Polymeric Systems for the Controlled Release of Flavonoids
Abstract
1. Introduction
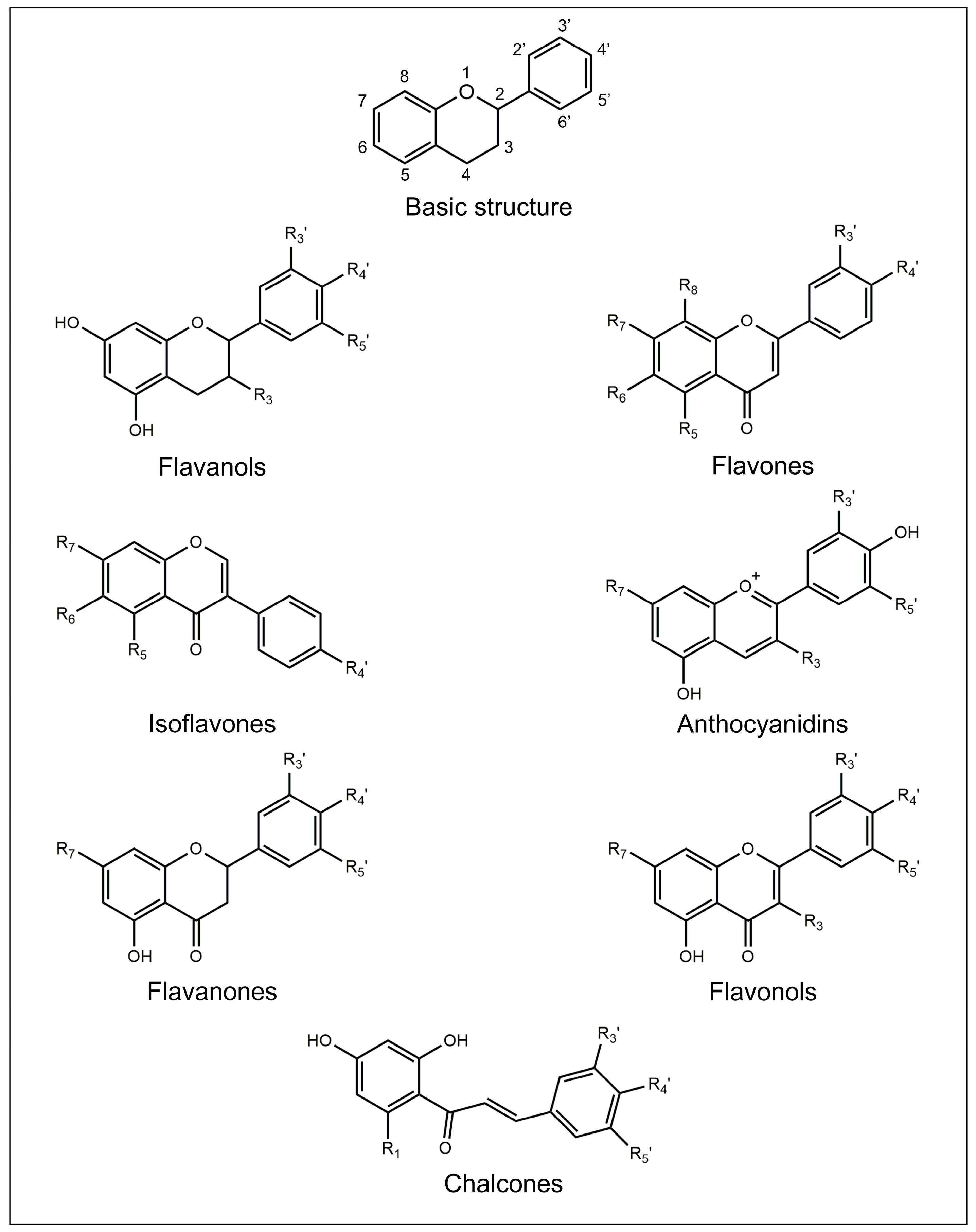
2. Flavonoids
2.1. Flavanols
2.2. Flavones
2.3. Isoflavones
2.4. Anthocyanidins
2.5. Flavanones
2.6. Flavonols
2.7. Chalcones
2.8. Bioactivity and Bioavailability of Flavonoids
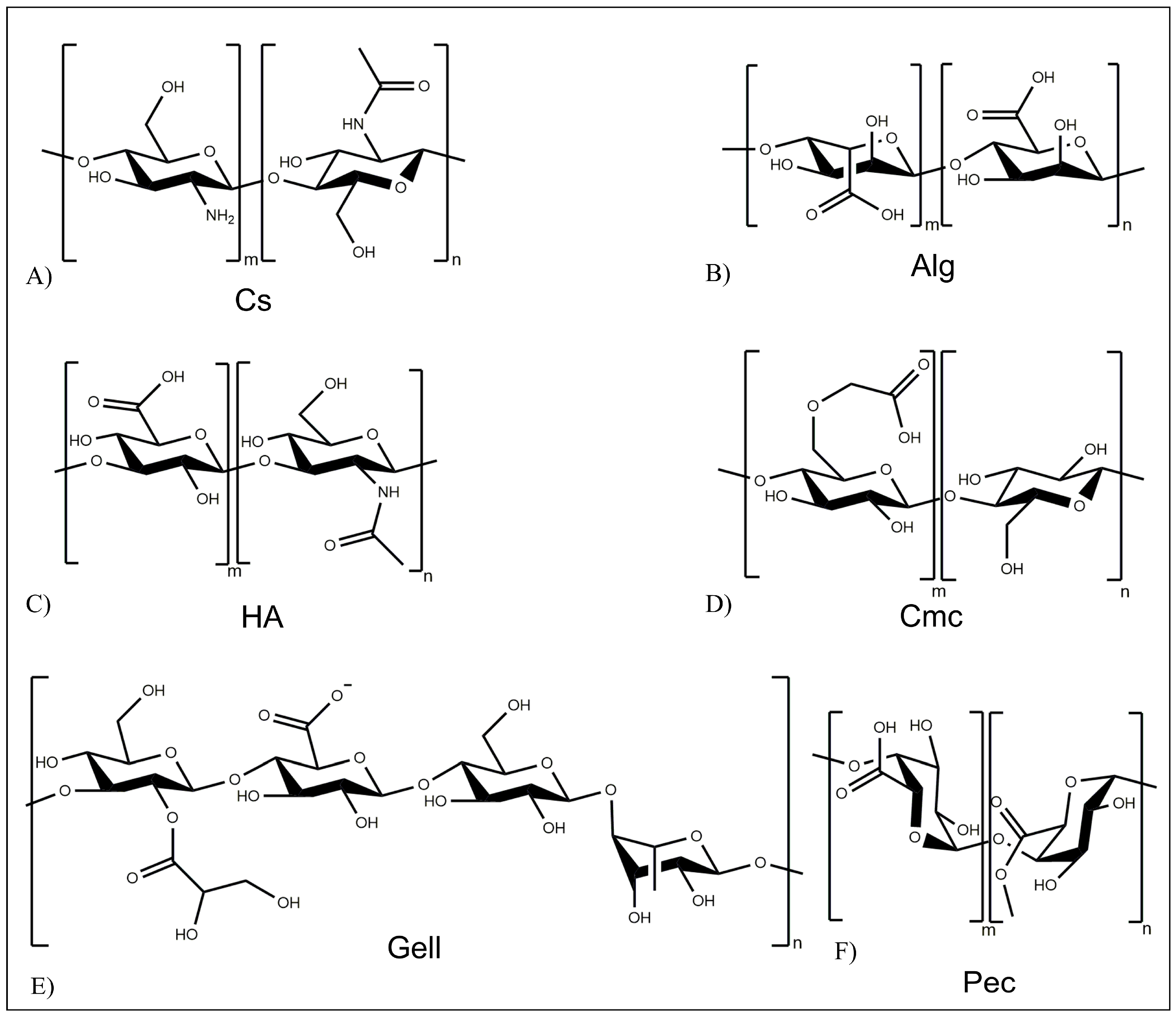
3. Polymeric Systems for Controlled Drug Release

4. Flavonoid-Based Commercial Products
5. Flavonoid-Loaded Polymeric Systems
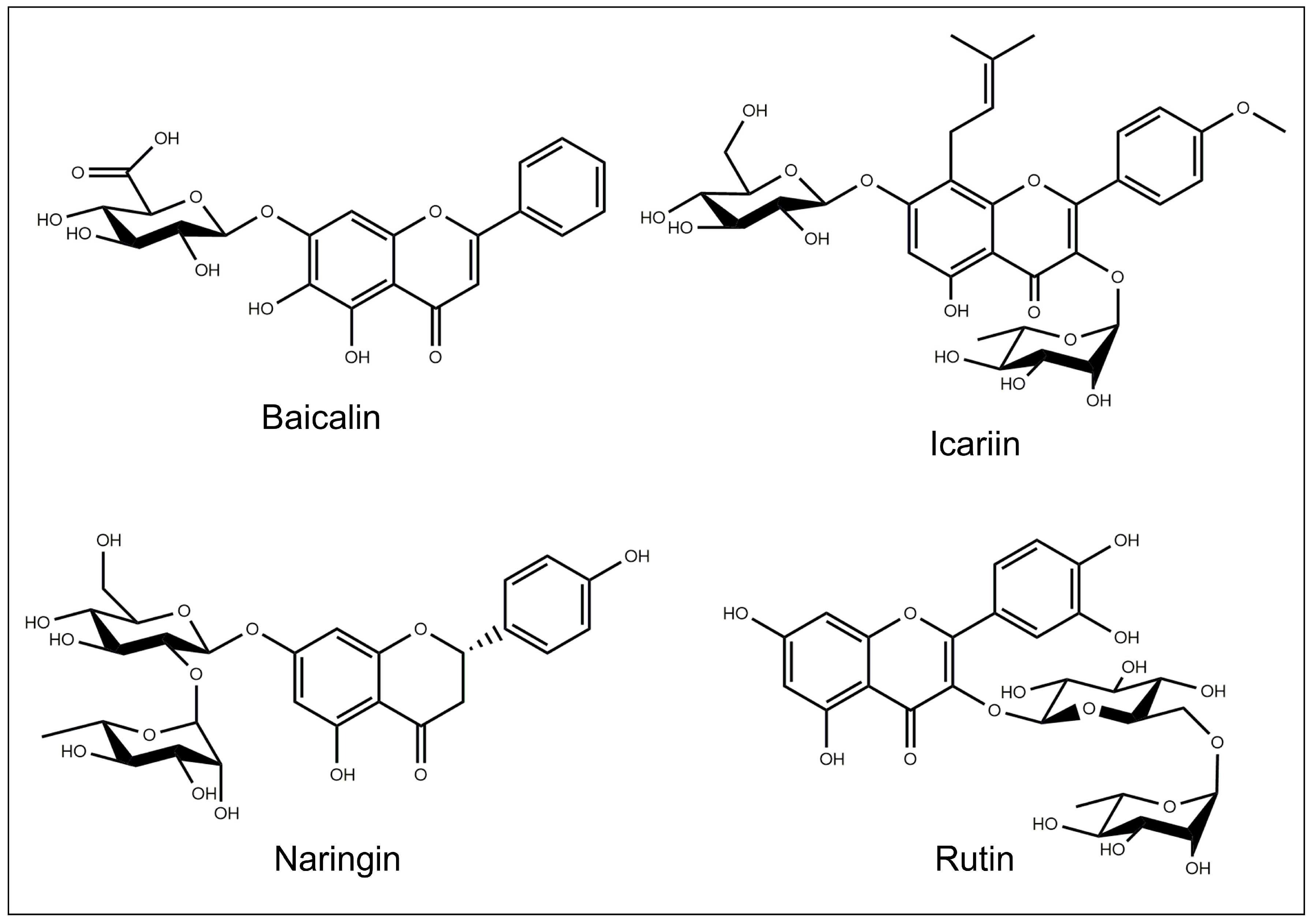
5.1. Baicalin
5.2. Cirsiliol
5.3. Chrysin
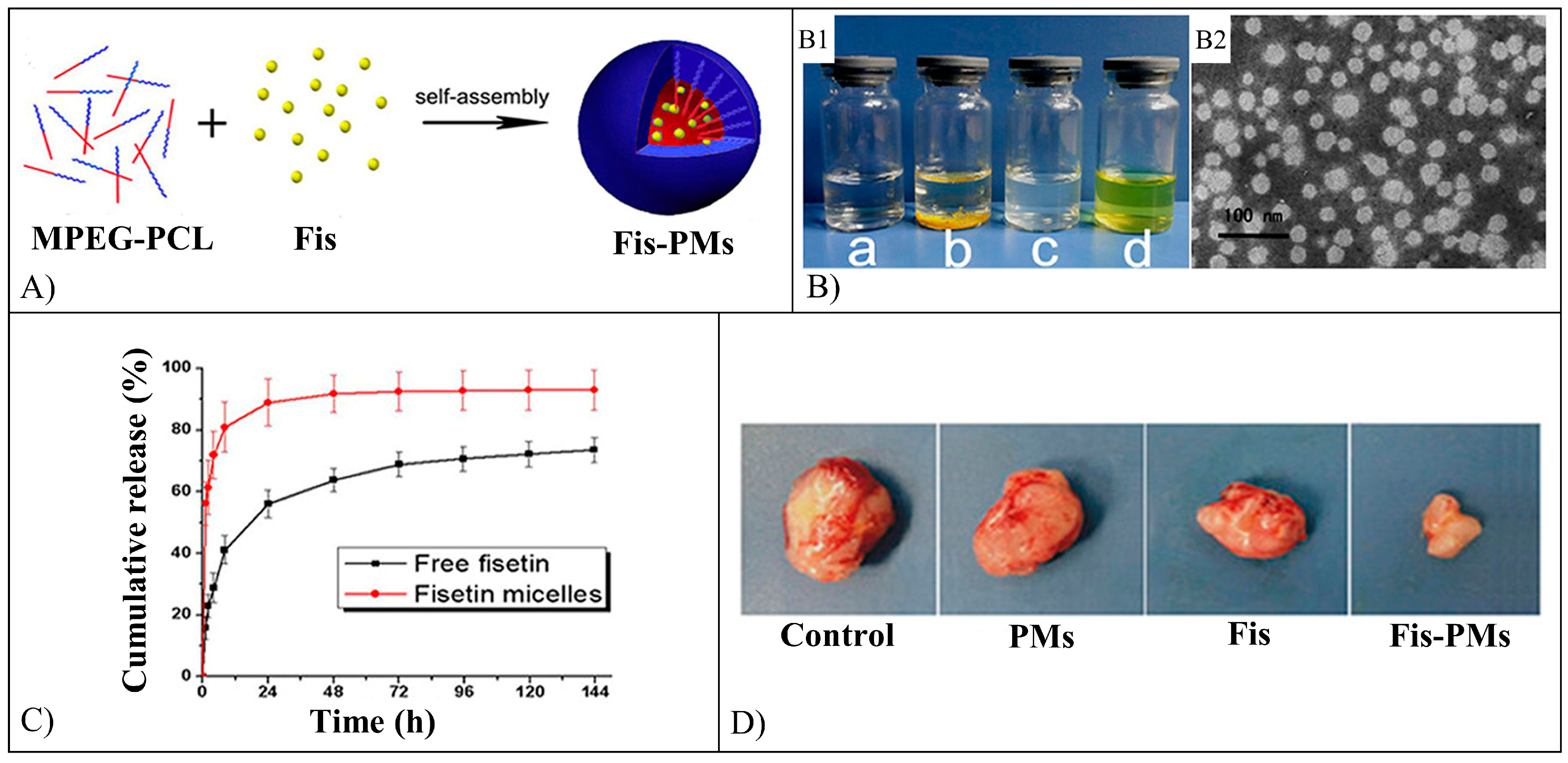
5.4. Fisetin
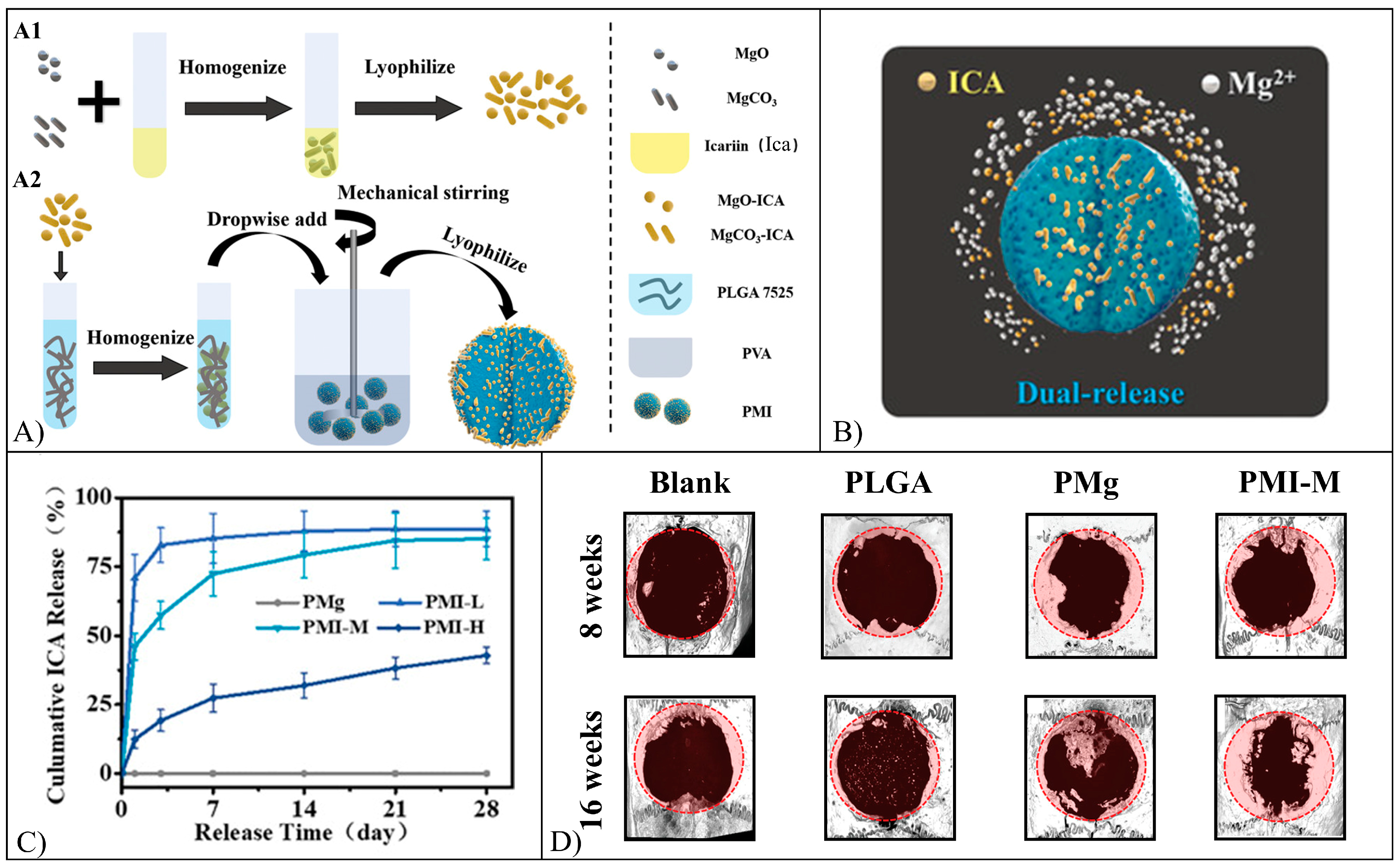
5.5. Icariin
5.6. Icaritin
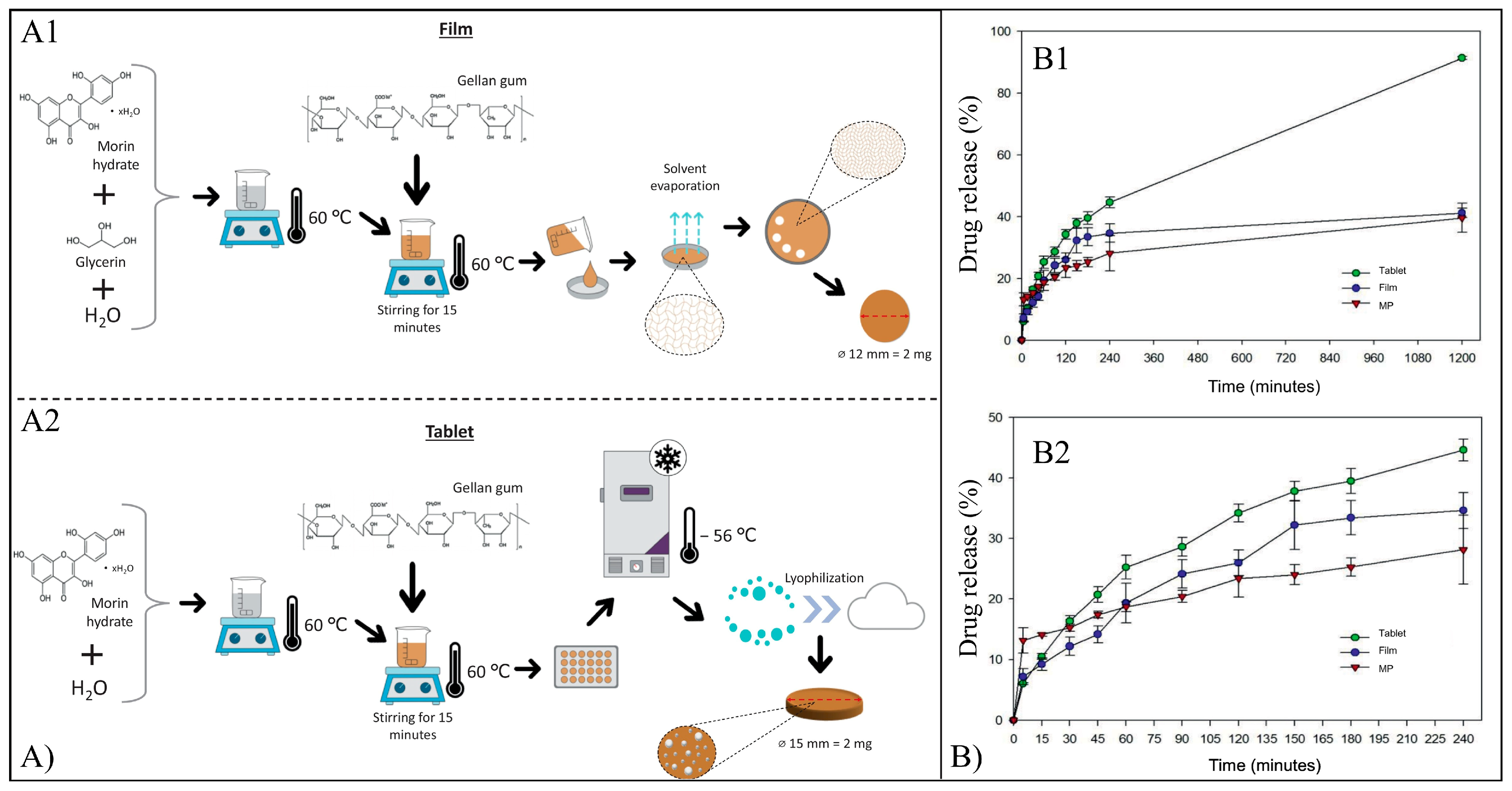
5.7. Morin
5.8. Myricetin
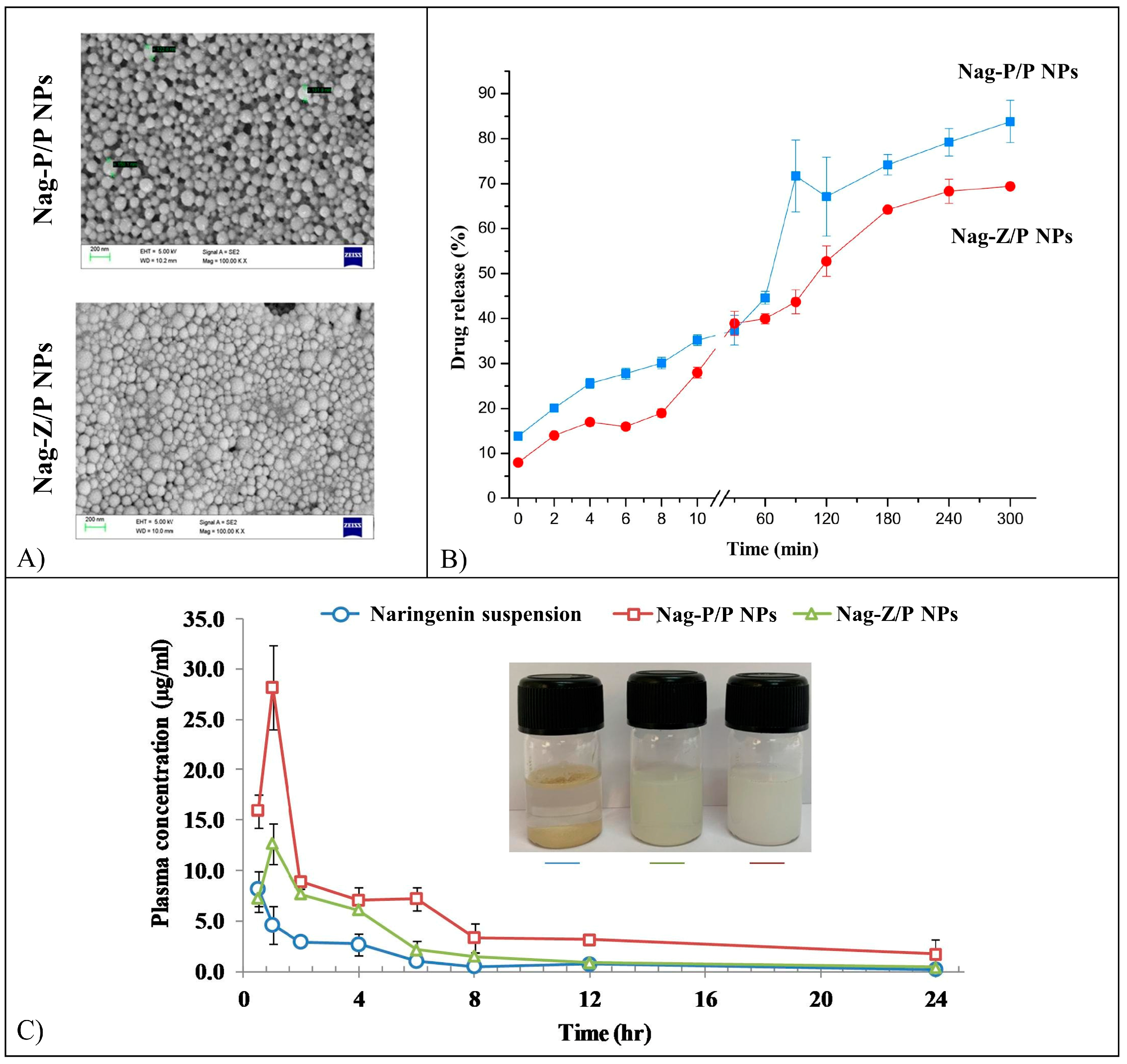
5.9. Naringenin
5.10. Naringin
5.11. Oroxylin A
5.12. Phloretin
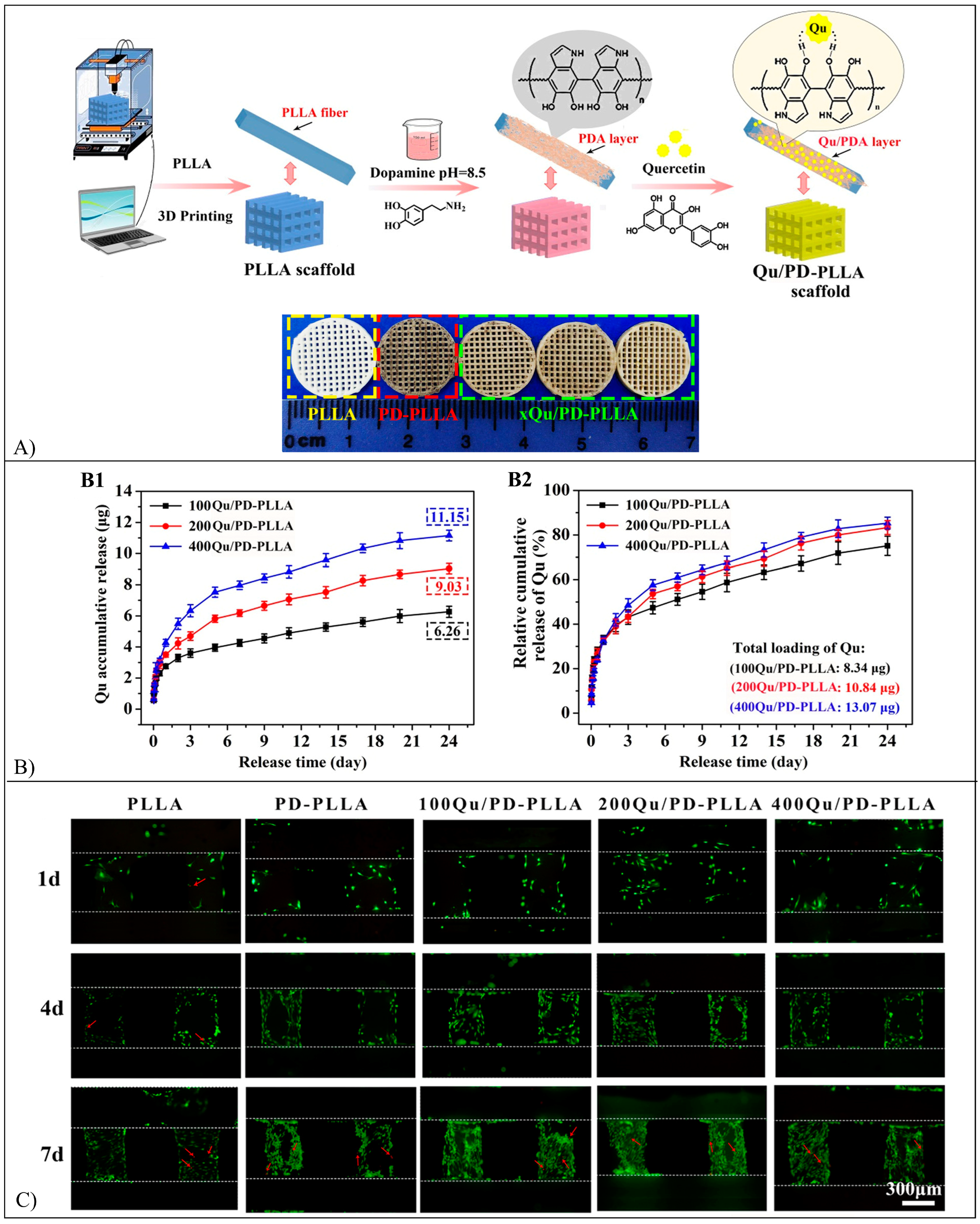
5.13. Quercetin
5.14. Rutin
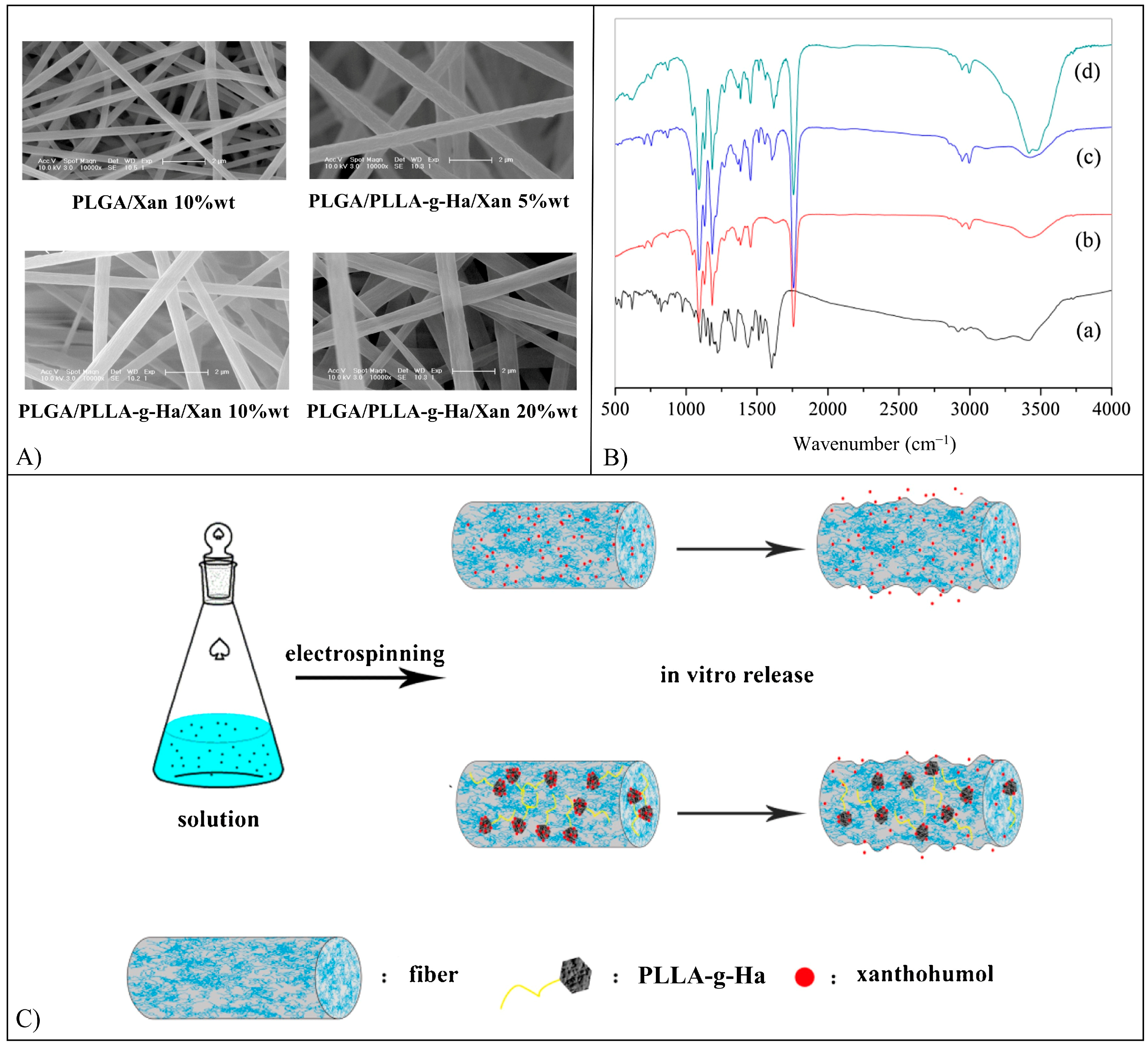
5.15. Xanthohumol
| Flavonoid | DDS a | Polymer (s) | Fabrication Technique (s) | In Vitro and In Vivo Results | Refs. |
|---|---|---|---|---|---|
| Baicalin | NCs | PLGA | Nanoprecipitation | Apoptotic effect on MCF-7, MDA-MB-231 and A549 cells; reduction of inflammatory markers in rats and in newborn skin pig. | [68,69,70,71,72] |
| Nanogels | Gell-cholesterol | Ionotropic gelation | |||
| NMs | Pluronic F68-SA | Thin film hydration | |||
| Transfersomes | Gell-cholesterol | Thin film hydration | |||
| Cirsiliol | NCs | PEG-PCL | Nanoprecipitation | Antiproliferative effect against MCF-7 cells. | [74] |
| Chrysin | NCs | PLGA | Nanoprecipitation | Reduction of serum blood glucose level in rats; reduction of growth of HGCCs c cells and of miR expression; anti-growth effect on T47D, MCF-7, Caco-2, HT29 cells. Repolarization of peritoneal macrophages to anti-inflammatory phenotype. Correction of drug-induced neurodegeneration. Increased viability of hADSCs b and expression of stemness genes, increased DPSCs d viability and proliferation and differentiation towards osteoblasts of MSCs e. | [84,85,86,87,88,89,90,91,92,93,94,95] |
| NPs | PLGA-PEG, PLGA-PEG-PLGA, BSA | Single/double emulsion solvent evaporation, nanoprecipitation | |||
| Nanovesicles | Cs | Ethanol injection | |||
| MHs | PCL-PEG-PCL | Electrospinning | |||
| SCs | PCL-Gel, Cmc/Cs/HA | Freeze drying, ionic gelation | |||
| Fisetin | NMs | PEG-PCL, PLA-TPGS | Self-assembly, nanoprecipitation | Inhibition of subcutaneous tumor growth, MCF-7 cell proliferation, and α-glucosidase activity. Antitumor effect against HCT116 cells, reduction of the tumor growth in rats. | [101,102,103,104] |
| NPs | PCL/PLGA-PEG-COOH, PLA | Single emulsion solvent evaporation | |||
| Icariin | NMs | PEG-PLGA | Nanoprecipitation | Cytotoxic effect against ASPC-1 cells. Increased proliferation of MG-63 and MC3T3-E1 cells, increased hADSCs and BMMSCs f differentiation, increased regeneration of bone in rats and in rabbits | [110,111,112,113,114,115,116,117,118,119,120,121,122] |
| SCs | PHBV, PLCL/SF, PCL/Col-Cs/Col, PLGA/, SIS, Cs/GP, ADA/Gel/MSN, Col, HA-MA/Col | Solvent casting salt leaching, coaxial electrospinning, freeze drying, low-temperature 3D printing, sol-gel transition, 3D printing, Solvent casting UV irradiation | |||
| MPs | PLGA | Single emulsion solvent evaporation | |||
| Icaritin | NMs | Soluplus®/Poloxamer 407 | ABS | Anti-HCC effect. Increased growth of BMMSCs and their differentiation into osteoblasts, healing of bone defects in rabbits and in adult emu. | [126,127,128,129,130] |
| NPs | PLGA-PEG | Nanoprecipitation | |||
| SCs | PLGA | Low-temperature 3D printing | |||
| Morin | NPs | HA-PBCA/TPGS | Dialysis | Cytotoxic effect against A549 cells. Antibacterial activity against A. naeslundii and S. mutans. | [134,135] |
| MPs | Alg/Gell | Ionotropic gelation | |||
| Films | Alg/Gell | Solvent casting | |||
| Tablets | Alg/Gell | Freeze drying | |||
| Myricetin | NPs | PDMAEMA-b-poly-(dimethylaminoethyl methacrylate-co-butyl methacrylate-co-propylacrylic acid)/PDMAEMA | Self-assembly | Antimicrobial and anti-biofilm properties; enhanced brain accumulation and penetration efficiency. | [136,137,138] |
