Doxorubicin Loading into Milk and Mesenchymal Stem Cells’ Extracellular Vesicles as Drug Delivery Vehicles
Abstract
1. Introduction
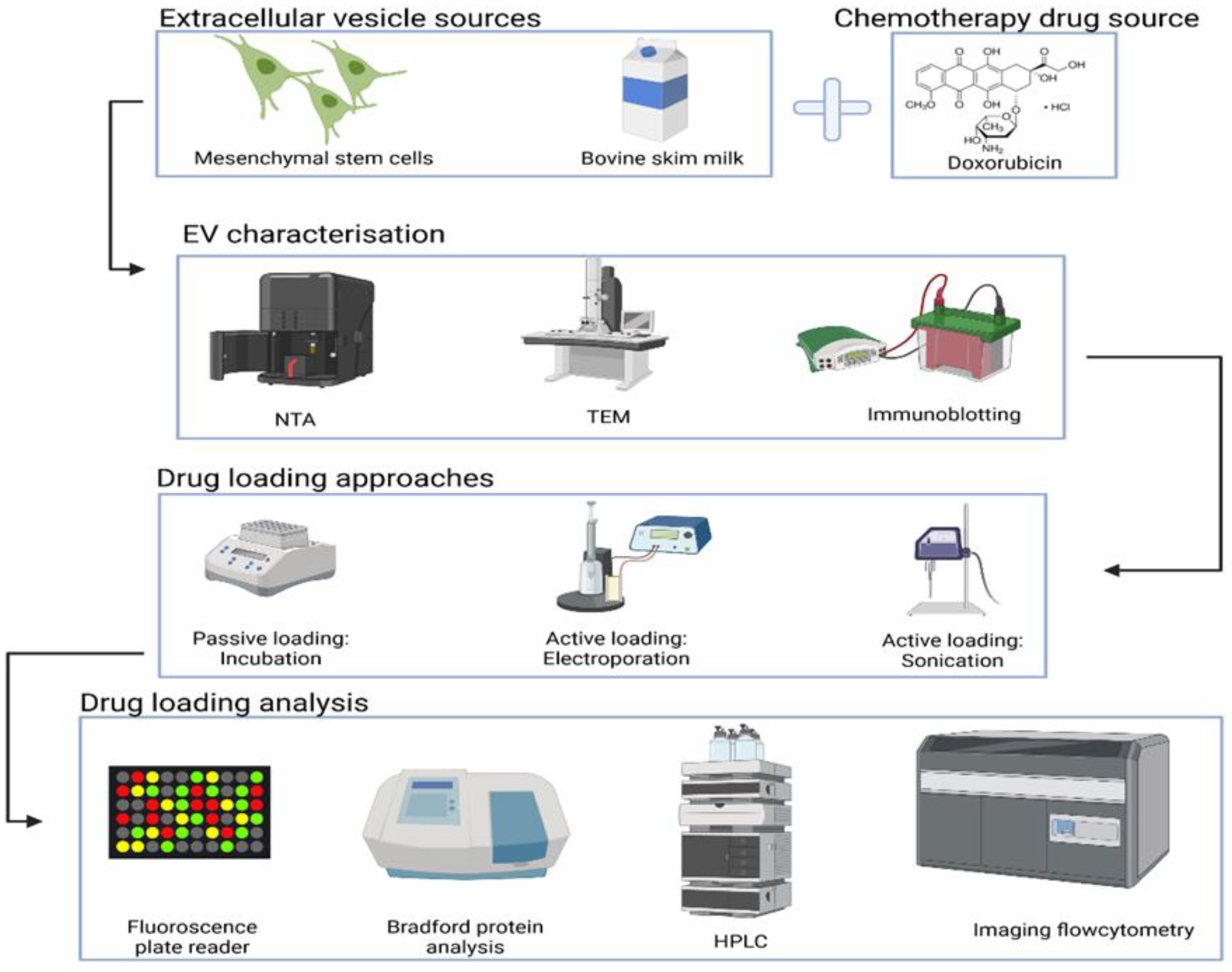
2. Materials and Methods
2.1. EVs Separation Methods
2.2. Fundamental Characterisation of EVs
2.2.1. Bradford Assay for Protein Quantification
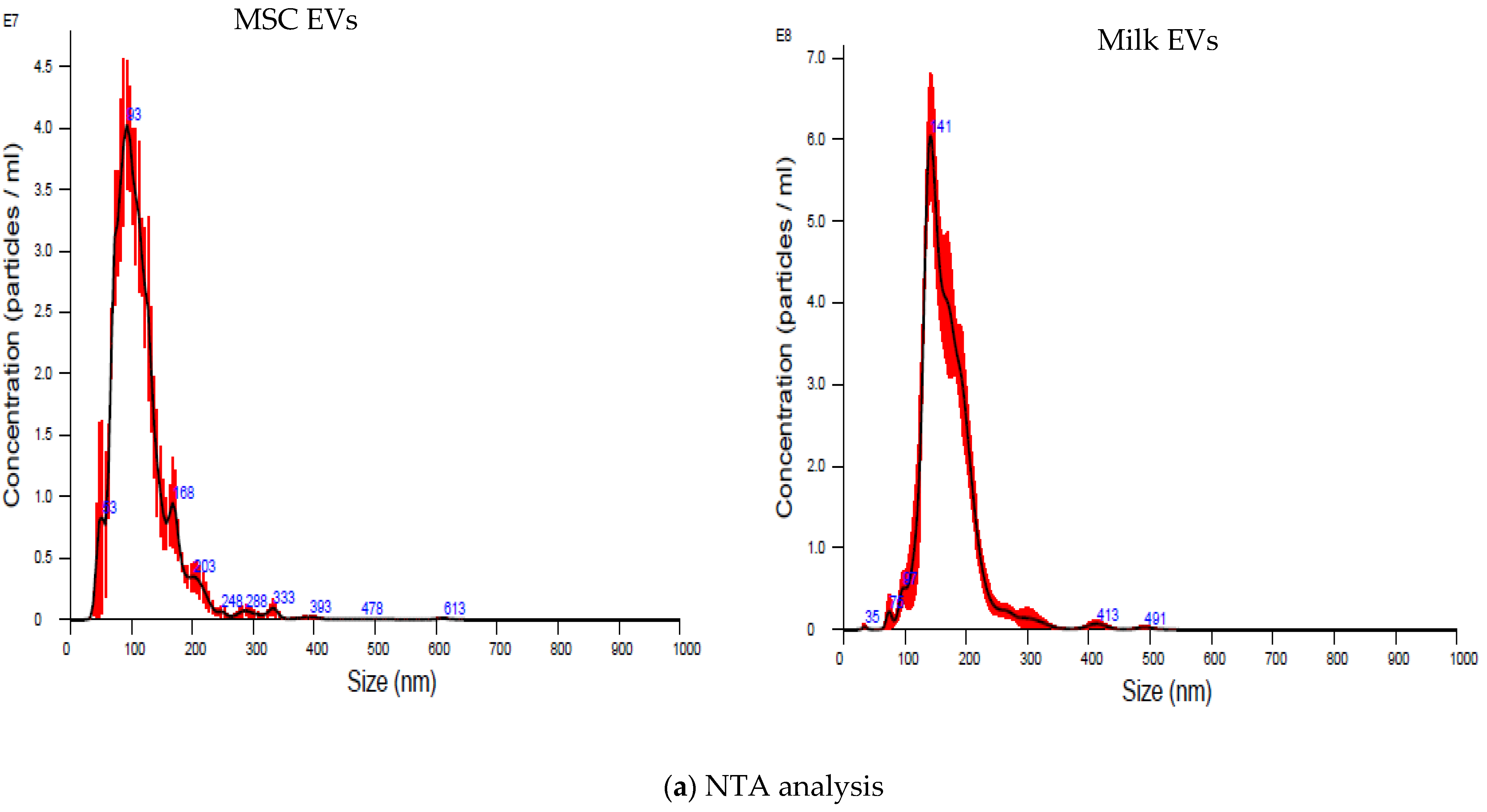
2.2.2. Nanoparticle Tracking Analysis
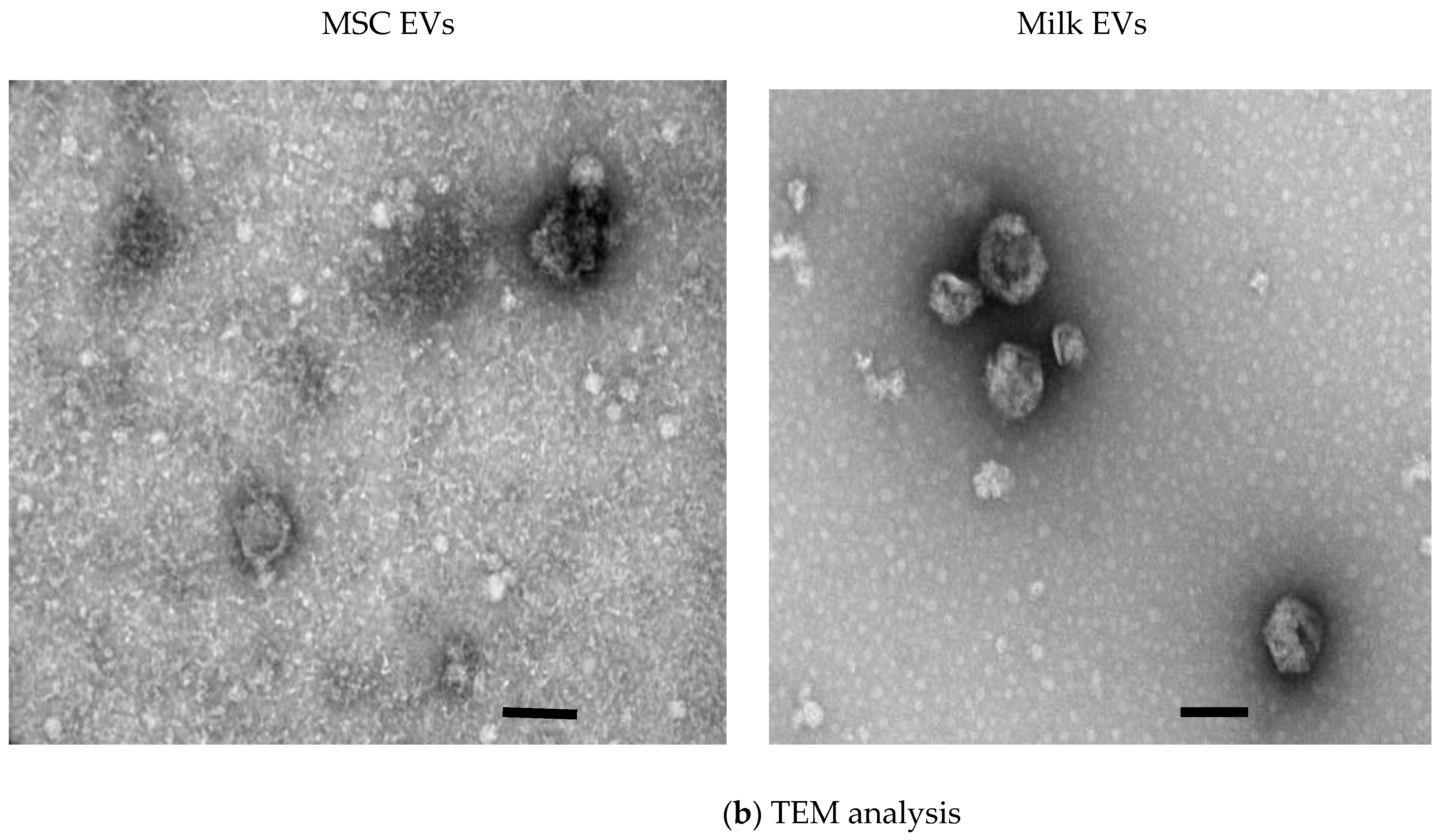
2.2.3. Transmission Electron Microscopy
