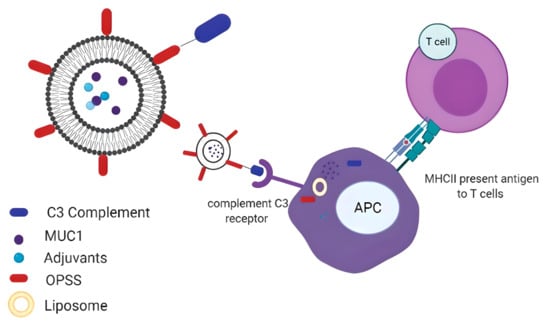
Enhancing T Cell and Antibody Response in Mucin-1 Transgenic Mice through Co-Delivery of Tumor-Associated Mucin-1 Antigen and TLR Agonists in C3-Liposomes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Transgenic Mouse Model, Vaccination Groups and Immunization
2.3. Liposome Preparation
2.4. HPLC-DAD Analysis of MUC1
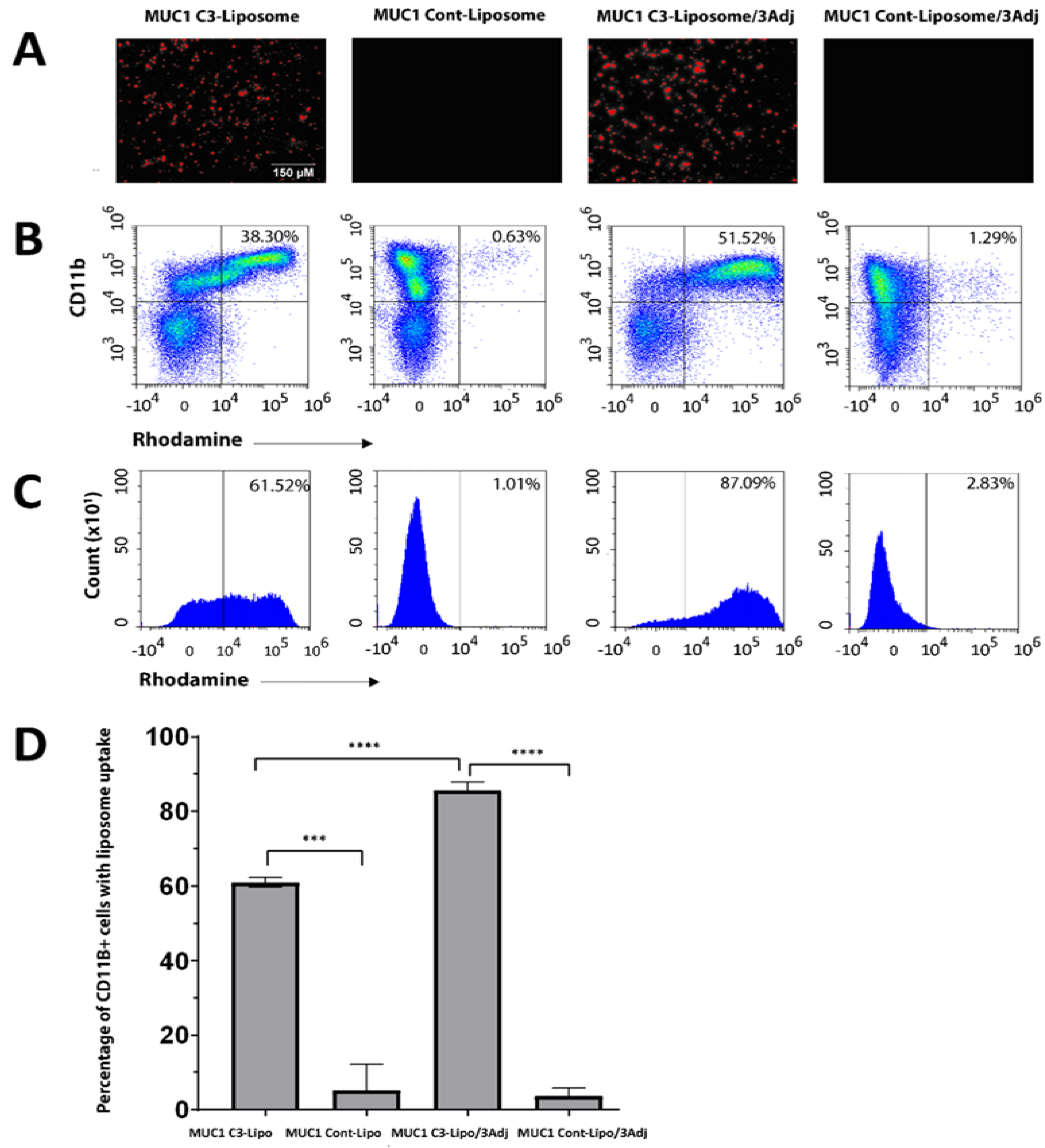
2.5. Targeting and Internalization of Liposomes
2.6. ELISA
2.7. ELISpot
2.8. Flow Cytometry Analyses on Spleen Cells
2.9. Statistical Analysis
3. Results
3.1. MUC1 C3-Liposomes Are Internalized by CD11b+ Immune Cells
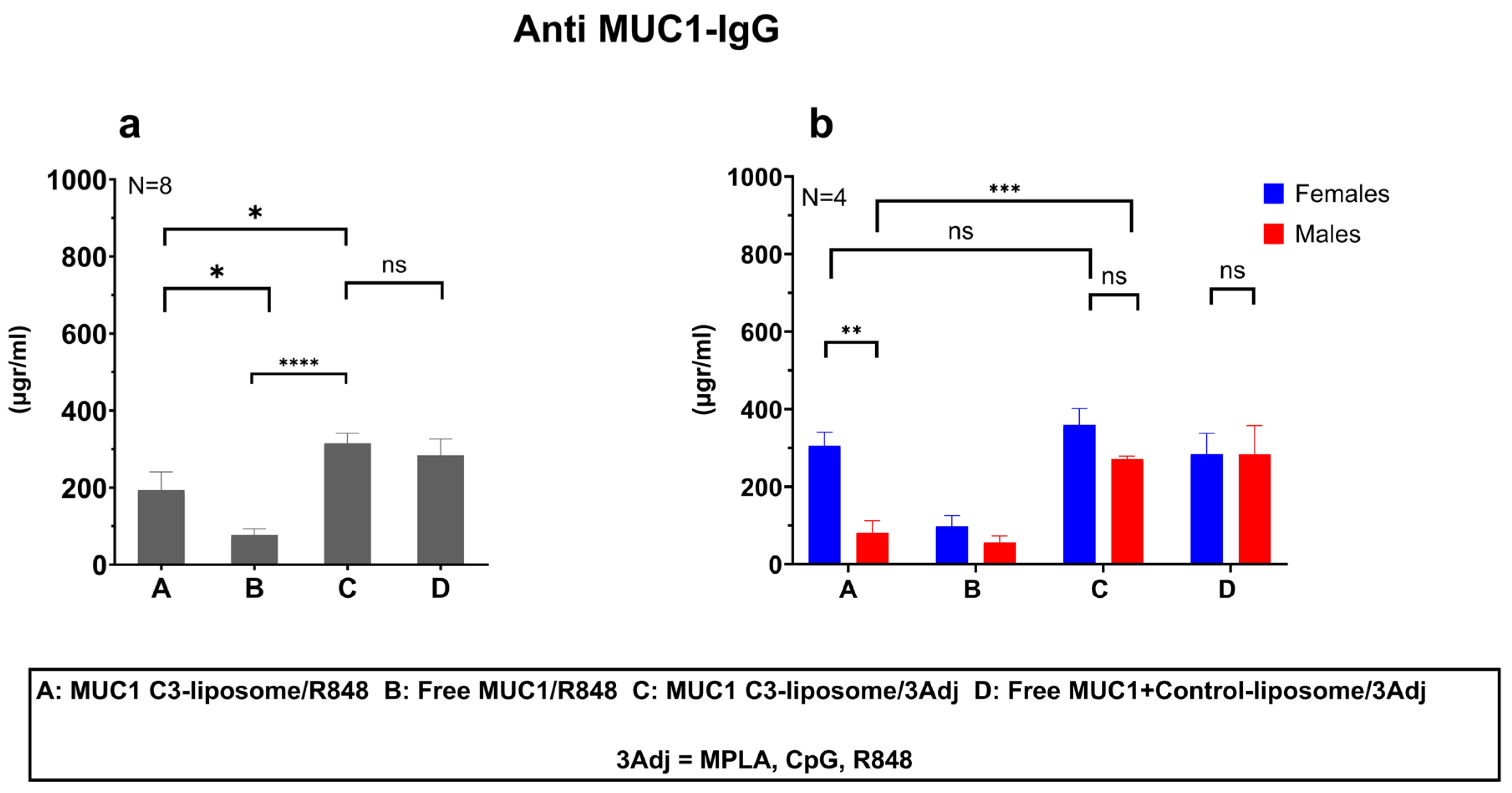
3.2. MUC1 C3-Liposomes with Encapsulated TLR Agonists Induce a MUC1-Specific IgG Antibody Response in MUC1 Transgenic Mice
3.3. Delivery of MUC1 with TLR Agonists in C3-liposomes Eliminated Sex Differences in the Anti-MUC1 Antibody Response
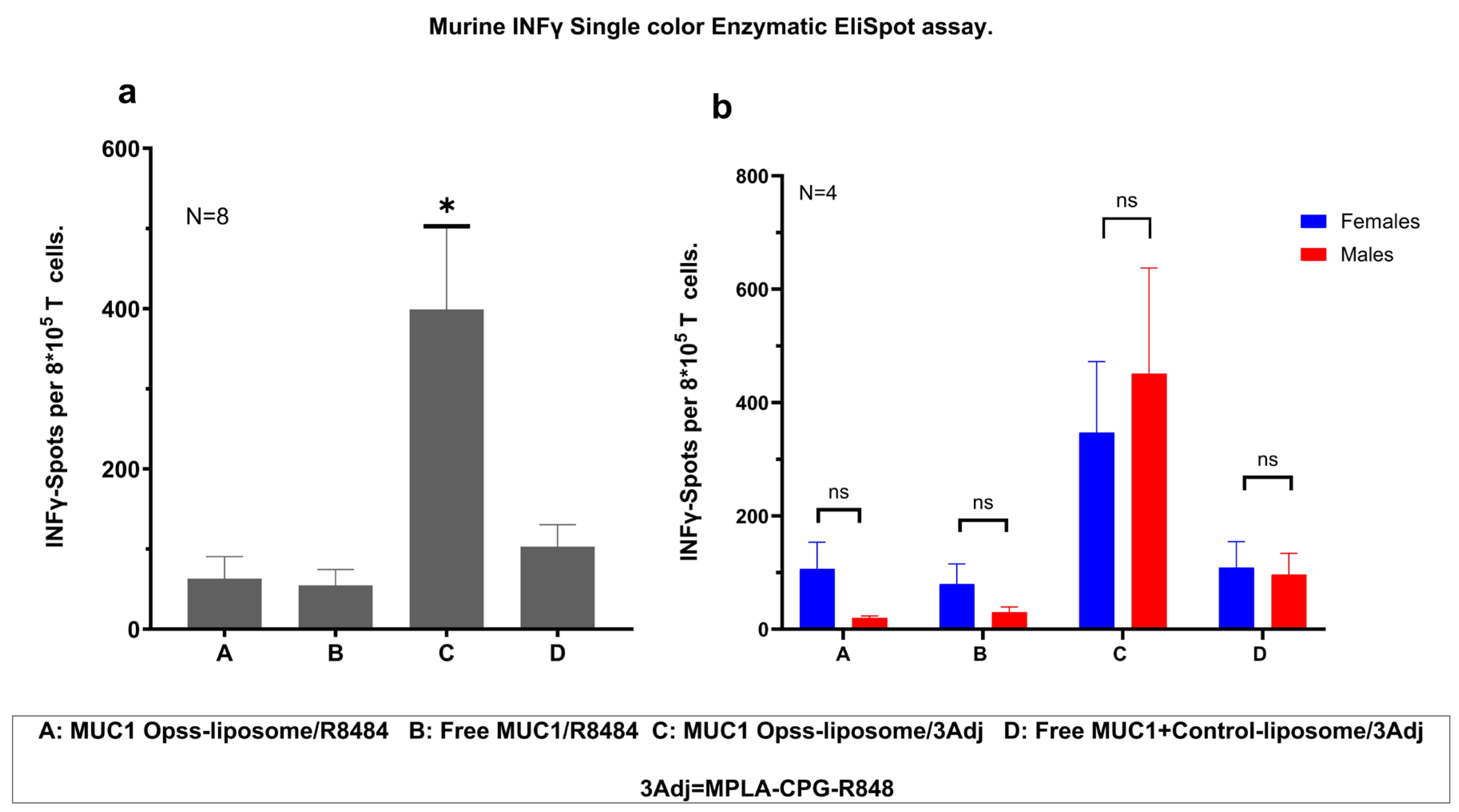
3.4. Vaccination with MUC1 C3-Liposomes and TLR Agonists Induces a Robust Antigen-Specific T Cell Response
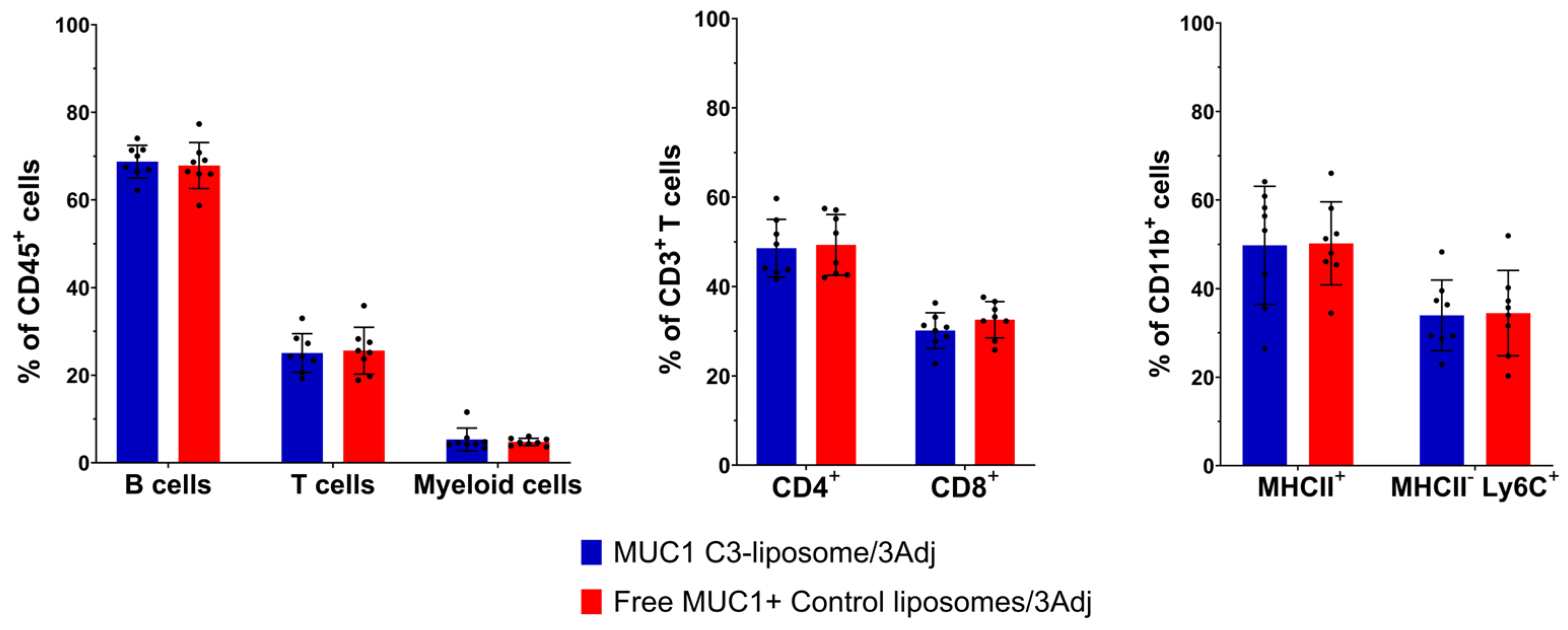
3.5. Analysis of Splenic Lymphocyte Populations in Vaccinated Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Han, K.C.; Park, D.; Ju, S.; Lee, Y.E.; Heo, S.H.; Kim, Y.A.; Lee, J.E.; Lee, Y.; Park, K.H.; Park, S.-H.; et al. Streamlined selection of cancer antigens for vaccine development through integrative multi-omics and high-content cell imaging. Sci. Rep. 2020, 10, 5885. [Google Scholar] [CrossRef] [PubMed]
- Farkona, S.; Diamandis, E.P.; Blasutig, M.I. Cancer immunotherapy: The beginning of the end of cancer? BMC Med. 2016, 14, 73. [Google Scholar] [CrossRef] [PubMed]
- Graziano, D.F.; Finn, O.J. Tumor antigens and tumor antigen discovery. Cancer Treat. Res. 2005, 123, 89–111. [Google Scholar] [PubMed]
