The Efficacy and Safety of Intranasal Formulations of Ketamine and Esketamine for the Treatment of Major Depressive Disorder: A Systematic Review
Abstract
:1. Introduction
- -
- Pharmacological strategies: switching strategies (concurrent switch, overlapping switch, or sequential switch) or combination strategy, i.e., adding another AD or an add-on strategy (with lithium or quetiapine, for example) to ongoing AD;
- -
- Psychotherapy approaches (supportive therapy and psychoeducational intervention) can be used too, but only in combination with an AD;
- -
- Brain stimulation techniques with a preferential choice for electroconvulsive therapy (ECT) and repetitive transcranial magnetic stimulation (rTMS) in monotherapy or in combination with the current AD.
2. Materials and Methods
2.1. Methods
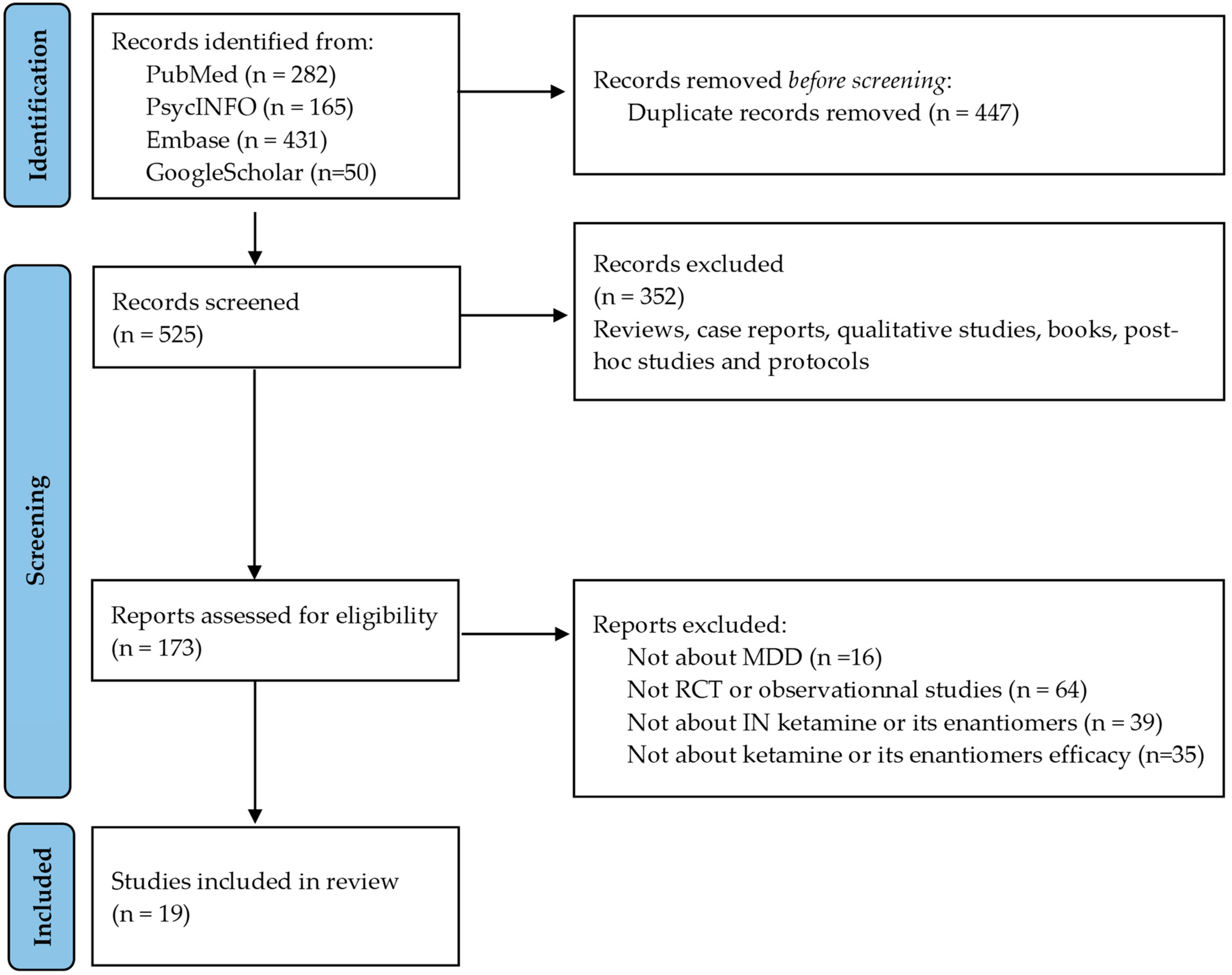
2.2. Literature Search
2.3. Study Selection Process
- -
- They included patients with an MDD diagnosis;
- -
- They included participants who were treated with IN ketamine or IN esketamine as an add-on or not;
- -
- They were randomized controlled trials (RCTs) or open-label or observational studies;
- -
- They measured the efficacy and safety of ketamine or esketamine treatment.
