Production and Utility of Extracellular Vesicles with 3D Culture Methods
Abstract
1. Introduction
2. Subtypes of Extracellular Vesicles
3. Exosomes as a Subset of Extracellular Vesicles—Their Origin and Characteristics
3.1. EVs in Diagnostics
3.2. Therapeutic Application of EVs
3.3. EVs as Drug Carriers
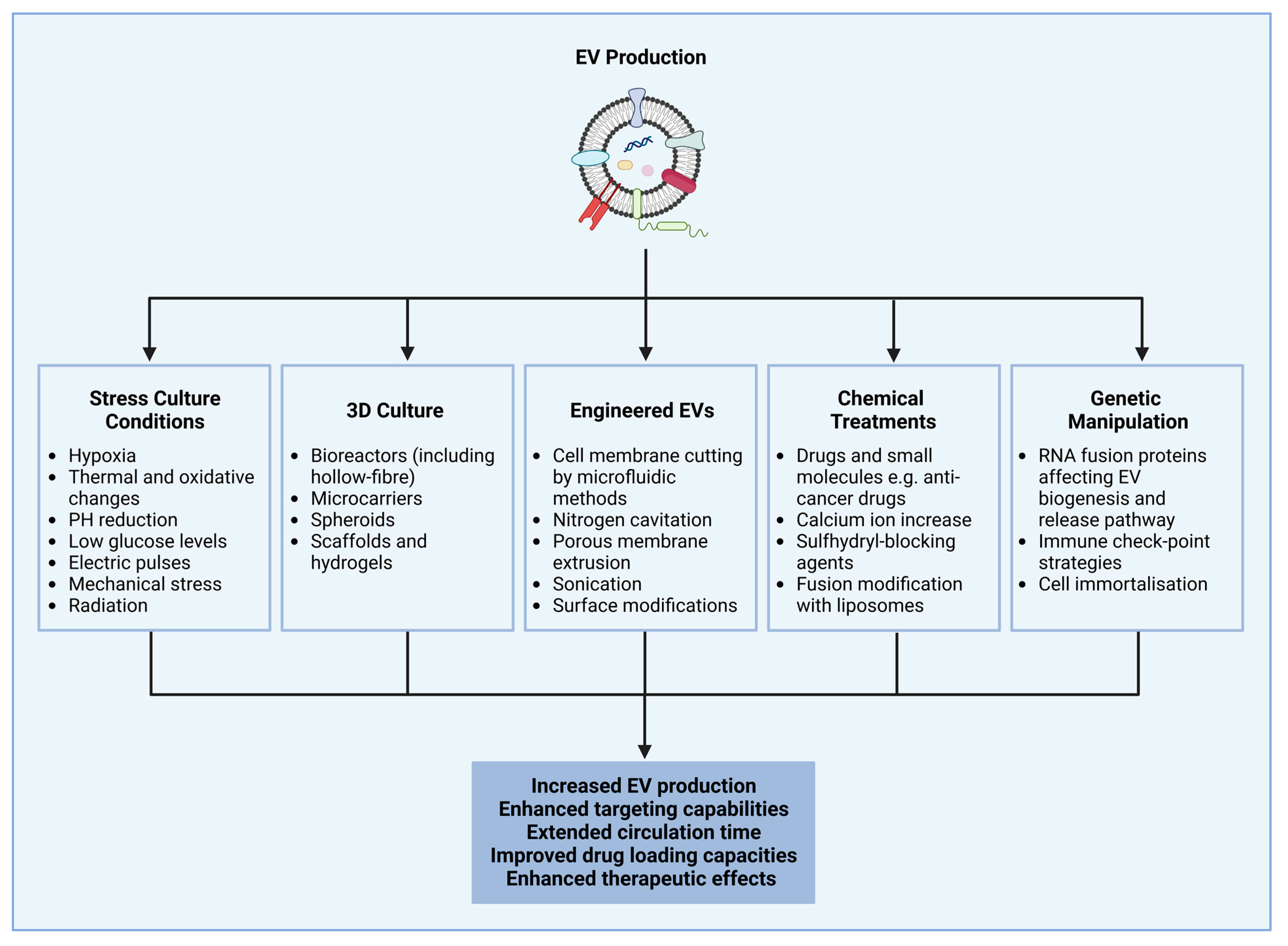
4. Extracellular Vesicle Production and Isolation Methods and Strategies to Enhance Cellular Production of Extracellular Vesicles
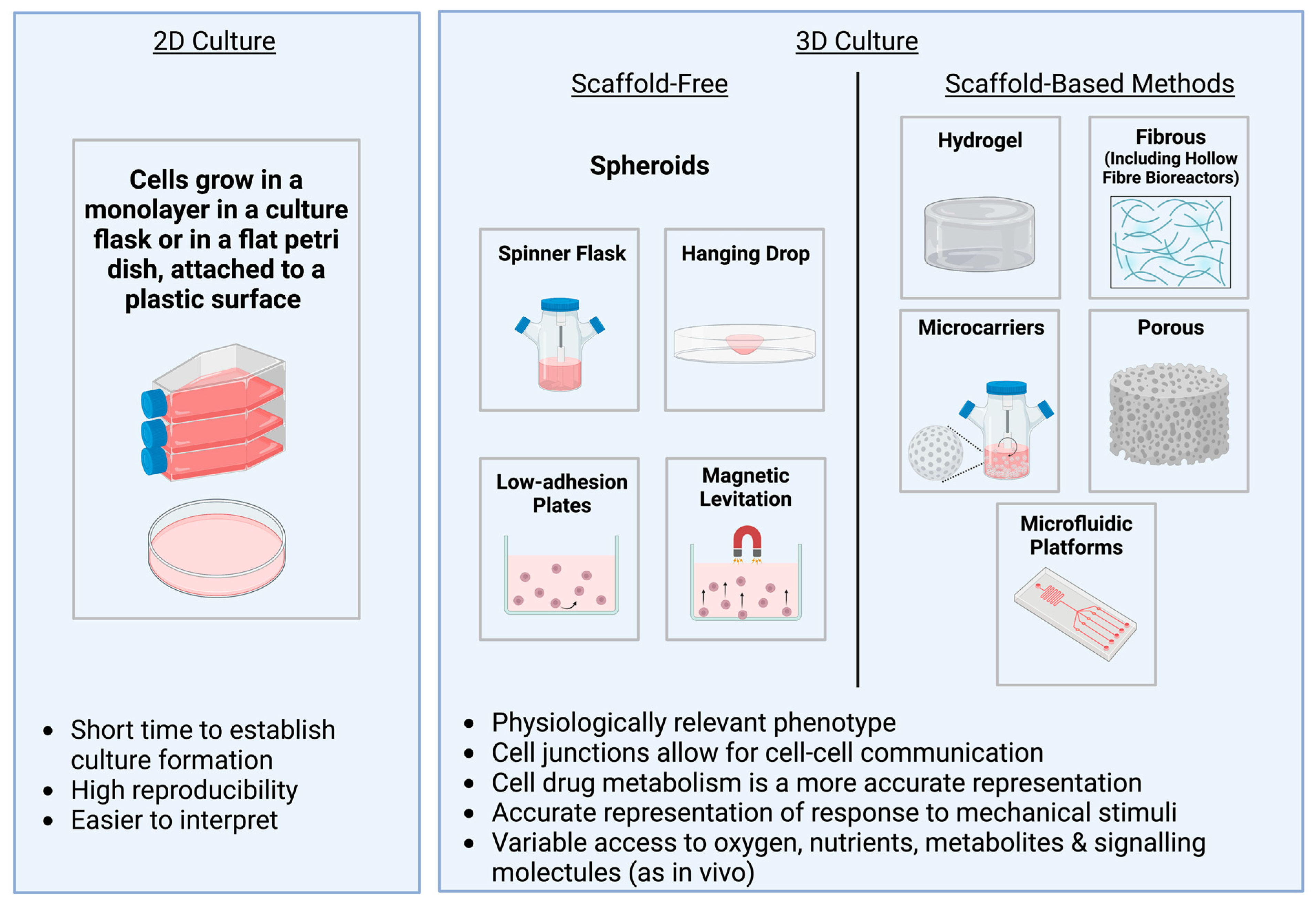
5. An Overview of Different 3D Culture Methods
6. Spheroids
7. Hydrogels
8. Hard Porous and Fibrous Scaffolds
| Type of Scaffold | Cell Type | Applications | References |
|---|---|---|---|
| Type I collagen and fibronectin matrix proteins | MSC | Augment performance of lineage specific differentiation of naïve MSCs in bone transplantation | [187] |
| Porous β-tricalcium phosphate | iPSC and MSC | Increased angiogenesis and osteogenesis | [188,189,190] |
| PCL | MSC and chondrogenic ATDC5 cells | Enhanced osteogenic differentiation | [191,192,193] |
| Mineral-doped PLA porous | hAD-MSC | Enhanced osteogenic MSC differentiation | [194] |
| Biodegradable PLGA | hAD-MSC | Enhanced osteogenic differentiation and enhanced mineralisation by endogenous cell recruitment | [195,196] |
| Tannic-acid-modified sulfonated SPEEK | BM-MSC | Enhanced osteoimmunomodulation by promotion of macrophage polarisation | [197] |
| 3D-printed porous Ti alloy (Ti6Al4V) | SC | Improved efficacy of Ti alloy scaffolds in bone repair | [198] |
| Calcium sulphate hydroxyapatite nanocement | MSC | Enhanced bone mineralisation | [199] |
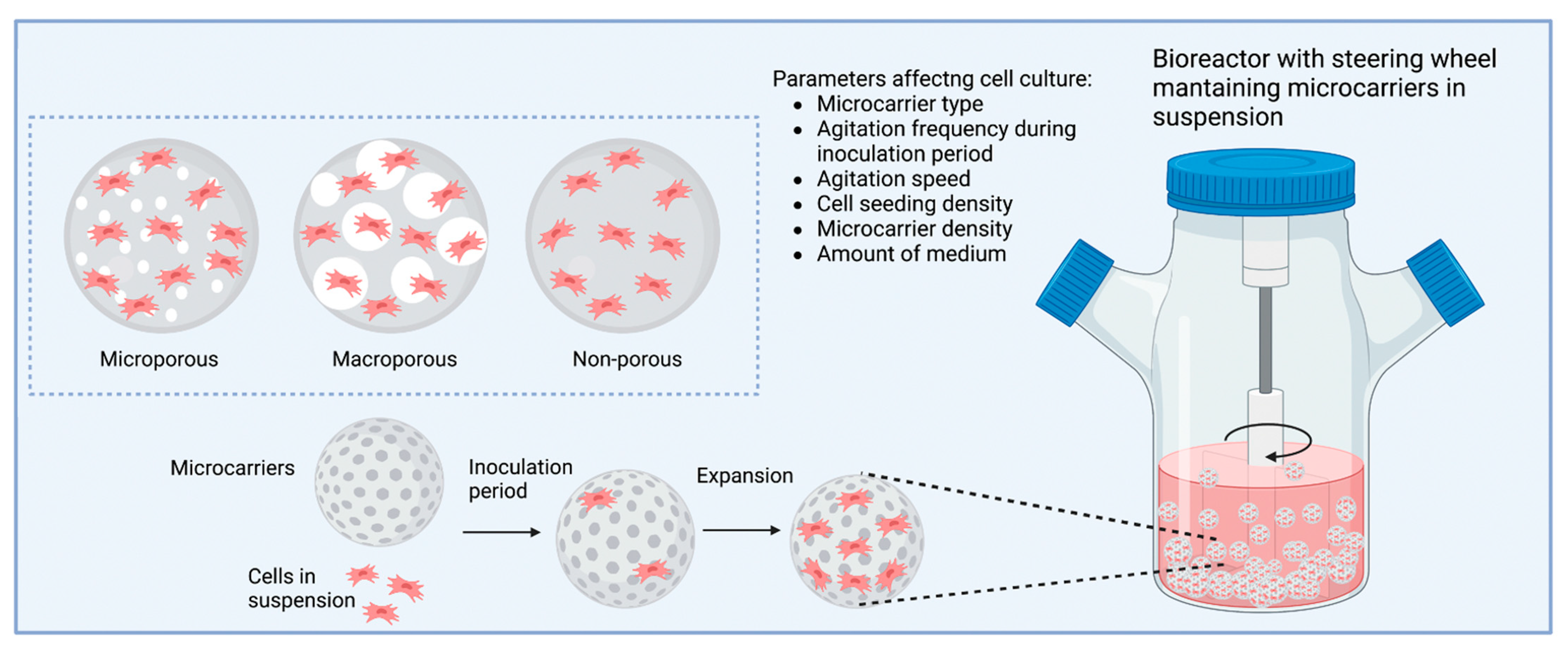
9. Microcarriers
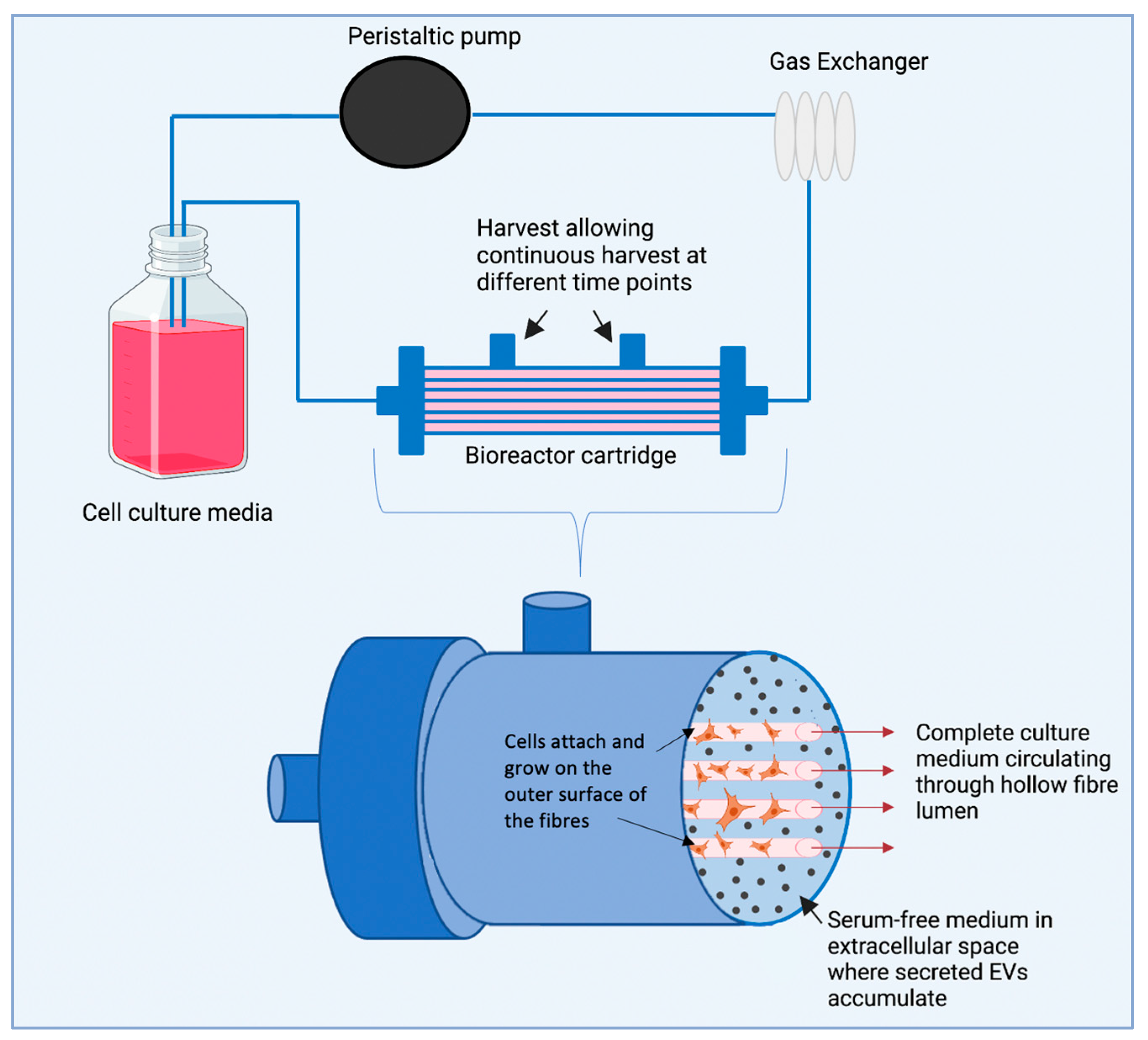
10. Hollow Fibre Bioreactor
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.; Catchpole, R.; Forterre, P. Extracellular membrane vesicles in the three domains of life and beyond. FEMS Microbiol. Rev. 2018, 43, 273–303. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: Biogenesis, biologic function and clinical potential. Cell Biosci. 2019, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Whiteside, T.L. Tumor-Derived Exosomes and Their Role in Cancer Progression. Adv. Clin. Chem. 2016, 74, 103–141. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.-Y.; Zhang, Q.-Y.; Tan, J.; Xie, H.-Q. Techniques for increasing the yield of stem cell-derived exosomes: What factors may be involved? Sci. China Life Sci. 2021, 65, 1325–1341. [Google Scholar] [CrossRef] [PubMed]
- Rashed, M.H.; Bayraktar, E.; Helal, G.K.; Abd-Ellah, M.F.; Amero, P.; Chavez-Reyes, A.; Rodriguez-Aguayo, C. Exosomes: From Garbage Bins to Promising Therapeutic Targets. Int. J. Mol. Sci. 2017, 18, 538. [Google Scholar] [CrossRef]
- Zhang, Q.; Jeppesen, D.K.; Higginbotham, J.N.; Graves-Deal, R.; Trinh, V.Q.; Ramirez, M.A.; Sohn, Y.; Neininger, A.C.; Taneja, N.; McKinley, E.T.; et al. Supermeres are functional extracellular nanoparticles replete with disease biomarkers and therapeutic targets. Nature 2021, 23, 1240–1254. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Zhang, Y.; Li, Y.; Luo, L.; Zhao, Y.; Yao, Y. Extracellular vesicles in cardiovascular diseases. Cell Death Discov. 2020, 6, 68. [Google Scholar] [CrossRef]
- Herrmann, I.K.; Wood, M.J.A.; Fuhrmann, G. Extracellular vesicles as a next-generation drug delivery platform. Nat. Nanotechnol. 2021, 16, 748–759. [Google Scholar] [CrossRef]
- Ciferri, M.; Quarto, R.; Tasso, R. Extracellular Vesicles as Biomarkers and Therapeutic Tools: From Pre-Clinical to Clinical Applications. Biology 2021, 10, 359. [Google Scholar] [CrossRef]
- Luo, R.; Liu, M.; Tan, T.; Yang, Q.; Wang, Y.; Men, L.; Zhao, L.; Zhang, H.; Wang, S.; Xie, T.; et al. Emerging Significance and Therapeutic Potential of Extracellular vesicles. Int. J. Biol. Sci. 2021, 17, 2476–2486. [Google Scholar] [CrossRef]
- Limongi, T.; Susa, F.; Dumontel, B.; Racca, L.; Perrone Donnorso, M.; Debellis, D.; Cauda, V. Extracellular vesicles tropism: A comparative study between passive innate tropism and the active engineered targeting capability of lymphocyte-derived evs. Membranes 2021, 11, 886. [Google Scholar] [CrossRef] [PubMed]
- Tai, Y.-L.; Chen, K.-C.; Hsieh, J.-T.; Shen, T.-L. Exosomes in cancer development and clinical applications. Cancer Sci. 2018, 109, 2364–2374. [Google Scholar] [CrossRef]
- Osaki, M.; Okada, F. Exosomes and Their Role in Cancer Progression. Yonago Acta Medica 2019, 62, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Logozzi, M.; Mizzoni, D.; Di Raimo, R.; Fais, S. Exosomes: A Source for New and Old Biomarkers in Cancer. Cancers 2020, 12, 2566. [Google Scholar] [CrossRef] [PubMed]
- Sumrin, A.; Moazzam, S.; Khan, A.A.; Ramzan, I.; Batool, Z.; Kaleem, S.; Ali, M.; Bashir, H.; Bilal, M. Exosomes as Biomarker of Cancer. Braz. Arch. Biol. Technol. 2018, 61, e18160730. [Google Scholar] [CrossRef]
- Liang, Y.; Duan, L.; Lu, J.; Xia, J. Engineering exosomes for targeted drug delivery. Theranostics 2021, 11, 3183–3195. [Google Scholar] [CrossRef]
- Soliman, H.M.; Ghonaim, G.A.; Gharib, S.M.; Chopra, H.; Farag, A.K.; Hassanin, M.H.; Nagah, A.; Emad-Eldin, M.; Hashem, N.E.; Yahya, G.; et al. Exosomes in Alzheimer’s Disease: From Being Pathological Players to Potential Diagnostics and Therapeutics. Int. J. Mol. Sci. 2021, 22, 10794. [Google Scholar] [CrossRef]
- Chen, J.; Li, P.; Zhang, T.; Xu, Z.; Huang, X.; Wang, R.; Du, L. Review on Strategies and Technologies for Exosome Isolation and Purification. Front. Bioeng. Biotechnol. 2022, 9, 811971. [Google Scholar] [CrossRef]
- Bordanaba-Florit, G.; Madarieta, I.; Olalde, B.; Falcón-Pérez, J.; Royo, F. 3D Cell Cultures as Prospective Models to Study Extracellular Vesicles in Cancer. Cancers 2021, 13, 307. [Google Scholar] [CrossRef]
- Wu, X.; Su, J.; Wei, J.; Jiang, N.; Ge, X. Recent Advances in Three-Dimensional Stem Cell Culture Systems and Applications. Stem Cells Int. 2021, 2021, 9477332. [Google Scholar] [CrossRef] [PubMed]
- Jensen, C.; Teng, Y. Is It Time to Start Transitioning From 2D to 3D Cell Culture? Front. Mol. Biosci. 2020, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Edmondson, R.; Broglie, J.; Adcock, A.; Yang, L. Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay Drug Dev. Technol. 2014, 12, 207–218. [Google Scholar] [CrossRef]
- Nooshabadi, V.T.; Khanmohammadi, M.; Shafei, S.; Banafshe, H.R.; Malekshahi, Z.V.; Ebrahimi-Barough, S.; Ai, J. Impact of atorvastatin loaded exosome as an anti-glioblastoma carrier to induce apoptosis of U87 cancer cells in 3D culture model. Biochem. Biophys. Rep. 2020, 23, 100792. [Google Scholar] [CrossRef] [PubMed]
- Thippabhotla, S.; Zhong, C.; He, M. 3D cell culture stimulates the secretion of in vivo like extracellular vesicles. Sci. Rep. 2019, 9, 13012. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, J.; Fu, H.; Kuang, S.; He, F.; Zhang, M.; Shen, Z.; Qin, W.; Lin, Z.; Huang, S. Exosomes derived from 3D-cultured MSCs improve therapeutic effects in periodontitis and experimental colitis and restore the Th17 cell/Treg balance in inflamed periodontium. Int. J. Oral Sci. 2021, 13, 43. [Google Scholar] [CrossRef]
- Yu, W.; Li, S.; Guan, X.; Zhang, N.; Xie, X.; Zhang, K.; Bai, Y. Higher yield and enhanced therapeutic effects of exosomes derived from MSCs in hydrogel-assisted 3D culture system for bone regeneration. Biomater. Adv. 2022, 133, 112646. [Google Scholar] [CrossRef]
- Cao, J.; Wang, B.; Tang, T.; Lv, L.; Ding, Z.; Li, Z.; Hu, R.; Wei, Q.; Shen, A.; Fu, Y.; et al. Three-dimensional culture of MSCs produces exosomes with improved yield and enhanced therapeutic efficacy for cisplatin-induced acute kidney injury. Stem Cell Res. Ther. 2020, 11, 206. [Google Scholar] [CrossRef]
