Abstract
Fermented plant extracts (FPEs) are functional liquids formed as a result of the fermentation of fresh plants by microorganisms, mainly bacteria and fungi. The appropriate selection of plants, microorganism strains, and conditions under which the fermentation process is carried out is very important in terms of obtaining a suitable matrix of biologically active compounds with different biological properties. The purpose of this review is to provide verified data on the current knowledge acquired regarding the biological activity of FPEs for cosmetic use and dermal applications. The antioxidant, antimicrobial, anti-inflammatory, anti-melanogenic, and wound-healing activity of FPEs, as well as their potential dermal applications, will be described.
1. Introduction
Fermented plant extracts (FPEs) are functional liquids formed as a result of the fermentation of fresh plants by microorganisms, mainly bacteria and fungi. The fermentation process enhances the biological activities of the substrate by converting high-molecular compounds into low-molecular structures, making fermented raw materials more compatible compared to unfermented ones [1]. Fermentation depends largely on the selection of the microorganism strain used and the conditions under which it is carried out. The appropriate choice of microorganisms and plants is critical to obtaining the desired matrix of biologically active compounds. The structural breakdown of plant cell walls and hydrolysis activity of the bacteria/fungi during fermentation increased polyphenols, flavonoids, organic acids, proteins, ceramides, amino acids, biological enzymes, and antioxidants in the fermentation medium [2,3]. Moreover, the product obtained after plant fermentation shows increased biological effectiveness and bioavailability with decreased cytotoxicity [2]. The fermentation of Camellia sinensis with mixture of Lactobacillus rhamnosus, Lactobacillus plantarum, and Saccharomyces cerevisiae [4], blueberry fruits with lactic acid bacteria (Lactobacillus plantarum, Lactobacillus fermentum) [5], and black tea with the kombucha [6] confirm the presence of phenolic compounds in fermentation medium. Moreover, fermentation time affects the content of bioactive compounds in bioferment. A fermentation medium with kombucha yerba mate extract showed that after 14 and 21 days of fermentation, the content of bioactive compounds, mainly polyphenols (chlorogenic acid, caffeoyl derivatives), as well as xanthines and flavonoids, may indicate a biological potential of fermented plant extract for dermatological use [7]. Recently, polyphenols have gained much more attention, owing to their possible beneficial implications in human health, such as in the treatment and prevention of cancer, cardiovascular disease, aging-associated mental deterioration, and neurodegeneration [8]. It was shown that fermented Magnolia denudata flower petal extract with Pediococcus acidilactici KCCM 11614 was higher than that of non-fermented plant extract against human gastric adenocarcinoma cell line (AGS), human cervical carcinoma (HeLa), and human colorectal carcinoma (LoVo) cells [9]. Also, Jo et al. [10] showed that ginseng extract fermented by Aspergillus usamii had better anticancerous activity against human hepatoma (HepG2), AGS, and human colon adenocarcinoma (DLD-1) cells in comparison to the non-fermented extract. Fermented Rhus verniciflua stem bark extracts showed anticancer activity in a colon cancer cell line HCT-116 and the ability to induce senescence or apoptosis and inhibit the hedgehog pathway [11]. The fermented Ophiopogon japonicas extract with Cordyceps militaris (first fermentation for 10 days), Lactobacillus plantarum, Enterococcus faecium, and Bifidobacterium longum (second fermentation for 2 days) can prevent cardiovascular diseases associated with the proliferation and migration of vascular smooth muscle cells (VSMCs) [12]. In turn, fermented Withania somnifera, Emblica officinalis, and Bacopa monnieri extract with Beauveria bassiana ATCC 7159 reduced chick embryo chorioallantoic membrane (CAM) vascularization, suggesting its anti-angiogenic potency [13]. This result poses application potential and may be used in many disorders due to uncontrolled vessel proliferation, such as atherosclerosis, diabetic retinopathy, rheumatoid arthritis, psoriasis, keratitis, glaucoma, and solid tumor development. Moreover, the fermented Withania somnifera, Emblica officinalis, and Bacopa monnieri extract were formulated for a nutraceutical anti-angiogenic treatment of age-related macular degeneration and commercialized in an oral form named Ethnodyne-VisioTM (Ethnodyne, France). The fermentation of Ginkgo biloba with Aspergillus niger enhances its neuro-protective role via antioxidant, anti-apoptotic and anti-inflammatory activities, leading to the amelioration of the stress hormones (catecholamines, epinephrine, norepinephrine, dopamine) compared to the non-fermented Ginkgo biloba leaf extract [14]. Fermented Carica papaya with yeast (commercialized as Immun’Age, Osato Research Institute, Gifu, Japan) significantly decreased 8-hydroxy-2′-deoxyguanosine (oxidative stress marker) in urine patients with Alzheimer’s disease [15] as well as reduced experimental ischemia-reperfusion-induced cerebral damage [16]. FPE, a rich source of bioactive compounds with various biological activities, may also gain interest in the cosmetics and pharmaceutical industries.
The purpose of this review is to provide verified data on the current knowledge acquired biological activity of fermented plant products for dermal applications.
2. Methods
2.1. Search Strategy
The PubMed, Scopus, and Google Scholar databases were used to search articles published from 2010 to 2023. Search terms included ‘fermented plant extract’, ‘fermented plant product’, ‘fermented plant extract biological activity’, and ‘fermented plant extract in dermal usage’. References from reviews regarding fermented plant products were also searched for additional articles and case reports. A manual search was also performed based on citations in the published literature.
2.2. Inclusion and Exclusion Criteria
All publications describing the biological activities of FPEs related to their dermal application have been included in this study. Other applications (e.g., food industry) of FPE than dermatology were excluded from this study. Publications in languages other than English were also excluded. Finally, 37 articles that meet the criteria were used for the review.
3. Biological Activity of Fermented Plant Extract for Dermal Applications
Some research showed that fermentation may improve the biological activities of the plant and enhance the production of bioactive compounds [3]. Fermented plant products showed varied biological activity, including antimicrobial, antioxidant, anti-inflammatory, anti-melanogenic, and wound-healing activity (Figure 1). FPE activities depend on the selection of microorganism strain used in the fermentation process in order to obtain a mixture of compounds characterized by the desired biological activity (Table 1).
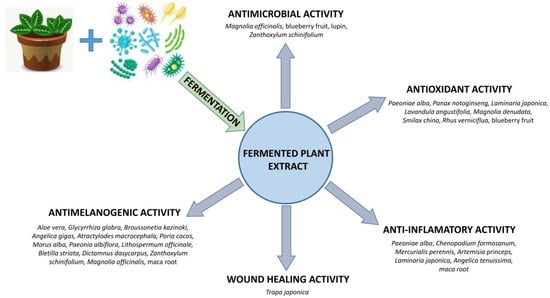
Figure 1.
Biological activity of FPE for dermal applications.

Table 1.
Fermented plant extract and their biological activity.