- -
- They were reviews, post hoc studies, case reports, qualitative studies, or protocols;
- -
- They were animal studies;
- -
- The treatment was not administered via an IN formulation;
- -
- They included participants who did not meet a diagnosis of MDD (i.e., bipolar disorder diagnosis).
2.4. Data Extraction
- -
- Article reference details, author, and year of publication;
- -
- Study characteristics: study design, primary outcomes, side effects, duration of study, main findings;
- -
- Population studied characteristics: sample size, mean age, % of female participants, clinical state, Montgomery–Asberg Depression Rating Scale (MADRS) score;
- -
- Treatment: ketamine, esketamine, dosage, add-on or not;
- -
- Efficacy characteristics: MADRS score;
- -
- Safety/tolerance characteristics: side effects, % of each side effect.
2.5. Methodological Quality Assessment
3. Results
3.1. Characteristics of Studies and Participants
| Author, Year | Study Design | Population Study | Primary Outcome | Duration | Study Drugs | Main Findings |
|---|---|---|---|---|---|---|
| MDD patients with TRD | ||||||
| Chen et al., 2023 [30] | Placebo-controlled RCT | 250 MDD patients with TRD | Change in MADRS total score from baseline to week 4 | 4 weeks | IN ESK 56–84 mg vs. placebo | No statistical difference in MADRS score was observed between ESK and placebo groups from baseline to week 4. |
| Reif et al., 2023 [41] | Active-controlled RCT (ESCAPE-TRD) | 676 MDD patients with TRD | Remission rate at week 8 | 32 weeks | IN ESK 28–84 mg vs. quetiapine | A significantly greater remission rate was observed in the ESK group compared to the quetiapine group at week 8 (27.1% vs. 17.6%, p = 0.003, respectively). |
| Takahashi et al., 2021 [32] | Placebo-controlled RCT | 202 MDD patients with TRD | Change in MADRS score from baseline to week 4 | 4 weeks (DB phase) 24 weeks (OL phase) | IN ESK 28, 56, and 84 mg vs. placebo | No statistical difference in MADRS score was observed between ESK and placebo groups from baseline to week 4. |
| Ochs Ross et al., 2020 [34] | Placebo-controlled RCT (TRANS FORM-3) | 138 MDD patients with TRD and ≥65 years old | Change in MADRS score from baseline to week 4 | 4 weeks | IN ESK 28–84 mg vs. placebo | No statistical difference in MADRS score was observed between ESK and placebo groups from baseline to week 4. |
| Wajs et al., 2020 [42] | OL trial (SUSTAIN-2) | 802 MDD patients with TRD | Change in MADRS score from baseline to week 4 (IND phase) and from week 5 to week 52 (OP/MAINT phase) | 52 weeks | IN ESK 28–84 mg | MADRS score decreased in the IND phase (baseline to week 4) (mean [SD] change: −16.4 [8.76]) and persisted during the OP/MAINT phase (week 5 to week 52) (mean [SD] change: 0.3 [8.12]). |
| Daly et al., 2019 [36] | Placebo-controlled RCT | 297 MDD patients with TRD | Time to relapse in patients with stable remission after IND phase (week 4) | Until relapse | IN ESK 56–84 mg vs. placebo | Significant delay to relapse was observed in the ESK group compared to the placebo group (HR, 0.49; 95% CI 0.29–0.84; p = 0.003). |
| Fedgchin et al., 2019 [37] | Placebo-controlled RCT (TRANS FORM-1) | 342 MDD patients with TRD | Change in MADRS score from baseline to week 4 | 4 weeks | IN ESK 56 and 84 mg vs. placebo | No statistical difference in MADRS score was observed between ESK and placebo groups from baseline to week 4. |
| Popova et al., 2019 [38] | Placebo-controlled RCT (TRANS FORM-2) | 227 MDD patients with TRD | Change in MADRS score from baseline to week 4 | 4 weeks | IN ESK 56–84 mg vs. placebo | Significantly greater improvement in MADRS score was observed in the ESK group compared to the placebo group from baseline to week 4 (LS mean difference [SE]: −4.0 [1.69], p = 0.02). |
