Oral Administration as a Potential Alternative for the Delivery of Small Extracellular Vesicles
Abstract
1. Introduction
2. Challenges of Orally Administered sEVs
3. Biodistribution, Stability, and Safety of Oral Delivery of Native and Drug Loaded sEVs
4. sEVs Attributes for an Efficient Oral Administration
5. Cellular and Molecular Mediators for sEVs Uptake after Oral Administration
6. Food Derived Vesicles (FDVs)-Based Nutraceutical Perspectives in Infant and Elderly Health
7. Limitation, Future Direction and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hua, S. Advances in Oral Drug Delivery for Regional Targeting in the Gastrointestinal Tract—Influence of Physiological, Pathophysiological and Pharmaceutical Factors. Front. Pharmacol. 2020, 11, 524. [Google Scholar] [CrossRef] [PubMed]
- Homayun, B.; Lin, X.; Choi, H.-J. Challenges and Recent Progress in Oral Drug Delivery Systems for Biopharmaceuticals. Pharmaceutics 2019, 11, 129. [Google Scholar] [CrossRef]
- Warren, M.R.; Zhang, C.; Vedadghavami, A.; Bokvist, K.; Dhal, P.K.; Bajpayee, A.G. Milk Exosomes with Enhanced Mucus Penetrability for Oral Delivery of SiRNA. Biomater. Sci. 2021, 9, 4260–4277. [Google Scholar] [CrossRef]
- Siew, A.; Le, H.; Thiovolet, M.; Gellert, P.; Schatzlein, A.; Uchegbu, I. Enhanced Oral Absorption of Hydrophobic and Hydrophilic Drugs Using Quaternary Ammonium Palmitoyl Glycol Chitosan Nanoparticles. Mol. Pharm. 2012, 9, 14–28. [Google Scholar] [CrossRef] [PubMed]
- Din, F.U.; Aman, W.; Ullah, I.; Qureshi, O.S.; Mustapha, O.; Shafique, S.; Zeb, A. Effective Use of Nanocarriers as Drug Delivery Systems for the Treatment of Selected Tumors. Int. J. Nanomed. 2017, 12, 7291–7309. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Chen, Y.; Shi, J. Exosome Biochemistry and Advanced Nanotechnology for Next-Generation Theranostic Platforms. Adv. Mater. 2019, 31, e1802896. [Google Scholar] [CrossRef] [PubMed]
- Figueroa-Valdés, A.I.; de la Fuente, C.; Hidalgo, Y.; Vega-Letter, A.M.; Tapia-Limonchi, R.; Khoury, M.; Alcayaga-Miranda, F. A Chemically Defined, Xeno- and Blood-Free Culture Medium Sustains Increased Production of Small Extracellular Vesicles from Mesenchymal Stem Cells. Front. Bioeng. Biotechnol. 2021, 9, 619930. [Google Scholar] [CrossRef] [PubMed]
- Alcayaga-Miranda, F.; Varas-Godoy, M.; Khoury, M. Harnessing the Angiogenic Potential of Stem Cell-Derived Exosomes for Vascular Regeneration. Stem Cells Int. 2016, 2016, 3409169. [Google Scholar] [CrossRef]
- Schuh, C.M.A.P.; Cuenca, J.; Alcayaga-Miranda, F.; Khoury, M. Exosomes on the Border of Species and Kingdom Intercommunication. Transl. Res. 2019, 210, 80–98. [Google Scholar] [CrossRef]
- Zavala, G.; Ramos, M.-P.; Figueroa-Valdés, A.I.; Cisternas, P.; Wyneken, U.; Hernández, M.; Toa, P.; Salmons, B.; Dangerfield, J.; Gunzburg, W.H.; et al. Semipermeable Cellulose Beads Allow Selective and Continuous Release of Small Extracellular Vesicles (SEV) from Encapsulated Cells. Front. Pharmacol. 2020, 11, 679. [Google Scholar] [CrossRef]
- Rosenberger, L.; Ezquer, M.; Lillo-Vera, F.; Pedraza, P.L.; Ortúzar, M.I.; González, P.L.; Figueroa-Valdés, A.I.; Cuenca, J.; Ezquer, F.; Khoury, M.; et al. Stem Cell Exosomes Inhibit Angiogenesis and Tumor Growth of Oral Squamous Cell Carcinoma. Sci. Rep. 2019, 9, 663. [Google Scholar] [CrossRef] [PubMed]
