Orodispersible Films: Current Innovations and Emerging Trends
Abstract
1. Introduction
2. Formulation Components
2.1. APIs
2.2. Polymers
2.2.1. Chitosan
2.2.2. Pectin
2.2.3. Polyvinyl Alcohol (PVA)
2.2.4. Alginic Acid
2.2.5. Hydrolysed Collagen and Gelatin
2.2.6. Pullulan
2.2.7. Starch
2.2.8. Maltodextrins (MDXs)
2.3. Plasticizers
2.4. Sweetening and Flavoring Agents
2.5. Saliva-Stimulating Agent
2.6. Coloring Agents
2.7. Stabilizing Agents
3. Manufacturing Methods
3.1. Solvent Casting
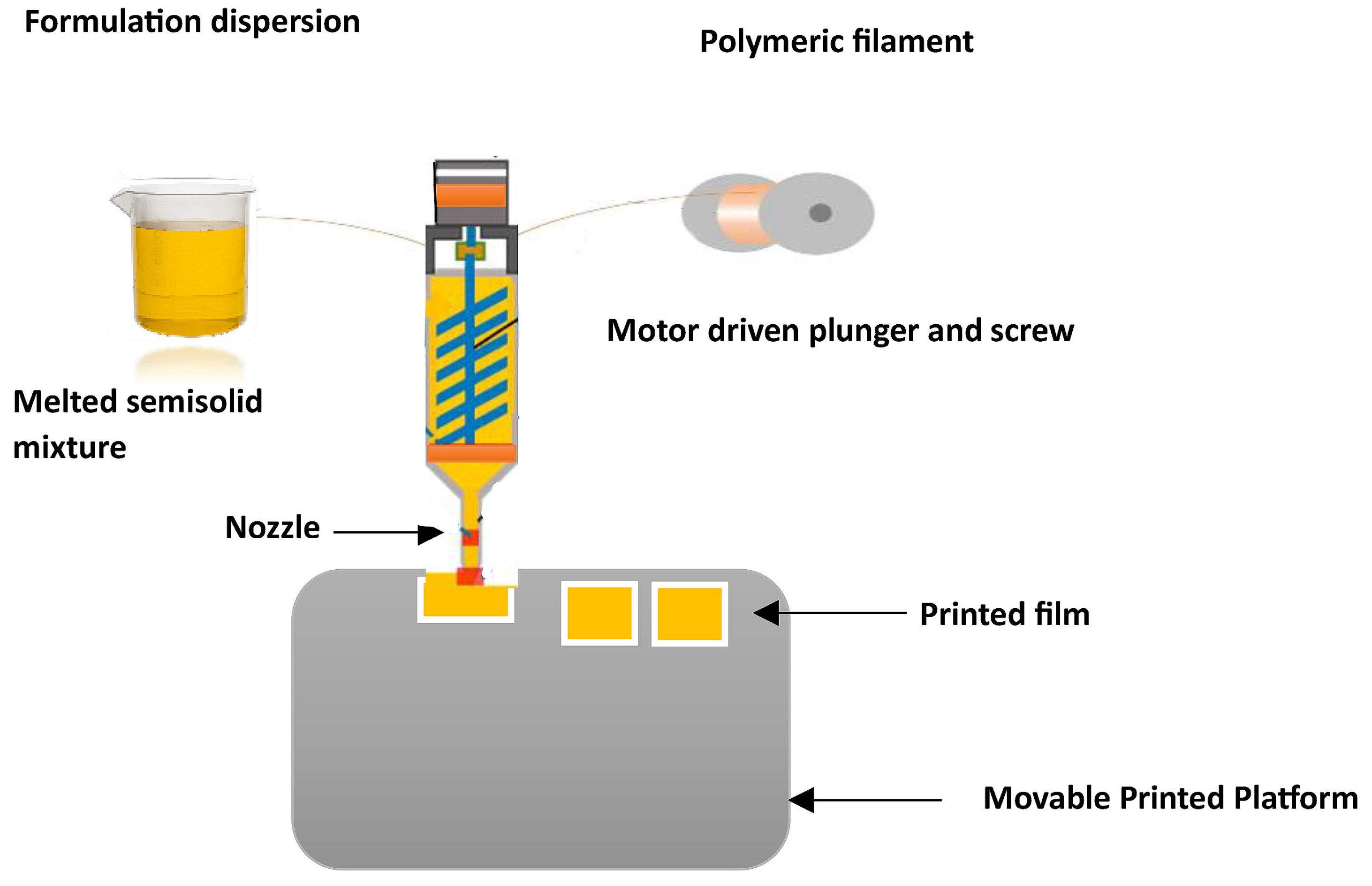
3.2. Three-Dimensional Printing (3DP) Method
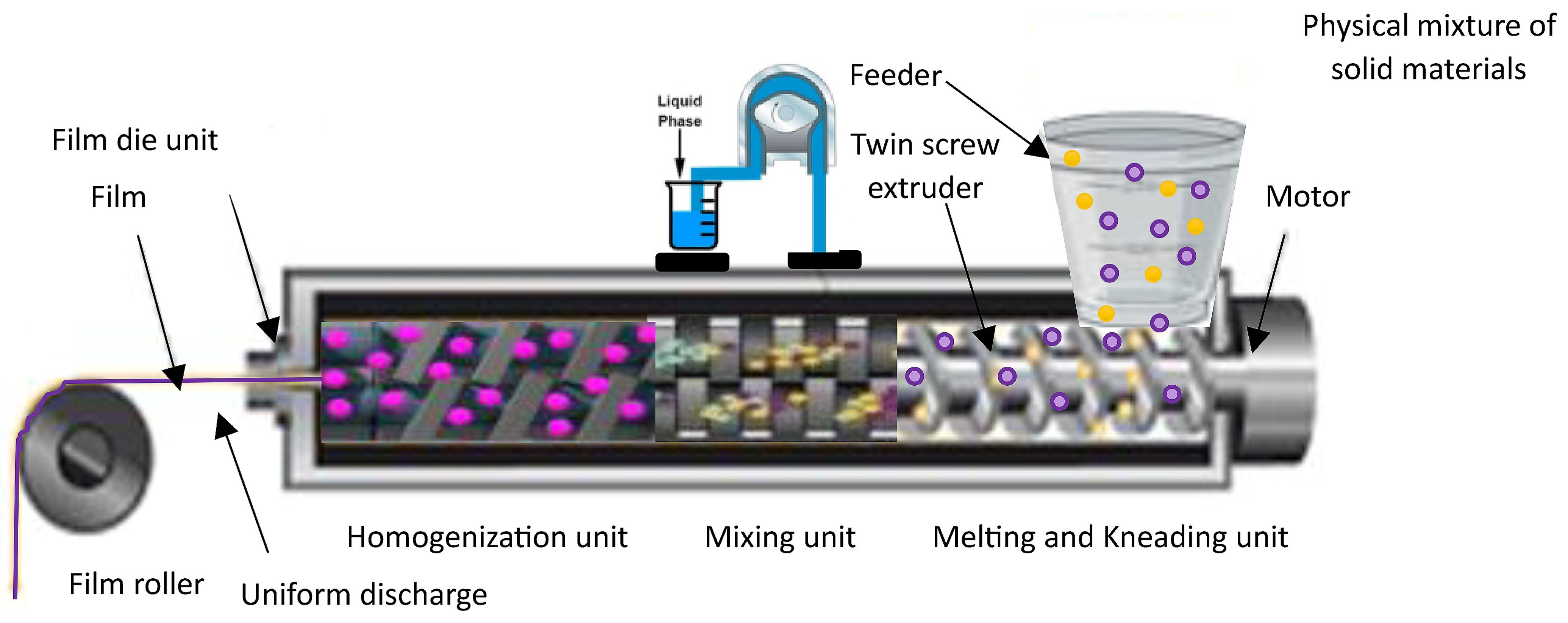
3.3. Hot-Melt Extrusion (HME)
3.4. Electrostatic Spray Deposition
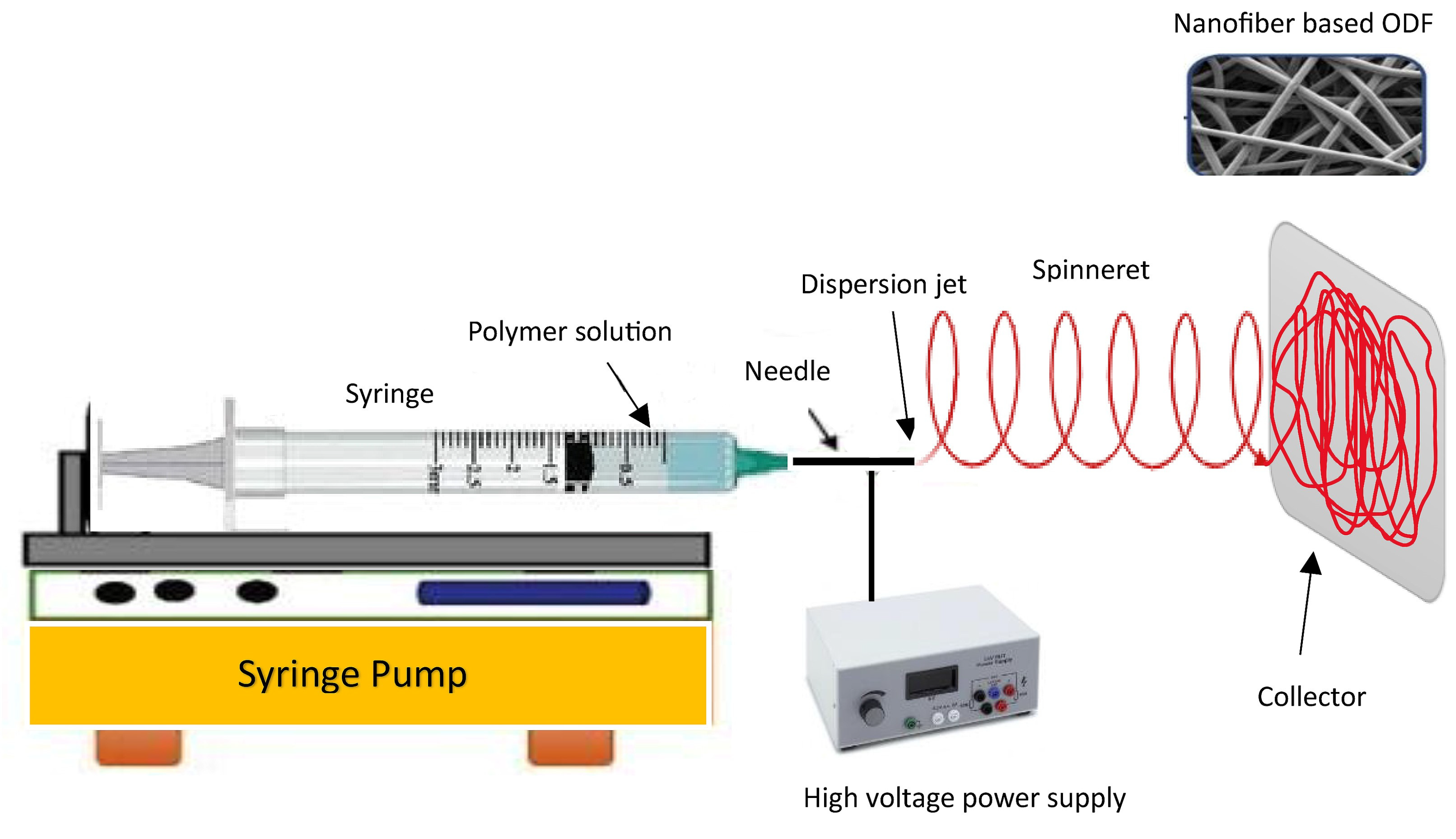
3.5. Electrospinning Method
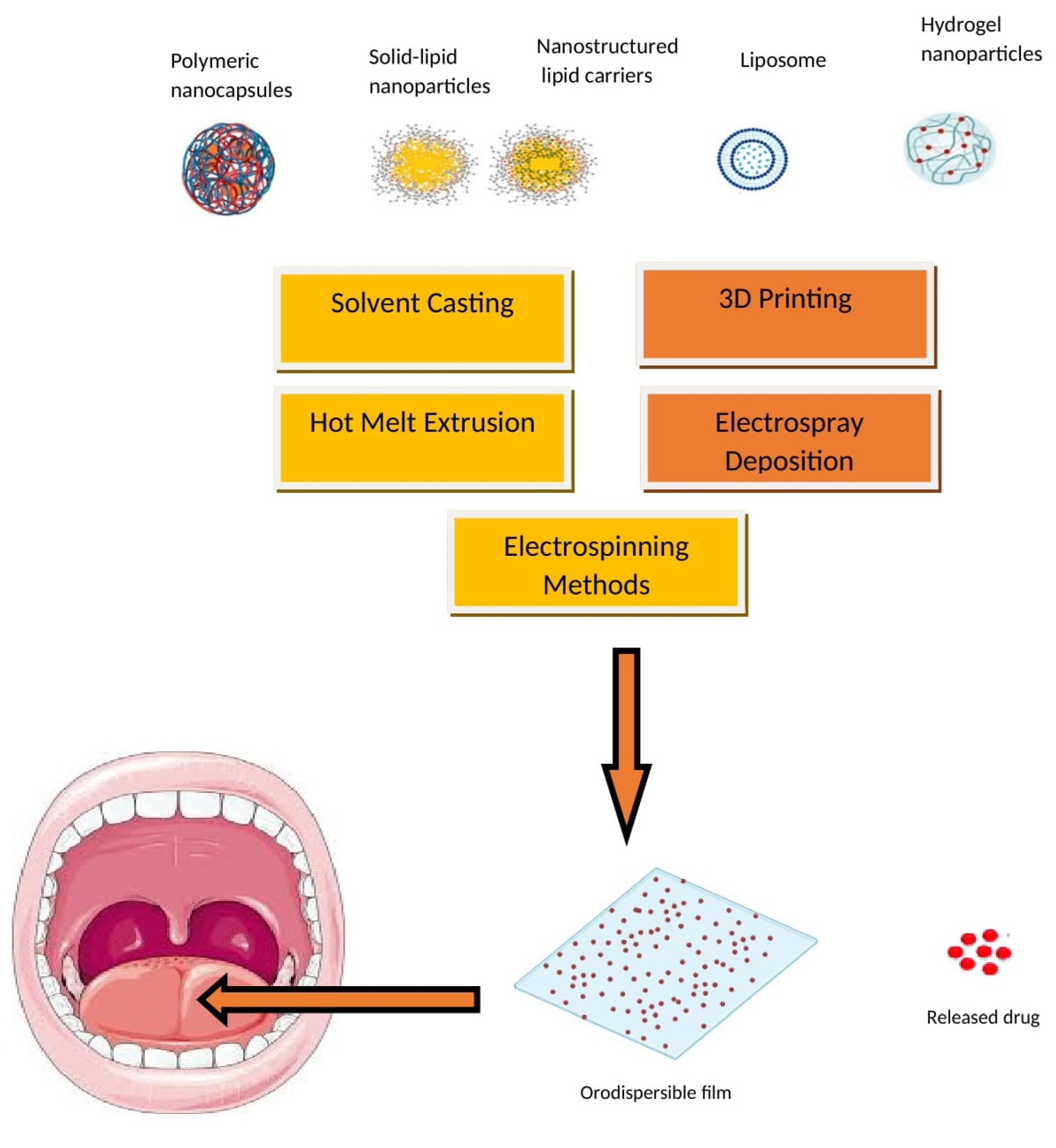
4. Nanoparticle-Embedded ODFs
5. In Vitro and In Vivo Evaluation Methods
| Technique | Principle | Evaluation Parameters | Ranges Units | References |
|---|---|---|---|---|
| Mechanical characteristics | ||||
| Tensile strength | Measure of a film’s ability to resist being pulled apart or stretched under tension without breaking or undergoing permanent deformation. | Tensile strength = Maximum load (N)/Original cross-sectional area (cm2) of the film | 1–33 Megapascals (MPa) | [64] |
| Young’s modulus | Also known as the elastic modulus or modulus of elasticity, it measures the stiffness, toughness, or rigidity of a material. | Young modulus = Stress (σ)/Strain (ε) | 50–1800 MPa | [138,148] |
| Percentage at break | Also known as elongation at break or strain at break; it is a measure of the amount of deformation a material undergoes before it fractures or breaks. | Strain at break = [(Final length − Original length)/Original length] × 100 | 0.3–38% | [148,149] |
| Strain energy | Elastic energy or deformation energy is the energy stored within a material when it undergoes deformation or strain. | Strain energy = (1/2) × Stress (σ) × Strain (ε) × Volume (V) | Joules (J) or Newton-meters (N·m) | [15] |
| Fracture energy or toughness | Measure of the amount of energy needed to cause complete failure or fracture of the material. | Energy to break = ∫ Stress (Applied stress) × d Strain (incremental change in strain) | J or N·m | [9] |
| Puncture strength | Also referred to as puncture resistance and is a measure of a material’s ability to withstand the penetration or puncturing force applied to it without rupturing or tearing. | Material’s resistance to the formation of holes or punctures or toughness | 0.2–13 mJ | [150] |
| Indentation test | Measures the hardness or material response to localized deformation; it involves applying a controlled force or load to the surface of a material using an indenter, typically a hardened ball or a diamond tip, and measures the resulting depth or indentation formed. | Hardness and Young’s modulus | 1 mPa and ~100 mPa | [151] |
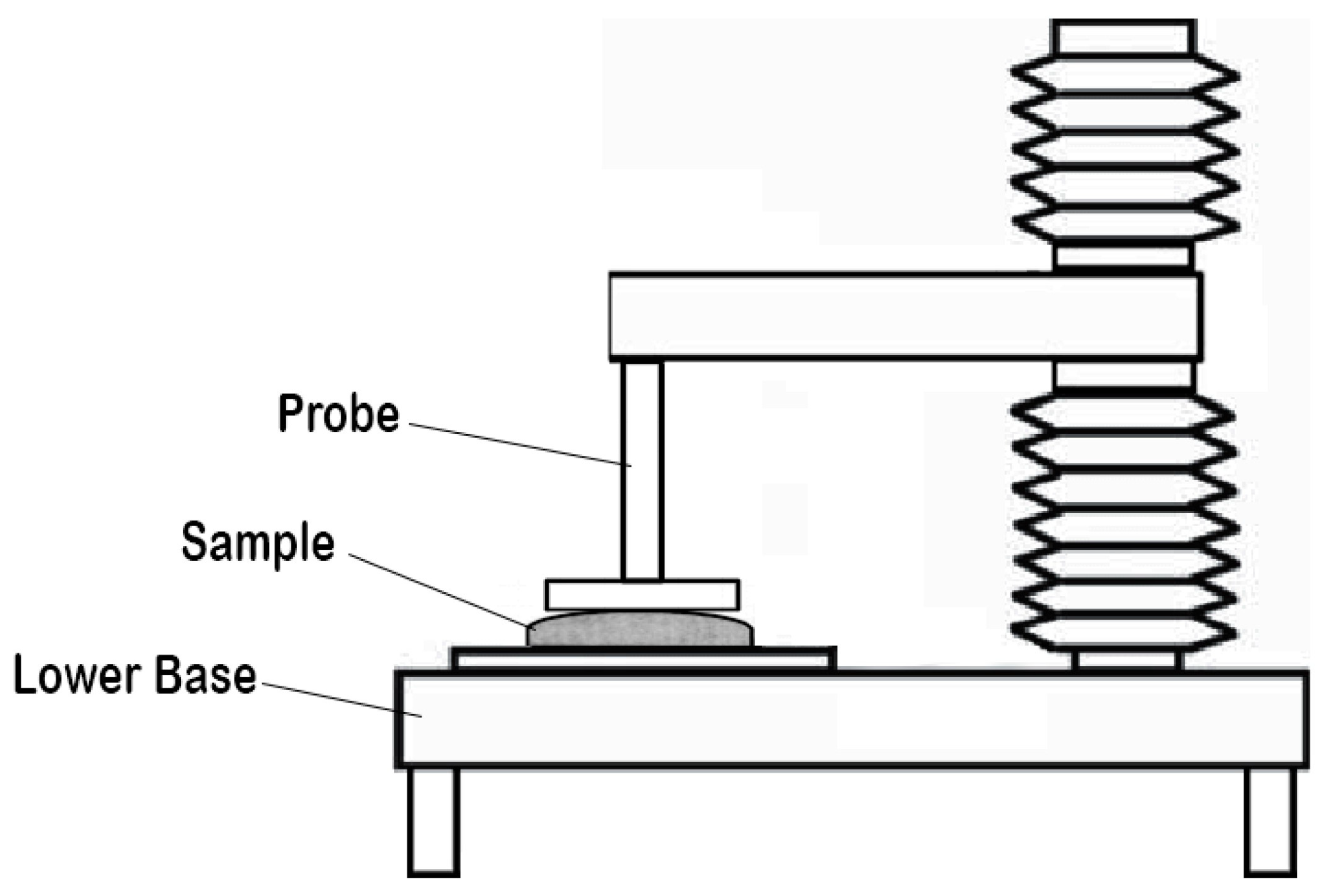
| Folding endurance | Refers to the number of times the sample strip of the film can be folded front and back at a 180° angle at the same plane without breaking. | Flexibility and durability | ~300 count | [152] |
| Film properties | ||||
| Water absorption capacity | The swelling capacity of an oral film evaluates its bioadhesion behavior, drug release, and physical integrity by measuring its ability to absorb water and expand in size or volume. | The percentage hydration of an oral film is determined using the equation [(X2 − X1) × 100/X1], wherein X1 represents the initial weight of the film, and X2 represents the weight of the film after hydration in simulated salivary fluid for a specified duration. | 5–25% | [153] |
| Thickness | The thickness of the film is inversely proportional to its disintegration time. The thinner films are generally more comfortable for patients and can affect the rate of drug release and absorption. Maintaining a consistent thickness is crucial for ensuring content uniformity across different dosage units. | Thickness measurement is carried out at the center and four corners of the film using a digital micrometer or vernier calipers. | 50–500 µm | [154] |
| Surface area | The surface area of the film can impact the rate of disintegration and dissolution of the API, which may contribute to absorption and bioavailability. | The surface area depends on its shape calculated using the appropriate geometric formula. For a square film, the total surface area is determined by squaring the length of any of its sides, and to determine the overall surface area of a rectangular film, simply multiply its length by its width. | 2–5 cm2 | [154] |
| Weight variation | Weight variation estimates the degree of variation or deviation in the weight of individual film units cut from the center and four corners within a batch or sample. | Weight variation is determined by calculating the difference between the weight of each film unit and the average weight of the film batch and then dividing it by the mean weight of the film. | <50 mg | [154] |
| Content uniformity | It ensures that the API is distributed uniformly throughout the dosage unit, minimizing variability between individual units. | Reliable and validated analytical method for the quantification of API. The individual film unit conforms to USP <905> uniformity of dosage units. | - | [154] |
| Surface characterization | ||||
| Surface morphology | Cutting a small section of the film, affixing to stubs utilizing adhesive tape, sputter-coating with a conducting material in an inert gas, and scanning it in a scanning electron microscope. | Surface features, roughness, presence of pores, cracks, surface irregularities, thickness, particle size, and other surface-related properties. | - | [155] |
| Surface pH | Subjected to a brief (<2 h) contact with distilled water at a temperature of 37 ± 0.5 °C, facilitating its swelling. It provides information about the acidity or alkalinity of the film’s surface | pH at the area of application, potential irritancy, or compatibility of the film with the oral tissues. | 6.0–7.5 | [156] |
| Film analysis | ||||
| Crystallinity and amorphous nature | Test material is placed in the ample holder of the X-ray diffractometer and scanned using specific voltage and current. Diffraction patterns were obtained at various 2θ values at a uniform scan speed with a particular step width. | Provide valuable information about the composition, crystallinity, amorphous nature, and physical properties of the sample. | % | [157] |
| Thermal scanning | The sample was sealed in the aluminum pan and heated at a uniform rate within the specified temperature range under an inert nitrogen atmosphere. | Examine the presence of phase transformation, recrystallization, thermal properties, and material characterization within the film. | °C | [158] |
| Infrared spectroscopy | Test sample and potassium bromide compressed at a certain pressure and scanned, or ground solid sample into a fine powder or placed into a liquid sample on an appropriate IR-transparent substrate, such as a salt plate or a diamond crystal. | Compatibility between drug and various inactive pharmaceutical ingredients in the formulation. | cm−1 | [159] |
| Release properties | ||||
| Disintegration test | Tested in 10 mL of simulated salivary fluid (pH 6.4) placed in a beaker maintained at 37 ± 0.5 °C and stirred at 10 rpm. | Assess the film’s ability to disintegrate/dissolve when in contact with simulated salivary fluid or other suitable fluid. | 30–60 s | [160] |
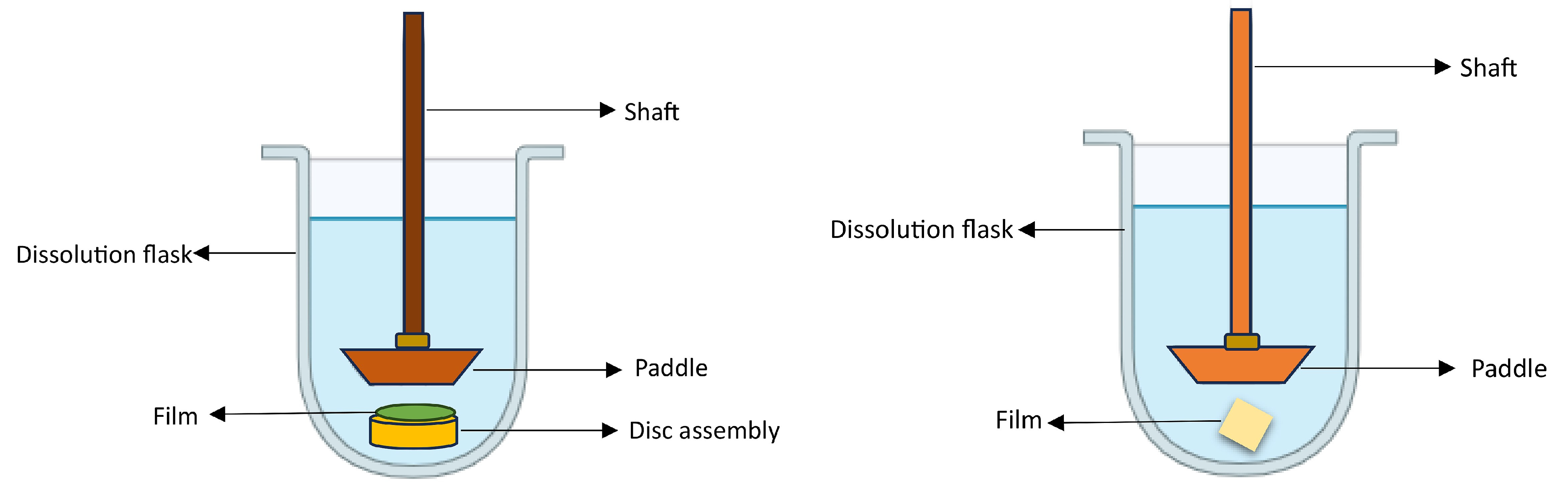
| In vitro drug release | Synthetic membrane using Franz diffusion cell, or utilizing USPXXIV Type 2 apparatus, USP Type V paddle-over-disk apparatus. | Drug release from the developed film using simulated salivary fluid (pH 6.2) or other suitable media. Acceptance criteria are similar to immediate-release conventional dosage forms. | % | [161,162] |
| Ex vivo permeation | Investigation carried out on freshly isolated buccal mucosa obtained from rabbit, pig, or chicken utilizing Franz diffusion cell, continuous flow through diffusion cell, Ussing chamber, or cell culture model using human oral epithelial cell line (TR-146). | Understand the transportation mechanism of a drug through oral epithelial tissues using parameters such as flux and permeability coefficient. | Flux (J) = μg/cm2/h permeability coefficient (P) = cm/h | [162] |
| Biocompatibility | ||||
| Cell cytotoxicity | Assess the potential toxicity of a substance or material on living cells. In vitro model of buccal mucosa, TR-146 cells cultured in Dulbecco’s modified eagle’s medium in optimum conditions at 37 °C in an atmosphere of 5% CO2 in plastic cell culture flasks with minimum passages of 20 and 28. Other techniques include MTT assay, lactate dehydrogenase release assay, live/dead staining, and cell proliferation assays. | Determine the impact of the ODF on cell viability, cell membrane integrity, and overall cellular function. | Number of viable cells | [163] |