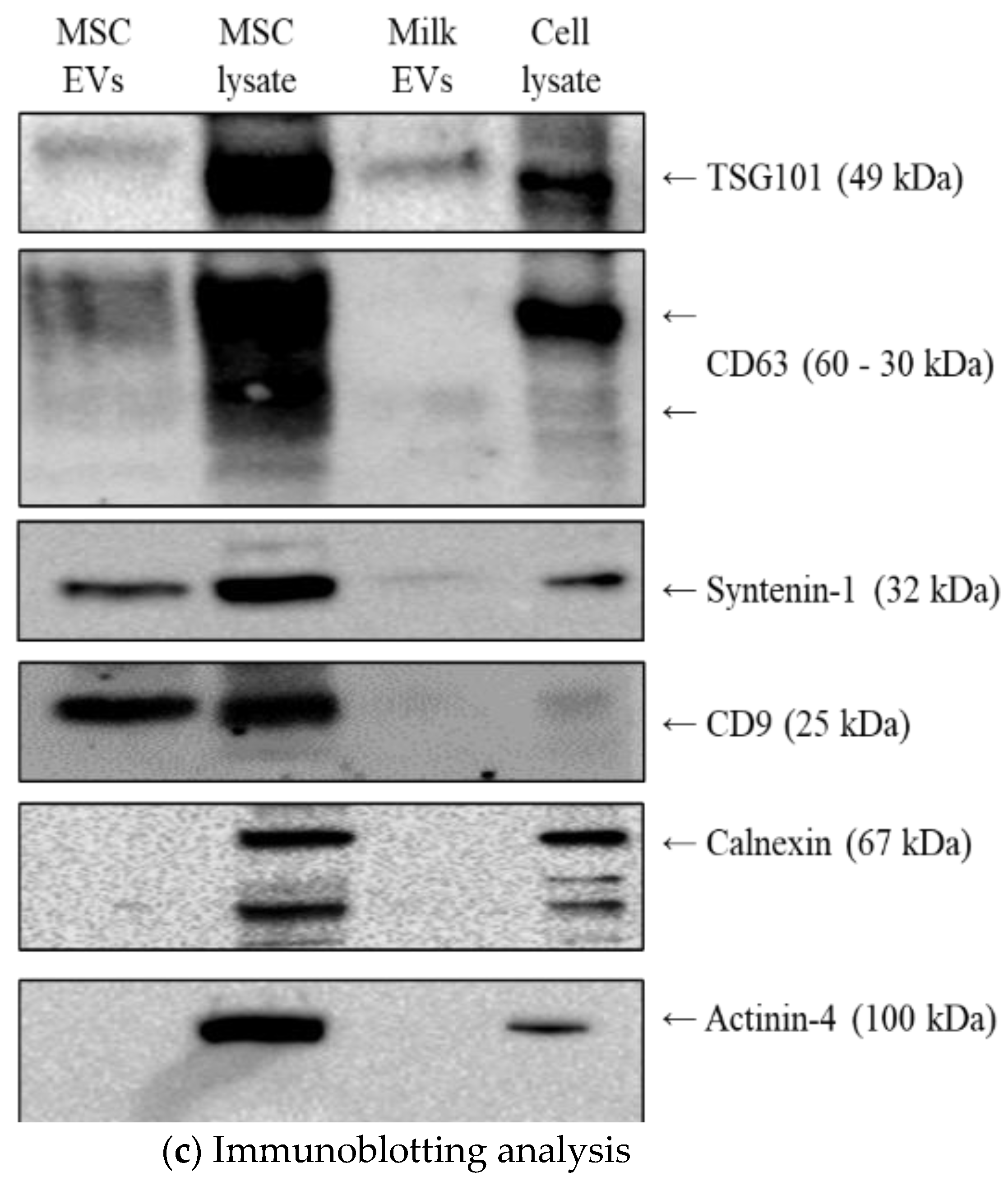
2.2.4. Immunoblotting
2.3. Doxorubicin Loading into EVs
2.3.1. Passive Loading
2.3.2. Active Loading
- (i)
- Electroporation: The electroporation of MSC EVs and milk EVs to load Dox was performed using a Neon™ Transfection System (Thermo Fisher Scientific, Dublin, Ireland). A total of 3 mL of Electrolytic buffer E was added to Neon™ tube and fixed to the tube stand. A total of 250 µg of Dox was mixed with 125 µg of MSC EVs or milk EVs and placed on ice. A 100 µL Neon™ Tip was attached to the Neon™ pipette and 100 µL of EV-Dox mix was pipetted up, the pipette was fixed to the Neon™ pipette station and electroporation protocol was performed using the program set on the Neon™ device at 1500 volts. The electroporated sample was collected in a fresh tube, and equal volume of Resuspension buffer R was added and incubated at 37 °C for 1 h for recovery of the EVs membrane.