| Daly et al., 2018 [39] | Placebo-controlled RCT | 67 MDD patients with TRD | Change in MADRS score from baseline to week 1 and week 2 | 2 weeks (DB phase) d15–d74 (OL phase) | IN ESK 28, 56, and 84 mg vs. placebo | Significantly greater improvement in MADRS score was observed in ESK groups compared to the placebo group in both periods (mean difference from placebo [SE]: ESK 28 mg: −4.2 [2.09], p = 0.02; ESK 56 mg: −6.3 [2.07], p = 0.001; ESK 84 mg: −9.0 [2.13], p < 0.001). |
| Galves et al., 2018 [40] | Active-controlled RCT | 5 MDD patients with TRD | Feasibility and safety of repeated IN ketamine | 4 weeks | IN ketamine 100 mg vs. midazolam 4.5 mg | The study was stopped early due to poor tolerability after the inclusion of five patients. |
| Lapidus et al., 2014 [23] | Placebo-controlled RCT | 20 MDD patients with TRD | Change in MADRS score from baseline to 24 h | 7 days | IN ketamine 50 mg vs. placebo | Significantly greater improvement in MADRS score was observed in the ketamine group compared to the placebo group from baseline to 24 h (t-test = 4.39, p < 0.001). |
| MDD patients with active suicidal ideation | ||||||
| Ionescu et al., 2021 [31] | Placebo-controlled RCT (ASPIRE II) | 230 MDD patients with ASI | Change in MADRS score from baseline to 24 h | 4 weeks | IN ESK 84 mg vs. placebo | Significantly greater improvement in MADRS score was observed in the ESK group compared to the placebo group from baseline to 24 h (LS mean difference [SE]: −3.9 [1.39]; −1.11; p = 0.006). |
| Fu et al., 2020 [33] | Placebo-controlled RCT (ASPIRE I) | 226 MDD patients with ASI | Change in MADRS score from baseline to 24 h | 4 weeks | IN ESK 84 mg vs. placebo | Significantly greater improvement in MADRS score was observed in the ESK group compared to the placebo group from baseline to 24 h (LS mean difference [SE]: −3.8 [1.39]; p = 0.006). |
| Canuso et al., 2019 [35] | Placebo-controlled RCT | 68 MDD patients with imminent suicide risk | Change in MADRS score from baseline to 4 h | 4 weeks | IN ESK 84 mg vs. placebo | Significantly greater improvement in MADRS score was observed in the ESK group compared to the placebo group from baseline to 4 h (LS mean difference [SE]: −5.3 [2.10], p = 0.015). |
| Author, Year | Study Design | Population Study | Main Outcomes | Duration | Study Drug | Main Findings |
|---|---|---|---|---|---|---|
| Estrade et al., 2023 [43] | OS | 105 MDD patients with TRD | Define trajectories of ESK response using MADRS score | 5 months | IN ESK 28–84 mg | After two ESK administrations, the MADRS score predicted the 90-day trajectories of response with an accuracy of 80%. |
| Singh et al., 2023 [44] | OS | 62 MDD patients with TRD | Change in QIDS-SR score and time to achieve response and remission | Up to 6 weeks | Ketamine IV 0.5 mg/kg vs. IN ESK 56–84 mg | There was no significant difference in response and remission rates between IV and IN routes. A significantly faster time to remission was observed in the ketamine IV group compared to the IN ESK group (HR = 5.0, p = 0.02). |
| Brendle et al., 2022 [45] | OS | 171 MDD patients | Change in GAD-7 and PHQ-9 scores | 1 to 71 treatment sessions | IN ESK 56–84 mg | Significant reduction in PHQ-9 and GAD-7 scores was observed between baseline and last available treatment (PHQ-9 = mean [SD]: 16.7 [5.82] vs. 12 [6.38], p < 0.001; GAD-7 = mean [SD]: 12.0 [5.8] vs. 8.7 [5.62], p < 0.001). |
| Martinotti et al., 2022 [46] | OS | 116 MDD patients with TRD | Change in MADRS score and response and remission rates | 3 months | ESK 28–84 mg | Significant improvement in MADRS score was observed at months 1 (T1) and 3 (T2) (Student t-test baseline vs. T1: t −15.79, p < 0.0001; T2 vs. baseline: t 18.07, p < 0.0001). |
| Samalin et al., 2022, [47] | OS | 66 MDD patients with TRD | Characteristics of TRD patients receiving IN ESK, change in MADRS score | 30 days (median treatment exposure) | ESK 28–84 mg | A decrease in MADRS score by 36% was observed from baseline to week 4 (mean MADRS score: 30.9 vs. 19.5, respectively). |
3.2. Characteristics of Interventions, Drugs, and Control Conditions
3.3. Efficacy of IN Ketamine and IN Esketamine
3.3.1. Efficacy in MDD Patients with TRD
