Abstract
Treating burns remains a challenge for modern medicine, especially in developing countries that cannot afford expensive, advanced therapies. This review article summarises clinical and animal model studies of botanical preparations and their mixtures in treating burn wounds and sunburn. Articles available in electronic databases such as PubMed, Scopus, Web of Science, Science Direct and Google Scholar, published in English in 2010–2022, were considered. In the described clinical trials, it was shown that some herbal preparations have better effectiveness in treating burn wounds, including shortening the healing time and reducing inflammation, than the conventional treatment used hitherto. These herbal preparations contained extracts from Albizia julibrissin, Alkanna tinctoria, Aloe vera, Arnebia euchroma, Betula pendula and Betula pubescens, Centella asiatica, Hippophaë rhamnoides, Juglans regia, Lawsonia inermis, and mixtures of Matricaria chamomilla and Rosa canina. Research on animal models shows that many extracts may potentially benefit the treatment of burn wounds and sunburn. Due to the diverse mechanism of action, antibacterial activity, the safety of use and cost-effectiveness, herbal preparations can compete with conventional treatment. The growing interest in alternative medicine and herbal medicine encourages further research. Not only single preparations but also their mixtures should be taken into account because the research conducted so far often suggests a synergistic effect of the ingredients.
1. Introduction
According to the World Health Organisation, burn injuries affect more than 11 million people yearly. More than 180,000 people die, and approximately 95% of deaths occur in low-income and developing countries. Due to high costs, modern therapies are available almost exclusively in developed countries. Poorly designed healthcare systems and low health expenditures per capita in low-income countries correlate with higher post-injury complications, which can lead to disability and death. Therefore, treating burn wounds remains challenging, particularly as cost-effective therapy [1,2].
Burns can be defined as tissue damage due to the action, in most cases, of high temperature, electricity, chemicals, and radiation. The classification of burns is based on their depth and size. There are four degrees based on the severity of the injury. Superficial burns, also known as first-degree burns, involve only the epidermis. They are characterised by redness and discomfort, sometimes pain, but usually do not require medical intervention. Second-degree burns can be divided into two subcategories, superficial partial-thickness burns and deep partial-thickness burns. A superficial partial-thickness burn covers the epidermis and part of the dermis. It is usually manifested by the appearance of blisters filled with serous fluid. It is painful and requires wound care and dressing but usually does not cause scarring. A deep partial-thickness burn covers the epidermis and dermis. It is deeper but usually less painful due to damage to the pain receptors. It leads to scarring and sometimes requires surgical intervention. Third-degree burns, or full-thickness burns, involve the entire skin. They are usually not painful because the nerve endings are damaged. Blood vessels and subcutaneous tissue are also damaged. Treatment is long and requires surgical removal of necrotic tissue, administration of antibiotics and usually a skin graft. In addition to the skin and subcutaneous tissue, fourth-degree burns involve muscles and bones. Charring is a characteristic picture. The changes are irreversible. They usually lead to amputation of the affected limb or death of the patients [3].
Burns are characterised by high susceptibility to bacterial infections. A damaged skin barrier, easy access to nutrients in the wound environment, damage to the wound vascularisation, lack of epithelialisation of the basal epidermal tissue, or systemic disorders leading to immunosuppression facilitate the entry of pathogens [4]. Therefore, topical antimicrobial agents are still very often used in treating burns. The most commonly used topical formulation and considered the standard therapy is 1% silver sulfadiazine (SSD) cream. However, in recent years, numerous disadvantages of this preparation have been described, including delaying healing or a cytotoxic effect on various host cells [5,6]. Therefore, new preparations containing, or no silver ions are introduced to the market, mainly in ready-to-use dressings. Studies show that burn wounds treated with new dressings heal faster and are easier to use. In addition, burns treated with the new dressings are less susceptible to secondary infection than treatment with 1% SSD cream [7,8].
Superficial and partial-thickness burns are the most common in pharmaceutical practice. The ideal dressing that we would like to recommend to a patient with a burn wound should, in addition to healing properties, have the following features: infection prevention, pain relief, moisture control, exudation removal, gas exchange, low skin adhesion, mechanical stable, reducing wound necrosis, cost-effective, non-toxic, biocompatible, and biodegradable [9]. However, individual regional needs or desires and the patient’s perspective and economic constraints must also be considered [10]. The answer to the requirements could be dressings or pharmaceutical preparations containing products of natural origin [11].
This review aimed to summarize knowledge on the use of plant preparations in the treatment of burn wounds. The paper includes articles from 2010 to 2022 describing clinical trials for single herbal preparations or their mixtures and animal studies of plants or plant extracts and their mixtures.
2. Materials and Methods
Electronic databases, including PubMed, Scopus, Web of Science, Science Direct, and Google Scholar, were searched for materials for this review. Articles published in English in the years 2010–2022 were selected. Search terms were “burn” and “sunburn” in the title or abstract, and “plant extract”, “plant”, and “herbal” in the abstract and full text.
Original papers describing the effect of individual plant preparations and mixtures on treating burns and sunburn were included. The paper describes clinical trials and studies on animal models in separate sections. In addition, the results for single formulations and mixtures are described separately. The descriptions or tables provide the scientific names of individual plant species or plants included in the mixture and the plant part used. Studies on single chemical compounds isolated from plants were not taken into account.
3. Results
3.1. Clinical Trials—Single Preparations
In 2010–2022, several clinical trials were conducted to check the effectiveness of single-plant preparations in treating burns. Studies have been conducted on the Persian silk tree (Albizia julibrissin), aloe (Aloe vera), pink Arnebia (Arnebia euchroma), silver birch (Betula pendula) and downy birch (Betula pubescens), tea plant (Camelia sinensis), gotu kola (Centella asiatica), sea buckthorn (Hippophaë rhamnoides), and common walnut (Juglans regia). Studies for individual species are described below. A summary of the results is provided in Supplementary Materials in Table S1.
3.1.1. Albizia julibrissin
Albizia julibrissin from the Fabaceae family was originally found in South and East Asia, from Iran and Azerbaijan to China and Korea. In local folk medicine, the Albizia species has been used to treat melancholy, insomnia, fever, headaches and abdominal pain, diabetes, and rheumatism, but also to treat wounds, snake bites, haemorrhoids, abscesses, erysipelas, and leprosy. The main chemical components are triterpene saponins, but flavonoids, lignans, alkaloids, and phenolic glycosides are also present [12].
A prospective, randomised, double-blind clinical trial investigated the efficacy of a gel containing 5% (w/w) Albizia julibrissin extract in treating burns [13]. The extract was prepared by extracting the stem bark of Albizia julibrissin with 60% (v/v) ethanol. Forty patients with second and third-degree burns participated in the study. Patients were randomly assigned to two equal groups where 20 were treated with 5% A. julibrissin gel and the remaining 20 with 1% SSD cream. The wounds were washed once daily with a saline solution, and treatment was applied. The treatment was continued for 30 days. The study compared clinical parameters such as inflammation, pain, itching, erythema, oedema, purulent discharge, and skin discolouration. Before the beginning of the study, there were no significant differences in those parameters between patients from the two groups.
After 15 days of treatment, a statistically significant reduction in pain, inflammatory reaction and purulent discharge were observed in the group treated with 5% A. julibrissin gel compared to the group treated with 1% SSD cream. There were no significant differences between groups in itching, erythema, oedema, and skin discolouration. At the end of treatment, after 30 days, a reduction in inflammation and pain sensation was observed in the group treated with the 5% A. julibrissin gel. However, the other assessed parameters found no statistically significant differences between the groups. Treatment with 5% A. julibrissin gel shortened epithelialisation time in second and third-degree burns by 33.3 and 43.78%, respectively, compared to treatment with 1% SSD cream. The patients rated the colour, odour, and stability of 5% A. julibrissin gel worse than the 1% SSD cream.
3.1.2. Aloe vera
Aloe vera (Asphodelaceae) has a long history of medicinal use, dating back to ancient cultures such as Chinese, Egyptian, and Indian. Over the years, several studies have been conducted on its pharmacological use. Its therapeutic activity includes, among others, antibacterial, antiviral, anticancer, antioxidant, anti-inflammatory, skin protective, wound healing, and regulating blood glucose and cholesterol levels. Aloe vera is known primarily for its beneficial effects on the skin, mainly its mucous gel filling the leaves, which is used in many cosmetic and pharmaceutical preparations [14].
The effectiveness of Aloe vera cream in treating second-degree burns was tested in a randomised and controlled clinical trial [15]. The study involved 30 patients with two thermal burns of similar size and depth on two different but similar body areas (such as hands or feet). The burn must have occurred within 24 h of treatment initiation and not affected more than 40% of the total body surface area. After cleaning the wound with saline solution, a base cream containing 0.5% pure spray-dried aloe powder (Zarband Phytopharmaceuticals, Teheran, Iran) was applied to one burn. A 1% SSD cream was applied to the second burn. Dressings were changed, and the cream was applied twice a day. Treatment was continued until complete epithelialisation of the burn.
Mean times to complete healing were 15.9 ± 2 and 18.73 ± 2.65 days for a burn treated with aloe cream and 1% SSD cream, respectively. The time to complete healing was statistically significantly shorter in the case of treatment with aloe cream. Additionally, the size of the wound treated with the aloe cream was significantly smaller after 10, 13, and 16 days. After days 3, 7, and 13, no microbial contamination was observed. This clinical study showed that aloe cream might be more effective in treating burns than 1% SSD cream, significantly reducing wound healing time and surface area.
Another randomised clinical trial compared the effectiveness of 98% Aloe vera gel with that of 1% SSD cream in treating second-degree burns [16]. The study involved 50 patients with second-degree heat burns that developed within 24 hours of starting treatment and did not exceed 25% of the total body surface area. The wound was washed with pyodine scrub and saline, and then aloe gel or 1% SSD cream was applied. Dressings were changed twice a day. Treatment was continued until the wound was completely healed and re-epithelialised.
The study compared the size and area of the wound as well as the patients’ subjective perception of pain. The mean wound epithelialisation time was 11 ± 4.18 and 24.24 ± 11.16 days for the aloe gel and 1% SSD cream treated groups, respectively, and was significantly shorter for those treated with aloe vera gel. There were no differences in the infection of the wounds of both groups. In the group treated with aloe vera gel, the time to complete pain relief was 21 days and was significantly shorter than in the group treated with 1% SSD cream, which was 26 days. The study showed that aloe vera gel significantly shortens the re-epithelialisation time, alleviates pain symptoms, and is more cost-effective.