| Organoleptic properties | ||||
| Taste perception | Tribology tests using a Bio-Tribometer aim to assess friction in a simulated tongue–palate interaction, taking into account the mechanical factors in the oral cavity and utilizing artificial (polydimethylsiloxane) models of biological tissues such as the tongue, palate, and saliva as test samples. A toolbox of techniques comprising Petri dish and oral cavity model methods for testing disintegration time and bio-tribology tests for the disintegration and oral perception of ODFs. | The test mimics the motion of the tongue and palate by employing a blend of a wide contact area and a relatively brief stroke length. Combined techniques to enable formulators to create, evaluate, and reformulate ODF. | Coefficient of friction (N) | [164] |
| Sensory panel studies | Perception of taste is a complex and intricate phenomenon characterized by individual variations in sensitivity and response, influenced by various physiological and environmental factors. | Each film strip is placed on the tongue and examined for a duration of 10 s using the swirl and spit technique. Standardized neutralization is conducted between successive samples. Bitterness intensity is promptly evaluated on an 11-point scale, ranging from 0 (not bitter) to 11 (bitter) | Bitterness score | [165] |
| Electronic tongue | It serves as a taste sensor equipped with a set of multichannel detectors designed to replicate the conditions of taste buds found on human tongues. | It assesses taste by gauging the detectors’ response to diverse compounds, delivering a swift and impartial taste evaluation. The evaluation parameters typically include factors such as taste intensity, sweetness, bitterness, sourness, and sometimes additional characteristics like saltiness or umami. | Bitterness score | [166] |
6. Clinical Translation and Future Prospects
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alqahtani, M.S.; Kazi, M.; Alsenaidy, M.A.; Ahmad, M.Z. Advances in Oral Drug Delivery. Front. Pharmacol. 2021, 12, 618411. [Google Scholar] [CrossRef]
- He, M.; Zhu, L.; Yang, N.; Li, H.; Yang, Q. Recent advances of oral film as platform for drug delivery. Int. J. Pharm. 2021, 604, 120759. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kim, K.; Kim, M.; Choi, D.H.; Jeong, S.H. Orally disintegrating films focusing on formulation, manufacturing process, and characterization. J. Pharm. Investig. 2017, 47, 183–201. [Google Scholar] [CrossRef]
- Jacob, S.; Nair, A.B.; Boddu, S.H.S.; Gorain, B.; Sreeharsha, N.; Shah, J. An Updated Overview of the Emerging Role of Patch and Film-Based Buccal Delivery Systems. Pharmaceutics 2021, 13, 1206. [Google Scholar] [CrossRef]
- Food and Drug Administration. Guidance for Industry: Orally Disintegrating Tablets; Food and Drug Administration: Silver Spring, MD, USA, 2008.
- Agency, E.M. Guideline on Pharmaceutical Development of Medicines for Paediatric Use; European Medicines Agency: London, UK, 2013. [Google Scholar]
- Karki, S.; Kim, H.; Na, S.-J.; Shin, D.; Jo, K.; Lee, J. Thin films as an emerging platform for drug delivery. Asian J. Pharm. Sci. 2016, 11, 559–574. [Google Scholar] [CrossRef]
- Borges, A.F.; Silva, C.; Coelho, J.F.; Simões, S. Oral films: Current status and future perspectives: I—Galenical development and quality attributes. J. Control. Release 2015, 206, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Alaei, S.; Omidian, H. Mucoadhesion and Mechanical Assessment of Oral Films. Eur. J. Pharm. Sci. 2021, 159, 105727. [Google Scholar] [CrossRef] [PubMed]
- Steiner, D.; Finke, J.H.; Kwade, A. SOFTs—Structured orodispersible film templates. Eur. J. Pharm. Biopharm. 2019, 137, 209–217. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. Quality by Design for Andas: An Example For Immediate-Release Dosage Forms; US Department of Health and Human Service—FDA: Rockville, MD, USA, 2012.
- Tian, Y.; Lin, J.; Jing, H.; Wang, Q.; Wu, Z.; Duan, Y. Recent progress in orodispersible films-mediated therapeutic applications: A review. MedComm Biomater. Appl. 2023, 2, e34. [Google Scholar] [CrossRef]
- Nishigaki, M.; Kawahara, K.; Nawa, M.; Futamura, M.; Nishimura, M.; Matsuura, K.; Kitaichi, K.; Kawaguchi, Y.; Tsukioka, T.; Yoshida, K.; et al. Development of fast dissolving oral film containing dexamethasone as an antiemetic medication: Clinical usefulness. Int. J. Pharm. 2012, 424, 12–17. [Google Scholar] [CrossRef]
- Irfan, M.; Rabel, S.; Bukhtar, Q.; Qadir, M.I.; Jabeen, F.; Khan, A. Orally disintegrating films: A modern expansion in drug delivery system. Saudi Pharm. J. 2016, 24, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Castro, P.M.; Fonte, P.; Oliveira, A.; Madureira, A.R.; Sarmento, B.; Pintado, M.E. Optimization of two biopolymer-based oral films for the delivery of bioactive molecules. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 76, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Maciel, V.B.; Remedio, L.N.; Yoshida, C.M.; Carvalho, R.A. Carboxymethyl cellulose-based orally disintegrating films enriched with natural plant extract for oral iron delivery. J. Drug Deliv. Sci. Technol. 2021, 66, 102852. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Umemura, K.; Tahara, K.; Takeuchi, H. Formulation design of hydroxypropyl cellulose films for use as orally disintegrating dosage forms. J. Drug Deliv. Sci. Technol. 2018, 46, 93–100. [Google Scholar] [CrossRef]
- Lai, K.L.; Fang, Y.; Han, H.; Li, Q.; Zhang, S.; Li, H.Y.; Chow, S.F.; Lam, T.N.; Lee, W.Y.T. Orally-dissolving film for sublingual and buccal delivery of ropinirole. Colloids Surf. B Biointerfaces 2018, 163, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Rani, K.C.; Parfati, N.; Aryani, N.L.D.; Winantari, A.N.; Fitriani, E.W.; Pradana, A.T.; Nawatila, R.; Putranti, A.R.; Irine, F.; Angelica, F.; et al. Development, Evaluation, and Molecular Docking of Oral Dissolving Film of Atenolol. Pharmaceutics 2021, 13, 1727. [Google Scholar] [CrossRef] [PubMed]
- Abdelbary, A.; Bendas, E.R.; Ramadan, A.A.; Mostafa, D.A. Pharmaceutical and pharmacokinetic evaluation of a novel fast dissolving film formulation of flupentixol dihydrochloride. AAPS Pharmscitech 2014, 15, 1603–1610. [Google Scholar] [CrossRef]
- Bharti, K.; Mittal, P.; Mishra, B. Formulation and characterization of fast dissolving oral films containing buspirone hydrochloride nanoparticles using design of experiment. J. Drug Deliv. Sci. Technol. 2019, 49, 420–432. [Google Scholar] [CrossRef]
- Panraksa, P.; Udomsom, S.; Rachtanapun, P.; Chittasupho, C.; Ruksiriwanich, W.; Jantrawut, P. Hydroxypropyl Methylcellulose E15: A Hydrophilic Polymer for Fabrication of Orodispersible Film Using Syringe Extrusion 3D Printer. Polymers 2020, 12, 2666. [Google Scholar] [CrossRef]
- Dahmash, E.Z.; Iyire, A.; Alyami, H.S. Development of orally dissolving films for pediatric-centric administration of anti-epileptic drug topiramate—A design of experiments (DoE) study. Saudi Pharm. J. 2021, 29, 635–647. [Google Scholar] [CrossRef]
- Al-Mogherah, A.I.; Ibrahim, M.A.; Hassan, M.A. Optimization and evaluation of venlafaxine hydrochloride fast dissolving oral films. Saudi Pharm. J. 2020, 28, 1374–1382. [Google Scholar] [CrossRef] [PubMed]
- Elbl, J.; Gajdziok, J.; Kolarczyk, J. 3D printing of multilayered orodispersible films with in-process drying. Int. J. Pharm. 2020, 575, 118883. [Google Scholar] [CrossRef]
- Lai, F.; Franceschini, I.; Corrias, F.; Sala, M.C.; Cilurzo, F.; Sinico, C.; Pini, E. Maltodextrin fast dissolving films for quercetin nanocrystal delivery. A feasibility study. Carbohydr. Polym. 2015, 121, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Pimparade, M.B.; Vo, A.; Maurya, A.S.; Bae, J.; Morott, J.T.; Feng, X.; Kim, D.W.; Kulkarni, V.I.; Tiwari, R.; Vanaja, K.; et al. Development and evaluation of an oral fast disintegrating anti-allergic film using hot-melt extrusion technology. Eur. J. Pharm. Biopharm. 2017, 119, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Quan, P.; Fang, L. Preparation of an oral thin film containing meclizine hydrochloride: In vitro and in vivo evaluation. Int. J. Pharm. 2015, 496, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Visser, J.C.; Klever, J.S.; Woerdenbag, H.J.; Frijlink, H.W.; Hinrichs, W.L.J. Orodispersible films based on blends of trehalose and pullulan for protein delivery. Eur. J. Pharm. Biopharm. 2018, 133, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Shariatinia, Z. Pharmaceutical applications of chitosan. Adv. Colloid. Interface Sci. 2019, 263, 131–194. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.Y.; Jia, X.W.; Liu, Q.; Kong, B.H.; Wang, H. Fast dissolving oral films for drug delivery prepared from chitosan/pullulan electrospinning nanofibers. Int. J. Biol. Macromol. 2019, 137, 224–231. [Google Scholar] [CrossRef]