- (ii)
- Sonication: The sonication procedure was performed using a microson ultrasonic cell disruptor with a 0.25 tip (Misonix Inc., New York, NY, USA) with the following settings: 20% amplitude, and 6 cycles of 30 s on/off for 4 min with a 2 min cooling period between each cycle. The sonicated samples were incubated at 37 °C for 1 h to allow for recovery of the EVs membrane.
2.4. Fluorescence Analysis of Doxorubicin
2.5. High-Performance Liquid Chromatography Analysis
2.6. Imaging Flow Cytometry Analysis
2.7. Data Analysis
3. Results and Discussion
3.1. MSC EVs and Milk EVs Separation and Characterisation
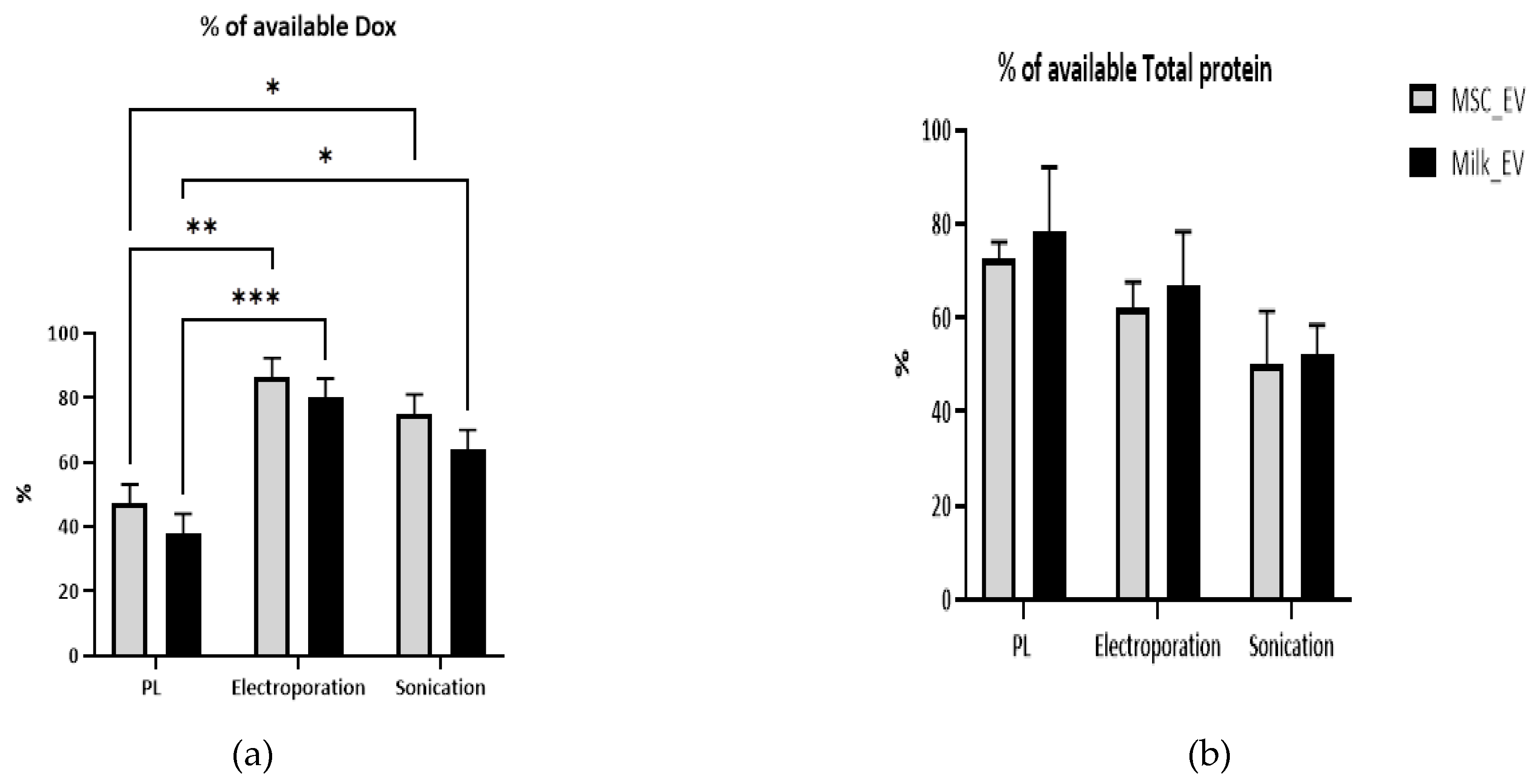
3.2. Comparison of Doxorubicin Retention and Protein Analysis Post-Drug Loading
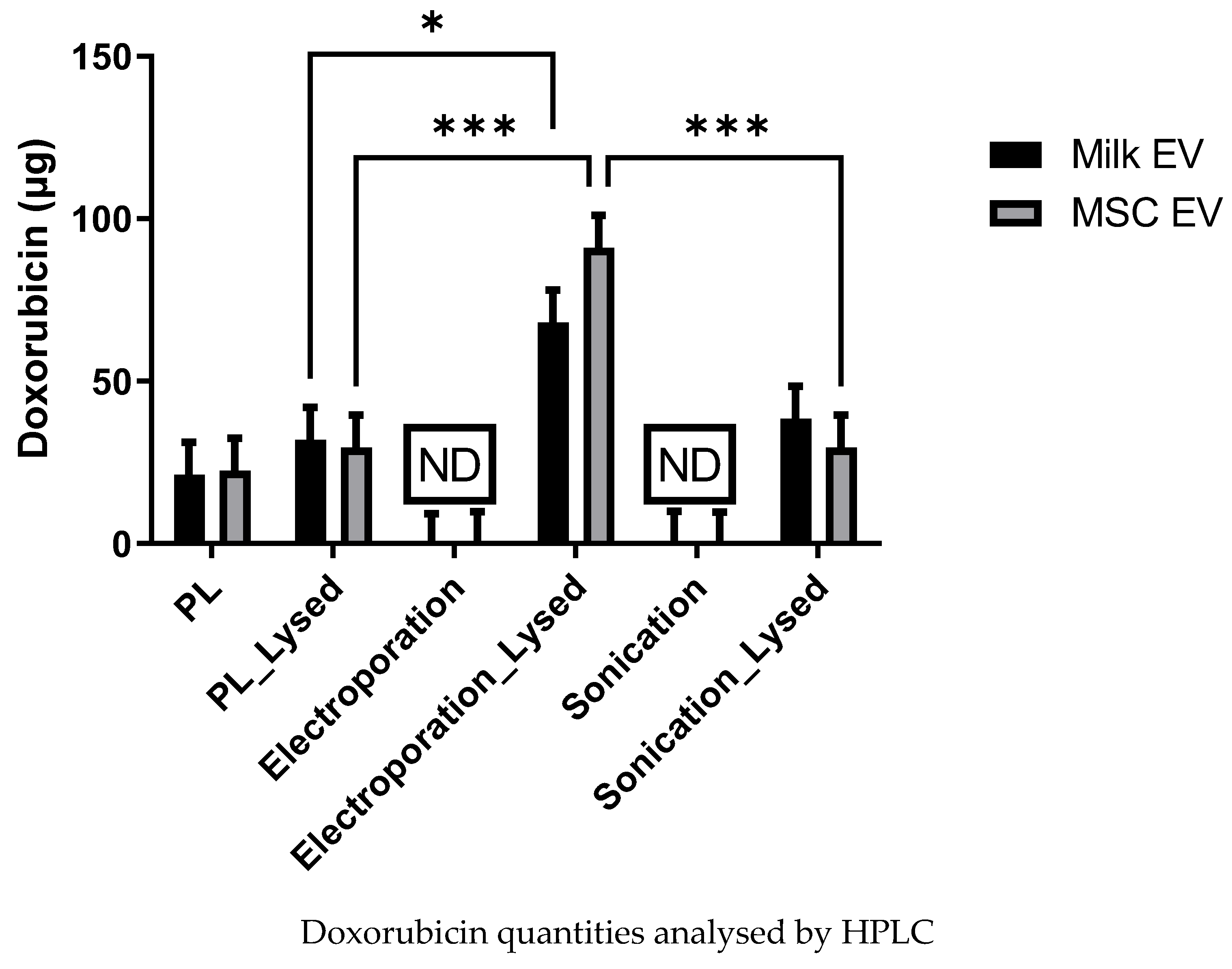
3.3. Analysis of Dox Quantity Loaded in MSC and Milk Evs by HPLC
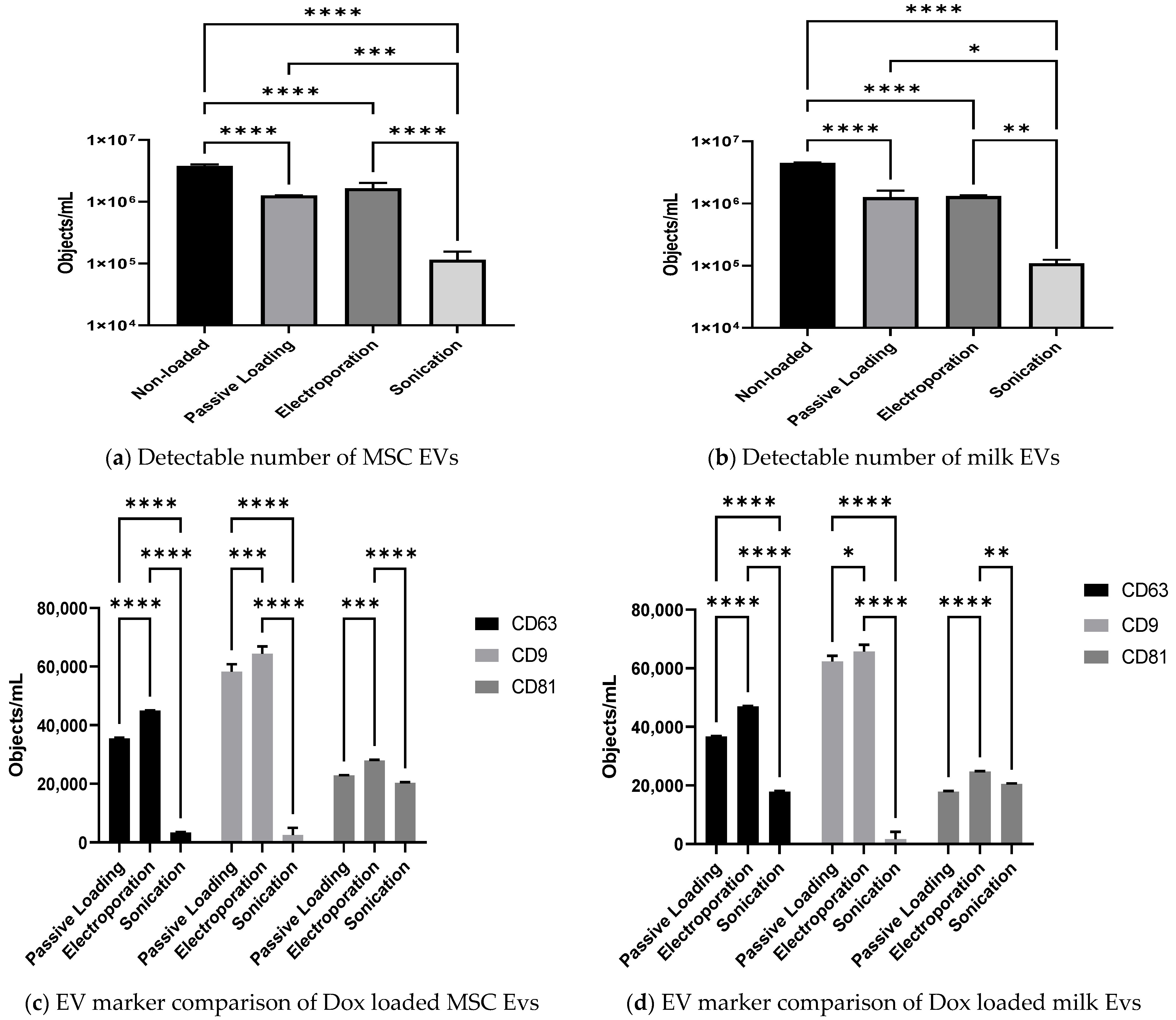
3.4. Effect of Passive and Active Loading Approaches on EVs Surface Markers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Daraee, H.; Etemadi, A.; Kouhi, M.; Alimirzalu, S.; Akbarzadeh, A. Application of liposomes in medicine and drug delivery. Samira Alimirzalu Abolfazl Akbarzadeh 2016, 44, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Busatto, S.; Pham, A.; Suh, A.; Shapiro, S.; Wolfram, J. Organotropic drug delivery: Synthetic nanoparticles and extracellular vesicles. Biomed Microdevices 2019, 21, 46. [Google Scholar] [CrossRef]
- Desai, N. Challenges in development of nanoparticle-based therapeutics. AAPS J. 2012, 14, 282–295. [Google Scholar] [CrossRef] [PubMed]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and challenges of liposome assisted drug delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef]
- Beltrán-Gracia, E.; López-Camacho, A.; Higuera-Ciapara, I.; Velázquez-Fernández, J.B.; Vallejo-Cardona, A.A. Nanomedicine review: Clinical developments in liposomal applications. Cancer Nanotechnol. 2019, 101, 10. [Google Scholar] [CrossRef]
- Walker, S.; Busatto, S.; Pham, A.; Tian, M.; Suh, A.; Carson, K.; Quintero, A.; Lafrence, M.; Malik, H.; Santana, M.X.; et al. Extracellular vesicle-based drug delivery systems for cancer treatment. Theranostics 2019, 9, 8001–8017. [Google Scholar] [CrossRef] [PubMed]
