Electrospinning of Potential Medical Devices (Wound Dressings, Tissue Engineering Scaffolds, Face Masks) and Their Regulatory Approach
Abstract
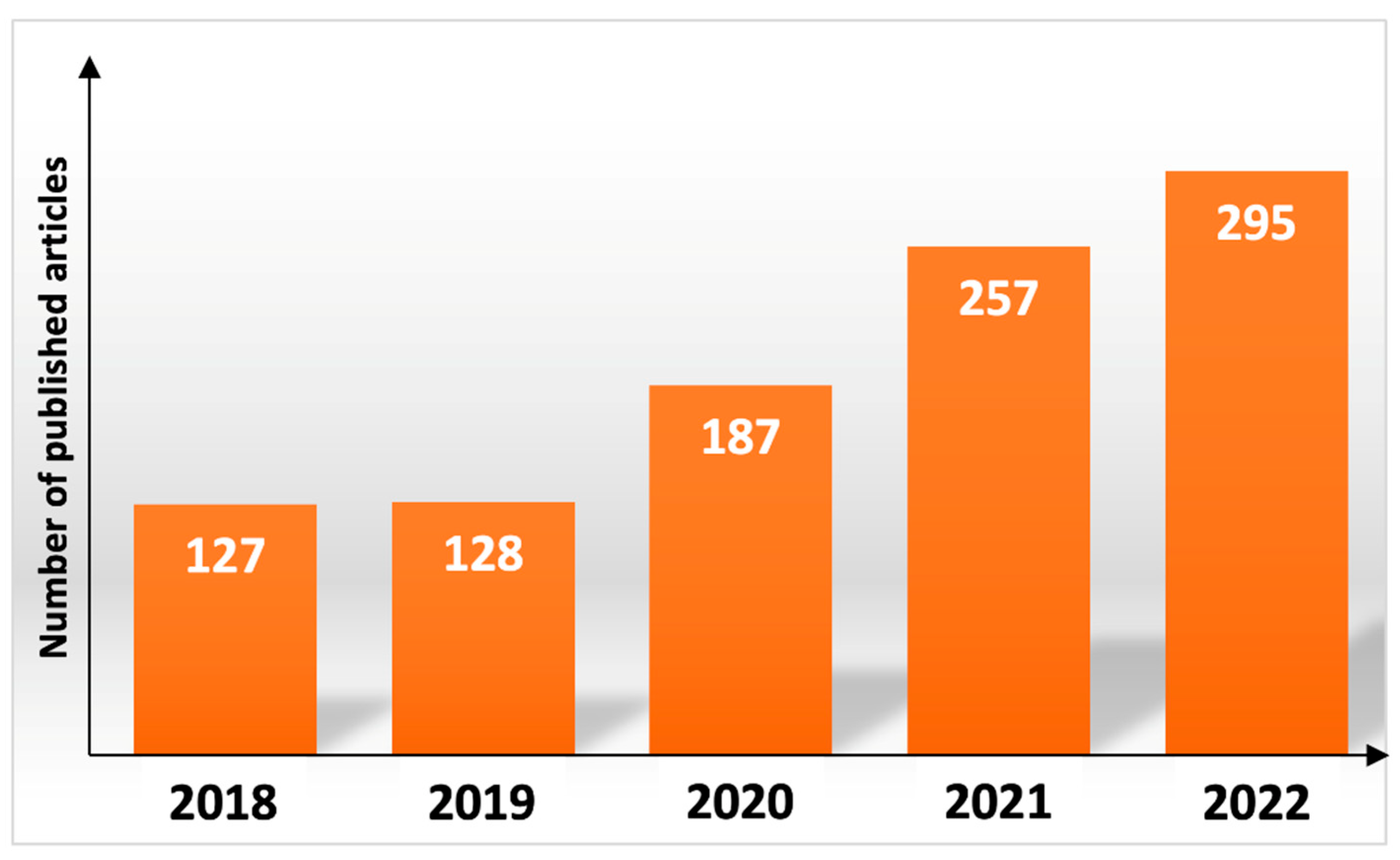
1. Introduction
2. Electrospinning
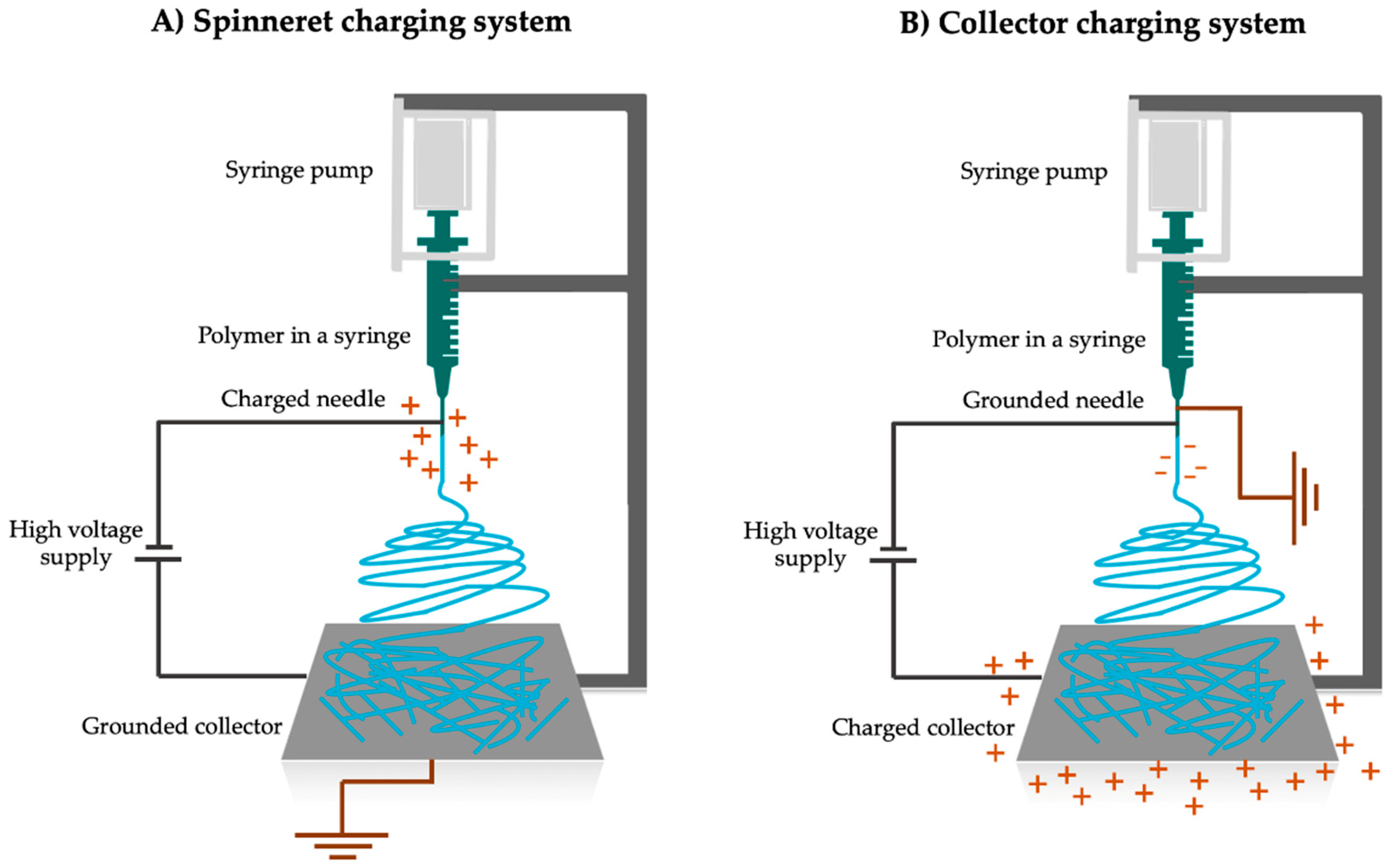
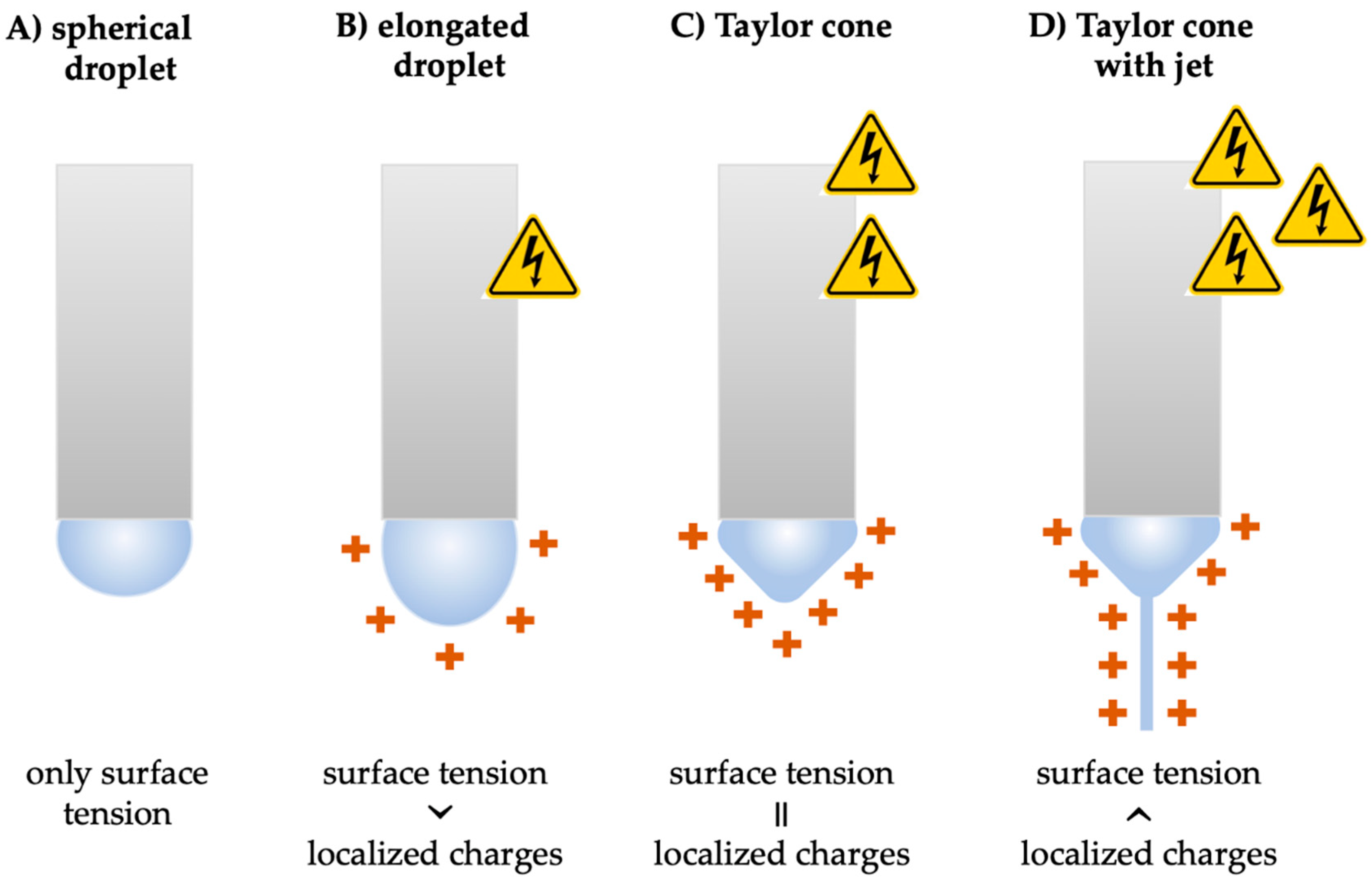
2.1. Principles of Electrospinning
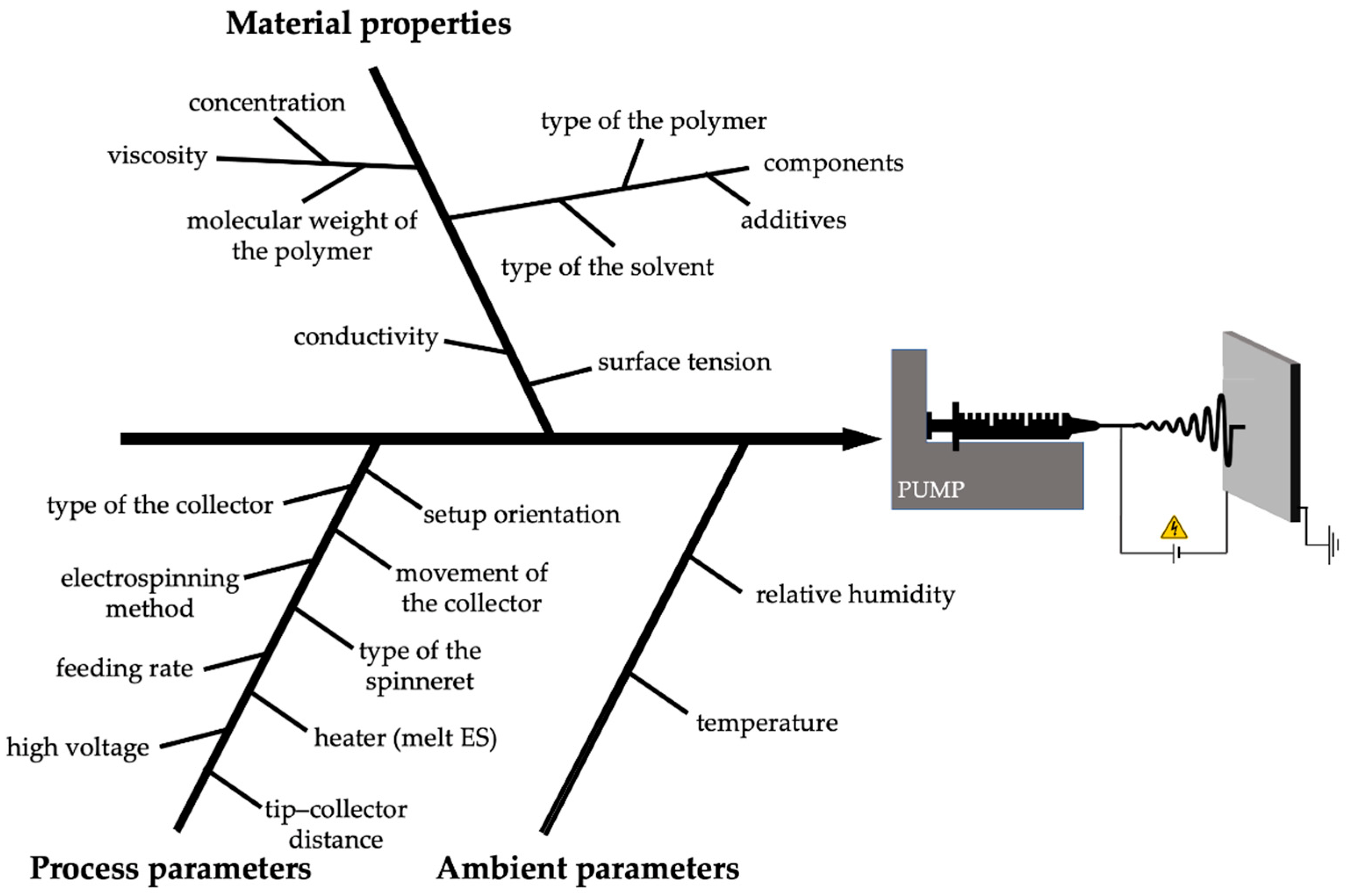
2.2. Effects of Different Electrospinning Parameters
- Process parameters (high voltage, flow rate, and distance between the Taylor cone and the collector);
- Material properties (viscosity—related to the molecular weight and concentration of the polymer, surface tension, conductivity, and volatility of the solvent);
- Ambient parameters (temperature and humidity).
2.2.1. Process Parameters
2.2.2. Material Properties
2.2.3. Ambient Parameters
2.3. Electrospinning Methods
2.3.1. Solution Electrospinning
Nozzle-Based Methods
Nozzle-Free Methods
2.3.2. Emulsion Electrospinning
2.3.3. Melt Electrospinning
Syringe-Based Method
Syringe-Free Methods
3. Biomedical Applications of Electrospun Nanofibers
4. Nanofibers as Medical Devices
5. Regulatory Aspects
- Free nanomaterials added to a medical device (e.g., nano-silver in wound dressings);
- Fixed nanomaterials form a coating on implants to increase biocompatibility (e.g., nano-hydroxyapatite) or to prevent infection (e.g., nano-silver);
- Embedded nanomaterials to strengthen biomaterials (e.g., carbon nanotubes in a catheter wall) [154].
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kannan, B.; Cha, H.; Hosie, I.C. Electrospinning—Commercial Applications, Challenges and Opportunities. In Nano-Size Polymers: Preparation, Properties, Applications; Fakirov, S., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 309–342. [Google Scholar]
- Bowers, S.; Franco, E. Chronic Wounds: Evaluation and Management. Am. Fam. Physician 2020, 101, 159–166. [Google Scholar]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Wu, S.; Dong, T.; Li, Y.; Sun, M.; Qi, Y.; Liu, J.; Kuss, M.A.; Chen, S.; Duan, B. State-of-the-Art Review of Advanced Electrospun Nanofiber Yarn-Based Textiles for Biomedical Applications. Appl. Mater. Today 2022, 27, 101473. [Google Scholar] [CrossRef]
- Foulkes, R.; Man, E.; Thind, J.; Yeung, S.; Joy, A.; Hoskins, C. The Regulation of Nanomaterials and Nanomedicines for Clinical Application: Current and Future Perspectives. Biomater. Sci. 2020, 8, 4653–4664. [Google Scholar] [CrossRef]
- Collins, G.; Federici, J.; Imura, Y.; Catalani, L.H. Charge Generation, Charge Transport, and Residual Charge in the Electrospinning of Polymers: A Review of Issues and Complications. J. Appl. Phys. 2012, 111, 044701. [Google Scholar] [CrossRef]
- Wu, C.-M.; Chiou, H.-G.; Lin, S.-L.; Lin, J.-M. Effects of Electrostatic Polarity and the Types of Electrical Charging on Electrospinning Behavior. J. Appl. Polym. Sci. 2012, 126, E89–E97. [Google Scholar] [CrossRef]
- Taylor, S.G. Disintegration of Water Drops in an Electric Field. Proc. R. Soc. Lond. Ser. A Math. Phys. R. 1964, 280, 383–397. [Google Scholar]
- Hartman, R.P.A.; Brunner, D.J.; Camelot, D.M.A.; Marijnissen, J.C.M.; Scarlett, B. Electrohydrodynamic Atomization in the Cone-Jet Mode Physical Modeling of the Liquid Cone and Jet. J. Aerosol Sci. 1999, 30, 823–849. [Google Scholar] [CrossRef]
- Reneker, D.H.; Yarin, A.L. Electrospinning Jets and Polymer Nanofibers. Polymer 2008, 49, 2387–2425. [Google Scholar] [CrossRef]
- Zhmayev, E.; Cho, D.; Lak Joo, Y. Electrohydrodynamic Quenching in Polymer Melt Electrospinning. Phys. Fluids 2011, 23, 73102. [Google Scholar] [CrossRef]
- Buchko, C.J.; Chen, L.C.; Shen, Y.; Martin, D.C. Processing and Microstructural Characterization of Porous Biocompatible Protein Polymer Thin Films. Polymer 1999, 40, 7397–7407. [Google Scholar] [CrossRef]
- Lee, J.S.; Choi, K.H.; Ghim, H.D.; Kim, S.S.; Chun, D.H.; Kim, H.Y.; Lyoo, W.S. Role of Molecular Weight of Atactic Poly(Vinyl Alcohol) (PVA) in the Structure and Properties of PVA Nanofabric Prepared by Electrospinning. J. Appl. Polym. Sci. 2004, 93, 1638–1646. [Google Scholar] [CrossRef]
- Tong, H.-W.; Wang, M. Negative Voltage Electrospinning and Positive Voltage Electrospinning of Tissue Engineering Scaffolds: A Comparative Study and Charge Retention on Scaffolds. Nano LIFE 2012, 2, 1250004. [Google Scholar] [CrossRef]
- Zhang, C.; Yuan, X.; Wu, L.; Han, Y.; Sheng, J. Study on Morphology of Electrospun Poly(Vinyl Alcohol) Mats. Eur. Polym. J. 2005, 41, 423–432. [Google Scholar] [CrossRef]
- Yarin, A.L.; Kataphinan, W.; Reneker, D.H. Branching in Electrospinning of Nanofibers. J. Appl. Phys. 2005, 98, 64501. [Google Scholar] [CrossRef]
- Suresh, S.; Becker, A.; Glasmacher, B. Impact of Apparatus Orientation and Gravity in Electrospinning—A Review of Empirical Evidence. Polymers 2020, 12, 2448. [Google Scholar] [CrossRef]
- Sill, T.J.; von Recum, H.A. Electrospinning: Applications in Drug Delivery and Tissue Engineering. Biomaterials 2008, 29, 1989–2006. [Google Scholar] [CrossRef]
- Rogina, A. Electrospinning Process: Versatile Preparation Method for Biodegradable and Natural Polymers and Biocomposite Systems Applied in Tissue Engineering and Drug Delivery. Appl. Surf. Sci. 2014, 296, 221–230. [Google Scholar] [CrossRef]
- Tan, S.M.; Teoh, X.Y.; Le Hwang, J.; Khong, Z.P.; Sejare, R.; Almashhadani, A.Q.; Assi, R.A.; Chan, S.Y. Electrospinning and Its Potential in Fabricating Pharmaceutical Dosage Form. J. Drug. Deliv. Sci. Technol. 2022, 76, 103761. [Google Scholar] [CrossRef]
- Alfaro De Prá, M.A.; Ribeiro-do-Valle, R.M.; Maraschin, M.; Veleirinho, B. Effect of Collector Design on the Morphological Properties of Polycaprolactone Electrospun Fibers. Mater. Lett. 2017, 193, 154–157. [Google Scholar] [CrossRef]
- Smit, E.; Bűttner, U.; Sanderson, R.D. Continuous Yarns from Electrospun Fibers. Polymer 2005, 46, 2419–2423. [Google Scholar] [CrossRef]