- Sizílio, R.H.; Galvão, J.G.; Trindade, G.G.G.; Pina, L.T.S.; Andrade, L.N.; Gonsalves, J.; Lira, A.A.M.; Chaud, M.V.; Alves, T.F.R.; Arguelho, M.; et al. Chitosan/pvp-based mucoadhesive membranes as a promising delivery system of betamethasone-17-valerate for aphthous stomatitis. Carbohydr. Polym. 2018, 190, 339–345. [Google Scholar] [CrossRef]
- Kumria, R.; Al-Dhubiab, B.E.; Shah, J.; Nair, A.B. Formulation and evaluation of chitosan-based buccal bioadhesive films of zolmitriptan. J. Pharm. Innov. 2018, 13, 133–143. [Google Scholar] [CrossRef]
- AnjiReddy, K.; Karpagam, S. In vitro and in vivo evaluation of oral disintegrating nanofiber and thin-film contains hyperbranched chitosan/donepezil for active drug delivery. J. Polym. Environ. 2021, 29, 922–936. [Google Scholar] [CrossRef]
- Dharmasthala, S.; Shabaraya, A.R.; Andrade, G.S.; Shriram, R.G.; Hebbar, S.; Dubey, A. Fast dissolving oral film of piroxicam: Solubility enhancement by forming an inclusion complex with β-cyclodextrin, formulation and evaluation. J. Young Pharm. 2019, 11, 1. [Google Scholar] [CrossRef]
- Timur, S.S.; Yüksel, S.; Akca, G.; Şenel, S. Localized drug delivery with mono and bilayered mucoadhesive films and wafers for oral mucosal infections. Int. J. Pharm. 2019, 559, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.; Gupta, R.; Vasanti, S. In vitro controlled release of alfuzosin hydrochloride using HPMC-based matrix tablets and its comparison with marketed product. Pharm. Dev. Technol. 2007, 12, 621–625. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Singla, Y.P.; Narang, R.S.; Pandita, D.; Singh, S.; Narang, J.K. Frovatriptan loaded hydroxy propyl methyl cellulose/treated chitosan based composite fast dissolving sublingual films for management of migraine. J. Drug Deliv. Sci. Technol. 2018, 47, 230–239. [Google Scholar] [CrossRef]
- De Moraes, M.A.; Da Silva, C.F.; Vieira, R.S. Biopolymer Membranes and Films: Health, Food, Environment, and Energy Applications; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Minzanova, S.T.; Mironov, V.F.; Arkhipova, D.M.; Khabibullina, A.V.; Mironova, L.G.; Zakirova, Y.M.; Milyukov, V.A. Biological Activity and Pharmacological Application of Pectic Polysaccharides: A Review. Polymers 2018, 10, 1407. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.S.; Murthy, K.R. Formulation and evaluation of oral fast dissolving films of poorly soluble drug ezetimibe using transcutol Hp. Indian J. Pharm. Educ. Res. 2018, 52, 398–407. [Google Scholar] [CrossRef]
- Sharma, R.; Kamboj, S.; Singh, G.; Rana, V. Development of aprepitant loaded orally disintegrating films for enhanced pharmacokinetic performance. Eur. J. Pharm. Sci. 2016, 84, 55–69. [Google Scholar] [CrossRef]
- Arefian, M.; Hojjati, M.; Tajzad, I.; Mokhtarzade, A.; Mazhar, M.; Jamavari, A. A review of Polyvinyl alcohol/Carboxymethyl cellulose (PVA/CMC) composites for various applications. J. Compos. Compd. 2020, 2, 69–76. [Google Scholar]
- Panraksa, P.; Tipduangta, P.; Jantanasakulwong, K.; Jantrawut, P. Formulation of Orally Disintegrating Films as an Amorphous Solid Solution of a Poorly Water-Soluble Drug. Membranes 2020, 10, 376. [Google Scholar] [CrossRef]
- Shamma, R.; Elkasabgy, N. Design of freeze-dried Soluplus/polyvinyl alcohol-based film for the oral delivery of an insoluble drug for the pediatric use. Drug Deliv. 2016, 23, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Khames, A. Hexyl alginate derivative, an amphiphilic innovative buccal film-forming material of promising mechanical and release characteristics for the improvement of repaglinide bioavailability. Drug Des. Dev. Ther. 2019, 13, 925–940. [Google Scholar] [CrossRef] [PubMed]
- Vijayanand, P.; Patil, J.; Reddy, M.V. Formulation and comparative pharmacokinetic evaluation of orodispersible tablets and films of nebivolol hydrochloride. J. Pharm. Investig. 2015, 45, 237–247. [Google Scholar] [CrossRef]
- Pamlényi, K.; Kristó, K.; Jójárt-Laczkovich, O.; Regdon, G., Jr. Formulation and Optimization of Sodium Alginate Polymer Film as a Buccal Mucoadhesive Drug Delivery System Containing Cetirizine Dihydrochloride. Pharmaceutics 2021, 13, 619. [Google Scholar] [CrossRef] [PubMed]
- El-Bary, A.A.; Al Sharabi, I.; Haza’a, B.S. Effect of casting solvent, film-forming agent and solubilizer on orodispersible films of a polymorphic poorly soluble drug: An in vitro/in silico study. Drug Dev. Ind. Pharm. 2019, 45, 1751–1769. [Google Scholar] [CrossRef]
- Shi, L.L.; Xu, W.J.; Cao, Q.R.; Yang, M.; Cui, J.H. Preparation, characterization and in vitro evaluation of a polyvinyl alcohol/sodium alginate based orodispersible film containing sildenafil citrate. Pharmazie 2014, 69, 327–334. [Google Scholar] [PubMed]
- Dos Santos Garcia, V.A.; Borges, J.G.; Maciel, V.B.V.; Mazalli, M.R.; das Graças Lapa-Guimaraes, J.; Vanin, F.M.; de Carvalho, R.A. Gelatin/starch orally disintegrating films as a promising system for vitamin C delivery. Food Hydrocoll. 2018, 79, 127–135. [Google Scholar] [CrossRef]
- Borges, J.G.; Silva, A.G.; Cervi-Bitencourt, C.M.; Vanin, F.M.; Carvalho, R.A. Lecithin, gelatin and hydrolyzed collagen orally disintegrating films: Functional properties. Int. J. Biol. Macromol. 2016, 86, 907–916. [Google Scholar] [CrossRef]
- Dos Santos Garcia, V.A.; Gonçalves Borges, J.; Mazalli, M.R.; Lapa-Guimarães, J.d.G.; Vanin, F.M.; de Carvalho, R.A. Gelatin and pregelatinized starch orally disintegrating films: Properties and stability of vitamin C. J. Appl. Polym. Sci. 2017, 134, 44841. [Google Scholar] [CrossRef]
- Dos Santos Garcia, V.A.; Borges, J.G.; Osiro, D.; Vanin, F.M.; de Carvalho, R.A. Orally disintegrating films based on gelatin and pregelatinized starch: New carriers of active compounds from acerola. Food Hydrocoll. 2020, 101, 105518. [Google Scholar] [CrossRef]
- Heinemann, R.J.B.; Vanin, F.M.; Carvalho, R.A.d.; Trindade, M.A.; Fávaro-Trindade, C.S. Characterization of low cost orally disintegrating film (ODF). Polímeros 2017, 27, 48–54. [Google Scholar] [CrossRef]
- Tedesco, M.P.; Monaco-Lourenço, C.A.; Carvalho, R.A. Gelatin/hydroxypropyl methylcellulose matrices—Polymer interactions approach for oral disintegrating films. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 69, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Kwak, H.W.; Woo, H.; Kim, I.-C.; Lee, K.H. Fish gelatin nanofibers prevent drug crystallization and enable ultrafast delivery. RSC Adv. 2017, 7, 40411–40417. [Google Scholar] [CrossRef]
- Pezik, E.; Gulsun, T.; Sahin, S.; Vural, İ. Development and characterization of pullulan-based orally disintegrating films containing amlodipine besylate. Eur. J. Pharm. Sci. 2021, 156, 105597. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.S.; Kumar, T.P.; Reddy, D.; Pathak, K.; Gowda, D.V.; Babu, A.V.N.; Aodah, A.H.; Khafagy, E.S.; Alotaibi, H.F.; Abu Lila, A.S.; et al. Development and Characterization of Pullulan-Based Orodispersible Films of Iron. Pharmaceutics 2023, 15, 1027. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Bhide, Y.C.; Woerdenbag, H.J.; Huckriede, A.L.W.; Frijlink, H.W.; Hinrichs, W.L.J.; Visser, J.C. Development of an Orodispersible Film Containing Stabilized Influenza Vaccine. Pharmaceutics 2020, 12, 245. [Google Scholar] [CrossRef] [PubMed]
- Krull, S.M.; Ma, Z.; Li, M.; Davé, R.N.; Bilgili, E. Preparation and characterization of fast dissolving pullulan films containing BCS class II drug nanoparticles for bioavailability enhancement. Drug Dev. Ind. Pharm. 2016, 42, 1073–1085. [Google Scholar] [CrossRef] [PubMed]
- Shah, U.; Naqash, F.; Gani, A.; Masoodi, F.A. Art and Science behind Modified Starch Edible Films and Coatings: A Review. Compr. Rev. Food Sci. Food Saf. 2016, 15, 568–580. [Google Scholar] [CrossRef]
- Limpongsa, E.; Jaipakdee, N. Physical modification of Thai rice starch and its application as orodispersible film former. Carbohydr. Polym. 2020, 239, 116206. [Google Scholar] [CrossRef]
- Bodini, R.B.; Guimarães, J.d.G.L.; Monaco-Lourenço, C.A.; de Carvalho, R.A. Effect of starch and hydroxypropyl methylcellulose polymers on the properties of orally disintegrating films. J. Drug Deliv. Sci. Technol. 2019, 51, 403–410. [Google Scholar] [CrossRef]
- Liew, K.B.; Odeniyi, M.A.; Peh, K.K. Application of freeze-drying technology in manufacturing orally disintegrating films. Pharm. Dev. Technol. 2016, 21, 346–353. [Google Scholar] [CrossRef]
- Sha, H.; Yuan, C.; Cui, B.; Zhao, M.; Wang, J. Pre-gelatinized cassava starch orally disintegrating films: Influence of β-Cyclodextrin. Food Hydrocoll. 2022, 123, 107196. [Google Scholar] [CrossRef]
- Tamanini, F.; Moraes, B.S.; Amaral, C.S.T.; Carvalho, A.J.F.; Trovatti, E. Starch-based orodispersible film for diclofenac release. Braz. J. Pharm. Sci. 2023, 59, e211019. [Google Scholar] [CrossRef]
