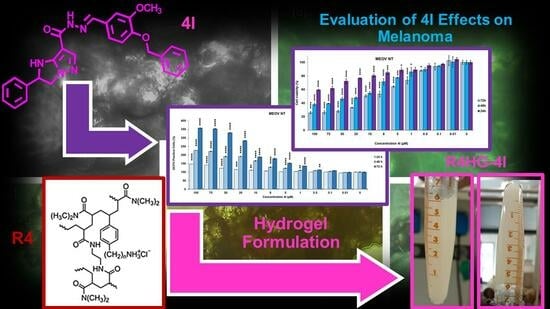
Antiproliferative Imidazo-Pyrazole-Based Hydrogel: A Promising Approach for the Development of New Treatments for PLX-Resistant Melanoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Instruments
2.2. Chemistry
2.2.1. Preparation of R4HG and R4-HG-4I
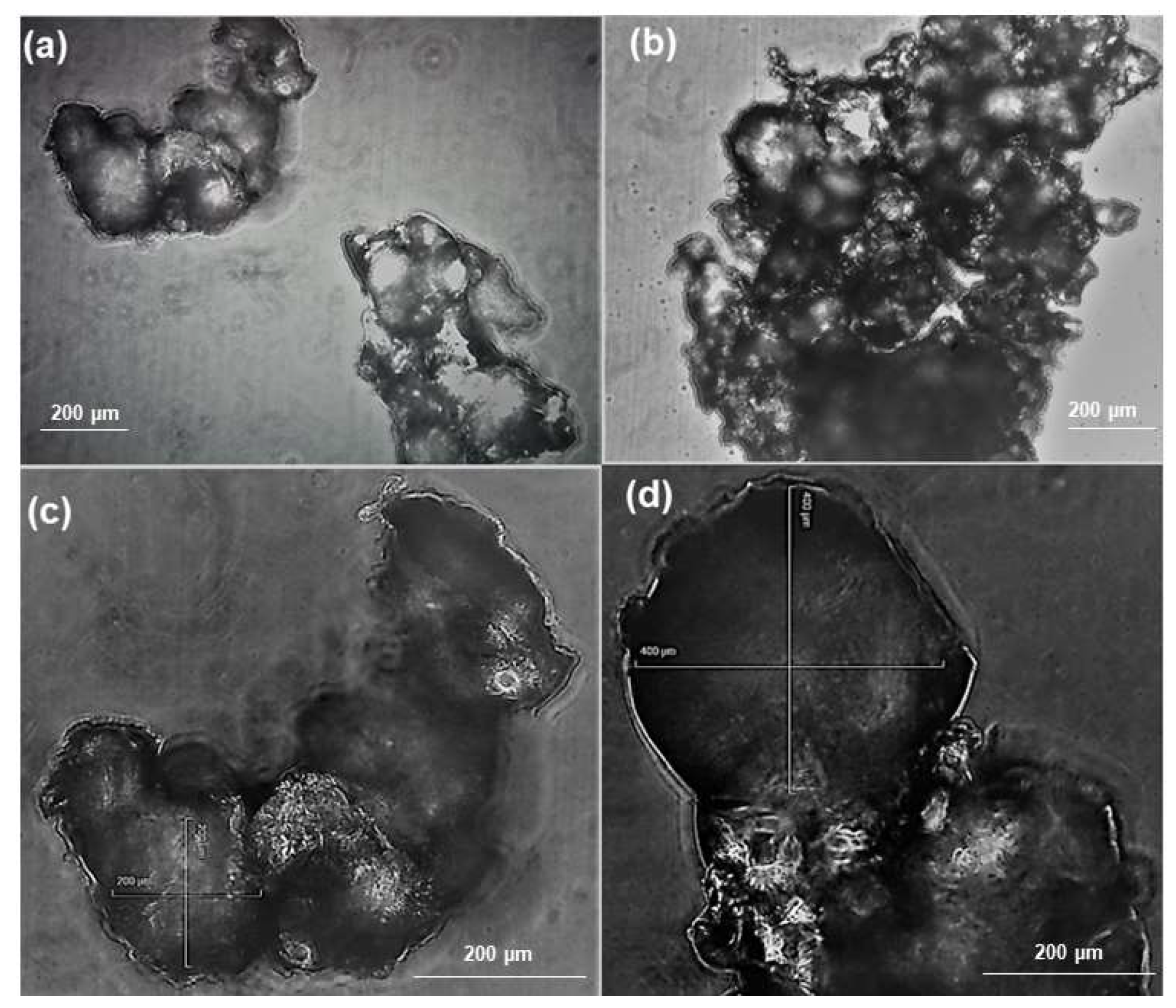
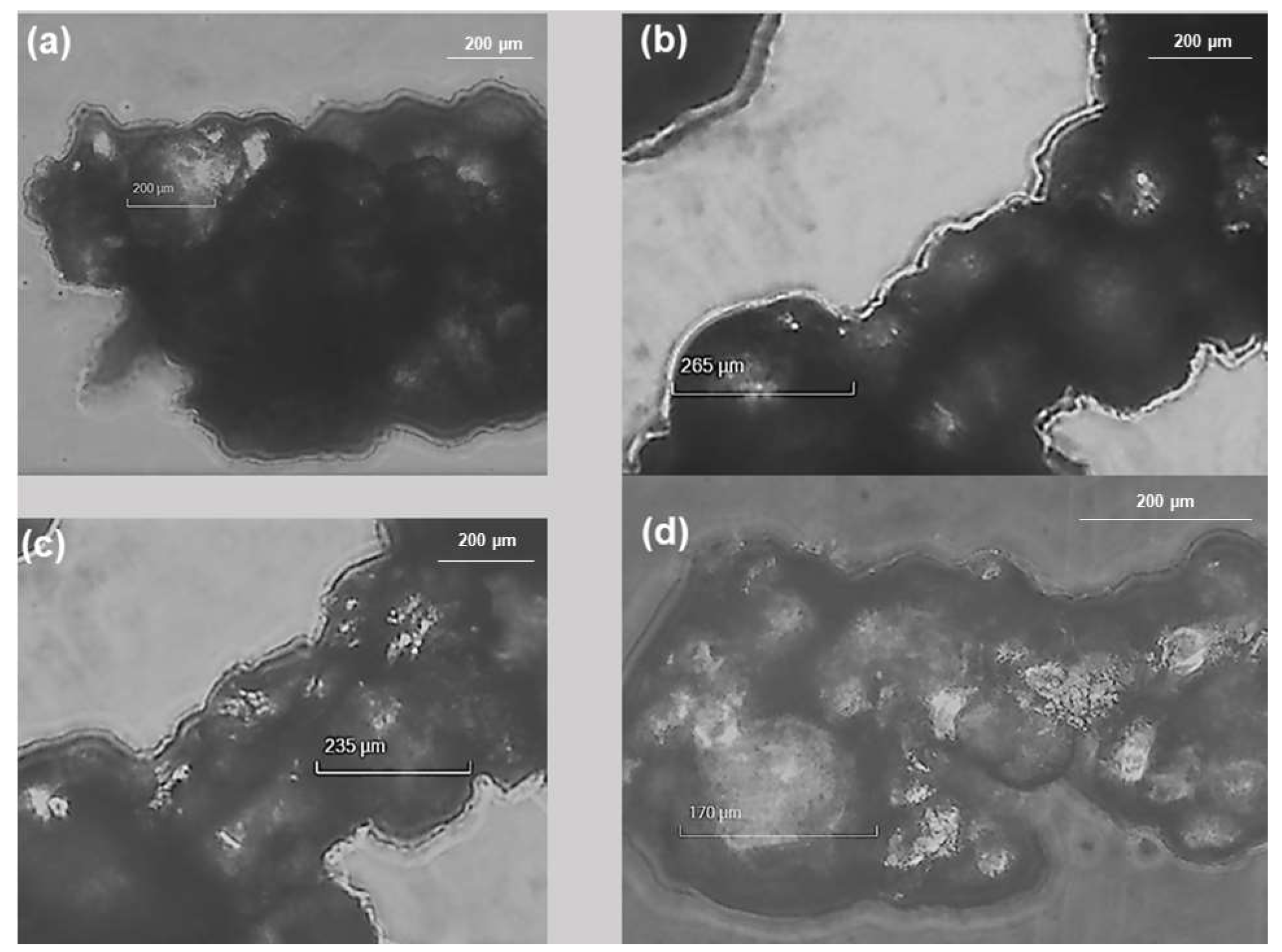
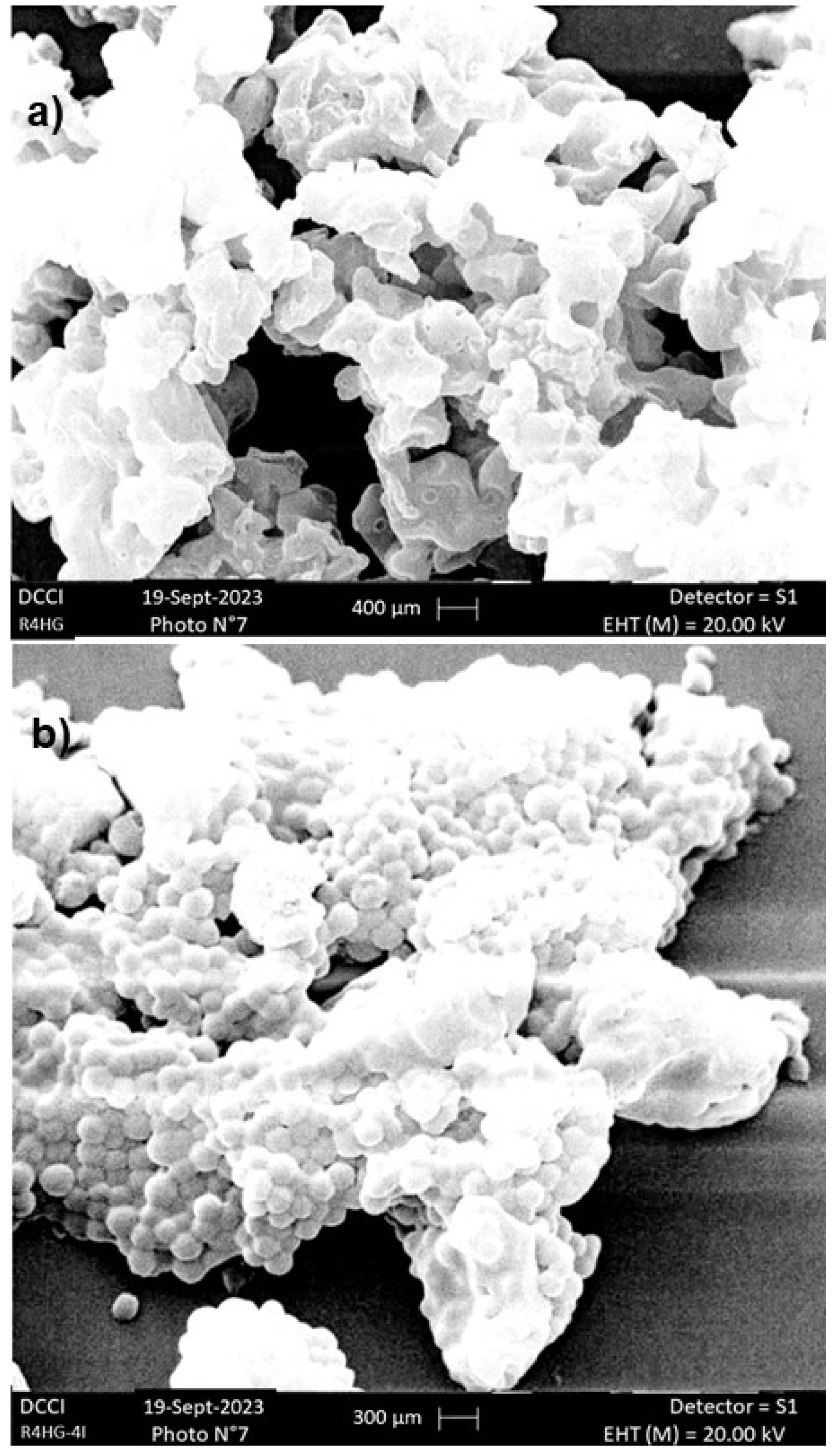
2.2.2. Scanning Electron Microscopy (SEM)
2.2.3. Chemometric-Assisted ATR-FTIR Spectroscopy