| NCs | Eudragit RS100® | Nanoprecipitation | |||
| NMs | Cs-Pluronic P123/F68 | Thin film hydration | |||
| Naringenin | NPs | PLGA, PLA/PVA, zein/pectin, Eudragit E100®, Cs/Alg, Cs | Nanoprecipitation, single emulsion solvent evaporation, ionotropic gelation | Increased brain accumulation, diabetogenic effects in rats, suppression of colorectal cancer growth. | [141,142,143,144,145,146,147] |
| NXs | PVP | Thin film hydration | |||
| Naringin | MPs | CAP | Spray drying | Reduction of chronic arthritis in rats. | [150,151] |
| NPs | PLGA | Single emulsion solvent evaporation | |||
| Oroxylin A | PDots | Conjugated polymer | Nanoprecipitation | Cytotoxic effect against A431 cells and preferential targeting of tumor tissue. | [153] |
| Phloretin | NPs | Cs | Ionic gelation | Cytotoxic effect against KB and SKMEL cells. | [156,157] |
| NCs | PCL | Nanoprecipitation | |||
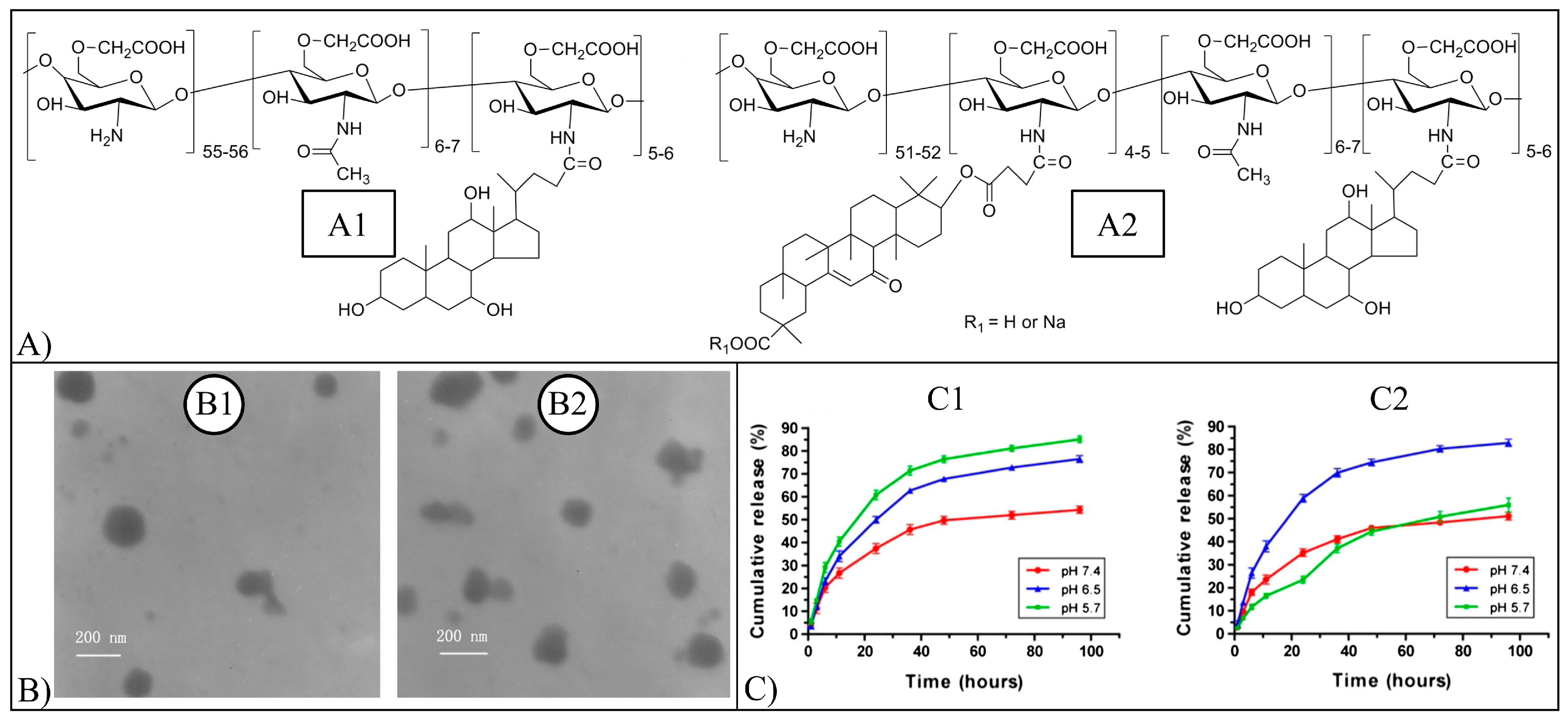
| Quercetin | NPs | PLGA, PCL, PCL-TPGS, BSA, BSA/PPG-PEG-PPG, Cs, NCS/Alg, CMCA, CMCA-GA, lecithin/Cs, HA | Nanoprecipitation, electrospraying, single/double emulsion solvent evaporation, ionic gelation, dialysis, microfluidic | Cytotoxic activity against A549, SKBR3, HepG2, A172, T98MG, MCF-7, MCF-7/ADR, SKOV-3, MDA-MB-231 cells. Increase of diuretic action in rats, adhesion and proliferation of H9c2 heart cells. Reduction of Alzheimer’s disease malignancy, sodium oxalate-induced cytotoxicity on Maldin-Darby canine kidney epithelial cells. Increase of proliferation and differentiation of MC3T3-E1 cells and proliferation of hPDLSCs g | [13,14,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177] |
| NMs | Pluronic-TPGS, Pluronic P123/L92/P407 | Thin film evaporation | |||
| SCs | PLLA, Cs/Col | Fused deposition modeling, sol-gel transitions | |||
| Rutin | MPs | CAP, CAT | Spray drying | Antioxidant effect on C-28 and NCTC2544 cells | [179,180,181] |
| NPs | PLGA, zein, Cs | Nanoprecipitation, ionotropic gelation | |||
| Xanthohumol | MHs | PLGA | Electrospinning | Ability to support MC3T3-E1 cells viability | [192] |
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 2D | Two dimensional |
| 3D | Three dimensional |
| 5-Fu | 5-fluorouracil |
| ABS | Acid-base shift |
| ABTS•+ | 3-ethylbezothiazoline-6-sulfonic acid |
| ADA | Alginate dialdehyde |
| Alg | Alginate |
| ALP | Alkaline lysis phosphatase |
| ASCs/ADSCs | Adipose-derived stem cells |
| Aβ | Amiloid-β peptide |
| Bai | Baicalin |
| BBB | Blood-brain barrier |
| BMSCs/BMMSCs | Bone marrow mesenchymal stem cells |
| BSA | Bovine serum albumin |
| CAP | Cellulose acetate phthalate |
| CAT | Cellulose acetate trimellitate |
| Chr | Chrysin |
| Cir | Cirsiliol |
| CLSM | Confocal laser scanning microscopy |
| Cmc | Carboxymethylcellulose |
| CMCA | O-carboxymethylated chitosan modified with cholic acid |
| Col | Collagen |
| Cs | Chitosan |
| Cur | Curcumin |
| DCF | Dichlorofluorescein |
| DDS | Drug delivery system |
| DMAEMA | 2-(dimethylamino)ethyl methacrylate |
| DMSO | Dimethyl sulfoxide |
| dMyr | Dihydromyricetin |
| DOPA | Dopamine |
| Dox | Doxorubicin |
| DPSCs | Dental pulp stem cells |
| ECM | Extracellular matrix |
| EMA | Ethyl methacrylate |
| FA | Folic acid |
| FDA | Food and drug administration |
| Fis | Fisetin |
| FT-IR | Fourier transformed infra red |
| GA | Glycirrhetinic acid |
| Gel | Gelatin |
| Gell | Gellan gum |
| Gly | Glycine |
| GP | Glycerophosphate |
| HA | Hyaluronic acid |
| Ha | Hydroxyapatite |
| HA-MA | Hyaluronic acid methacrylate |
| HAS | Human serum albumin |
| HGCCs | Human gastric carcinoma cells |
| HNT | Halloysite nanotubes |
| HPLC | High performance liquid chromatography |
| Hyp | Hydroxyproline |
| IC50 | Half maximal inhibitory concentration |
| Ica | Icariin |
| Ica-MA | Icariin methacrylate |
| Ict | Icaritin |
| IL-1β | Interleukin-1β |
| LPHNPs | Lipid/polymer hybrid NPs |
| MAA | Methacrylic acid |
| MH | Morin hydrate |
| MHs | Meshes |
| MI | Icariin-loaded microparticles |
| miR | Micro-ribonucleic acid |
| MMA | Methyl methacrylate |
| mNPs | Mixed polymers-based nanoparticles |
| Mor | Morin |
| MPs | Microparticles |
| MSCs | Mesenchymal stem cells |