- Finn, O.J. The dawn of vaccines for cancer prevention. Nat. Rev. Immunol. 2017, 18, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Tagliamonte, M.; Petrizzo, A.; Tornesello, M.L.; Buonaguro, F.M.; Buonaguro, L. Antigen-specific vaccines for cancer treatment. Hum. Vaccines Immunother. 2014, 10, 3332–3346. [Google Scholar] [CrossRef]
- Taylor-Papadimitriou, J.; Burchell, J.M.; Graham, R.; Beatson, R. Latest developments in MUC1 immunotherapy. Biochem. Soc. Trans. 2018, 46, 659–668. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, Z.; Zhang, S.; Zhu, P.; Ko, J.K.; Yung, K.K. MUC1: Structure, Function, and Clinic Application in Epithelial Cancers. Int. J. Mol. Sci. 2021, 22, 6567. [Google Scholar] [CrossRef]
- Cheever, M.A.; Allison, J.P.; Ferris, A.S.; Finn, O.J.; Hastings, B.M.; Hecht, T.T.; Mellman, I.; Prindiville, S.A.; Viner, J.L.; Weiner, L.M.; et al. The Prioritization of Cancer Antigens: A National Cancer Institute Pilot Project for the Acceleration of Translational Research. Clin. Cancer Res. 2009, 15, 5323–5337. [Google Scholar] [CrossRef]
- Gao, T.; Cen, Q.; Lei, H. A review on development of MUC1-based cancer vaccine. Biomed. Pharmacother. 2020, 132, 110888. [Google Scholar] [CrossRef]
- Kimura, T.; Finn, O.J. MUC1 immunotherapy is here to stay. Expert Opin. Biol. Ther. 2013, 13, 35–49. [Google Scholar] [CrossRef]
- Roulois, D.; Grégoire, M.; Fonteneau, J.F. MUC1-specific cytotoxic T lymphocytes in cancer therapy: Induction and challenge. BioMed Res. Int. 2013, 2013, 871936. [Google Scholar] [CrossRef] [PubMed]
- Nabavinia, M.S.; Gholoobi, A.; Charbgoo, F.; Nabavinia, M.; Ramezani, M.; Abnous, K. Anti-MUC1 aptamer: A potential opportunity for cancer treatment. Med. Res. Rev. 2017, 37, 1518–1539. [Google Scholar] [CrossRef] [PubMed]
