A Diphenylalanine Based Pentapeptide with Fibrillating Self-Assembling Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Peptide Synthesis
2.3. Reversed-Phase-High Performance Liquid Chromatography
2.4. MALDI-ToF Mass Spectrometry
2.5. Spectrophotometric and Spectrofluorimetric Studies
2.6. Atomic Force Microscopy
2.7. Replica Exchange and Coarse-Grain Molecular Dynamics Simulations
2.8. Data Analysis
3. Results and Discussion
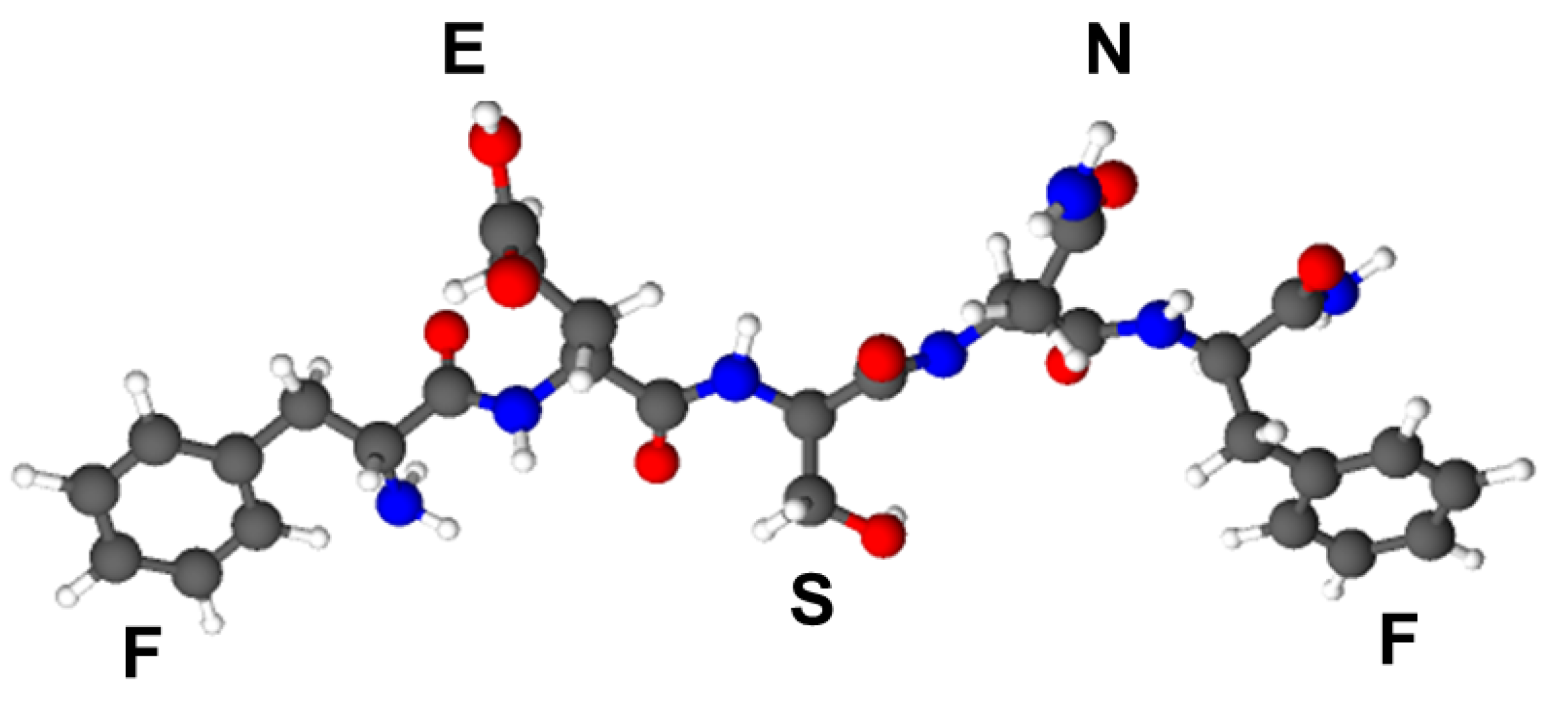
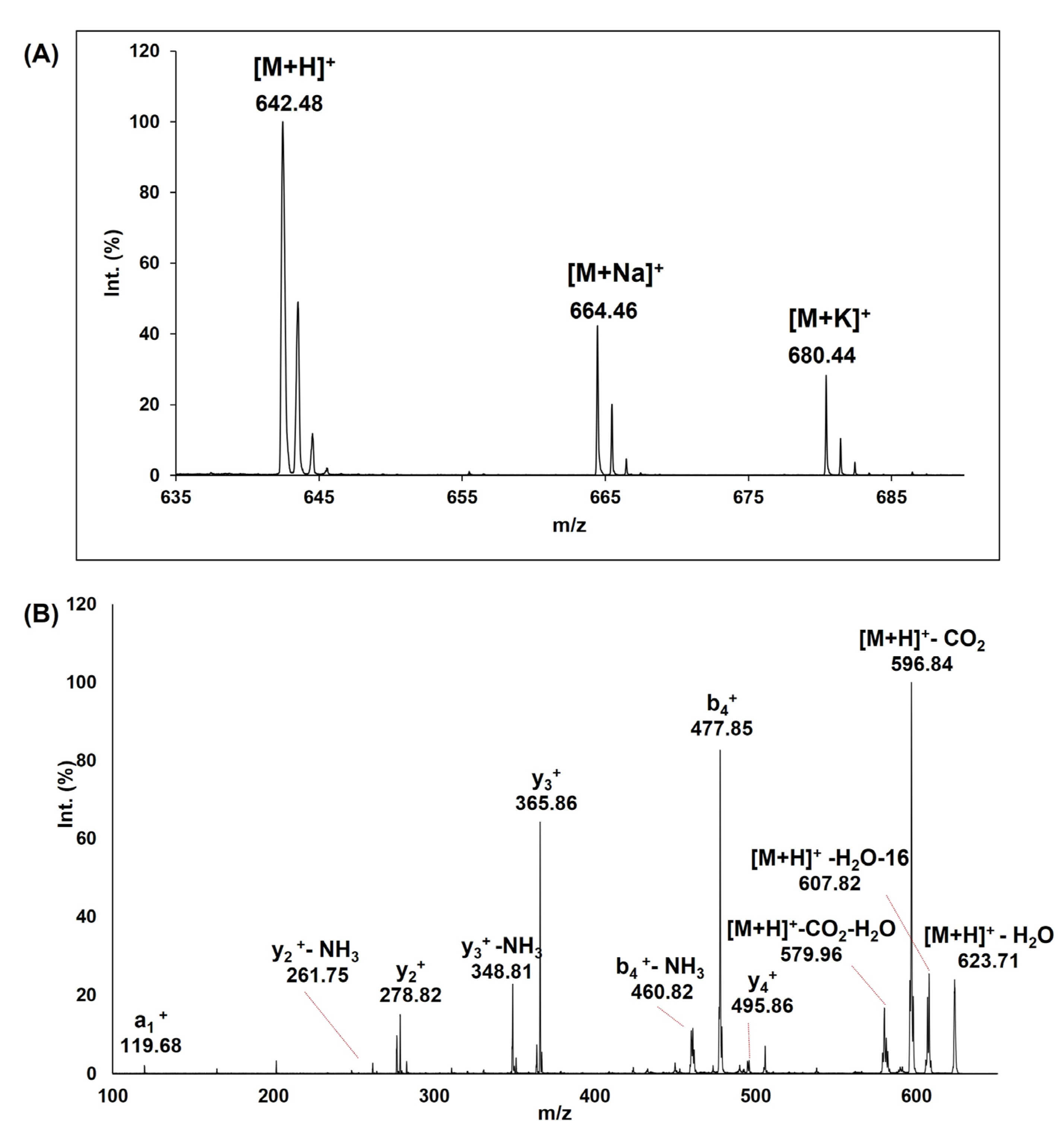
3.1. Peptide Synthesis, Purification and MS Characterization
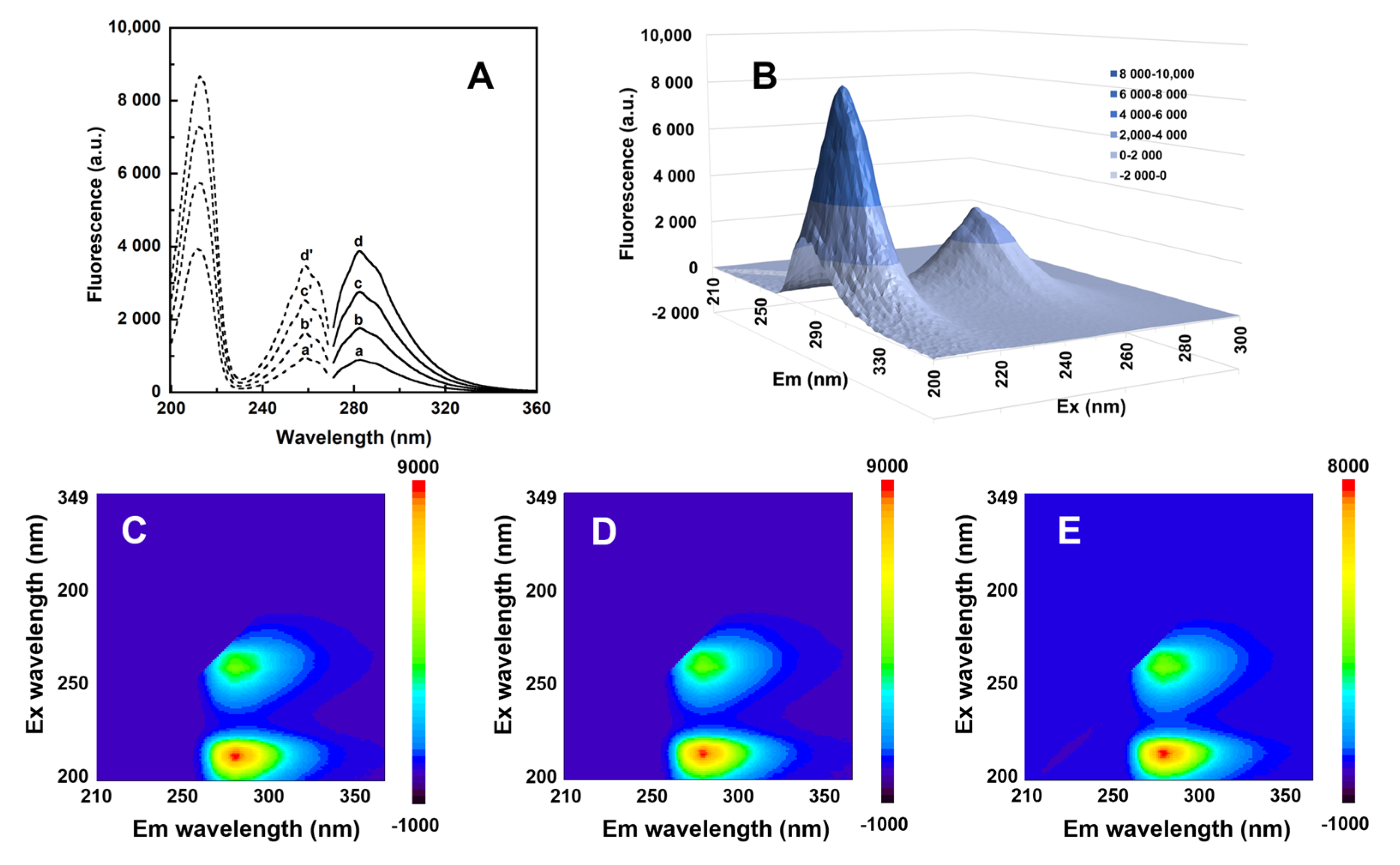
3.2. Spectral Properties Studies
3.3. Peptide Aggregation Studies
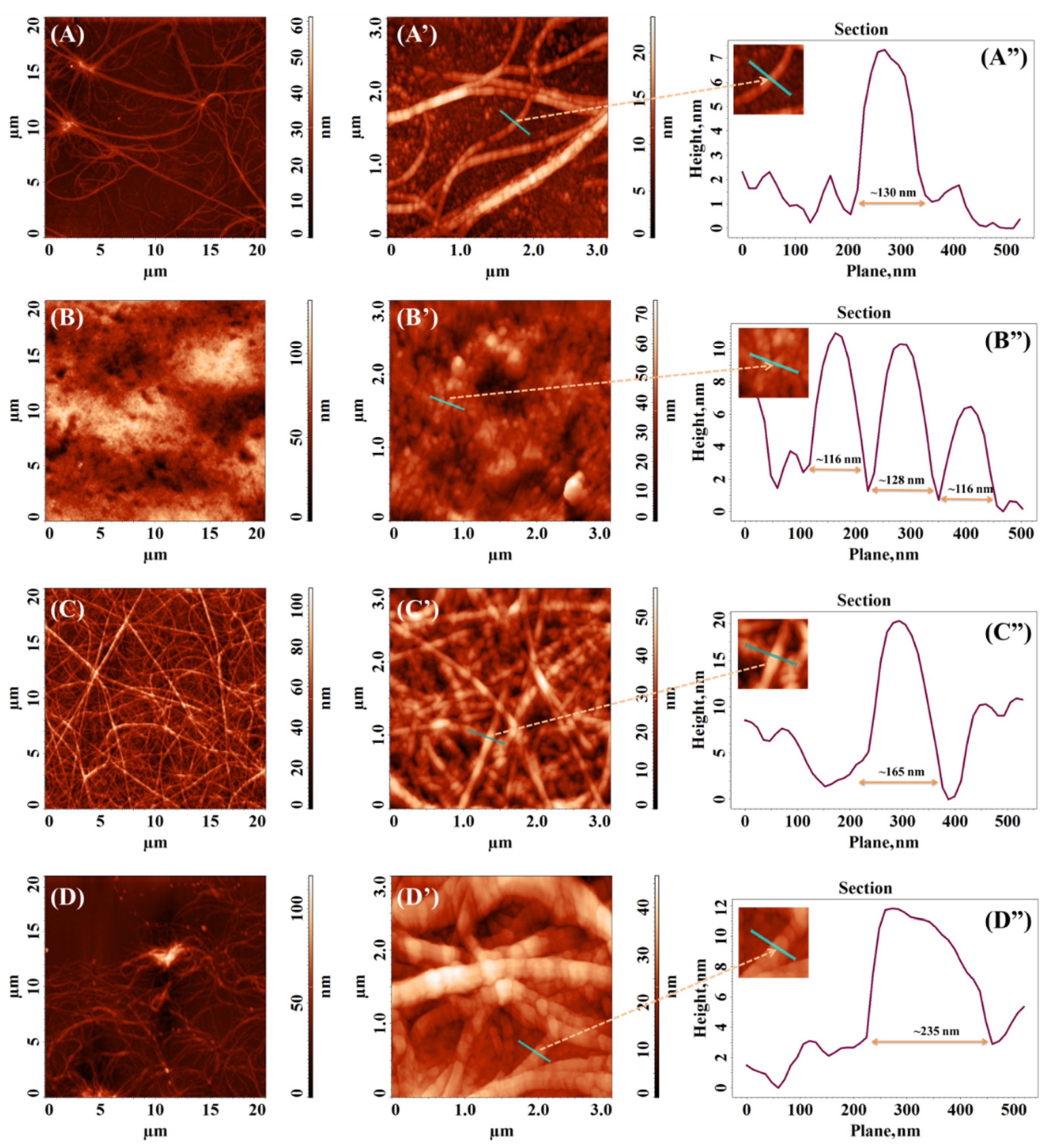
3.4. Atomic Force Microscopy (AFM)
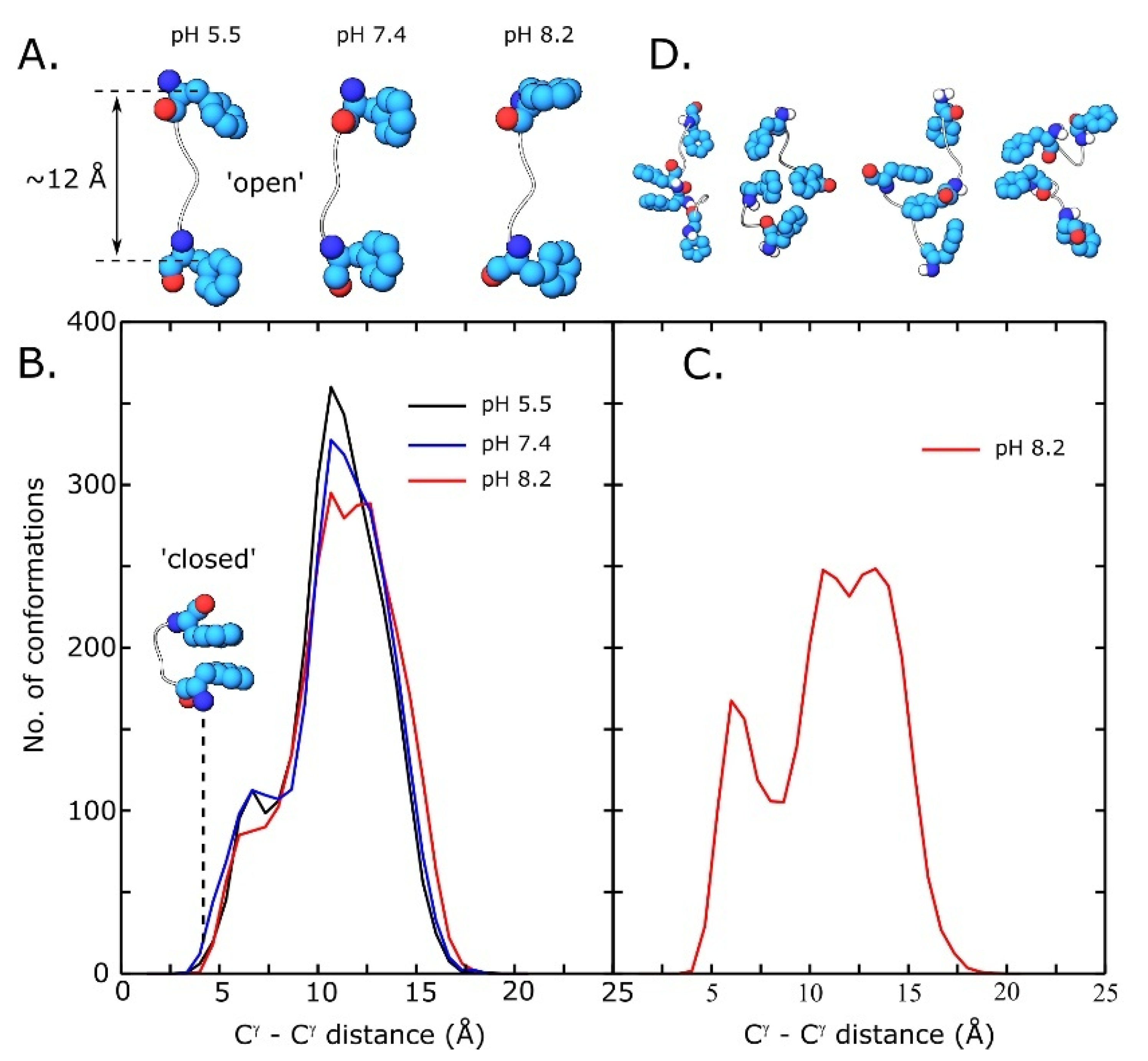
3.5. Replica Exchange Molecular Dynamics Simulations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, J.; An, H.-W.; Wang, H. Self-Assembled Peptide Drug Delivery Systems. ACS Appl. Bio Mater. 2021, 4, 24–46. [Google Scholar] [CrossRef]
- La Manna, S.; Di Natale, C.; Onesto, V.; Marasco, D. Self-Assembling Peptides: From Design to Biomedical Applications. Int. J. Mol. Sci. 2021, 22, 12662. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Feng, Z.; Xu, B. Supramolecular Assemblies of Peptides or Nucleopeptides for Gene Delivery. Theranostics 2019, 9, 3213–3222. [Google Scholar] [CrossRef] [PubMed]
- Ferraboschi, P.; Ciceri, S.; Grisenti, P. Applications of Lysozyme, an Innate Immune Defense Factor, as an Alternative Antibiotic. Antibiotics 2021, 10, 1534. [Google Scholar] [CrossRef] [PubMed]
- Chaari, A.; Fahy, C.; Chevillot-Biraud, A.; Rholam, M. Insights into Kinetics of Agitation-Induced Aggregation of Hen Lysozyme under Heat and Acidic Conditions from Various Spectroscopic Methods. PLoS ONE 2015, 10, e0142095. [Google Scholar] [CrossRef] [PubMed]
- Kummer, N.; Wu, T.; De France, K.J.; Zuber, F.; Ren, Q.; Fischer, P.; Campioni, S.; Nyström, G. Self-Assembly Pathways and Antimicrobial Properties of Lysozyme in Different Aggregation States. Biomacromolecules 2021, 22, 4327–4336. [Google Scholar] [CrossRef] [PubMed]
- van Dalen, M.; Post, J.; Karperien, M.; Claessens, M. Lysozyme Self-Assembles into Amyloid Networks That Support Cartilage Tissue Formation. Osteoarthr. Cartil. 2016, 24, S465. [Google Scholar] [CrossRef]