2.2.4. Content of 4I in R4HG-4I, Drug Loading (DL%) and Entrapment Efficiency (EE%)
Statistical Analysis
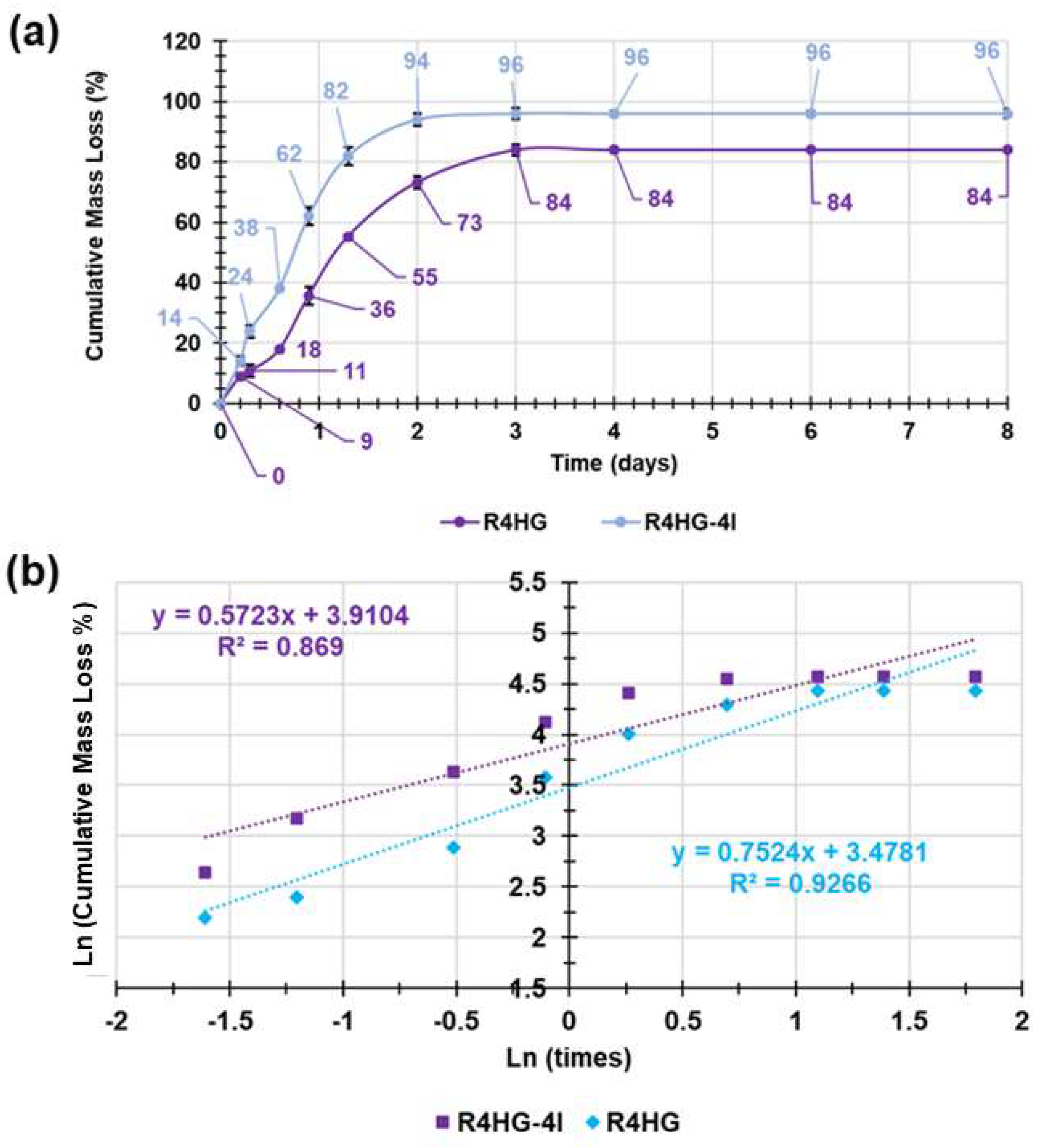
2.2.5. Biodegradability of R4HG and R4HG-4I over Time by In Vitro Mass Loss Experiments