- Alcayaga-Miranda, F.; González, P.L.; Lopez-Verrilli, A.; Varas-Godoy, M.; Aguila-Díaz, C.; Contreras, L.; Khoury, M. Prostate Tumor-Induced Angiogenesis Is Blocked by Exosomes Derived from Menstrual Stem Cells through the Inhibition of Reactive Oxygen Species. Oncotarget 2016, 7, 44462–44477. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; LeBleu, V.S. The Biology, Function, and Biomedical Applications of Exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, I.K.; Wood, M.J.A.; Fuhrmann, G. Extracellular Vesicles as a Next-Generation Drug Delivery Platform. Nat. Nanotechnol. 2021, 16, 748–759. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Jordan, V.; Blenkiron, C.; Chamley, L.W. Biodistribution of Extracellular Vesicles Following Administration into Animals: A Systematic Review. J. Extracell. Vesicles 2021, 10, e12085. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): A Position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Parada, N.; Romero-Trujillo, A.; Georges, N.; Alcayaga-Miranda, F. Camouflage Strategies for Therapeutic Exosomes Evasion from Phagocytosis. J. Adv. Res. 2021, 31, 61–74. [Google Scholar] [CrossRef]
- Azman, M.; Sabri, A.H.; Anjani, Q.K.; Mustaffa, M.F.; Hamid, K.A. Intestinal Absorption Study: Challenges and Absorption Enhancement Strategies in Improving Oral Drug Delivery. Pharmaceuticals 2022, 15, 975. [Google Scholar] [CrossRef]
- Reinholz, J.; Landfester, K.; Mailänder, V. The Challenges of Oral Drug Delivery via Nanocarriers. Drug Deliv. 2018, 25, 1694–1705. [Google Scholar] [CrossRef]
- Yu, M.; Yang, Y.; Zhu, C.; Guo, S.; Gan, Y. Advances in the Transepithelial Transport of Nanoparticles. Drug Discov. Today 2016, 21, 1155–1161. [Google Scholar] [CrossRef]
- Chen, M.-C.; Mi, F.-L.; Liao, Z.-X.; Hsiao, C.-W.; Sonaje, K.; Chung, M.-F.; Hsu, L.-W.; Sung, H.-W. Recent Advances in Chitosan-Based Nanoparticles for Oral Delivery of Macromolecules. Adv. Drug Deliv. Rev. 2013, 65, 865–879. [Google Scholar] [CrossRef] [PubMed]
- Tulkens, J.; Vergauwen, G.; Van Deun, J.; Geeurickx, E.; Dhondt, B.; Lippens, L.; De Scheerder, M.-A.; Miinalainen, I.; Rappu, P.; De Geest, B.G.; et al. Increased Levels of Systemic LPS-Positive Bacterial Extracellular Vesicles in Patients with Intestinal Barrier Dysfunction. Gut 2020, 69, 191–193. [Google Scholar] [CrossRef] [PubMed]
- Rubio, A.P.D.; Martínez, J.; Palavecino, M.; Fuentes, F.; López, C.M.S.; Marcilla, A.; Pérez, O.E.; Piuri, M. Transcytosis of Bacillus Subtilis Extracellular Vesicles through an in Vitro Intestinal Epithelial Cell Model. Sci. Rep. 2020, 10, 3120. [Google Scholar] [CrossRef] [PubMed]
- Cieślik, M.; Nazimek, K.; Bryniarski, K. Extracellular Vesicles—Oral Therapeutics of the Future. Int. J. Mol. Sci. 2022, 23, 7554. [Google Scholar] [CrossRef]
- Wu, L.; Wang, L.; Liu, X.; Bai, Y.; Wu, R.; Li, X.; Mao, Y.; Zhang, L.; Zheng, Y.; Gong, T.; et al. Milk-Derived Exosomes Exhibit Versatile Effects for Improved Oral Drug Delivery. Acta Pharm. Sin. B 2022, 12, 2029–2042. [Google Scholar] [CrossRef]
- Zhong, J.; Xia, B.; Shan, S.; Zheng, A.; Zhang, S.; Chen, J.; Liang, X.-J. High-Quality Milk Exosomes as Oral Drug Delivery System. Biomaterials 2021, 277, 121126. [Google Scholar] [CrossRef]