3.3.2. Efficacy in MDD Patients with Active Suicidal Ideation
3.4. Safety
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Charlson, F.; van Ommeren, M.; Flaxman, A.; Cornett, J.; Whiteford, H.; Saxena, S. New WHO Prevalence Estimates of Mental Disorders in Conflict Settings: A Systematic Review and Meta-Analysis. Lancet 2019, 394, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Hasin, D.S.; Sarvet, A.L.; Meyers, J.L.; Saha, T.D.; Ruan, W.J.; Stohl, M.; Grant, B.F. Epidemiology of Adult DSM-5 Major Depressive Disorder and Its Specifiers in the United States. JAMA Psychiatry 2018, 75, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Holma, K.M.; Melartin, T.K.; Haukka, J.; Holma, I.A.K.; Sokero, T.P.; Isometsä, E.T. Incidence and Predictors of Suicide Attempts in DSM-IV Major Depressive Disorder: A Five-Year Prospective Study. Am. J. Psychiatry 2010, 167, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Rush, A.J.; Thase, M.E.; Dubé, S. Research Issues in the Study of Difficult-to-Treat Depression. Biol. Psychiatry 2003, 53, 743–753. [Google Scholar] [CrossRef]
- Rush, A.J.; Trivedi, M.H.; Wisniewski, S.R.; Nierenberg, A.A.; Stewart, J.W.; Warden, D.; Niederehe, G.; Thase, M.E.; Lavori, P.W.; Lebowitz, B.D.; et al. Acute and Longer-Term Outcomes in Depressed Outpatients Requiring One or Several Treatment Steps: A STAR*D Report. Am. J. Psychiatry 2006, 163, 1905–1917. [Google Scholar] [CrossRef] [PubMed]
- Sforzini, L.; Worrell, C.; Kose, M.; Anderson, I.M.; Aouizerate, B.; Arolt, V.; Bauer, M.; Baune, B.T.; Blier, P.; Cleare, A.J.; et al. A Delphi-Method-Based Consensus Guideline for Definition of Treatment-Resistant Depression for Clinical Trials. Mol. Psychiatry 2022, 27, 1286–1299. [Google Scholar] [CrossRef] [PubMed]
- Bennabi, D.; Charpeaud, T.; Yrondi, A.; Genty, J.-B.; Destouches, S.; Lancrenon, S.; Alaïli, N.; Bellivier, F.; Bougerol, T.; Camus, V.; et al. Clinical Guidelines for the Management of Treatment-Resistant Depression: French Recommendations from Experts, the French Association for Biological Psychiatry and Neuropsychopharmacology and the Fondation FondaMental. BMC Psychiatry 2019, 19, 262. [Google Scholar] [CrossRef] [PubMed]
- Bosco-Lévy, P.; Grelaud, A.; Blin, P.; Astruc, B.; Falissard, B.; Llorca, P.-M.; Bernard, M.-A.; Lassalle, R.; Moore, N.; Droz-Perroteau, C. Treatment Resistant Depression Incidence and Prevalence Using the French Nationwide Claims Database. Pharmacoepidemiol. Drug Saf. 2021, 30, 169–177. [Google Scholar] [CrossRef]
- Reutfors, J.; Andersson, T.M.-L.; Brenner, P.; Brandt, L.; DiBernardo, A.; Li, G.; Hägg, D.; Wingård, L.; Bodén, R. Mortality in Treatment-Resistant Unipolar Depression: A Register-Based Cohort Study in Sweden. J. Affect. Disord. 2018, 238, 674–679. [Google Scholar] [CrossRef]
- Pérez-Sola, V.; Roca, M.; Alonso, J.; Gabilondo, A.; Hernando, T.; Sicras-Mainar, A.; Sicras-Navarro, A.; Herrera, B.; Vieta, E. Economic Impact of Treatment-Resistant Depression: A Retrospective Observational Study. J. Affect. Disord. 2021, 295, 578–586. [Google Scholar] [CrossRef]
- Cai, Q.; Sheehan, J.J.; Wu, B.; Alphs, L.; Connolly, N.; Benson, C. Descriptive Analysis of the Economic Burden of Treatment Resistance in a Major Depressive Episode. Curr. Med. Res. Opin. 2020, 36, 329–335. [Google Scholar] [CrossRef]
- Duman, R.S.; Sanacora, G.; Krystal, J.H. Altered Connectivity in Depression: GABA and Glutamate Neurotransmitter Deficits and Reversal by Novel Treatments. Neuron 2019, 102, 75–90. [Google Scholar] [CrossRef]
- Li, L.; Vlisides, P.E. Ketamine: 50 Years of Modulating the Mind. Front. Hum. Neurosci. 2016, 10, 612. [Google Scholar] [CrossRef]
- Natoli, S. The Multiple Faces of Ketamine in Anaesthesia and Analgesia. Drugs Context 2021, 10, 2020–12–18. [Google Scholar] [CrossRef]
- Peng, F.Z.; Fan, J.; Ge, T.T.; Liu, Q.Q.; Li, B.J. Rapid Anti-Depressant-like Effects of Ketamine and Other Candidates: Molecular and Cellular Mechanisms. Cell Prolif. 2020, 53, e12804. [Google Scholar] [CrossRef]