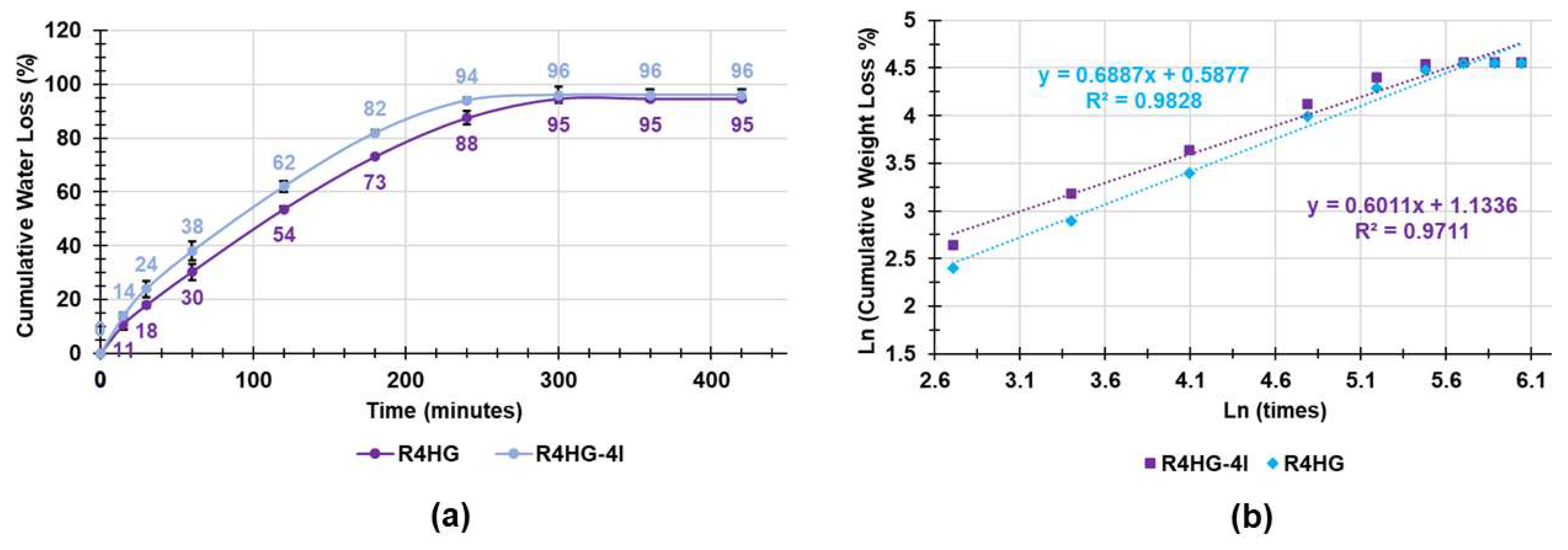
2.2.6. Water Loss Tests
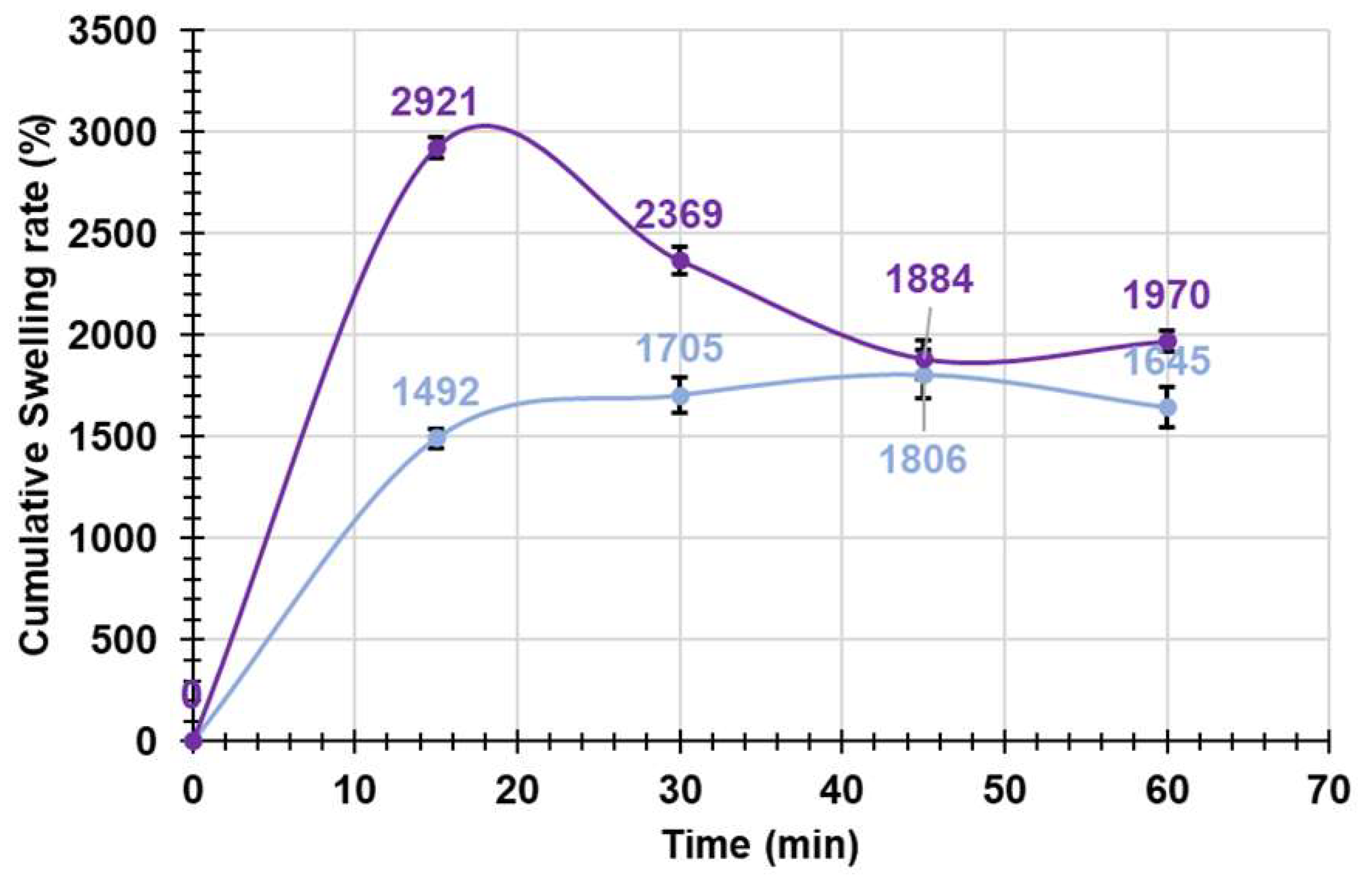
2.2.7. Equilibrium Swelling Rate
2.2.8. Potentiometric Titration of R4HG and R4HG-4I
2.2.9. Rheological Experiments
2.2.10. Evaluation of 4I in Vitro Releases