3.1. Antimicrobial Activity
Fermented Magnolia officinalis extracts with Aspergillus niger showed greater antibacterial activity against tested strains (Escherichia coli, Staphylococcus aureus, Bacillus subtilis, Staphylococcus epidermidis, Propionibacterium acnes, Epidermophyton floccosum, methicillin-resistant Staphylococcus aureus) and significantly increased 8–20-fold compared with that of the unfermented extracts [30]. The antibacterial activities against various bacterial strains, including methicillin-resistant Staphylococcus aureus (MRSA), were due to the enhancement of concentrations of antimicrobial compounds in the fermented Magnolia officinalis extracts (e.g., chlorogenic acid, magnolol, honokiol, and quercetin) and the production of new compounds with antimicrobial activity (e.g., catechin and ferulic acid) by Aspergillus niger fermentation [30]. Fermented Zanthoxylum schinifolium extract with Lactobacillus rhamnosus A6-5 showed greater antibacterial activity against Propionibacterium acnes and Staphylococcus epidermidis than the raw extract [36]. Fermented blueberry fruit extract with selected probiotic bacteria (Bacillus amyloliquefaciens and Lactobacillus brevis) and yeast (Starmerella bombicola) isolated from fermented starfish showed antibacterial activity against Brevibacterium linens, Propionibacterium acnes, Bacillus cereus, and Staphylococcus epidermidis [21]. Moreover, B. amyloliquefaciens and S. bombicola fermented blueberry fruit extracts appeared to possess a higher antimicrobial activity against skin bacteria and lower minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) values than L. brevis fermented extracts. Also, selenium nanoparticles synthesized by fermented aqueous extract of lupin by Aspergillus oryzae (ability to reduce selenium ion in the presence of gamma rays) were active towards Acinetobacter calcoaceticus, Staphylococcus aureus, Candida albicans and Aspergillus flavus [28]. Only a few studies show that FPE has antibacterial and antifungal properties [21,28,30,36]. Unfortunately, their antimicrobial mechanism of action remains unknown.
3.2. Antioxidant Activity
Fermentation also can improve the antioxidant activity of plant extracts associated with increased phytochemicals, mainly by polyphenols, antioxidant polysaccharides, and antioxidant peptides produced by microbial hydrolysis or biotransformation [37]. Fermented Paeoniae alba extract with plant-derived Lactobacillus brevis 174A significantly elevated the total phenolic content, reduced intracellular reactive oxygen species (ROS) levels, and inhibited the nitric oxide (NO) release [32]. The fermented Panax notoginseng polysaccharides (FPNP) with Saccharomyces cerevisiae CGMCC 17452 protect against the oxidative damage of collagen and elastin induced by H2O2 via TGF-β/Smad signaling pathway in human fibroblast cells [33]. Moreover, FPNP decreases ROS and malondialdehyde (MDA) contents, reversed the reduction in the antioxidant activity and the expression of the antioxidant enzyme catalase (CAT), glutathione peroxidase (GSH-Px), and superoxide dismutase (SOD) induced by H2O2. Furthermore, the upregulation in the expression of transforming growth factor-β (TGF-β), Smad2/3, and the downregulation in the expression of Smad7 in FPNP-treated groups showed that through the activation of the TGF-β/Smad signaling pathway, FPNP inhibited H2O2-induced collagen and elastin-injury in human fibroblast cells. Fermented Laminaria japonica extract with Saccharomyces cerevisiae has stronger antioxidant activity than unfermented Laminaria japonica extract [26]. Fermented Laminaria japonica extract possesses strong free radical scavenging ability via increases in the synthesis of antioxidant enzymes in human immortalized epidermal keratinocytes (HaCaT) exposed to UVB radiation. Also, Lavandula angustifolia extract fermented with Pediococcus pentosaceus DK1 showed higher inhibition of ROS generation than those treated with non-fermented extract [27].
The antioxidative effect of Magnolia denudata flower petal extract fermented by Pediococcus acidilactici KCCM 11614 was threefold higher than that of the (non-fermented) control [9]. Moreover, the 1,1-diphenyl-2-picryl-hydrazyl (DPPH) radical scavenging activity of fermented magnolia increased from 85.1% to 91.4% depending on the fermentation time, while those of the non-fermented plant extract were not significantly different. Fermented Rhus verniciflua bark methanol extract showed the highest DPPH radical-scavenging activity for ethyl acetate fraction and then beta hydroxy acid (BHA) and butylated hydroxytoluene (BHT) as control [11]. Fermented extract from Smilax china leaves with mixtures of Lactobacillus bulgaricus and Lactobacillus reuteri showed DPPH scavenging activity at 0.0625%, the same as ascorbic acid, and the maximum DPPH scavenging activity (92.44%) at 1% [34]. The 2, 2’-azino-bis (3-ethylbenzthiazoline-6-sulphonic acid (ABTS) and DPPH assays showed significant scavenging activity in fermented blueberry extract with Bacillus amyloliquefaciens, Lactobacillus brevis, and Starmerella bombicola [21]. Fermented for 28 days, green coffee beans with kombucha characterized the highest antioxidant capacity and may be a valuable source of bioactive substances used in cosmetic and dermatological products [25].
3.3. Anti-Inflammatory Activity
A large number of herbal products possess active constituents that retard the key steps of the inflammation pathway (nuclear factor kappa B (NF-κβ), lipoxygenase (LOX), and cyclooxygenase (COX)) [38]. The antioxidant compounds are involved in several functional properties of fermented plant products, such as neutralizing free radicals, regulating antioxidant enzyme activities, reducing oxidative stress, affecting inflammatory responses, and enhancing immune system performance [39].
Fermented Paeoniae alba extract with plant-derived Lactobacillus brevis 174A suppressed inflammatory cytokines interleukin (IL)—6, tumor necrosis factor-α (TNF-α), while simultaneously downregulating the gene expressions of inducible nitric oxide synthase (iNOS), IL-6, TNF-α, and IL-1 compared to the unfermented extract [32]. Fermented Mercurialis perennis extract with Lactobacteria (Lactobacillus plantarum and Pediococcus pentosaceus) and non-fermented extract enhanced NFκB and cytokine expression (IL-6, TNF, IL-8, and IL-1) in NFκB-THP-1 reporter cells, showing a concentration-dependent immunostimulatory effect [31]. Fermented Chenopodium formosanum leaf extract with Aspergillus oryzae increases anti-inflammatory activity via reduced nitric oxide (NO), IL-6, and TNF-α production in lipopolysaccharide (LPS)-stimulated RAW264.7 cells in a dose-dependent manner [23]. It has been found that the fermentation of Artemisia princeps plant extract with plant-derived Lactobacillus plantarum SN13T generates catechol and secotanapartholide C as IL-8 inhibitors [19]. Fermented maca root extracts with Lactobacillus strains, such as Lactobacillus plantarum, Lactobacillus rhamnosus, Lactobacillus casei, and Lactobacillus gasseri, exhibit higher anti-inflammatory activity than the non-fermented extracts at concentrations of 5% and 10% [29]. Fermented Laminaria japonica extract with Saccharomyces cerevisiae has stronger anti-inflammatory activity compared to unfermented Laminaria japonica extract [26]. Moreover, fermented Laminaria japonica extract inhibits the gene expression levels of pro-inflammatory factors (ILs, TNF-α, matrix metallopeptidase 9 (MMP-9)) and activates the nuclear factor 2-related factor 2 (Nrf2) signaling pathway in human immortalized epidermal keratinocytes (HaCaT) exposed to UVB radiation. Fermented Angelica tenuissima with Aspergillus oryzae was able to play a role in the attenuation of inflammatory responses caused by UVB irradiation via the upregulation of photo-protective hemeoxygease-1 and suppression of proinflammatory COX-2 expression [18].