- Tong, L.; Hao, H.; Zhang, Z.; Lv, Y.; Liang, X.; Liu, Q.; Liu, T.; Gong, P.; Zhang, L.; Cao, F.; et al. Milk-Derived Extracellular Vesicles Alleviate Ulcerative Colitis by Regulating the Gut Immunity and Reshaping the Gut Microbiota. Theranostics 2021, 11, 8570–8586. [Google Scholar] [CrossRef]
- Samuel, M.; Fonseka, P.; Sanwlani, R.; Gangoda, L.; Chee, S.H.; Keerthikumar, S.; Spurling, A.; Chitti, S.V.; Zanker, D.; Ang, C.-S.; et al. Oral Administration of Bovine Milk-Derived Extracellular Vesicles Induces Senescence in the Primary Tumor but Accelerates Cancer Metastasis. Nat. Commun. 2021, 12, 3950. [Google Scholar] [CrossRef]
- Betker, J.L.; Angle, B.M.; Graner, M.W.; Anchordoquy, T.J. The Potential of Exosomes from Cow Milk for Oral Delivery. J. Pharm. Sci. 2019, 108, 1496–1505. [Google Scholar] [CrossRef]
- Manca, S.; Upadhyaya, B.; Mutai, E.; Desaulniers, A.T.; Cederberg, R.A.; White, B.R.; Zempleni, J. Milk Exosomes Are Bioavailable and Distinct MicroRNA Cargos Have Unique Tissue Distribution Patterns. Sci. Rep. 2018, 8, 11321. [Google Scholar] [CrossRef]
- Munagala, R.; Aqil, F.; Jeyabalan, J.; Gupta, R.C. Bovine Milk-Derived Exosomes for Drug Delivery. Cancer Lett. 2016, 371, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Viennois, E.; Prasad, M.; Zhang, Y.; Wang, L.; Zhang, Z.; Han, M.K.; Xiao, B.; Xu, C.; Srinivasan, S.; et al. Edible Ginger-Derived Nanoparticles: A Novel Therapeutic Approach for the Prevention and Treatment of Inflammatory Bowel Disease and Colitis-Associated Cancer. Biomaterials 2016, 101, 321–340. [Google Scholar] [CrossRef] [PubMed]
- Umezu, T.; Takanashi, M.; Murakami, Y.; Ohno, S.-I.; Kanekura, K.; Sudo, K.; Nagamine, K.; Takeuchi, S.; Ochiya, T.; Kuroda, M. Acerola Exosome-Like Nanovesicles to Systemically Deliver Nucleic acid Medicine via Oral Administration. Mol. Ther.-Methods Clin. Dev. 2021, 21, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Ju, S.; Mu, J.; Dokland, T.; Zhuang, X.; Wang, Q.; Jiang, H.; Xiang, X.; Deng, Z.-B.; Wang, B.; Zhang, L.; et al. Grape Exosome-Like Nanoparticles Induce Intestinal Stem Cells and Protect Mice from DSS-Induced Colitis. Mol. Ther. 2013, 21, 1345–1357. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, K.; Mu, J.; Kumar, A.; Behera, J.; Lei, C.; Sriwastva, M.K.; Xu, F.; Dryden, G.W.; Zhang, L.; Chen, S.Y.; et al. Garlic Exosome-Like Nanoparticles Reverse High-Fat Diet Induced Obesity via the Gut/Brain Axis. Theranostics 2022, 12, 1220–1246. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zu, M.; Gong, H.; Ma, Y.; Sun, J.; Ran, S.; Shi, X.; Zhang, J.; Xiao, B. Tea Leaf-Derived Exosome-like Nanotherapeutics Retard Breast Tumor Growth by pro-Apoptosis and Microbiota Modulation. J. Nanobiotechnol. 2023, 21, 6. [Google Scholar] [CrossRef] [PubMed]
- Sriwastva, M.K.; Deng, Z.; Wang, B.; Teng, Y.; Kumar, A.; Sundaram, K.; Mu, J.; Lei, C.; Dryden, G.W.; Xu, F.; et al. Exosome-like Nanoparticles from Mulberry Bark Prevent DSS-Induced Colitis via the AhR/COPS8 Pathway. EMBO Rep. 2022, 23, e53365. [Google Scholar] [CrossRef]
- Wolf, T.; Baier, S.R.; Zempleni, J. The Intestinal Transport of Bovine Milk Exosomes Is Mediated by Endocytosis in Human Colon Carcinoma Caco-2 Cells and Rat Small Intestinal IEC-6 Cells. J. Nutr. 2015, 145, 2201–2206. [Google Scholar] [CrossRef]