| MSN | SiO2-CaO mesoporous nanoparticles |
| Myr | Myricetin |
| Nag | Naringenin |
| Nar | Naringin |
| NCs | Nanocapsules |
| NCS | N-succinyl chitosan |
| NMs | Nanomicelles |
| NPs | Nanoparticles |
| NXs | Nanocomplexes |
| OCMC | O-carboxymethylated chitosan |
| OrA | Oroxylin A |
| P/P NPs | Poly(lactic acid)/poly(vinyl alcohol) nanoparticles |
| P4HB | Poly(4-hydroxybutyrate) |
| PAA | Poly(acrylic acid) |
| PBCA | Poly(buthyl cyano acrylate) |
| PBS | Phosphate buffer saline |
| PCL | Poly(ε-caprolactone) |
| PDA | Poly(dopamine) |
| PDMAEMA | Poly(2-(dimethylamino)ethyl methacrylate) |
| Pec | Pectin |
| PEC | Polyelectrolite complex |
| PEG | Poly(ethylene glycol) |
| PEO | Poly(ethylene oxide) |
| PGA | Poly(glycolic acid) |
| P-gp | P-glycoprotein |
| PHA | Polyhydroxyalkanoates |
| PHB | Poly(3-hydroxybutyrate) |
| PHBHHx | Poly(3-hydroxybutyrate-co-3-hydroxyexanoate) |
| PHBV | Poly(3-hydroxybuthyrate-co-3-hydroxyvalerate) |
| Phl | Phloretin |
| PLA | Poly(lactic acid) |
| PLCL | Poly(lactic acid-co-ε-caprolactone) |
| PLGA | Poly(lactico-co-glycolic acid) |
| PLLA | Poly(L-lactic acid) |
| PMAA | Poly(methacrylic acid) |
| PMI | Poly(lactic-co-glycolic acid) microspheres containing icariin-loaded microparticles |
| PMI-H | PMI loaded with high amount of icariin |
| PMI-L | PMI loaded with low amount of icariin |
| PMI-M | PMI loaded with medium amount of icariin |
| PPO | Poly(propylene oxide) |
| Pro | Proline |
| PVA | Poly(vinyl alcohol) |
| PVP | Poly(vinyl pyrrolidone) |
| Que | Quercetin |
| ROS | Reactive oxygen species |
| Rut | Rutin |
| SA | Stearic acid |
| SAA | Surface active agent |
| SAON | Steroid associated osteonecrosis |
| SCd | Sulfobuthylether-β-cyclodextrin |
| SCs | Scaffolds |
| SEM | Scanning electron microscopy |
| SF | Silk fibroin |
| SGF | Simulated gastric fluid |
| SIF | Simulated intestinal fluid |
| SiN | Silica nanoparticles |
| SIS | Small intestine submucosa |
| SPC | Soybean phosphatidylcholine |
| TCP | Tricalcium phosphate |
| TEM | Transmission electron microscopy |
| THF | Tetrahydrofuran |
| TNF-α | Tumor necrosis factor-α |
| TPA | 12-O-tetradecanoylphorbol-13-acetate |
| TPGS | D-α-tocopheryl polyethylene glycol 1000 succinate |
| TPP | Sodium tripolyphosphate |
| UV | Ultraviolet |
| Xan | Xanthohumol |
| Z/P NPs | Zein/pectin nanoparticles |
| ε-CL | Ε-caprolactone |
| µ-CT | Micro-computed tomography |
References
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed]
- Kasprzak, M.M.; Erxleben, A.; Ochocki, J. Properties and applications of flavonoid metal complexes. RSC Adv. 2015, 5, 45853–45877. [Google Scholar] [CrossRef]
- Dias, M.C.; Pinto, D.C.G.A.; Silva, A.M.S. Plant Flavonoids: Chemical Characteristics and Biological Activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef] [PubMed]
- Iwashina, T. Flavonoid Function and Activity to Plants and Other Organisms. Biol. Sci. Space 2003, 17, 24–44. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Feng, Y.; Yu, S.; Fan, X.; Li, X.; Li, J.; Yin, H. The Flavonoid Biosynthesis Network in Plants. Int. J. Mol. Sci. 2021, 22, 5377. [Google Scholar] [CrossRef] [PubMed]
- Falcone Ferreyra, M.L.; Rius, S.P.; Casati, P. Flavonoids: Biosynthesis, biological functions, and biotechnological applications. Front. Plant Sci. 2012, 3, 222. [Google Scholar] [CrossRef]
- Morales, J.; Gunther, G.; Zanocco, A.L.; Lemp, E. Singlet oxygen reactions with flavonoids. A theoretical-experimental study. PLoS ONE 2012, 7, e40548. [Google Scholar] [CrossRef]
- Arct, J.; Pytkowska, K. Flavonoids as components of biologically active cosmeceuticals. Clin. Dermatol. 2008, 26, 347–357. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, D.; Hu, D.; Li, X.; Luo, J.; Duan, X.; Zhang, Y.; Wang, Y. Alkaloids and flavonoids exert protective effects against UVB-induced damage in a 3D skin model using human keratinocytes. Results Chem. 2022, 4, 100298. [Google Scholar] [CrossRef]
- Maity, S.; Acharyya, A.; Chakraborti, A. Flavonoid-based polymeric nanoparticles: A promising approach for cancer and diabetes treatment. Eur. Polym. J. 2022, 177, 111455. [Google Scholar] [CrossRef]