3.4. Melanogenic Inhibitory Effects
Skin pigmentation results from several processes, such as melanin synthesis, transport, and accumulation of melanin in keratinocytes [40]. Melanin is a pigment that plays an important role in providing coloration and protecting human skin from the harmful effects of UV light radiation [41]. Some studies showed that FPEs may be inhibitors of melanogenesis and skin-whitening compounds in cosmetics and dermatology (Figure 2).
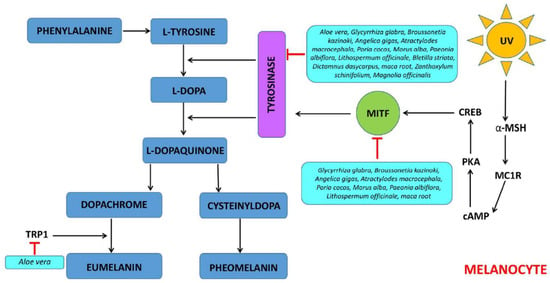
Figure 2.
Mechanism for inhibitory effect of FPE on melanogenesis. Legends: αMSH—α-melanocyte-stimulating hormone; MC1R—melanocortin 1 receptor; cAMP—cyclic adenosine monophosphate; PKA—protein kinase A; CREB—cAMP response element-binding protein; MITF—melanocyte-inducing transcription factor; TRP1—tyrosinase-related protein 1.
The fermented leaf skin of Aloe vera extract with Lactobacillus plantarum BN41 isolated from kimchi may be a natural ingredient that effectively inhibits skin melanogenesis [17]. It was shown that inhibition of tyrosinase activities and melanin synthesis at 0.3% (w/v) optimal dosage of fermented Aloe vera extract was much better than those of arbutin and aloesin, which are commercial skin-lightening ingredients. It was also proved that fermented Aloe vera extract effectively downregulated all microphthalmia-associated transcription factors (MITF), tyrosinase-related protein-1 (TYRP-1) and TYRP-2, and tyrosinase (TYR) gene expression, proposing melanogenesis inhibitory mechanism in the MITF/TYRP-1/TYRP-2/TYR pathway. The mixtures of fermented Glycyrrhiza glabra, Broussonetia kazinoki, Angelica gigas, Atractylodes macrocephala, Poria cocos, Morus alba (root bark), Paeonia albiflora, and Lithospermum officinale (2% each) with Phellinus linteus showed anti-melanogenic activity in tested cultured B16F0 mouse melanoma cells [24]. Mixtures of fermented plant extract inhibit melanogenesis through the activation of the phosphatidylinositol 3-kinase/Akt/glycogen synthase kinase-3beta signaling pathway and down-regulation of MITF. Moreover, mixtures of fermented plant extract in a dose-dependently manner inhibited melanin and tyrosinase activity and reduced melanogenesis-related proteins, including tyrosinase and MITF in B16F0 cells. The mixture of eight FPEs with Phellinus linteus (previously named 8-HsPLCB) showed a reduction in the melanin pigment in melanocytes and histological changes induced by UV irradiation in brown guinea pigs [42]. Moreover, the skin-lightening effect was comparable to arbutin, one of the most widely used ingredients in skin-whitening cosmetics. Fermented mixtures of Atractylodes macrocephala, Paeonia lactiflora, Bletilla striata, Poria cocos, Dictamnus dasycarpus, Ampelopsis japonica and Tribulus terrestris extract (FB-ChiBai) with Lactobacillus rhamnosus at concentrations ranging from 0.05% to 0.5% suppressed the CREB/MITF/tyrosinase melanogenic pathway without inducing cytotoxicity in B16F0 melanoma cells under α-MSH stimulation [20]. Furthermore, it was found that FB-ChiBai significantly attenuated melanin production, tyrosinase activities, and melanogenesis-related signaling pathways and reduced the nuclear translocation and promoter binding activities of MITF in B16F0 murine melanoma cells. Fermented maca root extracts with Lactobacillus strains, such as Lactobacillus plantarum, Lactobacillus rhamnosus, Lactobacillus casei, and Lactobacillus gasseri inhibiting tyrosinase activity, melanin synthesis, and melanogenesis by suppressing MITF-related mechanisms [29]. The fermented Zanthoxylum schinifolium extract with Lactobacillus rhamnosus A6-5 can be used as an ideal skin whitening agent via greater tyrosinase inhibitory activity and reduced melanin production compared with the raw extract [36]. Fermented Magnolia officinalis extracts with Aspergillus niger showed higher tyrosinase inhibitory activity than that of the unfermented extracts and the positive control, arbutin, but lower than that of kojic acid [30]. The fermented extracts showed higher inhibitory activity than non-fermented extracts, which may be owed, at least in part, to the combined effects of plant- and bacteria-derived active ingredients (e.g., kojic acid is naturally derived metabolites from Aspergillus sp.) [43].
3.5. Wound Healing Activity
Wound healing is a complex inter-related biological process at the molecular level, and it occurs in four stages or phases consisting of hemostasis, inflammation, proliferation, and finally epithelialization [44]. Herbal products and their active constituents through different mechanisms of action, including antimicrobial, anti-inflammatory, and antioxidant activity, the stimulation of angiogenesis, the production of cytokines and growth factors, keratinocytes, and fibroblast migration and proliferation, may be considered as an important support during conventional therapy or even as a substitute for synthetic drugs used for wounds treatment [45]. Some research found that FPEs may stimulate wound closure. Fermented hot-water Trapa japonica fruit extract with Bacillus methulotrophicus and Bacillus subtilis stimulate human dermal fibroblast (HDF) and keratinocytes (HaCaT) cells proliferation, and collagen synthesis via activating TGF-β1/GSK-3β/β-catenin pathway [35].
4. Fermented Plant Extracts for Dermal Applications
The cosmetics and pharmaceutical industries are looking for new products or improvements of existing products with innovative active principles. Herbal products and their active constituents are consistently popular with consumers. Among them are fermented plant products, especially popular in Asian countries and nowadays more noticed in the world markets. FPEs are a rich source of phytochemicals with different biological activities, which can be used as active ingredients in many pharmaceutical/cosmeceutical products [2,46]. Due to a wide spectrum of biological properties, FPEs could be used in several dermal applications like anti-aging and anti-photoaging, anti-wrinkle, skin whitening, moisturizing products, hair growth products, products for androgenic or diffuse alopecia treatment, and wound healing products (Figure 3). Most of them are based on in vitro studies (Table 2). Only a few have been transferred to animal models or clinical trials (Table 3).