- Doyle, L.; Wang, M. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef]
- Anand, S.; Samuel, M.; Mathivanan, S. Exomeres: A New Member of Extracellular Vesicles Family. New Front. Extracell. Vesicles 2021, 97, 89–97. [Google Scholar] [CrossRef]
- Gurung, S.; Perocheau, D.; Touramanidou, L.; Baruteau, J. The exosome journey: From biogenesis to uptake and intracellular signalling. Cell Commun. Signal. 2021, 19, 47. [Google Scholar] [CrossRef] [PubMed]
- De Palma, G.; Sallustio, F.; Schena, F.P. Clinical Application of Human Urinary Extracellular Vesicles in Kidney and Urologic Diseases. Int. J. Mol. Sci. 2016, 17, 1043. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, D.K.; Fenix, A.M.; Franklin, J.L.; Higginbotham, J.N.; Zhang, Q.; Zimmerman, L.J.; Liebler, D.C.; Ping, J.; Liu, Q.; Evans, R.; et al. Reassessment of Exosome Composition. Cell 2019, 177, 428–445.e18. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Hao, T.; Tian, J. Identification of exosomes and its signature miRNAs of male and female Cynoglossus semilaevis. Sci. Rep. 2017, 7, 860. [Google Scholar] [CrossRef]
- Paulaitis, M.; Agarwal, K.; Nana-Sinkam, P. Dynamic Scaling of Exosome Sizes. Langmuir 2018, 34, 9387–9393. [Google Scholar] [CrossRef]
- Schmidt, O.; Teis, D. The ESCRT machinery. Curr. Biol. 2012, 22, R116–R120. [Google Scholar] [CrossRef]
- Alcayaga-Miranda, F.; Varas-Godoy, M.; Khoury, M. Harnessing the Angiogenic Potential of Stem Cell-Derived Exosomes for Vascular Regeneration. Stem Cells Int. 2016, 2016, 3409169. [Google Scholar] [CrossRef]
- Zaborowski, M.P.; Balaj, L.; Breakefield, X.O.; Lai, C.P. Extracellular Vesicles: Composition, Biological Relevance, and Methods of Study. Bioscience 2015, 65, 783–797. [Google Scholar] [CrossRef]
- Zügel, U.; Kaufmann, S.H.E. Role of heat shock proteins in protection from and pathogenesis of infectious diseases. Clin. Microbiol. Rev. 1999, 12, 19–39. [Google Scholar] [CrossRef]
- Phuyal, S.; Hessvik, N.P.; Skotland, T.; Sandvig, K.; Llorente, A. Regulation of exosome release by glycosphingolipids and flotillins. FEBS J. 2014, 281, 2214–2227. [Google Scholar] [CrossRef]
- Joo, H.S.; Suh, J.H.; Lee, H.J.; Bang, E.S.; Lee, J.M. Current knowledge and future perspectives on mesenchymal stem cell-derived exosomes as a new therapeutic agent. Int. J. Mol. Sci. 2020, 21, 727. [Google Scholar] [CrossRef] [PubMed]
- Raposo, G.; Nijman, H.W.; Stoorvogel, W.; Liejendekker, R.; Harding, C.V.; Melief, C.J.; Geuze, H.J. B lymphocytes secrete antigen-presenting vesicles. J. Exp. Med. 1996, 183, 1161–1172. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tian, L.; Lu, J.; Ng, I.O.-L. Exosomes and cancer—Diagnostic and prognostic biomarkers and therapeutic vehicle. Oncogenesis 2022, 11, 54. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Kaslan, M.; Lee, S.H.; Yao, J.; Gao, Z. Progress in Exosome Isolation Techniques. Theranostics 2017, 7, 789–804. [Google Scholar] [CrossRef] [PubMed]
- Skotland, T.; Hessvik, N.P.; Sandvig, K.; Llorente, A. Exosomal lipid composition and the role of ether lipids and phosphoinositides in exosome biology. J. Lipid Res. 2019, 60, 9–18. [Google Scholar] [CrossRef]
- Li, I.; Nabet, B.Y. Exosomes in the tumor microenvironment as mediators of cancer therapy resistance. Mol. Cancer 2019, 18, 32. [Google Scholar] [CrossRef]
- Lin, J.; Li, J.; Huang, B.; Liu, J.; Chen, X.; Chen, X.-M.; Xu, Y.-M.; Huang, L.-F.; Wang, X.-Z. Exosomes: Novel Biomarkers for Clinical Diagnosis. Sci. World J. 2015, 2015, 657086. [Google Scholar] [CrossRef]
- Mathew, M.; Zade, M.; Mezghani, N.; Patel, R.; Wang, Y.; Momen-Heravi, F. Extracellular Vesicles as Biomarkers in Cancer Immunotherapy. Cancers 2020, 12, 2825. [Google Scholar] [CrossRef]
- Li, X.; Corbett, A.L.; Taatizadeh, E.; Tasnim, N.; Little, J.P.; Garnis, C.; Daugaard, M.; Guns, E.; Hoorfar, M.; Li, I.T.S. Challenges and opportunities in exosome research—Perspectives from biology, engineering, and cancer therapy. APL Bioeng. 2019, 3, 011503. [Google Scholar] [CrossRef]
- Tanaka, Y.; Kamohara, H.; Kinoshita, K.; Kurashige, J.; Ishimoto, T.; Iwatsuki, M.; Watanabe, M.; Baba, H. Clinical impact of serum exosomal microRNA-21 as a clinical biomarker in human esophageal squamous cell carcinoma. Cancer 2012, 119, 1159–1167. [Google Scholar] [CrossRef]
- Munagala, R.; Aqil, F.; Gupta, R.C. Exosomal miRNAs as biomarkers of recurrent lung cancer. Tumor Biol. 2016, 37, 10703–10714. [Google Scholar] [CrossRef] [PubMed]
- Machida, T.; Tomofuji, T.; Maruyama, T.; Yoneda, T.; Ekuni, D.; Azuma, T.; Miyai, H.; Mizuno, H.; Kato, H.; Tsutsumi, K.; et al. miR-1246 and miR-4644 in salivary exosome as potential biomarkers for pancreatobiliary tract cancer. Oncol. Rep. 2016, 36, 2375–2381. [Google Scholar] [CrossRef] [PubMed]
- Yuwen, D.-L.; Sheng, B.-B.; Liu, J.; Wenyu, W.; Shu, Y.-Q. MiR-146a-5p level in serum exosomes predicts therapeutic effect of cisplatin in non-small cell lung cancer. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 2650–2658. [Google Scholar] [PubMed]
- Wang, Y.; Niu, X.; Cheng, Y.; Zhang, Y.; Xia, L.; Xia, W.; Lu, S. Exosomal PD-L1 predicts response with immunotherapy in NSCLC patients. Clin. Exp. Immunol. 2022, 208, 316–322. [Google Scholar] [CrossRef]
- Soung, Y.H.; Ford, S.; Zhang, V.; Chung, J. Exosomes in Cancer Diagnostics. Cancers 2017, 9, 8. [Google Scholar] [CrossRef]
- Keller, S.; Ridinger, J.; Rupp, A.-K.; Janssen, J.W.G.; Altevogt, P. Body fluid derived exosomes as a novel template for clinical diagnostics. J. Transl. Med. 2011, 9, 86. [Google Scholar] [CrossRef]
- Chen, G.; Huang, A.C.; Zhang, W.; Zhang, G.; Wu, M.; Xu, W.; Yu, Z.; Yang, J.; Wang, B.; Sun, H.; et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature 2018, 560, 382–386. [Google Scholar] [CrossRef]
- Hoshino, A.; Costa-Silva, B.; Shen, T.-L.; Rodrigues, G.; Hashimoto, A.; Mark, M.T.; Molina, H.; Kohsaka, S.; Di Giannatale, A.; Ceder, S.; et al. Tumour exosome integrins determine organotropic metastasis. Nature 2015, 527, 329–335. [Google Scholar] [CrossRef]
- Hoshino, A.; Kim, H.S.; Bojmar, L.; Gyan, K.E.; Cioffi, M.; Hernandez, J.; Zambirinis, C.P.; Rodrigues, G.; Molina, H.; Heissel, S.; et al. Extracellular vesicle and particle biomarkers define multiple human cancers. Cell 2020, 182, 1044–1061.e18. [Google Scholar] [CrossRef]
- D’Anca, M.; Fenoglio, C.; Serpente, M.; Arosio, B.; Cesari, M.; Scarpini, E.A.; Galimberti, D. Exosome Determinants of Physiological Aging and Age-Related Neurodegenerative Diseases. Front. Aging Neurosci. 2019, 11, 232. [Google Scholar] [CrossRef]
- Dardet, J.P.; Serrano, N.; András, I.E.; Toborek, M. Overcoming Blood-Brain Barrier Resistance: Implications for Extracellular Vesicle-Mediated Drug Brain Delivery. Front. Drug Deliv. 2022, 2, 5. [Google Scholar] [CrossRef]
- Ebrahimkhani, S.; Vafaee, F.; Young, P.E.; Hur, S.S.J.; Hawke, S.; Devenney, E.; Beadnall, H.; Barnett, M.H.; Suter, C.M.; Buckland, M.E. Exosomal microRNA signatures in multiple sclerosis reflect disease status. Sci. Rep. 2017, 7, 14293. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Contreras, M.; Shah, S.H.; Tamayo, A.; Robbins, P.D.; Golberg, R.B.; Mendez, A.J.; Ricordi, C. Plasma-derived exosome characterization reveals a distinct microRNA signature in long duration Type 1 diabetes. Sci. Rep. 2017, 7, 5998. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Tao, Q.; Wu, X.; Zhang, L.; Liu, Q.; Wang, L. The Utility of Exosomes in Diagnosis and Therapy of Diabetes Mellitus and Associated Complications. Front. Endocrinol. 2021, 12, 1360. [Google Scholar] [CrossRef]