- Khan, H.; Ullah, H.; Martorell, M.; Valdes, S.E.; Belwal, T.; Tejada, S.; Sureda, A.; Kamal, M.A. Flavonoids nanoparticles in cancer: Treatment, prevention and clinical prospects. Semin. Cancer Biol. 2021, 69, 200–211. [Google Scholar] [CrossRef]
- Zverev, Y.F.; Rykunova, A.Y. Modern Nanocarriers as a Factor in Increasing the Bioavailability and Pharmacological Activity of Flavonoids. Appl. Biochem. Microbiol. 2022, 58, 1002–1020. [Google Scholar] [CrossRef] [PubMed]
- Arpornmaeklong, P.; Sareethammanuwat, M.; Apinyauppatham, K.; Boonyuen, S. Characteristics and biologic effects of thermosensitive quercetin-chitosan/collagen hydrogel on human periodontal ligament stem cells. J. Biomed. Mater. Res. B Appl. Biomater. 2021, 109, 1656–1670. [Google Scholar] [CrossRef]
- Chen, S.; Zhu, L.; Wen, W.; Lu, L.; Zhou, C.; Luo, B. Fabrication and Evaluation of 3D Printed Poly(l-lactide) Scaffold Functionalized with Quercetin-Polydopamine for Bone Tissue Engineering. ACS Biomater. Sci. Eng. 2019, 5, 2506–2518. [Google Scholar] [CrossRef] [PubMed]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef]
- Liu, X.; Le Bourvellec, C.; Guyot, S.; Renard, C.M.G.C. Reactivity of flavanols: Their fate in physical food processing and recent advances in their analysis by depolymerization. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4841–4880. [Google Scholar] [CrossRef]
- Braicu, C.; Ladomery, M.R.; Chedea, V.S.; Irimie, A.; Berindan-Neagoe, I. The relationship between the structure and biological actions of green tea catechins. Food Chem. 2013, 141, 3282–3289. [Google Scholar] [CrossRef] [PubMed]
- Hostetler, G.L.; Ralston, R.A.; Schwartz, S.J. Flavones: Food Sources, Bioavailability, Metabolism, and Bioactivity. Adv. Nutr. 2017, 8, 423–435. [Google Scholar] [CrossRef]
- Sohn, S.I.; Pandian, S.; Oh, Y.J.; Kang, H.J.; Cho, W.S.; Cho, Y.S. Metabolic Engineering of Isoflavones: An Updated Overview. Front. Plant Sci. 2021, 12, 670103. [Google Scholar] [CrossRef]
- Krizova, L.; Dadakova, K.; Kasparovska, J.; Kasparovsky, T. Isoflavones. Molecules 2019, 24, 1076. [Google Scholar] [CrossRef]
- Mohammed, H.A.; Khan, R.A. Anthocyanins: Traditional Uses, Structural and Functional Variations, Approaches to Increase Yields and Products’ Quality, Hepatoprotection, Liver Longevity, and Commercial Products. Int. J. Mol. Sci. 2022, 23, 2149. [Google Scholar] [CrossRef]
- Khan, M.; Zill, E.H.; Dangles, O. A comprehensive review on flavanones, the major citrus polyphenols. J. Food Compos. Anal. 2014, 33, 85–104. [Google Scholar] [CrossRef]
- Tomàs-Barberàn, F.A.; Clifford, M.N. Flavanones, chalcones and dihydrochalcones—Nature, occurrence and dietary burden. J. Sci. Food Agric. 2000, 80, 1073–1080. [Google Scholar] [CrossRef]
- Sharma, A.; Sharma, P.; Singh Tuli, H.; Sharma, A.K. Phytochemical and Pharmacological Properties of Flavonols; John and Wiley and Sons: Hoboken, NJ, USA, 2018; pp. 1–12. [Google Scholar] [CrossRef]
- Aherne, S.A.; O’brien, N.M. Dietary Flavonols: Chemistry, Food Content, and Metabolism. Nutrition 2002, 18, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Hollman, P.; Arts, I. Flavonols, flavones and flavanols—Nature, occurrence and dietary burden. J. Sci. Food Agric. 2000, 80, 1081–1093. [Google Scholar] [CrossRef]
- Zhuang, C.; Zhang, W.; Sheng, C.; Zhang, W.; Xing, C.; Miao, Z. Chalcone: A Privileged Structure in Medicinal Chemistry. Chem. Rev. 2017, 117, 7762–7810. [Google Scholar] [CrossRef] [PubMed]
- Rudrapal, M.; Khan, J.; Dukhyil, A.A.B.; Alarousy, R.m.i.i.; Attah, E.I.; Sharma, T.; Khairnar, S.J.; Bendale, A.R. Chalcone Scaffolds, Bioprecursors of Flavonoids: Chemistry, Bioactivities, and Pharmacokinetics. Molecules 2021, 26, 7177. [Google Scholar] [CrossRef]
- Juca, M.M.; Cysne Filho, F.M.S.; De Almeida, J.C.; Mesquita, D.D.S.; Barriga, J.R.M.; Dias, K.C.F.; Barbosa, T.M.; Vasconcelos, L.C.; Leal, L.k.a.m.; Ribeiro, J.E.; et al. Flavonoids: Biological activities and therapeutic potential. Nat. Prod. Res. 2020, 34, 692–705. [Google Scholar] [CrossRef]
- Code of Federal Regulations. Available online: https://www.ecfr.gov/current/title-21/chapter-I/subchapter-D/part-314/subpart-A/section-314.3 (accessed on 10 November 2022).