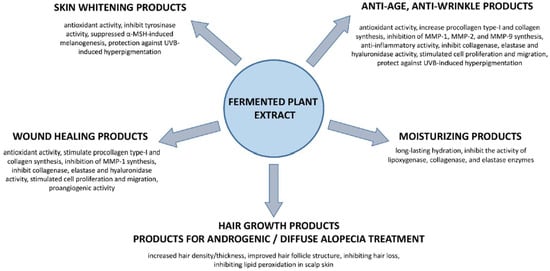
Figure 3.
FPE for dermal applications.

Table 2.
Fermented plant extract and their potential dermal applications—in vitro study.

Table 3.
Fermented plant extract and their dermal applications—in vivo study.
4.1. Anti-Aging Products
Skin aging is a complex biological process that is influenced by a combination of endogenous (genetics, cellular metabolism, hormones, and metabolic processes) and exogenous (chronic light exposure, pollution, ionizing radiation, chemicals, and poisons) factors [59]. Some research found that fermented plant products affect exogenous factors and pose anti-aging and anti-wrinkle activities.
Mixed root extracts of Taraxacum officinale rhizome/root, Arctium lappa, Anemarrhena asphodeloides, Pueraria lobata, and Nelumbo nucifera fermented with Saccharomyces cerevisiae enable human keratinocytes (HEKa) and fibroblasts (HDF) cells proliferation and migration, have anti-aging effects and can be used as active cosmetic raw materials [55]. Fermented Smilax china leaves extract with mixtures of Lactobacillus bulgaricus and Lactobacillus reuteri showed anti-pollution potential through their antioxidant activity and inhibited PGE2 production in HaCaT [34]. Saccharomyces cerevisiae-mediated fermented black ginseng has been reported for anti-wrinkle activity in cultured human fibroblasts (HS68) [50]. Moreover, fermented black ginseng increased the expression of type I procollagen and tissue inhibitor of MMP-2 and reduced the expression of MMP-1, MMP-2, and MMP-9 in HS68 cells. Fermented Magnolia officinalis extracts with Aspergillus niger inhibited skin aging-related enzymes such as collagenase, elastase, MMP-1, and MMP-2 [30]. Moreover, methanol-extracted M. officinalis fermented by A. niger for 72 h has the most active skincare or antiaging compounds for dermatological applications. Fermented leaves and branches of honeybush extracts indicate a high potential for anti-aging products use through strong antioxidant activity, significant ability to inhibit collagenase and hyaluronidase, and a weak influence on elastase activity, as well as medium photoprotection (sun protection factor, SPF) [53]. Artemisia vulgaris fermented solvent fraction showed anti-aging and anti-wrinkle effects via increased collagen synthesis and cell regeneration [49]. The fermented outer layers of the leaf skin of Aloe barbadensis at a concentration of 0.3% effectively scavenge cellular ROS generated from the oxidative stress of mitochondria, which results in the inhibition of skin wrinkling processes by increasing collagen production and decreasing MMP-1 production [48]. The fermented Triticum aestivum, Avena sativa, Helianthus tuberosus, Glycine max, and Smallanthus sonchifolius with Lactobacillus buchneri found in kimchi could be potential candidates for the protective effects against UVB-induced photoaging useful natural components of dermatological products [58]. Moreover, a mixture of fermented plant extracts decreased elastase and collagenase activity and increased type I collagen expression and MMP mRNA levels in UVB-induced photoaging of normal human dermal fibroblasts and epidermal keratinocytes. Furthermore, FPEs promoted the expression of moisture factor and anti-oxidant enzymes in UVB-induced photoaging in vitro models. Also, Lavandula angustifolia extract fermented with Pediococcus pentosaceus DK1 showed an MMP-1 expression lower than that in UVB-irradiated fibroblasts treated with non-fermented extract [27]. Moreover, fibroblasts treated with fermented L. angustifolia extract showed 20% less reduction in collagen production upon UVB irradiation than those treated with non-fermented extract. The fermented Angelica tenuissima root with Aspergillus oryzae showed anti-photoaging potential and could be utilized as an effective ingredient in anti-aging and anti-wrinkle products [18]. Fermented Angelica tenuissima was able to improve extracellular matrix impairment caused by UVB irradiation through the upregulation of procollagen type-1 synthesis and secretion as well as the suppression of MMP-1 and elastase expression in HaCaT (human keratinocyte) or Hs68 (human foreskin fibroblast) skin cells. The aqueous extract of Fructus arctii was fermented with Grifola frondosa UV-A exposed human dermal fibroblasts, showing reduced expressions of MMP-1 and collagen biosynthesis [52]. Citrus unshiu peel aqueous extracts were fermented by Schizophyllum commune, reducing the expression of MMP-1 and collagen biosynthetic activity in a dose-dependent manner after UV-A exposed human dermal fibroblasts [51]. The fermented Aloe arborescens extract with Lactobacillus plantarum enhanced anti-skin wrinkling due to synergistic effects between the barbaloin and the low-molecular-weight polysaccharides retained after the fermentation process [47].
The fermented Trapa japonica fruit extract stimulated the synthesis of collagen, reduced TNF-α-induced gene expression of MMPs in human dermal fibroblast cells, and promoted wound recovery in HaCaT cells [56]. Moreover, a randomized and double-blind clinical trial showed that eye cream with 0.5% peptide isolated from fermented Trapa japonica extract application on the eye twice a day for 8 weeks by participants (22 healthy women aged 41 to 57 years) significantly reduced skin wrinkles.
4.2. Skin Whitening Products
The fermented Aloe vera extract with Lactobacillus plantarum BN41 in a concentration of 0.3% (w/v) can be a natural ingredient with fewer side effects for replacement of many synthetic and chemical skin-lightening components in pharmaceutical and dermatological products [17]. The fermented mixtures of Atractylodes macrocephala, Paeonia lactiflora, Bletilla striata, Poria cocos, Dictamnus dasycarpus, Ampelopsis japonica, and Tribulus terrestris extract (FB-ChiBai) with Lactobacillus rhamnosus can protect against UV-B irradiation and that it might be used as an agent in products to protect against UVB-induced hyperpigmentation [20]. In the in vivo experiments, FB-ChiBai was topically applied to the dorsal skin of C57BL/6J nude mice and concurrently irradiated with UVB three times a week for 8 weeks. The results indicated that FB-ChiBai alleviated UVB-induced hyperpigmentation by reducing epidermal hyperplasia and inhibiting the CREB/MITF/tyrosinase pathway.
4.3. The Moisturizing Products
The fermented Yerba Mate extract with Kombucha showed antioxidant activity, a strong ability to inhibit collagenase and elastase enzymes in vitro study, and long-lasting hydration and reduced transepidermal water loss (TEWL) after application on the volunteers’ forearm skin (0.2 mL of 100 µg/mL FPE) in in vivo study [7].
