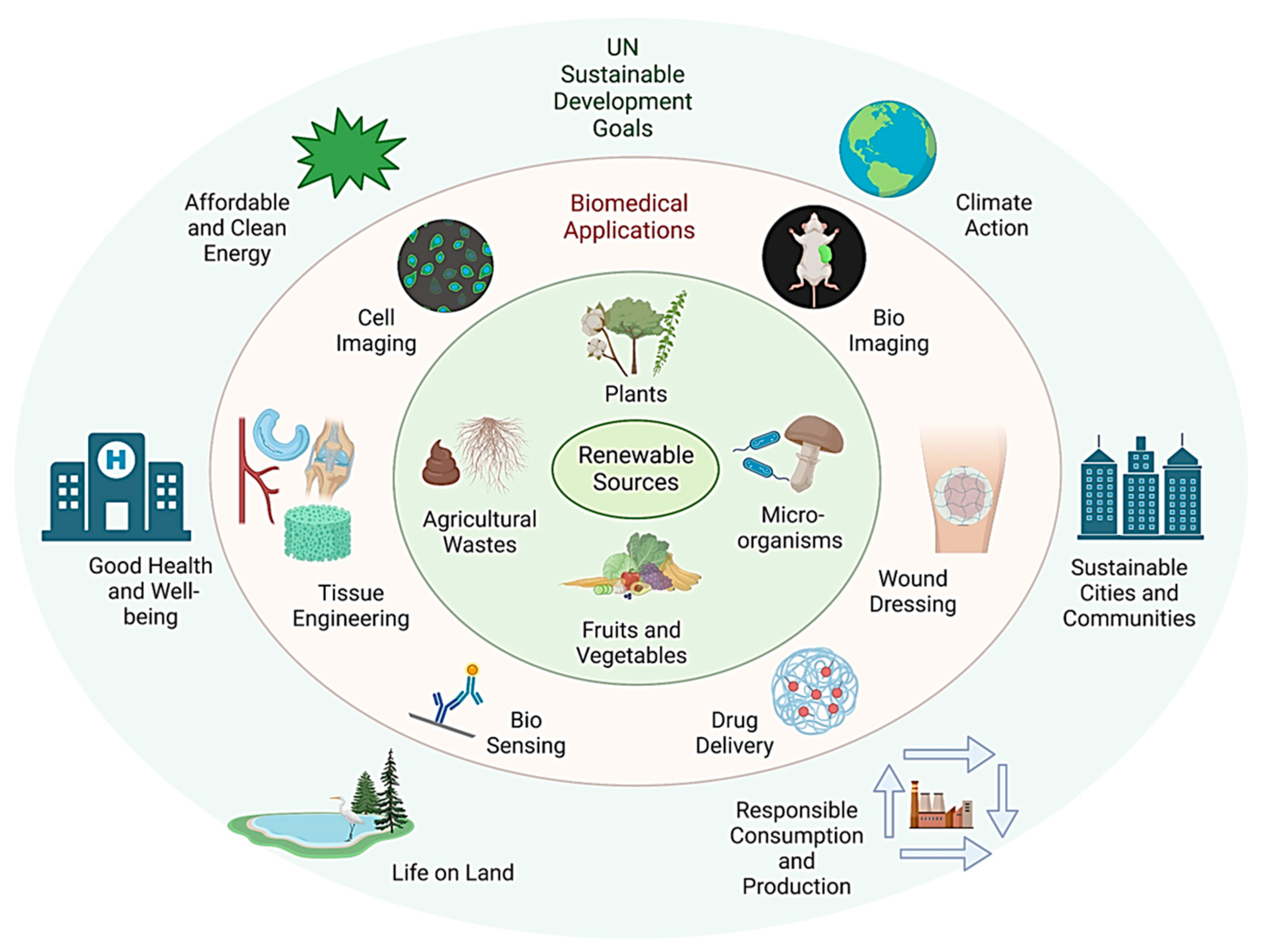
2. Design Framework for Sustainable Nanomaterials
3. Sustainable Nanomaterials for Biomedical Applications
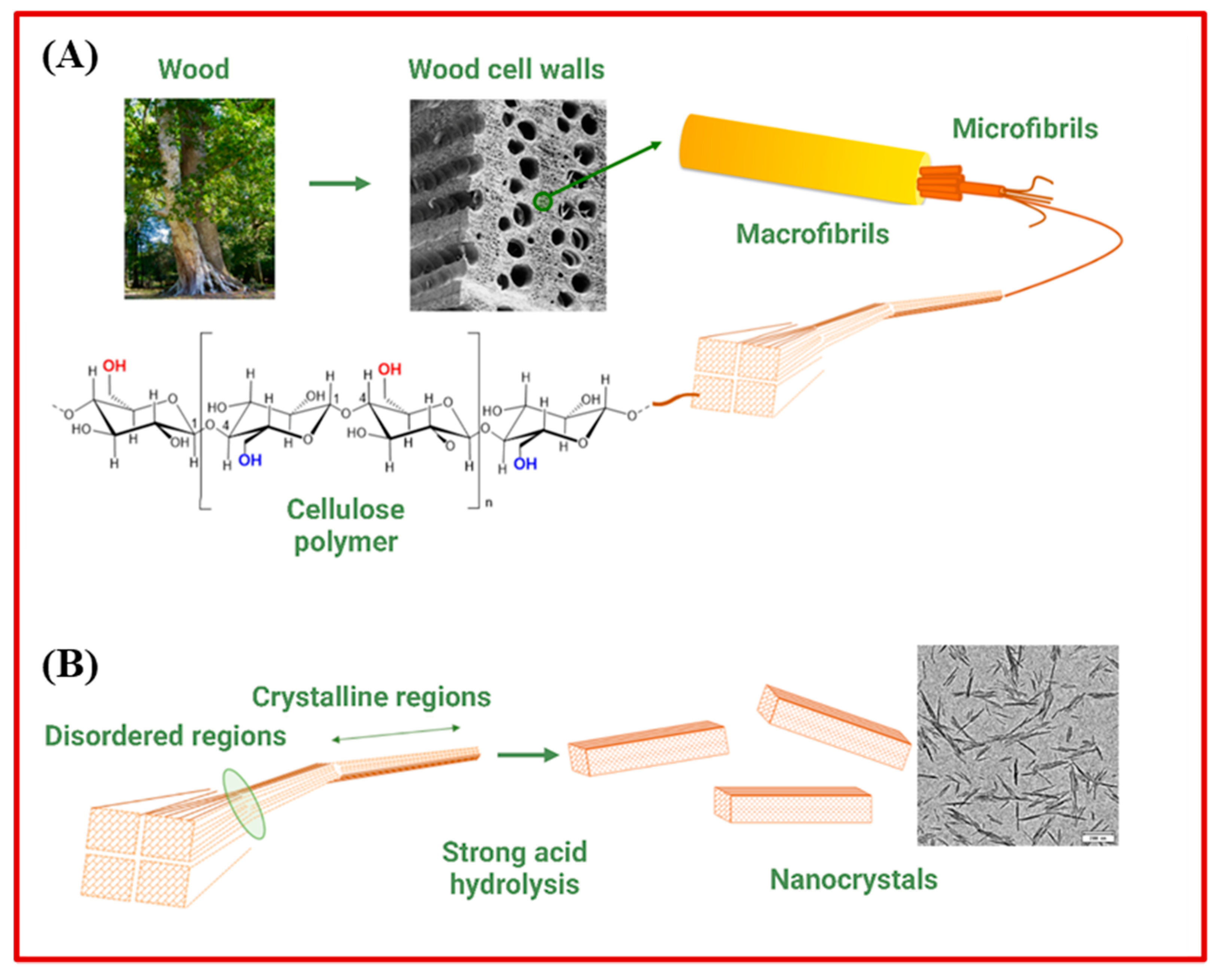
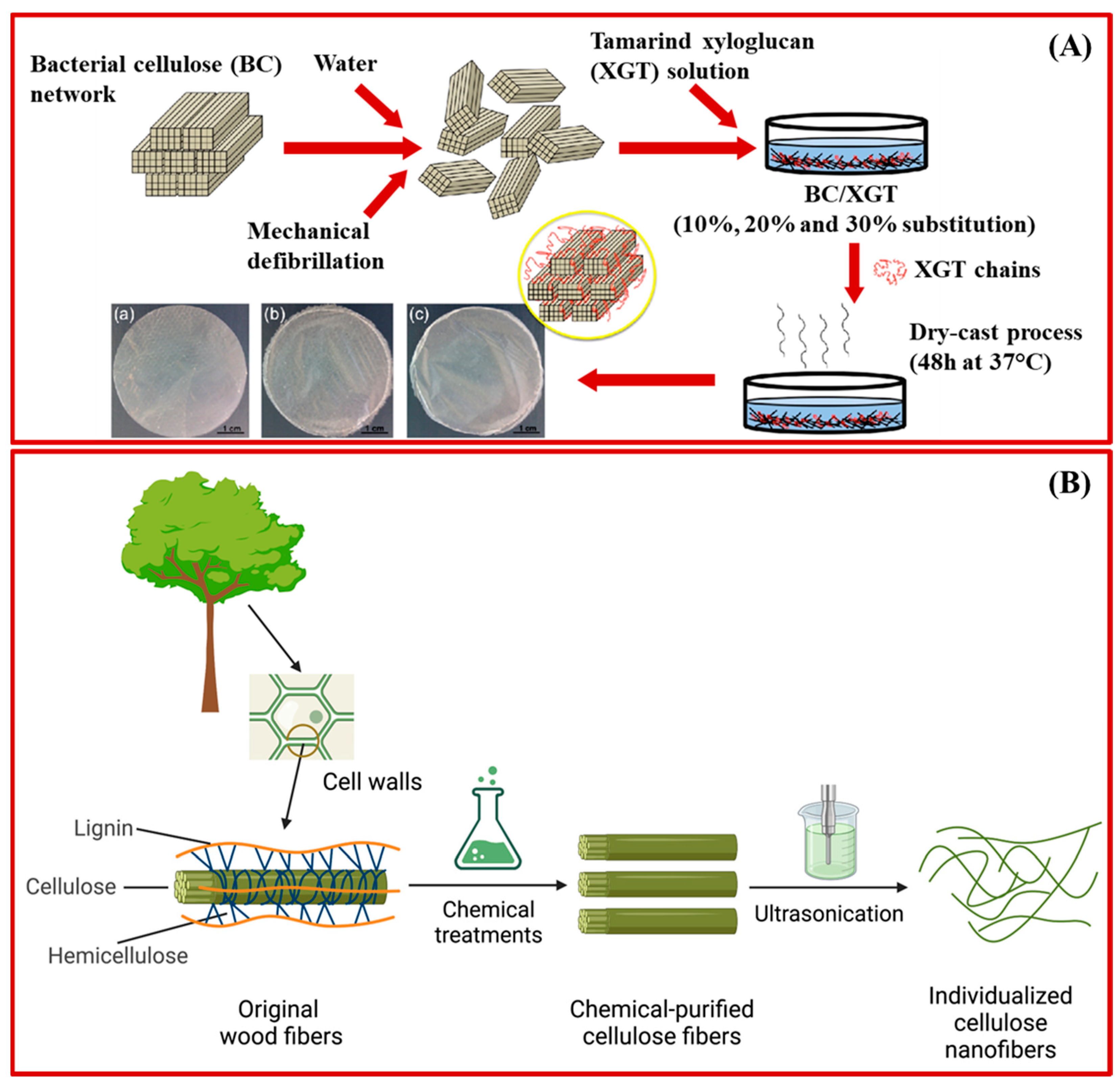
3.1. Sustainable Polymeric Nanomaterials
| Sustainable Nanomaterials | Natural Source | Biomedical Application |
|---|---|---|
| Cellulose Nanocrystals (CNCs) | Wood, cotton, and other cellulose-rich sources | Antimicrobial purpose [59,60,61,66] Bioimaging (osteoblasts cellular imaging [67]) Biosensing (DNA sensing [68]) Tissue engineering (fibroblasts proliferation [69]) Drug delivery [70,71] |
| Cellulose Nanofibers (CNFs) | Antimicrobial purpose [72,73,74] Tissue engineering scaffold (bone [75], ligament and tendon [29]) Ion-exchange membrane (DNA extraction and hemodialysis [76]) Reinforcing agent [77] Drug delivery [78] | |
| Bacterial Nanocellulose (BNC) | Glucose molecule | Functionalized wound dressing (antimicrobial [64,65]) Soft tissue engineering (cartilage, [79,80], bone [81], vessels [79]) |
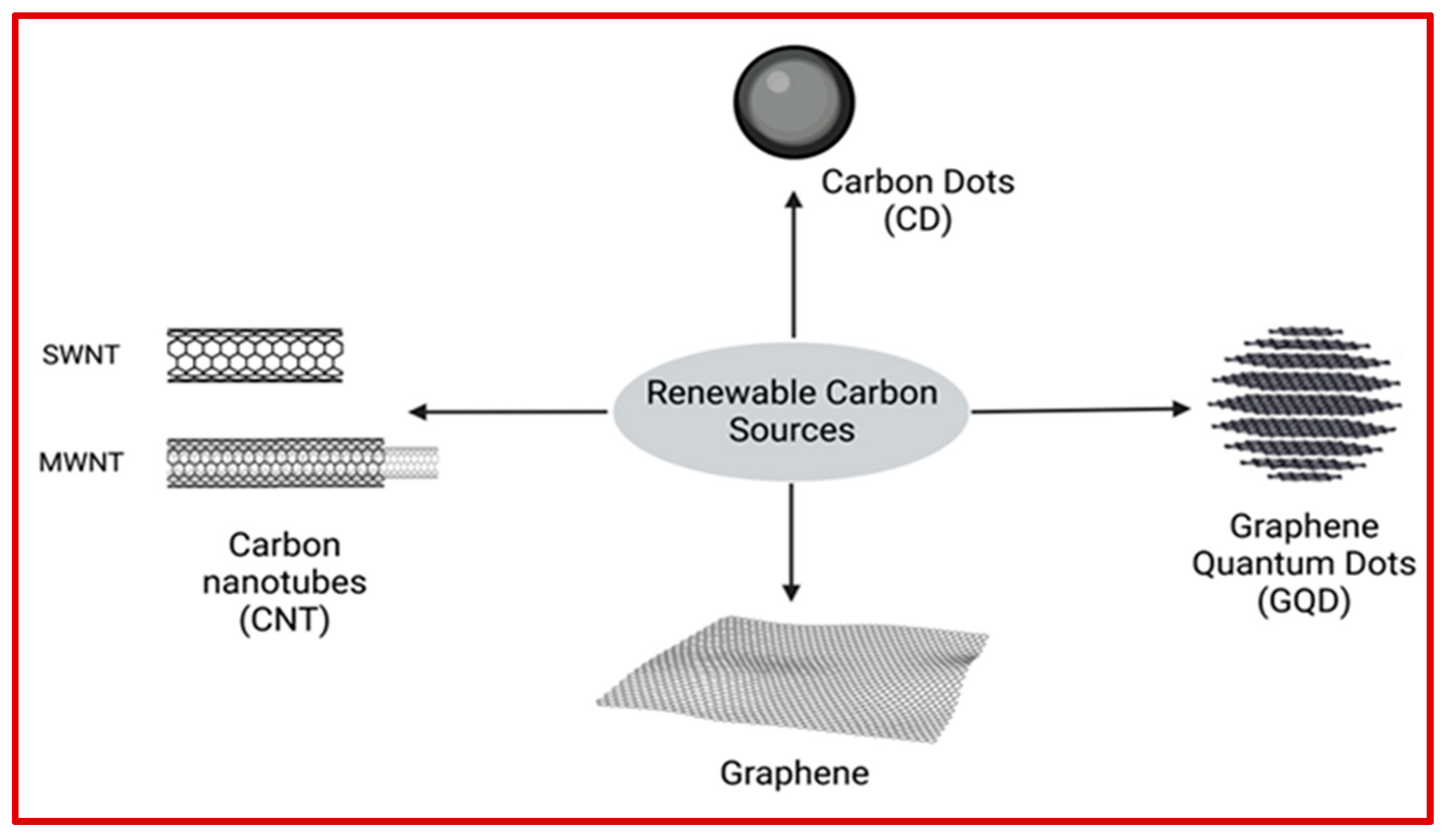
3.2. Carbonaceous Sustainable Nanomaterials
3.2.1. Carbon Dots and Graphene Quantum Dots
| Sustainable Nanomaterials | Sustainable Source | Biomedical Application |
|---|---|---|
| Carbon dots (CDs) | Organic pollutants/wastes, vegetables, fruits, woolds, cottons, oyster mushroom | Biosensing (DNA [101,103,104,105], protein and disease-related biomolecules [106]) Cellular bioimaging (bone regeneration monitoring [107]) Drug delivery [108] |
| Graphene quantum dots (GDs) | Biosensing (DNA, protein, disease-related biomolecules) [109] Cellular bioimaging [110] Drug delivery [108] | |
| Graphene | Organic carbonaceous wastes (bone, bagasse newspapers), plants, and natural | Limited due to aggregation in tissue |
| Graphene oxide (GOX) | Drug delivery [111] and transdermal drug delivery [112] Microelectrodes [113] Biosensing and tissue engineering | |
| Carbon nanotubes (CNTs) | plants (leaves, seeds, roots, and stem), waste cooking oil | Electromechanical actuators [114] Biosensors (miRNA [115]) Cellular bioimaging [116] Drug delivery [117] Photothermal cancer therapy [118] Nano tweezers [119,120] |
3.2.2. Graphene
3.2.3. Carbon Nanotubes
3.3. Sustainable Bioceramics
4. Conclusions and Future Opportunities
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Horejs, C. Lasting impact of lipid nanoparticles. Nat. Rev. Mater. 2021, 6, 1071. [Google Scholar] [CrossRef]
- Jayathilaka, W.A.D.M.; Qi, K.; Qin, Y.; Chinnappan, A.; Serrano-García, W.; Baskar, C.; Wang, H.; He, J.; Cui, S.; Thomas, S.W.; et al. Significance of Nanomaterials in Wearables: A Review on Wearable Actuators and Sensors. Adv. Mater. 2019, 31, 1805921. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Trotsyuk, A.A.; Niu, S.; Henn, D.; Chen, K.; Shih, C.-C.; Larson, M.R.; Mermin-Bunnell, A.M.; Mittal, S.; Lai, J.-C.; et al. Wireless, closed-loop, smart bandage with integrated sensors and stimulators for advanced wound care and accelerated healing. Nat. Biotechnol. 2022, 9, 57. [Google Scholar] [CrossRef]
- Ruggeri, M.; Bianchi, E.; Rossi, S.; Vigani, B.; Bonferoni, M.C.; Caramella, C.; Sandri, G.; Ferrari, F. Nanotechnology-Based Medical Devices for the Treatment of Chronic Skin Lesions: From Research to the Clinic. Pharmaceutics 2020, 12, 815. [Google Scholar] [CrossRef]
- Seo, G.; Lee, G.; Kim, M.J.; Baek, S.-H.; Choi, M.; Ku, K.B.; Lee, C.-S.; Jun, S.; Park, D.; Kim, H.G.; et al. Rapid Detection of COVID-19 Causative Virus (SARS-CoV-2) in Human Nasopharyngeal Swab Specimens Using Field-Effect Transistor-Based Biosensor. ACS Nano 2020, 14, 5135–5142. [Google Scholar] [CrossRef]
- Chopra, D.; Gulati, K.; Ivanovski, S. Understanding and optimizing the antibacterial functions of anodized nano-engineered titanium implants. Acta Biomater. 2021, 127, 80–101. [Google Scholar] [CrossRef] [PubMed]
- Hald Albertsen, C.; Kulkarni, J.A.; Witzigmann, D.; Lind, M.; Petersson, K.; Simonsen, J.B. The role of lipid components in lipid nanoparticles for vaccines and gene therapy. Adv. Drug Deliv. Rev. 2022, 188, 114416. [Google Scholar] [CrossRef] [PubMed]
- Poon, K.; Lu, Z.; De Deene, Y.; Ramaswamy, Y.; Zreiqat, H.; Singh, G. Tuneable manganese oxide nanoparticle based theranostic agents for potential diagnosis and drug delivery. Nanoscale Adv. 2021, 3, 4052–4061. [Google Scholar] [CrossRef]
- Harper-Harris, J.; Kant, K.; Singh, G. Oleic Acid-Assisted Synthesis of Tunable High-Aspect-Ratio Multiply-Twinned Gold Nanorods for Bioimaging. ACS Appl. Nano Mater. 2021, 4, 3325–3330. [Google Scholar] [CrossRef]
- Ouyang, C.; Zhang, S.; Xue, C.; Yu, X.; Xu, H.; Wang, Z.; Lu, Y.; Wu, Z.-S. Precision-Guided Missile-Like DNA Nanostructure Containing Warhead and Guidance Control for Aptamer-Based Targeted Drug Delivery into Cancer Cells in Vitro and in Vivo. J. Am. Chem. Soc. 2020, 142, 1265–1277. [Google Scholar] [CrossRef] [PubMed]