- Zein, H.F.; Alam, I.; Asanithi, P.; Sutthibutpong, T. Molecular Dynamics Study on the Effects of Charged Amino Acid Distribution under Low PH Condition to the Unfolding of Hen Egg White Lysozyme and Formation of Beta Strands. PLoS ONE 2022, 17, e0249742. [Google Scholar] [CrossRef]
- Morozova-Roche, L.A. Equine Lysozyme: The Molecular Basis of Folding, Self-Assembly and Innate Amyloid Toxicity. FEBS Lett. 2007, 581, 2587–2592. [Google Scholar] [CrossRef]
- Tokunaga, Y.; Sakakibara, Y.; Kamada, Y.; Watanabe, K.; Sugimoto, Y. Analysis of Core Region from Egg White Lysozyme Forming Amyloid Fibrils. Int. J. Biol. Sci. 2013, 9, 219–227. [Google Scholar] [CrossRef]
- Yang, L.; Li, H.; Yao, L.; Yu, Y.; Ma, G. Amyloid-Based Injectable Hydrogel Derived from Hydrolyzed Hen Egg White Lysozyme. ACS Omega 2019, 4, 8071–8080. [Google Scholar] [CrossRef] [PubMed]
- Memarpoor-Yazdi, M.; Asoodeh, A.; Chamani, J. A Novel Antioxidant and Antimicrobial Peptide from Hen Egg White Lysozyme Hydrolysates. J. Funct. Foods 2012, 4, 278–286. [Google Scholar] [CrossRef]
- Carrillo, W.; Ramos, M. Identification of Antimicrobial Peptides of Native and Heated Hydrolysates from Hen Egg White Lysozyme. J. Med. Food 2018, 21, 915–926. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-Y.; Pownall, H.J.; Gotto, A.M. Identification of Peptides Containing Tryptophan, Tyrosine, and Phenylalanine Using Photodiode-Array Spectrophotometry. Anal. Biochem. 1985, 145, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Radotić, K.; Melø, T.B.; Leblanc, R.M.; Yousef, Y.A.; Naqvi, K.R. Fluorescence and Phosphorescence of Tryptophan in Peptides of Different Length and Sequence. J. Photochem. Photobiol. B Biol. 2016, 157, 120–128. [Google Scholar] [CrossRef]
- Lee, J.; Ju, M.; Cho, O.H.; Kim, Y.; Nam, K.T. Tyrosine-Rich Peptides as a Platform for Assembly and Material Synthesis. Adv. Sci. 2019, 6, 1801255. [Google Scholar] [CrossRef]
- Żamojć, K.; Kamrowski, D.; Zdrowowicz, M.; Wyrzykowski, D.; Wiczk, W.; Chmurzyński, L.; Makowska, J. A Pentapeptide with Tyrosine Moiety as Fluorescent Chemosensor for Selective Nanomolar-Level Detection of Copper(II) Ions. Int. J. Mol. Sci. 2020, 21, 743. [Google Scholar] [CrossRef]