4.4. The Hair Growth Products
Bacillus/Trapa japonica fruit ferment filtrate extracts (TJFs) enhance human hair follicle dermal papilla (HDP) cell proliferation and migration via the Akt/ERK/GSK-3β signaling pathway, suggesting a potential treatment for alopecia [57]. The TJFs also induced cell cycle progression, inhibited type I 5α-reductase, decreased apoptosis, and enhanced angiogenesis via increased vascular endothelial growth factor (VEGF) and vascular expansion in CAM assay. Moreover, insulin-like growth factor-1 and keratinocyte growth factor, stimulating hair growth were detected in the human dermal papilla. Also, shampoo and lotion for hair care containing fermented papaya, fermented mangosteen, and caffeine were applied to 154 subjects of both sexes with clinically confirmed androgenic or diffuse alopecia for 3 months in a randomized double-blind clinical trial [54]. The hair care products significantly inhibited hair loss, increased hair density/thickness, and improved hair follicle structure versus placebo and caffeine controls. The products with fermented papaya and fermented mangosteen substantially normalized the microbiota pattern and increased ATP content in hair follicles, while inhibiting lipid peroxidation in the scalp skin, and SH-group formation in the hair shaft.
4.5. Wound Healing Products
Fermented Carica papaya preparation after 8 weeks of oral supplementation (0.2 g/kg body weight) improved wound healing activity in adult obese diabetic (db/db) mice [22]. Diabetic mice supplemented with fermented papaya showed a higher abundance of CD68 as well as CD31 at the wound site, suggesting effective recruitment of monocytes and an improved proangiogenic response.
5. Toxicological Aspect
Fermented plants have a long history of safe human consumption, while research into other beneficial effects of bioferments in skin care products and pharmaceuticals is still ongoing. Some research found that fermentation increased the biological effectiveness and bioavailability of fermented plants and decreased cytotoxicity compared to non-fermented plants [2]. The significant increase in cell viability of fibroblasts and keratinocytes for Yerba Mate ferments was observed in 500–1000 μL/mL, while Yerba Mate extracts at the highest concentration used (1000 µg/mL) caused a cytotoxic effect on fibroblasts [7]. The viability of a HaCaT cell was not reduced during treatment with 0.125–1% of fermented Smilax china leaves extract, but the viability was significantly reduced from 2% of FPE [34]. Fermented Zanthoxylum schinifolium extracts (<500 mg/mL) have no cytotoxic effect [36]. However, cell viability fermented Zanthoxylum schinifolium extracts beyond the concentration of 500 mg/mL dropped from 100% to 89.98% [36]. Aloe vera fermented extract showed no cytotoxicity against murine melanoma cells at concentrations below 0.5% (w/v) [17]. Fermented Angelica tenuissima (125–1000 μg/mL) treatment did not show any significant cytotoxic or cell proliferative effect on Hs68 or HaCaT cells [18]. Fermentation plant mixtures FB-ChiBai were found to have no cytotoxic effect on B16F0 cells at a concentration of 0.5% [20]. Fermented Chenopodium formosanum leaf extract (≤400 mg/L) was not cytotoxic [23]. Also, fermented Aloe arborescens [47] and Artemisia vulgaris [49] showed no cytotoxicity in the MTT test.
Laminaria japonica fermented freeze-dried powder is non-irritating to the eyes, has high safety, and can be added to skin care products as a functional raw material [26]. The aqueous and Lactobacterial-fermented Mercurialis perennis extracts were tested for micronuclei formation in THP-1 cells and toxicity in luminescent bacteria (V. fischeri), whereby no mutagenic or toxic effects were detected, which corroborates their safe use in pharmaceutical remedies [31]. Unfortunately, most of the studies presented in this paper do not include toxicological tests, which should be crucial for FPE and their use in cosmetics and drugs applied topically to the skin.
6. Future Research Directions
Fermentation could be considered a significant technique to obtain bioactive compounds with a broad spectrum of structural diversity and different biological activities useful for dermal applications. Fermentation is a feasible strategy for enhancing the bioactivity of herbal medicines via breaking down or converting the undesirable substrates into compatible components under the action of microbial enzymes, thereby improving the substrate properties via the production and enrichment of bioactive compounds [60]. Fermentation significantly increases the phenolic and anthocyanin contents, which reveals stronger antioxidant activities of FPE compared to non-fermented plants [9,11,26,27,33]. Bacteria and fungi have great potential for the production of antioxidants through enzymatic hydrolysis of phenolic glycosides to free polyphenols [61]. Polyphenols also enhance antimicrobial [62], anti-inflammatory [63] and wound healing activities [64]. Unfortunately, although FPE showed many desirable biological activities, their practical use in the cosmetic and pharmaceutical industries is negligible. Technological development of the fermentation process requires not only the appropriate selection of plants and starter cultures of microorganisms but also the optimization of fermentation conditions (temperature, pH, time of fermentation, etc.) which will collectively affect the presence of active compounds and their biological activities. Moreover, there are some important problems in the production of FPE, such as the possible generation of methanol, formaldehyde, biogenic amines, and nitrite during fermentation, as well as storage stability [65]. Also, the determination of the phytochemical composition of fermented plant extract brings many difficulties. The extraction of bioactive compounds from fermented plants and their quantitative and qualitative estimation is important for the exploration of new biocompounds to be used by the pharmaceutical industry both directly and/or indirectly as lead molecules used to synthesize more potent molecules. The isolation, purification, and identification of compounds responsible for the biological activity remain a great challenge in the drug discovery process. The various techniques involving the applications of chromatographic techniques such as HPLC (High-Performance Liquid Chromatography), TLC (Thin Layer Chromatography), HPTLC (High-Performance Thin Layer Chromatography), OPLC (Optimum Performance Laminar Chromatography), GC (Gas Chromatography), PC (Paper Chromatography), CC (Column Chromatography) and detection through Fourier Transform Infra-Red spectroscopy (FTIR), Nuclear Magnetic Resonance (NMR), and Mass Spectrometry (MS) offers enormous possibilities, but require experience in working with plant material and specialist knowledge in operating the above devices [66]. The selection of the isolation and identification methods depends on the properties of the bioactive substance and is a difficult and time-consuming process [65]. Therefore, most research on fermented plants is limited to determining the group of compounds found in the sample (e.g., polysaccharides, polyphenols, flavonoids). Most of the described research is conducted on cell lines in vitro methods (Table 2) and only concerns potential dermal applications. Little research has been transferred to animal models or clinical trials (Table 3), which are expensive and require appropriate permits. Fermented plant products have many valuable biological activities that could be used by the cosmetic and pharmaceutical industries but require further research.
7. Conclusions
FPE are innovative ingredients formed from plant raw materials during the fermentation process with appropriate strains of microorganisms. FPE showed different biological activities, including antimicrobial, antioxidant, anti-inflammatory, anti-melanogenic, and wound healing activity. Their biological activities may be used for several dermatological applications, including anti-aging and anti-photoaging products, skin whitening products, moisturizing products, hair growth products, products for androgenic or diffuse alopecia treatment, and wound healing products.