2.3. Biological Screening
2.3.1. Cell Culture Conditions
2.3.2. Treatments
2.3.3. Cell Viability Assay
2.3.4. Detection of Hydrogen Peroxide (H2O2) Production
2.3.5. Statistical Analyses
3. Results and Discussion
3.1. Chemistry
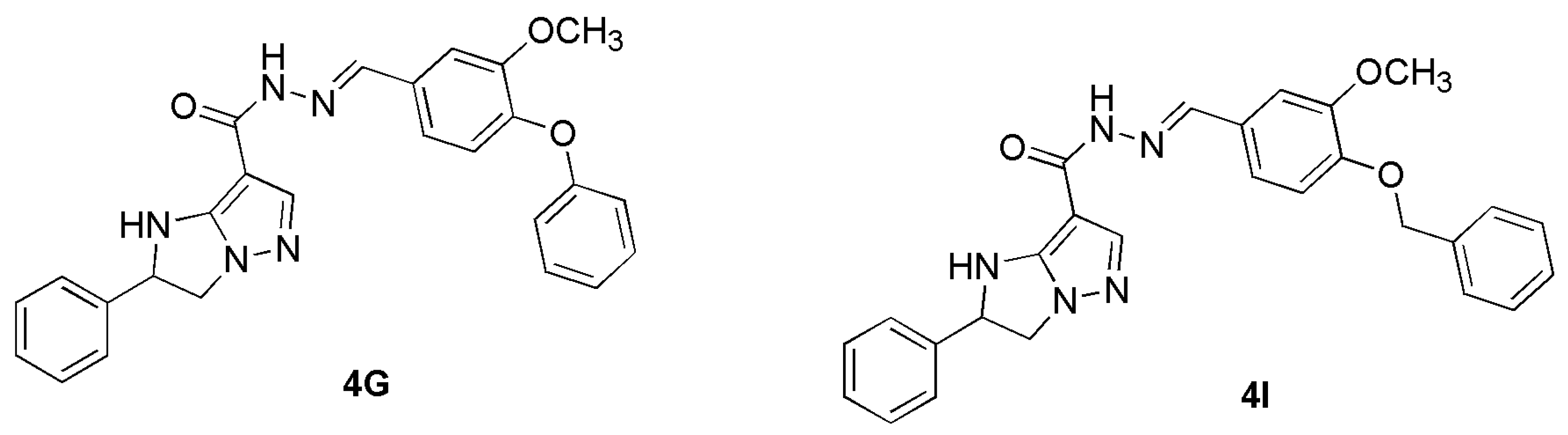
3.1.1. Synthesis and Characterization of R4
3.1.2. Preparation of R4HG and R4HG-4I
3.1.3. SEM Analysis
3.1.4. Chemometric-Assisted ATR-FTIR Analyses
Principal Components Analysis (PCA)
3.1.5. Determination of the Exact Amount of 4I Loaded in R4HG-4I