- Kalos, M.; June, C.H. Adoptive T cell transfer for cancer immunotherapy in the era of synthetic biology. Immunity 2013, 30, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Marasini, N.; Ghaffar, K.A.; Skwarczynski, M.; Toth, I. Liposomes as a Vaccine Delivery System. In Micro and Nanotechnology in Vaccine Development; William Andrew Publishing: Norwich, NY, USA, 2017; pp. 221–239. [Google Scholar]
- Fan, Y.; Moon, J.J. Nanoparticle Drug Delivery Systems Designed to Improve Cancer Vaccines and Immunotherapy. Vaccines 2015, 3, 662–685. [Google Scholar] [CrossRef] [PubMed]
- Joshi, M.D.; Unger, W.J.; Storm, G.; van Kooyk, Y.; Mastrobattista, E. Targeting tumor antigens to dendritic cells using particulate carriers. J. Control. Release 2012, 161, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Anchordoquy, T. Drug delivery trends in clinical trials and translational medicine: Challenges and opportunities in the delivery of nucleic acid-based therapeutics. J. Pharm. Sci. 2011, 100, 38–52. [Google Scholar] [CrossRef] [PubMed]
- Kullberg, M.; Martinson, H.; Mann, K.; Anchordoquy, T.J. Complement C3 mediated targeting of liposomes to granulocytic myeloid derived suppressor cells. Nanomedicine 2015, 11, 1355–1363. [Google Scholar] [CrossRef]
- Francian, A.; Mann, K.; Kullberg, M. Complement C3-dependent uptake of targeted liposomes into human macrophages, B cells, dendritic cells, neutrophils, and MDSCs. Int. J. Nanomed. 2017, 12, 5149–5161. [Google Scholar] [CrossRef]
- Francian, A.; Namen, S.; Stanley, M.; Mann, K.; Martinson, H.; Kullberg, M. Intratumoral delivery of antigen with complement C3-bound liposomes reduces tumor growth in mice. Nanomedicine 2019, 18, 326–335. [Google Scholar] [CrossRef]