- Sonseca, A.; Sahay, R.; Stepien, K.; Bukala, J.; Wcislek, A.; McClain, A.; Sobolewski, P.; Sui, X.; Puskas, J.E.; Kohn, J.; et al. Architectured Helically Coiled Scaffolds from Elastomeric Poly(Butylene Succinate) (PBS) Copolyester via Wet Electrospinning. Mater. Sci. Eng. C 2020, 108, 110505. [Google Scholar] [CrossRef]
- Tang, X.-P.; Si, N.; Xu, L.; Liu, H.-Y. Effect of Flow Rate on Diameter of Electrospun Nanoporous Fibers. Therm. Sci. 2014, 18, 1447–1449. [Google Scholar] [CrossRef]
- Uhljar, L.É.; Kan, S.Y.; Radacsi, N.; Koutsos, V.; Szabó-Révész, P.; Ambrus, R. In Vitro Drug Release, Permeability, and Structural Test of Ciprofloxacin-Loaded Nanofibers. Pharmaceutics 2021, 13, 556. [Google Scholar] [CrossRef]
- Zuo, W.; Zhu, M.; Yang, W.; Yu, H.; Chen, Y.; Zhang, Y. Experimental Study on Relationship between Jet Instability and Formation of Beaded Fibers during Electrospinning. Polym. Eng. Sci. 2005, 45, 704–709. [Google Scholar] [CrossRef]
- Angammana, C.J.; Jayaram, S.H. Analysis of the Effects of Solution Conductivity on Electrospinning Process and Fiber Morphology. IEEE Trans. Ind. Appl. 2011, 47, 1109–1117. [Google Scholar] [CrossRef]
- Colmenares Roldán, G.J.; Quintero Martínez, Y.; Agudelo Gómez, L.M.; Rodríguez Vinasco, L.F.; Hoyos Palacio, L.M. Influence of the Molecular Weight of Polymer, Solvents and Operational Condition in the Electrospinning of Polycaprolactone. Rev. Fac. Ing. Univ. Antioq. 2017, 84, 35–45. [Google Scholar] [CrossRef]
- Uhljar, L.É.; Alshweiat, A.; Katona, G.; Chung, M.; Radacsi, N.; Kókai, D.; Burián, K.; Ambrus, R. Comparison of Nozzle-Based and Nozzle-Free Electrospinning for Preparation of Fast-Dissolving Nanofibers Loaded with Ciprofloxacin. Pharmaceutics 2022, 14, 1559. [Google Scholar] [CrossRef] [PubMed]
- Mit-uppatham, C.; Nithitanakul, M.; Supaphol, P. Ultrafine Electrospun Polyamide-6 Fibers: Effect of Solution Conditions on Morphology and Average Fiber Diameter. Macromol. Chem. Phys. 2004, 205, 2327–2338. [Google Scholar] [CrossRef]
- Feng, Z.-Q.; Leach, M.K.; Chu, X.-H.; Wang, Y.-C.; Tian, T.; Shi, X.-L.; Ding, Y.-T.; Gu, Z.-Z. Electrospun Chitosan Nanofibers for Hepatocyte Culture. J. Biomed. Nanotechnol. 2010, 6, 658–666. [Google Scholar] [CrossRef]
- Song, Z.; Chiang, S.W.; Chu, X.; Du, H.; Li, J.; Gan, L.; Xu, C.; Yao, Y.; He, Y.; Li, B.; et al. Effects of Solvent on Structures and Properties of Electrospun Poly(Ethylene Oxide) Nanofibers. J. Appl. Polym. Sci. 2018, 135, 45787. [Google Scholar] [CrossRef]
- De Vrieze, S.; Van Camp, T.; Nelvig, A.; Hagström, B.; Westbroek, P.; De Clerck, K. The Effect of Temperature and Humidity on Electrospinning. J. Mater. Sci. 2009, 44, 1357–1362. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A Fascinating Fiber Fabrication Technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef]
- Pelipenko, J.; Kocbek, P.; Kristl, J. Critical Attributes of Nanofibers: Preparation, Drug Loading, and Tissue Regeneration. Int. J. Pharm. 2015, 484, 57–74. [Google Scholar] [CrossRef]
- Cai, Y.; Gevelber, M. The Effect of Relative Humidity and Evaporation Rate on Electrospinning: Fiber Diameter and Measurement for Control Implications. J. Mater. Sci. 2013, 48, 7812–7826. [Google Scholar] [CrossRef]
- Pelipenko, J.; Kocbek, P.; Kristl, J. Nanofiber Diameter as a Critical Parameter Affecting Skin Cell Response. Eur. J. Pharm. Sci. 2015, 66, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Mattoso, L.; Offeman, R.; Wood, D.; Orts, W.J.; Medeiros, E. Effect of Relative Humidity on the Morphology of Electrospun Polymer Fibers. Can. J. Chem. 2008, 86, 590–599. [Google Scholar] [CrossRef]
- Huang, L.; Bui, N.-N.; Manickam, S.S.; McCutcheon, J.R. Controlling Electrospun Nanofiber Morphology and Mechanical Properties Using Humidity. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 1734–1744. [Google Scholar] [CrossRef]
- Fuchs, A.; Youssef, A.; Seher, A.; Hartmann, S.; Brands, R.C.; Müller-Richter, U.D.A.; Kübler, A.C.; Linz, C. A New Multilayered Membrane for Tissue Engineering of Oral Hard- and Soft Tissue by Means of Melt Electrospinning Writing and Film Casting—An in Vitro Study. J. Cranio-Maxillofac. Surg. 2019, 47, 695–703. [Google Scholar] [CrossRef]
- Calori, I.R.; Braga, G.; de Jesus, P.D.C.C.; Bi, H.; Tedesco, A.C. Polymer Scaffolds as Drug Delivery Systems. Eur. Polym. J. 2020, 129, 109621. [Google Scholar] [CrossRef]
- Feng, X.; Li, J.; Zhang, X.; Liu, T.; Ding, J.; Chen, X. Electrospun Polymer Micro/Nanofibers as Pharmaceutical Repositories for Healthcare. J. Control. Release 2019, 302, 19–41. [Google Scholar] [CrossRef] [PubMed]
- Mickova, A.; Buzgo, M.; Benada, O.; Rampichova, M.; Fisar, Z.; Filova, E.; Tesarova, M.; Lukas, D.; Amler, E. Core/Shell Nanofibers with Embedded Liposomes as a Drug Delivery System. Biomacromolecules 2012, 13, 952–962. [Google Scholar] [CrossRef] [PubMed]
- Abdulkadhim, M.K.; Habeeb, S.A. The Possibility of Producing Uniform Nanofibers from Blends of Natural Biopolymers. Matls. Perf. Charact. 2022, 11, 20220045. [Google Scholar] [CrossRef]
- Maleki, M.; Latifi, M.; Amani-Tehran, M.; Mathur, S. Electrospun Core–Shell Nanofibers for Drug Encapsulation and Sustained Release. Polym. Eng. Sci. 2013, 53, 1770–1779. [Google Scholar] [CrossRef]
- Li, D.; Xia, Y. Direct Fabrication of Composite and Ceramic Hollow Nanofibers by Electrospinning. Nano Lett. 2004, 4, 933–938. [Google Scholar] [CrossRef]
- Li, D.; McCann, J.T.; Xia, Y. Use of Electrospinning to Directly Fabricate Hollow Nanofibers with Functionalized Inner and Outer Surfaces. Small 2005, 1, 83–86. [Google Scholar] [CrossRef]
- Li, B.; Li, D.; Chen, J.; Liu, Z.; Wang, G.; Zhang, X.; Xu, F.; Lu, Y. Hollow Core Micro-Fiber for Optical Wave Guiding and Microfluidic Manipulation. Sens. Actuators B Chem. 2018, 262, 953–957. [Google Scholar] [CrossRef]
- Zheng, Y.; Gong, R.H.; Zeng, Y. Multijet Motion and Deviation in Electrospinning. RSC Adv. 2015, 5, 48533–48540. [Google Scholar] [CrossRef]