- Żuk, M.; Podgórski, R.; Ruszczyńska, A.; Ciach, T.; Majkowska-Pilip, A.; Bilewicz, A.; Krysiński, P. Multifunctional Nanoparticles Based on Iron Oxide and Gold-198 Designed for Magnetic Hyperthermia and Radionuclide Therapy as a Potential Tool for Combined HER2-Positive Cancer Treatment. Pharmaceutics 2022, 14, 1680. [Google Scholar] [CrossRef] [PubMed]
- Colvin, V.L. The potential environmental impact of engineered nanomaterials. Nat. Biotechnol. 2003, 21, 1166–1170. [Google Scholar] [CrossRef]
- Li, X.; Wu, X.; Yang, H.; Li, L.; Ye, Z.; Rao, Y. A nuclear targeted Dox-aptamer loaded liposome delivery platform for the circumvention of drug resistance in breast cancer. Biomed. Pharma. 2019, 117, 109072. [Google Scholar] [CrossRef]
- Tran, H.-V.; Ngo, N.M.; Medhi, R.; Srinoi, P.; Liu, T.; Rittikulsittichai, S.; Lee, T.R. Multifunctional Iron Oxide Magnetic Nanoparticles for Biomedical Applications: A Review. Materials 2022, 15, 503. [Google Scholar] [CrossRef] [PubMed]
- Hadden, M.; Martinez-Martin, D.; Yong, K.-T.; Ramaswamy, Y.; Singh, G. Recent Advancements in the Fabrication of Functional Nanoporous Materials and Their Biomedical Applications. Materials 2022, 15, 2111. [Google Scholar] [CrossRef] [PubMed]
- Gaur, M.; Misra, C.; Yadav, A.B.; Swaroop, S.; Maolmhuaidh, F.Ó.; Bechelany, M.; Barhoum, A. Biomedical Applications of Carbon Nanomaterials: Fullerenes, Quantum Dots, Nanotubes, Nanofibers, and Graphene. Materials 2021, 14, 5978. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Z.; Pan, Z.; Liu, Y. Advanced bioactive nanomaterials for biomedical applications. Exploration 2021, 1, 20210089. [Google Scholar] [CrossRef]
- Singh, G.; Jami, H.; Lesani, P.; Bhattacharya, S.; Ramaswamy, Y.; Bisht, P.B.; Zreiqat, H. Evolution of stellated gold nanoparticles: New conceptual insights into controlling the surface processes. Nano Res. 2022, 15, 1260–1268. [Google Scholar] [CrossRef]
- Singh, G.; Agrawal, T.; Lesani, P.; Bisht, P.B.; Zreiqat, H. Tuning the size, concaveness, and aspect ratio of concave cubic gold nanoparticles produced with high reproducibility. Mater. Today Chem. 2022, 23, 100657. [Google Scholar] [CrossRef]
- McDonagh, B.H.; Staudinger, C.; Normile, P.S.; De Toro, J.A.; Bandyopadhyay, S.; Glomm, W.R.; Singh, G. New insights into controlling the twin structure of magnetic iron oxide nanoparticles. Appl. Mater. Today 2021, 24, 101084. [Google Scholar] [CrossRef]
- Khor, S.Y.; Quinn, J.F.; Whittaker, M.R.; Truong, N.P.; Davis, T.P. Controlling Nanomaterial Size and Shape for Biomedical Applications via Polymerization-Induced Self-Assembly. Macromol. Rapid Commun. 2019, 40, 1800438. [Google Scholar] [CrossRef] [PubMed]
- Masa, J.; Andronescu, C.; Schuhmann, W. Electrocatalysis as the Nexus for Sustainable Renewable Energy: The Gordian Knot of Activity, Stability, and Selectivity. Angew. Chem. Int. Ed. 2020, 59, 15298–15312. [Google Scholar] [CrossRef]
- Chen, K.; Kong, J.; Zhu, J.; Ermann, N.; Predki, P.; Keyser, U.F. Digital Data Storage Using DNA Nanostructures and Solid-State Nanopores. Nano Lett. 2019, 19, 1210–1215. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Pillai, S.C.; Ho, S.-H.; Zeng, J.; Li, Y.; Dionysiou, D.D. Plasmonic-based nanomaterials for environmental remediation. Appl. Catal. B Environ. 2018, 237, 721–741. [Google Scholar] [CrossRef]
- Yang, J.; Hou, B.; Wang, J.; Tian, B.; Bi, J.; Wang, N.; Li, X.; Huang, X. Nanomaterials for the Removal of Heavy Metals from Wastewater. Nanomaterials 2019, 9, 424. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Kim, K.Y.; Lee, S.H.; Kim, K.K.; Lee, K.; Lee, W.; Jeon, H.; Ko, S.H. Recent Advances in 1D Nanomaterial-Based Bioelectronics for Healthcare Applications. Adv. NanoBiomed. Res. 2022, 2, 2100111. [Google Scholar] [CrossRef]
- Zhang, S.; Jiang, S.-F.; Huang, B.-C.; Shen, X.-C.; Chen, W.-J.; Zhou, T.-P.; Cheng, H.-Y.; Cheng, B.-H.; Wu, C.-Z.; Li, W.-W.; et al. Sustainable production of value-added carbon nanomaterials from biomass pyrolysis. Nat. Sustain. 2020, 3, 753–760. [Google Scholar] [CrossRef]
- Esposito, D.; Antonietti, M. Redefining biorefinery: The search for unconventional building blocks for materials. Chem. Soc. Rev. 2015, 44, 5821–5835. [Google Scholar] [CrossRef]
- Vásquez-Garay, F.; Carrillo-Varela, I.; Vidal, C.; Reyes-Contreras, P.; Faccini, M.; Teixeira Mendonça, R. A Review on the Lignin Biopolymer and Its Integration in the Elaboration of Sustainable Materials. Sustainability 2021, 13, 697. [Google Scholar] [CrossRef]
- Chee, P.L.; Toh, W.L.; Yew, P.Y.; Peng, S.; Kai, D. Chapter 1: Introduction of Nanotechnology and Sustainability. In Sustainable Nanotechnology; The Royal Society of Chemistry: London, UK, 2022; pp. 1–32. [Google Scholar]
- Papageorgiou, G.Z. Thinking Green: Sustainable Polymers from Renewable Resources. Polymers 2018, 10, 952. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Romain, C.; Williams, C.K. Sustainable polymers from renewable resources. Nature 2016, 540, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Chung, I.M.; Park, I.; Seung-Hyun, K.; Thiruvengadam, M.; Rajakumar, G. Plant-Mediated Synthesis of Silver Nanoparticles: Their Characteristic Properties and Therapeutic Applications. Nanoscale Res. Lett. 2016, 11, 40. [Google Scholar] [CrossRef]