- Liu, J.; Sun, X.; Zhang, F.-L.; Jin, H.; Yan, X.-L.; Huang, S.; Guo, Z.-N.; Yang, Y. Clinical Potential of Extracellular Vesicles in Type 2 Diabetes. Front. Endocrinol. 2021, 11, 1058. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Dong, T.; Chen, T.; Sun, J.; Luo, J.; He, J.; Wei, L.; Zeng, B.; Zhang, H.; Li, W.; et al. Hepatic exosome-derived miR-130a-3p attenuates glucose intolerance via suppressing PHLPP2 gene in adipocyte. Metabolism 2019, 103, 154006. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Du, X.; Xu, J.; Zhang, Y.; Tian, Y.; Liu, G.; Wang, X.; Ma, M.; Du, W.; Liu, Y.; et al. Pancreatic β cell microRNA-26a alleviates type 2 diabetes by improving peripheral insulin sensitivity and preserving β cell function. PLoS Biol. 2020, 18, e3000603. [Google Scholar] [CrossRef]
- Liu, Q.; Piao, H.; Wang, Y.; Zheng, D.; Wang, W. Circulating exosomes in cardiovascular disease: Novel carriers of biological information. Biomed. Pharmacother. 2021, 135, 111148. [Google Scholar] [CrossRef]
- Chong, S.Y.; Lee, C.K.; Huang, C.; Ou, Y.H.; Charles, C.J.; Richards, A.M.; Neupane, Y.R.; Pavon, M.V.; Zharkova, O.; Pastorin, G.; et al. Extracellular Vesicles in Cardiovascular Diseases: Alternative Biomarker Sources, Therapeutic Agents, and Drug Delivery Carriers. Int. J. Mol. Sci. 2019, 20, 3272. [Google Scholar] [CrossRef]
- Zampetaki, A.; Willeit, P.; Tilling, L.; Drozdov, I.; Prokopi, M.; Renard, J.-M.; Mayr, A.; Weger, S.; Schett, G.; Shah, A.; et al. Prospective study on circulating microRNAs and risk of myocardial infarction. J. Am. Coll. Cardiol. 2012, 60, 290–299. [Google Scholar] [CrossRef]
- Zhang, X.; Borg, E.G.F.; Liaci, A.M.; Vos, H.R.; Stoorvogel, W. A novel three step protocol to isolate extracellular vesicles from plasma or cell culture medium with both high yield and purity. J. Extracell. Vesicles 2020, 9, 1791450. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, M.Z.; Ratajczak, J. Extracellular microvesicles/exosomes: Discovery, disbelief, acceptance, and the future? Leukemia 2020, 34, 3126–3135. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Ao, Q.; Wang, X.; Cao, Y.; Liu, Y.; Zheng, S.G.; Tian, X. Mesenchymal Stem Cell–Derived Exosomes: A Promising Biological Tool in Nanomedicine. Front. Pharmacol. 2021, 11, 1954. [Google Scholar] [CrossRef] [PubMed]
- Muthu, S.; Bapat, A.; Jain, R.; Jeyaraman, N.; Jeyaraman, M. Exosomal therapy—A new frontier in regenerative medicine. Stem Cell Investig. 2021, 8, 7. [Google Scholar] [CrossRef]
- Fuloria, S.; Subramaniyan, V.; Dahiya, R.; Dahiya, S.; Sudhakar, K.; Kumari, U.; Sathasivam, K.; Meenakshi, D.; Wu, Y.; Sekar, M.; et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles: Regenerative Potential and Challenges. Biology 2021, 10, 172. [Google Scholar] [CrossRef]
- Musiał-Wysocka, A.; Kot, M.; Majka, M. The Pros and Cons of Mesenchymal Stem Cell-Based Therapies. Cell Transplant. 2019, 28, 801–812. [Google Scholar] [CrossRef]
- Yin, K.; Wang, S.; Zhao, R.C. Exosomes from mesenchymal stem/stromal cells: A new therapeutic paradigm. Biomark. Res. 2019, 7, 8. [Google Scholar] [CrossRef]
- Wang, K.; Jiang, Z.; Webster, K.A.; Chen, J.; Hu, H.; Zhou, Y.; Zhao, J.; Wang, L.; Wang, Y.; Zhong, Z.; et al. Enhanced Cardioprotection by Human Endometrium Mesenchymal Stem Cells Driven by Exosomal MicroRNA-21. Stem Cells Transl. Med. 2016, 6, 209–222. [Google Scholar] [CrossRef]
- Ma, Y.; Ge, S.; Zhang, J.; Zhou, D.; Li, L.; Wang, X.; Su, J. Mesenchymal stem cell-derived extracellular vesicles promote nerve regeneration after sciatic nerve crush injury in rats. Int. J. Clin. Exp. Pathol. 2017, 10, 10032–10039. [Google Scholar]
- Rezaie, J.; Feghhi, M.; Etemadi, T. A review on exosomes application in clinical trials: Perspective, questions, and challenges. Cell Commun. Signal. 2022, 20, 1–13. [Google Scholar] [CrossRef]
- Lai, J.J.; Chau, Z.L.; Chen, S.; Hill, J.J.; Korpany, K.V.; Liang, N.; Lin, L.; Lin, Y.; Liu, J.K.; Liu, Y.; et al. Exosome Processing and Characterization Approaches for Research and Technology Development. Adv. Sci. 2022, 9, 2103222. [Google Scholar] [CrossRef] [PubMed]
- N’Diaye, E.-R.; Orefice, N.S.; Ghezzi, C.; Boumendjel, A. Chemically Modified Extracellular Vesicles and Applications in Radiolabeling and Drug Delivery. Pharmaceutics 2022, 14, 653. [Google Scholar] [CrossRef] [PubMed]
- Dryden, G.W. Study Investigating the Ability of Plant Exosomes to Deliver Curcumin to Normal and Colon Cancer Tissue—Full Text View—ClinicalTrials.gov Identifier NCT01294072. Available online: https://clinicaltrials.gov/ct2/show/NCT01294072 (accessed on 6 January 2023).
- Kim, M.S.; Haney, M.J.; Zhao, Y.; Mahajan, V.; Deygen, I.; Klyachko, N.L.; Inskoe, E.; Piroyan, A.; Sokolsky, M.; Okolie, O.; et al. Development of exosome-encapsulated paclitaxel to overcome mdr in cancer cells. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Martins-Marques, T.; Pinho, M.J.; Zuzarte, M.; Oliveira, C.; Pereira, P.; Sluijter, J.P.G.; Gomes, C.; Girao, H. Presence of Cx43 in extracellular vesicles reduces the cardiotoxicity of the anti-tumour therapeutic approach with doxorubicin. J. Extracell. Vesicles 2016, 5, 32538. [Google Scholar] [CrossRef]
- Kim, M.S.; Haney, M.J.; Zhao, Y.; Yuan, D.; Deygen, I.; Klyachko, N.L.; Kabanov, A.V.; Batrakova, E.V. Engineering macrophage-derived exosomes for targeted paclitaxel delivery to pulmonary metastases: In vitro and in vivo evaluations. Nanomedicine 2018, 14, 195–204. [Google Scholar] [CrossRef]
- Kalimuthu, S.; Gangadaran, P.; Rajendran, R.L.; Zhu, L.; Oh, J.M.; Lee, H.W.; Gopal, A.; Baek, S.H.; Jeong, S.Y.; Lee, S.-W.; et al. A New Approach for Loading Anticancer Drugs Into Mesenchymal Stem Cell-Derived Exosome Mimetics for Cancer Therapy. Front. Pharmacol. 2018, 9, 1116. [Google Scholar] [CrossRef]
- Li, Y.-J.; Wu, J.-Y.; Wang, J.-M.; Hu, X.-B.; Cai, J.-X.; Xiang, D.-X. Gemcitabine loaded autologous exosomes for effective and safe chemotherapy of pancreatic cancer. Acta Biomater. 2019, 101, 519–530. [Google Scholar] [CrossRef]
- Qu, M.; Lin, Q.; Huang, L.; Fu, Y.; Wang, L.; He, S.; Fu, Y.; Yang, S.; Zhang, Z.; Zhang, L.; et al. Dopamine-loaded blood exosomes targeted to brain for better treatment of Parkinson’s disease. J. Control. Release 2018, 287, 156–166. [Google Scholar] [CrossRef]
- De Jong, O.G.; Kooijmans, S.A.A.; Murphy, D.E.; Jiang, L.; Evers, M.J.W.; Sluijter, J.P.G.; Vader, P.; Schiffelers, R.M. Drug Delivery with Extracellular Vesicles: From Imagination to Innovation. Acc. Chem. Res. 2019, 52, 1761–1770. [Google Scholar] [CrossRef]
- Sidhom, K.; Obi, P.; Saleem, A. A Review of Exosomal Isolation Methods: Is Size Exclusion Chromatography the Best Option? Int. J. Mol. Sci. 2020, 21, 6466. [Google Scholar] [CrossRef]
- Villa, F.; Quarto, R.; Tasso, R. Extracellular Vesicles as Natural, Safe and Efficient Drug Delivery Systems. Pharmaceutics 2019, 11, 557. [Google Scholar] [CrossRef] [PubMed]
- Meng, W.; He, C.; Hao, Y.; Wang, L.; Li, L.; Zhu, G. Prospects and challenges of extracellular vesicle-based drug delivery system: Considering cell source. Drug Deliv. 2020, 27, 585–598. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Guo, J.; Zhang, Z.; Duan, M.; Wang, G.; Qian, Y.; Zhao, H.; Yang, Z.; Jiang, X. Application of engineered extracellular vesicles for targeted tumor therapy. J. Biomed. Sci. 2022, 29, 14. [Google Scholar] [CrossRef] [PubMed]
- Luan, X.; Sansanaphongpricha, K.; Myers, I.; Chen, H.; Yuan, H.; Sun, D. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol. Sin. 2017, 38, 754–763. [Google Scholar] [CrossRef]