- Thilakarathna, S.; Rupasinghe, H. Flavonoid Bioavailability and Attempts for Bioavailability Enhancement. Nutrients 2013, 5, 3367–3387. [Google Scholar] [CrossRef] [PubMed]
- Rahaman, S.; Mondal, S. Flavonoids: A vital resource in healthcare and medicine. Pharm. Pharmacol. Int. J. 2020, 8, 91–104. [Google Scholar] [CrossRef]
- Kopustinskiene, D.M.; Jakstas, V.; Savickas, A.; Bernatoniene, J. Flavonoids as Anticancer Agents. Nutrients 2020, 12, 457. [Google Scholar] [CrossRef]
- Dos Santos Lima, B.; Shanmugam, S.; De Souza Siqueira Quintans, J.; Quintans-Júnior, L.; De Souza Araújo, A. Inclusion complex with cyclodextrins enhances the bioavailability of flavonoid compounds: A systematic review. Phytochem. Rev. 2019, 18, 1337–1359. [Google Scholar] [CrossRef]
- Ferreira, M.; Costa, D.; Sousa, A. Flavonoids-Based Delivery Systems towards Cancer Therapies. Bioengineering 2022, 9, 197. [Google Scholar] [CrossRef] [PubMed]
- Mojtaba, Y.; Mahdi, S.; Sara, S.; Nasim, K.; Mortazavian, A. Encapsulation Systems for Delivery of Flavonoids: A Review. Biointerface Res. Appl. Chem. 2021, 11, 13934–13951. [Google Scholar] [CrossRef]
- Sung, Y.K.; Kim, S.W. Recent advances in polymeric drug delivery systems. Biomater. Res. 2020, 24, 12. [Google Scholar] [CrossRef] [PubMed]
- Vargason, A.M.; Anselmo, A.C.; Mitragotri, S. The evolution of commercial drug delivery technologies. Nat. Biomed. Eng. 2021, 5, 951–967. [Google Scholar] [CrossRef]
- Puppi, D.; Chiellini, F. Biodegradable Polymers for Biomedical Additive Manufacturing. Appl. Mater. Today 2020, 20, 100700. [Google Scholar] [CrossRef]
- Jao, D.; Xue, Y.; Medina, J.; Hu, X. Protein-Based Drug-Delivery Materials. Materials 2017, 10, 517. [Google Scholar] [CrossRef]
- Kianfar, E. Protein nanoparticles in drug delivery: Animal protein, plant proteins and protein cages, albumin nanoparticles. J. Nanobiotechnology 2021, 19, 159. [Google Scholar] [CrossRef]
- Larsen, M.T.; Kuhlmann, M.; Hvam, M.L.; Howard, K.A. Albumin-based drug delivery: Harnessing nature to cure disease. Mol. Cell. Ther. 2016, 4, 3. [Google Scholar] [CrossRef]
- Puppi, D.; Chiellini, F.; Piras, A.M.; Chiellini, E. Polymeric materials for bone and cartilage repair. Prog. Polym. Sci. 2010, 35, 403–440. [Google Scholar] [CrossRef]
- Volkov, V.; Ferreira, A.; Cavaco-Paulo, A. On the Routines of Wild-Type Silk Fibroin Processing Toward Silk-Inspired Materials: A Review. Macromol. Mater. Eng. 2015, 300, 1199–1216. [Google Scholar] [CrossRef]
- Prasher, P.; Sharma, M.; Mehta, M.; Satija, S.; Aljabali, A.A.; Tambuwala, M.M.; Anand, K.; Sharma, N.; Dureja, H.; Jha, N.K.; et al. Current-status and applications of polysaccharides in drug delivery systems. Colloid Interface Sci. Commun. 2021, 42, 100418. [Google Scholar] [CrossRef]
- Prakash, P.; Lee, W.H.; Loo, C.Y.; Wong, H.S.J.; Parumasivam, T. Advances in Polyhydroxyalkanoate Nanocarriers for Effective Drug Delivery: An Overview and Challenges. Nanomaterials 2022, 12, 175. [Google Scholar] [CrossRef]
- Puppi, D.; Pecorini, G.; Chiellini, F. Biomedical Processing of Polyhydroxyalkanoates. Bioengineering 2019, 6, 108. [Google Scholar] [CrossRef] [PubMed]
- Michalak, M.; Kurcok, P.; Hakkarainen, M. Polyhydroxyalkanoate-based drug delivery systems. Polym. Int. 2017, 66, 617–622. [Google Scholar] [CrossRef]
- Bodratti, A.M.; Alexandridis, P. Formulation of Poloxamers for Drug Delivery. J. Funct. Biomater. 2018, 9, 11. [Google Scholar] [CrossRef]
- Delplace, V.; Nicolas, J. Degradable vinyl polymers for biomedical applications. Nat. Chem. 2015, 7, 771–784. [Google Scholar] [CrossRef]
- Pereira, P.; Serra, A.; Coelho, J. Vinyl Polymer-based technologies towards the efficient delivery of chemotherapeutic drugs. Prog. Polym. Sci. 2021, 121, 101432. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, M.P.; Chavali, M.S. Recent advances in biomaterials for 3D scaffolds: A review. Bioact. Mater. 2019, 4, 271–292. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, P.; Truong-Dinh Tran, T.; Zhang, J.; Kong, L. Manufacturing Techniques and Surface Engineering of Polymer Based Nanoparticles for Targeted Drug Delivery to Cancer. Nanomaterials 2016, 6, 26. [Google Scholar] [CrossRef] [PubMed]
- Jenjob, R.; Phakkeeree, T.; Seidi, F.; Theerasilp, M.; Crespy, D. Emulsion Techniques for the Production of Pharmacological Nanoparticles. Macromol. Biosci. 2019, 19, 1900063. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef] [PubMed]
- Pecorini, G.; Chiellini, F.; Puppi, D. Mechanical Characterization of Additive Manufactured Polymeric Scaffolds for Tissue Engineering. In Biomimetic Biomaterials for Tissue Regeneration and Drug Delivery; Dash, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2022. [Google Scholar]
- Asmatulu, R. Highly Hydrophilic Electrospun Polyacrylonitrile/Polyvinypyrrolidone Nanofibers Incorporated with Gentamicin as Filter Medium for Dam Water and Wastewater Treatment. J. Membr. Sep. Technol. 2016, 5, 38–56. [Google Scholar] [CrossRef]
- Mota, C.; Puppi, D.; Chiellini, F.; Chiellini, E. Additive manufacturing techniques for the production of tissue engineering constructs. J. Tissue Eng. Regen. Med. 2015, 9, 174–190. [Google Scholar] [CrossRef]
- Amawi, H.; Ashby, C.R., Jr.; Tiwari, A.K. Cancer chemoprevention through dietary flavonoids: What’s limiting? Chin. J. Cancer 2017, 36, 50. [Google Scholar] [CrossRef]
- Zhou, J.; Du, G.; Chen, J. Novel fermentation processes for manufacturing plant natural products. Curr. Opin. Biotechnol. 2014, 25, 17–23. [Google Scholar] [CrossRef]
- Zamora-Ros, R.; Barupal, D.K.; Rothwell, J.A.; Jenab, M.; Fedirko, V.; Romieu, I.; Aleksandrova, K.; Overvad, K.; Kyro, C.; Tjonneland, A.; et al. Dietary flavonoid intake and colorectal cancer risk in the European prospective investigation into cancer and nutrition (EPIC) cohort. Int. J. Cancer 2017, 140, 1836–1844. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef] [PubMed]
- Drugbank Online. Available online: https://go.drugbank.com/categories/DBCAT001281 (accessed on 19 January 2023).