- Koul, B.; Poonia, A.K.; Yadav, D.; Jin, J.O. Microbe-Mediated Biosynthesis of Nanoparticles: Applications and Future Prospects. Biomolecules 2021, 11, 886. [Google Scholar] [CrossRef]
- Uzair, B.; Liaqat, A.; Iqbal, H.; Menaa, B.; Razzaq, A.; Thiripuranathar, G.; Rana, N.F.; Menaa, F. Green and Cost-Effective Synthesis of Metallic Nanoparticles by Algae: Safe Methods for Translational Medicine. Bioengineering 2020, 7, 129. [Google Scholar] [CrossRef] [PubMed]
- Padrela, L.; Rodrigues, M.A.; Duarte, A.; Dias, A.M.A.; Braga, M.E.M.; de Sousa, H.C. Supercritical carbon dioxide-based technologies for the production of drug nanoparticles/nanocrystals—A comprehensive review. Adv. Drug Deliv. Rev. 2018, 131, 22–78. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Yan, J.; Liu, D.; Li, X.; Kang, Q.; You, W.; Zhang, J.; Wang, L.; Tian, Z.; Lu, W.; et al. A nano-predator of pathological MDMX construct by clearable supramolecular gold(I)-thiol-peptide complexes achieves safe and potent anti-tumor activity. Theranostics 2021, 11, 6833–6846. [Google Scholar] [CrossRef]
- Yan, J.; Yao, Y.; Yan, S.; Gao, R.; Lu, W.; He, W. Chiral Protein Supraparticles for Tumor Suppression and Synergistic Immunotherapy: An Enabling Strategy for Bioactive Supramolecular Chirality Construction. Nano Lett. 2020, 20, 5844–5852. [Google Scholar] [CrossRef]
- Yan, J.; He, W.X.; Yan, S.Q.; Niu, F.; Liu, T.Y.; Ma, B.H.; Shao, Y.P.; Yan, Y.W.; Yang, G.; Lu, W.Y.; et al. Self-Assembled Peptide-Lanthanide Nanoclusters for Safe Tumor Therapy: Overcoming and Utilizing Biological Barriers to Peptide Drug Delivery. ACS Nano 2018, 12, 2017–2026. [Google Scholar] [CrossRef]
- Chen, Y.F. Nanofabrication by electron beam lithography and its applications: A review. Microelect. Eng. 2015, 135, 57–72. [Google Scholar] [CrossRef]
- Calderon, B.; Smith, F.; Aracil, I.; Fullana, A. Green Synthesis of Thin Shell Carbon-Encapsulated Iron Nanoparticles via Hydrothermal Carbonization. ACS Sustain. Chem. Eng. 2018, 6, 7995–8002. [Google Scholar] [CrossRef]
- Saloga, P.E.J.; Thunemann, A.F. Microwave-Assisted Synthesis of Ultrasmall Zinc Oxide Nanoparticles. Langmuir 2019, 35, 12469–12482. [Google Scholar] [CrossRef] [PubMed]
- Alshehri, A.A.; Malik, M.A. Phytomediated Photo-Induced Green Synthesis of Silver Nanoparticles Using Matricaria chamomilla L. and Its Catalytic Activity against Rhodamine B. Biomolecules 2020, 10, 1604. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, G.; da Costa Sousa, M.G.; Rezende, T.M.B.; Franco, O.L. Can metallic nanomaterials be green and sustainable? Curr. Opin. Environ. Sci. Health 2021, 24, 100292. [Google Scholar] [CrossRef]
- Ghosh, S.; Ahmad, R.; Zeyaullah, M.; Khare, S.K. Microbial Nano-Factories: Synthesis and Biomedical Applications. Front. Chem. 2021, 9, 626834. [Google Scholar] [CrossRef] [PubMed]
- Abitbol, T.; Rivkin, A.; Cao, Y.; Nevo, Y.; Abraham, E.; Ben-Shalom, T.; Lapidot, S.; Shoseyov, O. Nanocellulose, a tiny fiber with huge applications. Curr. Opin. Biotechnol. 2016, 39, 76–88. [Google Scholar] [CrossRef]
- Morán, J.I.; Alvarez, V.A.; Cyras, V.P.; Vázquez, A. Extraction of cellulose and preparation of nanocellulose from sisal fibers. Cellulose 2008, 15, 149–159. [Google Scholar] [CrossRef]
- Das, S.; Ghosh, B.; Sarkar, K. Nanocellulose as sustainable biomaterials for drug delivery. Sens. Int. 2022, 3, 100135. [Google Scholar] [CrossRef]
- Rudin, A.; Choi, P. Chapter 13: Biopolymers. In The Elements of Polymer Science & Engineering, 3rd ed.; Rudin, A., Choi, P., Eds.; Academic Press: Boston, MA, USA, 2013; pp. 521–535. [Google Scholar]
- Jorfi, M.; Foster, E.J. Recent advances in nanocellulose for biomedical applications. J. Appl. Polym. Sci. 2015, 132, 41719. [Google Scholar] [CrossRef]
- Dumanli, A.G. Nanocellulose and its Composites for Biomedical Applications. Curr. Med. Chem. 2017, 24, 512–528. [Google Scholar] [CrossRef]
- Randhawa, A.; Dutta, S.D.; Ganguly, K.; Patil, T.V.; Patel, D.K.; Lim, K.-T. A Review of Properties of Nanocellulose, Its Synthesis, and Potential in Biomedical Applications. Appl. Sci. 2022, 12, 7090. [Google Scholar] [CrossRef]
- Orsolini, P.; Michen, B.; Huch, A.; Tingaut, P.; Caseri, W.R.; Zimmermann, T. Characterization of Pores in Dense Nanopapers and Nanofibrillated Cellulose Membranes: A Critical Assessment of Established Methods. ACS Appl. Mater. Interfaces 2015, 7, 25884–25897. [Google Scholar] [CrossRef] [PubMed]
- Czaja, W.K.; Young, D.J.; Kawecki, M.; Brown, R.M., Jr. The future prospects of microbial cellulose in biomedical applications. Biomacromolecules 2007, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Barja, F. Bacterial nanocellulose production and biomedical applications. J. Biomed. Res. 2021, 35, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Rai, R.; Dhar, P. Biomedical engineering aspects of nanocellulose: A review. Nanotechnology 2022, 33, 362001. [Google Scholar] [CrossRef] [PubMed]