- Persano, L.; Camposeo, A.; Tekmen, C.; Pisignano, D. Industrial Upscaling of Electrospinning and Applications of Polymer Nanofibers: A Review. Macromol. Mater. Eng. 2013, 298, 504–520. [Google Scholar] [CrossRef]
- Thoppey, N.M.; Bochinski, J.R.; Clarke, L.I.; Gorga, R.E. Edge Electrospinning for High Throughput Production of Quality Nanofibers. Nanotechnology 2011, 22, 345301. [Google Scholar] [CrossRef]
- Jiang, G.; Zhang, S.; Qin, X. High Throughput of Quality Nanofibers via One Stepped Pyramid-Shaped Spinneret. Mater. Lett. 2013, 106, 56–58. [Google Scholar] [CrossRef]
- Thoppey, N.M.; Bochinski, J.R.; Clarke, L.I.; Gorga, R.E. Unconfined Fluid Electrospun into High Quality Nanofibers from a Plate Edge. Polymer 2010, 51, 4928–4936. [Google Scholar] [CrossRef]
- Partheniadis, I.; Nikolakakis, I.; Laidmäe, I.; Heinämäki, J. A Mini-Review: Needleless Electrospinning of Nanofibers for Pharmaceutical and Biomedical Applications. Processes 2020, 8, 673. [Google Scholar] [CrossRef]
- Keirouz, A.; Zakharova, M.; Kwon, J.; Robert, C.; Koutsos, V.; Callanan, A.; Chen, X.; Fortunato, G.; Radacsi, N. High-Throughput Production of Silk Fibroin-Based Electrospun Fibers as Biomaterial for Skin Tissue Engineering Applications. Mater. Sci. Eng. C 2020, 112, 110939. [Google Scholar] [CrossRef] [PubMed]
- Hodgyai, N.; Farmos, R.L.; Gergely, A. The Design and Implementation of a Disk Electrospinning Device. Műszaki Tudományos Közlemények 2020, 13, 72–76. [Google Scholar] [CrossRef]
- Wang, X.; Niu, H.; Lin, T.; Wang, X. Needleless Electrospinning of Nanofibers with a Conical Wire Coil. Polym. Eng. Sci. 2009, 49, 1582–1586. [Google Scholar] [CrossRef]
- Niu, H.; Wang, X.; Lin, T. Needleless Electrospinning: Influences of Fibre Generator Geometry. J. Text. Inst. 2012, 103, 787–794. [Google Scholar] [CrossRef]
- Luraghi, A.; Peri, F.; Moroni, L. Electrospinning for Drug Delivery Applications: A Review. J. Control. Release 2021, 334, 463–484. [Google Scholar] [CrossRef]
- Avossa, J.; Herwig, G.; Toncelli, C.; Itel, F.; Michel Rossi, R. Electrospinning Based on Benign Solvents: Current Definitions, Implications and Strategies. Green Chem. 2022, 24, 2347–2375. [Google Scholar] [CrossRef]
- Lanaro, M.; Booth, L.; Powell, S.K.; Woodruff, M.A. Electrofluidodynamic Technologies for Biomaterials and Medical Devices: Melt Electrospinning. In Electrofluidodynamic Technologies (EFDTs) for Biomaterials and Medical Devices: Principles and Advances; Woodhead Publishing Series in Biomaterials; Woodhead Publishing: Duxford, UK, 2011; pp. 37–70. ISBN 978-1-84569-741-9. [Google Scholar]
- Alghoraibi, I.; Alomari, S. Different Methods for Nanofiber Design and Fabrication. In Handbook of Nanofibers; Barhoum, A., Bechelany, M., Makhlouf, A., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–46. ISBN 978-3-319-42789-8. [Google Scholar]
- Hengsawas Surasarang, S.; Keen, J.M.; Huang, S.; Zhang, F.; McGinity, J.W.; Williams, R.O. Hot Melt Extrusion versus Spray Drying: Hot Melt Extrusion Degrades Albendazole. Drug Dev. Ind. Pharm. 2017, 43, 797–811. [Google Scholar] [CrossRef]
- Brown, T.D.; Dalton, P.D.; Hutmacher, D.W. Melt Electrospinning Today: An Opportune Time for an Emerging Polymer Process. Prog. Polym. Sci. 2016, 56, 116–166. [Google Scholar] [CrossRef]
- Zhou, H.; Green, T.B.; Joo, Y.L. The Thermal Effects on Electrospinning of Polylactic Acid Melts. Polymer 2006, 47, 7497–7505. [Google Scholar] [CrossRef]
- Lyons, J.; Li, C.; Ko, F. Melt-Electrospinning Part I: Processing Parameters and Geometric Properties. Polymer 2004, 45, 7597–7603. [Google Scholar] [CrossRef]
- Li, X.; Liu, H.; Wang, J.; Li, C. Preparation and Characterization of PLLA/NHA Nonwoven Mats via Laser Melt Electrospinning. Mater. Lett. 2012, 73, 103–106. [Google Scholar] [CrossRef]
- Malakhov, S.N.; Belousov, S.I.; Shcherbina, M.A.; Meshchankina, M.Y.; Chvalun, S.N.; Shepelev, A.D. Effect of Low Molecular Additives on the Electrospinning of Nonwoven Materials from a Polyamide-6 Melt. Polym. Sci. Ser. A 2016, 58, 236–245. [Google Scholar] [CrossRef]
- Morikawa, K.; Green, M.; Naraghi, M. A Novel Approach for Melt Electrospinning of Polymer Fibers. Procedia Manuf. 2018, 26, 205–208. [Google Scholar] [CrossRef]
- Balogh, A.; Farkas, B.; Faragó, K.; Farkas, A.; Wagner, I.; Van Assche, I.; Verreck, G.; Nagy, Z.K.; Marosi, G. Melt-Blown and Electrospun Drug-Loaded Polymer Fiber Mats for Dissolution Enhancement: A Comparative Study. J. Pharm. Sci. 2015, 104, 1767–1776. [Google Scholar] [CrossRef] [PubMed]
- Um, I.C.; Fang, D.; Hsiao, B.S.; Okamoto, A.; Chu, B. Electro-Spinning and Electro-Blowing of Hyaluronic Acid. Biomacromolecules 2004, 5, 1428–1436. [Google Scholar] [CrossRef]
- Zhmayev, E.; Cho, D.; Joo, Y.L. Nanofibers from Gas-Assisted Polymer Melt Electrospinning. Polymer 2010, 51, 4140–4144. [Google Scholar] [CrossRef]
- Hu, X.; Liu, S.; Zhou, G.; Huang, Y.; Xie, Z.; Jing, X. Electrospinning of Polymeric Nanofibers for Drug Delivery Applications. J. Control. Release 2014, 185, 12–21. [Google Scholar] [CrossRef]
- Tottoli, E.M.; Dorati, R.; Genta, I.; Chiesa, E.; Pisani, S.; Conti, B. Skin Wound Healing Process and New Emerging Technologies for Skin Wound Care and Regeneration. Pharmaceutics 2020, 12, 735. [Google Scholar] [CrossRef] [PubMed]
- Rasouli, R.; Barhoum, A.; Bechelany, M.; Dufresne, A. Nanofibers for Biomedical and Healthcare Applications. Macromol. Biosci. 2019, 19, 1800256. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, S.; Spriano, S.; Scalia, A.C.; Cochis, A.; Rimondini, L.; Cruz-Maya, I.; Guarino, V.; Varesano, A.; Vineis, C. Topographical and Biomechanical Guidance of Electrospun Fibers for Biomedical Applications. Polymers 2020, 12, 2896. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yokota, T.; Someya, T. Electrospun Nanofiber-Based Soft Electronics. NPG Asia Mater. 2021, 13, 22. [Google Scholar] [CrossRef]
- Ullah, S.; Ullah, A.; Lee, J.; Jeong, Y.; Hashmi, M.; Zhu, C.; Joo, K.I.; Cha, H.J.; Kim, I.S. Reusability Comparison of Melt-Blown vs Nanofiber Face Mask Filters for Use in the Coronavirus Pandemic. ACS Appl. Nano Mater. 2020, 3, 7231–7241. [Google Scholar] [CrossRef]