- Pascucci, L.; Coccè, V.; Bonomi, A.; Ami, D.; Ceccarelli, P.; Ciusani, E.; Viganò, L.; Locatelli, A.; Sisto, F.; Doglia, S.M.; et al. Paclitaxel is incorporated by mesenchymal stromal cells and released in exosomes that inhibit in vitro tumor growth: A new approach for drug delivery. J. Control. Release 2014, 192, 262–270. [Google Scholar] [CrossRef]
- Vader, P.; Mol, E.A.; Pasterkamp, G.; Schiffelers, R.M. Extracellular vesicles for drug delivery. Adv. Drug Deliv. Rev. 2016, 106, 148–156. [Google Scholar] [CrossRef]
- Haney, M.J.; Klyachko, N.L.; Zhao, Y.; Gupta, R.; Plotnikova, E.G.; He, Z.; Patel, T.; Piroyan, A.; Sokolsky, M.; Kabanov, A.V.; et al. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J. Control. Release 2015, 207, 18–30. [Google Scholar] [CrossRef]
- Fuhrmann, G.; Chandrawati, R.; Parmar, P.A.; Keane, T.J.; Maynard, S.A.; Bertazzo, S.; Stevens, M.M. Engineering Extracellular Vesicles with the Tools of Enzyme Prodrug Therapy. Adv. Mater. 2018, 30, e1706616. [Google Scholar] [CrossRef]
- Yang, Z.; Shi, J.; Xie, J.; Wang, Y.; Sun, J.; Liu, T.; Zhao, Y.; Zhao, X.; Wang, X.; Ma, Y.; et al. Large-scale generation of functional mRNA-encapsulating exosomes via cellular nanoporation. Nat. Biomed. Eng. 2020, 4, 69–83. [Google Scholar] [CrossRef]
- Hahm, J.; Kim, J.; Park, J. Strategies to Enhance Extracellular Vesicle Production. Tissue Eng. Regen. Med. 2021, 18, 513–524. [Google Scholar] [CrossRef]
- Syromiatnikova, V.; Prokopeva, A.; Gomzikova, M. Methods of the Large-Scale Production of Extracellular Vesicles. Int. J. Mol. Sci. 2022, 23, 10522. [Google Scholar] [CrossRef] [PubMed]
- Du, C.R.; Wang, L. Zhu, Y. Yang. Extracellular Vesicles as Delivery Vehicles for Therapeutic Nucleic Acids in Cancer Gene Therapy: Progress and Challenges. Pharmaceutics 2022, 14, 2236. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Yang, Y.; Zeng, Y.; He, M. A microfluidic ExoSearch chip for multiplexed exosome detection towards blood-based ovarian cancer diagnosis. Lab. Chip 2016, 16, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Lei, Y.; Wang, J.; Zhu, L.; Wu, Y.; Zhang, H.; Wu, L.; Zhang, P.; Yang, C. Microfluidic-Based Exosome Analysis for Liquid Biopsy. Small Methods 2021, 5, 2001131. [Google Scholar] [CrossRef]
- Contreras-Naranjo, J.C.; Wu, H.-J.; Ugaz, V.M. Microfluidics for exosome isolation and analysis: Enabling liquid biopsy for personalized medicine. Lab Chip 2017, 17, 3558–3577. [Google Scholar] [CrossRef]
- He, M.; Crow, J.; Roth, M.; Zeng, Y.; Godwin, A. From chip-in-a-lab to lab-on-a-chip: Towards a single handheld electronic system for multiple application-specific lab-on-a-chip (ASLOC). Lab Chip 2014, 19, 3773. [Google Scholar] [CrossRef]
- Kanwar, S.S.; Dunlay, C.J.; Simeone, D.M.; Nagrath, S. Microfluidic device (ExoChip) for on-chip isolation, quantification and characterization of circulating exosomes. Lab Chip 2014, 14, 1891–1900. [Google Scholar] [CrossRef]
- Lim, J.; Kang, B.; Son, H.Y.; Mun, B.; Huh, Y.-M.; Rho, H.W.; Kang, T.; Moon, J.; Lee, J.-J.; Seo, S.B.; et al. Microfluidic device for one-step detection of breast cancer-derived exosomal mRNA in blood using signal-amplifiable 3D nanostructure. Biosens. Bioelectron. 2022, 197, 113753. [Google Scholar] [CrossRef]
- Ko, J.; Bhagwat, N.; Yee, S.S.; Ortiz, N.; Sahmoud, A.; Black, T.; Aiello, N.M.; McKenzie, L.; O’Hara, M.; Redlinger, C.; et al. Combining Machine Learning and Nanofluidic Technology To Diagnose Pancreatic Cancer Using Exosomes. ACS Nano 2017, 11, 11182–11193. [Google Scholar] [CrossRef]
- Ibsen, S.D.; Wright, J.; Lewis, J.M.; Kim, S.; Ko, S.-Y.; Ong, J.; Manouchehri, S.; Vyas, A.; Akers, J.; Chen, C.C.; et al. Rapid Isolation and Detection of Exosomes and Associated Biomarkers from Plasma. ACS Nano 2017, 11, 6641–6651. [Google Scholar] [CrossRef]
- Tian, F.; Liu, C.; Deng, J.; Sun, J. Microfluidic Separation, Detection, and Engineering of Extracellular Vesicles for Cancer Diagnostics and Drug Delivery. Acc. Mater. Res. 2022, 3, 498–510. [Google Scholar] [CrossRef]
- Białkowska, K.; Komorowski, P.; Bryszewska, M.; Miłowska, K. Spheroids as a Type of Three-Dimensional Cell Cultures—Examples of Methods of Preparation and the Most Important Application. Int. J. Mol. Sci. 2020, 21, 6225. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Abdalla, A.M.; Xiao, L.; Yang, G. Biopolymer-Based Microcarriers for Three-Dimensional Cell Culture and Engineered Tissue Formation. Int. J. Mol. Sci. 2020, 21, 1895. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wang, X.; Wang, Y.; Gou, W.; Yuan, X.; Peng, J.; Guo, Q.; Lu, S. Past, present, and future of microcarrier-based tissue engineering. J. Orthop. Transl. 2015, 3, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Zhang, Q.; Jiang, L. Current Knowledge on Exosome Biogenesis, Cargo-Sorting Mechanism and Therapeutic Implications. Membranes 2022, 12, 498. [Google Scholar] [CrossRef]
- Valdoz, J.C.; Johnson, B.C.; Jacobs, D.J.; Franks, N.A.; Dodson, E.L.; Sanders, C.; Cribbs, C.G.; Van Ry, P.M. The ECM: To Scaffold, or Not to Scaffold, That Is the Question. Int. J. Mol. Sci. 2021, 22, 12690. [Google Scholar] [CrossRef]
- Carletti, E.; Motta, A.; Migliaresi, C. Scaffolds for tissue engineering and 3D cell culture. Methods Mol. Biol. 2010, 695, 17–39. [Google Scholar]
- Habanjar, O.; Diab-Assaf, M.; Caldefie-Chezet, F.; Delort, L. 3D Cell Culture Systems: Tumor Application, Advantages, and Disadvantages. Int. J. Mol. Sci. 2021, 22, 12200. [Google Scholar] [CrossRef]
- Minchinton, A.I.; Tannock, I.F. Drug penetration in solid tumours. Nat. Rev. Cancer 2006, 6, 583–592. [Google Scholar] [CrossRef]
- Nederman, T.; Norling, B.; Glimelius, B.; Carlsson, J.; Brunk, U. Demonstration of an extracellular matrix in multicellular tumor spheroids. Cancer Res. 1984, 44, 3090–3097. [Google Scholar]
- Jorgenson, A.; Choi, K.; Sicard, D.; Smith, K.; Hiemer, S.; Varelas, X.; Tschumperlin, D. TAZ activation drives fibroblast spheroid growth, expression of profibrotic paracrine signals, and context-dependent ECM gene expression. Am. J. Physiol. Cell Physiol. 2017, 312, C277–C285. [Google Scholar] [CrossRef] [PubMed]
- Salmenperä, P.; Kankuri, E.; Bizik, J.; Sirén, V.; Virtanen, I.; Takahashi, S.; Leiss, M.; Fässler, R.; Vaheri, A. Formation and activation of fibroblast spheroids depend on fibronectin–integrin interaction. Exp. Cell Res. 2008, 314, 3444–3452. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-W.; Tzeng, S.-C.; Chen, J.-K.; Sun, J.-S.; Lin, F.-H. A Dynamic Hanging-Drop System for Mesenchymal Stem Cell Culture. Int. J. Mol. Sci. 2020, 21, 4298. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Chen, S.; Naing, M.W. A review of manufacturing capabilities of cell spheroid generation technologies and future development. Biotechnol. Bioeng. 2020, 118, 542–554. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lee, J.W. 3D Spheroid Cultures of Stem Cells and Exosome Applications for Cartilage Repair. Life 2022, 12, 939. [Google Scholar] [CrossRef]
- Lewis, N.S.; EL Lewis, E.; Mullin, M.; Wheadon, H.; Dalby, M.J.; Berry, C.C. Magnetically levitated mesenchymal stem cell spheroids cultured with a collagen gel maintain phenotype and quiescence. J. Tissue Eng. 2017, 8, 2041731417704428. [Google Scholar] [CrossRef]
- Faruqu, F.N.; Liam-Or, R.; Zhou, S.; Nip, R.; Al-Jamal, K.T. Defined serum-free three-dimensional culture of umbilical cord-derived mesenchymal stem cells yields exosomes that promote fibroblast proliferation and migration in vitro. FASEB J. 2020, 35, e21206. [Google Scholar] [CrossRef]
- Kim, M.; Yun, H.-W.; Park, D.Y.; Choi, B.H.; Min, B.-H. Three-Dimensional Spheroid Culture Increases Exosome Secretion from Mesenchymal Stem Cells. Tissue Eng. Regen. Med. 2018, 15, 427–436. [Google Scholar] [CrossRef]