- Mehryab, F.; Rabbani, S.; Shahhosseini, S.; Shekari, F.; Fatahi, Y.; Baharvand, H.; Haeri, A. Exosomes as a next-generation drug delivery system: An update on drug loading approaches, characterization, and clinical application challenges. Acta Biomater. 2020, 113, 42–62. [Google Scholar] [CrossRef]
- Herrmann, I.K.; Wood, M.J.A.; Fuhrmann, G. Extracellular vesicles as a next-generation drug delivery platform. Nat. Nanotechnol. 2021, 16, 748–759. [Google Scholar] [CrossRef]
- O’Driscoll, L. Expanding on exosomes and ectosomes in cancer. N. Engl. J. Med. 2015, 372, 2359–2362. [Google Scholar] [CrossRef]
- Vader, P.; Mol, E.A.; Pasterkamp, G.; Schiffelers, R.M. Extracellular vesicles for drug delivery. Adv. Drug. Deliv. Rev. 2016, 106, 148–156. [Google Scholar] [CrossRef]
- O’Brien, K.; Lowry, M.C.; Corcoran, C.; Martinez, V.G.; Daly, M.; Rani, S.; Gallagher, W.M.; Radomski, M.W.; MacLeod, R.A.; O’Driscoll, L. miR-134 in extracellular vesicles reduces triple-negative breast cancer aggression and increases drug sensitivity. Oncotarget 2015, 6, 32774–32789. [Google Scholar] [CrossRef]
- Melzer, C.; Rehn, V.; Yang, Y.; Bähre, H.; von der Ohe, J.; Hass, R. Taxol-Loaded MSC-Derived Exosomes Provide a Therapeutic Vehicle to Target Metastatic Breast Cancer and Other Carcinoma Cells. Cancers 2019, 11, 798. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Chen, J.; Wang, S.; Fu, F.; Zhu, X.; Wu, C.; Liu, Z.; Zhong, G.; Lin, J. A Nanodrug Consisting Of Doxorubicin And Exosome Derived From Mesenchymal Stem Cells For Osteosarcoma Treatment In Vitro. Int. J. Nanomed. 2019, 14, 8603. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Sun, J.; Qiu, J.; Chen, G.; Wang, X.; Mu, Y.; Li, K.; Wang, W. Antitumor Activity of Cabazitaxel and MSC-TRAIL Derived Extracellular Vesicles in Drug-Resistant Oral Squamous Cell Carcinoma. Cancer Manag. Res. 2020, 12, 10809–10820. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, W.; Chen, X.; Wang, Q.; Li, C.; Chen, Q.; Zhang, Y.; Lu, Y.; Ding, X.; Jiang, C. Bone marrow mesenchymal stem cells-derived exosomes for penetrating and targeted chemotherapy of pancreatic cancer. Acta Pharm. Sin. B 2020, 10, 1563–1575. [Google Scholar] [CrossRef]