- Agrawal, A.K.; Aqil, F.; Jeyabalan, J.; Spencer, W.A.; Beck, J.; Gachuki, B.W.; Alhakeem, S.S.; Oben, K.; Munagala, R.; Bondada, S.; et al. Milk-Derived Exosomes for Oral Delivery of Paclitaxel. Nanomedicine 2017, 13, 1627–1636. [Google Scholar] [CrossRef]
- Kim, M.S.; Haney, M.J.; Zhao, Y.; Mahajan, V.; Deygen, I.; Klyachko, N.L.; Inskoe, E.; Piroyan, A.; Sokolsky, M.; Okolie, O.; et al. Development of Exosome-Encapsulated Paclitaxel to Overcome MDR in Cancer Cells. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 655–664. [Google Scholar] [CrossRef]
- Lai, P.; Weng, J.; Guo, L.; Chen, X.; Du, X. Novel Insights into MSC-EVs Therapy for Immune Diseases. Biomark. Res. 2019, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Bardonnet, P.L.; Faivre, V.; Pugh, W.J.; Piffaretti, J.C.; Falson, F. Gastroretentive Dosage Forms: Overview and Special Case of Helicobacter Pylori. J. Control. Release 2006, 111, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Aminzadeh, M.A.; Fournier, M.; Akhmerov, A.; Jones-Ungerleider, K.C.; Valle, J.B.; Marbán, E. Casein-Enhanced Uptake and Disease-Modifying Bioactivity of Ingested Extracellular Vesicles. J. Extracell. Vesicles 2021, 10, e12045. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Jiang, W.; Tan, Y.; Zou, S.; Zhang, H.; Mao, F.; Gong, A.; Qian, H.; Xu, W. HucMSC Exosome-Derived GPX1 Is Required for the Recovery of Hepatic Oxidant Injury. Mol. Ther. 2017, 25, 465–479. [Google Scholar] [CrossRef] [PubMed]
- Melzer, C.; von der Ohe, J.; Hass, R. Anti-Tumor Effects of Exosomes Derived from Drug-Incubated Permanently Growing Human MSC. Int. J. Mol. Sci. 2020, 21, 7311. [Google Scholar] [CrossRef] [PubMed]
- Nazimek, K.; Askenase, P.W.; Bryniarski, K. Antibody Light Chains Dictate the Specificity of Contact Hypersensitivity Effector Cell Suppression Mediated by Exosomes. Int. J. Mol. Sci. 2018, 19, 2656. [Google Scholar] [CrossRef] [PubMed]
- Collins, R.A.; Habpeb, A.E.; Scheeibee, M.; Elvehjem, C.A. The Folio Acid and Vitamin B12 Content of the Milk of Various Species1. J. Nutr. 1951, 43, 313–321. [Google Scholar] [CrossRef]
- Nazimek, K.; Bryniarski, K.; Ptak, W.; Kormelink, T.G.; Askenase, P.W. Orally Administered Exosomes Suppress Mouse Delayed-Type Hypersensitivity by Delivering Mirna-150 to Antigen-Primed Macrophage Apc Targeted by Exosome-Surface Anti-Peptide Antibody Light Chains. Int. J. Mol. Sci. 2020, 21, 5540. [Google Scholar] [CrossRef]
- Wąsik, M.; Nazimek, K.; Nowak, B.; Askenase, P.W.; Bryniarski, K. Delayed-Type Hypersensitivity Underlying Casein Allergy Is Suppressed by Extracellular Vesicles Carrying Mirna-150. Nutrients 2019, 11, 907. [Google Scholar] [CrossRef]
- Zhang, M.; Johnson-Stephenson, T.K.; Wang, W.; Wang, Y.; Li, J.; Li, L.; Zen, K.; Chen, X.; Zhu, D. Mesenchymal Stem Cell-Derived Exosome-Educated Macrophages Alleviate Systemic Lupus Erythematosus by Promoting Efferocytosis and Recruitment of IL-17+ Regulatory T Cell. Stem Cell Res. Ther. 2022, 13, 484. [Google Scholar] [CrossRef]
- Imai, T.; Takahashi, Y.; Nishikawa, M.; Kato, K.; Morishita, M.; Yamashita, T.; Matsumoto, A.; Charoenviriyakul, C.; Takakura, Y. Macrophage-Dependent Clearance of Systemically Administered B16BL6-Derived Exosomes from the Blood Circulation in Mice. J. Extracell. Vesicles 2015, 4, 26238. [Google Scholar] [CrossRef] [PubMed]