- World Health Organization. Global Atlas of Medical Devices; WHO Medical Device Technical Series; World Health Organization: Geneva, Switzerland, 2017; ISBN 978-92-4-151231-2. [Google Scholar]
- Mistry, P.; Chhabra, R.; Muke, S.; Narvekar, A.; Sathaye, S.; Jain, R.; Dandekar, P. Fabrication and Characterization of Starch-TPU Based Nanofibers for Wound Healing Applications. Mater. Sci. Eng. C 2021, 119, 111316. [Google Scholar] [CrossRef]
- Anisiei, A.; Rosca, I.; Sandu, A.-I.; Bele, A.; Cheng, X.; Marin, L. Imination of Microporous Chitosan Fibers—A Route to Biomaterials with “On Demand” Antimicrobial Activity and Biodegradation for Wound Dressings. Pharmaceutics 2022, 14, 117. [Google Scholar] [CrossRef]
- Vigani, B.; Rossi, S.; Sandri, G.; Bonferoni, M.C.; Milanesi, G.; Bruni, G.; Ferrari, F. Coated Electrospun Alginate-Containing Fibers as Novel Delivery Systems for Regenerative Purposes. Int. J. Nanomed. 2018, 13, 6531–6550. [Google Scholar] [CrossRef]
- Venault, A.; Lin, K.-H.; Tang, S.-H.; Dizon, G.V.; Hsu, C.-H.; Maggay, I.V.B.; Chang, Y. Zwitterionic Electrospun PVDF Fibrous Membranes with a Well-Controlled Hydration for Diabetic Wound Recovery. J. Membr. Sci. 2020, 598, 117648. [Google Scholar] [CrossRef]
- Wu, G.; Ma, X.; Fan, L.; Gao, Y.; Deng, H.; Wang, Y. Accelerating Dermal Wound Healing and Mitigating Excessive Scar Formation Using LBL Modified Nanofibrous Mats. Mater. Des. 2020, 185, 108265. [Google Scholar] [CrossRef]
- Movahedi, M.; Asefnejad, A.; Rafienia, M.; Khorasani, M.T. Potential of Novel Electrospun Core-Shell Structured Polyurethane/Starch (Hyaluronic Acid) Nanofibers for Skin Tissue Engineering: In Vitro and in Vivo Evaluation. Int. J. Biol. Macromol. 2020, 146, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Bazmandeh, A.Z.; Mirzaei, E.; Ghasemi, Y.; Kouhbanani, M.A.J. Hyaluronic Acid Coated Electrospun Chitosan-Based Nanofibers Prepared by Simultaneous Stabilizing and Coating. Int. J. Biol. Macromol. 2019, 138, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Fadaie, M.; Mirzaei, E.; Asvar, Z.; Azarpira, N. Stabilization of Chitosan Based Electrospun Nanofibers through a Simple and Safe Method. Mater. Sci. Eng. C 2019, 98, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Cao, R.; Zhang, Y.; Chen, R. Metallic Ions Encapsulated in Electrospun Nanofiber for Antibacterial and Angiogenesis Function to Promote Wound Repair. Front. Cell Dev. Biol. 2021, 9, 660571. [Google Scholar] [CrossRef]
- Adeli, H.; Khorasani, M.T.; Parvazinia, M. Wound Dressing Based on Electrospun PVA/Chitosan/Starch Nanofibrous Mats: Fabrication, Antibacterial and Cytocompatibility Evaluation and in Vitro Healing Assay. Int. J. Biol. Macromol. 2019, 122, 238–254. [Google Scholar] [CrossRef]
- Chanda, A.; Adhikari, J.; Ghosh, A.; Chowdhury, S.R.; Thomas, S.; Datta, P.; Saha, P. Electrospun Chitosan/Polycaprolactone-Hyaluronic Acid Bilayered Scaffold for Potential Wound Healing Applications. Int. J. Biol. Macromol. 2018, 116, 774–785. [Google Scholar] [CrossRef]
- Kianfar, P.; Vitale, A.; Dalle Vacche, S.; Bongiovanni, R. Photo-Crosslinking of Chitosan/Poly(Ethylene Oxide) Electrospun Nanofibers. Carbohydr. Polym. 2019, 217, 144–151. [Google Scholar] [CrossRef]
- Sun, L.; Gao, W.; Fu, X.; Shi, M.; Xie, W.; Zhang, W.; Zhao, F.; Chen, X. Enhanced Wound Healing in Diabetic Rats by Nanofibrous Scaffolds Mimicking the Basketweave Pattern of Collagen Fibrils in Native Skin. Biomater. Sci. 2018, 6, 340–349. [Google Scholar] [CrossRef]
- Zhang, K.; Bai, X.; Yuan, Z.; Cao, X.; Jiao, X.; Li, Y.; Qin, Y.; Wen, Y.; Zhang, X. Layered Nanofiber Sponge with an Improved Capacity for Promoting Blood Coagulation and Wound Healing. Biomaterials 2019, 204, 70–79. [Google Scholar] [CrossRef]
- Lukiev, I.V.; Antipina, L.S.; Goreninskii, S.I.; Tverdokhlebova, T.S.; Vasilchenko, D.V.; Nemoykina, A.L.; Goncharova, D.A.; Svetlichnyi, V.A.; Dambaev, G.T.; Bouznik, V.M.; et al. Antibacterial Ferroelectric Hybrid Membranes Fabricated via Electrospinning for Wound Healing. Membranes 2021, 11, 986. [Google Scholar] [CrossRef]
- Grumezescu, A.M.; Stoica, A.E.; Dima-Bălcescu, M.-Ș. Electrospun Polyethylene Terephthalate Nanofibers Loaded with Silver Nanoparticles: Novel Approach in Anti-Infective Therapy. J. Clin. Med. 2019, 8, 1039. [Google Scholar] [CrossRef] [PubMed]
- Timnak, A.; Gerstenhaber, J.A.; Dong, K.; Har-El, Y.-E.; Lelkes, P.I. Gradient Porous Fibrous Scaffolds: A Novel Approach to Improving Cell Penetration in Electrospun Scaffolds. Biomed Mater. 2018, 13, 65010. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Zhu, J.; Wang, C.; Chen, R.; Sun, L.; Ru, C. Wrinkle-Free, Sandwich, Electrospun PLGA/SF Nanofibrous Scaffold for Skin Tissue Engineering. IEEE Trans. Nanotechnol. 2018, 17, 675–679. [Google Scholar] [CrossRef]
- Miguel, S.P.; Cabral, C.S.D.; Moreira, A.F.; Correia, I.J. Production and Characterization of a Novel Asymmetric 3D Printed Construct Aimed for Skin Tissue Regeneration. Colloids Surf. B Biointerfaces 2019, 181, 994–1003. [Google Scholar] [CrossRef]
- Chen, S.; Wang, H.; Su, Y.; John, J.V.; McCarthy, A.; Wong, S.L.; Xie, J. Mesenchymal Stem Cell-Laden, Personalized 3D Scaffolds with Controlled Structure and Fiber Alignment Promote Diabetic Wound Healing. Acta Biomater. 2020, 108, 153–167. [Google Scholar] [CrossRef]
- Dehghan-Manshadi, N.; Fattahi, S.; Hadizadeh, M.; Nikukar, H.; Moshtaghioun, S.M.; Aflatoonian, B. The Influence of Elastomeric Polyurethane Type and Ratio on the Physicochemical Properties of Electrospun Polyurethane/Silk Fibroin Hybrid Nanofibers as Potential Scaffolds for Soft and Hard Tissue Engineering. Eur. Polym. J. 2019, 121, 109294. [Google Scholar] [CrossRef]