- Zhao, H.; Lv, X.; Huang, J.; Huang, S.; Zhou, H.; Wang, H.; Xu, Y.; Wang, J.; Wang, J.; Liu, Z. Two-phase releasing immune-stimulating composite orchestrates protection against microbial infections. Biomaterials 2021, 277, 121106. [Google Scholar] [CrossRef]
- Shimosaton, T.; Kitazawa, H.; Tohno, M.; Katoh, S.; Kawai, Y.; Saito, T. Development of immune assay system for both CpG and non-CpG DNA from lactic acid bacteria using a transfectant of swine Toll-like receptor 9. Anim. Sci. J. 2004, 75, 377–382. [Google Scholar] [CrossRef]
- Van Aalst, S.; Jansen, M.A.A.; Ludwig, I.S.; van der Zee, R.; van Eden, W.; Broere, F. Routing dependent immune responses after experimental R848-adjuvated vaccination. Vaccine 2018, 36, 1405–1413. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, R.K.; Lee, K.M.; McKolanis, J.; Hitbold, E.; Schraut, W.; Moser, A.J.; Warnick, E.; Whiteside, T.; Osborne, J.; Kim, H.; et al. Phase I study of a MUC1 vaccine composed of different doses of MUC1 peptide with SB-AS2 adjuvant in resected and locally advanced pancreatic cancer. Cancer Immunol. Immunother. 2005, 54, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; McKolanis, J.R.; Dzubinski, L.A.; Islam, K.; Potter, D.M.; Salazar, A.M.; Schoen, R.E.; Finn, O.J. MUC1 vaccine for individuals with advanced adenoma of the colon: A cancer immunoprevention feasibility study. Cancer Prev. Res. 2013, 6, 18. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, Y.; Ni, W.; Wu, X.; Zhou, J.; Zhang, Z.; Zhou, H.; Zhang, N.; Jiang, M.; Sang, Q.; et al. TRAF6-overexpressing dendritic cells loaded with MUC1 peptide enhance anti-tumor activity in B16-MUC1 melanoma-bearing mice. Int. Immunopharmacol. 2022, 107, 108667. [Google Scholar] [CrossRef] [PubMed]