- Madhu, K.; Carole, F.; Grégory, C.; Jean-Luc, P.; Audrey, M. Transmission Electron Microscopy for the Characterization of Cellulose Nanocrystals. In The Transmission Electron Microscope; Khan, M., Ed.; IntechOpen: Rijeka, Croatia, 2015; Chapter 6. [Google Scholar]
- Picheth, G.F.; Pirich, C.L.; Sierakowski, M.R.; Woehl, M.A.; Sakakibara, C.N.; de Souza, C.F.; Martin, A.A.; da Silva, R.; de Freitas, R.A. Bacterial cellulose in biomedical applications: A review. Int. J. Biol. Macromol. 2017, 104, 97–106. [Google Scholar] [CrossRef]
- Mohite, B.V.; Patil, S.V. In situ development of nanosilver-impregnated bacterial cellulose for sustainable released antimicrobial wound dressing. J. Appl. Biomater. Funct. Mater. 2016, 14, 53–58. [Google Scholar] [CrossRef]
- Sharma, C.; Bhardwaj, N.K. Bacterial nanocellulose: Present status, biomedical applications and future perspectives. Mater. Sci. Eng. C 2019, 104, 109963. [Google Scholar] [CrossRef]
- Reshmy, R.; Philip, E.; Paul, S.A.; Madhavan, A.; Sindhu, R.; Binod, P.; Pandey, A.; Sirohi, R. Nanocellulose-based products for sustainable applications-recent trends and possibilities. Rev. Environ. Sci. Biotechnol. 2020, 19, 779–806. [Google Scholar] [CrossRef]
- Salvo, J.; Sandoval, C. Role of copper nanoparticles in wound healing for chronic wounds: Literature review. Burn. Trauma 2022, 10, 47. [Google Scholar] [CrossRef]
- Sandoval, C.; Ríos, G.; Sepúlveda, N.; Salvo, J.; Souza-Mello, V.; Farías, J. Effectiveness of Copper Nanoparticles in Wound Healing Process Using In Vivo and In Vitro Studies: A Systematic Review. Pharmaceutics 2022, 14, 1838. [Google Scholar] [CrossRef]
- Taheri, P.; Jahanmardi, R.; Koosha, M.; Abdi, S. Physical, mechanical and wound healing properties of chitosan/gelatin blend films containing tannic acid and/or bacterial nanocellulose. Int. J. Biol. Macromol. 2020, 154, 421–432. [Google Scholar] [CrossRef]
- Wang, X.; Tang, J.; Huang, J.; Hui, M. Production and characterization of bacterial cellulose membranes with hyaluronic acid and silk sericin. Colloids Surf. B Biointer. 2020, 195, 111273. [Google Scholar] [CrossRef]
- Islam, M.S.; Chen, L.; Sisler, J.; Tam, K.C. Cellulose nanocrystal (CNC)–inorganic hybrid systems: Synthesis, properties and applications. J. Mater. Chem. B 2018, 6, 864–883. [Google Scholar] [CrossRef]
- Fan, X.-M.; Yu, H.-Y.; Wang, D.-C.; Yao, J.; Lin, H.; Tang, C.-X.; Tam, K.C. Designing Highly Luminescent Cellulose Nanocrystals with Modulated Morphology for Multifunctional Bioimaging Materials. ACS Appl. Mater. Interfaces 2019, 11, 48192–48201. [Google Scholar] [CrossRef]
- Ganguly, K.; Patel, D.K.; Dutta, S.D.; Lim, K.-T. TEMPO-Cellulose Nanocrystal-Capped Gold Nanoparticles for Colorimetric Detection of Pathogenic DNA. ACS Omega 2021, 6, 12424–12431. [Google Scholar] [CrossRef]
- Rueda, L.; Saralegi, A.; Bidegain, B.; Zhou, Q.; Alonso-Varona, A.; Berglund, L.; Mondragon, I.; Corcuera, M.; Eceiza, A. In situ polymerization and characterization of elastomeric polyurethane-cellulose nanocrystal nanocomposites: Cell response evaluation. Cellulose 2013, 2013, 1819–1828. [Google Scholar] [CrossRef]
- Lemarchand, C.; Gref, R.; Couvreur, P. Polysaccharide-decorated nanoparticles. Eur. J. Pharm. Biopharm. 2004, 58, 327–341. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.-H.; Lee, S.Y.; Hwang, C.; Yang, M.; Lee, J.; Lee, S.-H.; Cho, H.-J. Multi-layered cellulose nanocrystal system for CD44 receptor-positive tumor-targeted anticancer drug delivery. Int. J. Biol. Macromol. 2020, 162, 798–809. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.S.; Jiang, F.; Hsieh, Y.-L.; Nitin, N. Cellulose nanofibrils improve dispersibility and stability of silver nanoparticles and induce production of bacterial extracellular polysaccharides. J. Mater. Chem. B 2014, 2, 6226–6235. [Google Scholar] [CrossRef]
- Martins, N.C.T.; Freire, C.S.R.; Neto, C.P.; Silvestre, A.J.D.; Causio, J.; Baldi, G.; Sadocco, P.; Trindade, T. Antibacterial paper based on composite coatings of nanofibrillated cellulose and ZnO. Colloids Surf. A Physicochem. Eng. Asp. 2013, 417, 111–119. [Google Scholar] [CrossRef]
- Xiao, W.; Xu, J.; Liu, X.; Hu, Q.; Huang, J. Antibacterial hybrid materials fabricated by nanocoating of microfibril bundles of cellulose substance with titania/chitosan/silver-nanoparticle composite films. J. Mater. Chem. B 2013, 1, 3477–3485. [Google Scholar] [CrossRef]
- Carlström, I.E.; Rashad, A.; Campodoni, E.; Sandri, M.; Syverud, K.; Bolstad, A.I.; Mustafa, K. Cross-linked gelatin-nanocellulose scaffolds for bone tissue engineering. Mater. Lett. 2020, 264, 127326. [Google Scholar] [CrossRef]
- Rubino, S.; Razaq, A.; Nyholm, L.; Strømme, M.; Leifer, K.; Mihranyan, A. Spatial mapping of elemental distributions in polypyrrole-cellulose nanofibers using energy-filtered transmission electron microscopy. J. Phys. Chem. B 2010, 114, 13644–13649. [Google Scholar] [CrossRef]
- Maharjan, B.; Park, J.; Kaliannagounder, V.K.; Awasthi, G.P.; Joshi, M.K.; Park, C.H.; Kim, C.S. Regenerated cellulose nanofiber reinforced chitosan hydrogel scaffolds for bone tissue engineering. Carbohy. Polym. 2021, 251, 117023. [Google Scholar] [CrossRef]