- Zanos, P.; Moaddel, R.; Morris, P.J.; Riggs, L.M.; Highland, J.N.; Georgiou, P.; Pereira, E.F.R.; Albuquerque, E.X.; Thomas, C.J.; Zarate, C.A.; et al. Ketamine and Ketamine Metabolite Pharmacology: Insights into Therapeutic Mechanisms. Pharmacol. Rev. 2018, 70, 621–660. [Google Scholar] [CrossRef]
- Phillips, J.L.; Norris, S.; Talbot, J.; Birmingham, M.; Hatchard, T.; Ortiz, A.; Owoeye, O.; Batten, L.A.; Blier, P. Single, Repeated, and Maintenance Ketamine Infusions for Treatment-Resistant Depression: A Randomized Controlled Trial. Focus (Am. Psychiatr. Publ.) 2020, 18, 236–243. [Google Scholar] [CrossRef]
- Murrough, J.W.; Iosifescu, D.V.; Chang, L.C.; Al Jurdi, R.K.; Green, C.E.; Perez, A.M.; Iqbal, S.; Pillemer, S.; Foulkes, A.; Shah, A.; et al. Antidepressant Efficacy of Ketamine in Treatment-Resistant Major Depression: A Two-Site Randomized Controlled Trial. Am. J. Psychiatry 2013, 170, 1134–1142. [Google Scholar] [CrossRef]
- Zarate, C.A.; Singh, J.B.; Carlson, P.J.; Brutsche, N.E.; Ameli, R.; Luckenbaugh, D.A.; Charney, D.S.; Manji, H.K. A Randomized Trial of an N-Methyl-D-Aspartate Antagonist in Treatment-Resistant Major Depression. Arch. Gen. Psychiatry 2006, 63, 856–864. [Google Scholar] [CrossRef]
- Chong, C.; Schug, S.A.; Page-Sharp, M.; Jenkins, B.; Ilett, K.F. Development of a Sublingual/Oral Formulation of Ketamine for Use in Neuropathic Pain: Preliminary Findings from a Three-Way Randomized, Crossover Study. Clin. Drug Investig. 2009, 29, 317–324. [Google Scholar] [CrossRef]
- Erdő, F.; Bors, L.A.; Farkas, D.; Bajza, Á.; Gizurarson, S. Evaluation of Intranasal Delivery Route of Drug Administration for Brain Targeting. Brain Res. Bull. 2018, 143, 155–170. [Google Scholar] [CrossRef]
- Panek, M.; Kawalec, P.; Pilc, A.; Lasoń, W. Developments in the Discovery and Design of Intranasal Antidepressants. Expert Opin. Drug Discov. 2020, 15, 1145–1164. [Google Scholar] [CrossRef]
- Lapidus, K.A.B.; Levitch, C.F.; Perez, A.M.; Brallier, J.W.; Parides, M.K.; Soleimani, L.; Feder, A.; Iosifescu, D.V.; Charney, D.S.; Murrough, J.W. A Randomized Controlled Trial of Intranasal Ketamine in Major Depressive Disorder. Biol. Psychiatry 2014, 76, 970–976. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Hashimoto, K. An Update on Ketamine and Its Two Enantiomers as Rapid-Acting Antidepressants. Expert Rev. Neurother. 2019, 19, 83–92. [Google Scholar] [CrossRef]
- Pochwat, B.; Krupa, A.J.; Siwek, M.; Szewczyk, B. New Investigational Agents for the Treatment of Major Depressive Disorder. Expert Opin. Investig. Drugs 2022, 31, 1053–1066. [Google Scholar] [CrossRef] [PubMed]
- Leal, G.C.; Souza-Marques, B.; Mello, R.P.; Bandeira, I.D.; Caliman-Fontes, A.T.; Carneiro, B.A.; Faria-Guimarães, D.; Guerreiro-Costa, L.N.F.; Jesus-Nunes, A.P.; Silva, S.S.; et al. Arketamine as Adjunctive Therapy for Treatment-Resistant Depression: A Placebo-Controlled Pilot Study. J. Affect. Disord. 2023, 330, 7–15. [Google Scholar] [CrossRef]
- Leal, G.C.; Bandeira, I.D.; Correia-Melo, F.S.; Telles, M.; Mello, R.P.; Vieira, F.; Lima, C.S.; Jesus-Nunes, A.P.; Guerreiro-Costa, L.N.F.; Marback, R.F.; et al. Intravenous Arketamine for Treatment-Resistant Depression: Open-Label Pilot Study. Eur. Arch. Psychiatry Clin. Neurosci. 2021, 271, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Perez-Ruixo, C.; Rossenu, S.; Zannikos, P.; Nandy, P.; Singh, J.; Drevets, W.C.; Perez-Ruixo, J.J. Population Pharmacokinetics of Esketamine Nasal Spray and Its Metabolite Noresketamine in Healthy Subjects and Patients with Treatment-Resistant Depression. Clin. Pharmacokinet. 2021, 60, 501–516. [Google Scholar] [CrossRef]
- Traynor, K. Esketamine Nasal Spray Approved for Treatment-Resistant Depression. Am. J. Health Syst. Pharm. 2019, 76, 573. [Google Scholar] [CrossRef]