- Nathanael, J.G.; Gamon, L.F.; Cordes, M.; Rablen, P.R.; Bally, T.; Fromm, K.M.; Giese, B.; Wille, U. Amide Neighbouring-Group Effects in Peptides: Phenylalanine as Relay Amino Acid in Long-Distance Electron Transfer. ChemBioChem 2018, 19, 922–926. [Google Scholar] [CrossRef]
- Ketnawa, S.; Wickramathilaka, M.; Liceaga, A.M. Changes on Antioxidant Activity of Microwave-Treated Protein Hydrolysates after Simulated Gastrointestinal Digestion: Purification and Identification. Food Chem. 2018, 254, 36–46. [Google Scholar] [CrossRef]
- Thomas, A.; Meurisse, R.; Charloteaux, B.; Brasseur, R. Aromatic Side-Chain Interactions in Proteins. I. Main Structural Features. Proteins Struct. Funct. Genet. 2002, 48, 628–634. [Google Scholar] [CrossRef]
- Pérez-Madrigal, M.M.; Gil, A.M.; Casanovas, J.; Jiménez, A.I.; Macor, L.P.; Alemán, C. Self-Assembly Pathways in a Triphenylalanine Peptide Capped with Aromatic Groups. Colloids Surf. B Biointerfaces 2022, 216, 112522. [Google Scholar] [CrossRef] [PubMed]
- Mayans, E.; Alemán, C. Revisiting the Self-Assembly of Highly Aromatic Phenylalanine Homopeptides. Molecules 2020, 25, 6037. [Google Scholar] [CrossRef] [PubMed]
- Pashuck, E.T.; Cui, H.; Stupp, S.I. Tuning Supramolecular Rigidity of Peptide Fibers through Molecular Structure. J. Am. Chem. Soc. 2010, 132, 6041–6046. [Google Scholar] [CrossRef] [PubMed]
- Hamley, I.W.; Castelletto, V.; Moulton, C.M.; Rodríguez-Pérez, J.; Squires, A.M.; Eralp, T.; Held, G.; Hicks, M.R.; Rodger, A. Alignment of a Model Amyloid Peptide Fragment in Bulk and at a Solid Surface. J. Phys. Chem. B 2010, 114, 8244–8254. [Google Scholar] [CrossRef] [PubMed]
- Catalini, S.; Perinelli, D.R.; Sassi, P.; Comez, L.; Palmieri, G.F.; Morresi, A.; Bonacucina, G.; Foggi, P.; Pucciarelli, S.; Paolantoni, M. Amyloid Self-Assembly of Lysozyme in Self-Crowded Conditions: The Formation of a Protein Oligomer Hydrogel. Biomacromolecules 2021, 22, 1147–1158. [Google Scholar] [CrossRef]
- Maurer-Stroh, S.; Debulpaep, M.; Kuemmerer, N.; de la Paz, M.L.; Martins, I.C.; Reumers, J.; Morris, K.L.; Copland, A.; Serpell, L.; Serrano, L.; et al. Exploring the Sequence Determinants of Amyloid Structure Using Position-Specific Scoring Matrices. Nat. Methods 2010, 7, 237–242. [Google Scholar] [CrossRef]