- Samuel, J.; Budzynski, W.A.; Reddish, M.A.; Ding, L.; Zimmermann, G.L.; Krantz, M.J.; Koganty, R.R.; Longenecker, B.M. Immunogenicity and antitumor activity of a liposomal MUC1 peptide-based vaccine. Int. J. Cancer 1998, 75, 295–302. [Google Scholar] [CrossRef]
- Loveland, B.E.; Zhao, A.; White, S.; Gan, H.; Hamilton, K.; Xing, P.X.; Pietersz, G.A.; Apostolopoulos, V.; Vaughan, H.; Karanikas, V.; et al. Mannan-MUC1-pulsed dendritic cell immunotherapy: A phase I trial in patients with adenocarcinoma. Clin. Cancer Res. 2006, 12, 869–877. [Google Scholar] [CrossRef]
- Pecher, G.; Häring, A.; Kaiser, L.; Thiel, E. Mucin gene (MUC1) transfected dendritic cells as vaccine: Results of a phase I/II clinical trial. Cancer Immunol. Immunother. 2002, 51, 669–673. [Google Scholar] [CrossRef]
- Goydos, J.S.; Elder, E.; Whiteside, T.L.; Finn, O.J.; Lotze, M.T. A phase I trial of a synthetic mucin peptide vaccine: Induction of specific immune reactivity in patients with adenocarcinoma. J. Surg. Res. 1996, 63, 298–304. [Google Scholar] [CrossRef]
- Beatty, P.L.; Narayanan, S.; Gariépy, J.; Ranganathan, S.; Finn, O.J. Vaccine against MUC1 antigen expressed in inflammatory bowel disease and cancer lessens colonic inflammation and prevents progression to colitis-associated colon cancer. Cancer Prev. Res. 2010, 3, 438–446. [Google Scholar] [CrossRef]
- Dougan, M.; Dranoff, G. Immune therapy for cancer. Annu. Rev. Immunol. 2009, 27, 83–117. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Bernstein, M.B.; Hodge, J.W. Enhancing immune responses to tumor-associated antigens. Cancer Biol. Ther. 2009, 8, 1440. [Google Scholar] [CrossRef] [PubMed]