- Wang, B.; Yang, L.; Wang, B.; Luo, C.; Wang, Y.; Wang, H.; Chen, F.; Xiang, X. Development, In Vitro and In Vivo Evaluation of Racecadotril Orodispersible Films for Pediatric Use. AAPS PharmSciTech 2021, 22, 15. [Google Scholar] [CrossRef]
- Cupone, I.E.; Sansone, A.; Marra, F.; Giori, A.M.; Jannini, E.A. Orodispersible Film (ODF) Platform Based on Maltodextrin for Therapeutical Applications. Pharmaceutics 2022, 14, 2011. [Google Scholar] [CrossRef] [PubMed]
- Pechová, V.; Gajdziok, J.; Muselík, J.; Vetchý, D. Development of Orodispersible Films Containing Benzydamine Hydrochloride Using a Modified Solvent Casting Method. AAPS PharmSciTech 2018, 19, 2509–2518. [Google Scholar] [CrossRef] [PubMed]
- Cilurzo, F.; Montanari, L.; Minghetti, P. Self Supporting Film for Pharmaceutical and Food. Use. Patent EP16893472004, 27 October 2004. [Google Scholar]
- Radicioni, M.; Castiglioni, C.; Giori, A.; Cupone, I.; Frangione, V.; Rovati, S. Bioequivalence study of a new sildenafil 100 mg orodispersible film compared to the conventional film-coated 100 mg tablet administered to healthy male volunteers. Drug Des. Dev. Ther. 2017, 11, 1183–1192. [Google Scholar] [CrossRef]
- Radicioni, M.; Caverzasio, C.; Rovati, S.; Giori, A.M.; Cupone, I.; Marra, F.; Mautone, G. Comparative Bioavailability Study of a New Vitamin D3 Orodispersible Film Versus a Marketed Oral Solution in Healthy Volunteers. Clin. Drug Investig. 2022, 42, 151–161. [Google Scholar] [CrossRef]
- Cupone, I.E.; Roselli, G.; Marra, F.; Riva, M.; Angeletti, S.; Dugo, L.; Spoto, S.; Fogolari, M.; Giori, A.M. Orodispersible Film Based on Maltodextrin: A Convenient and Suitable Method for Iron Supplementation. Pharmaceutics 2023, 15, 1575. [Google Scholar] [CrossRef]
- Franceschini, I.; Selmin, F.; Pagani, S.; Minghetti, P.; Cilurzo, F. Nanofiller for the mechanical reinforcement of maltodextrins orodispersible films. Carbohydr. Polym. 2016, 136, 676–681. [Google Scholar] [CrossRef]
- Teixeira, S.C.; Silva, R.R.A.; de Oliveira, T.V.; Stringheta, P.C.; Pinto, M.R.M.R.; Soares, N.d.F.F. Glycerol and triethyl citrate plasticizer effects on molecular, thermal, mechanical, and barrier properties of cellulose acetate films. Food Biosci. 2021, 42, 101202. [Google Scholar] [CrossRef]
- Aloui, H.; Baraket, K.; Sendon, R.; Silva, A.S.; Khwaldia, K. Development and characterization of novel composite glycerol-plasticized films based on sodium caseinate and lipid fraction of tomato pomace by-product. Int. J. Biol. Macromol. 2019, 139, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Tarique, J.; Sapuan, S.M.; Khalina, A. Effect of glycerol plasticizer loading on the physical, mechanical, thermal, and barrier properties of arrowroot (Maranta arundinacea) starch biopolymers. Sci. Rep. 2021, 11, 13900. [Google Scholar] [CrossRef] [PubMed]
- Vuddanda, P.R.; Montenegro-Nicolini, M.; Morales, J.O.; Velaga, S. Effect of plasticizers on the physico-mechanical properties of pullulan based pharmaceutical oral films. Eur. J. Pharm. Sci. 2017, 96, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, J.; Cheng, Y.; Wang, C.; Liu, H.; Bian, H.; Pan, Y.; Sun, J.; Han, W. Application of Protein-Based Films and Coatings for Food Packaging: A Review. Polymers 2019, 11, 2039. [Google Scholar] [CrossRef] [PubMed]
- Briddick, A.; Li, P.; Hughes, A.; Courchay, F.; Martinez, A.; Thompson, R.L. Surfactant and Plasticizer Segregation in Thin Poly(vinyl alcohol) Films. Langmuir 2016, 32, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Aydın, A.A.; Ilberg, V. Effect of different polyol-based plasticizers on thermal properties of polyvinyl alcohol:starch blends. Carbohydr. Polym. 2016, 136, 441–448. [Google Scholar] [CrossRef]
- Olechno, K.; Basa, A.; Winnicka, K. “Success Depends on Your Backbone”-About the Use of Polymers as Essential Materials Forming Orodispersible Films. Materials 2021, 14, 4872. [Google Scholar] [CrossRef]
- Lin, S.Y.; Chen, K.S.; Run-Chu, L. Organic esters of plasticizers affecting the water absorption, adhesive property, glass transition temperature and plasticizer permanence of eudragit acrylic films. J. Control. Release 2000, 68, 343–350. [Google Scholar] [CrossRef]
- Honary, S.; Orafai, H. The effect of different plasticizer molecular weights and concentrations on mechanical and thermomechanical properties of free films. Drug Dev. Ind. Pharm. 2002, 28, 711–715. [Google Scholar] [CrossRef]
- Mascia, L.; Kouparitsas, Y.; Nocita, D.; Bao, X. Antiplasticization of Polymer Materials: Structural Aspects and Effects on Mechanical and Diffusion-Controlled Properties. Polymers 2020, 12, 769. [Google Scholar] [CrossRef] [PubMed]
- Vishvakarma, V.; Kaur, M.; Nagpal, M.; Arora, S. Role of Nanotechnology in Taste Masking: Recent Updates. Curr. Drug Res. Rev. 2023, 15, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Joshi, U.; Singh, A.; Saharan, V.A. Lipids for Taste masking and Taste assessment in pharmaceutical formulations. Chem. Phys. Lipids 2021, 235, 105031. [Google Scholar] [CrossRef] [PubMed]
- Graebin, C.S. The pharmacological activities of glycyrrhizinic acid (“glycyrrhizin”) and glycyrrhetinic acid. Sweeteners 2018, 245–261. [Google Scholar] [CrossRef]
- Mathur, S.; Bulchandani, N.; Parihar, S.; Shekhawat, G.S. Critical review on steviol glycosides: Pharmacological, toxicological and therapeutic aspects of high potency zero caloric sweetener. Int. J. Pharmacol. 2017, 13, 916–928. [Google Scholar] [CrossRef]
- O’Donnell, K.; Kearsley, M. Sweeteners and Sugar Alternatives in Food Technology; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- DuBois, G.E.; Prakash, I. Non-caloric sweeteners, sweetness modulators, and sweetener enhancers. Annu. Rev. Food Sci. Technol. 2012, 3, 353–380. [Google Scholar] [CrossRef] [PubMed]
- Homler, B.E. Aspartame: Implications for the food scientist. In Aspartame; CRC Press: Boca Raton, FL, USA, 2020; pp. 247–262. [Google Scholar]
- Czarnecka, K.; Pilarz, A.; Rogut, A.; Maj, P.; Szymańska, J.; Olejnik, Ł.; Szymański, P. Aspartame-True or False? Narrative Review of Safety Analysis of General Use in Products. Nutrients 2021, 13, 1957. [Google Scholar] [CrossRef]
- Borges, J.G.; De Carvalho, R.A. Orally disintegrating films containing propolis: Properties and release profile. J. Pharm. Sci. 2015, 104, 1431–1439. [Google Scholar] [CrossRef]
- Cocco, F.; Cagetti, M.G.; Majdub, O.; Campus, G. Concentration in saliva and antibacterial effect of xylitol chewing gum: In vivo and in vitro study. Appl. Sci. 2020, 10, 2900. [Google Scholar] [CrossRef]
- Nagaraju, T.; Gowthami, R.; Rajashekar, M.; Sandeep, S.; Mallesham, M.; Sathish, D.; Kumar, Y.S. Comprehensive review on oral disintegrating films. Curr. Drug Deliv. 2013, 10, 96–108. [Google Scholar] [CrossRef]
- Manda, P.; Popescu, C.; Juluri, A.; Janga, K.; Kakulamarri, P.R.; Narishetty, S.; Narasimha Murthy, S.; Repka, M.A. Micronized Zaleplon Delivery via Orodispersible Film and Orodispersible Tablets. AAPS PharmSciTech 2018, 19, 1358–1366. [Google Scholar] [CrossRef] [PubMed]
- Turković, E.; Vasiljević, I.; Drašković, M.; Parojčić, J. Orodispersible films—Pharmaceutical development for improved performance: A review. J. Drug Deliv. Sci. Technol. 2022, 75, 103708. [Google Scholar] [CrossRef]
- Allen, L.V., Jr. Basics of Compounding: Compounding Films. Int. J. Pharm. Compd. 2016, 20, 298–305. [Google Scholar] [PubMed]
- Bülbül, E.Ö.; Mesut, B.; Cevher, E.; Öztaş, E.; Özsoy, Y. Product transfer from lab-scale to pilot-scale of quetiapine fumarate orodispersible films using quality by design approach. J. Drug Deliv. Sci. Technol. 2019, 54, 101358. [Google Scholar] [CrossRef]
- Foo, W.C.; Khong, Y.M.; Gokhale, R.; Chan, S.Y. A novel unit-dose approach for the pharmaceutical compounding of an orodispersible film. Int. J. Pharm. 2018, 539, 165–174. [Google Scholar] [CrossRef]
- Zayed, G.M.; Rasoul, S.A.; Ibrahim, M.A.; Saddik, M.S.; Alshora, D.H. In vitro and in vivo characterization of domperidone-loaded fast dissolving buccal films. Saudi Pharm. J. 2020, 28, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Barhart, S.; Rathborne, N.; Hadgraft, J.; Roberts, M. Thin film oral dosage form in modified release drug delivery technology. Drug Pharm. Sci. 2008, 2, 209–216. [Google Scholar]
- Thabet, Y.; Breitkreutz, J. Orodispersible films: Product transfer from lab-scale to continuous manufacturing. Int. J. Pharm. 2018, 535, 285–292. [Google Scholar] [CrossRef]
- Alsofany, J.M.; Hamza, M.Y.; Abdelbary, A.A. Fabrication of Nanosuspension Directly Loaded Fast-Dissolving Films for Enhanced Oral Bioavailability of Olmesartan Medoxomil: In Vitro Characterization and Pharmacokinetic Evaluation in Healthy Human Volunteers. AAPS PharmSciTech 2018, 19, 2118–2132. [Google Scholar] [CrossRef]
- Speer, I.; Preis, M.; Breitkreutz, J. Prolonged drug release properties for orodispersible films by combining hot-melt extrusion and solvent casting methods. Eur. J. Pharm. Biopharm. 2018, 129, 66–73. [Google Scholar] [CrossRef]