- Chen, X.; Hou, X.; Bai, D.; Lane, R.; Zhang, C.; Canuso, C.; Wang, G.; Fu, D.-J. Efficacy and Safety of Flexibly Dosed Esketamine Nasal Spray Plus a Newly Initiated Oral Antidepressant in Adult Patients with Treatment-Resistant Depression: A Randomized, Double-Blind, Multicenter, Active-Controlled Study Conducted in China and USA. Neuropsychiatr. Dis. Treat. 2023, 19, 693–707. [Google Scholar] [CrossRef]
- Ionescu, D.F.; Fu, D.-J.; Qiu, X.; Lane, R.; Lim, P.; Kasper, S.; Hough, D.; Drevets, W.C.; Manji, H.; Canuso, C.M. Esketamine Nasal Spray for Rapid Reduction of Depressive Symptoms in Patients with Major Depressive Disorder Who Have Active Suicide Ideation with Intent: Results of a Phase 3, Double-Blind, Randomized Study (ASPIRE II). Int. J. Neuropsychopharmacol. 2021, 24, 22–31. [Google Scholar] [CrossRef]
- Takahashi, N.; Yamada, A.; Shiraishi, A.; Shimizu, H.; Goto, R.; Tominaga, Y. Efficacy and Safety of Fixed Doses of Intranasal Esketamine as an Add-on Therapy to Oral Antidepressants in Japanese Patients with Treatment-Resistant Depression: A Phase 2b Randomized Clinical Study. BMC Psychiatry 2021, 21, 526. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.-J.; Ionescu, D.F.; Li, X.; Lane, R.; Lim, P.; Sanacora, G.; Hough, D.; Manji, H.; Drevets, W.C.; Canuso, C.M. Esketamine Nasal Spray for Rapid Reduction of Major Depressive Disorder Symptoms in Patients Who Have Active Suicidal Ideation With Intent: Double-Blind, Randomized Study (ASPIRE I). J. Clin. Psychiatry 2020, 81, 19m13191. [Google Scholar] [CrossRef] [PubMed]
- Ochs-Ross, R.; Daly, E.J.; Zhang, Y.; Lane, R.; Lim, P.; Morrison, R.L.; Hough, D.; Manji, H.; Drevets, W.C.; Sanacora, G.; et al. Efficacy and Safety of Esketamine Nasal Spray Plus an Oral Antidepressant in Elderly Patients With Treatment-Resistant Depression-TRANSFORM-3. Am. J. Geriatr. Psychiatry 2020, 28, 121–141. [Google Scholar] [CrossRef]
- Canuso, C.M.; Singh, J.B.; Fedgchin, M.; Alphs, L.; Lane, R.; Lim, P.; Pinter, C.; Hough, D.; Sanacora, G.; Manji, H.; et al. Efficacy and Safety of Intranasal Esketamine for the Rapid Reduction of Symptoms of Depression and Suicidality in Patients at Imminent Risk for Suicide: Results of a Double-Blind, Randomized, Placebo-Controlled Study. Focus (Am. Psychiatr. Publ.) 2019, 17, 55–65. [Google Scholar] [CrossRef]
- Daly, E.J.; Trivedi, M.H.; Janik, A.; Li, H.; Zhang, Y.; Li, X.; Lane, R.; Lim, P.; Duca, A.R.; Hough, D.; et al. Efficacy of Esketamine Nasal Spray Plus Oral Antidepressant Treatment for Relapse Prevention in Patients With Treatment-Resistant Depression: A Randomized Clinical Trial. JAMA Psychiatry 2019, 76, 893–903. [Google Scholar] [CrossRef]
- Fedgchin, M.; Trivedi, M.; Daly, E.J.; Melkote, R.; Lane, R.; Lim, P.; Vitagliano, D.; Blier, P.; Fava, M.; Liebowitz, M.; et al. Efficacy and Safety of Fixed-Dose Esketamine Nasal Spray Combined With a New Oral Antidepressant in Treatment-Resistant Depression: Results of a Randomized, Double-Blind, Active-Controlled Study (TRANSFORM-1). Int. J. Neuropsychopharmacol. 2019, 22, 616–630. [Google Scholar] [CrossRef]
- Popova, V.; Daly, E.J.; Trivedi, M.; Cooper, K.; Lane, R.; Lim, P.; Mazzucco, C.; Hough, D.; Thase, M.E.; Shelton, R.C.; et al. Efficacy and Safety of Flexibly Dosed Esketamine Nasal Spray Combined With a Newly Initiated Oral Antidepressant in Treatment-Resistant Depression: A Randomized Double-Blind Active-Controlled Study. Am. J. Psychiatry 2019, 176, 428–438. [Google Scholar] [CrossRef]
- Daly, E.J.; Singh, J.B.; Fedgchin, M.; Cooper, K.; Lim, P.; Shelton, R.C.; Thase, M.E.; Winokur, A.; Van Nueten, L.; Manji, H.; et al. Efficacy and Safety of Intranasal Esketamine Adjunctive to Oral Antidepressant Therapy in Treatment-Resistant Depression: A Randomized Clinical Trial. JAMA Psychiatry 2018, 75, 139–148. [Google Scholar] [CrossRef]
- Gálvez, V.; Li, A.; Huggins, C.; Glue, P.; Martin, D.; Somogyi, A.A.; Alonzo, A.; Rodgers, A.; Mitchell, P.B.; Loo, C.K. Repeated Intranasal Ketamine for Treatment-Resistant Depression—The Way to Go? Results from a Pilot Randomised Controlled Trial. J. Psychopharmacol. 2018, 32, 397–407. [Google Scholar] [CrossRef]