Funding
This work was supported by Warsaw University of Technology [“NChem.4” grant number 504/04846/1020/43.122303], Warsaw, Poland.
Conflicts of Interest
The authors declare that they have no known competing financial interest or personal relationship that could have appeared to influence the work reported in this paper.
References
- Wang, C.Y.; Ng, C.C.; Lin, H.T.; Shyu, Y.T. Free radical-scavenging and tyrosinase-inhibiting activities of extracts from sorghum distillery residue. J. Biosci. Bioeng. 2011, 111, 554–556. [Google Scholar] [CrossRef] [PubMed]
- Majchrzak, W.; Motyl, I.; Śmigielski, K. Biological and cosmetical importance of fermented raw materials: An overview. Molecules 2022, 27, 4845. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Zhang, M.; Mujumdar, A.S.; Gao, Z. Recent research process of fermented plant extract: A review. Trends Food Sci. Technol. 2017, 65, 40–48. [Google Scholar] [CrossRef]
- Makhamrueang, N.; Raiwa, A.; Jiaranaikulwanitch, J.; Kaewarsar, E.; Butrungrod, W.; Sirilun, S. Beneficial bio-extract of Camellia sinensis var. assamica fermented with a combination of probiotics as a potential ingredient for skin care. Cosmetics 2023, 10, 85. [Google Scholar] [CrossRef]
- Li, S.; Tao, Y.; Li, D.; Wen, G.; Zhou, J.; Manickam, S.; Han, Y.; Chai, W.S. Fermentation of blueberry juices using autochthonous lactic acid bacteria isolated from fruit environment: Fermentation characteristics and evolution of phenolic profiles. Chemosphere 2021, 276, 130090. [Google Scholar] [CrossRef] [PubMed]
- Villarreal-Soto, S.A.; Beaufort, S.; Bouajila, J.; Souchard, J.P.; Renard, T.; Rollan, S.; Taillandier, P. Impact of fermentation conditions on the production of bioactive compounds with anticancer, anti-inflammatory and antioxidant properties in kombucha tea extracts. Process Biochem. 2019, 83, 44–54. [Google Scholar] [CrossRef]
- Ziemlewska, A.; Nizioł-Łukaszewska, Z.; Bujak, T.; Zagórska-Dziok, M.; Wójciak, M.; Sowa, I. Effect of fermentation time on the content of bioactive compounds with cosmetic and dermatological properties in Kombucha Yerba Mate extracts. Sci. Rep. 2021, 11, 18792. [Google Scholar] [CrossRef] [PubMed]
- Leri, M.; Scuto, M.; Ontario, M.L.; Calabrese, V.; Calabrese, E.J.; Bucciantini, M.; Stefani, M. Healthy effects of plant polyphenols: Molecular mechanisms. Int. J. Molec. Sci. 2020, 21, 1250. [Google Scholar] [CrossRef]
- Park, E.H.; Kim, H.S.; Eom, S.J.; Kim, K.T.; Paik, H.D. Antioxidative and anticanceric activities of magnolia (Magnolia denudata) flower petal extract fermented by Pediococcus acidilactici KCCM 11614. Molecules 2015, 20, 12154–12165. [Google Scholar] [CrossRef]
- Jo, M.N.; Jung, J.E.; Lee, J.H.; Park, S.H.; Yoon, H.J.; Kim, K.T.; Paik, H.D. Cytotoxicity of the white ginseng extract and red ginseng extract treated with partially purified β-glucosidase from Aspergillus usamii KCTC 6954. Food Sci. Biotechnol. 2014, 23, 215–219. [Google Scholar] [CrossRef]
- Kim, M.; Yang, J.; Kwon, Y.S.; Kim, M.J. Antioxidant and anticancer effects of fermented Rhus verniciflua stem bark extracts in HCT-116 cells. Sci. Asia 2015, 41, 2306. [Google Scholar] [CrossRef]
- Song, J.H.; Jeong, G.H.; Park, S.L.; Won, S.Y.; Paek, N.S.; Lee, B.H.; Moon, S.K. Inhibitory effects of fermented extract of Ophiopogon japonicas on thrombin-induced vascular smooth muscle cells. Mol. Med. Rep. 2016, 13, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Rabhi, C.; Arcile, G.; Cariel, L.; Lenoir, C.; Bignon, J.; Wdzieczak-Bakala, J.; Ouazzani, J. Antiangiogenic-like properties of fermented extracts of ayurvedic medicinal plants. J. Med. Food 2015, 18, 1065–1072. [Google Scholar] [CrossRef]
- Ismail, A.F.; El-Sonbaty, S.M. Fermentation enhances the protective role of Ginkgo biloba on gamma-irradiation-induced neuroinflammatory gene expression and stress hormones in rat brain. J. Photochem. Photobiol. B Biol. 2016, 158, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo, M.; Marotta, F.; Dominguez, L.J. Oxidative stress in patients with Alzheimer’s disease: Effect of extracts of fermented papaya powder. Mediat. Inflamm. 2015, 2015, 624801. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Mori, A.; Chen, Q.; Zhao, B. Fermented papaya preparation attenuates β-amyloid precursor protein: β-amyloid-mediated copper neurotoxicity in β-amyloid precursor protein and β-amyloid precursor protein Swedish mutation overexpressing SH-SY5Y cells. Neuroscience 2006, 143, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Jeon, G.; Ro, H.S.; Kim, G.R.; Lee, H.Y. Enhancement of melanogenic inhibitory effects of the leaf skin extracts of Aloe barbadensis Miller by the fermentation process. Fermentation 2022, 8, 580. [Google Scholar] [CrossRef]
- Park, Y.A.; Lee, S.R.; Lee, J.W.; Koo, H.J.; Jang, S.A.; Yun, S.W.; Kim, H.J.; Woo, J.S.; Park, M.R.; Kang, S.C.; et al. Suppressive effect of fermented Angelica tenuissima root extract against photoaging: Possible involvement of hemeoxygenase-1. J. Microbiol. Biotechnol. 2018, 28, 1391–1400. [Google Scholar] [CrossRef]
- Okamoto, T.; Sugimoto, S.; Noda, M.; Yokooji, T.; Danshiitsoodol, N.; Higashikawa, F.; Sugiyama, M. Interleukin-8 release inhibitors generated by fermentation of Artemisia princeps Pampanini herb extract with Lactobacillus plantarum SN13T. Front. Microbiol. 2020, 11, 1159. [Google Scholar] [CrossRef]
- Ho, C.C.; Ng, S.C.; Chuang, H.L.; Chen, J.Y.; Wen, S.Y.; Kuo, C.H.; Mahalakshmi, B.; Le, Q.W.; Huang, C.Y.; Kuo, W.W. Seven traditional Chinese herbal extracts fermented by Lactobacillus rhamnosus provide anti-pigmentation effects by regulating the CREB /MITF/ tyrosinase pathway. Environ. Toxicol. 2021, 36, 654–664. [Google Scholar] [CrossRef]