- Cailleaux, S.; Sanchez-Ballester, N.M.; Gueche, Y.A.; Bataille, B.; Soulairol, I. Fused Deposition Modeling (FDM), the new asset for the production of tailored medicines. J. Control. Release 2021, 330, 821–841. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.; Nair, A.B.; Patel, V.; Shah, J. 3D Printing Technologies: Recent Development and Emerging Applications in Various Drug Delivery Systems. AAPS PharmSciTech 2020, 21, 220. [Google Scholar] [CrossRef] [PubMed]
- Tracy, T.; Wu, L.; Liu, X.; Cheng, S.; Li, X. 3D printing: Innovative solutions for patients and pharmaceutical industry. Int. J. Pharm. 2023, 631, 122480. [Google Scholar] [CrossRef] [PubMed]
- Vuddanda, P.R.; Alomari, M.; Dodoo, C.C.; Trenfield, S.J.; Velaga, S.; Basit, A.W.; Gaisford, S. Personalisation of warfarin therapy using thermal ink-jet printing. Eur. J. Pharm. Sci. 2018, 117, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Eleftheriadis, G.K.; Monou, P.K.; Bouropoulos, N.; Boetker, J.; Rantanen, J.; Jacobsen, J.; Vizirianakis, I.S.; Fatouros, D.G. Fabrication of Mucoadhesive Buccal Films for Local Administration of Ketoprofen and Lidocaine Hydrochloride by Combining Fused Deposition Modeling and Inkjet Printing. J. Pharm. Sci. 2020, 109, 2757–2766. [Google Scholar] [CrossRef] [PubMed]
- Sjöholm, E.; Sandler, N. Additive manufacturing of personalized orodispersible warfarin films. Int. J. Pharm. 2019, 564, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Phadke, A.; Amin, P. Orally Disintegrating Film of High-Dose BCS II Drug by Hot Melt Extrusion through Design of Experiment. J. Pharm. Innov. 2023, 18, 247–261. [Google Scholar] [CrossRef]
- Alshehri, S.M.; Park, J.-B.; Alsulays, B.B.; Tiwari, R.V.; Almutairy, B.; Alshetaili, A.S.; Morott, J.; Shah, S.; Kulkarni, V.; Majumdar, S. Mefenamic acid taste-masked oral disintegrating tablets with enhanced solubility via molecular interaction produced by hot melt extrusion technology. J. Drug Deliv. Sci. Technol. 2015, 27, 18–27. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, A.; Thakkar, R.; Zhang, Y.; Maniruzzaman, M. Development and Evaluation of Amorphous Oral Thin Films Using Solvent-Free Processes: Comparison between 3D Printing and Hot-Melt Extrusion Technologies. Pharmaceutics 2021, 13, 1613. [Google Scholar] [CrossRef]
- Speer, I.; Lenhart, V.; Preis, M.; Breitkreutz, J. Prolonged release from orodispersible films by incorporation of diclofenac-loaded micropellets. Int. J. Pharm. 2019, 554, 149–160. [Google Scholar] [CrossRef]
- Sauer, D.; Cerea, M.; DiNunzio, J.; McGinity, J. Dry powder coating of pharmaceuticals: A review. Int. J. Pharm. 2013, 457, 488–502. [Google Scholar] [CrossRef] [PubMed]
- Prasad, L.K.; Keen, J.M.; LaFountaine, J.S.; Maincent, J.; Williams, R.O., III; McGinity, J.W. Electrostatic powder deposition to prepare films for drug delivery. J. Drug Deliv. Sci. Technol. 2015, 30, 501–510. [Google Scholar] [CrossRef]
- Prasad, L.K.; LaFountaine, J.S.; Keen, J.M.; Williams, R.O., 3rd; McGinity, J.W. Influence of process parameters on the preparation of pharmaceutical films by electrostatic powder deposition. Int. J. Pharm. 2016, 515, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Nangare, S.; Jadhav, N.; Ghagare, P.; Muthane, T. Pharmaceutical Applications of Electrospinning. In Annales Pharmaceutiques Francaises; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–11. [Google Scholar]
- Yu, D.G.; Zhou, J. How can Electrospinning Further Service Well for Pharmaceutical Researches? J. Pharm. Sci. 2023, 112, 2719–2723. [Google Scholar] [CrossRef] [PubMed]
- Sofi, H.S.; Abdal-Hay, A.; Ivanovski, S.; Zhang, Y.S.; Sheikh, F.A. Electrospun nanofibers for the delivery of active drugs through nasal, oral and vaginal mucosa: Current status and future perspectives. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 111, 110756. [Google Scholar] [CrossRef] [PubMed]
- AnjiReddy, K.; Karpagam, S. Hyperbranched cellulose polyester of oral thin film and nanofiber for rapid release of donepezil; preparation and in vivo evaluation. Int. J. Biol. Macromol. 2019, 124, 871–887. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Li, Y.; Zhang, C.; Feng, F.; Zhang, H. Sequential electrospinning of multilayer ethylcellulose/gelatin/ethylcellulose nanofibrous film for sustained release of curcumin. Food Chem. 2020, 308, 125599. [Google Scholar] [CrossRef]
- Guo, X.; Cun, D.; Wan, F.; Bera, H.; Song, Q.; Tian, X.; Chen, Y.; Rantanen, J.; Yang, M. Comparative assessment of in vitro/in vivo performances of orodispersible electrospun and casting films containing rizatriptan benzoate. Eur. J. Pharm. Biopharm. 2020, 154, 283–289. [Google Scholar] [CrossRef]
- Chachlioutaki, K.; Tzimtzimis, E.K.; Tzetzis, D.; Chang, M.W.; Ahmad, Z.; Karavasili, C.; Fatouros, D.G. Electrospun Orodispersible Films of Isoniazid for Pediatric Tuberculosis Treatment. Pharmaceutics 2020, 12, 470. [Google Scholar] [CrossRef]
- Ravasi, E.; Melocchi, A.; Arrigoni, A.; Chiappa, A.; Gennari, C.G.M.; Uboldi, M.; Bertarelli, C.; Zema, L.; Briatico Vangosa, F. Electrospinning of pullulan-based orodispersible films containing sildenafil. Int. J. Pharm. 2023, 643, 123258. [Google Scholar] [CrossRef]
- Edmans, J.G.; Murdoch, C.; Santocildes-Romero, M.E.; Hatton, P.V.; Colley, H.E.; Spain, S.G. Incorporation of lysozyme into a mucoadhesive electrospun patch for rapid protein delivery to the oral mucosa. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 112, 110917. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.; Nair, A.B.; Shah, J.; Gupta, S.; Boddu, S.H.S.; Sreeharsha, N.; Joseph, A.; Shinu, P.; Morsy, M.A. Lipid Nanoparticles as a Promising Drug Delivery Carrier for Topical Ocular Therapy-An Overview on Recent Advances. Pharmaceutics 2022, 14, 533. [Google Scholar] [CrossRef]
- Nair, A.B.; Shah, J.; Al-Dhubiab, B.E.; Patel, S.S.; Morsy, M.A.; Patel, V.; Chavda, V.; Jacob, S.; Sreeharsha, N.; Shinu, P. Development of asialoglycoprotein receptor-targeted nanoparticles for selective delivery of gemcitabine to hepatocellular carcinoma. Molecules 2019, 24, 4566. [Google Scholar] [CrossRef] [PubMed]
- Kotta, S.; Aldawsari, H.M.; Badr-Eldin, S.M.; Nair, A.B.; Yt, K. Progress in Polymeric Micelles for Drug Delivery Applications. Pharmaceutics 2022, 14, 1636. [Google Scholar] [CrossRef] [PubMed]
- Scioli Montoto, S.; Muraca, G.; Ruiz, M.E. Solid Lipid Nanoparticles for Drug Delivery: Pharmacological and Biopharmaceutical Aspects. Front. Mol. Biosci. 2020, 7, 587997. [Google Scholar] [CrossRef] [PubMed]
- Kamboj, S.; Bala, S.; Nair, A.B. Solid lipid nanoparticles: An effective lipid based technology for poorly water soluble drugs. Int. J. Pharm. Sci. Rev. Res. 2010, 5, 78–90. [Google Scholar]
- Vinarov, Z.; Abrahamsson, B.; Artursson, P.; Batchelor, H.; Berben, P.; Bernkop-Schnürch, A.; Butler, J.; Ceulemans, J.; Davies, N.; Dupont, D.; et al. Current challenges and future perspectives in oral absorption research: An opinion of the UNGAP network. Adv. Drug Deliv. Rev. 2021, 171, 289–331. [Google Scholar] [CrossRef] [PubMed]
- Tzanova, M.M.; Hagesaether, E.; Tho, I. Solid lipid nanoparticle-loaded mucoadhesive buccal films—Critical quality attributes and in vitro safety & efficacy. Int. J. Pharm. 2021, 592, 120100. [Google Scholar] [CrossRef]
- Steiner, D.; Emmendörffer, J.F.; Bunjes, H. Orodispersible Films: A Delivery Platform for Solid Lipid Nanoparticles? Pharmaceutics 2021, 13, 2162. [Google Scholar] [CrossRef]
- Talekar, S.D.; Haware, R.V.; Dave, R.H. Evaluation of self-nanoemulsifying drug delivery systems using multivariate methods to optimize permeability of captopril oral films. Eur. J. Pharm. Sci. 2019, 130, 215–224. [Google Scholar] [CrossRef]
- Shankar Raman, S.; Narayanan, V.H.B.; Durai, R. Lamotrigine Nanoparticle Laden Polymer Composite Oral Dissolving Films for Improving Therapeutic Potential of the Hydrophobic Antiepileptic Molecule. Assay Drug Dev. Technol. 2021, 19, 2–16. [Google Scholar] [CrossRef]
- Steiner, D.; Finke, J.H.; Kwade, A. Model-based description of disintegration time and dissolution rate of nanoparticle-loaded orodispersible films. Eur. J. Pharm. Sci. 2019, 132, 18–26. [Google Scholar] [CrossRef]
- Sinha, S.; Sonali; Garg, V.; Thapa, S.; Singh, S.; Chauhan, M.; Dutt, R.; Singh, R.P. Empagliflozin containing chitosan-alginate nanoparticles in orodispersible film: Preparation, characterization, pharmacokinetic evaluation and its in-vitro anticancer activity. Drug Dev. Ind. Pharm. 2022, 48, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Shah, H.G.; Rathod, V.; Basim, P.; Gajera, B.; Dave, R.H. Understanding the Impact of Multi-factorial Composition on Efficient Loading of the Stable Ketoprofen Nanoparticles on Orodispersible Films Using Box-Behnken Design. J. Pharm. Sci. 2022, 111, 1451–1462. [Google Scholar] [CrossRef]