- Liang, L.; Zhao, L.; Wang, Y.; Wang, Y. Treatment for Hepatocellular Carcinoma Is Enhanced When Norcantharidin Is Encapsulated in Exosomes Derived from Bone Marrow Mesenchymal Stem Cells. Mol. Pharm. 2021, 18, 1003–1013. [Google Scholar] [CrossRef] [PubMed]
- Aqil, F.; Jeyabalan, J.; Agrawal, A.K.; Kyakulaga, A.-H.; Munagala, R.; Parker, L.; Gupta, R.C. Exosomal Delivery of Berry Anthocyanidins for the Management of Ovarian Cancer. 2017. Available online: https://pubs.rsc.org/en/content/articlehtml/2017/fo/c7fo00882a (accessed on 9 October 2022).
- Munagala, R.; Aqil, F.; Jeyabalan, J. Exosomal Formulation of Anthocyanidins Against Multiple Cancer Types; Elsevier: Amsterdam, The Netherlands, 2017; Available online: https://www.sciencedirect.com/science/article/pii/S0304383517301040?casa_token=OsCOqVD_CQgAAAAA:1xLlN1_fsdnMHFj72F6r72_7r-XNeGTsO7LNrYrP7v_OwBPErAS8FDZnYLcmwovuBw3WCYDMsVY (accessed on 9 October 2022).
- Vashisht, M.; Rani, P.; Onteru, S.K.; Singh, D. Curcumin Encapsulated in Milk Exosomes Resists Human Digestion and Possesses Enhanced Intestinal Permeability in Vitro. Appl. Biochem. Biotechnol. 2017, 183, 993–1007. [Google Scholar] [CrossRef]
- Ludwig, A.-K.; De Miroschedji, K.; Doeppner, T.R.; Börger, V.; Ruesing, J.; Rebmann, V.; Durst, S.; Jansen, S.; Bremer, M.; Behrmann, E.; et al. Precipitation with polyethylene glycol followed by washing and pelleting by ultracentrifugation enriches extracellular vesicles from tissue culture supernatants in small and large scales. J. Extracell. Vesicles 2018, 7, 1528109. [Google Scholar] [CrossRef]
- Mukhopadhya, A.; Santoro, J.; Moran, B.; Useckaite, Z.; O’Driscoll, L. Optimisation and comparison of orthogonal methods for separation and characterisation of extracellular vesicles to investigate how representative infant milk formula is of milk. Food Chem. 2021, 353, 129309. [Google Scholar] [CrossRef]
- Mukhopadhya, A.; Santoro, J.; O’Driscoll, L. Extracellular vesicle separation from milk and infant milk formula using acid precipitation and ultracentrifugation. STAR Protoc. 2021, 2, 100821. [Google Scholar] [CrossRef]