3.1.3. Arnebia euchroma
Arnebia euchroma from the Boraginaceae family occurs naturally in high mountain regions, mainly in the Himalayas and other regions of Asia and North Africa. It is a source of many promising chemical compounds from the group of naphthoquinones, mainly ester derivatives of shikonin, alkannin, and isohexenylnaphthazarin. Potential medicinal properties include wound-healing, antibacterial, antiviral, antifungal, anti-inflammatory, and anticancer effects [17].
The effectiveness of Arnebia euchroma ointment was tested in a prospective, randomised, single-blind clinical study compared to the effectiveness of 1% SSD cream [18]. To prepare the ointment, chopped dried roots of A. euchroma were heated in goat fat, cow butter, and glycerin at 95–100 °C for 30 min. The mixture was sterilised, filtered, and then Eucerin, methylparaben, and propylene paraben were added. The weight ratio of A. euchroma roots to primary materials was 10%.
The study involved 45 patients who suffered second-degree burns on two parts of their body, covering no more than 15% of their total body surface area. The burn was to appear within 24 h of starting treatment. The injured parts were randomly assigned to treatment with A. euchroma ointment (AEO) or 1% SSD cream, which was continued until complete wound healing. The wound was washed daily with saline solution, and appropriate treatment was applied. On days 1, 3, 5, 7, 10, 13, 15, 20, 25, and 30, the wound was measured, and photographs were taken prior to the application of the cream.
By the fifth day of treatment, no significant differences were observed in the size of the wound treated with AEO and 1% SSD cream. In the following days, the area of the wound treated with AEO was significantly smaller than that of the wound treated with SSD. The average wound healing time was 13.9 ± 5.3 days for AEO and 17.5 ± 6.9 days for 1% SSD cream. It was significantly lower for wounds treated with AEO. Physicians’ treatment preferences overwhelmingly favoured AEO from day 15 of treatment. The mean global assessment of wound appearance by the experienced nurse did not differ between the groups. Burning sensation and pain sensation were lower, while warming of the injury area was higher for the part treated with AEO than the part treated with 1% SSD cream. Patients’ satisfaction with AEO treatment was significantly higher than with 1% SSD cream treatment.
3.1.4. Betula pendula, Betula pubescens
The leaves of Betula pendula and Betula pubescens, species of the Betulaceae family, rich in flavonoid compounds, have been used in traditional medicine as diuretics that increase urine flow, flush the urinary tract, and prevent the development of infections [19]. However, now researchers are interested in birch bark. Betulin, the main component of birch bark extract, was first described in 1788. Only in recent years has it gained importance as a pharmaceutical ingredient. Betulin has been found to have the ability to stabilize water-in-oil emulsions but not as a surfactant. In addition, it gels oils, thanks to which it creates thixotropic gels, the durability of which is higher at body temperature than at room temperature. After receiving excellent results in toxicology and pharmaceutical safety studies, the era of clinical trials began to clarify the indications where triterpene birch bark extract could be used [20].
Oleogel-S10 (tradename Episalvan®) is a sterile gel containing 10% birch bark extract and 90% sunflower oil. Triterpene birch extract, obtained from Betula pendula, Betula pubescens and mixtures of these species, is standardised for the content of betulin (72–88%), and it also contains, among others, betulinic acid, lupeol, oleanolic acid, and erythrodiol.
An open, blindly evaluated, randomised clinical trial was conducted to test the effectiveness of Oleogel-S10 in treating superficial partial-thickness burn wounds [21]. Based on the study set out below, the European Medicines Agency decided to approve Episalvan® for treating second-degree burns. The obtained results were compared to treatment with octenidine hydrochloride gel (Octenilin® wound gel, Schülke & Mayr GmbH, Germany). Patients with one superficial second-degree burn >80 cm2 and <25% of total body surface area or two comparable burns >40 cm2 and <12.5% of total body surface area were eligible for the study. After washing the wound with octenidine hydrochloride or polyhexanide, Oleogel-S10 was applied to one wound or half of a large burn, and Octenilin® was applied to the other, both approx. 1 mm thick, and then covered with gauze. The wounds were washed and dressed every 2 days for 21 days. Finally, treatment parameters were compared in 57 patients.
The study showed that 35 patients out of 57 had differences in the time required for wound closure. Among them, 30 patients (85.7%) treated with Oleogel-S10 had earlier healing than 5 (14.3%) treated with Octenilin®. The statistical analysis showed the advantage of treatment with Oleogel-S10 over treatment with Octenilin®. The percentage of wound epithelialisation on each analysed day was significantly higher for burns treated with Oleogel-S10 compared to Octenilin®. Oleogel-S10 was rated “better” or “significantly better” than Octenilin® by 73.7% of the investigators and 71.9% of the patients. Treatment with these preparations was considered comparable by 8.8% of the investigators and 12.3% of the patients. Only 1.8% of the investigators and none of the patients considered that treatment with Octenilin® was “better” or “much better”. At the end of the treatment, the tolerability of treatment with Oleogel-S10 and Octenilin® was assessed. Oleogel-S10 was rated as “better” or “much better” by 65.6% of the investigators and 65.6% of the patients. The treatment was considered comparable by 19.7% of the investigators and 18.0% of the patients. Only 1.6% of patients and none of the investigators considered the treatment with Octenilin® “better” or “much better”.
After 3 months of continuation in which 43 patients participated, the treatment with Oleogel-S10 was superior to treatment with Octenilin®. After 12 months of follow-up with 25 patients, the same result was obtained.
3.1.5. Camellia sinensis
Camellia sinensis from the Theaceae family is a rich source of compounds from the polyphenol group, mainly catechin, epicatechin, and their derivatives. It is known primarily for their strong antioxidant and anti-inflammatory properties. Many studies have proven its beneficial effects on the skin, including photoprotection, anti-ageing, and anti-cellulite. Moreover, they have been shown to improve the condition of hair and skin and its blood supply [22].
The effectiveness of a cream with 10% water extract from green tea leaves containing 85% catechins in the treatment of burns was tested in a clinical study compared to a 1% SSD cream [23]. The study involved 50 patients who developed second-degree thermal burns to less than 5% of their total body surface area within 24 hours of starting treatment. The patients were divided equally into two groups so that each group included patients with a similar body surface area affected by the injury. The wounds were cleaned with a saline solution, a cream with green tea extract (GT) or 1% SSD cream was applied directly to the burn, and a dressing was applied. The patients and those changing the dressing and making records did not know which cream had been applied. The wounds were cleaned, and cream was applied daily. Photographs of the burned area were taken just before applying the cream. Treatment and photographic documentation continued until complete epithelisation. Treatment progress was assessed daily using the Bates-Jensen assessment tool, which includes 13 parameters rated on a 5-point Likert scale. The parameters assessed included wound size, wound depth, wound edge, undermining, necrotic tissue type, necrotic type amount, exudate type, amount of exudate, surrounding skin colour, peripheral tissue induration, peripheral tissue oedema, granulation tissue, and epithelialisation.
There were no statistically significant differences in the assessment of treatment effectiveness in the GT cream and the 1% SSD cream groups, comparing the results between 2 and 14 days. Although in the 1% SSD cream group, only 2 patients had complete epithelialisation after 8 days, and as many as 7 patients in the GT cream group, finally, after 14 days of treatment, the number and time of complete epithelialisation did not differ. Moreover, slightly better (the difference was not statistically significant) patients from the GT cream group assessed the effect of treatment on peripheral oedema, the presence of granulation tissue and epithelialisation on days 8 to 12.
3.1.6. Centella asiatica
Centella asiatica is a plant from the Apiaceae family derived from traditional Chinese medicine. The main chemical compounds responsible for its action are terpenoids, mainly asiaticoside, asiatic acid, madecassoside, and madecassic acid. The potential therapeutic effect is mainly related to the influence on the nuclear factor kappa-light-chain-enchancer of activated B cells (NF-κB), migoten-activated protein kinase (MAPK), glycogen synthase kinase 3β (GSK-3β), phosphoinositide 3-kinases/protein kinase B (PI3K/AKT), transforming growth factor β1/Smad (TGF-β1/Smad), and Janus kinases/signal transducer and activator of transcription proteins (JAK/STAT) pathways. Clinical studies have proven, among others, the effect on improving cognitive functions, alleviating anxiety, supporting wound healing or having a beneficial effect on skin care [24].
In a prospective randomised clinical trial, Centiderm® ointment and 1% SSD cream were compared in parallel to treat second-degree burns [25]. Centiderm® ointment contains the butanolic fraction of ethanolic extract (approx. 3%) from Centella asiatica leaves and is made with Vaseline and glycerine. Patients with second-degree burns on a limb that covered no more than 10% of the total body surface and occurred within 48 hours of the start of treatment were eligible for the study. Finally, 60 patients randomly assigned to two equal groups were analysed. Centiderm® ointment or 1% SSD cream was applied to the burn once a day until complete healing. On days 0, 3, 7 and 14 of the study, objective (pliability, vascularity, pigmentation, heigh, visual acuity score (VAS) and scoring according to Vancouver Scar Scale (VSS)) and subjective (dryness, itching and irritation) indices were assessed. In addition, the time needed for re-epithelialisation and complete healing was assessed.
Statistically, significantly more favourable effects of Centiderm® ointment were observed compared to 1% SSD cream from the 3rd day of treatment. Pliability, height, vascularity, VAS and VSS were rated significantly better in the group treated with Centiderm® ointment. The exception was pigmentation, for which no differences in the assessment were observed on the seventh day. However, on the 3rd and 14th day, it was assessed more favourably in the Centiderm® group. Also, according to the patients’ subjective assessment of dryness, irritation and itching, the use of Centiderm® ointment was more effective and prevailed over 1% SSD cream. The mean time to re-epithelialisation of 13.7 ± 1.48 and 20.67 ± 2.02 days for the Centiderm® group and the 1% SSD cream group, respectively, was significantly shorter for the Centiderm® group. On average, complete wound healing was 14.67 ± 1.78 days in Centiderm® versus 21.53 ± 1.65 days in 1% SSD cream, which was a statistically significant difference.
3.1.7. Hippophaë rhamnoides
Hippophaë rhamnoides is a plant of the Elaeagnaceae family that has been cultivated and harvested for its nutritional and medicinal properties since ancient times. It is used mainly due to its anti-diabetic, anti-obesity, and cardiovascular-improving properties. When used topically on the skin, its protective effect against solar radiation is emphasised [26,27].