- Thomas, M.S.; Pillai, P.K.S.; Faria, M.; Cordeiro, N.; Barud, H.; Thomas, S.; Pothen, L.A. Electrospun Polylactic Acid-Chitosan Composite: A Bio-Based Alternative for Inorganic Composites for Advanced Application. J. Mater. Sci. Mater. Med. 2018, 29, 137. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, K.; Solorzano-Vargas, R.S.; Lin, P.-Y.; Walthers, C.M.; Thomas, A.-L.; Martín, M.G.; Dunn, J.C.Y. Bioengineered Intestinal Muscularis Complexes with Long-Term Spontaneous and Periodic Contractions. PLoS ONE 2018, 13, e0195315. [Google Scholar] [CrossRef]
- Jing, X.; Li, H.; Mi, H.-Y.; Liu, Y.-J.; Tan, Y.-M. Fabrication of Three-Dimensional Fluffy Nanofibrous Scaffolds for Tissue Engineering via Electrospinning and CO2 Escaping Foaming. Ind. Eng. Chem. Res. 2019, 58, 9412–9421. [Google Scholar] [CrossRef]
- Maleknia, L.; Dilamian, M.; Pilehrood, M.; Sadeghi-Aliabadi, H.; Hekmati, A. Preparation, Process Optimization and Characterization of Core-Shell Polyurethane/Chitosan Nanofibers as a Potential Platform for Bioactive Scaffolds. Res. Pharm. Sci. 2018, 13, 273. [Google Scholar] [CrossRef]
- Augustine, R.; Dalvi, Y.B.; Yadu Nath, V.K.; Varghese, R.; Raghuveeran, V.; Hasan, A.; Thomas, S.; Sandhyarani, N. Yttrium Oxide Nanoparticle Loaded Scaffolds with Enhanced Cell Adhesion and Vascularization for Tissue Engineering Applications. Mater. Sci. Eng. C 2019, 103, 109801. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, H.; McCarthy, A.; Yan, Z.; Kim, H.J.; Carlson, M.A.; Xia, Y.; Xie, J. Three-Dimensional Objects Consisting of Hierarchically Assembled Nanofibers with Controlled Alignments for Regenerative Medicine. Nano Lett. 2019, 19, 2059–2065. [Google Scholar] [CrossRef] [PubMed]
- Iglesias-Echevarria, M.; Durante, L.; Johnson, R.; Rafuse, M.; Ding, Y.; Bonani, W.; Maniglio, D.; Tan, W. Coaxial PCL/PEG-Thiol-Ene Microfiber with Tunable Physico-Chemical Properties for Regenerative Scaffolds. Biomater. Sci. 2019, 7, 3640–3651. [Google Scholar] [CrossRef]
- Vong, M.; Speirs, E.; Klomkliang, C.; Akinwumi, I.; Nuansing, W.; Radacsi, N. Controlled Three-Dimensional Polystyrene Micro- and Nano-Structures Fabricated by Three-Dimensional Electrospinning. RSC Adv. 2018, 8, 15501–15512. [Google Scholar] [CrossRef]
- Eivazi Zadeh, Z.; Solouk, A.; Shafieian, M.; Haghbin Nazarpak, M. Electrospun Polyurethane/Carbon Nanotube Composites with Different Amounts of Carbon Nanotubes and Almost the Same Fiber Diameter for Biomedical Applications. Mater. Sci. Eng. C 2021, 118, 111403. [Google Scholar] [CrossRef]
- Feng, C.; Liu, C.; Liu, S.; Wang, Z.; Yu, K.; Zeng, X. Electrospun Nanofibers with Core–Shell Structure for Treatment of Bladder Regeneration. Tissue Eng. Part A 2019, 25, 1289–1299. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, K.R.; Jeon, S.H.; Jung, A.R.; Kim, I.G.; Kim, G.E.; Park, Y.H.; Kim, S.H.; Lee, J.Y. Stem Cells Seeded on Multilayered Scaffolds Implanted into an Injured Bladder Rat Model Improves Bladder Function. Tissue Eng. Regen. Med. 2019, 16, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B.; Anderson, Z.; Han, D.; Nebor, I.; Forbes, J.; Steckl, A.J. Electrospinning of Cyanoacrylate Tissue Adhesives for Human Dural Repair in Endonasal Surgery. J. Biomed. Mater. Res. Part B Appl. Biomater. 2022, 110, 660–667. [Google Scholar] [CrossRef]
- Zhao, Z.; Wu, T.; Cui, Y.; Zhao, R.; Wan, Q.; Xu, R. Design and Fabrication of Nanofibrous Dura Mater with Antifibrosis and Neuroprotection Effects on SH-SY5Y Cells. Polymers 2022, 14, 1882. [Google Scholar] [CrossRef]
- Su, Y.; Li, Z.; Zhu, H.; He, J.; Wei, B.; Li, D. Electrohydrodynamic Fabrication of Triple-Layered Polycaprolactone Dura Mater Substitute with Antibacterial and Enhanced Osteogenic Capability. Chin. J. Mech. Eng. Addit. Manuf. Front. 2022, 1, 100026. [Google Scholar] [CrossRef]
- Yao, T.; Chen, H.; Samal, P.; Giselbrecht, S.; Baker, M.B.; Moroni, L. Self-Assembly of Electrospun Nanofibers into Gradient Honeycomb Structures. Mater. Des. 2019, 168, 107614. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, H.; Xiong, J.; Li, J.; Miao, X.; Lan, X.; Liu, X.; Wang, W.; Cai, N.; Tang, Y. Fabrication and in Vitro Evaluation of PCL/Gelatin Hierarchical Scaffolds Based on Melt Electrospinning Writing and Solution Electrospinning for Bone Regeneration. Mater. Sci. Eng. C 2021, 128, 112287. [Google Scholar] [CrossRef]
- Shao, Z.; Yu, L.; Xu, L.; Wang, M. High-Throughput Fabrication of Quality Nanofibers Using a Modified Free Surface Electrospinning. Nanoscale Res. Lett. 2017, 12, 470. [Google Scholar] [CrossRef] [PubMed]
- Bachimam, K.; Emul, E.; Saglam, N.; Korkusuz, F. Baicalein Nanofiber Scaffold Containing Hyaluronic Acid and Polyvinyl Alcohol: Preparation and Evaluation. Turk. J. Med. Sci. 2020, 50, 1139–1146. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yin, C.; Cheng, Y.; Huang, X.; Liu, K.; Cheng, G.; Li, Z. Electrospinning Nanofiber-Reinforced Aerogels for the Treatment of Bone Defects. Adv. Wound Care 2020, 9, 441. [Google Scholar] [CrossRef]
- Eilenberg, M.; Enayati, M.; Ehebruster, D.; Grasl, C.; Walter, I.; Messner, B.; Baudis, S.; Potzmann, P.; Kaun, C.; Podesser, B.K.; et al. Long Term Evaluation of Nanofibrous, Bioabsorbable Polycarbonate Urethane Grafts for Small Diameter Vessel Replacement in Rodents. Eur. J. Vasc. Endovasc. Surg. 2020, 59, 643–652. [Google Scholar] [CrossRef]
- Montoya, Y.; Cardenas, J.; Bustamante, J.; Valencia, R. Effect of Sequential Electrospinning and Co-Electrospinning on Morphological and Fluid Mechanical Wall Properties of Polycaprolactone and Bovine Gelatin Scaffolds, for Potential Use in Small Diameter Vascular Grafts. Biomater. Res. 2021, 25, 38. [Google Scholar] [CrossRef]
- Do, T.M.; Yang, Y.; Deng, A. Porous Bilayer Vascular Grafts Fabricated from Electrospinning of the Recombinant Human Collagen (RHC) Peptide-Based Blend. Polymers 2021, 13, 4042. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Gu, H.; Zhang, H.; Wang, H.; Xia, H.; Mo, X.; Wu, J. Covalent Grafting of PEG and Heparin Improves Biological Performance of Electrospun Vascular Grafts for Carotid Artery Replacement. Acta Biomater. 2021, 119, 211–224. [Google Scholar] [CrossRef]