- Löbmann, K.; Svagan, A.J. Cellulose nanofibers as excipient for the delivery of poorly soluble drugs. Int. J. Pharm. 2017, 533, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Bäckdahl, H.; Helenius, G.; Bodin, A.; Nannmark, U.; Johansson, B.R.; Risberg, B.; Gatenholm, P. Mechanical properties of bacterial cellulose and interactions with smooth muscle cells. Biomaterials 2006, 27, 2141–2149. [Google Scholar] [CrossRef] [PubMed]
- Apelgren, P.; Karabulut, E.; Amoroso, M.; Mantas, A.; Martínez Ávila, H.; Kölby, L.; Kondo, T.; Toriz, G.; Gatenholm, P. In Vivo Human Cartilage Formation in Three-Dimensional Bioprinted Constructs with a Novel Bacterial Nanocellulose Bioink. ACS Biomater. Sci. Eng. 2019, 5, 2482–2490. [Google Scholar] [CrossRef]
- Andrade, F.K.; Costa, R.; Domingues, L.; Soares, R.; Gama, M. Improving bacterial cellulose for blood vessel replacement: Functionalization with a chimeric protein containing a cellulose-binding module and an adhesion peptide. Acta Biomater. 2010, 6, 4034–4041. [Google Scholar] [CrossRef] [PubMed]
- Starborg, T.; Kalson, N.S.; Lu, Y.; Mironov, A.; Cootes, T.F.; Holmes, D.F.; Kadler, K.E. Using transmission electron microscopy and 3View to determine collagen fibril size and three-dimensional organization. Nat. Prot. 2013, 8, 1433–1448. [Google Scholar] [CrossRef]
- Bao, L.; Hong, F.F.; Li, G.; Hu, G.; Chen, L. Improved Performance of Bacterial Nanocellulose Conduits by the Introduction of Silk Fibroin Nanoparticles and Heparin for Small-Caliber Vascular Graft Applications. Biomacromolecules 2021, 22, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Pitkänen, M.; Kangas, H.; Laitinen, O.; Sneck, A.; Lahtinen, P.; Peresin, M.; Niinimaki, J. Characteristics and safety of nano-sized cellulose fibrils. Cellulose 2014, 21, 3871–3886. [Google Scholar] [CrossRef]
- Vartiainen, J.; Pöhler, T.; Sirola, K.; Pylkkänen, L.; Alenius, H.; Hokkinen, J.; Tapper, U.; Lahtinen, P.; Kapanen, A.; Putkisto, K.; et al. Health and environmental safety aspects of friction grinding and spray drying of microfibrillated cellulose. Cellulose 2011, 18, 775–786. [Google Scholar] [CrossRef]
- Lopes, V.R.; Sanchez-Martinez, C.; Strømme, M.; Ferraz, N. In vitro biological responses to nanofibrillated cellulose by human dermal, lung and immune cells: Surface chemistry aspect. Part. Fibre Toxicol. 2017, 14, 1. [Google Scholar] [CrossRef]
- Xue, Y.; Mou, Z.; Xiao, H. Nanocellulose as a sustainable biomass material: Structure, properties, present status and future prospects in biomedical applications. Nanoscale 2017, 9, 14758–14781. [Google Scholar] [CrossRef] [PubMed]
- Shukla, M.K.; Dong, W.-L.; Azizov, S.; Singh, K.R.B.; Kumar, D.; Singh, R.P.; Singh, J. Trends of bioderived carbonaceous materials for futuristic biomedical applications. Mater. Lett. 2022, 311, 131606. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z.; Wang, J.; Li, J.; Lin, Y. Graphene and graphene oxide: Biofunctionalization and applications in biotechnology. Trends Biotechnol. 2011, 29, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Panwar, N.; Soehartono, A.M.; Chan, K.K.; Zeng, S.; Xu, G.; Qu, J.; Coquet, P.; Yong, K.-T.; Chen, X. Nanocarbons for Biology and Medicine: Sensing, Imaging, and Drug Delivery. Chem. Rev. 2019, 119, 9559–9656. [Google Scholar] [CrossRef] [PubMed]
- Mamidi, N.; Velasco Delgadillo, R.M.; Barrera, E.V.; Ramakrishna, S.; Annabi, N. Carbonaceous nanomaterials incorporated biomaterials: The present and future of the flourishing field. Comp. Part B Eng. 2022, 243, 110150. [Google Scholar] [CrossRef]
- Lesani, P.; Lu, Z.; Singh, G.; Mursi, M.; Mirkhalaf, M.; New, E.J.; Zreiqat, H. Influence of carbon dot synthetic parameters on photophysical and biological properties. Nanoscale 2021, 13, 11138–11149. [Google Scholar] [CrossRef]
- Sagar, V.K.; Dey, S.; Bhattacharya, S.; Lesani, P.; Ramaswamy, Y.; Singh, G.; Zreiqat, H.; Bisht, P.B. Probing heteroatoms co-doped graphene quantum dots for energy transfer and 2-photon assisted applications. J. Photochem. Photobiol. A Chem. 2022, 423, 113618. [Google Scholar] [CrossRef]
- Lesani, P.; Singh, G.; Lu, Z.; Mirkhalaf, M.; New, E.J.; Zreiqat, H. Two-photon ratiometric carbon dot-based probe for real-time intracellular pH monitoring in 3D environment. Chem. Eng. J. 2022, 433, 133668. [Google Scholar] [CrossRef]
- Goswami, A.D.; Trivedi, D.H.; Jadhav, N.L.; Pinjari, D.V. Sustainable and green synthesis of carbon nanomaterials: A review. J. Environ. Chem. Eng. 2021, 9, 106118. [Google Scholar] [CrossRef]
- Chen, W.; Shen, J.; Wang, Z.; Liu, X.; Xu, Y.; Zhao, H.; Astruc, D. Turning waste into wealth: Facile and green synthesis of carbon nanodots from pollutants and applications to bioimaging. Chem. Sci. 2021, 12, 11722–11729. [Google Scholar] [CrossRef]
- Shen, J.; Xu, Y.; Wang, Z.; Chen, W.; Zhao, H.; Liu, X. Facile and green synthesis of carbon nanodots from environmental pollutants for cell imaging and Fe3+ detection. N. J. Chem. 2022, 46, 12581–12588. [Google Scholar] [CrossRef]