In a randomised, triple-blind clinical trial, the effectiveness of sea buckthorn cream and 1% SSD cream in treating burns was investigated and compared [28]. The study involved 30 patients with second-degree thermal burns that affected no more than 10% of their total body surface area and occurred within 6 hours of arrival at the hospital. Patients were randomly assigned to two equal groups. The burns of the first group were treated with 1% SSD cream, and the burns of the second group with sea buckthorn cream. Sea buckthorn cream in 100 g contained 40 g of active ingredients from fresh fruits of Hippophaë rhamnoides. Once a day, after washing the wound with sterile normal saline, the cream was applied to a thickness of 3 mm. The study was completed by 27 patients in the first group and 28 in the second group.
The average wound healing time in the group treated with sea buckthorn cream was 6.7 ± 2.1 days and was statistically significantly lower than in the group treated with 1% SSD cream, which was 11.2 ± 2.3 days.
3.1.8. Juglans regia
Walnuts (Juglans regia) from the Juglandaceae family are valued primarily in Asia and Europe for their nutritional properties. They are a rich source of unsaturated fatty acids, proteins, vitamins, and minerals. Phytosterols, flavonoids, and polyphenols are also present. Due to their antioxidant, anti-inflammatory, and antibacterial properties, walnuts have been used in folk medicine to treat acne and eczema [29].
The Department of Burns and Plastic Surgery of the General Hospital of Ningxia Medical University (Ningxia, China) developed a walnut-based ointment decades ago that was successfully used to treat non-healing burn wounds. In a retrospective evaluation of cases, it was decided to compare the effectiveness of treatment with walnut ointment, conventional treatment, and surgery [29]. The study enrolled 411 patients with burn wounds covering 0.1 to 7% of the total body surface area, which were classified as non-healing. In the experimental group (49 patients), the burns were covered with a layer of 1–2 mm thick walnut ointment, prepared by crushing the nuts, heating them for 30 min, and grinding them into a paste. In 165 patients receiving conventional treatment, wounds were treated with an antimicrobial agent and recombinant human epidermal growth factor (rhEGF), 88 patients were treated with silver ion dressing + rhEGF, 42 patients were treated with Polymyxin B + rhEGF, and 35 patients were treated with Gentamicin + rhEGF. Patients qualified for the surgical group (197 people) received wound debridement and skin autograft.
The successful cure was reported for 76.60% of cases in the experimental group, 75.13% in the surgical group, and only 9.70% in the conventional treatment group. Treatment with walnut ointment was statistically as effective as surgery, and both treatments were superior to conventional treatment. The time necessary for complete wound closure was 19.87 ± 9.10 days for the experimental group and 22.71 ± 11.77 days for the surgical group, and it was significantly shorter than in the conventional treatment group, where it was 36.67 ± 10.18 days.
3.2. Clinical Trials—Mixtures of Natural Products
From 2010–2022, six clinical trials were also conducted to test the effectiveness of treating burn wounds with mixtures of plant-origin preparations. They are described below and summarised in Table S2 in Supplementary Materials.
3.2.1. Alkanna tinctoria, Olive Oil, and Beeswax
The effectiveness of a mixture of Alkanna tinctoria, beeswax and olive oil in the treatment of burn wounds was tested in a clinical study [30]. The mixture was prepared by adding 30 g of beeswax to 1000 mL of medical olive oil brought to the boiling point (200–210 °C), and then, after its complete melting, 50 g of Alkanna tinctoria (the part of the plant used was not specified) was added and heated for 5 min. Afterwards, the mixture was filtered and dispensed into bottles which were then sterilised. Dressings were prepared immediately before application by saturating a sterile sponge with the mixture.
The study ultimately compared the results of 64 patients (33 in the control group and 31 in the experimental group) with thermal burns caused, in most cases, by boiling liquids within 24 h of admission to the hospital. There were no statistically significant differences in injury characteristics at the start of the study. Dressings were changed every two days using aseptic techniques under sterile conditions. The wound was washed with normal saline and 0.1% chlorhexidine digluconate, and dressings were applied. In the experimental group, it was a dressing saturated with a previously prepared mixture, while in the control group, a standard dressing used in this hospital for burns with nitrofurazone and rifamycin was used.
The time to start re-epithelialisation was 3.0 ± 0.85 days in the experimental group and was significantly shorter than in the control group, which was 6.79 ± 1.77 days. The average pain experienced by patients was significantly lower in the experimental group (8.12 ± 1.38 points) than in the control group (9.39 ± 1.05 points). In addition, the use of treatment with a natural mixture significantly reduced the duration of hospitalisation. In the control group, it was 14.42 ± 7.79 days, and in the experimental group, it was only 8.22 ± 3.05 days.
3.2.2. Aloe vera and Centella asiatica
A randomised, prospective clinical trial compared the effectiveness of treatment of second-degree burns with a dressing containing Aloe vera and Centella asiatica and a commercial Bactigras® (Smith & Nephew, Hull, UK) dressing [31]. Thirty-five patients with second-degree burns covering at least 20% of their total body surface area were randomly divided into two groups. The experimental group was treated with a dressing impregnated with lipocolloids, 5% of Centella asiatica cream (Cosmelene®), 2.5% spray-dried powder of Aloe vera gel, and the standard group with a dressing impregnated with soft paraffin and 0.5% chlorhexidine acetate. Dressings were changed every 3 days until complete wound healing. Each time, the wound surface was measured, and the patient’s pain was assessed 30 minutes after applying a new dressing.
The time to complete healing in the group treated with dressings with herbal extracts was 18.53 ± 1.66 days and was significantly shorter than in the group treated with a standard dressing (20.06 ± 2.51 days). Moreover, the hospital patients’ stay was significantly shortened, from 22.78 ± 2.58 days in the standard group to 21.12 ± 1.83 days in the experimental group. The percentage of epithelialisation was significantly higher in the experimental group from day 15, and the mean pain the patient experienced was lower than the standard group.
There was one Pseudomonas aeruginosa infection in the experimental group on day 7. Therefore, the patient received standard treatment and dropped out of the clinical trial. No alarming symptoms or side effects of treatment with Aloe vera and Centella asiatica-impregnated dressings were observed.
3.2.3. Aloe vera, Lavandula stoechas, and Pelargonium roseum
A randomised, double-blind clinical trial investigated the effectiveness of a herbal mixture containing Aloe vera gel, Lavandula stoechas, and Pelargonium roseum essential oils in treating burns [32]. The exact composition of the preparation has not been provided. The results were compared with standard treatment with 1% SSD cream. The study enrolled 120 patients with second-degree burns that developed within 48 h of treatment and covered less than 5% of the total body surface area. The treatment consisted of daily dressing changes preceded by cleaning the wound with an antimicrobial solution and applying a cream (5 g per 10 cm2 of the injured area). Patients were assessed for pain intensity, skin dryness, and infection.
The study was completed by 111 patients. There were 56 people in the experimental group and 55 in the standard group. There were no statistically significant differences in the occurrence of dry skin between the groups at any time. Both groups had a reduction in pain compared to the first day. In the experimental group, pain intensity was significantly lower on the 7th day than in the standard group. There was only one case of infection in the experimental group that resolved during continued treatment.
3.2.4. Azadirachta indica Oil and Hypericum perforatum Oil
In a retrospective, non-controlled study, the effectiveness of a plant preparation in a spray (1 Primary Wound Dressing®; Phytoceuticals AG, Zurich, Switzerland) in treating burn wounds was checked [33]. The product contains hypericum oil (Hypericum perforatum) and neem oil (Azadirachta indica), which, when applied directly to the wound, creates a mist that provides an appropriate wound healing environment and does not adhere to the wound. The review was performed on 9 paediatric patients with 18 wounds in total. Granulation tissue formation, epithelisation, wound surface, pain sensation, and time to healing were assessed.
After a few days of using the preparation, granulation tissue formation and epithelisation were induced. The average time needed for wound healing was 16.6 ± 4.69 days. In six patients, a strong relief of pain was observed (from about 7–8 out of 10 points to 0) in the first week of using the preparation. In the remaining patients, the pain subsided within the second or third week. No adverse effects of the therapy, such as an allergic reaction or infection, were observed.
3.2.5. Lawsonia inermis and Beeswax
Originating from Iran, the herbal ointment Fundermol, which contains Lawsonia inermis and beeswax, has been used to treat severe burns. The exact composition of the preparation has not been provided. Its effectiveness was tested in a clinical study for treating second-degree burns compared to 1% SSD cream [34]. The study involved 50 patients with burns covering 1 to 10% of the total body surface, which resulted from contact with a heater or hot liquid within 6 hours of arrival at the clinic. Patients were randomly assigned to two equal groups, treated once daily with Fundermol ointment or 1% SSD cream, respectively.
The average wound healing time in the group treated with Fundermol ointment was 4.4 ± 1.87 days and was significantly shorter than in the group treated with 1% SSD cream, which was 5.9 ± 2.20 days.
3.2.6. Matricaria chamomilla, Rosa canina and Beeswax
Adibderm® ointment is a herbal preparation which includes chamomile and rose extracts, ascorbic acid, beeswax, and oleic and linoleic acids. The formulation activity was tested in a randomised clinical trial involving 60 patients with second-degree burns covering 1–10% of the total body surface area, which occurred within 2 h of admission to the emergency room [35]. Patients were randomly assigned to two groups where patients applied herbal ointment and 1% SSD cream every six hours. The average wound healing time was 7.53 ± 2.28 days in the herbal ointment group and 11.83 ± 2.32 days in the 1% SSD cream group. The difference was statistically significant.
Patients’ satisfaction with herbal treatment was significantly higher than conventionally treated patients. In the group treated with Adibderm® ointment, there were no cases of infection, but 7 patients developed irritation, while in the group treated with 1% SSD cream, there was one case of infection and no irritation.
3.3. In Vivo Studies on Animal Models of Burn
In order to better understand the physiological and pathophysiological mechanisms associated with burn injury, in vivo models are used in which animals, mainly mice, rats, guinea pigs, rabbits, hamsters, and sometimes pigs, are used. None of these could be considered better than the others. Rather, they should be considered complementary and show basic mechanisms that may not always reflect the pathology of a burn in humans. Mice and rats are the most commonly used models as they are cheap and have a high reproductive rate. However, there are many differences compared to humans, including their size, anatomy, and metabolic characteristics. It has been shown that, compared to mice or rats, guinea pig skin is anatomically and physiologically more similar to human skin. Due to, among other things, the thickness of the epidermis, the guinea pig burn model better reflects the thermal skin of a human wound [36]. The model closest to humans is pigs. However, they are very expensive to maintain, require increased care and carry a higher risk of infections [37].