- Visnovitz, T.; Osteikoetxea, X.; Sódar, B.W.; Mihály, J.; Lőrincz, P.; Vukman, K.V.; Tóth, E.; Koncz, A.; Székács, I.; Horváth, R.; et al. An improved 96 well plate format lipid quantification assay for standardisation of experiments with extracellular vesicles. J. Extracell. Vesicles 2019, 8, 1565263. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, K.; Rani, S.; Corcoran, C.; Wallace, R.; Hughes, L.; Friel, A.M.; McDonnell, S.; Crown, J.; Radomski, M.W.; O’Driscoll, L. Exosomes from triple-negative breast cancer cells can transfer phenotypic traits representing their cells of origin to secondary cells. Eur. J. Cancer 2013, 49, 1845–1859. [Google Scholar] [CrossRef]
- Li, D.; Yao, S.; Zhou, Z.; Shi, J.; Huang, Z.; Wu, Z. Hyaluronan decoration of milk exosomes directs tumor-specific delivery of doxorubicin. Carbohydr. Res. 2020, 493, 108032. [Google Scholar] [CrossRef]
- Zhang, Q.; Xiao, Q.; Yin, H.; Xia, C.; Pu, Y.; He, Z.; Hu, Q.; Wang, J.; Wang, Y. Milk-exosome based pH/light sensitive drug system to enhance anticancer activity against oral squamous cell carcinoma. RSC Adv. 2020, 10, 28314–28323. [Google Scholar] [CrossRef]
- O’Regan, J.; Ennis, M.P.; Mulvihill, D.M. “Milk Proteins,” in Handbook of Hydrocolloids; Elsevier: Amsterdam, The Netherlands, 2009; pp. 298–358. [Google Scholar]
- Gomari, H.; Moghadam, M.F.; Soleimani, M.; Ghavami, M.; Khodashenas, S. Targeted delivery of doxorubicin to HER2 positive tumor models. Int. J. Nanomed. 2019, 14, 5679–5690. [Google Scholar] [CrossRef]
- Bagheri, E.; Abnous, K.; Farzad, S.A.; Taghdisi, S.M.; Ramezani, M.; Alibolandi, M. Targeted doxorubicin-loaded mesenchymal stem cells-derived exosomes as a versatile platform for fighting against colorectal cancer. Life Sci. 2020, 261, 118369. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Oller, L.; Seras-Franzoso, J.; Andrade, F.; Rafael, D.; Abasolo, I.; Gener, P.; Schwartz, S., Jr. Extracellular Vesicles as Drug Delivery Systems in Cancer. Pharmaceutics 2020, 12, 1146. [Google Scholar] [CrossRef] [PubMed]
- Stam, J.; Bartel, S.; Bischoff, R.; Wolters, J.C. Isolation of extracellular vesicles with combined enrichment methods. J. Chromatogr. B 2021, 1169, 122604. [Google Scholar] [CrossRef] [PubMed]
- Rankin-Turner, S.; Vader, P.; O’Driscoll, L.; Giebel, B.; Heaney, L.M.; Davies, O.G. A call for the standardised reporting of factors affecting the exogenous loading of extracellular vesicles with therapeutic cargos. Adv. Drug Deliv. Rev. 2021, 173, 479–491. [Google Scholar] [CrossRef]