- Xie, L.; Mao, M.; Zhou, L.; Zhang, L.; Jiang, B. Signal Factors Secreted by 2D and Spheroid Mesenchymal Stem Cells and by Cocultures of Mesenchymal Stem Cells Derived Microvesicles and Retinal Photoreceptor Neurons. Stem Cells Int. 2017, 2017, 2730472. [Google Scholar] [CrossRef]
- Miceli, V.; Pampalone, M.; Vella, S.; Carreca, A.P.; Amico, G.; Conaldi, P.G. Comparison of Immunosuppressive and Angiogenic Properties of Human Amnion-Derived Mesenchymal Stem Cells between 2D and 3D Culture Systems. Stem Cells Int. 2019, 2019, 7486279. [Google Scholar] [CrossRef]
- Hu, S.; Li, Z.; Lutz, H.; Huang, K.; Su, T.; Cores, J.; Dinh, P.-U.C.; Cheng, K. Dermal exosomes containing miR-218-5p promote hair regeneration by regulating β-catenin signaling. Sci. Adv. 2020, 6, eaba1685. [Google Scholar] [CrossRef]
- Hu, S.; Li, Z.; Cores, J.; Huang, K.; Su, T.; Dinh, P.-U.; Cheng, K. Needle-Free Injection of Exosomes Derived from Human Dermal Fibroblast Spheroids Ameliorates Skin Photoaging. ACS Nano 2019, 13, 11273–11282. [Google Scholar] [CrossRef] [PubMed]
- Tu, J.; Luo, X.; Liu, H.; Zhang, J.; He, M. Cancer spheroids derived exosomes reveal more molecular features relevant to progressed cancer. Biochem. Biophys. Rep. 2021, 26, 101026. [Google Scholar] [CrossRef] [PubMed]
- Naseri, M.; Zöller, M.; Hadjati, J.; Ghods, R.; Pirmardan, E.R.; Kiani, J.; Eini, L.; Bozorgmehr, M.; Madjd, Z. Dendritic cells loaded with exosomes derived from cancer stem cell-enriched spheroids as a potential immunotherapeutic option. J. Cell. Mol. Med. 2021, 25, 3312–3326. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ma, L.; Hua, F.; Min, Z.; Zhan, Y.; Zhang, W.; Yao, J. Exosomal circCARM1 from spheroids reprograms cell metabolism by regulating PFKFB2 in breast cancer. Oncogene 2022, 41, 2012–2025. [Google Scholar] [CrossRef] [PubMed]
- Dinh, P.-U.C.; Paudel, D.; Brochu, H.; Popowski, K.D.; Gracieux, M.C.; Cores, J.; Huang, K.; Hensley, M.T.; Harrell, E.; Vandergriff, A.C.; et al. Inhalation of lung spheroid cell secretome and exosomes promotes lung repair in pulmonary fibrosis. Nat. Commun. 2020, 11, 1064. [Google Scholar] [CrossRef]
- Choi, S.-Y.; Kang, S.; Oh, S.; Lee, K.; Lee, H.-J.; Gum, S.; Kwon, T.-G.; Kim, J.-W.; Lee, S.-T.; Hong, Y.; et al. Differential Angiogenic Potential of 3-Dimension Spheroid of HNSCC Cells in Mouse Xenograft. Int. J. Mol. Sci. 2021, 22, 8245. [Google Scholar] [CrossRef]
- Verdera, H.C.; Gitz-Francois, J.J.; Schiffelers, R.M.; Vader, P. Cellular uptake of extracellular vesicles is mediated by clathrin-independent endocytosis and macropinocytosis. J. Control. Release 2017, 266, 100–108. [Google Scholar] [CrossRef]
- Abdollahi, S. Extracellular vesicles from organoids and 3D culture systems. Biotechnol. Bioeng. 2020, 118, 1029–1049. [Google Scholar] [CrossRef]
- Khayambashi, P.; Iyer, J.; Pillai, S.; Upadhyay, A.; Zhang, Y.; Tran, S. Hydrogel Encapsulation of Mesenchymal Stem Cells and Their Derived Exosomes for Tissue Engineering. Int. J. Mol. Sci. 2021, 22, 684. [Google Scholar] [CrossRef]
- Langhans, S.A. Three-Dimensional in Vitro Cell Culture Models in Drug Discovery and Drug Repositioning. Front. Pharmacol. 2018, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, M.P.; Chavali, M.S. Recent advances in biomaterials for 3D scaffolds: A review. Bioact. Mater. 2019, 4, 271–292. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.J.; Jeong, J.H.; Chan, V.; Cha, C.; Baek, K.; Lai, M.-H.; Bashir, R.; Kong, H. Tailoring the Dependency between Rigidity and Water Uptake of a Microfabricated Hydrogel with the Conformational Rigidity of a Polymer Cross-Linker. Biomacromolecules 2013, 14, 1361–1369. [Google Scholar] [CrossRef] [PubMed]
- Tibbitt, M.W.; Anseth, K.S. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol. Bioeng. 2009, 103, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Martinez-Garcia, F.D.; Fischer, T.; Hayn, A.; Mierke, C.T.; Burgess, J.K.; Harmsen, M.C. A Beginner’s Guide to the Characterization of Hydrogel Microarchitecture for Cellular Applications. Gels 2022, 8, 535. [Google Scholar] [CrossRef] [PubMed]
- Akther, F.; Little, P.; Li, Z.; Nguyen, N.-T.; Ta, H.T. Hydrogels as artificial matrices for cell seeding in microfluidic devices. RSC Adv. 2020, 10, 43682–43703. [Google Scholar] [CrossRef]
- Radulescu, D.-M.; Neacsu, I.A.; Grumezescu, A.-M.; Andronescu, E. New Insights of Scaffolds Based on Hydrogels in Tissue Engineering. Polymers 2022, 14, 799. [Google Scholar] [CrossRef]
- Lin, X.; Zhao, X.; Xu, C.; Wang, L.; Xia, Y. Progress in the mechanical enhancement of hydrogels: Fabrication strategies and underlying mechanisms. J. Polym. Sci. 2022, 60, 2525–2542. [Google Scholar] [CrossRef]
- Patwardhan, S.; Mahadik, P.; Shetty, O.; Sen, S. ECM stiffness-tuned exosomes drive breast cancer motility through thrombospondin-1. Biomaterials 2021, 279, 121185. [Google Scholar] [CrossRef]
- Han, M.; Yang, H.; Lu, X.; Li, Y.; Liu, Z.; Li, F.; Shang, Z.; Wang, X.; Li, X.; Li, J.; et al. Three-Dimensional-Cultured MSC-Derived Exosome-Hydrogel Hybrid Microneedle Array Patch for Spinal Cord Repair. Nano Lett. 2022, 22, 6391–6401. [Google Scholar] [CrossRef]
- Safari, B.; Aghazadeh, M.; Davaran, S.; Roshangar, L. Exosome-loaded hydrogels: A new cell-free therapeutic approach for skin regeneration. Eur. J. Pharm. Biopharm. 2021, 171, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.; Hu, Y.; Yang, P.; Xie, X.; Fang, B. Extracellular vesicle-loaded hydrogels for tissue repair and regeneration. Mater. Today Bio 2023, 18, 100522. [Google Scholar] [CrossRef]
- Chen, C.W.; Wang, L.L.; Zaman, S.; Gordon, J.; Arisi, M.F.; Venkataraman, C.M.; Chung, J.J.; Hung, G.; Gaffey, A.C.; Spruce, L.A.; et al. Sustained release of endothelial progenitor cell-derived extracellular vesicles from shear-thinning hydrogels improves angiogenesis and promotes function after myocardial infarction. Cardiovasc. Res. 2018, 114, 1029–1040. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Hu, S.; Yang, H.; Li, Z.; Huang, K.; Su, T.; Wang, S.; Cheng, K. Hyaluronic Acid Hydrogel Integrated with Mesenchymal Stem Cell-Secretome to Treat Endometrial Injury in a Rat Model of Asherman’s Syndrome. Adv. Health Mater. 2019, 8, e1900411. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.-C.; Tao, S.-C.; Yin, W.-J.; Qi, X.; Yuan, T.; Zhang, C.-Q. Exosomes derived from platelet-rich plasma promote the re-epithelization of chronic cutaneous wounds via activation of YAP in a diabetic rat model. Theranostics 2017, 7, 81–96. [Google Scholar] [CrossRef] [PubMed]
- Shafei, S.; Khanmohammadi, M.; Heidari, R.; Ghanbari, H.; Taghdiri Nooshabadi, V.; Farzamfar, S.; Akbariqomi, M.; Sanikhani, N.S.; Absalan, M.; Tavoosidana, G. Exosome loaded alginate hydrogel promotes tissue regeneration in full-thickness skin wounds: An in vivo study. J. Biomed. Mater. Res. Part A 2020, 108, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Zhou, J.; Liu, B.; Liang, C.; Pan, X.; Zhang, Y.; Zhang, Y.; Wang, Y.; Shao, L.; Zhu, B.; et al. Delivery of miR-675 by stem cell-derived exosomes encapsulated in silk fibroin hydrogel prevents aging-induced vascular dysfunction in mouse hindlimb. Mater. Sci. Eng. C 2019, 99, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Qian, Z.; Liu, D.; Sun, J.; Wang, X.; Liu, H.; Xu, J.; Guo, X. GMSC-Derived Exosomes Combined with a Chitosan/Silk Hydrogel Sponge Accelerates Wound Healing in a Diabetic Rat Skin Defect Model. Front. Physiol. 2017, 8, 904. [Google Scholar] [CrossRef]
- Wang, C.; Liang, C.; Wang, R.; Yao, X.; Guo, P.; Yuan, W.; Liu, Y.; Song, Y.; Li, Z.; Xie, X. The fabrication of a highly efficient self-healing hydrogel from natural biopolymers loaded with exosomes for the synergistic promotion of severe wound healing. Biomater. Sci. 2020, 8, 313–324. [Google Scholar] [CrossRef]