- Rousseau, F.; Schymkowitz, J.; Serrano, L. Protein Aggregation and Amyloidosis: Confusion of the Kinds? Curr. Opin. Struct. Biol. 2006, 16, 118–126. [Google Scholar] [CrossRef]
- Gugasyan, R.; Vidavsky, I.; Nelson, C.A.; Gross, M.L.; Unanue, E.R. Isolation and Quantitation of a Minor Determinant of Hen Egg White Lysozyme Bound to I-Ak by Using Peptide-Specific Immunoaffinity. J. Immunol. 1998, 161, 6074–6083. [Google Scholar] [CrossRef]
- Xie, B.; Sharp, J.S. Relative Quantification of Sites of Peptide and Protein Modification Using Size Exclusion Chromatography Coupled with Electron Transfer Dissociation. J. Am. Soc. Mass Spectrom. 2016, 27, 1322–1327. [Google Scholar] [CrossRef]
- Thakur, K.S.; Eswaran, S.V. ESI-MS and Stavrox 3.6.0.1 Investigations of Crosslinking by an Aryl-Azido-NHS-Heterobifunctional Crosslinker. J. Anal. Bioanal. Tech 2018, 9, 1000402. [Google Scholar] [CrossRef]
- Jayawarna, V.; Richardson, S.M.; Hirst, A.R.; Hodson, N.W.; Saiani, A.; Gough, J.E.; Ulijn, R.V. Introducing Chemical Functionality in Fmoc-Peptide Gels for Cell Culture. Acta Biomater. 2009, 5, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Hansen, P.R.; Oddo, A. Fmoc Solid-Phase Peptide Synthesis. In Peptide Antibodies; Humana Press: New York, NY, USA, 2015; pp. 33–50. [Google Scholar]
- Habasescu, L.; Jureschi, M.; Petre, B.-A.; Mihai, M.; Gradinaru, R.-V.; Murariu, M.; Drochioiu, G. Histidine-Lacked Aβ(1–16) Peptides: pH-Dependent Conformational Changes in Metal Ion Binding. Int. J. Pept. Res. Ther. 2020, 26, 2529–2546. [Google Scholar] [CrossRef]
- Mocanu, C.S.; Petre, B.A.; Darie-Ion, L.; Drochioiu, G.; Niculaua, M.; Stoica, I.; Homocianu, M.; Nita, L.E.; Gradinaru, V.R. Structural Characterization of a New Collagen Biomimetic Octapeptide with Nanoscale Self-Assembly Potential: Experimental and Theoretical Approaches. ChemPlusChem 2022, 87, e202100462. [Google Scholar] [CrossRef]
- Mergler, M.; Durieux, J.P. The Bachem Practice of SPPS. Tips and Tricks from the Experts at Bachem-Hand Book; BACHEM A.G.: Bubendorf, Switzerland, 2000. [Google Scholar]
- Pignataro, M.F.; Herrera, M.G.; Dodero, V.I. Evaluation of Peptide/Protein Self-Assembly and Aggregation by Spectroscopic Methods. Molecules 2020, 25, 4854. [Google Scholar] [CrossRef] [PubMed]