- Cook, I.F. Sexual dimorphism of humoral immunity with human vaccines. Vaccine 2008, 26, 3551–3555. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, K.L.; Fink, A.L.; Plebanski, M.; Klein, S.L. Sex and Gender Differences in the Outcomes of Vaccination over the Life Course. Annu. Rev. Cell Dev. Biol. 2017, 33, 577–599. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, Y.; Ran, S.; Segal, S. Sex-associated differences in the regulation of immune responses controlled by the MHC of the mouse. J. Immunol. 1984, 132, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Kovats, S. Estrogen receptors regulate innate immune cells and signaling pathways. Cell. Immunol. 2015, 294, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Libert, C.; Dejager, L.; Pinheiro, I. The X chromosome in immune functions: When a chromosome makes the difference. Nat. Rev. Immunol. 2010, 10, 594–604. [Google Scholar] [CrossRef]
- Laffont, S.; Rouquie, N.; Azar, P.; Seillet, C.; Plumas, J.; Aspord, C.; Guéry, J.-C. X-Chromosome complement and estrogen receptor signaling independently contribute to the enhanced TLR7-mediated IFN-alpha production of plasmacytoid dendritic cells from women. J. Immunol. 2014, 193, 5444–5452. [Google Scholar] [CrossRef]
- Furman, D.; Hejblum, B.P.; Simon, N.; Jojic, V.; Dekker, C.L.; Thiébaut, R.; Tibshirani, R.J.; Davis, M.M. Systems analysis of sex differences reveals an immunosuppressive role for testosterone in the response to influenza vaccination. Proc. Natl. Acad. Sci. USA 2014, 111, 869–874. [Google Scholar] [CrossRef]
- Markle, J.G.; Fish, E.N. SeXX matters in immunity. Trends Immunol. 2014, 35, 97–104. [Google Scholar] [CrossRef]
- Luchner, M.; Reinke, S.; Milicic, A. TLR Agonists as Vaccine Adjuvants Targeting Cancer and Infectious Diseases. Pharmaceutics 2021, 13, 142. [Google Scholar] [CrossRef] [PubMed]