- Donoso-Meneses, D.; Figueroa-Valdés, A.I.; Georges, N.; Tobar, H.E.; Alcayaga-Miranda, F. Turning Adversity into Opportunity: Small Extracellular Vesicles as Nanocarriers for Tumor-Associated Macrophages Re-Education. Bioeng. Transl. Med. 2022, 8, e10349. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, C.; Wagner, C.; Bonnardel, J.; Gorvel, J.-P.; Lelouard, H. The Peyer’s Patch Mononuclear Phagocyte System at Steady State and during Infection. Front. Immunol. 2017, 8, 1254. [Google Scholar] [CrossRef]
- Dillon, A.; Lo, D.D. M Cells: Intelligent Engineering of Mucosal Immune Surveillance. Front. Immunol. 2019, 10, 1499. [Google Scholar] [CrossRef] [PubMed]
- Garinot, M.; Fiévez, V.; Pourcelle, V.; Stoffelbach, F.; des Rieux, A.; Plapied, L.; Theate, I.; Freichels, H.; Jérôme, C.; Marchand-Brynaert, J.; et al. PEGylated PLGA-Based Nanoparticles Targeting M Cells for Oral Vaccination. J. Control. Release 2007, 120, 195–204. [Google Scholar] [CrossRef]
- De Coen, R.; Vanparijs, N.; Risseeuw, M.D.P.; Lybaert, L.; Louage, B.; De Koker, S.; Kumar, V.; Grooten, J.; Taylor, L.; Ayres, N.; et al. pH-Degradable Mannosylated Nanogels for Dendritic Cell Targeting. Biomacromolecules 2016, 17, 2479–2488. [Google Scholar] [CrossRef] [PubMed]
- Chionh, Y.-T.; Wee, J.L.K.; Every, A.L.; Ng, G.Z.; Sutton, P. M-Cell Targeting of Whole Killed Bacteria Induces Protective Immunity against Gastrointestinal Pathogens. Infect. Immun. 2009, 77, 2962–2970. [Google Scholar] [CrossRef]
- Sakhon, O.S.; Ross, B.; Gusti, V.; Pham, A.J.; Vu, K.; Lo, D.D. M Cell-Derived Vesicles Suggest a Unique Pathway for Trans-Epithelial Antigen Delivery. Tissue Barriers 2015, 3, e1004975. [Google Scholar] [CrossRef]
- Ito, Y.; Taniguchi, K.; Kuranaga, Y.; Eid, N.; Inomata, Y.; Lee, S.-W.; Uchiyama, K. Uptake of Micrornas from Exosome-Like Nanovesicles of Edible Plant Juice by Rat Enterocytes. Int. J. Mol. Sci. 2021, 22, 3749. [Google Scholar] [CrossRef]
- Mitsuhashi, S.; Feldbrügge, L.; Csizmadia, E.; Mitsuhashi, M.; Robson, S.C.; Moss, A.C. Luminal Extracellular Vesicles (EVs) in Inflammatory Bowel Disease (IBD) Exhibit Proinflammatory Effects on Epithelial Cells and Macrophages. Inflamm. Bowel Dis. 2016, 22, 1587–1595. [Google Scholar] [CrossRef]
- Chen, T.; Xie, M.-Y.; Sun, J.-J.; Ye, R.-S.; Cheng, X.; Sun, R.-P.; Wei, L.-M.; Li, M.; Lin, D.-L.; Jiang, Q.-Y.; et al. Porcine Milk-Derived Exosomes Promote Proliferation of Intestinal Epithelial Cells. Sci. Rep. 2016, 6, 33862. [Google Scholar] [CrossRef]
- Schuh, C.M.A.P.; Aguayo, S.; Zavala, G.; Khoury, M. Exosome-Like Vesicles in Apis mellifera Bee Pollen, Honey and Royal Jelly Contribute to Their Antibacterial and Pro-Regenerative Activity. J. Exp. Biol. 2019, 222, jeb208702. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, B.; Li, X.; An, T.T.; Zhou, Y.; Li, G.; Wu-Smart, J.; Alvarez, S.; Naldrett, M.J.; Eudy, J.; et al. Identification of Anti-Inflammatory Vesicle-like Nanoparticles in Honey. J. Extracell. Vesicles 2021, 10, e12069. [Google Scholar] [CrossRef] [PubMed]
- Munir, J.; Lee, M.; Ryu, S. Exosomes in Food: Health Benefits and Clinical Relevance in Diseases. Adv. Nutr. Int. Rev. J. 2020, 11, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Paredes, P.T.; Gutzeit, C.; Johansson, S.; Admyre, C.; Stenius, F.; Alm, J.; Scheynius, A.; Gabrielsson, S. Differences in Exosome Populations in Human Breast Milk in Relation to Allergic Sensitization and Lifestyle. Allergy Eur. J. Allergy Clin. Immunol. 2014, 69, 463–471. [Google Scholar] [CrossRef] [PubMed]