- Rajan, R.; Ahmed, S.; Sharma, N.; Kumar, N.; Debas, A.; Matsumura, K. Review of the Current State of Protein Aggregation Inhibition from a Materials Chemistry Perspective: Special Focus on Polymeric Materials. Mater. Adv. 2021, 2, 1139–1176. [Google Scholar] [CrossRef]
- Vlad, C.; Lindner, K.; Karreman, C.; Schildknecht, S.; Leist, M.; Tomczyk, N.; Rontree, J.; Langridge, J.; Danzer, K.; Ciossek, T.; et al. Autoproteolytic Fragments Are Intermediates in the Oligomerization/Aggregation of the Parkinson’s Disease Protein Alpha-Synuclein as Revealed by Ion Mobility Mass Spectrometry. ChemBioChem 2011, 12, 2740–2744. [Google Scholar] [CrossRef]
- Zhu, K.; Day, T.; Warshaviak, D.; Murrett, C.; Friesner, R.; Pearlman, D. Antibody Structure Determination Using a Combination of Homology Modeling, Energy-Based Refinement, and Loop Prediction. Proteins Struct. Funct. Bioinforma. 2014, 82, 1646–1655. [Google Scholar] [CrossRef]
- Banks, J.L.; Beard, H.S.; Cao, Y.; Cho, A.E.; Damm, W.; Farid, R.; Felts, A.K.; Halgren, T.A.; Mainz, D.T.; Maple, J.R.; et al. Integrated Modeling Program, Applied Chemical Theory (IMPACT). J. Comput. Chem. 2005, 26, 1752–1780. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of Simple Potential Functions for Simulating Liquid Water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Wang, L.; Friesner, R.A.; Berne, B.J. Replica Exchange with Solute Scaling: A More Efficient Version of Replica Exchange with Solute Tempering (REST2). J. Phys. Chem. B 2011, 115, 9431–9438. [Google Scholar] [CrossRef]
- Bowers, K.J.; Chow, D.E.; Xu, H.; Dror, R.O.; Eastwood, M.P.; Gregersen, B.A.; Klepeis, J.L.; Kolossvary, I.; Moraes, M.A.; Sacerdoti, F.D.; et al. Scalable Algorithms for Molecular Dynamics Simulations on Commodity Clusters. In Proceedings of the ACM/IEEE SC 2006 Conference (SC’06), Tampa, FL, USA, 11–17 November 2006; IEEE: Piscataway, NJ, USA, 2006; p. 43. [Google Scholar]
- Martyna, G.J.; Klein, M.L.; Tuckerman, M. Nosé–Hoover Chains: The Canonical Ensemble via Continuous Dynamics. J. Chem. Phys. 1992, 97, 2635–2643. [Google Scholar] [CrossRef]