Burns are usually formed on the previously hairless backs of animals and cover 5 to 30% of the total body surface area [36]. Animals are obligatorily anaesthetised with pharmacological agents such as ketamine, xylazine, diazepam, midazolam, thiopental and others, or mixtures thereof, and a burn is induced [38]. The most important animal models of burn wound formation include the gas flame burn model, burning ethanol bath burn model, pre-heated single metal plate/bar burn models, boiling or hot water burn models, and pre-heated double brass blocks burn model [36]. The assessment of burn healing is based on the time of epithelisation, wound closure, and histopathological analysis. Biochemical parameters can also be assessed, including the activity of superoxide dismutase, catalase, glutathione S-transferase, hydroxyproline content, or total protein content. Occasionally, the degree of hair regrowth in the injured area can be assessed [38].
In vivo studies in animal models of the effectiveness of preparations of plant origin in treating burn wounds in the single form are presented in Table 1, while mixtures of preparations are presented in Table 2.

Table 1.
In vivo studies on animal models—single preparations.

Table 2.
In vivo studies on animal models—a mixture of preparations.
4. Discussion
Burns significantly affect the quality of life of patients. Although they are common worldwide, they are a significant problem in developing countries, mainly because health care in every country cannot provide access to the latest, most effective therapies. Treatment, for example using only creams with antibacterial agents such as 1% silver sulfadiazine cream, can prolong healing time, lead to complications, and increase antibiotic resistance. Therefore, the search for new, safe, effective, and cost-effective preparations supporting burn healing is ongoing [2,107]. Burn injury affects the patient’s physical health, quality of life, and mental health. Therefore, the challenge is not only the wound treatment itself, which should be effective, but also possible in the patient and long-term care and rehabilitation in more serious cases [3].
Burn is accompanied by an inflammatory and immune reaction, metabolic changes, and distributive shock, especially in the case of severe burns. These symptoms can be difficult to manage, leading to multiple organ failure. An important factor in assessing the treatment needs of a burn injury patient is the wound’s depth [108]. In severe burns, highly deregulated inflammation develops, characterised by the release of inflammatory cytokines, chemokines, and acute phase proteins. During this violent phase, the immune system is stimulated and reacts inadequately to stimuli. Therefore, the anti-inflammatory effect of preparations on burn wounds is highly recommended [109,110]. The inflammatory phase is followed by the proliferation phase, during which primarily keratinocytes and fibroblasts are stimulated to rebuild tissue and vessels. In the final phase of healing, the wound remodels. Collagen and elastin are deposited, and fibroblasts are transformed into myofibroblasts. In the case of incorrect and fibrous location of collagen fibres and imbalance in the re-epithelialisation process, scar formation may occur [3,111].
Although several therapeutic activities have been demonstrated for plant-derived products, which are also beneficial in the treatment of burn wounds and sunburn, their preparation is associated with certain limitations. The chemical composition of plants and extracts made from them may be subject to certain deviations due to many factors. The place and time of harvesting, the level of insolation, the geographical altitude, the method of drying and fragmentation are some of the factors that have a significant impact on the chemical composition of the plant material [112,113]. Then, the method of extraction, selection of solvents, the ratio of plant material to solvent, time, and temperature of extraction, as well as the method of its drying and purification affect the composition of the extract. Therefore, it is necessary to introduce certain procedures for standardisation and assessment of the chemical composition when working with plant material [114].
The next step that has a significant impact on the activity is the appropriate formulation of the finished product that will achieve the intended effect in the biological system. Most of the formulations presented in this review are traditional products, such as ointments and creams, containing previously prepared extracts. However, in recent decades, the approach to wound care and dressing has completely changed. Patients more and more often use ready-made dressings, e.g., polymer or hydrogel, which contain incorporated active substances released in a controlled manner. This method of the formulation is also increasingly used for herbal products [115,116]. Another new way of preparing natural preparations is nanoformulation. The development of methods using nanotechnology can support the effectiveness of herbal products. In the treatment of dermatological diseases, various novel drug delivery systems can be used, which contain compounds or extracts of interest in the form of, among others, nanoparticles, liposomes, nanoparticle polymers, nanohydrogels, and nanofibres. Some studies have confirmed that they may be more effective than conventional systems [117,118]. Plant extracts prepared in the form of nanoparticles are characterised by higher bioavailability and penetration of biological membranes, as well as a controlled release at the target site [119]. The role of natural products in different stages of wound healing is presented in Figure 1. It can be assumed that soon, natural compounds used in the form of nanopreparations will be the basis for the creation of new pharmaceutical drugs. However, it is necessary to conduct clinical trials that would verify these theses. In addition, studies are needed to compare the effectiveness compared to standard treatment, as well as to check whether isolated chemical compounds or extracts work better in specific clinical situations.
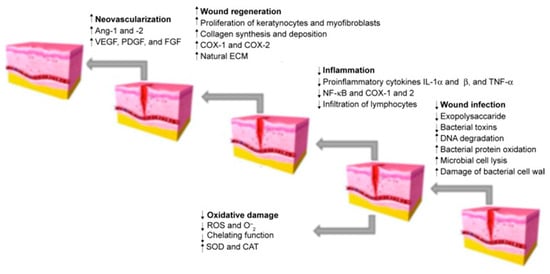
Figure 1.
Stages of wound healing. Adapted from Hajialyani, M.; Tewari, D.; Sobarzo-Sánchez, E.; Nabavi, S.M.; Farzaei, M.H.; Abdollahi, M. Natural Product-Based Nanomedicines for Wound Healing Purposes: Therapeutic Targets and Drug Delivery Systems [119].
This review focuses on plant extracts and their mixtures that have been tested in animal models and clinical trials. Extracts, as mixtures of chemical compounds, can show different directions of action affecting the various stages of wound healing (Figure 1). In the inflammation phase, compounds with antibacterial, antioxidant, and anti-inflammatory effects will be particularly important. These include mainly polyphenols and flavonoid compounds, as well as essential oils [120,121]. They may have an antibacterial effect, as well as modulate the inflammatory response by regulating the secretion of cytokines and chemokines, such as interleukin 1β or interleukin 8, or tumour necrosis factor α (TNF-α). Studies on the effect of extracts and chemical compounds on the inflammatory phase are quite popular and there are more and more publications describing this issue [122]. However, less is known about the impact of individual chemical compounds on the proliferation and remodelling phase of wound healing. It is assumed that compounds from the groups of alkaloids, tannins, flavonoids and terpenes are of the greatest importance [11,120]. Important for the proliferative phase will be compounds that affect the formation of the extracellular matrix and stimulate re-epithelialisation, angiogenesis, or the formation of granulation tissue through, among others, stimulating cell proliferation and increasing the expression of proteins such as transforming growth factor β (TGF-β) or vascular endothelial growth factor (VEGF). Finally, in the remodelling phase, chemicals that affect, for example, stimulation of collagen deposition and elastin fibres [11,123] will be necessary. Thanks to the diverse mechanisms of action, potential antibacterial effect and safety of use, natural preparations compete with conventional treatment, all the more that the public’s interest in traditional medicine and herbal medicine is growing [124]. The chemical structures of the compounds involved in the wound-healing process are presented in Figure 2.
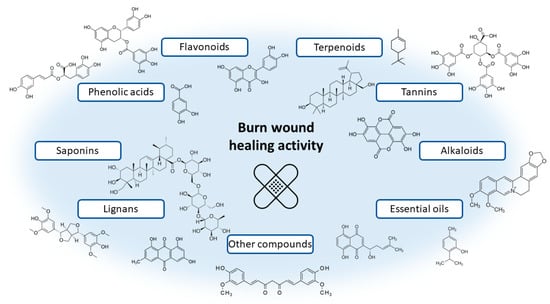
Figure 2.
Examples of chemical structures of naturally occurring compounds involved in the wound healing process.
The results of clinical trials show that herbal preparations may work as well or better than conventional drugs. Preparations containing extracts from Albizia julibrissin, Aloe vera, Arnebia euchroma, Centella asiatica, Hippophaë rhamnoides, Lawsonia inermis, and the mixture of Matricaria chamomilla and Rosa canina work significantly better than 1% silver sulfadiazine cream, which was considered the “gold standard” of burn treatment until recently. Preparations containing extracts from Betula, Juglans regia, Alkanna tinctoria, Aloe vera, and Centella asiatica have shown in clinical trials an advantage over other conventional drugs, mainly containing antimicrobial agents. Considering the above results, the possibility of using herbal preparations, such as the marketed drug Episalvan® containing birch bark extract, should be considered to be on par with standard drugs. Additionally,, in many studies of herbal formulations in animal models listed in Table 1 and Table 2, their superiority over treatment with antimicrobial agents, mainly 1% silver sulfadiazine cream, was demonstrated. Therefore, it is important to continue searching for new plant substances that potentially benefit burn wound healing. First, it is necessary to conduct clinical trials for promising formulations. Such research can contribute to introducing new drugs with documented beneficial effects that are safer and more affordable and would be available to all patients worldwide. In addition, considering the results in Table 2 and Table S2 (Supplementary Materials), the benefits of combining several plant substances in preparations should be considered. Many studies suggest a synergistic effect and may be more beneficial in treating burn wounds.
Scientific, clinical, and animal studies should be well-planned. In some of the studies presented above, a positive control group treated with a standard preparation with known therapeutic activity was not designed. That makes it difficult to interpret the results and unambiguously assess the tested preparation’s effectiveness. In addition, the tests performed should be described in detail. The lack of information about the research model, such as the conditions of creating a burn wound, make it impossible to reproduce the study in another centre, for example, to compare the results. In the case of market preparations, some authors did not provide information on their exact composition and manufacturer. Any deficiencies were noted in the individual studies in the tables. Appropriate interpretation of the obtained results and a correctly performed statistical analysis is essential. Some of the studies lacked statistical comparisons between the individual study groups, which made it difficult to analyse the results.
Researchers studying preparations of natural origin should pay particular attention to the accurate representation of the plant material under study. The chemical composition of plants changes depending on many factors, such as geographical altitude or insolation in the natural site [113]. Changes in chemical composition determine changes in therapeutic activity, which is why it is important to standardize the obtained extracts. Not all authors paid sufficient attention to describe and characterize the tested products adequately. Some studies lacked basic information, such as the final concentration or the amount of preparation applied to the burn.