- Hanten, J.A.; Vasilakos, J.P.; Riter, C.L.; Neys, L.; Lipson, K.E.; Alkan, S.S.; Birmachu, W. Comparison of human B cell activation by TLR7 and TLR9 agonists. BMC Immunol. 2008, 9, 39. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Wang, H.; Zhou, H. The Role of TLR4 on B Cell Activation and Anti-β2GPI Antibody Production in the Anti-phospholipid Syndrome. J. Immunol. Res. 2016, 2016, 1719720. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arabi, A.; Aria, S.; Maniaci, B.; Mann, K.; Martinson, H.; Kullberg, M. Enhancing T Cell and Antibody Response in Mucin-1 Transgenic Mice through Co-Delivery of Tumor-Associated Mucin-1 Antigen and TLR Agonists in C3-Liposomes. Pharmaceutics 2023, 15, 2774. https://doi.org/10.3390/pharmaceutics15122774
Arabi A, Aria S, Maniaci B, Mann K, Martinson H, Kullberg M. Enhancing T Cell and Antibody Response in Mucin-1 Transgenic Mice through Co-Delivery of Tumor-Associated Mucin-1 Antigen and TLR Agonists in C3-Liposomes. Pharmaceutics. 2023; 15(12):2774. https://doi.org/10.3390/pharmaceutics15122774
Chicago/Turabian StyleArabi, Ameneh, Shahab Aria (Soltani), Brandon Maniaci, Kristine Mann, Holly Martinson, and Max Kullberg. 2023. "Enhancing T Cell and Antibody Response in Mucin-1 Transgenic Mice through Co-Delivery of Tumor-Associated Mucin-1 Antigen and TLR Agonists in C3-Liposomes" Pharmaceutics 15, no. 12: 2774. https://doi.org/10.3390/pharmaceutics15122774
APA StyleArabi, A., Aria, S., Maniaci, B., Mann, K., Martinson, H., & Kullberg, M. (2023). Enhancing T Cell and Antibody Response in Mucin-1 Transgenic Mice through Co-Delivery of Tumor-Associated Mucin-1 Antigen and TLR Agonists in C3-Liposomes. Pharmaceutics, 15(12), 2774. https://doi.org/10.3390/pharmaceutics15122774