- Martyna, G.J.; Tobias, D.J.; Klein, M.L. Constant Pressure Molecular Dynamics Algorithms. J. Chem. Phys. 1994, 101, 4177–4189. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Monticelli, L.; Kandasamy, S.K.; Periole, X.; Larson, R.G.; Tieleman, D.P.; Marrink, S.-J. The MARTINI Coarse-Grained Force Field: Extension to Proteins. J. Chem. Theory Comput. 2008, 4, 819–834. [Google Scholar] [CrossRef] [PubMed]
- Van Der Spoel, D.; Lindahl, E.; Hess, B.; Groenhof, G.; Mark, A.E.; Berendsen, H.J.C. GROMACS: Fast, Flexible, and Free. J. Comput. Chem. 2005, 26, 1701–1718. [Google Scholar] [CrossRef] [PubMed]
- Khatun, J.; Ramkissoon, K.; Giddings, M.C. Fragmentation Characteristics of Collision-Induced Dissociation in MALDI TOF/TOF Mass Spectrometry. Anal. Chem. 2007, 79, 3032–3040. [Google Scholar] [CrossRef]
- Sun, S.; Yu, C.; Qiao, Y.; Lin, Y.; Dong, G.; Liu, C.; Zhang, J.; Zhang, Z.; Cai, J.; Zhang, H.; et al. Deriving the Probabilities of Water Loss and Ammonia Loss for Amino Acids from Tandem Mass Spectra. J. Proteome Res. 2008, 7, 202–208. [Google Scholar] [CrossRef]
- Stathopoulos, P.; Papas, S.; Tsikaris, V. C-TerminalN-Alkylated Peptide Amides Resulting from the Linker Decomposition of the Rink Amide Resin. A New Cleavage Mixture Prevents Their Formation. J. Pept. Sci. 2006, 12, 227–232. [Google Scholar] [CrossRef]
- Rodger, A. UV Absorbance Spectroscopy of Biological Macromolecules. In Encyclopedia of Biophysics; Springer: Berlin/Heidelberg, Germany, 2013; pp. 2714–2718. [Google Scholar]
- Barazzouk, S.; Daneault, C. Amino Acid and Peptide Immobilization on Oxidized Nanocellulose: Spectroscopic Characterization. Nanomaterials 2012, 2, 187–205. [Google Scholar] [CrossRef]
- Lakowicz, J. Principles of Fluorescence Spectroscopy; Springer: New York, NY, USA, 2006; ISBN 978-0-387-31278-1. [Google Scholar]
- Bortolotti, A.; Wong, Y.H.; Korsholm, S.S.; Bahring, N.H.B.; Bobone, S.; Tayyab, S.; van de Weert, M.; Stella, L. On the Purported “Backbone Fluorescence” in Protein Three-Dimensional Fluorescence Spectra. RSC Adv. 2016, 6, 112870–112876. [Google Scholar] [CrossRef]
- Zapadka, K.L.; Becher, F.J.; Gomes dos Santos, A.L.; Jackson, S.E. Factors Affecting the Physical Stability (Aggregation) of Peptide Therapeutics. Interface Focus 2017, 7, 20170030. [Google Scholar] [CrossRef] [PubMed]