- Meng, W.; Bai, X.; Wang, B.; Liu, Z.; Lu, S.; Yang, B. Biomass-Derived Carbon Dots and Their Applications. Ener. Environ. Mater. 2019, 2, 172–192. [Google Scholar] [CrossRef]
- Xu, H.; Yan, L.; Nguyen, V.; Yu, Y.; Xu, Y. One-step synthesis of nitrogen-doped carbon nanodots for ratiometric pH sensing by femtosecond laser ablation method. Appl. Surf. Sci. 2017, 414, 238–243. [Google Scholar] [CrossRef]
- de Menezes, F.D.; dos Reis, S.R.R.; Pinto, S.R.; Portilho, F.L.; do Vale Chaves e Mello, F.; Helal-Neto, E.; da Silva de Barros, A.O.; Alencar, L.M.R.; de Menezes, A.S.; dos Santos, C.C.; et al. Graphene quantum dots unraveling: Green synthesis, characterization, radiolabeling with 99mTc, in vivo behavior and mutagenicity. Mater. Sci. Eng. C 2019, 102, 405–414. [Google Scholar] [CrossRef]
- Boobalan, T.; Sethupathi, M.; Sengottuvelan, N.; Kumar, P.; Balaji, P.; Gulyás, B.; Padmanabhan, P.; Selvan, S.T.; Arun, A. Mushroom-Derived Carbon Dots for Toxic Metal Ion Detection and as Antibacterial and Anticancer Agents. ACS Appl. Nano Mater. 2020, 3, 5910–5919. [Google Scholar] [CrossRef]
- Sangam, S.; Gupta, A.; Shakeel, A.; Bhattacharya, R.; Sharma, A.K.; Suhag, D.; Chakrabarti, S.; Garg, S.K.; Chattopadhyay, S.; Basu, B.; et al. Sustainable synthesis of single crystalline sulphur-doped graphene quantum dots for bioimaging and beyond. Green Chem. 2018, 20, 4245–4259. [Google Scholar] [CrossRef]
- Guo, R.; Chen, B.; Li, F.; Weng, S.; Zheng, Z.; Chen, M.; Wu, W.; Lin, X.; Yang, C. Positive carbon dots with dual roles of nanoquencher and reference signal for the ratiometric fluorescence sensing of DNA. Sens. Act. B Chem. 2018, 264, 193–201. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Ge, S.; Wang, S.; Yan, M.; Zang, D.; Yu, J. Facile and sensitive paper-based chemiluminescence DNA biosensor using carbon dots dotted nanoporous gold signal amplification label. Anal. Meth. 2013, 5, 1328–1336. [Google Scholar] [CrossRef]
- Hamd-Ghadareh, S.; Salimi, A.; Fathi, F.; Bahrami, S. An amplified comparative fluorescence resonance energy transfer immunosensing of CA125 tumor marker and ovarian cancer cells using green and economic carbon dots for bio-applications in labeling, imaging and sensing. Biosens. Bioelect. 2017, 96, 308–316. [Google Scholar] [CrossRef]
- Das, S.; Kaushik, R.; Goswami, P. Multifaceted Interaction Studies between Carbon Dots and Proteins of Clinical Importance for Optical Sensing Signals. ACS Appl. Bio Mater. 2022, 5, 889–896. [Google Scholar] [CrossRef]
- Saranti, A.; Tiron-Stathopoulos, A.; Papaioannou, L.; Gioti, C.; Ioannou, A.; Karakassides, M.A.; Avgoustakis, K.; Koutselas, I.; Dimos, K. 3D-printed bioactive scaffolds for bone regeneration bearing carbon dots for bioimaging purposes. Smart Mater. Med. 2022, 3, 12–19. [Google Scholar] [CrossRef]
- Calabrese, G.; De Luca, G.; Nocito, G.; Rizzo, M.G.; Lombardo, S.P.; Chisari, G.; Forte, S.; Sciuto, E.L.; Conoci, S. Carbon Dots: An Innovative Tool for Drug Delivery in Brain Tumors. Int. J. Mol. Sci. 2021, 22, 11783. [Google Scholar] [CrossRef]
- Iannazzo, D.; Espro, C.; Celesti, C.; Ferlazzo, A.; Neri, G. Smart Biosensors for Cancer Diagnosis Based on Graphene Quantum Dots. Cancers 2021, 13, 3194. [Google Scholar] [CrossRef]
- Gong, L.; Sun, J.; Zheng, P.; Liu, Y.; Yang, G. Yellow Fluorescent Nitrogen and Bromine Co-doped Graphene Quantum Dots for Bioimaging. ACS Appl. Nano Mater. 2021, 4, 8564–8571. [Google Scholar] [CrossRef]
- Prabakaran, S.; Jeyaraj, M.; Nagaraj, A.; Sadasivuni, K.K.; Rajan, M. Polymethyl methacrylate–ovalbumin @ graphene oxide drug carrier system for high anti-proliferative cancer drug delivery. Appl. Nanosci. 2019, 9, 1487–1500. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, Y.; Xian, Y.; Singh, P.; Feng, J.; Cui, S.; Carrier, A.; Oakes, K.; Luan, T.; Zhang, X. Multifunctional Graphene-Oxide-Reinforced Dissolvable Polymeric Microneedles for Transdermal Drug Delivery. ACS Appl. Mater. Interfaces 2020, 12, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Lyu, H.; Richardson, A.G.; Lucas, T.H.; Kuzum, D. Flexible Neural Electrode Array Based-on Porous Graphene for Cortical Microstimulation and Sensing. Sci. Rep. 2016, 6, 33526. [Google Scholar] [CrossRef] [PubMed]
- Ru, J.; Bian, C.; Zhu, Z.; Wang, Y.; Zhang, J.; Horiuchi, T.; Sugino, T.; Liu, X.; Chen, H.; Asaka, K. Controllable and durable ionic electroactive polymer actuator based on nanoporous carbon nanotube film electrode. Smart Mater. Struct. 2019, 28, 085032. [Google Scholar] [CrossRef]
- Li, T.; Liang, Y.; Li, J.; Yu, Y.; Xiao, M.-M.; Ni, W.; Zhang, Z.; Zhang, G.-J. Carbon Nanotube Field-Effect Transistor Biosensor for Ultrasensitive and Label-Free Detection of Breast Cancer Exosomal miRNA21. Anal. Chem. 2021, 93, 15501–15507. [Google Scholar] [CrossRef]
- Singh, V.; Chatterjee, S.; Palecha, M.; Sen, P.; Ateeq, B.; Verma, V. Chickpea peel waste as sustainable precursor for synthesis of fluorescent carbon nanotubes for bioimaging application. Carbon Lett. 2021, 31, 117–123. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Z.; Zhang, Y. The application of carbon nanotubes in target drug delivery systems for cancer therapies. Nanoscale Res. Lett. 2011, 6, 555. [Google Scholar] [CrossRef] [PubMed]