- Jacob, S.; Nair, A.B.; Shah, J. Emerging role of nanosuspensions in drug delivery systems. Biomater. Res. 2020, 24, 3. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chang, D.; Zhang, X.; Sui, H.; Kong, Y.; Zhu, R.; Wang, W. Oral fast-dissolving films containing lutein nanocrystals for improved bioavailability: Formulation development, in vitro and in vivo evaluation. AAPS PharmSciTech 2017, 18, 2957–2964. [Google Scholar] [CrossRef] [PubMed]
- Van Nguyen, K.; Nguyen, H.T.; Nghiem, L.H.T.; Van Can, M.; Tran, T.H. Nanosized-Loratadine Embedded Orodispersible Films for Enhanced Bioavailability: Scalable Preparations and Characterizations. AAPS PharmSciTech 2022, 23, 78. [Google Scholar] [CrossRef]
- Steiner, D.; Tidau, M.; Finke, J.H. Embedding of Poorly Water-Soluble Drugs in Orodispersible Films-Comparison of Five Formulation Strategies. Pharmaceutics 2022, 15, 17. [Google Scholar] [CrossRef]
- Khalid, G.M.; Selmin, F.; Musazzi, U.M.; Gennari, C.G.M.; Minghetti, P.; Cilurzo, F. Trends in the Characterization Methods of Orodispersible Films. Curr. Drug Deliv. 2021, 18, 935–946. [Google Scholar] [CrossRef]
- Borges, A.F.; Silva, C.; Coelho, J.F.; Simões, S. Outlining critical quality attributes (CQAs) as guidance for the development of orodispersible films. Pharm. Dev. Technol. 2017, 22, 237–245. [Google Scholar] [CrossRef]
- Tsai, W.; Tsai, H.; Wong, Y.; Hong, J.; Chang, S.; Lee, M. Preparation and characterization of gellan gum/glucosamine/clioquinol film as oral cancer treatment patch. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 82, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Speer, I.; Steiner, D.; Thabet, Y.; Breitkreutz, J.; Kwade, A. Comparative study on disintegration methods for oral film preparations. Eur. J. Pharm. Biopharm. 2018, 132, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Jovanović, M.; Tomić, N.; Cvijić, S.; Stojanović, D.; Ibrić, S.; Uskoković, P. Mucoadhesive Gelatin Buccal Films with Propranolol Hydrochloride: Evaluation of Mechanical, Mucoadhesive, and Biopharmaceutical Properties. Pharmaceutics 2021, 13, 273. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, Y.; Ikeda, N.; Tahara, K.; Takeuchi, H. Mechanical characteristics of orally disintegrating films: Comparison of folding endurance and tensile properties. Int. J. Pharm. 2020, 589, 119876. [Google Scholar] [CrossRef] [PubMed]
- Kumria, R.; Nair, A.B.; Goomber, G.; Gupta, S. Buccal films of prednisolone with enhanced bioavailability. Drug Deliv. 2016, 23, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Bala, R.; Pawar, P.; Khanna, S.; Arora, S. Orally dissolving strips: A new approach to oral drug delivery system. Int. J. Pharm. Investig. 2013, 3, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Li, Y.; Wu, J.; Shi, Z.; Zhao, P.; Su, H.; Wang, Q.; Jin, L. Nanofiber orodispersible films based on carboxymethyl curdlan and PEO: New delivery system for amlodipine besylate. Colloids Surf. A Physicochem. Eng. Asp. 2022, 635, 128096. [Google Scholar] [CrossRef]
- Nair, A.B.; Al-ghannam, A.A.; Al-Dhubiab, B.E.; Hasan, A.A. Mucoadhesive Film Embedded with Acyclovir Loaded Biopolymeric Nanoparticles: In vitro Studies. J. Young Pharm. 2017, 9, 100. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, C.; Song, I.O.; Lee, B.J.; Kang, C.Y.; Park, J.B. Investigation of Patient-Centric 3D-Printed Orodispersible Films Containing Amorphous Aripiprazole. Pharmaceuticals 2022, 15, 895. [Google Scholar] [CrossRef]
- Nair, A.B.; Al-Dhubiab, B.E.; Shah, J.; Jacob, S.; Saraiya, V.; Attimarad, M.; SreeHarsha, N.; Akrawi, S.H.; Shehata, T.M. Mucoadhesive buccal film of almotriptan improved therapeutic delivery in rabbit model. Saudi Pharm. J. 2020, 28, 201–209. [Google Scholar] [CrossRef]
- Park, H.-R.; Seok, S.H.; Hwang, K.-M.; Kim, J.-Y.; Park, C.-W.; Park, E.-S. Formulation of sustained-release orodispersible film containing drug–resin complexes of donepezil hydrochloride. J. Pharm. Investig. 2022, 52, 259–272. [Google Scholar] [CrossRef]
- Ouda, G.I.; Dahmash, E.Z.; Alyami, H.; Iyire, A. A Novel Technique to Improve Drug Loading Capacity of Fast/Extended Release Orally Dissolving Films with Potential for Paediatric and Geriatric Drug Delivery. AAPS PharmSciTech 2020, 21, 126. [Google Scholar] [CrossRef] [PubMed]
- Jug, M.; Hafner, A.; Lovrić, J.; Kregar, M.L.; Pepić, I.; Vanić, Ž.; Cetina-Čižmek, B.; Filipović-Grčić, J. An overview of in vitro dissolution/release methods for novel mucosal drug delivery systems. J. Pharm. Biomed. Anal. 2018, 147, 350–366. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.B.; Kumria, R.; Harsha, S.; Attimarad, M.; Al-Dhubiab, B.E.; Alhaider, I.A. In vitro techniques to evaluate buccal films. J. Control. Release 2013, 166, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Pamlényi, K.; Regdon, G., Jr.; Nemes, D.; Fenyvesi, F.; Bácskay, I.; Kristó, K. Stability, permeability and cytotoxicity of buccal films in allergy treatment. Pharmaceutics 2022, 14, 1633. [Google Scholar] [CrossRef] [PubMed]
- Samaras, G.; Bikos, D.; Vieira, J.; Hartmann, C.; Charalambides, M.; Hardalupas, Y.; Masen, M.; Cann, P. Measurement of molten chocolate friction under simulated tongue-palate kinematics: Effect of cocoa solids content and aeration. Curr. Res. Food Sci. 2020, 3, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Ranmal, S.R.; Nhouchi, Z.; Keeley, A.; Adler, L.; Lavarde, M.; Pensé-Lhéritier, A.M.; Tuleu, C. Taste assessment for paediatric drug Development: A comparison of bitterness taste aversion in children versus Naïve and expert young adult assessors. Int. J. Pharm. 2023, 647, 123494. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, Y.; Usui, R.; Ikezaki, H.; Tahara, K.; Takeuchi, H. An advanced technique using an electronic taste-sensing system to evaluate the bitterness of orally disintegrating films and the evaluation of model films. Int. J. Pharm. 2017, 531, 179–190. [Google Scholar] [CrossRef]
- Mohd Basri, M.S.; Mohd Jais, N.; Sulaiman, A.; Mohd Nor, M.Z.; Abdul Karim Shah, N.N.; Ariffin, S.H. Optimizing the processing factor and formulation of oat-based cookie dough for enhancement in stickiness and moisture content using response surface methodology and superimposition. Processes 2020, 8, 797. [Google Scholar] [CrossRef]
- Hossain, M.L.; Nguyen, M.; Benington, L.; Lim, L.Y.; Hammer, K.; Hettiarachchi, D.; Locher, C. Application of a Customised Franz-Type Cell Coupled with HPTLC to Monitor the Timed Release of Bioactive Components in Complex Honey Matrices. Methods Protoc. 2023, 6, 70. [Google Scholar] [CrossRef]
- Jacob, S.; Nair, A.B.; Morsy, M.A. Dose conversion between animals and humans: A practical solution. Indian J. Pharm. Educ. Res. 2022, 56, 600–607. [Google Scholar] [CrossRef]
- Berben, P.; Bauer-Brandl, A.; Brandl, M.; Faller, B.; Flaten, G.E.; Jacobsen, A.C.; Brouwers, J.; Augustijns, P. Drug permeability profiling using cell-free permeation tools: Overview and applications. Eur. J. Pharm. Sci. 2018, 119, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Desai, N.; Masen, M.; Cann, P.; Hanson, B.; Tuleu, C.; Orlu, M. Modernising Orodispersible Film Characterisation to Improve Palatability and Acceptability Using a Toolbox of Techniques. Pharmaceutics 2022, 14, 732. [Google Scholar] [CrossRef] [PubMed]
- Serrano, D.R.; Fernandez-Garcia, R.; Mele, M.; Healy, A.M.; Lalatsa, A. Designing Fast-Dissolving Orodispersible Films of Amphotericin B for Oropharyngeal Candidiasis. Pharmaceutics 2019, 11, 369. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Shen, B.; Xu, H.; Bai, J.; Dai, L.; Lv, Q.; Han, J.; Yuan, H. Formulation and optimization of a novel oral fast dissolving film containing drug nanoparticles by Box-Behnken design-response surface methodology. Drug Dev. Ind. Pharm. 2014, 40, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Al-Nemrawi, N.K.; Dave, R.H. Formulation and characterization of acetaminophen nanoparticles in orally disintegrating films. Drug Deliv. 2016, 23, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.S.; Gowda, D.V.; Kumar, T.P.; Rosenholm, J.M. A Comprehensive Review of Patented Technologies to Fabricate Orodispersible Films: Proof of Patent Analysis (2000–2020). Pharmaceutics 2022, 14, 820. [Google Scholar] [CrossRef]
- Gupta, M.S.; Kumar, T.P.; Gowda, D.V. Orodispersible Thin Film: A new patient-centered innovation. J. Drug Deliv. Sci. Technol. 2020, 59, 101843. [Google Scholar] [CrossRef]



| Aspects | Advantages | Drawbacks |
|---|---|---|
| Ease of administration | Convenient, easy, and safe for administration | May not be suitable for certain patient populations experiencing swallowing difficulties |
| Patient compliance | Enhanced patient compliance, particularly for geriatric, pediatric, disabled, and dysphagic populations | Formulation might possess a bitter taste and may include allergens or excipients, potentially affecting patient acceptability |
| Rapid onset of action | Quick disintegration | Limited capacity for delivering modified-release formulations |