- Housmans, J.A.J.; Wu, G.; Schymkowitz, J.; Rousseau, F. A Guide to Studying Protein Aggregation. FEBS J. 2021. [Google Scholar] [CrossRef] [PubMed]
- Anand, B.G.; Dubey, K.; Shekhawat, D.S.; Kar, K. Intrinsic Property of Phenylalanine to Trigger Protein Aggregation and Hemolysis Has a Direct Relevance to Phenylketonuria. Sci. Rep. 2017, 7, 11146. [Google Scholar] [CrossRef] [PubMed]
- Zaguri, D.; Zimmermann, M.R.; Meisl, G.; Levin, A.; Rencus-Lazar, S.; Knowles, T.P.J.; Gazit, E. Kinetic and Thermodynamic Driving Factors in the Assembly of Phenylalanine-Based Modules. ACS Nano 2021, 15, 18305–18311. [Google Scholar] [CrossRef] [PubMed]
- Barrera, E.E.; Zonta, F.; Pantano, S. Dissecting the Role of Glutamine in Seeding Peptide Aggregation. Comput. Struct. Biotechnol. J. 2021, 19, 1595–1602. [Google Scholar] [CrossRef]
- Shattuck, J.E.; Waechter, A.C.; Ross, E.D. The Effects of Glutamine/Asparagine Content on Aggregation and Heterologous Prion Induction by Yeast Prion-like Domains. Prion 2017, 11, 249–264. [Google Scholar] [CrossRef]
- Ziaunys, M.; Smirnovas, V. Emergence of Visible Light Optical Properties of L-Phenylalanine Aggregates. PeerJ 2019, 7, e6518. [Google Scholar] [CrossRef]
- Arad, E.; Green, H.; Jelinek, R.; Rapaport, H. Revisiting Thioflavin T (ThT) Fluorescence as a Marker of Protein Fibrillation—The Prominent Role of Electrostatic Interactions. J. Colloid Interface Sci. 2020, 573, 87–95. [Google Scholar] [CrossRef]
- Bernson, D.; Mecinovic, A.; Abed, M.T.; Limé, F.; Jageland, P.; Palmlöf, M.; Esbjörner, E.K. Amyloid Formation of Bovine Insulin Is Retarded in Moderately Acidic pH and by Addition of Short-Chain Alcohols. Eur. Biophys. J. 2020, 49, 145–153. [Google Scholar] [CrossRef]
- Zelenovskiy, P.; Kornev, I.; Vasilev, S.; Kholkin, A. On the Origin of the Great Rigidity of Self-Assembled Diphenylalanine Nanotubes. Phys. Chem. Chem. Phys. 2016, 18, 29681–29685. [Google Scholar] [CrossRef]
- Bachem Peptide Calculator. Available online: http://www.bachem.com/service-support/peptide-calculator/ (accessed on 10 August 2022).