- Oh, B.T.; Jeong, S.Y.; Velmurugan, P.; Park, J.H.; Jeong, D.Y. Probiotic-mediated blueberry (Vaccinium corymbosum L.) fruit fermentation to yield functionalized products for augmented antibacterial and antioxidant activity. J. Biosci. Bioeng. 2017, 124, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Collard, E.; Roy, S. Improved function of diabetic wound-site macrophages and accelerated wound closure in response to oral supplementation of a fermented papaya preparation. Antioxid. Redox Signal. 2010, 13, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.M.; Chung, Y.C.; Chen, P.Y.; Chang, Y.C.; Chen, W.L. Fermentation of Chenopodium formosanum leaf extract with Aspergillus oryzae significantly enhanced its physiological activities. Appl. Sci. 2023, 13, 2917. [Google Scholar] [CrossRef]
- Cha, J.Y.; Yang, H.J.; Moon, H.I.; Cho, Y.S. Inhibitory effect and mechanism on melanogenesis from fermented herbal composition for medical or food uses. Food Res. Int. 2012, 45, 225–231. [Google Scholar] [CrossRef]
- Nizioł-Łukaszewska, Z.; Ziemlewska, A.; Bujak, T.; Zagórska-Dziok, M.; Zarębska, M.; Hordyjewicz-Baran, Z.; Wasilewski, T. Effect of fermentation time on antioxidant and anti-ageing properties of green coffee Kombucha ferments. Molecules 2020, 25, 5394. [Google Scholar]
- Sun, Q.; Fang, J.; Wang, Z.; Song, Z.; Geng, J.; Wang, D.; Wang, C.; Li, M. Two Laminaria japonica fermentation broths alleviate oxidative stress and inflammatory response caused by UVB damage: Photoprotective and reparative effects. Mar. Drugs 2022, 20, 650. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.H.; Kim, A.R.; Lee, K.S.; Xuan, S.H.; Kang, H.C.; Lee, D.H.; Cha, M.Y.; Kim, H.J.; An, M.; Park, S.N. Anti-aging activity of Lavandula angustifolia extract fermented with Pediococcus pentosaceus DK1 isolated from Diospyros kaki fruit in UVB-irradiated human skin fibroblasts and analysis of principal components. J. Microbiol. Biotechnol. 2019, 29, 21–29. [Google Scholar] [CrossRef]
- Mosallam, F.M.; El-Sayyad, G.S.; Fathy, R.M.; El-Batal, A.I. Biomolecules-mediated synthesis of selenium nanoparticles using Aspergillus oryzae fermented Lupin extract and gamma radiation for hindering the growth of some multidrug-resistant bacteria and pathogenic fungi. Microbial Pathog. 2018, 122, 108–116. [Google Scholar] [CrossRef]
- Yang, J.; Cho, H.; Gil, M.; Kim, K.E. Anti-inflammation and anti-melanogenic effects of maca root extracts fermented using Lactobacillus strains. Antioxidants 2023, 12, 798. [Google Scholar] [CrossRef]
- Wu, L.; Chen, C.; Cheng, C.; Dai, H.; Ai, Y.; Lin, C.; Chung, Y. Evaluation of tyrosinase inhibitory, antioxidant, antimicrobial, and antiaging activities of Magnolia officinalis extracts after Aspergillus niger fermentation. BioMed Res. Int. 2018, 2018, 5201786. [Google Scholar] [CrossRef]
- Lorenz, P.; Zilkowski, I.; Mailänder, L.K.; Klaiber, I.; Nicolay, S.; Garcia-Käufer, M.; Zimmermann-Klemd, A.M.; Turek, C.; Stintzing, F.C.; Kammerer, D.R.; et al. Comparison of aqueous and Lactobacterial-fermented Mercurialis perennis L. (Dog’s Mercury) extracts with respect to their immunostimulating activity. Fermentation 2023, 9, 190. [Google Scholar] [CrossRef]
- Shakya, S.; Danshiitsoodol, N.; Sugimoto, S.; Noda, M.; Sugiyama, M. Anti-oxidant and anti-inflammatory substance generated newly in Paeoniae Radix Alba extract fermented with plant-derived Lactobacillus brevis 174A. Antioxidants 2021, 10, 1071. [Google Scholar] [CrossRef] [PubMed]
- You, S.; Shi, X.; Yu, D.; Zhao, D.; An, Q.; Wang, D.; Zhang, J.; Li, M.; Wang, C. Fermentation of Panax notoginseng root extract polysaccharides attenuates oxidative stress and promotes type I procollagen synthesis in human dermal fibroblast cells. BMC Complement. Med. Ther. 2021, 21, 34. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Kang, D.J. Anti-pollution activity, antioxidant and anti-inflammatory effects of fermented extract from Smilax china leaf in macrophages and keratinocytes. Cosmetics 2022, 9, 120. [Google Scholar] [CrossRef]
- Nam, G.H.; Kawk, H.W.; Kim, S.Y.; Kim, Y.M. Solvent fractions of fermented Trapa japonica fruit extract stimulate collagen synthesis through TGF-β1/GSK-3β/β-catenin pathway in human dermal fibroblasts. J. Cosmet. Dermatol. 2020, 19, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Lim, J.M.; Mohan, H.; Seralathan, K.K.; Park, Y.J.; Lee, J.H.; Oh, B.T. Enhanced bioactivity of Zanthoxylum schinifolium fermented extract: Anti-inflammatory, anti-bacterial, and anti-melanogenic activity. J. Biosci. Bioeng. 2020, 129, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.S.; Eweys, A.S.; Zhang, J.Y.; Zhu, Y.; Bai, J.; Darwesh, O.M.; Zhang, H.B.; Xiao, X. Fermentation affects the antioxidant activity of plant-based food material through the release and production of bioactive components. Antioxidants 2021, 10, 2004. [Google Scholar] [CrossRef] [PubMed]
- Chouhan, S.; Guleria, S. Anti-inflammatory activity of medicinal plants: Present status and future perspectives. Bot. Leads Drug Discov. 2020, 67–92. [Google Scholar] [CrossRef]
- Shahbazi, R.; Sharifzad, F.; Bagheri, R.; Alsadi, N.; Yasavoli-Sharahi, H.; Matar, C. Anti-inflammatory and immunomodulatory properties of fermented plant foods. Nutrients 2021, 13, 1516. [Google Scholar] [CrossRef]
- Lin, J.Y.; Fisher, D.E. Melanocyte biology and skin pigmentation. Nature 2007, 445, 843–850. [Google Scholar] [CrossRef]
- Uyen, L.D.P.; Nguyen, D.H.; Kim, E.K. Mechanism of skin pigmentation. Biotech. Bioproc. Eng. 2008, 13, 383–395. [Google Scholar] [CrossRef]