| Dosage accuracy | Precise dosing | Restricted ability to accommodate high-dose medications |
| Bioavailability | Improved bioavailability due to potential oromucosal absorption | Stability concerns, especially for certain drug formulations |
| Dosing | Feasibility of personalized dosing | Storage and handling requirements may be more stringent |
| Market acceptance | Emerging popularity in the market | Expensive compared to conventional dosage forms |
| Critical Quality Attributes | Target | |
|---|---|---|
| Physical attributes | Size | Length, width, and thickness allow the film to be convenient for placement on the surface of the tongue. The size of the film is 1 cm × 1 cm, and the thickness is 100 µm |
| Mechanical characteristics | High tensile strength, high elongation at break, and low Young’s modulus | |
| Identification | Positive for drug | |
| Assay | 100% w/w of label claim | |
| Content uniformity | Conforms to USP <905> uniformity of dosage units | |
| Disintegration time | Not more than 60 s | |
| Dissolution | Acceptance criteria similar to the conventional immediate-release solid dosage forms | |
| Polymer/Plasticizer | Drug | Method | Highlights | Reference |
|---|---|---|---|---|
| Carboxymethyl cellulose and gelatin type A/D-sorbitol | Caffeine | Solvent casting | Gelatin type A proved to be more efficient in regulating caffeine release, with a peak value of 97.4% ± 0.95 occurring after 20 min. Conversely, carboxymethyl cellulose is more suitable for immediate release, as it resulted in a maximum caffeine release of 81.1% ± 2.14 within 4 min. The ex vivo permeability assessment was consistent with the USP dissolution assay, where 42.0% ± 7.79 of caffeine was released from carboxymethyl cellulose and 15.3% ± 4.0 from Gelatin type A oral films. | [15] |
| Carboxymethyl cellulose/sorbitol | Ora-pro-nobis extract | Tape casting technique | Fast disintegration (<50 s) combined with the release of iron, occurring within a maximum of 26 min in CMC-based ODF microparticles and 50 min with CMC-based ODF containing ora-pro-nobis (OPN) extract. Release kinetics indicated the Fickian diffusion mechanism Developed systems have the potential capacity to serve as innovative means for delivering oral iron intake, beneficial for iron deficiency. | [16] |
| Hydroxypropyl cellulose SL | Ibuprofen/Calcium carbonate | Solvent casting method | Disintegration time decreased as the film thickness decreased. The incorporation of ibuprofen into the film extended the disintegration time, whereas embedding calcium carbonate particles in the film reduced it. Drug loading led to a reduction in tensile strength as well as elastic modulus. | [17] |
| HPMC 603/PEG 400 | Ropinirole | Solvent casting | Ropinirole reached systemic circulation in less than 15 min. Bioavailability was tremendously enhanced by higher Cmax from sublingual (188.9 ± 25.1 ng/mL) and buccal films (166.7 ± 55.2 ng/mL) compared to the oral tablet (29.9 ± 16.2 ng/mL). Physical stability was demonstrated for at least 28 days. | [18] |
| HPMC E5/glycerin | Atenolol | Solvent casting | HPMC-based ODF demonstrated superior attributes in terms of uniformity, drug content, and dissolution properties. Molecular docking results, confirmed through FTIR spectral studies, indicated the presence of hydrogen bonding between atenolol and the film formers. pH measurement indicated neutrality, suggesting that the films would not sensitize oral mucosa. | [19] |
| HPMC E5/glycerin | Flupentixol dihydrochloride | Solvent casting method | ODF containing 2% HPMC demonstrated remarkable consistency and stability of flupentixol aside from rapid disintegration. ODF of flupentixol exhibited a rapid absorption rate, resulting in a relative bioavailability of 151.06% in comparison to the marketed product. | [20] |
| HPMC E5 and PEG 400/propylene glycol | Quetiapine fumarate | Solvent casting method | The mean thickness of the film was between 0.159–0.172 mm. The dry film thickness exhibited an inverse correlation with both the plasticizer quantity and drying temperature. Estimated disintegration times were in the range of 128.0–136.3 s. ODFs with higher propylene glycol and those dried at elevated temperatures demonstrated a notably faster dissolution rate when compared to other films. | [21] |
| HPMC E15/glycerin | Phenytoin | Syringe extrusion 3D printing | Optimized phenytoin-loaded ODFs displayed good physical appearance, acceptable mechanical strength, rapid disintegration (<5 s), and immediate drug release (80% < 10 min). Additionally, an enhancement in the solubility and dissolution rates was observed. | [22] |
| HPMC E15 and PVA (Polyvinyl alcohol) | Buspirone hydrochloride | Solvent casting method | The nanoprecipitation technique was utilized to prepare poly(lactic-co-glycolic acid) nanoparticles and embed them into the polymeric dispersion-containing plasticizer. Particle size, zeta potential, and entrapment efficiency of the nanoparticles measured were 189.23 ± 0.95 nm, −21.56 ± 0.56 mV, and 68.28 ± 3.69%, respectively. XRD studies showed the existence of the drug in an amorphous state. The in vitro drug release demonstrated prolonged delivery for 48 h and followed the Korsmeyer–Peppas model. | [21] |
| HPMC K100/glycerin | Topiramate | Solvent casting | The D-optimal statistical experimental design showed that independent variables related to polymer and plasticizer had a predominant impact on dependent variables like disintegration time, assay, and film thickness. The fabricated film released 98% of topiramate in just 10 min while preserving the drug’s physicochemical stability. | [23] |
| HPMC LVE3/glycerin | Venlafaxine HCl | Solvent casting | The film formula with moderate levels of HPMC and glycerol, along with the highest concentration of sodium starch glycolate, resulted in the highest observed swelling index at 3.64 ± 0.59. Optimized ODF composition, based on the statistical approach, indicated 2% HPMC, 5% sodium starch glycolate, and 1% glycerol. | [24] |
| Maltodextrins/sorbitol | Benzydamine hydrochloride | Semisolid extrusion 3D printing method | Modifying the formulation’s viscosity enabled direct printing into thin layers, followed by in-process drying. The dose could be controlled by adjusting the thickness or overall volume of the digital model. The modified printing method holds potential for personalized film dosage production and compartmentalization of drugs aside from the potential for taste masking or release control ability. | [25] |
| Maltodextrins/glycerin | Quercetin | Modified solvent casting method | Quercetin nanosuspensions were prepared by high-pressure homogenization. The average particle size was 753 nm, and the polydispersity index was 0.31. The dissolution profile of the drug, loaded in fast-dissolving film, was significantly faster than that of the free freeze-dried nanocrystals. | [26] |
| Modified starch/glycerin | Chlorpheniramine maleate | Hot-melt extrusion technology | Glycerol lowers the melt viscosity and enhances the free volume of starch chains, thereby facilitating the extrusion process. The absence of peaks in melt-extruded formulation confirms the presence of the drug in an amorphous state. ODF formulations created via hot-melt extrusion technology exhibited rapid disintegration times (6 to 11 s), achieving over 95% dissolution within 5 min. The human panel study and animal model confirmed a reduction in bitterness in the films compared to the pure drug and physical mixture. | [27] |
| Polyvinyl alcohol 4 -88/PEG 400 | Meclizine hydrochloride | Solvent casting | The thickness and tensile strength of the meclizine ODF was 0.116 ± 0.004 mm and 17.37 ± 1.54 N mm−2, respectively. Drug dissolution exceeded 80% in less than 5 min, both in distilled water and 0.1 M hydrochloric acid. ATR-FTIR analysis revealed that the drug molecules were incorporated into the polymer’s network structure, leading to the prevention of drug recrystallization. The Cmax values for Zentrip® (Tokyo, Japan) and meclizine OTF were 1.46 ± 0.44 μg/mL and 1.91 ± 0.51 μg/mL, while their respective AUC values were 10.38 ± 2.93 μg h/mL and 13.74 ± 3.23 μg h/mL. | [28] |
| Pullulan/glycerin | Ovalbumin, β-galactosidase and Lysozyme | Solvent casting method | Loading ovalbumin had no significant impact on the mechanical characteristics of freeze-dried ODFs, while introducing it into air-dried ODFs resulted in a notable decrease in tensile strength. The stability of lysozyme remained unaffected by changes in the trehalose/pullulan ratio, whereas higher ratios led to improved stability of β-galactosidase. Freeze-drying was more suitable for process stability, and air-drying was better for storage stability. | [29] |
| Clinical Trials | Indication | Phase | Enrolment | Identifier |
|---|---|---|---|---|