- Li, M.; Ke, Q.-F.; Tao, S.-C.; Guo, S.-C.; Rui, B.-Y.; Guo, Y.-P. Fabrication of hydroxyapatite/chitosan composite hydrogels loaded with exosomes derived from miR-126-3p overexpressed synovial mesenchymal stem cells for diabetic chronic wound healing. J. Mater. Chem. B 2016, 4, 6830–6841. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhao, X.; Chen, X.; Wei, Y.; Du, W.; Wang, Y.; Liu, L.; Zhao, W.; Han, Z.; Kong, D.; et al. Enhanced Therapeutic Effects of Mesenchymal Stem Cell-Derived Exosomes with an Injectable Hydrogel for Hindlimb Ischemia Treatment. ACS Appl. Mater Interfaces 2018, 10, 30081–30091. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liu, Y.; Jia, P.; Cheng, H.; Wang, C.; Chen, S.; Huang, H.; Han, Z.; Han, Z.-C.; Marycz, K.; et al. Chitosan hydrogel-loaded MSC-derived extracellular vesicles promote skin rejuvenation by ameliorating the senescence of dermal fibroblasts. Stem Cell Res. Ther. 2021, 12, 196. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.-C.; Guo, S.-C.; Li, M.; Ke, Q.-F.; Guo, Y.-P.; Zhang, C.-Q. Chitosan Wound Dressings Incorporating Exosomes Derived from MicroRNA-126-Overexpressing Synovium Mesenchymal Stem Cells Provide Sustained Release of Exosomes and Heal Full-Thickness Skin Defects in a Diabetic Rat Model. STEM CELLS Transl. Med. 2016, 6, 736–747. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhu, B.; Yin, P.; Zhao, L.; Wang, Y.; Fu, Z.; Dang, R.; Xu, J.; Zhang, J.; Wen, N. Integration of Human Umbilical Cord Mesenchymal Stem Cells-Derived Exosomes with Hydroxyapatite-Embedded Hyaluronic Acid-Alginate Hydrogel for Bone Regeneration. ACS Biomater. Sci. Eng. 2020, 6, 1590–1602. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, M.; Xu, T.; Zhang, X.; Lin, C.; Gao, W.; Xu, H.; Lei, B.; Mao, C. Engineering Bioactive Self-Healing Antibacterial Exosomes Hydrogel for Promoting Chronic Diabetic Wound Healing and Complete Skin Regeneration. Theranostics 2019, 9, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Shang, Y.; Chen, X.; Midgley, A.C.; Wang, Z.; Zhu, D.; Wu, J.; Chen, P.; Wu, L.; Wang, X.; et al. Supramolecular Nanofibers Containing Arginine-Glycine-Aspartate (RGD) Peptides Boost Therapeutic Efficacy of Extracellular Vesicles in Kidney Repair. ACS Nano 2020, 14, 12133–12147. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, Y.; Mu, J.; Chen, J.; Zhang, C.; Cao, H.; Gao, J.; Gao, J. Transplantation of Human Mesenchymal Stem-Cell-Derived Exosomes Immobilized in an Adhesive Hydrogel for Effective Treatment of Spinal Cord Injury. Nano Lett. 2020, 20, 4298–4305. [Google Scholar] [CrossRef]
- Mardpour, S.; Ghanian, M.H.; Sadeghi-Abandansari, H.; Mardpour, S.; Nazari, A.; Shekari, F.; Baharvand, H. Hydrogel-Mediated Sustained Systemic Delivery of Mesenchymal Stem Cell-Derived Extracellular Vesicles Improves Hepatic Regeneration in Chronic Liver Failure. ACS Appl. Mater. Interfaces 2019, 11, 37421–37433. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, Z.; Gao, R.; Yuan, J.; Zhang, J.; Li, H.; Xie, Z.; Wang, Y. Controlled release of MSC-derived small extracellular vesicles by an injectable Diels-Alder crosslinked hyaluronic acid/PEG hydrogel for osteoarthritis improvement. Acta Biomater. 2021, 128, 163–174. [Google Scholar] [CrossRef]
- DiCerbo, M.; Benmassaoud, M.M.; Vega, S.L. Porous Scaffold-Hydrogel Composites Spatially Regulate 3D Cellular Mechanosensing. Front. Med. Technol. 2022, 4, 22. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Holzwarth, J.M.; Ma, P.X. Functionalized Synthetic Biodegradable Polymer Scaffolds for Tissue Engineering. Macromol. Biosci. 2012, 12, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Rider, P.; Kačarević, P.; Alkildani, S.; Retnasingh, S.; Barbeck, M. Bioprinting of tissue engineering scaffolds. J. Tissue Eng. 2018, 9, 2041731418802090. [Google Scholar] [CrossRef] [PubMed]
- Lv, D.; Hu, Z.; Lu, L.; Lu, H.; Xu, X. Three-dimensional cell culture: A powerful tool in tumor research and drug discovery (Review). Oncol. Lett. 2017, 14, 6999–7010. [Google Scholar] [CrossRef]
- Kadir, N.D.; Yang, Z.; Hassan, A.; Denslin, V.; Lee, E.H. Electrospun fibers enhanced the paracrine signaling of mesenchymal stem cells for cartilage regeneration. Stem Cell Res. Ther. 2021, 12, 3. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Chiarini, A.; Wu, J.; Freddi, G.; Nie, K.; Armato, U.; Dal Prà, I. Exosomes of adult human fibroblasts cultured on 3D silk fibroin nonwovens intensely stimulate neoangiogenesis. Burns Trauma 2021, 9, tkab003. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Liang, T.; He, R.; Ren, J.; Yao, H.; Wang, K.; Zhu, L.; Xu, Y. Exosomes from 3D culture of marrow stem cells enhances endothelial cell proliferation, migration, and angiogenesis via activation of the HMGB1/AKT pathway. Stem Cell Res. 2020, 50, 102122. [Google Scholar] [CrossRef] [PubMed]
- Man, K.; Brunet, M.Y.; Louth, S.; Robinson, T.E.; Fernandez-Rhodes, M.; Williams, S.; Federici, A.S.; Davies, O.G.; Hoey, D.A.; Cox, S.C. Development of a Bone-Mimetic 3D Printed Ti6Al4V Scaffold to Enhance Osteoblast-Derived Extracellular Vesicles’ Therapeutic Efficacy for Bone Regeneration. Front. Bioeng. Biotechnol. 2021, 9, 1003. [Google Scholar] [CrossRef]
- Liu, X.; Wang, J.; Wang, P.; Zhong, L.; Wang, S.; Feng, Q.; Wei, X.; Zhou, L. Hypoxia-pretreated mesenchymal stem cell-derived exosomes-loaded low-temperature extrusion 3D-printed implants for neural regeneration after traumatic brain injury in canines. Front. Bioeng. Biotechnol. 2022, 10, 1828. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, D.; Nie, Z.; Lu, L.; Lin, Z.; Zhou, D.; Zhang, Y.; Long, X.; Fan, S.; Xu, T. A scalable coaxial bioprinting technology for mesenchymal stem cell microfiber fabrication and high extracellular vesicle yield. Biofabrication 2022, 14, 015012. [Google Scholar] [CrossRef]
- Born, L.J.; McLoughlin, S.T.; Dutta, D.; Mahadik, B.; Jia, X.; Fisher, J.P.; Jay, S.M. Sustained released of bioactive mesenchymal stromal cell-derived extracellular vesicles from 3D-printed gelatin methacrylate hydrogels. J. Biomed. Mater. Res. Part A 2022, 110, 1190–1198. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Zheng, L.; Wang, Y.; Tao, M.; Xie, Z.; Xia, C.; Gu, C.; Chen, J.; Qiu, P.; Mei, S.; et al. Desktop-stereolithography 3D printing of a radially oriented extracellular matrix/mesenchymal stem cell exosome bioink for osteochondral defect regeneration. Theranostics 2019, 9, 2439–2459. [Google Scholar] [CrossRef] [PubMed]
- Di Bella, M.A.; Brucato, V.; Blanda, V.; Zummo, F.; Vitrano, I.; Di Liegro, C.M.; Ghersi, G.; Di Liegro, I.; Schiera, G. A 3D-scaffold of PLLA induces the morphological differentiation and migration of primary astrocytes and promotes the production of extracellular vesicles. Mol. Med. Rep. 2019, 20, 1288–1296. [Google Scholar] [CrossRef]
- Zhang, Y.; Chopp, M.; Zhang, Z.G.; Katakowski, M.; Xin, H.; Qu, C.; Ali, M.; Mahmood, A.; Xiong, Y. Systemic administration of cell-free exosomes generated by human bone marrow derived mesenchymal stem cells cultured under 2D and 3D conditions improves functional recovery in rats after traumatic brain injury. Neurochem. Int. 2017, 111, 69–81. [Google Scholar] [CrossRef]
- Zhang, L.; Fan, C.; Hao, W.; Zhuang, Y.; Liu, X.; Zhao, Y.; Chen, B.; Xiao, Z.; Chen, Y.; Dai, J. NSCs Migration Promoted and Drug Delivered Exosomes-Collagen Scaffold via a Bio-Specific Peptide for One-Step Spinal Cord Injury Repair. Adv. Health Mater. 2021, 10, e2001896. [Google Scholar] [CrossRef]