- García-Martínez, J.; Pérez-Castillo, M.; Salto, R.; López-Pedrosa, J.M.; Rueda, R.; Girón, M.D. Beneficial Effects of Bovine Milk Exosomes in Metabolic Interorgan Cross-Talk. Nutrients 2022, 14, 1442. [Google Scholar] [CrossRef] [PubMed]
- Maity, S.; Bhat, A.H.; Giri, K.; Ambatipudi, K. BoMiProt: A Database of Bovine Milk Proteins. J. Proteom. 2020, 215, 103648. [Google Scholar] [CrossRef]
- Grossen, P.; Portmann, M.; Koller, E.; Duschmalé, M.; Minz, T.; Sewing, S.; Pandya, N.J.; van Geijtenbeek, S.K.; Ducret, A.; Kusznir, E.-A.; et al. Evaluation of Bovine Milk Extracellular Vesicles for the Delivery of Locked Nucleic Acid Antisense Oligonucleotides. Eur. J. Pharm. Biopharm. 2021, 158, 198–210. [Google Scholar] [CrossRef]
- Karlsson, O.; Rodosthenous, R.S.; Jara, C.; Brennan, K.J.; Wright, R.O.; Baccarelli, A.A.; Wright, R.J. Detection of Long Non-Coding RNAs in Human Breastmilk Extracellular Vesicles: Implications for Early Child Development. Epigenetics 2016, 11, 721–729. [Google Scholar] [CrossRef]
- Leiferman, A.; Shu, J.; Upadhyaya, B.; Cui, J.; Zempleni, J. Storage of Extracellular Vesicles in Human Milk, and MicroRNA Profiles in Human Milk Exosomes and Infant Formulas. J. Craniof. Surg. 2019, 69, 235–238. [Google Scholar] [CrossRef]
- Aguilar-Lozano, A.; Baier, S.; Grove, R.; Shu, J.; Giraud, D.; Leiferman, A.; Mercer, K.E.; Cui, J.; Badger, T.M.; Adamec, J.; et al. Concentrations of Purine Metabolites Are Elevated in Fluids from Adults and Infants and in Livers from Mice Fed Diets Depleted of Bovine Milk Exosomes and Their RNA Cargos. J. Nutr. 2018, 148, 1886–1894. [Google Scholar] [CrossRef] [PubMed]
- Mutai, E.; Ngu, A.K.H.; Zempleni, J. Preliminary Evidence That Lectins in Infant Soy Formula Apparently BIND bovine Milk Exosomes and Prevent Their Absorption in Healthy Adults. BMC Nutr. 2022, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Wiklander, O.P.B.; Nordin, J.Z.; O’Loughlin, A.; Gustafsson, Y.; Corso, G.; Mäger, I.; Vader, P.; Lee, Y.; Sork, H.; Seow, Y.; et al. Extracellular Vesicle in Vivo Biodistribution Is Determined by Cell Source, Route of Administration and Targeting. J. Extracell. Vesicles 2015, 4, 26316. [Google Scholar] [CrossRef] [PubMed]
- Albanese, M.; Chen, Y.-F.A.; Hüls, C.; Gärtner, K.; Tagawa, T.; Mejias-Perez, E.; Keppler, O.T.; Göbel, C.; Zeidler, R.; Shein, M.; et al. MicroRNAs Are Minor Constituents of Extracellular Vesicles That Are Rarely Delivered to Target Cells. PLoS Genet. 2021, 17, e1009951. [Google Scholar] [CrossRef] [PubMed]
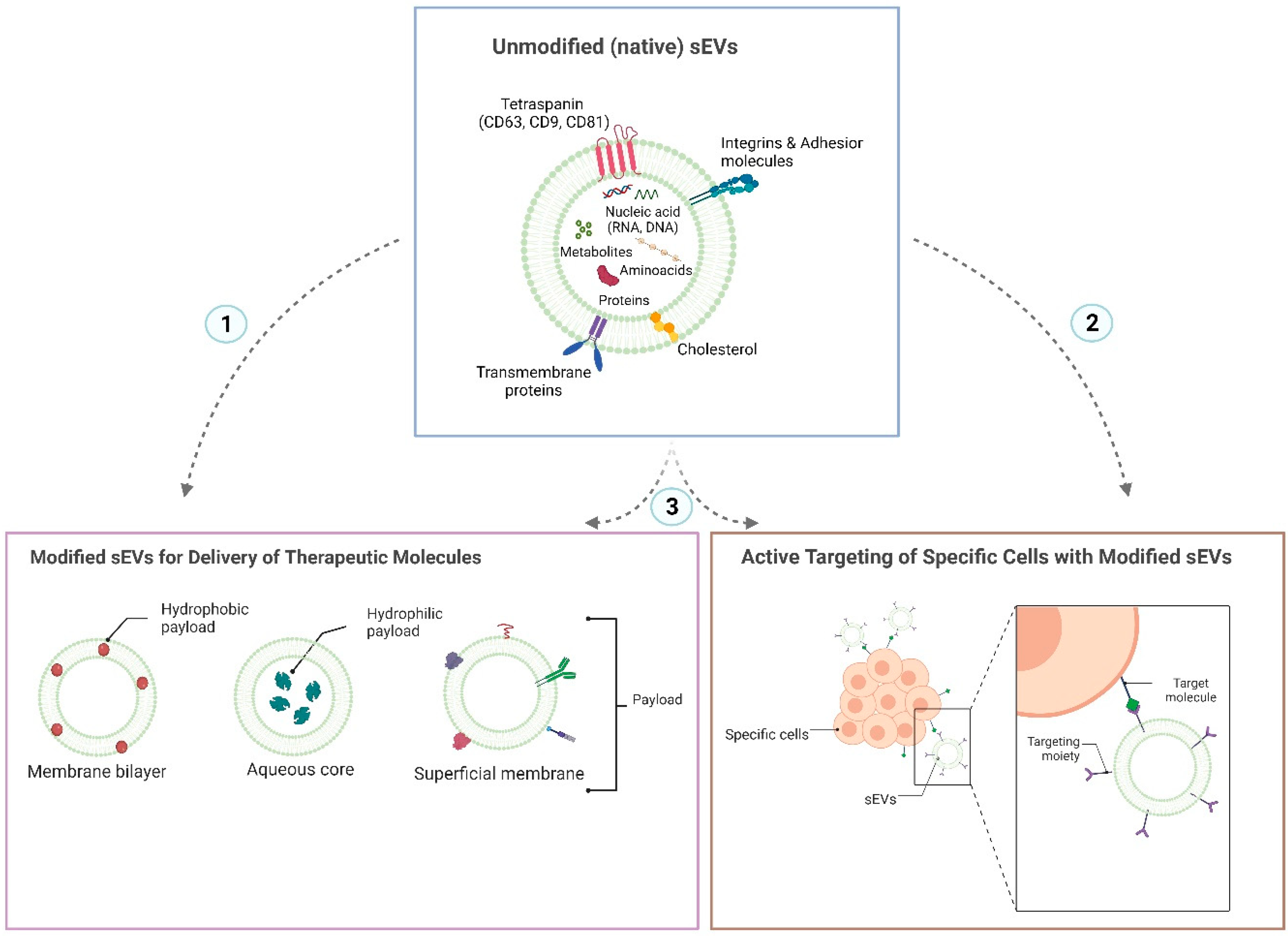

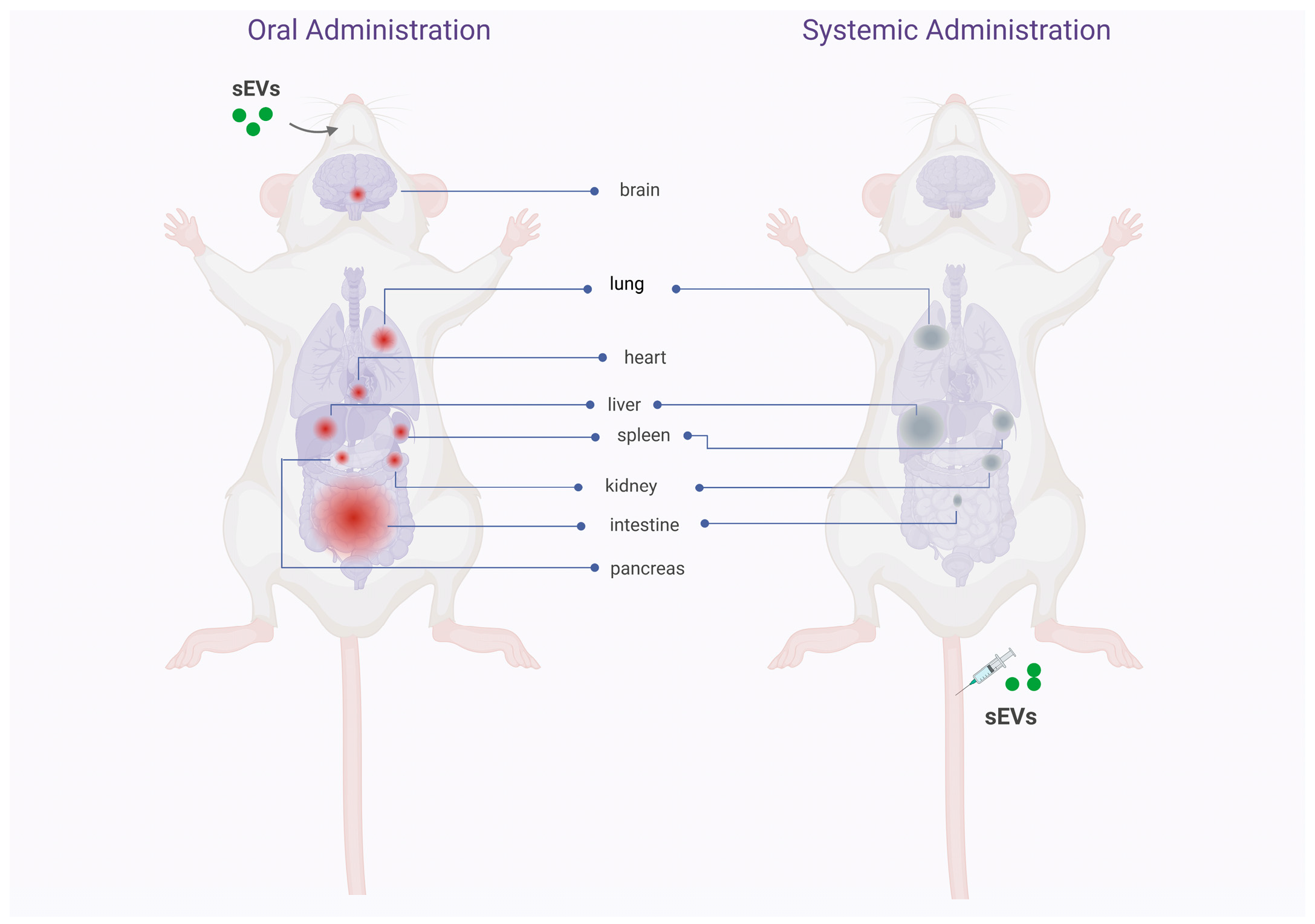
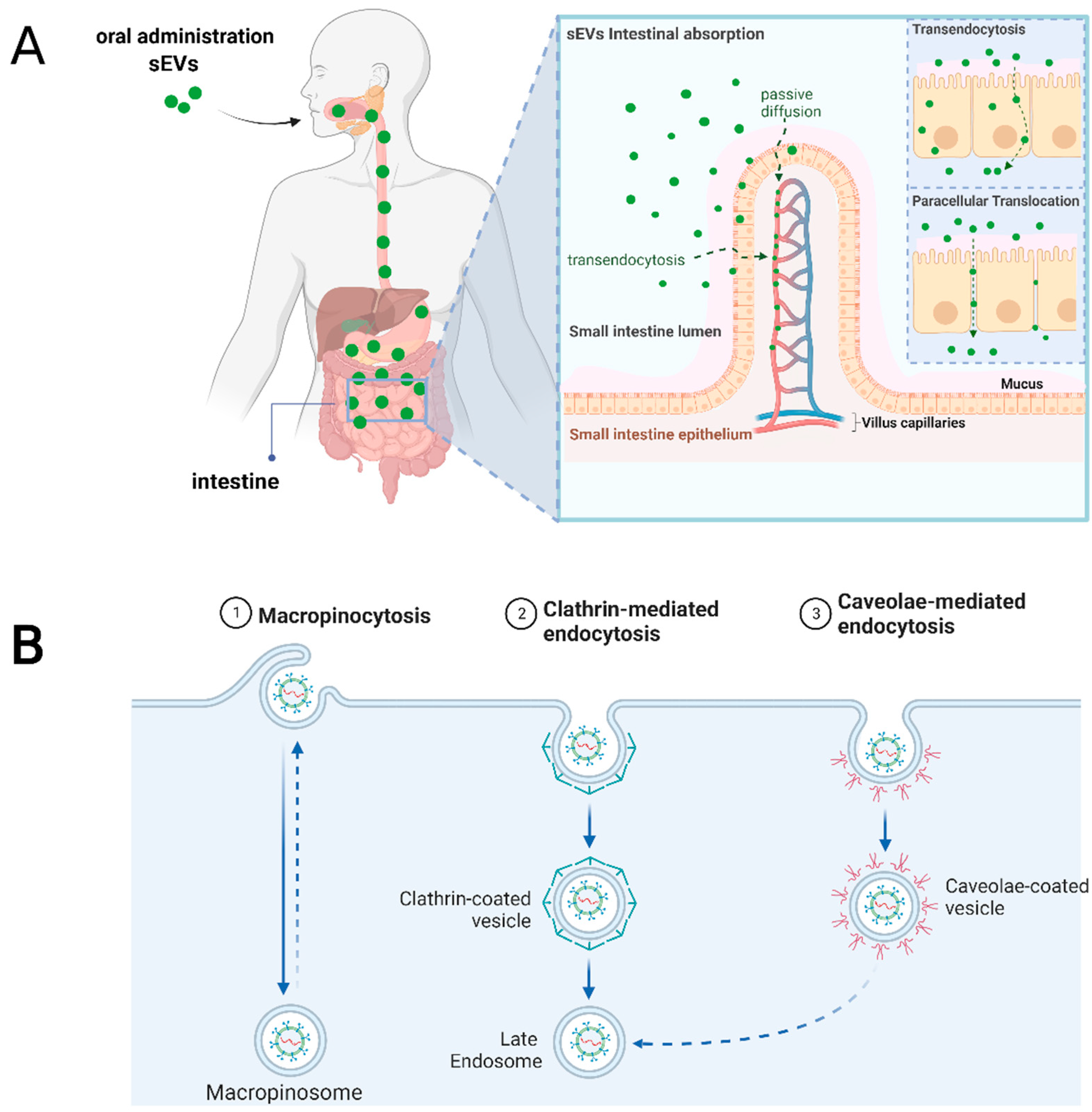
| Type of sEV | Cell Source | Labeling Method | Dose | Mouse Strain | Time of Detection and Tissue Distribution | Reference |
|---|---|---|---|---|---|---|
| sEVs | Bovine milk | DiR | 0.5 mg prot/mouse | C57BL/6 mice with DSS-induced ulcerative colitis | 1 h Small intestine 6 h Colon | [27] |
| Exosomes | Bovine milk | DiR | 1 × 1012 part/gr of mouse | Balb/c mice | 24 h Intestine, lung, and liver | [30] |
| Exosomes | Bovine milk | DiR | 40 mg prot/kg of mouse | Balb/c mice | 30 min Blood 6 h Liver, spleen, kidney, heart, and lung | [29] |