What is more, sometimes the authors did not provide the method of preparation and did not even specify the part of the plant used. Such errors and oversights lead to a loss of credibility and trust among other scientists and doctors [125]. In conclusion, new research is needed for promising herbal products, but they should be carried out following all guidelines for this type of research.
5. Conclusions
Due to the diverse mechanism of action, antibacterial activity, and safety, herbal preparations compete with conventional treatment in treating burns and sunburn. The growing interest in alternative therapies and herbal medicine is also generating demand for such products. However, there is still a lack of clinical trials that would check the effectiveness of preparations showing beneficial effects on animal models of burns. Creating an ideal dressing for burn wounds that could replace the common use of antibacterial agents is a challenge for modern medicine, and the research presented in this review suggests that formulations based on herbal products are a strong competition for synthetic compounds.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pharmaceutics15020633/s1, Table S1: Summary of clinical trials—single preparations; Table S2: Summary of clinical trials—mixtures of preparations.
Author Contributions
Conceptualisation, W.S.; methodology, W.S.; investigation, W.S.; resources, W.S.; data curation, W.S.; writing—original draft preparation, W.S.; writing—review and editing, A.B.; visualisation, A.B. and W.S.; supervision, A.B.; project administration, A.B.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Markiewicz-Gospodarek, A.; Kozioł, M.; Tobiasz, M.; Baj, J.; Radzikowska-Büchner, E.; Przekora, A. Burn Wound Healing: Clinical Complications, Medical Care, Treatment, and Dressing Types: The Current State of Knowledge for Clinical Practice. Int. J. Environ. Res. Public Health 2022, 19, 1338. [Google Scholar] [CrossRef] [PubMed]
- Yakupu, A.; Zhang, J.; Dong, W.; Song, F.; Dong, J.; Lu, S. The Epidemiological Characteristic and Trends of Burns Globally. BMC Public Health 2022, 22, 1596. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, M.G.; van Baar, M.E.; Choudhry, M.A.; Chung, K.K.; Gibran, N.S.; Logsetty, S. Burn Injury. Nat. Rev. Dis. Prim. 2020, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.; Kharkwal, G.B.; Tanaka, M.; Huang, Y.-Y.; Bil de Arce, V.J.; Hamblin, M.R. Animal Models of External Traumatic Wound Infections. Virulence 2011, 2, 296–315. [Google Scholar] [CrossRef] [PubMed]
- Atiyeh, B.S.; Costagliola, M.; Hayek, S.N.; Dibo, S.A. Effect of Silver on Burn Wound Infection Control and Healing: Review of the Literature. Burns 2007, 33, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Aziz, Z.; Abu, S.F.; Chong, N.J. A Systematic Review of Silver-Containing Dressings and Topical Silver Agents (Used with Dressings) for Burn Wounds. Burns 2012, 38, 307–318. [Google Scholar] [CrossRef]
- Heyneman, A.; Hoeksema, H.; Vandekerckhove, D.; Pirayesh, A.; Monstrey, S. The Role of Silver Sulphadiazine in the Conservative Treatment of Partial Thickness Burn Wounds: A Systematic Review. Burns 2016, 42, 1377–1386. [Google Scholar] [CrossRef]
- Nímia, H.H.; Carvalho, V.F.; Isaac, C.; Souza, F.Á.; Gemperli, R.; Paggiaro, A.O. Comparative Study of Silver Sulfadiazine with Other Materials for Healing and Infection Prevention in Burns: A Systematic Review and Meta-Analysis. Burns 2019, 45, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Rezvani Ghomi, E.; Khalili, S.; Nouri Khorasani, S.; Esmaeely Neisiany, R.; Ramakrishna, S. Wound Dressings: Current Advances and Future Directions. J. Appl. Polym. Sci. 2019, 136, 47738. [Google Scholar] [CrossRef]
- Opriessnig, E.; Luze, H.; Smolle, C.; Draschl, A.; Zrim, R.; Giretzlehner, M.; Kamolz, L.-P.; Nischwitz, S.P. Epidemiology of Burn Injury and the Ideal Dressing in Global Burn Care—Regional Differences Explored. Burns 2022, 49, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Yazarlu, O.; Iranshahi, M.; Kashani, H.R.K.; Reshadat, S.; Habtemariam, S.; Iranshahy, M.; Hasanpour, M. Perspective on the Application of Medicinal Plants and Natural Products in Wound Healing: A Mechanistic Review. Pharmacol. Res. 2021, 174, 105841. [Google Scholar] [CrossRef]
- He, Y.; Wang, Q.; Ye, Y.; Liu, Z.; Sun, H. The Ethnopharmacology, Phytochemistry, Pharmacology and Toxicology of Genus Albizia: A Review. J. Ethnopharmacol. 2020, 257, 112677. [Google Scholar] [CrossRef] [PubMed]
- Asgarirad, H.; Chabra, A.; Rahimnejad, M.; Zaghi Hosseinzadeh, A.; Davoodi, A.; Azadbakht, M. Comparison of Albizia Julibressin and Silver Sulfadiazine in Healing of Second and Third Degree Burns. World J. Plast. Surg. 2018, 7, 34–44. [Google Scholar]
- Gao, Y.; Kuok, K.I.; Jin, Y.; Wang, R. Biomedical Applications of Aloe Vera. Crit. Rev. Food Sci. Nutr. 2019, 59, S244–S256. [Google Scholar] [CrossRef] [PubMed]
- Khorasani, G.; Hosseinimehr, S.J.; Azadbakht, M.; Zamani, A.; Mahdavi, M.R. Aloe versus Silver Sulfadiazine Creams for Second-Degree Burns: A Randomized Controlled Study. Surg. Today 2009, 39, 587–591. [Google Scholar] [CrossRef]
- Shahzad, M.N.; Ahmed, N. Effectiveness of Aloe Vera Gel Compared with 1% Silver Sulphadiazine Cream as Burn Wound Dressing in Second Degree Burns. J. Pak. Med. Assoc. 2010, 63, 225–230. [Google Scholar]
- Ashkani-Esfahani, S.; Imanieh, M.H.; Khoshneviszadeh, M.; Meshksar, A.; Noorafshan, A.; Geramizadeh, B.; Ebrahimi, S.; Handjani, F.; Tanideh, N. The Healing Effect of Arnebia Euchroma in Second Degree Burn Wounds in Rat as an Animal Model. Iran. Red Crescent Med. J. 2012, 14, 70–74. [Google Scholar]
- Nasiri, E.; Hosseinimehr, S.J.; Zaghi Hosseinzadeh, A.; Azadbakht, M.; Akbari, J.; Azadbakht, M. The Effects of Arnebia Euchroma Ointment on Second-Degree Burn Wounds: A Randomized Clinical Trial. J. Ethnopharmacol. 2016, 189, 107–116. [Google Scholar] [CrossRef]
- European Medicines Agency. European Union Herbal Monograph on Betula Pendula Roth and/or Betula Pubescens Ehrh. as Well as Hybrids of Both Species, Folium; European Medicines Agency: London, UK, 2014; Volume 44.
- Scheffler, A. The Wound Healing Properties of Betulin from Birch Bark from Bench to Bedside. Planta Med. 2019, 85, 524–527. [Google Scholar] [CrossRef]
- Frew, Q.; Rennekampff, H.-O.; Dziewulski, P.; Moiemen, N.; Zahn, T.; Hartmann, B. Betulin Wound Gel Accelerated Healing of Superficial Partial Thickness Burns: Results of a Randomized, Intra-individually Controlled, Phase III Trial with 12-months Follow-up. Burns 2019, 45, 876–890. [Google Scholar] [CrossRef]
- Koch, W.; Zagórska, J.; Marzec, Z.; Kukula-Koch, W. Kukula-Koch Applications of Tea (Camellia Sinensis) and Its Active Constituents in Cosmetics. Molecules 2019, 24, 4277. [Google Scholar] [CrossRef]
- Pipelzadeh, M.; Siahpoosh, A.; Sheikhi, A.R.; Jafarzadeh, E. Effectiveness of Green Tea Cream in Comparison with Silver Sulfadiazine Cream in the Treatment of Second Degree Burn in Human Subjects. J. Herb. Med. 2022, 32, 100533. [Google Scholar] [CrossRef]
- Sun, B.; Wu, L.; Wu, Y.; Zhang, C.; Qin, L.; Hayashi, M.; Kudo, M.; Gao, M.; Liu, T. Therapeutic Potential of Centella Asiatica and Its Triterpenes: A Review. Front. Pharmacol. 2020, 11, 568032. [Google Scholar] [CrossRef]
- Saeidinia, A.; Keihanian, F.; Lashkari, A.P.; Lahiji, H.G.; Mobayyen, M.; Heidarzade, A.; Golchai, J. Partial-Thickness Burn Wounds Healing by Topical Treatment. Medicine 2017, 96, e6168. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Xu, Z.; Liao, X. Bioactive Compounds, Health Benefits and Functional Food Products of Sea Buckthorn: A Review. Crit. Rev. Food Sci. Nutr. 2022, 62, 6761–6782. [Google Scholar] [CrossRef]