- Reif, A.; Bitter, I.; Buyze, J.; Cebulla, K.; Frey, R.; Fu, D.-J.; Ito, T.; Kambarov, Y.; Llorca, P.-M.; Oliveira-Maia, A.J.; et al. Esketamine Nasal Spray versus Quetiapine for Treatment-Resistant Depression. N. Engl. J. Med. 2023, 389, 1298–1309. [Google Scholar] [CrossRef]
- Wajs, E.; Aluisio, L.; Holder, R.; Daly, E.J.; Lane, R.; Lim, P.; George, J.E.; Morrison, R.L.; Sanacora, G.; Young, A.H.; et al. Esketamine Nasal Spray Plus Oral Antidepressant in Patients With Treatment-Resistant Depression: Assessment of Long-Term Safety in a Phase 3, Open-Label Study (SUSTAIN-2). J. Clin. Psychiatry 2020, 81, 19m12891. [Google Scholar] [CrossRef]
- Estrade, I.; Petit, A.-C.; Sylvestre, V.; Danon, M.; Leroy, S.; Perrain, R.; Vinckier, F.; Mekaoui, L.; Gaillard, R.; Advenier-Iakovlev, E.; et al. Early Effects Predict Trajectories of Response to Esketamine in Treatment-Resistant Depression. J. Affect. Disord. 2023, 342, 166–176. [Google Scholar] [CrossRef]
- Singh, B.; Kung, S.; Pazdernik, V.; Schak, K.M.; Geske, J.; Schulte, P.J.; Frye, M.A.; Vande Voort, J.L. Comparative Effectiveness of Intravenous Ketamine and Intranasal Esketamine in Clinical Practice Among Patients With Treatment-Refractory Depression: An Observational Study. J. Clin. Psychiatry 2023, 84, 22m14548. [Google Scholar] [CrossRef]
- Brendle, M.; Ahuja, S.; Valle, M.D.; Moore, C.; Thielking, P.; Malone, D.C.; Robison, R. Safety and Effectiveness of Intranasal Esketamine for Treatment-Resistant Depression: A Real-World Retrospective Study. J. Comp. Eff. Res. 2022, 11, 1323–1336. [Google Scholar] [CrossRef]
- Martinotti, G.; Vita, A.; Fagiolini, A.; Maina, G.; Bertolino, A.; Dell’Osso, B.; Siracusano, A.; Clerici, M.; Bellomo, A.; Sani, G.; et al. Real-World Experience of Esketamine Use to Manage Treatment-Resistant Depression: A Multicentric Study on Safety and Effectiveness (REAL-ESK Study). J. Affect. Disord. 2022, 319, 646–654. [Google Scholar] [CrossRef]
- Samalin, L.; Rothärmel, M.; Mekaoui, L.; Gaudré-Wattinne, E.; Codet, M.-A.; Bouju, S.; Sauvaget, A. Esketamine Nasal Spray in Patients with Treatment-Resistant Depression: The Real-World Experience in the French Cohort Early Access Programme. Int. J. Psychiatry Clin. Pract. 2022, 26, 352–362. [Google Scholar] [CrossRef]
- Jollant, F.; Colle, R.; Nguyen, T.M.L.; Corruble, E.; Gardier, A.M.; Walter, M.; Abbar, M.; Wagner, G. Ketamine and Esketamine in Suicidal Thoughts and Behaviors: A Systematic Review. Ther. Adv. Psychopharmacol. 2023, 13, 1–25. [Google Scholar] [CrossRef]
- Nikolin, S.; Rodgers, A.; Schwaab, A.; Bahji, A.; Zarate, C.; Vazquez, G.; Loo, C. Ketamine for the Treatment of Major Depression: A Systematic Review and Meta-Analysis. eClinicalMedicine 2023, 62, 102–127. [Google Scholar] [CrossRef]
- Domany, Y.; McCullumsmith, C.B. Single, Fixed-Dose Intranasal Ketamine for Alleviation of Acute Suicidal Ideation. An Emergency Department, Trans-Diagnostic Approach: A Randomized, Double-Blind, Placebo-Controlled, Proof-of-Concept Trial. Arch. Suicide Res. 2022, 26, 1250–1265. [Google Scholar] [CrossRef]
- Zaki, N.; Chen, L.N.; Lane, R.; Doherty, T.; Drevets, W.C.; Morrison, R.L.; Sanacora, G.; Wilkinson, S.T.; Popova, V.; Fu, D.-J. Long-Term Safety and Maintenance of Response with Esketamine Nasal Spray in Participants with Treatment-Resistant Depression: Interim Results of the SUSTAIN-3 Study. Neuropsychopharmacology 2023, 48, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- Nijs, M.; Wajs, E.; Aluisio, L.; Turkoz, I.; Daly, E.; Janik, A.; Borentain, S.; Singh, J.B.; DiBernardo, A.; Wiegand, F. Managing Esketamine Treatment Frequency toward Successful Outcomes: Analysis of Phase 3 Data. Int. J. Neuropsychopharmacol. 2020, 23, 426–433. [Google Scholar] [CrossRef]
- European Medicines Agency. SPRAVATO® Assessment Report. 2019. Available online: https://www.ema.europa.eu/en/documents/assessment-report/spravato-epar-public-assessment-report_en.pdf (accessed on 27 November 2023).