- Wakuda, Y.; Nishimoto, S.; Suye, S.; Fujita, S. Native Collagen Hydrogel Nanofibres with Anisotropic Structure Using Core-Shell Electrospinning. Sci. Rep. 2018, 8, 6248. [Google Scholar] [CrossRef]
- Wu, T.; Zhang, J.; Wang, Y.; Li, D.; Sun, B.; El-Hamshary, H.; Yin, M.; Mo, X. Fabrication and Preliminary Study of a Biomimetic Tri-Layer Tubular Graft Based on Fibers and Fiber Yarns for Vascular Tissue Engineering. Mater. Sci. Eng. C 2018, 82, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Chalony, C.; Erik Aguilar, L.; Hee Park, C.; Sang Kim, C. Drug Free Anti-Cell Proliferative and Anti-Platelet Adhesion Coating for Vascular Stents via Polymeric Electrospun Fibers. Mater. Lett. 2021, 291, 129545. [Google Scholar] [CrossRef]
- Putzu, M.; Causa, F.; Parente, M.; González de Torre, I.; Rodriguez-Cabello, J.C.; Netti, P.A. Silk-ELR Co-Recombinamer Covered Stents Obtained by Electrospinning. Regen. Biomater. 2019, 6, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Nazarkina, Z.K.; Chelobanov, B.P.; Kuznetsov, K.A.; Shutov, A.V.; Romanova, I.V.; Karpenko, A.A.; Laktionov, P.P. Influence of Elongation of Paclitaxel-Eluting Electrospun-Produced Stent Coating on Paclitaxel Release and Transport through the Arterial Wall after Stenting. Polymers 2021, 13, 1165. [Google Scholar] [CrossRef] [PubMed]
- Kersani, D.; Mougin, J.; Lopez, M.; Degoutin, S.; Tabary, N.; Cazaux, F.; Janus, L.; Maton, M.; Chai, F.; Sobocinski, J.; et al. Stent Coating by Electrospinning with Chitosan/Poly-Cyclodextrin Based Nanofibers Loaded with Simvastatin for Restenosis Prevention. Eur. J. Pharm. Biopharm. 2020, 150, 156–167. [Google Scholar] [CrossRef]
- Lee, C.-H.; Hsieh, M.-J.; Chang, S.-H.; Hung, K.-C.; Wang, C.-J.; Hsu, M.-Y.; Juang, J.-H.; Hsieh, I.-C.; Wen, M.-S.; Liu, S.-J. Nanofibrous Vildagliptin-Eluting Stents Enhance Re-Endothelialization and Reduce Neointimal Formation in Diabetes: In Vitro and in Vivo. Int. J. Nanomed. 2019, 14, 7503–7513. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, X.; Liu, R.; Li, Y.; Zeng, J.; Zhou, M.; Zhao, Y. Bioinspired Vascular Stents with Microfluidic Electrospun Multilayer Coatings for Preventing In-Stent Restenosis. Adv. Healthc. Mater. 2022, 11, 2200965. [Google Scholar] [CrossRef]
- Mounesan, M.; Akbari, S.; Brycki, B.E. Needleless Electrospun Mats Based on Polyamidoamine Dendritic Polymers for Encapsulation of Essential Oils in Personal Respiratory Equipment. J. Ind. Text. 2022, 51, 6333S–6352S. [Google Scholar] [CrossRef]
- Leung, W.W.F.; Sun, Q. Electrostatic Charged Nanofiber Filter for Filtering Airborne Novel Coronavirus (COVID-19) and Nano-Aerosols. Sep. Purif. Technol. 2020, 250, 116886. [Google Scholar] [CrossRef]
- Khandaker, M.; Progri, H.; Arasu, D.T.; Nikfarjam, S.; Shamim, N. Use of Polycaprolactone Electrospun Nanofiber Mesh in a Face Mask. Materials 2021, 14, 4272. [Google Scholar] [CrossRef]
- Huang, C.; Xu, X.; Fu, J.; Yu, D.-G.; Liu, Y. Recent Progress in Electrospun Polyacrylonitrile Nanofiber-Based Wound Dressing. Polymers 2022, 14, 3266. [Google Scholar] [CrossRef]
- Miguel, S.P.; Figueira, D.R.; Simões, D.; Ribeiro, M.P.; Coutinho, P.; Ferreira, P.; Correia, I.J. Electrospun Polymeric Nanofibres as Wound Dressings: A Review. Colloids Surf. B Biointerfaces 2018, 169, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Augustine, R.; Rehman, S.R.U.; Ahmed, R.; Zahid, A.A.; Sharifi, M.; Falahati, M.; Hasan, A. Electrospun Chitosan Membranes Containing Bioactive and Therapeutic Agents for Enhanced Wound Healing. Int. J. Biol. Macromol. 2020, 156, 153–170. [Google Scholar] [CrossRef] [PubMed]
- Alven, S.; Aderibigbe, B.A. Fabrication of Hybrid Nanofibers from Biopolymers and Poly (Vinyl Alcohol)/Poly (ε-Caprolactone) for Wound Dressing Applications. Polymers 2021, 13, 2104. [Google Scholar] [CrossRef] [PubMed]
- Maurmann, N.; Sperling, L.-E.; Pranke, P. Electrospun and Electrosprayed Scaffolds for Tissue Engineering. In Cutting-Edge Enabling Technologies for Regenerative Medicine; Advances in Experimental Medicine and, Biology; Chun, H.J., Park, C.H., Kwon, I.K., Khang, G., Eds.; Springer: Singapore, 2018; pp. 79–100. ISBN 9789811309502. [Google Scholar]
- Tao, F.; Cheng, Y.; Shi, X.; Zheng, H.; Du, Y.; Xiang, W.; Deng, H. Applications of Chitin and Chitosan Nanofibers in Bone Regenerative Engineering. Carbohydr. Polym. 2020, 230, 115658. [Google Scholar] [CrossRef] [PubMed]
- Aoki, K.; Haniu, H.; Kim, Y.A.; Saito, N. The Use of Electrospun Organic and Carbon Nanofibers in Bone Regeneration. Nanomaterials 2020, 10, E562. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Yan, J.; Zhang, K.; Lin, F.; Xiang, L.; Deng, L.; Guan, Z.; Cui, W.; Zhang, H. Pharmaceutical Electrospinning and 3D Printing Scaffold Design for Bone Regeneration. Adv. Drug Deliv. Rev. 2021, 174, 504–534. [Google Scholar] [CrossRef]
- Rickel, A.P.; Deng, X.; Engebretson, D.; Hong, Z. Electrospun Nanofiber Scaffold for Vascular Tissue Engineering. Mater. Sci. Eng. C 2021, 129, 112373. [Google Scholar] [CrossRef]
- Lategan, M.; Kumar, P.; Choonara, Y.E. Functionalizing Nanofibrous Platforms for Neural Tissue Engineering Applications. Drug Discov. Today 2022, 27, 1381–1403. [Google Scholar] [CrossRef]
- Gonçalves, A.M.; Moreira, A.; Weber, A.; Williams, G.R.; Costa, P.F. Osteochondral Tissue Engineering: The Potential of Electrospinning and Additive Manufacturing. Pharmaceutics 2021, 13, 983. [Google Scholar] [CrossRef]
- Suh, T.C.; Amanah, A.Y.; Gluck, J.M. Electrospun Scaffolds and Induced Pluripotent Stem Cell-Derived Cardiomyocytes for Cardiac Tissue Engineering Applications. Bioengineering 2020, 7, 105. [Google Scholar] [CrossRef]
- Zamani, M.; Shakhssalim, N.; Ramakrishna, S.; Naji, M. Electrospinning: Application and Prospects for Urologic Tissue Engineering. Front. Bioeng. Biotechnol. 2020, 8, 579925. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Medical Devices. Available online: https://www.ema.europa.eu/en/human-regulatory/overview/medical-devices (accessed on 29 October 2022).