- Takahashi, H.; Chen, M.C.; Pham, H.; Angst, E.; King, J.C.; Park, J.; Brovman, E.Y.; Ishiguro, H.; Harris, D.M.; Reber, H.A.; et al. Baicalein, a component of Scutellaria baicalensis, induces apoptosis by Mcl-1 down-regulation in human pancreatic cancer cells. Biochim. Biophys. Acta 2011, 1813, 1465–1474. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Zhan, S.; Wang, Y.; Zhou, G.; Liang, H.; Chen, X.; Shen, H. Baicalin, the major component of traditional Chinese medicine Scutellaria baicalensis induces colon cancer cell apoptosis through inhibition of oncomiRNAs. Sci. Rep. 2018, 8, 14477. [Google Scholar] [CrossRef]
- Li, B.Q.; Fu, T.; Gong, W.-H.; Dunlop, N.; Kung, H.-F.; Yan, Y.; Kang, J.; Wang, J.M. The flavonoid baicalin exhibits anti-inflammatory activity by binding to chemokines. Immunopharmacology 2000, 49, 295–306. [Google Scholar] [CrossRef]
- El-Gogary, R.I.; Gaber, S.A.A.; Nasr, M. Polymeric nanocapsular baicalin: Chemometric optimization, physicochemical characterization and mechanistic anticancer approaches on breast cancer cell lines. Sci. Rep. 2019, 9, 11064. [Google Scholar] [CrossRef]
- Manconi, M.; Manca, M.L.; Caddeo, C.; Cencetti, C.; Di Meo, C.; Zoratto, N.; Nacher, A.; Fadda, A.M.; Matricardi, P. Preparation of gellan-cholesterol nanohydrogels embedding baicalin and evaluation of their wound healing activity. Eur. J. Pharm. Biopharm. 2018, 127, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Jangid, A.K.; Agraval, H.; Rai, D.B.; Jain, P.; Yadav, U.C.; Pooja, D.; Kulhari, H. Baicalin encapsulating lipid-surfactant conjugate based nanomicelles: Preparation, characterization and anticancer activity. Chem. Phys. Lipids 2020, 233, 104978. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, O.; Nasr, M.; Nebsen, M.; Said, A.M.A.; Sammour, O. In vitro stabilization and in vivo improvement of ocular pharmacokinetics of the multi-therapeutic agent baicalin: Delineating the most suitable vesicular systems. Int. J. Pharm. 2018, 539, 83–94. [Google Scholar] [CrossRef]
- Manconi, M.; Manca, M.L.; Caddeo, C.; Valenti, D.; Cencetti, C.; Diez-Sales, O.; Nacher, A.; Mir-Palomo, S.; Terencio, M.C.; Demurtas, D.; et al. Nanodesign of new self-assembling core-shell gellan-transfersomes loading baicalin and in vivo evaluation of repair response in skin. Nanomedicine 2018, 14, 569–579. [Google Scholar] [CrossRef]
- Carlini, L.; Tancreda, G.; Iobbi, V.; Caicci, F.; Bruno, S.; Esposito, A.; Calzia, D.; Benini, S.; Bisio, A.; Manni, L.; et al. The Flavone Cirsiliol from Salvia x jamensis Binds the F1 Moiety of ATP Synthase, Modulating Free Radical Production. Cells 2022, 11, 3169. [Google Scholar] [CrossRef]
- Al-Shalabi, E.; Alkhaldi, M.; Sunoqrot, S. Development and evaluation of polymeric nanocapsules for cirsiliol isolated from Jordanian Teucrium polium L. as a potential anticancer nanomedicine. J. Drug Deliv. Sci. Technol. 2020, 56, 101544. [Google Scholar] [CrossRef]
- Mani, R.; Natesan, V. Chrysin: Sources, beneficial pharmacological activities, and molecular mechanism of action. Phytochemistry 2018, 145, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Filho, C.B.; Jesse, C.R.; Donato, F.; Del Fabbro, L.; De Gomes, M.G.; Goes, A.T.R.; Souza, L.C.; Giacomeli, R.; Antunes, M.; Luchese, C.; et al. Neurochemical factors associated with the antidepressant-like effect of flavonoid chrysin in chronically stressed mice. Eur. J. Pharmacol. 2016, 791, 284–296. [Google Scholar] [CrossRef]
- Pushpavalli, G.; Kalaiarasi, P.; Veeramani, C.; Pugalendi, K.V. Effect of chrysin on hepatoprotective and antioxidant status in D-galactosamine-induced hepatitis in rats. Eur. J. Pharmacol. 2010, 631, 36–41. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, Z.; Wu, X.; Zhang, X.; Geng, F.; Wang, Q.; Geng, Z.; Yu, C.; Li, Z. Chrysin alleviates lipopolysaccharide-induced neuron damage and behavioral deficits in mice through inhibition of Fyn. Int. Immunopharmacol. 2022, 111, 109118. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Espinosa, J.J.; Saldana-Rios, J.; Garcia-Jimenez, S.; Villalobos-Molina, R.; Avila-Villarreal, G.; Rodriguez-Ocampo, A.N.; Bernal-Fernandez, G.; Estrada-Soto, S. Chrysin Induces Antidiabetic, Antidyslipidemic and Anti-Inflammatory Effects in Athymic Nude Diabetic Mice. Molecules 2017, 23, 67. [Google Scholar] [CrossRef]
- Yao, J.; Jiang, M.; Zhang, Y.; Liu, X.; Du, Q.; Feng, G. Chrysin alleviates allergic inflammation and airway remodeling in a murine model of chronic asthma. Int. Immunopharmacol. 2016, 32, 24–31. [Google Scholar] [CrossRef]
- Modi, F.; Bhavsar, S.K.; Patel, J.H.; Varia, R.D.; Modi, L.C.; Kale, N. Evaluation of Pharmacokinetics, Antibacterial and Anti-Inflammatory Activities of Chrysin in Rat. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 1494–1503. [Google Scholar] [CrossRef]
- Khoo, B.Y.; Chua, S.L.; Balaram, P. Apoptotic effects of chrysin in human cancer cell lines. Int. J. Mol. Sci. 2010, 11, 2188–2199. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Mishra, P.S.; Bandopadhyay, R.; Khurana, N.; Angelopoulou, E.; Paudel, Y.N.; Piperi, C. Neuroprotective Potential of Chrysin: Mechanistic Insights and Therapeutic Potential for Neurological Disorders. Molecules 2021, 26, 6456. [Google Scholar] [CrossRef] [PubMed]
- El-Hussien, D.; El-Zaafarany, G.M.; Nasr, M.; Sammour, O. Chrysin nanocapsules with dual anti-glycemic and anti-hyperlipidemic effects: Chemometric optimization, physicochemical characterization and pharmacodynamic assessment. Int. J. Pharm. 2021, 592, 120044. [Google Scholar] [CrossRef] [PubMed]
- Mohammadian, F.; Pilehvar-Soltanahmadi, Y.; Mofarrah, M.; Dastani-Habashi, M.; Zarghami, N. Down regulation of miR-18a, miR-21 and miR-221 genes in gastric cancer cell line by chrysin-loaded PLGA-PEG nanoparticles. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1972–1978. [Google Scholar] [CrossRef]
- Mohammadian, F.; Pilehvar-Soltanahmadi, Y.; Zarghami, F.; Akbarzadeh, A.; Zarghami, N. Upregulation of miR-9 and Let-7a by nanoencapsulated chrysin in gastric cancer cells. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1201–1206. [Google Scholar] [CrossRef]
- Anari, E.; Akbarzadeh, A.; Zarghami, N. Chrysin-loaded PLGA-PEG nanoparticles designed for enhanced effect on the breast cancer cell line. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1410–1416. [Google Scholar] [CrossRef]
- Lotfi-Attari, J.; Pilehvar-Soltanahmadi, Y.; Dadashpour, M.; Alipour, S.; Farajzadeh, R.; Javidfar, S.; Zarghami, N. Co-Delivery of Curcumin and Chrysin by Polymeric Nanoparticles Inhibit Synergistically Growth and hTERT Gene Expression in Human Colorectal Cancer Cells. Nutr. Cancer 2017, 69, 1290–1299. [Google Scholar] [CrossRef] [PubMed]
- Khaledi, S.; Jafari, S.; Hamidi, S.; Molavi, O.; Davaran, S. Preparation and characterization of PLGA-PEG-PLGA polymeric nanoparticles for co-delivery of 5-Fluorouracil and Chrysin. J. Biomater. Sci. Polym. Ed. 2020, 31, 1107–1126. [Google Scholar] [CrossRef] [PubMed]
- Firouzi-Amandi, A.; Dadashpour, M.; Nouri, M.; Zarghami, N.; Serati-Nouri, H.; Jafari-Gharabaghlou, D.; Karzar, B.H.; Mellatyar, H.; Aghebati-Maleki, L.; Babaloo, Z.; et al. Chrysin-nanoencapsulated PLGA-PEG for macrophage repolarization: Possible application in tissue regeneration. Biomed. Pharm. 2018, 105, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Nosrati, H.; Abbasi, R.; Charmi, J.; Rakhshbahar, A.; Aliakbarzadeh, F.; Danafar, H.; Davaran, S. Folic acid conjugated bovine serum albumin: An efficient smart and tumor targeted biomacromolecule for inhibition folate receptor positive cancer cells. Int. J. Biol. Macromol. 2018, 117, 1125–1132. [Google Scholar] [CrossRef]
- Ibrahim, S.S.; Abo Elseoud, O.G.; Mohamedy, M.H.; Amer, M.M.; Mohamed, Y.Y.; Elmansy, S.A.; Kadry, M.M.; Attia, A.A.; Fanous, R.A.; Kamel, M.S.; et al. Nose-to-brain delivery of chrysin transfersomal and composite vesicles in doxorubicin-induced cognitive impairment in rats: Insights on formulation, oxidative stress and TLR4/NF-kB/NLRP3 pathways. Neuropharmacology 2021, 197, 108738. [Google Scholar] [CrossRef] [PubMed]
- Deldar, Y.; Zarghami, F.; Pilehvar-Soltanahmadi, Y.; Dadashpour, M.; Zarghami, N. Antioxidant effects of chrysin-loaded electrospun nanofibrous mats on proliferation and stemness preservation of human adipose-derived stem cells. Cell Tissue Bank 2017, 18, 475–487. [Google Scholar] [CrossRef]
- Alipour, M.; Pouya, B.; Aghazadeh, Z.; Samadikafil, H.; Ghorbani, M.; Alizadeh, S.; Aghazadeh, M.; Dalir Abdolahinia, E. The Antimicrobial, Antioxidative, and Anti-Inflammatory Effects of Polycaprolactone/Gelatin Scaffolds Containing Chrysin for Regenerative Endodontic Purposes. Stem Cells Int. 2021, 2021, 3828777. [Google Scholar] [CrossRef]
- Menon, A.H.; Soundarya, S.P.; Sanjay, V.; Chandran, S.V.; Balagangadharan, K.; Selvamurugan, N. Sustained release of chrysin from chitosan-based scaffolds promotes mesenchymal stem cell proliferation and osteoblast differentiation. Carbohydr. Polym. 2018, 195, 356–367. [Google Scholar] [CrossRef]
- Imran, M.; Saeed, F.; Gilani, S.A.; Shariati, M.A.; Imran, A.; Afzaal, M.; Atif, M.; Tufail, T.; Anjum, F.M. Fisetin: An anticancer perspective. Food Sci. Nutr. 2021, 9, 3–16. [Google Scholar] [CrossRef]
- Molagoda, I.M.N.; Jayasingha, J.; Choi, Y.H.; Jayasooriya, R.; Kang, C.H.; Kim, G.Y. Fisetin inhibits lipopolysaccharide-induced inflammatory response by activating beta-catenin, leading to a decrease in endotoxic shock. Sci. Rep. 2021, 11, 8377. [Google Scholar] [CrossRef] [PubMed]
- Gabor, M.; Eperjessy, E. Antibacterial Effect of Fisetin and Fisetinidin. Nature 1966, 212, 1273. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, B.; Lu, J.; Shi, H.; Gong, S.; Wang, Y.; Hamdy, R.C.; Chua, B.H.L.; Yang, L.; Xu, X. Fisetin provides antidepressant effects by activating the tropomyosin receptor kinase B signal pathway in mice. J. Neurochem. 2017, 143, 561–568. [Google Scholar] [CrossRef]
- Khan, N.; Syed, D.N.; Ahmad, N.; Mukhtar, H. Fisetin: A dietary antioxidant for health promotion. Antioxid. Redox Signal. 2013, 19, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wu, Q.; Song, L.; He, T.; Li, Y.; Li, L.; Su, W.; Liu, L.; Qian, Z.; Gong, C. Polymeric micelles encapsulating fisetin improve the therapeutic effect in colon cancer. ACS Appl. Mater. Interfaces 2015, 7, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, D.Z.; Wang, Y.X. Bioflavonoid Fisetin Loaded alpha-Tocopherol-Poly(lactic acid)-Based Polymeric Micelles for Enhanced Anticancer Efficacy in Breast Cancers. Pharm. Res. 2017, 34, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Sechi, M.; Syed, D.N.; Pala, N.; Mariani, A.; Marceddu, S.; Brunetti, A.; Mukhtar, H.; Sanna, V. Nanoencapsulation of dietary flavonoid fisetin: Formulation and in vitro antioxidant and alpha-glucosidase inhibition activities. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 68, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Yuan, X.; Chu, K.; Zhang, H.; Ji, W.; Rui, M. Preparation and optimization of poly (lactic acid) nanoparticles loaded with fisetin to improve anti-cancer therapy. Int. J. Biol. Macromol. 2019, 125, 700–710. [Google Scholar] [CrossRef]
- Khezri, M.R.; Nazari-Khanamiri, F.; Mohammadi, T.; Moloodsouri, D.; Ghasemnejad-Berenji, M. Potential effects of icariin, the Epimedium-derived bioactive compound in the treatment of COVID-19: A hypothesis. Naunyn Schmiedebergs Arch. Pharm. 2022, 395, 1019–1027. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Chen, Y.; Wang, Y.; Liu, J. Roles of the antioxidant properties of icariin and its phosphorylated derivative in the protection against duck virus hepatitis. BMC Vet. Res. 2014, 10, 226. [Google Scholar] [CrossRef]