- Narayanan, R.; Huang, C.-C.; Ravindran, S. Hijacking the Cellular Mail: Exosome Mediated Differentiation of Mesenchymal Stem Cells. Stem Cells Int. 2016, 2016, 3808674. [Google Scholar] [CrossRef]
- Qi, X.; Zhang, J.; Yuan, H.; Xu, Z.; Li, Q.; Niu, X.; Hu, B.; Wang, Y.; Li, X. Exosomes Secreted by Human-Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells Repair Critical-Sized Bone Defects through Enhanced Angiogenesis and Osteogenesis in Osteoporotic Rats. Int. J. Biol. Sci. 2016, 12, 836–849. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Li, H.; Chen, C.; Bin Hu, B.; Niu, X.; Li, Q.; Zhao, B.; Xie, Z.; Wang, Y. Exosomes/tricalcium phosphate combination scaffolds can enhance bone regeneration by activating the PI3K/Akt signaling pathway. Stem Cell Res. Ther. 2016, 7, 136. [Google Scholar] [CrossRef]
- Ying, C.; Wang, R.; Wang, Z.; Tao, J.; Yin, W.; Zhang, J.; Yi, C.; Qi, X.; Han, D. BMSC-Exosomes Carry Mutant HIF-1α for Improving Angiogenesis and Osteogenesis in Critical-Sized Calvarial Defects. Front. Bioeng. Biotechnol. 2020, 8, 565561. [Google Scholar] [CrossRef]
- Sanchez, M.A.; Felice, B.; Sappia, L.D.; Moura, S.L.; Martí, M.; Pividori, M.I. Osteoblastic exosomes. A non-destructive quantitative approach of alkaline phosphatase to assess osteoconductive nanomaterials. Mater. Sci. Eng. C 2020, 115, 110931. [Google Scholar] [CrossRef]
- Wang, X.; Ao, J.; Lu, H.; Zhao, Q.; Ma, Y.; Zhang, J.; Ren, H.; Zhang, Y. Osteoimmune Modulation and Guided Osteogenesis Promoted by Barrier Membranes Incorporated with S-Nitrosoglutathione (GSNO) and Mesenchymal Stem Cell-Derived Exosomes. Int. J. Nanomed. 2020, 15, 3483–3496. [Google Scholar] [CrossRef] [PubMed]
- Zha, Y.; Li, Y.; Lin, T.; Chen, J.; Zhang, S.; Wang, J. Progenitor cell-derived exosomes endowed with VEGF plasmids enhance osteogenic induction and vascular remodeling in large segmental bone defects. Theranostics 2021, 11, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Gandolfi, M.G.; Gardin, C.; Zamparini, F.; Ferroni, L.; Degli Esposti, M.; Parchi, G.; Ercan, B.; Manzoli, L.; Fava, F.; Fabbri, P.; et al. Mineral-Doped Poly(L-lactide) Acid Scaffolds Enriched with Exosomes Improve Osteogenic Commitment of Human Adipose-Derived Mesenchymal Stem Cells. Nanomaterials 2020, 10, 432. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, Y.; Zhang, P.; Tang, Y.; Zhou, M.; Jiang, W.; Zhang, X.; Wu, G.; Zhou, Y. Tissue-Engineered Bone Immobilized with Human Adipose Stem Cells-Derived Exosomes Promotes Bone Regeneration. ACS Appl. Mater. Interfaces 2018, 10, 5240–5254. [Google Scholar] [CrossRef] [PubMed]
- Swanson, W.B.; Zhang, Z.; Xiu, K.; Gong, T.; Eberle, M.; Wang, Z.; Ma, P.X. Scaffolds with controlled release of pro-mineralization exosomes to promote craniofacial bone healing without cell transplantation. Acta Biomater. 2020, 118, 215–232. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Guan, P.; Xiao, C.; Wen, H.; Wang, Q.; Liu, C.; Luo, Y.; Ma, L.; Tan, G.; Yu, P.; et al. Exosome-functionalized polyetheretherketone-based implant with immunomodulatory property for enhancing osseointegration. Bioact. Mater. 2021, 6, 2754–2766. [Google Scholar] [CrossRef]
- Wu, Z.; Pu, P.; Su, Z.; Zhang, X.; Nie, L.; Chang, Y. Schwann Cell-derived exosomes promote bone regeneration and repair by enhancing the biological activity of porous Ti6Al4V scaffolds. Biochem. Biophys. Res. Commun. 2020, 531, 559–565. [Google Scholar] [CrossRef]
- Teotia, A.K.; Qayoom, I.; Singh, P.; Mishra, A.; Jaiman, D.; Seppälä, J.; Lidgren, L.; Kumar, A. Exosome-Functionalized Ceramic Bone Substitute Promotes Critical-Sized Bone Defect Repair in Rats. ACS Appl. Bio Mater. 2021, 4, 3716–3726. [Google Scholar] [CrossRef]
- Koh, B.; Sulaiman, N.; Fauzi, M.B.; Law, J.X.; Ng, M.H.; Idrus, R.B.H.; Yazid, M.D. Three dimensional microcarrier system in mesenchymal stem cell culture: A systematic review. Cell Biosci. 2020, 10, 75. [Google Scholar] [CrossRef]
- Patel, D.B.; Luthers, C.R.; Lerman, M.J.; Fisher, J.P.; Jay, S.M. Enhanced extracellular vesicle production and ethanol-mediated vascularization bioactivity via a 3D-printed scaffold-perfusion bioreactor system. Acta Biomater. 2018, 95, 236–244. [Google Scholar] [CrossRef]
- Fuzeta, M.D.A.; Bernardes, N.; Oliveira, F.D.; Costa, A.C.; Fernandes-Platzgummer, A.; Farinha, J.P.; Rodrigues, C.A.V.; Jung, S.; Tseng, R.-J.; Milligan, W.; et al. Scalable Production of Human Mesenchymal Stromal Cell-Derived Extracellular Vesicles Under Serum-/Xeno-Free Conditions in a Microcarrier-Based Bioreactor Culture System. Front. Cell Dev. Biol. 2020, 8, 553444. [Google Scholar] [CrossRef] [PubMed]
- Bellani, C.F.; Ajeian, J.; Duffy, L.; Miotto, M.; Groenewegen, L.; Connon, C.J. Scale-Up Technologies for the Manufacture of Adherent Cells. Front. Nutr. 2020, 7, 575146. [Google Scholar] [CrossRef] [PubMed]
- Bodiou, V.; Moutsatsou, P.; Post, M.J. Microcarriers for Upscaling Cultured Meat Production. Front. Nutr. 2020, 7, 10. [Google Scholar] [CrossRef]
- Nam, J.H.; Ermonval, M.; Sharfstein, S.T. Cell Attachment to Microcarriers Affects Growth, Metabolic Activity, and Culture Productivity in Bioreactor Culture. Biotechnol. Prog. 2008, 23, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Derakhti, S.; Safiabadi-Tali, S.H.; Amoabediny, G.; Sheikhpour, M. Attachment and detachment strategies in microcarrier-based cell culture technology: A comprehensive review. Mater. Sci. Eng. C 2019, 103, 109782. [Google Scholar] [CrossRef]
- Grinneli, F.; Feld, M.K. Spreading of human fibroblasts in serum-free medium: Inhibition by dithiothreitol and the effect of cold insoluble globulin (plasma fibronectin). J. Cell. Physiol. 1980, 104, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Healthcare, G.E.; Biosciences, A. Microcarrier Cell Culture: Principles and Methods; General Electric Company: Fairfield, CT, USA, 2005. [Google Scholar]
- Berthiaume, F.; Yarmush, M. Tissue Engineering, Encyclopedia of Physical Science and Technology. Tissue Eng. 2003, 3, 817–842. [Google Scholar] [CrossRef]
- Badenes, S.; Fernandes-Platzgummer, A.; Rodrigues, C.; Diogo, M.; da Silva, C.; Cabral, J. Microcarrier Culture Systems for Stem Cell Manufacturing. In Stem Cell Manufacturing; Elsevier: Amsterdam, the Netherlands, 2016; pp. 77–104. [Google Scholar] [CrossRef]
- Schmidt, J.J.; Jeong, J.; Kong, H. The Interplay Between Cell Adhesion Cues and Curvature of Cell Adherent Alginate Microgels in Multipotent Stem Cell Culture. Tissue Eng. Part A 2011, 17, 2687–2694. [Google Scholar] [CrossRef]
- Čebatariūnienė, A.; Kriaučiūnaitė, K.; Prunskaitė, J.; Tunaitis, V.; Pivoriūnas, A. Extracellular Vesicles Suppress Basal and Lipopolysaccharide-Induced NFκB Activity in Human Periodontal Ligament Stem Cells. Stem Cells Dev. 2019, 28, 1037–1049. [Google Scholar] [CrossRef]
- Xu, C.; Zhao, J.; Li, Q.; Hou, L.; Wang, Y.; Li, S.; Jiang, F.; Zhu, Z.; Tian, L. Exosomes derived from three-dimensional cultured human umbilical cord mesenchymal stem cells ameliorate pulmonary fibrosis in a mouse silicosis model. Stem Cell Res. Ther. 2020, 11, 503. [Google Scholar] [CrossRef]
- Haraszti, R.A.; Miller, R.; Stoppato, M.; Sere, Y.Y.; Coles, A.; Didiot, M.-C.; Wollacott, R.; Sapp, E.; Dubuke, M.L.; Li, X.; et al. Exosomes Produced from 3D Cultures of MSCs by Tangential Flow Filtration Show Higher Yield and Improved Activity. Mol. Ther. 2018, 26, 2838–2847. [Google Scholar] [CrossRef] [PubMed]