- Ahn, H.Y.; Choo, Y.M.; Cho, Y.S. Anti-pigmentation effects of eight Phellinus linteus-fermented traditional crude herbal extracts on brown guinea pigs of ultraviolet b-induced hyperpigmentation. J. Microbiol. Biotechnol. 2018, 28, 375–380. [Google Scholar] [CrossRef]
- Kim, K.; Huh, Y.; Lim, K.M. Anti-pigmentary natural compounds and their mode of action. Int. J. Molec. Sci. 2021, 22, 6206. [Google Scholar] [CrossRef]
- Gantwerker, E.A.; Hom, D.B. Skin: Histology and physiology of wound healing. Facial Plast. Surg. Clin. N. Am. 2011, 19, 441–453. [Google Scholar] [CrossRef]
- Herman, A.; Herman, A.P. Herbal products and their active constituents for diabetic wound healing—Preclinical and clinical studies: A systematic review. Pharmaceutics 2023, 15, 281. [Google Scholar] [CrossRef]
- Sivamaruthi, B.S.; Chaiyasut, C.; Kesika, P. Cosmeceutical importance of fermented plant extracts: A short review. Int. J. Appl. Pharm. 2018, 10, 31–34. [Google Scholar] [CrossRef]
- Ro, H.S.; Jang, H.J.; Kim, G.R.; Park, S.J.; Lee, H.Y. Enhancement of the anti-skin wrinkling effects of Aloe arborescens Miller extracts associated with lactic acid fermentation. Evid.-Based Complement. Altern. Med. 2020, 2020, 2743594. [Google Scholar] [CrossRef]
- Lee, H.; Choi, W.; Ro, H.; Kim, G.; Lee, H. Skin Antiaging effects of the fermented outer layers of leaf skin of Aloe barbadensis miller associated with the enhancement of mitochondrial activities of UVb-irradiated human skin fibroblasts. Appl. Sci. 2021, 11, 5660. [Google Scholar] [CrossRef]
- Park, Y.S.; Nam, G.H.; Jo, K.J.; Kawk, H.W.; Yoo, J.G.; Jang, J.D.; Kang, S.; Kim, S.Y.; Kim, Y.M. Adequacy of the anti-aging and anti-wrinkle effects of the Artemisia vulgaris fermented solvent fraction. KSBB J. 2019, 34, 199–206. [Google Scholar] [CrossRef]
- Pham, Q.L.; Jang, H.J.; Kim, K.B. Anti-wrinkle effect of fermented black ginseng on human fibroblasts. Int. J. Molec. Med. 2017, 39, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.T.; Ko, H.J.; Kim, G.B.; Pyo, H.B.; Lee, G.S. Protective effects of fermented Citrus Unshiu peel extract against ultraviolet-A-induced photoageing in human dermal fibrobolasts. Phytother. Res. 2012, 26, 1851–1856. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Bae, J.T.; Song, M.H.; Lee, G.S.; Choe, S.Y.; Pyo, H.B. Biological activities of Fructus arctii fermented with the basidiomycete Grifola frondosa. Arch. Pharm. Res. 2010, 33, 1943–1951. [Google Scholar] [CrossRef]
- Hering, A.; Stefanowicz-Hajduk, J.; Gucwa, M.; Wielgomas, B.; Ochocka, J.R. Photoprotection and antiaging activity of extracts from honeybush (Cyclopia sp.)—In vitro wound healing and inhibition of the skin extracellular matrix enzymes: Tyrosinase, collagenase, elastase and hyaluronidase. Pharmaceutics 2023, 15, 1542. [Google Scholar] [CrossRef] [PubMed]
- Mayer, W.; Weibel, M.; De Luca, C.; Ibragimova, G.; Trakhtman, I.; Kharaeva, Z.; Chandler, D.L.; Korkina, L. Biomolecules of fermented tropical fruits and fermenting microbes as regulators of human hair loss, hair quality, and scalp microbiota. Biomolecules 2023, 13, 699. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, Y.J.; Cheung, W.H. Biological effects of yeast-fermented plant root extract mixture on skin cells. Asian J. Beauty Cosmetol. 2022, 20, 349–359. [Google Scholar] [CrossRef]
- Jang, J.D.; Kim, M.; Nam, G.H.; Kim, Y.M.; Kang, S.M.; Lee, K.Y.; Park, Y.J. Antiaging activity of peptide identified from fermented Trapa Japonica fruit extract in human dermal fibroblasts. Evid. Based Compl. Altern. Med. 2020, 2020, 5895029. [Google Scholar] [CrossRef] [PubMed]
- Nam, G.H.; Jo, K.J.; Park, Y.S.; Kawk, H.W.; Yoo, J.G.; Jang, J.D.; Kang, S.M.; Kim, S.Y.; Kim, Y.M. Bacillus/Trapa japonica fruit extract ferment filtrate enhances human hair follicle dermal papilla cell proliferation via the Akt/ERK/GSK-3β signaling pathway. BMC Complement. Altern. Med. 2019, 19, 104. [Google Scholar] [CrossRef]
- Kang, Y.M.; Hong, C.H.; Kang, S.H.; Seo, D.S.; Kim, S.O.; Lee, H.Y.; Sim, H.J.; An, H.J. Anti-photoaging effect of plant extract fermented with Lactobacillus buchneri on CCD-986sk fibroblasts and HaCaT keratinocytes. J. Funct. Biomater. 2020, 11, 3. [Google Scholar] [CrossRef]
- Ganceviciene, R.; Liakou, A.I.; Theodoridis, A.; Makrantonaki, E.; Zouboulis, C.C. Skin anti-aging strategies. Derm.-Endocrinol. 2012, 4, 308–319. [Google Scholar] [CrossRef]
- Hussain, A.; Bose, S.; Wang, J.H.; Yadav, M.K.; Mahajan, G.B.; Kim, H. Fermentation is a feasible strategy for enhancing the bioactivity of herbal medicines. Food Res. Int. 2016, 81, 1–16. [Google Scholar] [CrossRef]
- Hur, S.J.; Lee, S.Y.; Kim, Y.C.; Choi, I.; Kim, G.B. Effect of fermentation on the antioxidant activity in plant-based foods. Food Chem. 2014, 160, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotech. 2012, 23, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The immunomodulatory and anti-inflammatory role of polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef]
- Guimarães, I.; Baptista-Silva, S.; Pintado, M.; Oliveira, L.A. Polyphenols: A promising avenue in therapeutic solutions for wound care. Appl. Sci. 2021, 11, 1230. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, M.; Mujumdar, A.S. New technology to overcome defects in production of fermented plant products—A review. Trends Food Sci. Technol. 2021, 116, 829–841. [Google Scholar] [CrossRef]
- Ingle, K.P.; Deshmukh, A.G.; Padole, D.A.; Dudhare, M.S.; Moharil, M.P.; Khelurkar, V.C. Phytochemicals: Extraction methods, identification and detection of bioactive compounds from plant extracts. J. Pharmacogn. Phytochem. 2017, 6, 32–36. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).