- Suo, X.; Eldridge, B.N.; Zhang, H.; Mao, C.; Min, Y.; Sun, Y.; Singh, R.; Ming, X. P-Glycoprotein-Targeted Photothermal Therapy of Drug-Resistant Cancer Cells Using Antibody-Conjugated Carbon Nanotubes. ACS Appl. Mater. Interfaces 2018, 10, 33464–33473. [Google Scholar] [CrossRef] [PubMed]
- Raphey, V.R.; Henna, T.K.; Nivitha, K.P.; Mufeedha, P.; Sabu, C.; Pramod, K. Advanced biomedical applications of carbon nanotube. Mater. Sci. Eng. C 2019, 100, 616–630. [Google Scholar] [CrossRef]
- Seiji Akita, S.A.; Hidehiro Nishijima, H.N.; Takayoshi Kishida, T.K.; Yoshikazu Nakayama, Y.N. Influence of Force Acting on Side Face of Carbon Nanotube in Atomic Force Microscopy. Jpn. J. Appl. Phys. 2000, 39, 3724. [Google Scholar] [CrossRef]
- Miao, P.; Han, K.; Tang, Y.; Wang, B.; Lin, T.; Cheng, W. Recent advances in carbon nanodots: Synthesis, properties and biomedical applications. Nanoscale 2015, 7, 1586–1595. [Google Scholar] [CrossRef]
- Riley, P.R.; Narayan, R.J. Recent advances in carbon nanomaterials for biomedical applications: A review. Curr. Opin. Biomed. Eng. 2021, 17, 100262. [Google Scholar] [CrossRef] [PubMed]
- Ghosal, K.; Sarkar, K. Biomedical Applications of Graphene Nanomaterials and Beyond. ACS Biomater. Sci. Eng. 2018, 4, 2653–2703. [Google Scholar] [CrossRef] [PubMed]
- Hashemzadeh, H.; Raissi, H. Understanding loading, diffusion and releasing of Doxorubicin and Paclitaxel dual delivery in graphene and graphene oxide carriers as highly efficient drug delivery systems. Appl. Surf. Sci. 2020, 500, 144220. [Google Scholar] [CrossRef]
- Torres, F.G.; Troncoso, O.P.; Rodriguez, L.; De-la-Torre, G.E. Sustainable synthesis, reduction and applications of graphene obtained from renewable resources. Sustain. Mater. Technol. 2021, 29, e00310. [Google Scholar] [CrossRef]
- Akhavan, O.; Bijanzad, K.; Mirsepah, A. Synthesis of graphene from natural and industrial carbonaceous wastes. RSC Adv. 2014, 4, 20441–20448. [Google Scholar] [CrossRef]
- Wei, S.; Qiu, X.; An, J.; Chen, Z.; Zhang, X. Highly sensitive, flexible, green synthesized graphene/biomass aerogels for pressure sensing application. Comp. Sci. Technol. 2021, 207, 108730. [Google Scholar] [CrossRef]
- Somanathan, T.; Prasad, K.; Ostrikov, K.; Saravanan, A.; Krishna, V.M. Graphene Oxide Synthesis from Agro Waste. Nanomaterials 2015, 5, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Grüner, G.; Zhao, Y. Recent advancements of graphene in biomedicine. J. Mater. Chem. B 2013, 1, 2542–2567. [Google Scholar] [CrossRef]
- Yoon, H.; Nah, J.; Kim, H.; Ko, S.; Sharifuzzaman, M.; Barman, S.C.; Xuan, X.; Kim, J.; Park, J.Y. A chemically modified laser-induced porous graphene based flexible and ultrasensitive electrochemical biosensor for sweat glucose detection. Sens. Act. B Chem. 2020, 311, 127866. [Google Scholar] [CrossRef]
- Vukojević, V.; Djurdjić, S.; Ognjanović, M.; Fabián, M.; Samphao, A.; Kalcher, K.; Stanković, D.M. Enzymatic glucose biosensor based on manganese dioxide nanoparticles decorated on graphene nanoribbons. J. Electroanal. Chem. 2018, 823, 610–616. [Google Scholar] [CrossRef]
- Geetha Bai, R.; Muthoosamy, K.; Manickam, S.; Hilal-Alnaqbi, A. Graphene-based 3D scaffolds in tissue engineering: Fabrication, applications, and future scope in liver tissue engineering. Int. J. Nanomed. 2019, 14, 5753–5783. [Google Scholar] [CrossRef]
- Amani, H.; Mostafavi, E.; Arzaghi, H.; Davaran, S.; Akbarzadeh, A.; Akhavan, O.; Pazoki-Toroudi, H.; Webster, T.J. Three-Dimensional Graphene Foams: Synthesis, Properties, Biocompatibility, Biodegradability, and Applications in Tissue Engineering. ACS Biomater. Sci. Eng. 2019, 5, 193–214. [Google Scholar] [CrossRef] [PubMed]
- Makgabutlane, B.; Nthunya, L.N.; Maubane-Nkadimeng, M.S.; Mhlanga, S.D. Green synthesis of carbon nanotubes to address the water-energy-food nexus: A critical review. J. Environ. Chem. Eng. 2021, 9, 104736. [Google Scholar] [CrossRef]
- Tan, C.W.; Tan, K.H.; Ong, Y.T.; Mohamed, A.R.; Zein, S.H.S.; Tan, S.H. Energy and environmental applications of carbon nanotubes. Environ. Chem. Lett. 2012, 10, 265–273. [Google Scholar] [CrossRef]
- Mhlanga, S.D.; Mondal, K.C.; Carter, R.; Witcomb, M.J.; Coville, N.J. The effect of synthesis parameters on the catalytic synthesis of multiwalled carbon nanotubes using Fe-Co/CaCO3 catalysts. S. Afr. J. Chem. 2009, 62, 67–76. [Google Scholar]
- Duarte, M.P.; Silva, R.C.F.; de Medeiros, T.P.V.; Ardisson, J.D.; Cotta, A.A.C.; Naccache, R.; de Carvalho Teixeira, A.P. Carbon nanotubes derived from waste cooking oil for the removal of emerging contaminants. N. J. Chem. 2022, 46, 11315–11328. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, N.; Yin, P.; Zhang, C. Facile growth of carbon nanotubes using microwave ovens: The emerging application of highly efficient domestic plasma reactors. Nanoscale Adv. 2019, 1, 4546–4559. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Ruiz-Hernández, E. Bioceramics: From Bone Regeneration to Cancer Nanomedicine. Adv. Mater. 2011, 23, 5177–5218. [Google Scholar] [CrossRef] [PubMed]