- Żuchowski, J. Phytochemistry and Pharmacology of Sea Buckthorn (Elaeagnus Rhamnoides; Syn. Hippophae Rhamnoides): Progress from 2010 to 2021. Phytochem. Rev. 2022, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Abdullahzadeh, M.; Shafiee, S. To Compare the Effect of Sea Buckthorn and Silver Sulfadiazine Dressing on Period of Wound Healing in Patients with Second-degree Burns: A Randomized Triple-blind Clinical Trial. Wound Repair Regen. 2021, 29, 732–740. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Deng, X.; Qiang, L.; Yao, M.; Guan, L.; Xie, N.; Zhao, D.; Ma, J.; Ma, L.; Wu, Y.; et al. Investigating the Effects of Walnut Ointment on Non-Healing Burn Wounds. Burns 2021, 47, 455–465. [Google Scholar] [CrossRef]
- Gümüş, K.; Özlü, Z.K. The Effect of a Beeswax, Olive Oil and Alkanna Tinctoria (L.) Tausch Mixture on Burn Injuries: An Experimental Study with a Control Group. Complement. Ther. Med. 2017, 34, 66–73. [Google Scholar] [CrossRef]
- Muangman, P.; Praditsuktavorn, B.; Chinaroonchai, K.; Chuntrasakul, C. Clinical Efficacy Test of Polyester Containing Herbal Extract Dressings in Burn Wound Healing. Int. J. Low. Extrem. Wounds 2016, 15, 203–212. [Google Scholar] [CrossRef]
- Panahi, Y.; Beiraghdar, F.; Akbari, H.; Bekhradi, H.; Taghizadeh, M.; Sahebkar, A. A Herbal Cream Consisting of Aloe Vera, Lavandulastoechas, and Pelargonium Roseum as an Alternative for Silver Sulfadiazine in Burn Management. Asian Biomed. 2012, 6, 273–278. [Google Scholar] [CrossRef]
- Mainetti, S.; Carnevali, F. An Experience with Paediatric Burn Wounds Treated with a Plant-Derived Wound Therapeutic. J. Wound Care 2013, 22, 681–689. [Google Scholar] [CrossRef]
- Daryabeigi, R.; Heidari, M.; Hosseini, S.A.; Omranifar, M. Comparison of Healing Time of the 2 Degree Burn Wounds with Two Dressing Methods of Fundermol Herbal Ointment and 1% Silver Sulfadiazine Cream. Iran. J. Nurs. Midwifery Res. 2010, 15, 97–101. [Google Scholar]
- Ala, A.; Ebrahimi Bakhtavar, H.; Shams Vahdati, S.; Rahmani, F.; Azargoun, M.; Ebrahimi Bakhtavar, H. Effects of Silver Sulfadiazine and Adibderm® Herbal Ointments in Treatment of Patients with Second Degree Burns: A Randomized Clinical Trial. Trauma Mon. 2018, 23. [Google Scholar] [CrossRef]
- Mofazzal Jahromi, M.A.; Sahandi Zangabad, P.; Moosavi Basri, S.M.; Sahandi Zangabad, K.; Ghamarypour, A.; Aref, A.R.; Karimi, M.; Hamblin, M.R. Nanomedicine and Advanced Technologies for Burns: Preventing Infection and Facilitating Wound Healing. Adv. Drug Deliv. Rev. 2018, 123, 33–64. [Google Scholar] [CrossRef] [PubMed]
- Abdullahi, A.; Amini-Nik, S.; Jeschke, M.G. Animal Models in Burn Research. Cell. Mol. Life Sci. 2014, 71, 3241–3255. [Google Scholar] [CrossRef]
- Prakash, J.A.; Saad, M.; Mohan, M. A Review on Burn and Burn Models in Animals. J. Basic Pharmacol. Toxicol. 2017, 1, 1–8. [Google Scholar]
- Ghosh, D.; Mondal, S.; Ramakrishna, K. A Topical Ointment Formulation Containing Leaves Extract of Aegialitis Rotundifolia Roxb., Accelerates Excision, Incision and Burn Wound Healing in Rats. Wound Med. 2019, 26, 100168. [Google Scholar] [CrossRef]
- Jalali, F.S.S.; Tajik, H.; Hadian, M. Efficacy of Topical Application of Alcoholic Extract of Yarrow in the Healing Process of Experimental Burn Wounds in Rabbit. Comp. Clin. Path. 2012, 21, 177–181. [Google Scholar] [CrossRef]
- Barua, C.C.; Talukdar, A.; Begum, S.A.; Pathak, D.C.; Sarma, D.K.; Borah, R.S.; Gupta, A. In Vivo Wound-Healing Efficacy and Antioxidant Activity of Achyranthes Aspera in Experimental Burns. Pharm. Biol. 2012, 50, 892–899. [Google Scholar] [CrossRef]
- Mohajeri, G.; Masoudpour, H.; Heidarpour, M.; Khademi, E.F.; Ghafghazi, S.; Adibi, S.; Akbari, M. The Effect of Dressing with Fresh Kiwifruit on Burn Wound Healing. Surgery 2010, 148, 963–968. [Google Scholar] [CrossRef]
- Hafezi, F.; Rad, H.E.; Naghibzadeh, B.; Nouhi, A.; Naghibzadeh, G. Actinidia Deliciosa (Kiwifruit), a New Drug for Enzymatic Debridement of Acute Burn Wounds. Burns 2010, 36, 352–355. [Google Scholar] [CrossRef]
- Guevara-Vásquez, A.M.; Marín-Tello, C. Wound Healing Activity of Allium Cepa L. Bulbs in a Second-Degree Burn Wound Model in Holtzman Rats. Vitae 2021, 28. [Google Scholar] [CrossRef]
- Hosseinimehr, S.J.; Khorasani, G.; Azadbakht, M.; Zamani, P.; Ghasemi, M.; Ahmadi, A. Effect of Aloe Cream versus Silver Sulfadiazine for Healing Burn Wounds in Rats. Acta Dermatovenerol. Croat. 2010, 18, 2–7. [Google Scholar] [PubMed]
- Rahman, M.S.; Islam, R.; Rana, M.M.; Spitzhorn, L.-S.; Rahman, M.S.; Adjaye, J.; Asaduzzaman, S.M. Characterization of Burn Wound Healing Gel Prepared from Human Amniotic Membrane and Aloe Vera Extract. BMC Complement. Altern. Med. 2019, 19, 115. [Google Scholar] [CrossRef] [PubMed]
- Moayeri, A.; Ramz, K.; Karimi, E.; Azizi, M.; Abbasi, N.; Aidy, A.; Bahmani, M. Therapeutic Effects of Aloe Vera (L.) Burm.f. and Vitis Vinifera L. Combination Cream on Wound Healing in Second-Degree Burn Model in Rats: Quantification of Compounds and VEGF & TGFβ Gene Expression. Tradit. Integr. Med. 2022, 7, 52–63. [Google Scholar] [CrossRef]
- Yuniarti, W.M.; Lukiswanto, B.S. Effects of Herbal Ointment Containing the Leaf Extracts of Madeira Vine (Anredera Cordifolia (Ten.) Steenis) for Burn Wound Healing Process on Albino Rats. Vet. World 2017, 10, 808–813. [Google Scholar] [CrossRef] [PubMed]
- Ashkani-Esfahani, S.; Imanieh, M.H.; Meshksar, A.; Khoshneviszadeh, M.; Noorafshan, A.; Geramizadeh, B.; Ebrahimi, S.; Handjani, F.; Nadimi, E.; Seyed Jafari, S.M. Enhancement of Fibroblast Proliferation, Vascularization and Collagen Synthesis in the Healing Process of Third-Degree Burn Wounds by Topical Arnebia Euchroma, a Herbal Medicine. Galen Med. J. 2013, 1, 53–59. [Google Scholar] [CrossRef]
- Kakade, A.S. Evaluation of Wound Healing Activity of Polyherbal Gel Formulation. World J. Pharm. Res. 2017, 6, 501–509. [Google Scholar] [CrossRef]
- Ananth, K.V.; Asad, M.; Kumar, N.P.; Asdaq, S.M.B.; Rao, G.S. Evaluation of Wound Healing Potential of Bauhinia Purpurea Leaf Extracts in Rats. Indian J. Pharm. Sci. 2010, 72, 122–127. [Google Scholar] [PubMed]
- Hassanzadeh, G.; Hajmanouchehri, F.; Roi, A.B.; Hassanzadeh, N.; Shafigh, N.; Barzroudipour, M.; Baazm, M.; Choobineh, H. Comparing Effects of Silver Sulfadiazine, Sucralfate and Brassica Oleracea Extract on Burn Wound Healing. Life Sci. J. 2013, 10, 104–113. [Google Scholar]
- Karimi, M.; Parsaei, P.; Asadi, S.Y.; Ezzati, S.; Boroujeni, R.K.; Zamiri, A.; Rafieian-Kopaei, M. Effects of Camellia Sinensis Ethanolic Extract on Histometric and Histopathological Healing Process of Burn Wound in Rat. Middle East J. Sci. Res. 2013, 13, 14–19. [Google Scholar] [CrossRef]
- Fatemi, M.J.; Nikoomaram, B.; Rahimi, A.A.K.; Talayi, D.; Taghavi, S.; Ghavami, Y. Effect of Green Tea on the Second Degree Burn Wounds in Rats. Indian J. Plast. Surg. 2014, 47, 370–374. [Google Scholar] [CrossRef]
- Sanwal, R.; Chaudhary, A.K. Wound Healing and Antimicrobial Potential of Carissa Spinarum Linn. in Albino Mice. J. Ethnopharmacol. 2011, 135, 792–796. [Google Scholar] [CrossRef]
- Mayefis, D. Burn Wound Healing Activity of the Combination of Centella Asiatica Extract and Papaya Latex on Male White Mice. Int. J. Res. Pharm. Pharm. Sci. 2016, 1, 7–12. [Google Scholar]
- Somboonwong, J.; Kankaisre, M.; Tantisira, B.; Tantisira, M.H. Wound Healing Activities of Different Extracts of Centella Asiatica in Incision and Burn Wound Models: An Experimental Animal Study. BMC Complement. Altern. Med. 2012, 12, 103. [Google Scholar] [CrossRef] [PubMed]
- Tran, G.-B.; Le, N.-T.T.; Dam, S.-M. Potential Use of Essential Oil Isolated from Cleistocalyx Operculatus Leaves as a Topical Dermatological Agent for Treatment of Burn Wound. Dermatol. Res. Pract. 2018, 2018, 2730169. [Google Scholar] [CrossRef]
- Becker, G.; Brusco, I.; Casoti, R.; Marchiori, M.C.L.; Cruz, L.; Trevisan, G.; Oliveira, S.M. Copaiba Oleoresin Has Topical Antinociceptive Activity in a UVB Radiation-Induced Skin-Burn Model in Mice. J. Ethnopharmacol. 2020, 250, 112476. [Google Scholar] [CrossRef] [PubMed]
- Alemzadeh, E.; Oryan, A. Effectiveness of a Crocus Sativus Extract on Burn Wounds in Rats. Planta Med. 2018, 84, 1191–1200. [Google Scholar] [CrossRef]