- Bi, Z.; Zhang, W.; Yan, X. Anti-inflammatory and immunoregulatory effects of icariin and icaritin. Biomed Pharm. 2022, 151, 113180. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Yang, Y.; Liu, Y.; Jiang, S.; Di, S.; Hu, W.; Ma, Z.; Li, T.; Zhu, Y.; Xin, Z.; et al. Icariin displays anticancer activity against human esophageal cancer cells via regulating endoplasmic reticulum stress-mediated apoptotic signaling. Sci. Rep. 2016, 6, 21145. [Google Scholar] [CrossRef]
- Rui Li, L.; Sethi, G.; Zhang, X.; Liu Liu, C.; Huang, Y.; Liu, Q.; Xu Ren, B.; Ru Tang, F. The neuroprotective effects of icariin on ageing, various neurological, neuropsychiatric disorders, and brain injury induced by radiation exposure. Aging 2022, 14, 1562. [Google Scholar]
- Alhakamy, N.A. Development and Evaluation of Icariin-Loaded PLGA-PEG Nanoparticles for Potentiation the Proapoptotic Activity in Pancreatic Cancer Cells. AAPS PharmSciTech 2021, 22, 252. [Google Scholar] [CrossRef]
- Xia, L.; Li, Y.; Zhou, Z.; Dai, Y.; Liu, H.; Liu, H. Icariin delivery porous PHBV scaffolds for promoting osteoblast expansion in vitro. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 3545–3552. [Google Scholar] [CrossRef]
- Yuan, Z.; Wan, Z.; Wei, P.; Lu, X.; Mao, J.; Cai, Q.; Zhang, X.; Yang, X. Dual-Controlled Release of Icariin/Mg(2+) from Biodegradable Microspheres and Their Synergistic Upregulation Effect on Bone Regeneration. Adv. Healthc. Mater. 2020, 9, 2000211. [Google Scholar] [CrossRef]
- Yin, L.; Wang, K.; Lv, X.; Sun, R.; Yang, S.; Yang, Y.; Liu, Y.; Liu, J.; Zhou, J.; Yu, Z. The fabrication of an ICA-SF/PLCL nanofibrous membrane by coaxial electrospinning and its effect on bone regeneration in vitro and in vivo. Sci. Rep. 2017, 7, 8616. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Tang, J.; Zhou, D.; Weng, Y.; Qin, W.; Liu, C.; Lv, S.; Wang, W.; Zhao, X. Electrospun Icariin-Loaded Core-Shell Collagen, Polycaprolactone, Hydroxyapatite Composite Scaffolds for the Repair of Rabbit Tibia Bone Defects. Int. J. Nanomed. 2020, 15, 3039–3056. [Google Scholar] [CrossRef]
- Lai, Y.; Cao, H.; Wang, X.; Chen, S.; Zhang, M.; Wang, N.; Yao, Z.; Dai, Y.; Xie, X.; Zhang, P.; et al. Porous composite scaffold incorporating osteogenic phytomolecule icariin for promoting skeletal regeneration in challenging osteonecrotic bone in rabbits. Biomaterials 2018, 153, 1–13. [Google Scholar] [CrossRef]
- Li, M.; Gu, Q.; Chen, M.; Zhang, C.; Chen, S.; Zhao, J. Controlled delivery of icariin on small intestine submucosa for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 71, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Kazemi-Aghdam, F.; Jahed, V.; Dehghan-Niri, M.; Ganji, F.; Vasheghani-Farahani, E. Injectable chitosan hydrogel embedding modified halloysite nanotubes for bone tissue engineering. Carbohydr. Polym. 2021, 269, 118311. [Google Scholar] [CrossRef] [PubMed]
- Monavari, M.; Homaeigohar, S.; Fuentes-Chandia, M.; Nawaz, Q.; Monavari, M.; Venkatraman, A.; Boccaccini, A.R. 3D printing of alginate dialdehyde-gelatin (ADA-GEL) hydrogels incorporating phytotherapeutic icariin loaded mesoporous SiO2-CaO nanoparticles for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 131, 112470. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Yuan, T.; Zhang, X.; Xiao, Y.; Wang, R.; Fan, Y.; Zhang, X. Icariin: A potential promoting compound for cartilage tissue engineering. Osteoarthr. Cartil. 2012, 20, 1647–1656. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Yang, J.; Lu, J.; Xiao, Y.; Fan, Y.; Zhang, X. Preparation and characterization of a novel hyaluronic acid–icariin conjugate hydrogel. Mater. Lett. 2014, 136, 41–44. [Google Scholar] [CrossRef]
- Yuan, T.; He, L.; Yang, J.; Zhang, L.; Xiao, Y.; Fan, Y.; Zhang, X. Conjugated icariin promotes tissue-engineered cartilage formation in hyaluronic acid/collagen hydrogel. Process Biochem. 2015, 50, 2242–2250. [Google Scholar] [CrossRef]
- Yang, J.; Liu, Y.; He, L.; Wang, Q.; Wang, L.; Yuan, T.; Xiao, Y.; Fan, Y.; Zhang, X. Icariin conjugated hyaluronic acid/collagen hydrogel for osteochondral interface restoration. Acta Biomater 2018, 74, 156–167. [Google Scholar] [CrossRef]
- Tao, H.; Liu, M.; Wang, Y.; Luo, S.; Xu, Y.; Ye, B.; Zheng, L.; Meng, K.; Li, L. Icaritin Induces Anti-tumor Immune Responses in Hepatocellular Carcinoma by Inhibiting Splenic Myeloid-Derived Suppressor Cell Generation. Front. Immunol. 2021, 12, 609295. [Google Scholar] [CrossRef]
- Lai, X.; Ye, Y.; Sun, C.; Huang, X.; Tang, X.; Zeng, X.; Yin, P.; Zeng, Y. Icaritin exhibits anti-inflammatory effects in the mouse peritoneal macrophages and peritonitis model. Int. Immunopharmacol. 2013, 16, 41–49. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, S.Q. Antiosteoporosis Effects, Pharmacokinetics, and Drug Delivery Systems of Icaritin: Advances and Prospects. Pharmaceuticals 2022, 15, 397. [Google Scholar] [CrossRef]
- Tang, C.; Chen, X.; Yao, H.; Yin, H.; Ma, X.; Jin, M.; Lu, X.; Wang, Q.; Meng, K.; Yuan, Q. Enhanced Oral Absorption of Icaritin by Using Mixed Polymeric Micelles Prepared with a Creative Acid-Base Shift Method. Molecules 2021, 26, 3450. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Guo, J.; Hu, M.; Gao, Y.; Huang, L. Icaritin Exacerbates Mitophagy and Synergizes with Doxorubicin to Induce Immunogenic Cell Death in Hepatocellular Carcinoma. ACS Nano 2020, 14, 4816–4828. [Google Scholar] [CrossRef]
- Chen, S.H.; Wang, X.L.; Xie, X.H.; Zheng, L.Z.; Yao, D.; Wang, D.P.; Leng, Y.; Zhang, G.; Qin, L. Comparative study of osteogenic potential of a composite scaffold incorporating either endogenous bone morphogenetic protein-2 or exogenous phytomolecule icaritin: An in vitro efficacy study. Acta Biomater. 2012, 8, 3128–3137. [Google Scholar] [CrossRef]
- Wang, X.L.; Xie, X.H.; Zhang, G.; Chen, S.H.; Yao, D.; He, K.; Wang, X.H.; Yao, X.S.; Leng, Y.; Fung, K.P.; et al. Exogenous phytoestrogenic molecule icaritin incorporated into a porous scaffold for enhancing bone defect repair. J. Orthop. Res. 2013, 31, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Yao, D.; Zheng, L.; Liu, W.C.; Liu, Z.; Lei, M.; Huang, L.; Xie, X.; Wang, X.; Chen, Y.; et al. Phytomolecule icaritin incorporated PLGA/TCP scaffold for steroid-associated osteonecrosis: Proof-of-concept for prevention of hip joint collapse in bipedal emus and mechanistic study in quadrupedal rabbits. Biomaterials 2015, 59, 125–143. [Google Scholar] [CrossRef]
- Rajput, S.A.; Wang, X.Q.; Yan, H.C. Morin hydrate: A comprehensive review on novel natural dietary bioactive compound with versatile biological and pharmacological potential. Biomed Pharm. 2021, 138, 111511. [Google Scholar] [CrossRef]
- Ola, M.S.; Aleisa, A.M.; Al-Rejaie, S.S.; Abuohashish, H.M.; Parmar, M.Y.; Alhomida, A.S.; Ahmed, M.M. Flavonoid, morin inhibits oxidative stress, inflammation and enhances neurotrophic support in the brain of streptozotocin-induced diabetic rats. Neurol. Sci. 2014, 35, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- Hussain, J.; Ali, L.; Khan, A.L.; Rehman, N.U.; Jabeen, F.; Kim, J.S.; Al-Harrasi, A. Isolation and bioactivities of the flavonoids morin and morin-3-O-beta-D-glucopyranoside from Acridocarpus orientalis-A wild Arabian medicinal plant. Molecules 2014, 19, 17763–17772. [Google Scholar] [CrossRef]
- Abbad, S.; Wang, C.; Waddad, A.Y.; Lv, H.; Zhou, J. Preparation, in vitro and in vivo evaluation of polymeric nanoparticles based on hyaluronic acid-poly(butyl cyanoacrylate) and D-alpha-tocopheryl polyethylene glycol 1000 succinate for tumor-targeted delivery of morin hydrate. Int. J. Nanomed. 2015, 10, 305–320. [Google Scholar] [CrossRef]
- De Farias, A.L.; Meneguin, A.B.; Da Silva Barud, H.; Brighenti, F.L. The role of sodium alginate and gellan gum in the design of new drug delivery systems intended for antibiofilm activity of morin. Int. J. Biol. Macromol. 2020, 162, 1944–1958. [Google Scholar] [CrossRef]
- Sims, K.R., Jr.; He, B.; Koo, H.; Benoit, D.S.W. Electrostatic Interactions Enable Nanoparticle Delivery of the Flavonoid Myricetin. ACS Omega 2020, 5, 12649–12659. [Google Scholar] [CrossRef] [PubMed]
- Dalcin, A.J.F.; Santos, C.G.; Gundel, S.S.; Roggia, I.; Raffin, R.P.; Ourique, A.F.; Santos, R.C.V.; Gomes, P. Anti biofilm effect of dihydromyricetin-loaded nanocapsules on urinary catheter infected by Pseudomonas aeruginosa. Colloids Surf B Biointerfaces 2017, 156, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, J.J.; Tang, X.J.; Du, L.; Li, F. In vitro and in vivo evaluation of functionalized chitosan-Pluronic micelles loaded with myricetin on glioblastoma cancer. Nanomedicine 2016, 12, 1263–1278. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Shariati, M.A.; Imran, M.; Bashir, K.; Khan, S.A.; Mitra, S.; Emran, T.B.; Badalova, K.; Uddin, M.S.; Mubarak, M.S.; et al. Comprehensive review on naringenin and naringin polyphenols as a potent anticancer agent. Environ. Sci. Pollut. Res. Int. 2022, 29, 31025–31041. [Google Scholar] [CrossRef] [PubMed]
- Arafah, A.; Rehman, M.U.; Mir, T.M.; Wali, A.F.; Ali, R.; Qamar, W.; Khan, R.; Ahmad, A.; Aga, S.S.; Alqahtani, S.; et al. Multi-Therapeutic Potential of Naringenin (4’,5,7-Trihydroxyflavonone): Experimental Evidence and Mechanisms. Plants 2020, 9, 1784. [Google Scholar] [CrossRef]
- Bhandari, R.; Paliwal, J.; Kuhad, A. Naringenin and its nanocarriers as potential phytotherapy for autism spectrum disorders. J. Funct. Foods 2018, 47, 361–375. [Google Scholar] [CrossRef]
- Maity, S.; Chakraborti, A. Formulation, physico-chemical characterization and antidiabetic potential of naringenin-loaded poly D, L lactide-co-glycolide (N-PLGA) nanoparticles. Eur. Polym. J. 2020, 134, 109818. [Google Scholar] [CrossRef]
- Smruthi, M.R.; Nallamuthu, I.; Anand, T. A comparative study of optimized naringenin nanoformulations using nano-carriers (PLA/PVA and zein/pectin) for improvement of bioavailability. Food Chem. 2022, 369, 130950. [Google Scholar] [CrossRef]
- Chaurasia, S.; Patel, R.R.; Vure, P.; Mishra, B. Potential of Cationic-Polymeric Nanoparticles for Oral Delivery of Naringenin: In Vitro and In Vivo Investigations. J. Pharm. Sci. 2018, 107, 706–716. [Google Scholar] [CrossRef]
- Maity, S.; Mukhopadhyay, P.; Kundu, P.P.; Chakraborti, A.S. Alginate coated chitosan core-shell nanoparticles for efficient oral delivery of naringenin in diabetic animals-An in vitro and in vivo approach. Carbohydr. Polym. 2017, 170, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Liu, X.; Hu, W.; Bai, Y.; Zhang, L. Preparation and evaluation of naringenin-loaded sulfobutylether-beta-cyclodextrin/chitosan nanoparticles for ocular drug delivery. Carbohydr. Polym. 2016, 149, 224–230. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Yang, H.; Wang, J.; Li, Q.; Qu, R.; Wu, X. Nanocomplexes based polyvinylpyrrolidone K-17PF for ocular drug delivery of naringenin. Int. J. Pharm. 2020, 578, 119133. [Google Scholar] [CrossRef]
- Liu, W.; Zheng, W.; Cheng, L.; Li, M.; Huang, J.; Bao, S.; Xu, Q.; Ma, Z. Citrus fruits are rich in flavonoids for immunoregulation and potential targeting ACE2. Nat. Prod. Bioprospect 2022, 12, 4. [Google Scholar] [CrossRef]
- Chen, R.; Qi, Q.L.; Wang, M.T.; Li, Q.Y. Therapeutic potential of naringin: An overview. Pharm. Biol. 2016, 54, 3203–3210. [Google Scholar] [CrossRef]
- Lauro, M.R.; De Simone, F.; Sansone, F.; Iannelli, P.; Aquino, R.P. Preparations and release characteristics of naringin and naringenin gastro-resistant microparticles by spray-drying. J. Drug Deliv. Sci. Technol. 2007, 17, 119–124. [Google Scholar] [CrossRef]
- Mohanty, S.; Konkimalla, V.B.; Pal, A.; Sharma, T.; Si, S.C. Naringin as Sustained Delivery Nanoparticles Ameliorates the Anti-inflammatory Activity in a Freund’s Complete Adjuvant-Induced Arthritis Model. ACS Omega 2021, 6, 28630–28641. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Guo, Q.; Zhao, L. Overview of Oroxylin A: A Promising Flavonoid Compound. Phytother. Res. 2016, 30, 1765–1774. [Google Scholar] [CrossRef]