| iRGD-Exosomes | Bovine milk | DiR | 40 mg prot/kg of mouse | Tumor-bearing Balb/c mice | 4 h Tumor, liver, spleen, kidney, lung, and heart | [29] |
| Exosomes | Bovine milk | DiR | 60 mg prot/kg of mouse | Athymic nude mice | 4 d Liver, lung, kidney, pancreas, spleen, ovaries, colon, and brain | [31] |
| sEVs | Bovine milk | DiR | 25 mg prot/kg of mouse | Balb/c mice | 2 and 6 h Intestine 24 h GI tract, liver, spleen, lungs, kidney, and heart | [28] |
| sEVs | Bovine milk | DiR | 25 mg prot/kg of mouse/day × 38 days | Balb/c-Fox1nuAusb mice | 24 h Tumor tissue | [28] |
| sEVs | Yeast | DiR | 25 mg prot/kg of mouse | Not indicated | 24 h GI tract, liver, spleen, kidney, lungs, and heart | [28] |
| sEVs | Beer | DiR | Not indicated | Not indicated | Not bioavailable in the mice | [28] |
| Exosomes-like | Grape | DiR, PKH26 | 1 mg/mouse | C57BL/6 mice | 6 h Intestine | [34] |
| Exosomes-like | Acerola | PKH26 | 3 × 109 particles/mouse | C57BL/6 mice | 1 h Intestine, liver, and bladder, weak signal in brain | [33] |
| Exosomes-like | Ginger | DiR | 0.3 mg/mouse | C57BL/6 mice | 12 h Colon (in non-starved mice); 12 h Stomach and small intestine (in starved mice) | [32] |
| Exosomes-like | Garlic | DiR PKH26 | 1 × 1010 particles/mouse | C57BL/6 mice | 24 h Brain, liver, small intestine, and large intestine | [35] |
| Exosomes-like | Tea leaves | DiR | 3 mg/kg of mouse | Balb/c mice | 6 h Small intestine | [36] |
| Exosomes-like | Mulberry bark | DiR | 10 × 1010 particles/mouse | C57BL/6 mice | 3 h Small intestine, colon, cecum; small fraction was observed in spleen, liver, lung, kidney, heart, and blood | [37] |
| sEVs Type and Cell Source | Dose | Murine Strain | Time of Detection | Toxicity Profile | Reference |
|---|---|---|---|---|---|
| Cow’s milk-derived exosomes | 25 mg/kg (single administration) 25 mg/kg daily × 15 d | Sprague Dawley rats | 6 h 15 d | No changes in clinical signs, body weight, or dietary intake in animals. Biochemical (liver and kidney function) and hematological parameters remained unchanged except for triglycerides. No changes in cytokine profile (IL-1α, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12, IL-13, GM-CSF, IFN-γ and TNF-α), except for the anti-inflammatory cytokine GM-CSF. | [31] |
| Cow’s milk-derived sEVs | 2 mg/kg × 7 d | IRC mice | 7 d | No changes in body weight in animals. Biochemical (liver function) and hematological parameters remained unchanged. Histopathology examination (H&E staining) of the heart, liver, spleen, lung, kidney, and small intestine exhibited no pathological changes. | [25] |
| sEV Source | sEV Attribute/Modification/Treatment | Loaded Molecule | Biological Effect | Type of Study | References | |
|---|---|---|---|---|---|---|
| sEVs protection in transit trough digestive tract | Bovine milk and colorectal cancer cells | Calcium chloride addition | N/A | Enhanced EV stability after acidification (pH = 2) and boiling (105 °C) | In vitro | [28] |
| Human cardiosphere-derived stromal cells | Casein addition | N/A | Enhanced uptake and disease-modifying bioactivity | In vivo | [43] | |
| sEVs uptake by gut cells | Grape juice | Phosphatidic acids, Phosphatidylethanolamines | N/A | Dextrane sulfate sodium-induced colitis protection via induction of intestinal stem cells | In vivo | [34] |