- US Food and Drug Administration. Classify Your Medical Device. Available online: https://www.fda.gov/medical-devices/overview-device-regulation/classify-your-medical-device (accessed on 28 October 2022).
- US Food and Drug Administration. Premarket Approval (PMA). Available online: https://www.fda.gov/medical-devices/premarket-submissions-selecting-and-preparing-correct-submission/premarket-approval-pma (accessed on 28 October 2022).
- US Food and Drug Administration. Acceptance of Clinical Data to Support Medical Device Applications and Submissions: Frequently Asked Questions. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/acceptance-clinical-data-support-medical-device-applications-and-submissions-frequently-asked (accessed on 29 October 2022).
- European Commission. Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017 on Medical Devices, Amending Directive 2001/83/EC, Regulation (EC) No 178/2002 and Regulation (EC) No 1223/2009 and Repealing Council Directives 90/385/EEC and 93/42/EEC (Text with EEA Relevance.). Off. J. Eur. Union 2017, 117, 1–175. [Google Scholar]
- International Organization for Standardization. ISO 14155:2020(En), Clinical Investigation of Medical Devices for Human Subjects—Good Clinical Practice. Available online: https://www.iso.org/obp/ui/#iso:std:71690:en (accessed on 29 October 2022).
- European Commission—Scientific Committee on Emerging and Newly Identified Health Risks Directorate General for Health and Consumers. Guidance on the Determination of Potential Health Effects of Nanomaterials Used in Medical Devices; European Commission: Brussels, Belgium, 2015; ISBN 978-92-79-35590-5. [Google Scholar]
- US Food and Drug Administration. Coronavirus Disease 2019 (COVID-19) Emergency Use Authorizations for Medical Devices. Available online: https://www.fda.gov/medical-devices/emergency-use-authorizations-medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices (accessed on 29 October 2022).
- Naragund, V.S.; Panda, P.K. Electrospun Nanofiber-Based Respiratory Face Masks—A Review. Emergent Mater. 2022, 5, 261–278. [Google Scholar] [CrossRef] [PubMed]
- Fortin, W.; Bouchet, M.; Therasse, E.; Maire, M.; Héon, H.; Ajji, A.; Soulez, G.; Lerouge, S. Negative In Vivo Results Despite Promising In Vitro Data with a Coated Compliant Electrospun Polyurethane Vascular Graft. J. Surg. Res. 2022, 279, 491–504. [Google Scholar] [CrossRef] [PubMed]
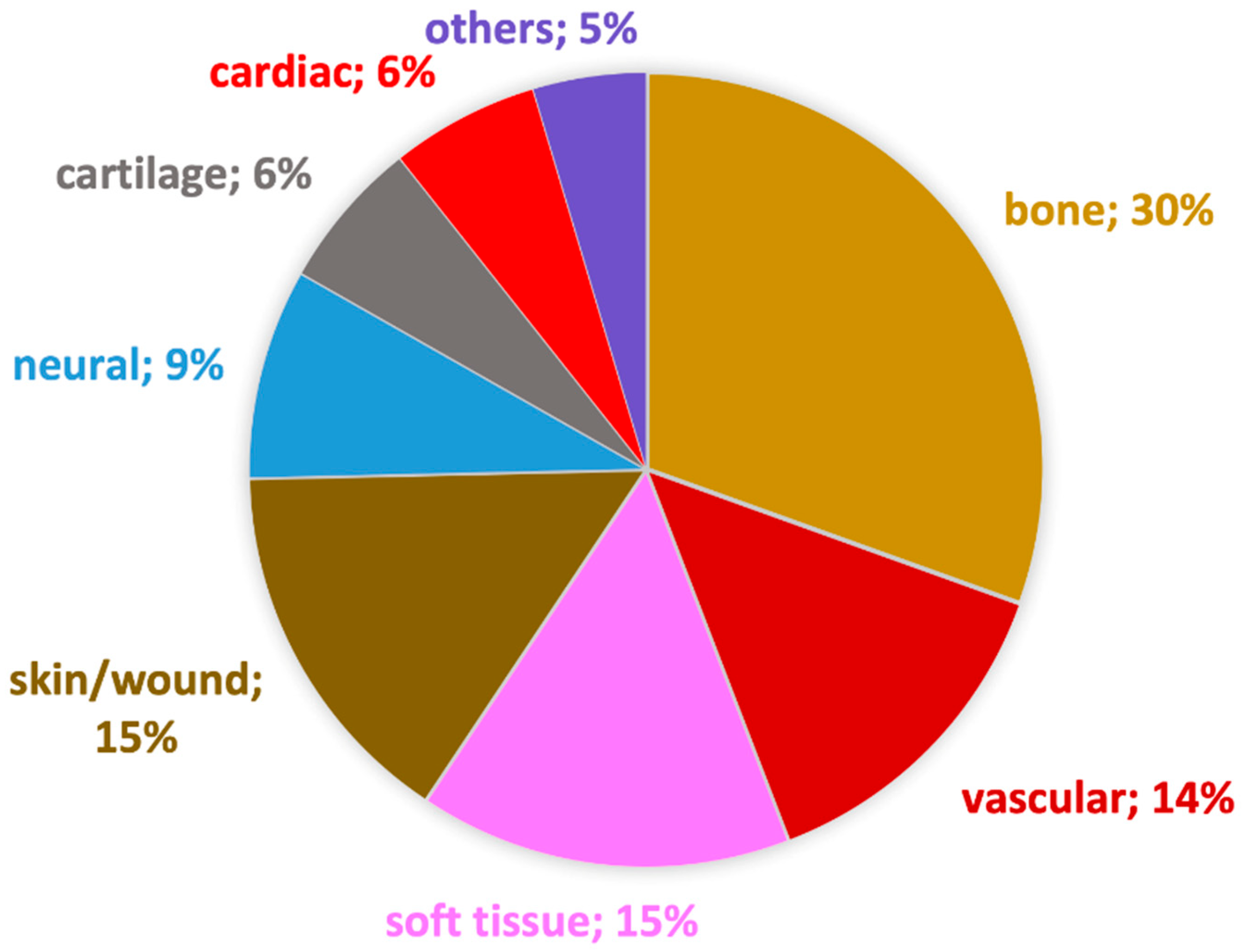
| Type of Use | Type of Production | Polymer | Solvent | Bioactive Agent | Excipients | Cell Line/Animal | Ref. |
|---|---|---|---|---|---|---|---|
| Wound dressing | Single-needle solution ES | Starch-TPU | DMSO, DMF | - | - | NHDFs, Sprague Dawley rats | [80] |
| Wound dressing | Single-needle solution ES | Functionalized CS | 80% AcOH | - | PEO (sacrificial polymer), 2-formylphenyl-boronic acid (imination reactant/reagent) | NHDFs | [81] |
| Wound dressing | Single-needle solution ES | Alginate/dextran and alginate/PEO | DW, pH 5.5 PBS | - | PBS (conductivity); P407 and TX100 (surface tension) | NHDFs | [82] |
| Wound dressing | Single-needle solution ES | PVDF, zP(S-r-4VP) zwitterionic copolymer | DMF, acetone | - | - | L929 mouse fibroblast cells, mice | [83] |
| Wound dressing | Single-needle solution ES | SF/PCL | HFIP | - | CS and COL type I (coat) | NHDFs | [84] |
| Wound dressing | Coaxial ES | DMF, DW | - | - | L929 mouse fibroblast cells, Albino Wister rats | [85] | |
| Wound dressing | Single-needle solution ES | CS/PEO | 80% AcOH | - | HA (coat) | NHDFs | [86] |
| Wound dressing | Single-needle solution ES | CS/PEO | 80% AcOH | - | - | NHDFs | [87] |
| Wound dressing | Single-needle solution ES | GEL/PCL | HFIP | Ag and Mg ions | - | NHDFs, HUVECs, Sprague Dawley rats | [88] |
| Wound dressing | Single-needle solution ES | PVA/CS/starch | DW, AcOH | - | - | L929 mouse fibroblast cells | [89] |
| Wound dressing | Single-needle solution ES | CS/PCL—HA/PEO bilayered scaffolds | Formic acid, acetone, DW | - | - | Vero cell (monkey epithelial cell line) | [90] |
| Wound dressing | Single-needle solution ES | CS/PEO | AcOH, DW | - | Benzophenone (photoinitiator for photo-crosslinking) | - | [91] |
| Wound dressing | Single-needle solution ES | PCL/COL | HFIP | - | - | Human foreskin fibroblasts, Sprague Dawley rats | [92] |
| Wound dressing | Single-needle solution ES | CS/PVA | 80% AcOH, DW | - | NaBH4 solution (3D layered NF sponge creation) | 3T3 mouse fibroblasts, JB6 epidermal cells, C57BL/6 mice | [93] |
| Wound dressing | Single-needle solution ES | VDF-TeFE/PVP | Acetone, isopropanol, DMF | ZnO | - | Wistar rats | [94] |
| Wound dressing or implant coating | Single-needle solution ES | PET | TFA, DCM | Ag nanoparticles | - | AFSCs, CD1 mice | [95] |
| Skin tissue scaffold | Co-ES + electroblowing | Soy protein isolate/PEO—PEO | HFIP, ethanol | - | - | HDFBs, RAW 264.7 murine macrophage cell line | [96] |
| Skin tissue scaffold | Hierarchical construction ES (Sandwich mode) | PLGA/SF | THF, DMF, formic acid | - | - | Human skin stem cells | [97] |
| Skin tissue scaffold | Single-needle solution ES | PCL/silk sericin | TFE, formic acid | - | 3D-printed CS/sodium alginate hydrogel (bottom layer) | NHDFs | [98] |
| Skin tissue scaffold | Single-needle solution ES | PCL | DCM, DMF | - | Poloxamer 407 | BMSCs (C57BL/6 mice) | [99] |
| Tissue engineering scaffolds | Single-needle solution ES | EPU/SF | TFA | - | - | Fibroblast cells from human neonatal foreskin | [100] |
| Tissue engineering scaffolds | Single-needle solution ES | PLA/CS | Chloroform, AcOH | - | - | GM07492 human fibroblast cells | [101] |
| Tissue engineering scaffolds | Single-needle solution ES | PCL | HFIP | - | Neutralized COL (coat) | C57BL/6 mice, de-identified healthy small intestine tissues from discarded surgical samples of infant, teenager or adult | [102] |
| Tissue engineering scaffolds | Wet ES (+CO2 foaming) | PLA | Chloroform, DMF | - | - | NIH 3T3 fibroblasts | [103] |
| Tissue engineering scaffolds | Coaxial ES | PU/CS | THF, DMF | - | PEO (co-spinning polymer of CS) | - | [104] |
| Tissue engineering scaffolds | Single-needle solution ES | PCL | Acetone | Y2O3 nanoparticles | - | L-929 mouse fibroblast cells, UMR-106 rat osteoblast-like cells, Sprague Dawley rats | [105] |
| Tissue engineering scaffolds | Single-needle solution ES | PCL | DCM, DMF | - | - | Green fluorescent protein (GFP)-labeled fibroblasts, rat neural progenitor cells, rats | [106] |