- Zhu, Y.; Guo, Y.; Liu, M.; Wei, L.; Wang, X. An oroxylin A-loaded aggregation-induced emission active polymeric system greatly increased the antitumor efficacy against squamous cell carcinoma. J. Mater. Chem. B 2020, 8, 2040–2047. [Google Scholar] [CrossRef] [PubMed]
- David, A.V.A.; Arulmoli, R.; Parasuraman, S. Overviews of Biological Importance of Quercetin: A Bioactive Flavonoid. Pharmacogn. Rev. 2016, 10, 84–89. [Google Scholar] [CrossRef]
- Nakhate, K.T.; Badwaik, H.; Choudhary, R.; Sakure, K.; Agrawal, Y.O.; Sharma, C.; Ojha, S.; Goyal, S.N. Therapeutic Potential and Pharmaceutical Development of a Multitargeted Flavonoid Phloretin. Nutrients 2022, 14, 3638. [Google Scholar] [CrossRef]
- Mariadoss, A.V.A.; Vinayagam, R.; Senthilkumar, V.; Paulpandi, M.; Murugan, K.; Xu, B.; Gothandam, K.M.; Kotakadi, S.; David, E. Phloretin loaded chitosan nanoparticles augments the pH-dependent mitochondrial-mediated intrinsic apoptosis in human oral cancer cells. Int. J. Biol. Macromol. 2019, 130, 997–1008. [Google Scholar] [CrossRef] [PubMed]
- Casarini, T.P.A.; Frank, L.A.; Benin, T.; Onzi, G.; Pohlmann, A.R.; Guterres, S.S. Innovative hydrogel containing polymeric nanocapsules loaded with phloretin: Enhanced skin penetration and adhesion. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 120, 111681. [Google Scholar] [CrossRef]
- Salehi, B.; Machin, L.; Monzote, L.; Sharifi-Rad, J.; Ezzat, S.; Salem, M.; Merghany, R.; El Mahdy, N.; Kılıç, C.; Sytar, O.; et al. Therapeutic Potential of Quercetin: New Insights and Perspectives for Human Health. ACS Omega 2020, 5, 11849–11872. [Google Scholar] [CrossRef]
- Pimple, S.; Manjappa, A.S.; Ukawala, M.; Murthy, R.S. PLGA nanoparticles loaded with etoposide and quercetin dihydrate individually: In vitro cell line study to ensure advantage of combination therapy. Cancer Nanotechnol. 2012, 3, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Giannouli, M.; Karagkiozaki, V.; Pappa, F.; Moutsios, I.; Gravalidis, C.; Logothetidis, S. Fabrication of quercetin-loaded PLGA nanoparticles via electrohydrodynamic atomization for cardiovascular disease. Mater. Today Proc. 2018, 5, 15998–16005. [Google Scholar] [CrossRef]
- Anwer, M.K.; Al-Mansoor, M.A.; Jamil, S.; Al-Shdefat, R.; Ansari, M.N.; Shakeel, F. Development and evaluation of PLGA polymer based nanoparticles of quercetin. Int. J. Biol. Macromol. 2016, 92, 213–219. [Google Scholar] [CrossRef]
- Sun, D.; Li, N.; Zhang, W.; Zhao, Z.; Mou, Z.; Huang, D.; Liu, J.; Wang, W. Design of PLGA-functionalized quercetin nanoparticles for potential use in Alzheimer’s disease. Colloids Surf B Biointerfaces 2016, 148, 116–129. [Google Scholar] [CrossRef]
- Fatma, S.; Talegaonkar, S.; Iqbal, Z.; Panda, A.K.; Negi, L.M.; Goswami, D.G.; Tariq, M. Novel flavonoid-based biodegradable nanoparticles for effective oral delivery of etoposide by P-glycoprotein modulation: An in vitro, ex vivo and in vivo investigations. Drug Deliv. 2016, 23, 500–511. [Google Scholar] [CrossRef]
- Wang, L.; Feng, M.; Li, Y.; Du, Y.; Wang, H.; Chen, Y.; Li, L. Fabrication of superparamagnetic nano-silica@ quercetin-encapsulated PLGA nanocomposite: Potential application for cardiovascular diseases. J. Photochem. Photobiol. B 2019, 196, 111508. [Google Scholar] [CrossRef] [PubMed]
- Dinesh, K.; Verma, P.; Singh, S. Development and evaluation of biodegradable polymeric nanoparticles for the effective delivery of quercetin using a quality by design approach. LWT—Food Sci. Technol. 2015, 61, 330–338. [Google Scholar] [CrossRef]
- Suksiriworapong, J.; Phoca, K.; Ngamsom, S.; Sripha, K.; Moongkarndi, P.; Junyaprasert, V.B. Comparison of poly(epsilon-caprolactone) chain lengths of poly(epsilon-caprolactone)-co-d-alpha-tocopheryl-poly(ethylene glycol) 1000 succinate nanoparticles for enhancement of quercetin delivery to SKBR3 breast cancer cells. Eur. J. Pharm. Biopharm. 2016, 101, 15–24. [Google Scholar] [CrossRef]
- Antonio, E.; Khalil, N.M.; Mainardes, R.M. Bovine Serum Albumin Nanoparticles Containing Quercetin: Characterization and Antioxidant Activity. J. Nanosci. Nanotechnol. 2016, 16, 1346–1353. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Kundu, J.; Verma, R.; Chowdhury, P. Albumin coated polymer nanoparticles loaded with plant extract derived quercetin for modulation of inflammation. Materialia 2020, 9, 100605. [Google Scholar] [CrossRef]
- Baksi, R.; Singh, D.P.; Borse, S.P.; Rana, R.; Sharma, V.; Nivsarkar, M. In vitro and in vivo anticancer efficacy potential of Quercetin loaded polymeric nanoparticles. Biomed Pharm. 2018, 106, 1513–1526. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Y.; Tang, K.; Hu, X.; Zou, G. Physicochemical characterization and antioxidant activity of quercetin-loaded chitosan nanoparticles. J. Appl. Polym. Sci. 2008, 107, 891–897. [Google Scholar] [CrossRef]
- Mukhopadhyay, P.; Maity, S.; Chakraborty, S.; Rudra, R.; Ghodadara, H.; Solanki, M.; Chakraborti, A.; Prajapati, A.K.; Kundu, P.P. Oral delivery of quercetin to diabetic animals using novel pH responsive carboxypropionylated chitosan/alginate microparticles. RSC Adv. 2016, 6, 73210–73221. [Google Scholar] [CrossRef]
- Du, H.; Liu, M.; Yang, X.; Zhai, G. The role of glycyrrhetinic acid modification on preparation and evaluation of quercetin-loaded chitosan-based self-aggregates. J. Colloid Interface Sci. 2015, 460, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.; Liu, W.; Guo, C.; Zhai, G. Preparation and evaluation of quercetin-loaded lecithin-chitosan nanoparticles for topical delivery. Int. J. Nanomed. 2011, 6, 1621–1630. [Google Scholar] [CrossRef]
- De Grandi, D.; Meghdadi, A.; Lutheryn, G.; Carugo, D. Facile production of quercetin nanoparticles using 3D printed centrifugal flow reactors. RSC Adv. 2022, 12, 20696–20713. [Google Scholar] [CrossRef]
- Barbarisi, M.; Iaffaioli, R.V.; Armenia, E.; Schiavo, L.; De Sena, G.; Tafuto, S.; Barbarisi, A.; Quagliariello, V. Novel nanohydrogel of hyaluronic acid loaded with quercetin alone and in combination with temozolomide as new therapeutic tool, CD44 targeted based, of glioblastoma multiforme. J. Cell. Physiol. 2018, 233, 6550–6564. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Shi, Y.; Zou, S.; Sun, M.; Lil, L.; Zhail, G. Formulation and in vitro evaluation of quercetin loaded polymeric micelles composed of pluronic P123 and D-a-tocopheryl polyethylene glycol succinate. J. Biomed. Nanotechnol. 2011, 7, 358–365. [Google Scholar] [CrossRef]
- Patra, A.; Satpathy, S.; Shenoy, A.K.; Bush, J.A.; Kazi, M.; Hussain, M.D. Formulation and evaluation of mixed polymeric micelles of quercetin for treatment of breast, ovarian, and multidrug resistant cancers. Int. J. Nanomed. 2018, 13, 2869–2881. [Google Scholar] [CrossRef]
- Gullón, B.; Lú-Chau, T.A.; Moreira, M.; Lema, J.M.; Eibes, G. Rutin: A review on extraction, identification and purification methods, biological activities and approaches to enhance its bioavailability. Trends Food Sci. Technol. 2017, 67, 220–235. [Google Scholar] [CrossRef]
- Ishak, R.A.H.; Mostafa, N.M.; Kamel, A.O. Stealth lipid polymer hybrid nanoparticles loaded with rutin for effective brain delivery—comparative study with the gold standard (Tween 80): Optimization, characterization and biodistribution. Drug Deliv. 2017, 24, 1874–1890. [Google Scholar] [CrossRef]
- Gagliardi, A.; Paolino, D.; Costa, N.; Fresta, M.; Cosco, D. Zein- vs PLGA-based nanoparticles containing rutin: A comparative investigation. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 118, 111538. [Google Scholar] [CrossRef] [PubMed]
- Lauro, M.R.; Maggi, L.; Conte, U.; De Simone, F.; Aquino, R.P. Rutin and quercetin gastro-resistant microparticles obtained by spray-drying technique. J. Drug Deliv. Sci. Technol. 2005, 15, 363–369. [Google Scholar] [CrossRef]
- Konecsni, K.; Low, N.H.; Nickerson, M.T. Chitosan-tripolyphosphate submicron particles as the carrier of entrapped rutin. Food Chem. 2012, 134, 1775–1779. [Google Scholar] [CrossRef] [PubMed]
- Zugravu, C.A.; Bohiltea, R.E.; Salmen, T.; Pogurschi, E.; Otelea, M.R. Antioxidants in Hops: Bioavailability, Health Effects and Perspectives for New Products. Antioxidants 2022, 11, 241. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.Y.; Hsieh, M.J.; Lo, Y.S.; Lin, C.C.; Chuang, Y.C.; Chen, M.K.; Chou, M.C. Xanthohumol targets the JNK1/2 signaling pathway in apoptosis of human nasopharyngeal carcinoma cells. Environ. Toxicol. 2022, 37, 1509–1520. [Google Scholar] [CrossRef]
- Li, X.; Jin, L.; Ma, Y.; Jiang, Z.; Tang, H.; Tong, X. Xanthohumol inhibits non-small cell lung cancer by activating PUMA-mediated apoptosis. Toxicology 2022, 470, 153141. [Google Scholar] [CrossRef]
- Vesaghhamedani, S.; Ebrahimzadeh, F.; Najafi, E.; Shabgah, O.G.; Askari, E.; Shabgah, A.G.; Mohammadi, H.; Jadidi-Niaragh, F.; Navashenaq, J.G. Xanthohumol: An underestimated, while potent and promising chemotherapeutic agent in cancer treatment. Prog. Biophys. Mol. Biol. 2022, 172, 3–14. [Google Scholar] [CrossRef]
- Niederau, C.; Bhargava, S.; Schneider-Kramman, R.; Jankowski, J.; Craveiro, R.B.; Wolf, M. Xanthohumol exerts anti-inflammatory effects in an in vitro model of mechanically stimulated cementoblasts. Sci. Rep. 2022, 12, 14970. [Google Scholar] [CrossRef] [PubMed]
- Lela, L.; Ponticelli, M.; Caddeo, C.; Vassallo, A.; Ostuni, A.; Sinisgalli, C.; Faraone, I.; Santoro, V.; De Tommasi, N.; Milella, L. Nanotechnological exploitation of the antioxidant potential of Humulus lupulus L. extract. Food Chem. 2022, 393, 133401. [Google Scholar] [CrossRef] [PubMed]
- Miranda, C.L.; Elias, V.D.; Hay, J.J.; Choi, J.; Reed, R.L.; Stevens, J.F. Xanthohumol improves dysfunctional glucose and lipid metabolism in diet-induced obese C57BL/6J mice. Arch. Biochem. Biophys. 2016, 599, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Bogdanova, K.; Roderova, M.; Kolar, M.; Langova, K.; Dusek, M.; Jost, P.; Kubelkova, K.; Bostik, P.; Olsovska, J. Antibiofilm activity of bioactive hop compounds humulone, lupulone and xanthohumol toward susceptible and resistant staphylococci. Res. Microbiol. 2018, 169, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Garcia, C.; Rancan, L.; Paredes, S.D.; Montero, C.; De La Fuente, M.; Vara, E.; Tresguerres, J.A.F. Xanthohumol exerts protective effects in liver alterations associated with aging. Eur. J. Nutr. 2019, 58, 653–663. [Google Scholar] [CrossRef]
- Qiao, T.; Jiang, S.; Song, P.; Song, X.; Liu, Q.; Wang, L.; Chen, X. Effect of blending HA-g-PLLA on xanthohumol-loaded PLGA fiber membrane. Colloids Surf B Biointerfaces 2016, 146, 221–227. [Google Scholar] [CrossRef]
- Sukumaran, S.T.; Sugathan, S.; Abdulhameed, S. Plant Metabolites Methods, Applications and Prospects; Springer: Singapore, 2020. [Google Scholar]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pecorini, G.; Ferraro, E.; Puppi, D. Polymeric Systems for the Controlled Release of Flavonoids. Pharmaceutics 2023, 15, 628. https://doi.org/10.3390/pharmaceutics15020628
Pecorini G, Ferraro E, Puppi D. Polymeric Systems for the Controlled Release of Flavonoids. Pharmaceutics. 2023; 15(2):628. https://doi.org/10.3390/pharmaceutics15020628
Chicago/Turabian StylePecorini, Gianni, Elisabetta Ferraro, and Dario Puppi. 2023. "Polymeric Systems for the Controlled Release of Flavonoids" Pharmaceutics 15, no. 2: 628. https://doi.org/10.3390/pharmaceutics15020628
APA StylePecorini, G., Ferraro, E., & Puppi, D. (2023). Polymeric Systems for the Controlled Release of Flavonoids. Pharmaceutics, 15(2), 628. https://doi.org/10.3390/pharmaceutics15020628