| sEVs targeting beyond gut mucosa | Bovine milk | Non modified | N/A | Tumor growth reduction and accelerated metastasis, xenograft | In vivo | [28] |
| Bovine milk | Folic acid functionalization | Withaferin A Anthocyanidins Curcumin Paclitaxel Docetxel | Tumor targeting, xenograft | In vivo | [31] | |
| Human umbilical cord | Non modified | N/A | Antioxidant and anti-apoptotic and rescue from liver failure | In vivo | [44] | |
| Human MSC544 cell line | Non modified | Taxol | Tumor reducing capabilities | In vivo | [45] | |
| Mouse suppresor T cells | Antibodies free light chains coating | miRNA-150 | Immune tolerance | In vivo | [46] |
| Cell Lineage | Target Cell Source | sEV Source | Findings | Type of Study | References |
|---|---|---|---|---|---|
| Microfold cells (M cells) | Several sources | N/A (Synthetic nanoparticles) | M cells possess reduced intracellular enzymatic activity, thinner mucus layer and glycocalyx, promoting easier access and intracellular transport. | In vitro In vivo | [1] |
| Macrophages | Ag-presenting macrophages | Ts cell-derived | Macrophage clodronate depletion abolishes anti-inflammatory effect of Ts derived sEVs observed in DTH model. | In vivo | [48] |
| Dendritic cells | Transgenic reporter mice | M cell-derived vesicles | M cell-derived vesicles are taken up by dendritic cells. | In vivo | [58] |
| Enterocytes | Rat intestinal epithelial cells (IEC-6) | Grapefruit juice | Plant EV’s miRNAs are taken up by rat intestinal enterocytes. | In vitro | [59] |
| Colonocytes | Human colonocyte cell line (DLD-1) Mouse intestinal epithelial cell line (CMT-93) | Colonic luminal fluid aspirates | sEVs mRNA was present within cells, showing take up. | In vitro | [60] |
| Enterocytes | Porcine intestinal cells (IPEC-J2) | Porcine milk | sEVs promoted enterocytes proliferation in vitro. increased villus height, crypt depth and ratio of villus length to crypt depth of intestinal tissues was observed in vivo. | In vitro In vivo | [61] |
| Enterocytes | Rat intestinal epithelial cells (IEC-6) Human colon carcinoma (Caco-2) | Bovine milk | sEVs uptake decreased when incubated at low temperature (4 °C), after proteinase K treatment, using endocytosis inhibitors or carbohydrate competitors. | In vitro | [38] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donoso-Meneses, D.; Figueroa-Valdés, A.I.; Khoury, M.; Alcayaga-Miranda, F. Oral Administration as a Potential Alternative for the Delivery of Small Extracellular Vesicles. Pharmaceutics 2023, 15, 716. https://doi.org/10.3390/pharmaceutics15030716
Donoso-Meneses D, Figueroa-Valdés AI, Khoury M, Alcayaga-Miranda F. Oral Administration as a Potential Alternative for the Delivery of Small Extracellular Vesicles. Pharmaceutics. 2023; 15(3):716. https://doi.org/10.3390/pharmaceutics15030716
Chicago/Turabian StyleDonoso-Meneses, Darío, Aliosha I. Figueroa-Valdés, Maroun Khoury, and Francisca Alcayaga-Miranda. 2023. "Oral Administration as a Potential Alternative for the Delivery of Small Extracellular Vesicles" Pharmaceutics 15, no. 3: 716. https://doi.org/10.3390/pharmaceutics15030716
APA StyleDonoso-Meneses, D., Figueroa-Valdés, A. I., Khoury, M., & Alcayaga-Miranda, F. (2023). Oral Administration as a Potential Alternative for the Delivery of Small Extracellular Vesicles. Pharmaceutics, 15(3), 716. https://doi.org/10.3390/pharmaceutics15030716