| Tissue engineering scaffolds | Coaxial ES | PCL—core; PEG-NB—shell | HFIP | - | Irgacure 2959 (photoinitiator for UV polymerization) | Bovine pulmonary artery endothelial cells, Sprague Dawley rats | [107] |
| Tissue engineering scaffolds | Single-nozzle solution ES combined with extrusion-based 3D-printing technology | PS | DMF, THF | - | 85% phosphoric acid solution (doping agent) | - | [108] |
| Tissue engineering scaffolds | Single-needle solution ES | PU/carbon nanotube composites | DMF | _ | _ | HUVECs | [109] |
| Bladder tissue engineering scaffolds | Coaxial ES | PLCL—core; HA—shell | HFIP, formic acid | - | - | Rat bladder smooth muscle cells, Sprague Dawley rats | [110] |
| Bladder tissue engineering scaffolds | Single-needle solution ES | PLCL | HFIP | - | COL type I (coat) | hADSCs, Sprague–Dawley rats | [111] |
| Dura mater substitute | Near-field solution ES | n-octyl-2-cyanoacrylate | - | - | - | Harvested dura | [112] |
| Dura mater substitute | Coaxial ES | Tetramethylpyrazine—core; PLGA—shell | Ethanol, HFIP | - | CS (PLGA/CS graft) | SH-SY5Y human neuroblastoma cells, fibroblasts | [113] |
| Dura mater substitute (triple-layered) | Single-needle solution ES—inner and middle layer; melt-based electrohydrodynamic printing—outer layer | PCL | HFIP | Gentamicin—inner layer; nano-hydroxyapatite—outer layer | - | NHDFs, MC3T3-E1 cells | [114] |
| Interface tissue engineering scaffolds | Single-needle solution ES | PCL | Chloroform, DMF | - | - | - | [115] |
| Oral hard- and soft-tissue engineering scaffolds | Melt ES writing | PCL | - | - | - | MG63 human osteoblast-like cells, HaCaT keratinocyte cells, L929 fibroblast cells | [40] |
| Bone tissue engineering scaffolds | Single-needle solution ES + melt ES writing | GEL—solution ES; PCL—melt ES writing | AcOH | - | - | Saos-2 cells | [116] |
| Bone tissue engineering scaffolds | Modified free surface (bubble) ES | PVA | DW | - | Sodium dodecyl benzene sulfonates (surfactant) | - | [117] |
| Bone tissue engineering scaffolds | Single-needle solution ES | HA/PEO, PVA | DW | TGF-β 2, Baicalein | - | - | [118] |
| Bone tissue engineering scaffolds | Single-needle solution ES | CA/PCL | HFIP | - | CS (aerogel) | MC3T3-E1 murine osteoblast cells | [119] |
| Artificial blood vessels | Single-needle solution ES | dPCU | HFIP | - | - | Sprague Dawley rats | [120] |
| Artificial blood vessels | Multi-nozzle solution ES and co-ES | Bovine GEL/PCL | 20% AcOH, DMF, DCM | - | - | 3T3 mouse fibroblasts | [121] |
| Artificial blood vessels | Single-needle solution ES—inner layer; co-ES—outer layer | RHC/PCL—inner layer; PCL—outer layer | HFIP, ethanol | - | PEO (sacrificial polymer) | HUVECs—inner layer; A7r5 rat smooth muscle cells—outer layer | [122] |
| Artificial blood vessels | Single-needle solution ES | PEUU | HFIP | Heparin | PEG (to earn PEUU@PEG-Hep grafts) | HUVECs, rats and New Zealand white rabbits | [123] |
| Artificial blood vessels | Coaxial ES | COL | DW | - | PVP (sacrificial polymer) | HUVECs | [124] |
| Artificial blood vessels | Single-needle solution ES + magnetic environment—inner layer; double-nozzle ES—middle layer; single-needle solution ES—outer layer | PLCL/COL–PLGA/SF–PLCL/COL tri-layer graft | HFIP | - | - | HUVECs, smooth muscle cells, male nude mice | [125] |
| Cardiovascular stent coating | Coaxial ES | PU—core; PECA—shell | THF, DMF, acetone, DMSO | - | - | NIH-3T3 mouse fibroblasts, platelet | [126] |
| Cardiovascular stent coating | Single-needle solution ES | Co-recombiner silk-elastin | TFE | - | - | HUVECs | [127] |
| Drug-eluting stent coating | Single-needle solution ES | PCL/HSA | HFIP | Paclitaxel | Triethylamine | Rabbit iliac artery (drug-release study) | [128] |
| Drug-eluting stent coating | Single-needle solution ES | CS/PEO/HPβCD | 90% AcOH | Simvastatin | - | HPMEC | [129] |
| Drug-eluting stent coating | Single-needle solution ES | PLGA | HFIP | Vildagliptin | - | HUVECs, New Zealand white rabbits | [130] |
| Drug-eluting stent coating | Microfluidic ES | GelMA/PEGDA—inner layer; PCL—outer layer | DW, methanol, DCM | Heparin, VEGF | Polydopamine (adherence enhancer), 2-hydroxy-2-methylpropiophenone (photoinitiator for photocrosslinking) | HUVECs, HUASMCs, New Zealand white rabbits | [131] |
| Respiratory mask | Nozzle-free ES (NTP120 setup) | PAN | DMF | Tea tree essential oil | Polyamidoamine dendritic polymers (drug delivery) | - | [132] |
| Respiratory mask | Corona ES | PVDF | DMF, acetone | - | - | - | [133] |
| Respiratory mask | Single-needle solution ES | PCL | Acetone | - | - | - | [134] |
| Brand Name | Intended Use | Approved |
|---|---|---|
| Bio Hygienic Mask | Compostable mask with FFP2-like filtration capacity | Spain |
| Bioweb™ | Stent coating composite | In the pipeline |
| Cerafix® Dura Substitute | Regenerative dural repair patch | USA |
| Covora™ | Soft-tissue engineering matrix | USA |
| EktoTherix™ | Soft-tissue scaffold | Completed clinical trial |
| Inofilter® 95/99 | Face mask | USA |
| PK Papyrus | Covered stent | USA |
| ReBOSSIS-J | Absorbent bone regenerated material | Japan |
| ReDura™ | Regenerative dural repair patch | Unknown status clinical trial |
| Restrata® Wound Matrix | Absorbable wound dressing | USA |
| Rivelin® plain patches | Wound patches | Completed clinical trial |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uhljar, L.É.; Ambrus, R. Electrospinning of Potential Medical Devices (Wound Dressings, Tissue Engineering Scaffolds, Face Masks) and Their Regulatory Approach. Pharmaceutics 2023, 15, 417. https://doi.org/10.3390/pharmaceutics15020417
Uhljar LÉ, Ambrus R. Electrospinning of Potential Medical Devices (Wound Dressings, Tissue Engineering Scaffolds, Face Masks) and Their Regulatory Approach. Pharmaceutics. 2023; 15(2):417. https://doi.org/10.3390/pharmaceutics15020417
Chicago/Turabian StyleUhljar, Luca Éva, and Rita Ambrus. 2023. "Electrospinning of Potential Medical Devices (Wound Dressings, Tissue Engineering Scaffolds, Face Masks) and Their Regulatory Approach" Pharmaceutics 15, no. 2: 417. https://doi.org/10.3390/pharmaceutics15020417
APA StyleUhljar, L. É., & Ambrus, R. (2023). Electrospinning of Potential Medical Devices (Wound Dressings, Tissue Engineering Scaffolds, Face Masks) and Their Regulatory Approach. Pharmaceutics, 15(2), 417. https://doi.org/10.3390/pharmaceutics15020417