- Guo, M.; Gorman, P.M.; Rico, M.; Chakrabartty, A.; Laurents, D.V. Charge Substitution Shows That Repulsive Electrostatic Interactions Impede the Oligomerization of Alzheimer Amyloid Peptides. FEBS Lett. 2005, 579, 3574–3578. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Lau, L.S.; Legge, R.L.; Chen, P. Critical self-assembly concentration of an ionic-complementary peptide EAK16-I. J. Adhes. 2004, 80, 913–931. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Grădinaru, V.R.; Apetrei, C. Development of a Chemically Modified Sensor Based on a Pentapeptide and Its Application for Sensitive Detection of Verbascoside in Extra Virgin Olive Oil. Int. J. Mol. Sci. 2022, 23, 15704. [Google Scholar] [CrossRef] [PubMed]
- Saiani, A.; Mohammed, A.; Frielinghaus, H.; Collins, R.; Hodson, N.; Kielty, C.M.; Sherratt, M.J.; Miller, A.F. Self-Assembly and Gelation Properties of α-Helix versus β-Sheet Forming Peptides. Soft Matter. 2009, 5, 193–202. [Google Scholar] [CrossRef]
- Marqusee, S.; Baldwin, R.L. Helix Stabilization by Glu-...Lys+ Salt Bridges in Short Peptides of de Novo Design. Proc. Natl. Acad. Sci. USA 1987, 84, 8898–8902. [Google Scholar] [CrossRef] [PubMed]
- Barducci, A.; Pfaendtner, J.; Bonomi, M. Tackling Sampling Challenges in Biomolecular Simulations. In Molecular Modeling of Proteins; Humana Press: New York, NY, USA, 2015; pp. 151–171. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jitaru, S.-C.; Neamtu, A.; Drochioiu, G.; Darie-Ion, L.; Stoica, I.; Petre, B.-A.; Gradinaru, V.-R. A Diphenylalanine Based Pentapeptide with Fibrillating Self-Assembling Properties. Pharmaceutics 2023, 15, 371. https://doi.org/10.3390/pharmaceutics15020371
Jitaru S-C, Neamtu A, Drochioiu G, Darie-Ion L, Stoica I, Petre B-A, Gradinaru V-R. A Diphenylalanine Based Pentapeptide with Fibrillating Self-Assembling Properties. Pharmaceutics. 2023; 15(2):371. https://doi.org/10.3390/pharmaceutics15020371
Chicago/Turabian StyleJitaru, Stefania-Claudia, Andrei Neamtu, Gabi Drochioiu, Laura Darie-Ion, Iuliana Stoica, Brindusa-Alina Petre, and Vasile-Robert Gradinaru. 2023. "A Diphenylalanine Based Pentapeptide with Fibrillating Self-Assembling Properties" Pharmaceutics 15, no. 2: 371. https://doi.org/10.3390/pharmaceutics15020371
APA StyleJitaru, S.-C., Neamtu, A., Drochioiu, G., Darie-Ion, L., Stoica, I., Petre, B.-A., & Gradinaru, V.-R. (2023). A Diphenylalanine Based Pentapeptide with Fibrillating Self-Assembling Properties. Pharmaceutics, 15(2), 371. https://doi.org/10.3390/pharmaceutics15020371