- Jalilian, E.; Massoumi, H.; Bigit, B.; Amin, S.; Katz, E.A.; Guaiquil, V.H.; Anwar, K.N.; Hematti, P.; Rosenblatt, M.I.; Djalilian, A.R. Bone marrow mesenchymal stromal cells in a 3D system produce higher concentration of extracellular vesicles (EVs) with increased complexity and enhanced neuronal growth properties. Stem Cell Res. Ther. 2022, 13, 425. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Li, X.; Xu, X.; Xu, L.; Wang, D.; Ouyang, K.; Liang, Y. Large-Scale Preparation of Synovial Fluid Mesenchymal Stem Cell-Derived Exosomes by 3D Bioreactor Culture. J. Vis. Exp. 2022, 185, e62221. [Google Scholar] [CrossRef]
- Jarmalavičiūtė, A.; Tunaitis, V.; Pivoraitė, U.; Venalis, A.; Pivoriūnas, A. Exosomes from dental pulp stem cells rescue human dopaminergic neurons from 6-hydroxy-dopamine–induced apoptosis. Cytotherapy 2015, 17, 932–939. [Google Scholar] [CrossRef] [PubMed]
- Hou, M.; Shi, L.; Zhou, Y.; Wang, J.; Jiang, J.; Jiang, J.; He, J. Expanding the codes: The development of density-encoded hydrogel microcarriers for suspension arrays. Biosens. Bioelectron. 2021, 181, 113133. [Google Scholar] [CrossRef] [PubMed]
- Phan, J.; Kumar, P.; Hao, D.; Gao, K.; Farmer, D.; Wang, A. Engineering mesenchymal stem cells to improve their exosome efficacy and yield for cell-free therapy. J. Extracell. Vesicles 2018, 7, 1522236. [Google Scholar] [CrossRef] [PubMed]
- Storm, M.P.; Sorrell, I.; Shipley, R.; Regan, S.; Luetchford, K.A.; Sathish, J.; Webb, S.; Ellis, M.J. Hollow Fiber Bioreactors for In Vivo-like Mammalian Tissue Culture. J. Vis. Exp. 2016, 111, e53431. [Google Scholar] [CrossRef]
- Gobin, J.; Muradia, G.; Mehic, J.; Westwood, C.; Couvrette, L.; Stalker, A.; Bigelow, S.; Luebbert, C.C.; Bissonnette, F.S.-D.; Johnston, M.J.W.; et al. Hollow-fiber bioreactor production of extracellular vesicles from human bone marrow mesenchymal stromal cells yields nanovesicles that mirrors the immuno-modulatory antigenic signature of the producer cell. Stem Cell Res. Ther. 2021, 12, 127. [Google Scholar] [CrossRef]
- Yan, I.K.; Shukla, N.; Borrelli, D.A.; Patel, T. Use of a Hollow Fiber Bioreactor to Collect Extracellular Vesicles from Cells in Culture. Extracell. RNA Methods Protoc. 2018, 35–41. [Google Scholar] [CrossRef]
- Yan, L.; Wu, X. Exosomes produced from 3D cultures of umbilical cord mesenchymal stem cells in a hollow-fiber bioreactor show improved osteochondral regeneration activity. Cell Biol. Toxicol. 2019, 36, 165–178. [Google Scholar] [CrossRef]
- Watson, D.C.; Bayik, D.; Srivatsan, A.; Bergamaschi, C.; Valentin, A.; Niu, G.; Bear, J.; Monninger, M.; Sun, M.; Morales-Kastresana, A.; et al. Efficient production and enhanced tumor delivery of engineered extracellular vesicles. Biomaterials 2016, 105, 195–205. [Google Scholar] [CrossRef] [PubMed]
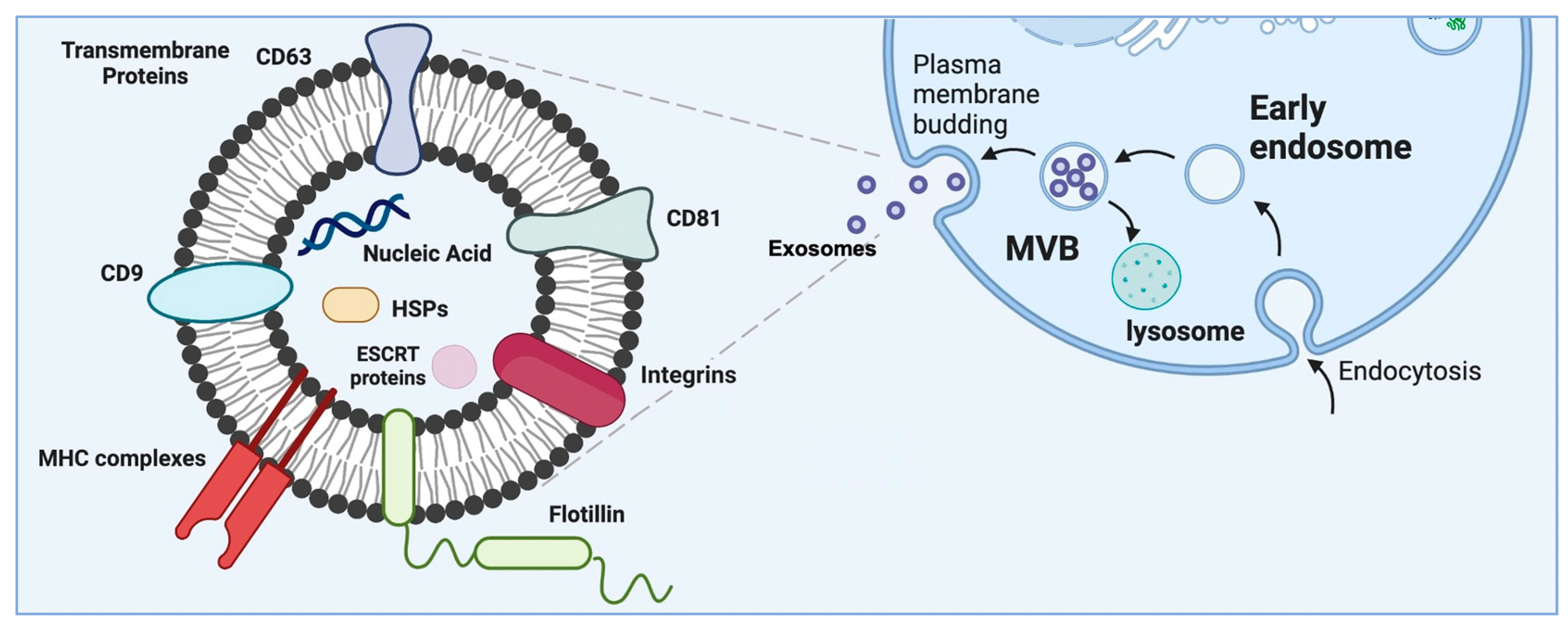
| Vesicle | Size | Origin | Contents | Markers | References |
|---|---|---|---|---|---|
| Exomeres | ≤50 nm | Unknown | DNA, RNA, miRNAs and lipids | Unclear—More studies are required | [7,30] |
| Exosomes | 30–200 nm * | Intra-luminal budding into MVBs and release by MVB fusion with cell membrane | Membrane proteins, different RNA species, lipids and DNA | Tetraspanins, heat-shock proteins, integrins, TSG101, flotillin, MFGE8 and ESCRT components. They include cell-type-specific proteins | [1,3,31,32] |
| MVs | 10–1000 nm | Outward budding or blebbing of cell plasma membrane | Membrane proteins, different RNA species, lipids, and DNA | Annexin A1 (on MVs that shed directly from the plasma membrane), integrins, selectins, and CD40 ligand, phosphatidylserine | [3,29,31,32,33] |
| Apoptotic bodies | 1–5 µm | Outward blebbing of apoptotic cell plasma membrane | Nuclear fractions, cell organelles and degraded proteins | Annexin V and high amounts of phosphatidylserine | [3,31,32] |
| Method | Principles & Materials | Advantages | Disadvantages | References |
|---|---|---|---|---|
| Differential Ultracentrifugation (dUC) | Physical—Components with imparity of size and density possess various sediment speeds | Gold standard Low cost Pure samples Suitable for large sample volumes | Time-consuming Low yield Repeated and high-speed steps might damage EVs | [19,41,44,91] |
| Density Gradient Centrifugation | Physical—Components with imparity of size and density possess various sediment speeds | Higher purity than dUC Maintains EVs intact | Time-consuming Low yield | [19,68] |
| Ultrafiltration (UF) | Physical—Filters particles with various sizes and molecular weights | Quick and simple High yield | Low purity EV deformation | [19,41,68,81,91] |
| Precipitation | Physical/Chemical—High hydrophilic polymers influence the solubility of EVs | High yield Easy Low cost Concentrates diluted samples | Low purity Potential contaminants (co-purifying protein aggregates) | [19,41,44,81,88] |
| Size Exclusion Chromatography (SEC) | Physical/Chemical—Columns packed with pore beads separate particles of various sizes and molecular weights | High yield Pure samples Maintains EVs intact | Potential contaminants (co-purifying protein aggregates) Samples can be diluted | [19,41,44,68,91] |
| Immunoaffinity Capture | Chemical—Uses antibodies to interact with specific membrane proteins | Quick High yield Pure samples | Expensive Lack of standardisation | [19,41,44,68,81,91] |
| Microfluidics | Physical/Chemical—Based on several principles including immunoaffinity, size and density | High yield Very pure samples | Expensive | [19,44,91] |
| Materials Used | Type of Cells/EVs | Applications | References |
|---|---|---|---|
| Hyaluronic Acid | MSC secretome | Asherman’s syndrome (injured endometrium) | [156] |
| Alginate | PPR exosomes/AD-MSC exosomes | Skin regeneration | [157,158] |
| Chitosan-based hydrogel with silk fibroin | hUC-MSC treated with miR-675-exosomes/gingival MSC exosomes | Aging-induced vascular disfunction/skin wound healing | [159,160] |
| Methylcellulose-chitosan | Placental MSC exosomes | Wound healing | [161] |
| Chitosan/chitosan-hyaluronic acid composite hydrogels | miR-126-3p overexpressing MSC exosomes/MSC exosomes/BM-MSC exosomes | Wound healing Ischaemia Skin regeneration | [162,163,164,165] |
| Hydroxyapatite embedded hyaluronic acid | UC-MSC exosomes | Bone regeneration | [166] |
| Polypetide-based FHE hydrogel | AD-MSC exosomes | Wound healing | [167] |
| RGD peptide/peptide-modified adhesive hydrogel | MSC exosomes | AKI repair/SCI treatment | [168,169] |
| Polyethylene glycol (PEG) hydrogel | MSC EVs | Chronic liver regeneration | [170] |
| Crosslinked hyaluronic acid/PEG hydrogel | MSC EVs | Osteoarthritis | [171] |
| Cellular Origin | Fold Increase | Characteristic Alteration | Reference |
|---|---|---|---|
| MSC (BM-, AD- and hUC-) | 5.7-fold increase | Increased purity | [202] |
| hUC-MSC | 18.38-fold increase | Increased inhibition of silica-induced PF | [213] |
| hUC-MSC | 20-fold increase | More potent siRNA transfer to neurons | [214] |
| BM-MSC | 24-fold increase | Increased neurite length in TG neurons | [215] |
| hSF-MSC | 1.6-fold increase | NA | [216] |
| SHED | NA | Induce apoptosis in dopaminergic neurons | [217] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casajuana Ester, M.; Day, R.M. Production and Utility of Extracellular Vesicles with 3D Culture Methods. Pharmaceutics 2023, 15, 663. https://doi.org/10.3390/pharmaceutics15020663
Casajuana Ester M, Day RM. Production and Utility of Extracellular Vesicles with 3D Culture Methods. Pharmaceutics. 2023; 15(2):663. https://doi.org/10.3390/pharmaceutics15020663
Chicago/Turabian StyleCasajuana Ester, Mar, and Richard M. Day. 2023. "Production and Utility of Extracellular Vesicles with 3D Culture Methods" Pharmaceutics 15, no. 2: 663. https://doi.org/10.3390/pharmaceutics15020663
APA StyleCasajuana Ester, M., & Day, R. M. (2023). Production and Utility of Extracellular Vesicles with 3D Culture Methods. Pharmaceutics, 15(2), 663. https://doi.org/10.3390/pharmaceutics15020663