| Comparative bioavailability studies of Riluzole orodispersible film (32.0 mm × 22.0 mm) vs. marketed Rilutek® Tablets (Strasbourg, France) containing an equivalent dose of 50 mg. | Amyotrophic lateral sclerosis | 1 | 54 | NCT04819438 |
| Single-center, single-dose, open-label study to assess the effect of a single 50 mg dose of Riluzole oral soluble film. | Amyotrophic lateral sclerosis | II | 9 | NCT03679975 |
| Prospective, interventional, multi-center, randomized, double-blind, fixed-dose, parallel-group clinical study to demonstrate the efficacy and safety of sildenafil oral film (50/75/100 mg) in comparison with placebo. | Erectile dysfunction | III | 600 | NCT05490680 |
| Open, monocentric, comparative, crossover study to evaluate bioavailability after a single oral dose of iron ODF vs. SiderAL® FORTE (Pisa, Italy) capsules in healthy women. | Iron supplement | I | 9 | NCT05660200 |
| Assess the pharmacokinetics and efficacy of insulin-loaded orally dissolved films. | Glucose management | 1 | 7 | NCT01446120 |
| Pharmacokinetic study in volunteer smokers with various doses (0/2/4 mg) of oral nicotine film, assessing for peak blood nicotine concentrations, safety, and subjective effects. | Oral nicotine replacement therapies | Interventional | 24 | NCT02239770 |
| Open-label, balanced, two-treatment, two-period, randomized study comparing ondansetron orally dissolving film strip with Zofran ODT. | Chemotherapy-induced and radiation-induced nausea and vomiting, postoperative nausea and vomiting | 1 and II | 48 | NCT01217190 |
| Open-label study to assess the safety and tolerability in opioid-dependent subjects maintained on a stabilized dose of Suboxone tablets or films. | Maintenance treatment of opioid dependence | II | 249 | NCT01666119 |
| Investigate the efficacy of oral thin film loaded with cholecalciferol in patients undergoing hematopoietic stem cell transplantation. | Vitamin D replacement therapy | Interventional | 24 | NCT04818957 |
| A single-dose, randomized, three-period, crossover comparative bioavailability study of aripiprazole oral soluble film 10 mg (test) vs. Abilify® (Tokyo, Japan) 10 mg tablet (reference) in healthy male volunteers. | Schizophrenia | I | 36 | NCT02501109 |
| Application ID | Title | Composition of Invention | Date of Publication |
|---|---|---|---|
| US 20230136398 A1 | Oral thin film with smooth fused film. | Comprising at least 40% w/w polyethylene oxide as a film-forming polymer with water-insoluble actives and utilizing solvent evaporation technique. | 4 May 2023 |
| US 20230133317 A1 | Taste-masked and rapidly disintegrating ultra-thin iron orodispersible film and a process thereof. | Consists of microencapsulated iron, beta-cyclodextrin, flavoring agent, calcium carboxy methyl cellulose, pullulan, mannitol, sweetening agent, polyethylene glycol, lecithin, malic acid, ascorbic acid, and kiwi flavor. | 4 May 2023 |
| US 20220331337 A1 | Orodispersible formulations. | Contains an active ingredient (0.5% w/w), an intragranular component (40–80% w/w) containing diluents, disintegrants, and binders, and an extragranular component containing diluents and disintegrants. ODF disintegrates within 60 s and has specific percentage ranges for each component. | 20 October 2022 |
| US 20170182105 A1 | ODF. | Describes film-forming suspensions with a plant extract and. method for producing ODFs. | 29 June 2017 |
| US 20170165315 A1 | ODF containing enalapril, designed for treating hypertension in pediatric patients. | Comprised of water-soluble polymer, preferably pullulan and modified starch such as Lycoat® (Lestrem, France) (50–80% w/w), in addition to plasticizers, fillers, sweetening agents, and an acidic agent. | 15 June 2017 |
| US 20170143623 A1 | Orodispersible films have quick dissolution times for therapeutic and food use. | Comprised of film-forming substances, such as maltodextrin (40–80% w/w), plasticizers (15–55% w/w), surfactant system (0.5–6% w/w), homopolymer or copolymer of vinyl acetate (1–20% w/w), and an active ingredient ranging between 0.05% and 30% w/w. | 25 May 2017 |
| US 11452698 B2 | Dissolvable gel-forming film for delivery of active agents. | Presents a dissolvable, gel-forming film made up of a water-soluble cellulose ether and an active proteolytic enzyme. | 27 September 2022 |
| Product Name | Active/Key Ingredients (Dose) | Indications | Category | Manufacturer |
|---|---|---|---|---|
| Benadryl® Allergy Quick Dissolve Strips | Diphenhydramine HCl (25 mg) | Allergic symptoms | OTC | McNeil-PPC, Inc., Fort Washington, PA, USA |
| Triaminic® Children’s Thin Strips | Dextromethorphan (3.67 mg) and phenylephrine HCl (2.5 mg) | Cough suppressant and nasal decongestant | OTC | Novartis Consumer Health, Basel, Switzerland |
| Gas-X Thin Strips | Simethicone (62.5 mg) | Ant flatulent | OTC | Novartis Consumer Health, Basel, Switzerland |
| Pedia-Lax Quick Dissolve Strips | Sennosides (8.6 mg) | Occasional constipation | OTC | C.B. Fleet Company, Lynchburg, VA, USA |
| Risperidon HEXAL_ SF Schmelzfilm | Risperidone (5 mg) | Schizophrenia | Rx | Hexal AG, Holzkirchen, Germany |
| Sudafed_ PE Quick dissolve strips | Phenylephrine HCl (10 mg) | Nasal decongestant | OTC | McNeil-PPC, Inc., Fort Washington, PA, USA |
| Zuplenz® | Ondansetron (4/8 mg) | Antiemetics | Rx | Strativa Pharmaceuticals, El Segundo, CA, USA |
| NiQuitin | Nicotine (2.5 mg) | Nicotine replacement therapy | Rx | Boots UK Ltd., Nottingham, UK |
| Zolmitriptan ODF | Zolmitriptan (2.5 mg) | Migraine | Rx | APR Applied Pharma Research and Labtec, Balerna, Switzerland |
| Sildenafil Sandoz Orodispersible Film | Sildenafil (25/50/100 mg) | Erectile dysfunction | Rx | Sandoz, Basel, Switzerland |
| Sympazan oral film | Clobazam (5/10/20 mg) | Seizures associated with Lennox-Gastaut syndrome | Rx | Otter Pharmaceuticals, Lake Forest, IL, USA |
| IVYFILM | Ivy leaves dried extracts (16 mg) | Antitussives and expectorants | OTC | Forrester Pharma, Cape Town, South Africa |
| Chloraseptic Sore Throat Relief Strips | Benzocaine (3 mg) | Soothes throat and relieves pain | OTC | Prestige, Melville, NY, USA |
| Theraflu® Thin Strips multi symptom | Diphenhydramine HCl (25 mg) | Allergic symptoms/Cough suppressant | OTC | Novartis Consumer Health, Basel, Switzerland |
| Theraflu®Thin Strips long acting cough | Dextromethorphan HBr (11 mg) | Cough suppressant | OTC | Novartis Consumer Health, Basel, Switzerland |
| Theraflu® Daytime thin strips | Dextromethorphan (14.8 mg) and Phenylephrine HCl (10 mg) | Temporary relief for nasal and sinus congestion as may occur with a cold | OTC | Novartis Consumer Health, Basel, Switzerland |
| Theraflu® Nighttime thin strips | Diphenhydramine HCl (25 mg) and phenylephrine HCl (10 mg) | Temporary relief for nasal and sinus congestion as may occur with a cold | OTC | Novartis Consumer Health, Basel, Switzerland |
| Triaminic® thin strips cold with a stuffy nose | Phenylephrine HCl (2.5 mg) | Temporary relief for nasal and sinus congestion as may occur with a cold | OTC | Novartis Consumer Health, Basel, Switzerland |
| Triaminic® Thin strips day time cold & cough | Dextromethorphan (3.67 mg) | Nasal decongestant | OTC | Novartis Consumer Health, Basel, Switzerland |
| Listerine PocketPak breath strips | Pullulan, xanthan menthol, methyl salicylate, eucalyptol, thymol, menthyl succinate | Mouth freshener | OTC | Johnson and Johnson Consumer Health Inc., New Brunswick, NJ, USA |
| Methylcobalamin orally disintegrating strips | Methylcobalamin (1500 µg) | Vitamin B12 supplement | OTC | Shilpa Medicare Ltd., Raichur, India |
| Tadalafil orally disintegrating strips | Tadalafil (10/20 mg) | Erectile dysfunction | Rx | D.K-Livkon Healthcare Pvt. Ltd., Mumbai, India |
| Levocetirizine orally disintegrating strips | Levocetirizine (5 mg) | Allergic symptoms | OTC | D.K-Livkon Healthcare Pvt. Ltd., Mumbai, India |
| Vitamin D3 orally disintegrating strips | Vitamin D3 (6000 IU) | Vitamin D3 supplement | OTC | D.K-Livkon Healthcare Pvt. Ltd., Mumbai, India |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jacob, S.; Boddu, S.H.S.; Bhandare, R.; Ahmad, S.S.; Nair, A.B. Orodispersible Films: Current Innovations and Emerging Trends. Pharmaceutics 2023, 15, 2753. https://doi.org/10.3390/pharmaceutics15122753
Jacob S, Boddu SHS, Bhandare R, Ahmad SS, Nair AB. Orodispersible Films: Current Innovations and Emerging Trends. Pharmaceutics. 2023; 15(12):2753. https://doi.org/10.3390/pharmaceutics15122753
Chicago/Turabian StyleJacob, Shery, Sai H. S. Boddu, Richie Bhandare, Samiullah Shabbir Ahmad, and Anroop B. Nair. 2023. "Orodispersible Films: Current Innovations and Emerging Trends" Pharmaceutics 15, no. 12: 2753. https://doi.org/10.3390/pharmaceutics15122753
APA StyleJacob, S., Boddu, S. H. S., Bhandare, R., Ahmad, S. S., & Nair, A. B. (2023). Orodispersible Films: Current Innovations and Emerging Trends. Pharmaceutics, 15(12), 2753. https://doi.org/10.3390/pharmaceutics15122753