- Bahramsoltani, R.; Farzaei, M.H.; Abdolghaffari, A.H.; Rahimi, R.; Samadi, N.; Heidari, M.; Esfandyari, M.; Baeeri, M.; Hassanzadeh, G.; Abdollahi, M.; et al. Evaluation of Phytochemicals, Antioxidant and Burn Wound Healing Activities of Cucurbita Moschata Duchesne Fruit Peel. Iran. J. Basic Med. Sci. 2017, 20, 799–806. [Google Scholar] [CrossRef]
- Tabatabaei, S.M.; Mohebi Far, A.; Saremi, Z.; Zandi, M.; Aghaei, S.; Gohari, M.; Asadollahi, H. The Effect of Sesame Oil and Cucurbita on Healing Wounds Caused by Third-Degree Burn. J. Ski. Stem Cell 2017, 4, e68333. [Google Scholar] [CrossRef]
- Kittana, N.; Abu-Rass, H.; Sabra, R.; Manasra, L.; Hanany, H.; Jaradat, N.; Hussein, F.; Zaid, A.N. Topical Aqueous Extract of Ephedra Alata Can Improve Wound Healing in an Animal Model. Chin. J. Traumatol. 2017, 20, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Ghlissi, Z.; Kallel, R.; Sila, A.; Harrabi, B.; Atheymen, R.; Zeghal, K.; Bougatef, A.; Sahnoun, Z. Globularia Alypum Methanolic Extract Improves Burn Wound Healing Process and Inflammation in Rats and Possesses Antibacterial and Antioxidant Activities. Biomed. Pharmacother. 2016, 84, 1488–1495. [Google Scholar] [CrossRef] [PubMed]
- Tanideh, N.; Rokhsari, P.; Mehrabani, D.; Mohammadi Samani, S.; Sabet Sarvestani, F.; Ashraf, M.J.; Koohi Hosseinabadi, O.; Shamsian, S.; Ahmadi, N. The Healing Effect of Licorice on Pseudomonas Aeruginosa Infected Burn Wounds in Experimental Rat Model. World J. Plast. Surg. 2014, 3, 99–106. [Google Scholar] [PubMed]
- Javanmardi, S.; Safari, I.; Aghaz, F.; Khazaei, M. Wound Healing Activities of Gundelia Tournefortii L Extract and Milk-Cream Ointment on Second-Degree Burns of Rat Skin. Int. J. Low. Extrem. Wounds 2021, 20, 272–281. [Google Scholar] [CrossRef]
- Upadhyay, N.K.; Kumar, R.; Siddiqui, M.S.; Gupta, A. Mechanism of Wound-Healing Activity of Hippophae Rhamnoides L. Leaf Extract in Experimental Burns. Evid.-Based Complement. Altern. Med. 2011, 2011, 659705. [Google Scholar] [CrossRef]
- Ito, H.; Asmussen, S.; Traber, D.L.; Cox, R.A.; Hawkins, H.K.; Connelly, R.; Traber, L.D.; Walker, T.W.; Malgerud, E.; Sakurai, H.; et al. Healing Efficacy of Sea Buckthorn (Hippophae Rhamnoides L.) Seed Oil in an Ovine Burn Wound Model. Burns 2014, 40, 511–519. [Google Scholar] [CrossRef]
- Priyadarshi, A.; Keshri, G.K.; Gupta, A. Hippophae Rhamnoides L. Leaf Extract Diminishes Oxidative Stress, Inflammation and Ameliorates Bioenergetic Activation in Full-Thickness Burn Wound Healing. Phytomedicine Plus 2022, 2, 100292. [Google Scholar] [CrossRef]
- Seyhan, N. Evaluation of the Healing Effects of Hypericum Perforatum and Curcumin on Burn Wounds in Rats. Evid.-Based Complement. Altern. Med. 2020, 2020, 6462956. [Google Scholar] [CrossRef]
- Beroual, K.; Agabou, A.; Abdeldjelil, M.-C.; Boutaghane, N.; Haouam, S.; Hamdi-Pacha, Y. Evaluation of Crude Flaxseed (Linum Usitatissimum L.) Oil in Burn Wound Healing in New Zealand Rabbits. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 280–286. [Google Scholar] [CrossRef]
- Samdavid Thanapaul, R.J.R.; Ranjan, A.; Manikandan, S.K.; Nadar, M.S.A.M. Efficacy of Lobelia Alsinoides Lam Ethanolic Extract on a Third-degree Burn: An Experimental Study on Rats. Dermatol. Ther. 2020, 33, e14242. [Google Scholar] [CrossRef]
- Nasiri, E.; Hosseinimehr, S.J.; Azadbakht, M.; Akbari, J.; Enayati-Fard, R.; Azizi, S. Effect of Malva Sylvestris Cream on Burn Injury and Wounds in Rats. Avicenna J. Phytomedicine 2015, 5, 341–354. [Google Scholar]
- Shanbhag, T.; Kodidela, S.; Shenoy, S.; Amuthan, A.; Kurra, S. Effect of Michelia Champaca Linn Flowers on Burn Wound Healing in Wistar Rats. Int. J. Pharm. Sci. Rev. Res. 2011, 7, 112–115. [Google Scholar]
- Amutha, K.; Selvakumari, U. Wound Healing Activity of Methanolic Stem Extract of Musa Paradisiaca Linn. (Banana) in Wistar Albino Rats. Int. Wound J. 2016, 13, 763–767. [Google Scholar] [CrossRef] [PubMed]
- Ozcan, O.; Ipekci, H.; Alev, B.; Ustundag, U.V.; Ak, E.; Sen, A.; Alturfan, E.E.; Sener, G.; Yarat, A.; Cetinel, S.; et al. Protective Effect of Myrtle (Myrtus Communis) on Burn Induced Skin Injury. Burns 2019, 45, 1856–1863. [Google Scholar] [CrossRef] [PubMed]
- Yaman, I.; Durmus, A.; Ceribasi, S.; Yaman, M. Effects of Nigella Sativa and Silver Sulfadiazine on Burn Wound Healing in Rats. Vet. Med. 2010, 55, 619–624. [Google Scholar] [CrossRef]
- Gurfinkel, R.; Palivatkel-Naim, M.; Gleisinger, R.; Rosenberg, L.; Singer, A.J. Comparison of Purified Olive Oil and Silver Sulfadiazine in the Treatment of Partial Thickness Porcine Burns. Am. J. Emerg. Med. 2012, 30, 79–83. [Google Scholar] [CrossRef]
- Raisi, A.; Sefidbaf, E.; Farjanikish, G.; Esmaeili-Fard-Barzegar, P.; Abbasi, M. The Effects of Olive Leaf Extract Ointment on Third-Degree Burn in Rat. Iran. J. Vet. Surg. 2022, 17, 16–23. [Google Scholar] [CrossRef]
- Moghaddam, P.Z.; Zolfaghari, M.R.; Ghaemi, E.A.; Mazandarani, M.; Mansourian, A.R.; Taheri, S.A. Negative Performance of Root Extract of Onosma Dichroanthum Boiss. on the Burn Wound Healing in an Animal Model. Arch. Clin. Microbiol. 2011, 2, 1–5. [Google Scholar] [CrossRef]
- Hemmati, A.; Namjuyan, F.; Yousefi, S.; Housmand, G.; Khadem Haghighian, H.; Rezaei, A. The Healing Effect of N-Hexan- Dichloromethane Extract Root Onosma Bulbotrichum in Second Degree Burns. World J. Plast. Surg. 2018, 7, 25–33. [Google Scholar]
- Shanbhag, T.; Amuthan, A.; Shenoy, S.; Sudhakar Effect of Phyllanthus Niruri. Linn on Burn Wound in Rats. Asian Pac. J. Trop. Med. 2010, 3, 105–108. [Google Scholar] [CrossRef]
- Haghdoost, F.; Baradaran Mahdavi, M.M.; Zandifar, A.; Sanei, M.H.; Zolfaghari, B.; Javanmard, S.H. Pistacia Atlantica Resin Has a Dose-Dependent Effect on Angiogenesis and Skin Burn Wound Healing in Rat. Evid.-Based Complement. Altern. Med. 2013, 2013, 893425. [Google Scholar] [CrossRef] [PubMed]
- Fakour, S.; Heydari, S.; Akradi, L.; Rahymi Bane, R. Effect of Pistacia Atlantica Mastic Extract on Experimental Wound Healing and Various Biochemical Parameters of Blood Serum in Rabbit Models. J. Med. Plants 2017, 16, 78–91. [Google Scholar]
- Shahouzehi, B.; Sepehri, G.; Sadeghiyan, S.; Masoomi-Ardakani, Y. Effect of Pistacia Atlantica Resin Oil on Anti-Oxidant, Hydroxyprolin and VEGF Changes in Experimentally-Induced Skin Burn in Rat. WORLD J. Plast. Surg. 2018, 7, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Djerrou, J.; Maameri, Z.; Hamdo-Pacha, Y.; Serakta, M.; Riachi, F.; Djaalab, H.; Boukeloua, A. Effect of Virgin Fatty Oil of Pistacia Lentiscus on Experimental Burn Wound’s Healing in Rabbits. Afr. J. Tradit. Complement. Altern. Med. 2010, 7, 258–263. [Google Scholar] [CrossRef]
- Amini, M.; Kherad, M.; Mehrabani, D.; Azarpira, N.; Panjehshahin, M.R.; Tanideh, N. Effect of Plantago Major on Burn Wound Healing in Rat. J. Appl. Anim. Res. 2010, 37, 53–56. [Google Scholar] [CrossRef]
- Mohammed Haneefa, K.P.; Abid; Shahima Hanan, K.; Mohanta, G.P.; Nayar, C. Formulation and Evaluation of Herbal Emulgel of Pothos Scandens Linn for Burn Wound Healing Activity. J. Pharm. Sci. Res. 2014, 6, 63–67. [Google Scholar]
- Mo, J.; Panichayupakaranant, P.; Kaewnopparat, N.; Nitiruangjaras, A.; Reanmongkol, W. Wound Healing Activities of Standardized Pomegranate Rind Extract and Its Major Antioxidant Ellagic Acid in Rat Dermal Wounds. J. Nat. Med. 2014, 68, 377–386. [Google Scholar] [CrossRef]
- Lukiswanto, B.; Miranti, A.; Sudjarwo, S.; Primarizky, H.; Yuniarti, W. Evaluation of Wound Healing Potential of Pomegranate (Punica Granatum) Whole Fruit Extract on Skin Burn Wound in Rats (Rattus Norvegicus). J. Adv. Vet. Anim. Res. 2019, 6, 202. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, R.; Hu, Y.; Yang, Y.; Zhang, X.; He, B.; Shen, Z.; Yang, J.; Chen, P. Promoting Effect of Pomegranate Peel Extract on Second-Degree Burn Wound-Healing through VEGF-A and TGF-Β1 Regulation. Burns 2022, 48, 639–648. [Google Scholar] [CrossRef]
- Mogoşanu, G.D.; Popescu, F.C.; Busuioc, C.J.; Lascǎr, I.; Mogoantǎ, L. Comparative Study of Microvascular Density in Experimental Third-Degree Skin Burns Treated with Topical Preparations Containing Herbal Extracts. Rom. J. Morphol. Embryol. 2013, 54, 107–113. [Google Scholar]