- Salinas, A.J.; Esbrit, P.; Vallet-Regí, M. A tissue engineering approach based on the use of bioceramics for bone repair. Biomater. Sci. 2013, 1, 40–51. [Google Scholar] [CrossRef]
- Vallet-Regí, M. Bioceramics: From bone substitutes to nanoparticles for drug delivery. Pure Appl. Chem. 2019, 91, 687–706. [Google Scholar] [CrossRef]
- Abbas, Z.; Dapporto, M.; Tampieri, A.; Sprio, S. Toughening of Bioceramic Composites for Bone Regeneration. J. Comp. Sci. 2021, 5, 259. [Google Scholar] [CrossRef]
- Waheed, M.; Yousaf, M.; Shehzad, A.; Inam-Ur-Raheem, M.; Khan, M.K.I.; Khan, M.R.; Ahmad, N.; Abdullah; Aadil, R.M. Channelling eggshell waste to valuable and utilizable products: A comprehensive review. Trends Food Sci. Technol. 2020, 106, 78–90. [Google Scholar] [CrossRef]
- Lee, S.J.; Oh, S.H. Fabrication of calcium phosphate bioceramics by using eggshell and phosphoric acid. Mater. Lett. 2003, 57, 4570–4574. [Google Scholar] [CrossRef]
- Ononiwu, N.H.; Akinlabi, E.T. Effects of ball milling on particle size distribution and microstructure of eggshells for applications in metal matrix composites. Mater. Today Proceed. 2020, 26, 1049–1053. [Google Scholar] [CrossRef]
- Rivera, E.M.; Araiza, M.; Brostow, W.; Castaño, V.M.; Díaz-Estrada, J.R.; Hernández, R.; Rodríguez, J.R. Synthesis of hydroxyapatite from eggshells. Mater. Lett. 1999, 41, 128–134. [Google Scholar] [CrossRef]
- Siddharthan, A.; Seshadri, S.K.; Kumar, T.S.S. Microwave accelerated synthesis of nanosized calcium deficient hydroxyapatite. J. Mater. Sci. Mater. Med. 2004, 15, 1279–1284. [Google Scholar] [CrossRef]
- Siva Rama Krishna, D.; Siddharthan, A.; Seshadri, S.K.; Sampath Kumar, T.S. A novel route for synthesis of nanocrystalline hydroxyapatite from eggshell waste. J. Mater. Sci. Mater. Med. 2007, 18, 1735–1743. [Google Scholar] [CrossRef] [PubMed]
- Sampath Kumar, T.S.; Madhumathi, K.; Rajkamal, B.; Zaheatha, S.; Rajathi Malar, A.; Alamelu Bai, S. Enhanced protein delivery by multi-ion containing eggshell derived apatitic-alginate composite nanocarriers. Colloids Surf. B Biointerfaces 2014, 123, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kim, W.; Cho, J.H.; Oh, N.S.; Lee, M.H.; Lee, S.J. Comparison of Bone Formation in Rabbits Using Hydroxyapatite and β-Tricalcium Phosphate Scaffolds Fabricated From Egg Shells. Adv. Mater. Res. 2008, 47–50, 999–1002. [Google Scholar] [CrossRef]
- Falinski, M.M.; Plata, D.L.; Chopra, S.S.; Theis, T.L.; Gilbertson, L.M.; Zimmerman, J.B. A framework for sustainable nanomaterial selection and design based on performance, hazard, and economic considerations. Nat. Nanotechnol. 2018, 13, 708–714. [Google Scholar] [CrossRef]
- Plata, D.L.; Janković, N.Z. Achieving sustainable nanomaterial design though strategic cultivation of big data. Nat. Nanotechnol. 2021, 16, 612–614. [Google Scholar] [CrossRef]
| Physical Methods | Chemical Methods | Green Methods |
|---|---|---|
| Advantages | ||
| Production of nanomaterials with control over size and shape | Synthesis of nanomaterials from a range of materials | Sustainable and environmentally friendly methods |
| Fabricating nanomaterials with high purity | Control over size, shape, crystallinity, and surface functionality | Fabrication of biocompatible nanomaterials from non-toxic and renewable resources |
| Scalable | High throughput fabrication of nanomaterials | Less energy intensive method with minimal waste |
| Disadvantages | ||
| Fabrication of nanomaterials from limited materials | Energy intensive | Limited scalability |
| Energy intensive and expensive | Require toxic chemicals and generate hazardous waste as reaction byproduct | Limited control over size, shape, and physical properties |
| Some physical methods (e.g., etching) require toxic gases and chemicals | Require additional steps of modification to improve biocompatibility | Restricted to the fabrication of nanomaterials from few materials |
| Not eco-friendly | Not eco-friendly | Reproducibility |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Poon, K.; Masonsong, G.S.P.; Ramaswamy, Y.; Singh, G. Sustainable Nanomaterials for Biomedical Applications. Pharmaceutics 2023, 15, 922. https://doi.org/10.3390/pharmaceutics15030922
Zhang Y, Poon K, Masonsong GSP, Ramaswamy Y, Singh G. Sustainable Nanomaterials for Biomedical Applications. Pharmaceutics. 2023; 15(3):922. https://doi.org/10.3390/pharmaceutics15030922
Chicago/Turabian StyleZhang, Yuhang, Kingsley Poon, Gweneth Sofia P. Masonsong, Yogambha Ramaswamy, and Gurvinder Singh. 2023. "Sustainable Nanomaterials for Biomedical Applications" Pharmaceutics 15, no. 3: 922. https://doi.org/10.3390/pharmaceutics15030922
APA StyleZhang, Y., Poon, K., Masonsong, G. S. P., Ramaswamy, Y., & Singh, G. (2023). Sustainable Nanomaterials for Biomedical Applications. Pharmaceutics, 15(3), 922. https://doi.org/10.3390/pharmaceutics15030922