- Srivastava, A.; Amreddy, N.; Pareek, V.; Chinnappan, M.; Ahmed, R.; Mehta, M.; Razaq, M.; Munshi, A.; Ramesh, R. Progress in extracellular vesicle biology and their application in cancer medicine. Wiley Interdiscip. Rev. Nanomed. Nanobiotech. 2020, 12, e1621. [Google Scholar] [CrossRef]
- Nizamudeen, Z.A.; Xerri, R.; Parmenter, C.; Suain, K.; Markus, R.; Chakrabarti, L.; Sottile, V. Low-power sonication can alter extracellular vesicle size and properties. Cells 2021, 10, 2413. [Google Scholar] [CrossRef] [PubMed]
- De Jong, O.G.; Kooijmans, S.A.A.; Murphy, D.E.; Jiang, L.; Evers, M.J.W.; Sluijter, J.P.G.; Vader, P.; Schiffelers, R.M. Drug Delivery with Extracellular Vesicles: From Imagination to Innovation. Acc. Chem. Res. 2019, 52, 1761–1770. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Jones, T.W.; Dutta, S.; Zhu, Y.; Wang, X.; Narayanan, S.P.; Fagan, S.C.; Zhang, D. Overview and update on methods for cargo loading into extracellular vesicles. Processes 2021, 9, 356. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mukhopadhya, A.; Tsiapalis, D.; McNamee, N.; Talbot, B.; O’Driscoll, L. Doxorubicin Loading into Milk and Mesenchymal Stem Cells’ Extracellular Vesicles as Drug Delivery Vehicles. Pharmaceutics 2023, 15, 718. https://doi.org/10.3390/pharmaceutics15030718
Mukhopadhya A, Tsiapalis D, McNamee N, Talbot B, O’Driscoll L. Doxorubicin Loading into Milk and Mesenchymal Stem Cells’ Extracellular Vesicles as Drug Delivery Vehicles. Pharmaceutics. 2023; 15(3):718. https://doi.org/10.3390/pharmaceutics15030718
Chicago/Turabian StyleMukhopadhya, Anindya, Dimitrios Tsiapalis, Niamh McNamee, Brian Talbot, and Lorraine O’Driscoll. 2023. "Doxorubicin Loading into Milk and Mesenchymal Stem Cells’ Extracellular Vesicles as Drug Delivery Vehicles" Pharmaceutics 15, no. 3: 718. https://doi.org/10.3390/pharmaceutics15030718
APA StyleMukhopadhya, A., Tsiapalis, D., McNamee, N., Talbot, B., & O’Driscoll, L. (2023). Doxorubicin Loading into Milk and Mesenchymal Stem Cells’ Extracellular Vesicles as Drug Delivery Vehicles. Pharmaceutics, 15(3), 718. https://doi.org/10.3390/pharmaceutics15030718