- Said, A.; Wahid, F.; Bashir, K.; Rasheed, H.M.; Khan, T.; Hussain, Z.; Siraj, S. Sauromatum Guttatum Extract Promotes Wound Healing and Tissue Regeneration in a Burn Mouse Model via Up-Regulation of Growth Factors. Pharm. Biol. 2019, 57, 736–743. [Google Scholar] [CrossRef]
- Le, X.; Fan, Y. Healing Effect of Sanguisorba Officinalis L Extract on Second-Degree Burns in Rats. Trop. J. Pharm. Res. 2017, 16, 1045. [Google Scholar] [CrossRef][Green Version]
- Isaac, J.A.; Daburi, A.; Ifeanyi, B.; Ben-Umeh, K.C.; Adedokun, A.A.; Builders, P. Senna Podocarpa Emulgel: A Herbal Alternative for Chemical Burn Wound Treatment. Pharm. Front. 2022, 04, e30–e39. [Google Scholar] [CrossRef]
- Nasiri, E.; Hosseinimehr, S.J.; Azadbakht, M.; Akbari, J.; Enayati-fard, R.; Azizi, S. The Effect of Terminalia Chebula Extract vs. Silver Sulfadiazine on Burn Wounds in Rats. J. Complement. Integr. Med. 2015, 12, 127–135. [Google Scholar] [CrossRef]
- Heidari, M.; Bahramsoltani, R.; Abdolghaffari, A.H.; Rahimi, R.; Esfandyari, M.; Baeeri, M.; Hassanzadeh, G.; Abdollahi, M.; Farzaei, M.H. Efficacy of Topical Application of Standardized Extract of Tragopogon Graminifolius in the Healing Process of Experimental Burn Wounds. J. Tradit. Complement. Med. 2019, 9, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Piana, M.; Silva, M.A.; Trevisan, G.; de Brum, T.F.; Silva, C.R.; Boligon, A.A.; Oliveira, S.M.; Zadra, M.; Hoffmeister, C.; Rossato, M.F.; et al. Antiinflammatory Effects of Viola Tricolor Gel in a Model of Sunburn in Rats and the Gel Stability Study. J. Ethnopharmacol. 2013, 150, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-Q.; Kang, R.; Huo, J.-C.; Xie, Y.-H.; Wang, S.-W.; Cao, W. Wound-Healing Activity of Zanthoxylum Bungeanum Maxim Seed Oil on Experimentally Burned Rats. Pharmacogn. Mag. 2017, 13, 363. [Google Scholar] [CrossRef]
- Ait Abderrahim, L.; Taïbi, K.; Ait Abderrahim, N.; Boussaid, M.; Rios-Navarro, C.; Ruiz-Saurí, A. Euphorbia Honey and Garlic: Biological Activity and Burn Wound Recovery. Burns 2019, 45, 1695–1706. [Google Scholar] [CrossRef]
- Shukla, R.; Kashaw, V. Evaluation of Wound Healing Potential of Nerium Indicum, Artocarpus Heterophyllus, Murraya Koenigii and Punica Granatum Using Incision Wound Model in Rats. Asian J. Pharm. Pharmacol. 2019, 5, 625–633. [Google Scholar] [CrossRef]
- Akhoondinasab, M.R.; Khodarahmi, A.; Akhoondinasab, M.; Saberi, M.; Iranpour, M. Assessing Effect of Three Herbal Medicines in Second and Third Degree Burns in Rats and Comparison with Silver Sulfadiazine Ointment. Burns 2015, 41, 125–131. [Google Scholar] [CrossRef]
- Mehrabani, M.; Seyyedkazemi, S.M.; Nematollahi, M.H.; Jafari, E.; Mehrabani, M.; Mehdipour, M.; Sheikhshoaee, Z.; Mandegary, A. Accelerated Burn Wound Closure in Mice with a New Formula Based on Traditional Medicine. Iran. Red Crescent Med. J. 2016, 18, e59052. [Google Scholar] [CrossRef] [PubMed]
- Fahimi, S.; Abdollahi, M.; Mortazavi, S.A.; Hajimehdipoor, H.; Abdolghaffari, A.H.; Rezvanfar, M.A. Wound Healing Activity of a Traditionally Used Poly Herbal Product in a Burn Wound Model in Rats. Iran. Red Crescent Med. J. 2015, 17, e19960. [Google Scholar] [CrossRef]
- Shukla, R.; Kashaw, V. Wound Healing Prospective of Pongamia Glabra, Piper Nigrum and Momordica Charantia on Albino Rats Using Anemic Burn Wound Model. J. Drug Deliv. Ther. 2018, 8, 146–154. [Google Scholar] [CrossRef]
- Vaghardoost, R.; Mousavi Majd, S.G.; Tebyanian, H.; Babavalian, H.; Malaei, L.; Niazi, M.; Javdani, A. The Healing Effect of Sesame Oil, Camphor and Honey on Second Degree Burn Wounds in Rat. World J. Plast. Surg. 2018, 7, 67–71. [Google Scholar] [PubMed]
- Wang, Y.; Beekman, J.; Hew, J.; Jackson, S.; Issler-Fisher, A.C.; Parungao, R.; Lajevardi, S.S.; Li, Z.; Maitz, P.K.M. Burn Injury: Challenges and Advances in Burn Wound Healing, Infection, Pain and Scarring. Adv. Drug Deliv. Rev. 2018, 123, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, M.G.; Gauglitz, G.G.; Kulp, G.A.; Finnerty, C.C.; Williams, F.N.; Kraft, R.; Suman, O.E.; Mlcak, R.P.; Herndon, D.N. Long-Term Persistance of the Pathophysiologic Response to Severe Burn Injury. PLoS ONE 2011, 6, e21245. [Google Scholar] [CrossRef]
- Jeschke, M.G.; Chinkes, D.L.; Finnerty, C.C.; Kulp, G.; Suman, O.E.; Norbury, W.B.; Branski, L.K.; Gauglitz, G.G.; Mlcak, R.P.; Herndon, D.N. Pathophysiologic Response to Severe Burn Injury. Ann. Surg. 2008, 248, 387–401. [Google Scholar] [CrossRef]
- Rowan, M.P.; Cancio, L.C.; Elster, E.A.; Burmeister, D.M.; Rose, L.F.; Natesan, S.; Chan, R.K.; Christy, R.J.; Chung, K.K. Burn Wound Healing and Treatment: Review and Advancements. Crit. Care 2015, 19, 243. [Google Scholar] [CrossRef]
- Keck, M.; Herndon, D.H.; Kamolz, L.P.; Frey, M.; Jeschke, M.G. Pathophysiology of Burns. Wien. Med. Wochenschr. 2009, 159, 327–336. [Google Scholar] [CrossRef]
- Senica, M.; Stampar, F.; Veberic, R.; Mikulic-Petkovsek, M. The Higher the Better? Differences in Phenolics and Cyanogenic Glycosides in Sambucus Nigra Leaves, Flowers and Berries from Different Altitudes. J. Sci. Food Agric. 2017, 97, 2623–2632. [Google Scholar] [CrossRef] [PubMed]
- Gairola, S.; Shariff, N.M.; Bhatt, A.; Kala, C.P. Influence of Climate Change on Production of Secondary Chemicals in High Altitude Medicinal Plants: Issues Needs Immediate Attention. J. Med. Plants Res. 2010, 4, 1825–1829. [Google Scholar] [CrossRef]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.G.; Lightfoot, D.A. Phytochemicals: Extraction, Isolation, and Identification of Bioactive Compounds from Plant Extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Ajazuddin; Saraf, S. Applications of Novel Drug Delivery System for Herbal Formulations. Fitoterapia 2010, 81, 680–689. [Google Scholar] [CrossRef]
- Sandhiya, V.; Ubaidulla, U. A Review on Herbal Drug Loaded into Pharmaceutical Carrier Techniques and Its Evaluation Process. Futur. J. Pharm. Sci. 2020, 6, 51. [Google Scholar] [CrossRef]
- Qadir, A.; Jahan, S.; Aqil, M.; Warsi, M.H.; Alhakamy, N.A.; Alfaleh, M.A.; Khan, N.; Ali, A. Phytochemical-Based Nano-Pharmacotherapeutics for Management of Burn Wound Healing. Gels 2021, 7, 209. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Hu, J.; Qian, W.; Chen, L.; Zhang, D. Recent Advances in Nanotherapeutics for the Treatment of Burn Wounds. Burn. Trauma 2021, 9, tkab026. [Google Scholar] [CrossRef] [PubMed]
- Hajialyani, M.; Tewari, D.; Sobarzo-Sánchez, E.; Nabavi, S.M.; Farzaei, M.H.; Abdollahi, M. Natural Product-Based Nanomedicines for Wound Healing Purposes: Therapeutic Targets and Drug Delivery Systems. Int. J. Nanomed. 2018, 13, 5023–5043. [Google Scholar] [CrossRef] [PubMed]
- Selvakumar, P.M.; Rajkumar, S.R.J.; MSA, M.N. Phytochemicals as a Potential Source for Anti-Microbial, Anti-Oxidant and Wound Healing—A Review. MOJ Bioorganic Org. Chem. 2018, 2, 61–70. [Google Scholar] [CrossRef]
- El Ayadi, A.; Jay, J.W.; Prasai, A. Current Approaches Targeting the Wound Healing Phases to Attenuate Fibrosis and Scarring. Int. J. Mol. Sci. 2020, 21, 1105. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Amini-Nik, S. The Role of Phytochemicals in the Inflammatory Phase of Wound Healing. Int. J. Mol. Sci. 2017, 18, 1068. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.K.; Sharma, A.K.; Gupta, V.; Yashavarddhan, M.H. Pharmacological Control of Inflammation in Wound Healing. J. Tissue Viability 2019, 28, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Shedoeva, A.; Leavesley, D.; Upton, Z.; Fan, C. Wound Healing and the Use of Medicinal Plants. Evid.-Based Complement. Altern. Med. 2019, 2019, 2684108. [Google Scholar] [CrossRef] [PubMed]
- Glasziou, P.; Altman, D.G.; Bossuyt, P.; Boutron, I.; Clarke, M.; Julious, S.; Michie, S.; Moher, D.; Wager, E. Reducing Waste from Incomplete or Unusable Reports of Biomedical Research. Lancet 2014, 383, 267–276. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).