- Food Drugs Administration. SPRAVATO® US Prescribing Information. 2020. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/211243s004lbl.pdf (accessed on 27 November 2023).
- Lofts, A.; Abu-Hijleh, F.; Rigg, N.; Mishra, R.K.; Hoare, T. Using the Intranasal Route to Administer Drugs to Treat Neurological and Psychiatric Illnesses: Rationale, Successes, and Future Needs. CNS Drugs 2022, 36, 739–770. [Google Scholar] [CrossRef] [PubMed]
| TEAEs | Placebo n = 782 [30,31,32,33,34,35,37,38,39] | IN Esketamine 28 mg n = 60 [32,39] | IN Esketamine 56 mg n = 176 [32,37,39] | IN Esketamine 84 mg n = 435 [31,32,33,35,37,39] | IN Esketamine Flexible Dose n = 312 [30,34,38] | IN Esktamine Any Doses n = 983 [30,31,32,33,34,35,37,38,39] |
|---|---|---|---|---|---|---|
| Dizziness | 86 (11.0) | 15 (25.0) | 58 (33.0) | 148 (34.0) | 136 (43.6) | 357 (36.3) |
| Dissociation | 42 (5.4) | 14 (23.3) | 47 (26.7) | 146 (33.6) | 114 (36.5) | 321 (32.7) |
| Nausea | 81 (10.4) | 9 (15.0) | 42 (23.9) | 123 (28.3) | 96 (30.8) | 270 (27.5) |
| Headache | 114 (14.6) | 12 (20.0) | 31 (17.6) | 88 (20.2) | 48 (15.4) | 179 (18.2) |
| Somnolence | 70 (9.0) | 10 (16.7) | 37 (21.0) | 83 (19.1) | 35 (11.2) | 165 (16.8) |
| Increased blood pressure | 40 (5.1) | 12 (20.0) | 29 (16.5) | 57 (13.1) | 58 (18.6) | 156 (15.9) |
| Dysgeusia | 80 (10.2) | 2 (3.3) | 20 (11.4) | 81 (18.6) | 46 (14.7) | 149 (15.2) |
| Vertigo | 9 (1.2) | 6 (10.0) | 32 (18.2) | 51 (11.7) | 38 (12.2) | 127 (12.9) |
| Hypoesthesia | 12 (1.5) | 7 (11.7) | 22 (12.5) | 41 (9.4) | 37 (11.9) | 107 (10.9) |
| Vomiting | 22 (2.8) | 1 (1.7) | 10 (5.7) | 51 (11.7) | 39 (12.5) | 101 (10.3) |
| Blurred vision | 15 (1.9) | 0 (0.0) | 8 (4.5) | 38 (8.7) | 37 (11.9) | 83 (8.4) |
| Paresthesia | 14 (1.8) | 0 (0.0) | 19 (10.8) | 40 (9.2) | 17 (5.4) | 76 (7.7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boudieu, L.; Mennetrier, M.; Llorca, P.-M.; Samalin, L. The Efficacy and Safety of Intranasal Formulations of Ketamine and Esketamine for the Treatment of Major Depressive Disorder: A Systematic Review. Pharmaceutics 2023, 15, 2773. https://doi.org/10.3390/pharmaceutics15122773
Boudieu L, Mennetrier M, Llorca P-M, Samalin L. The Efficacy and Safety of Intranasal Formulations of Ketamine and Esketamine for the Treatment of Major Depressive Disorder: A Systematic Review. Pharmaceutics. 2023; 15(12):2773. https://doi.org/10.3390/pharmaceutics15122773
Chicago/Turabian StyleBoudieu, Ludivine, Myriam Mennetrier, Pierre-Michel Llorca, and Ludovic Samalin. 2023. "The Efficacy and Safety of Intranasal Formulations of Ketamine and Esketamine for the Treatment of Major Depressive Disorder: A Systematic Review" Pharmaceutics 15, no. 12: 2773. https://doi.org/10.3390/pharmaceutics15122773
APA StyleBoudieu, L., Mennetrier, M., Llorca, P.-M., & Samalin, L. (2023). The Efficacy and Safety of Intranasal Formulations of Ketamine and Esketamine for the Treatment of Major Depressive Disorder: A Systematic Review. Pharmaceutics, 15(12), 2773. https://doi.org/10.3390/pharmaceutics15122773