3.1.6. Evaluation of Biodegradability of R4HG and R4-HG-4I over Time by Mass Loss Experiments
3.1.7. Water Loss Experiments
3.1.8. Equilibrium Swelling Rate Experiments
Kinetic Studies
3.1.9. Potentiometric Titrations of R4HG and R4HG-4I
3.1.10. Rheological Studies
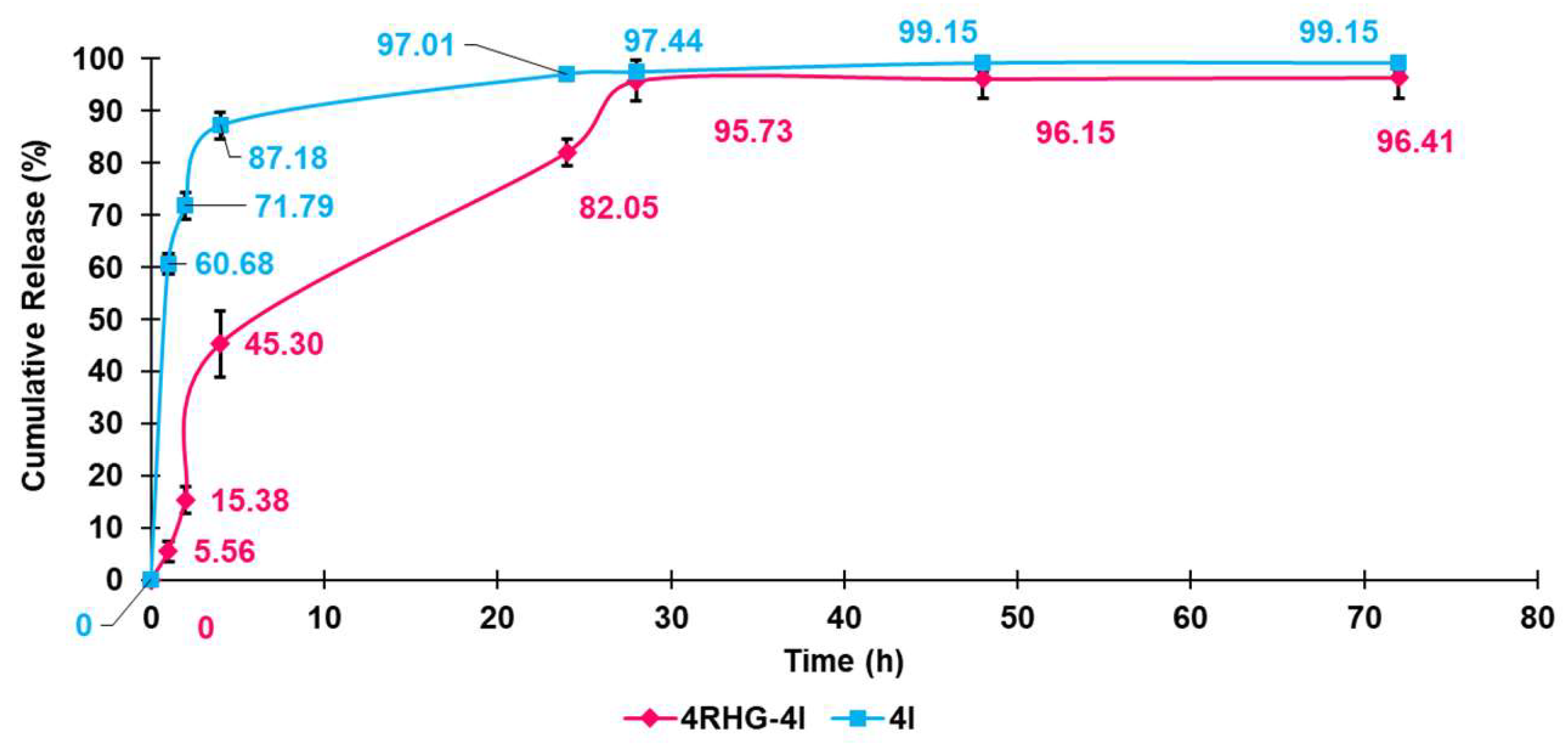
3.1.11. Evaluation of 4I in Vitro Release Profile
Kinetic Studies
3.2. Biological Evaluations
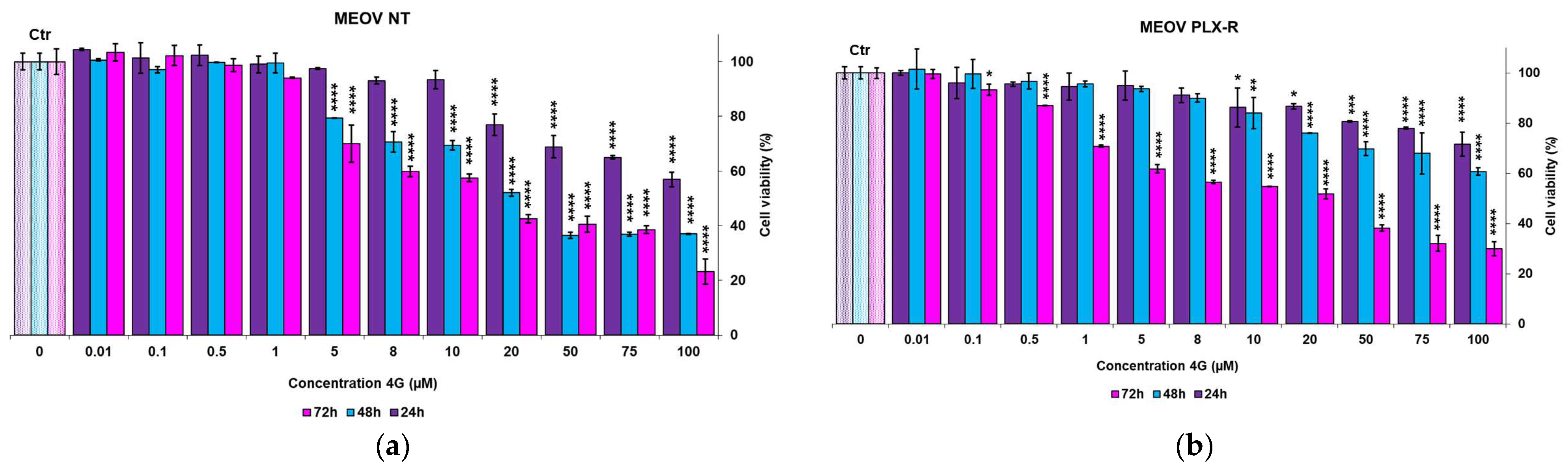
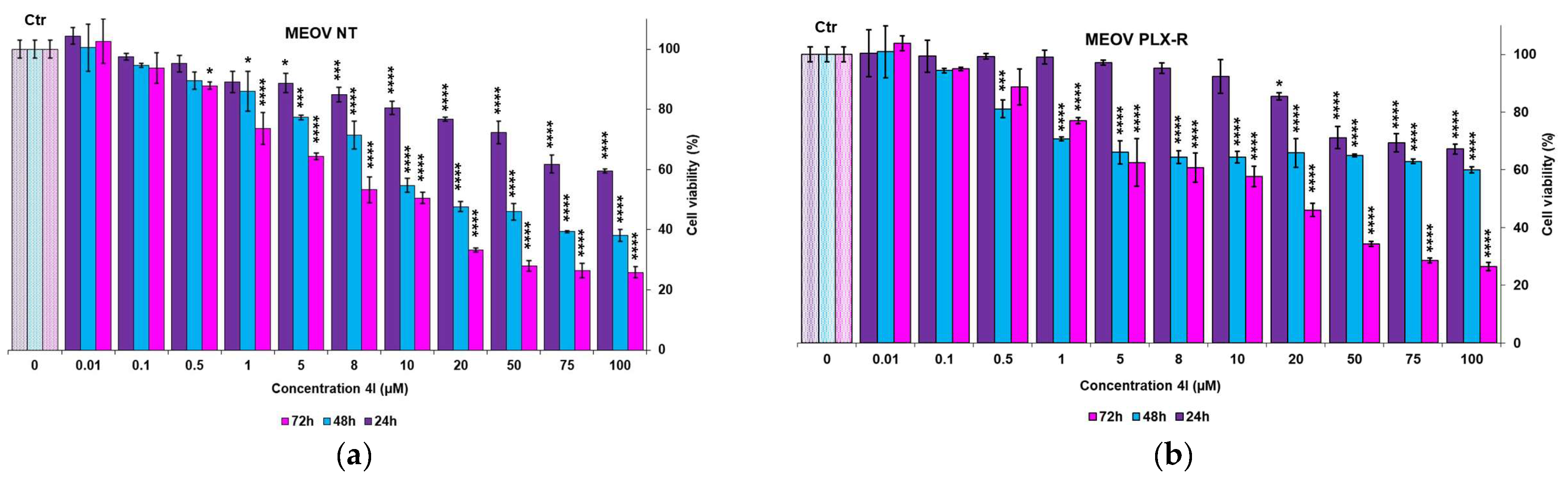
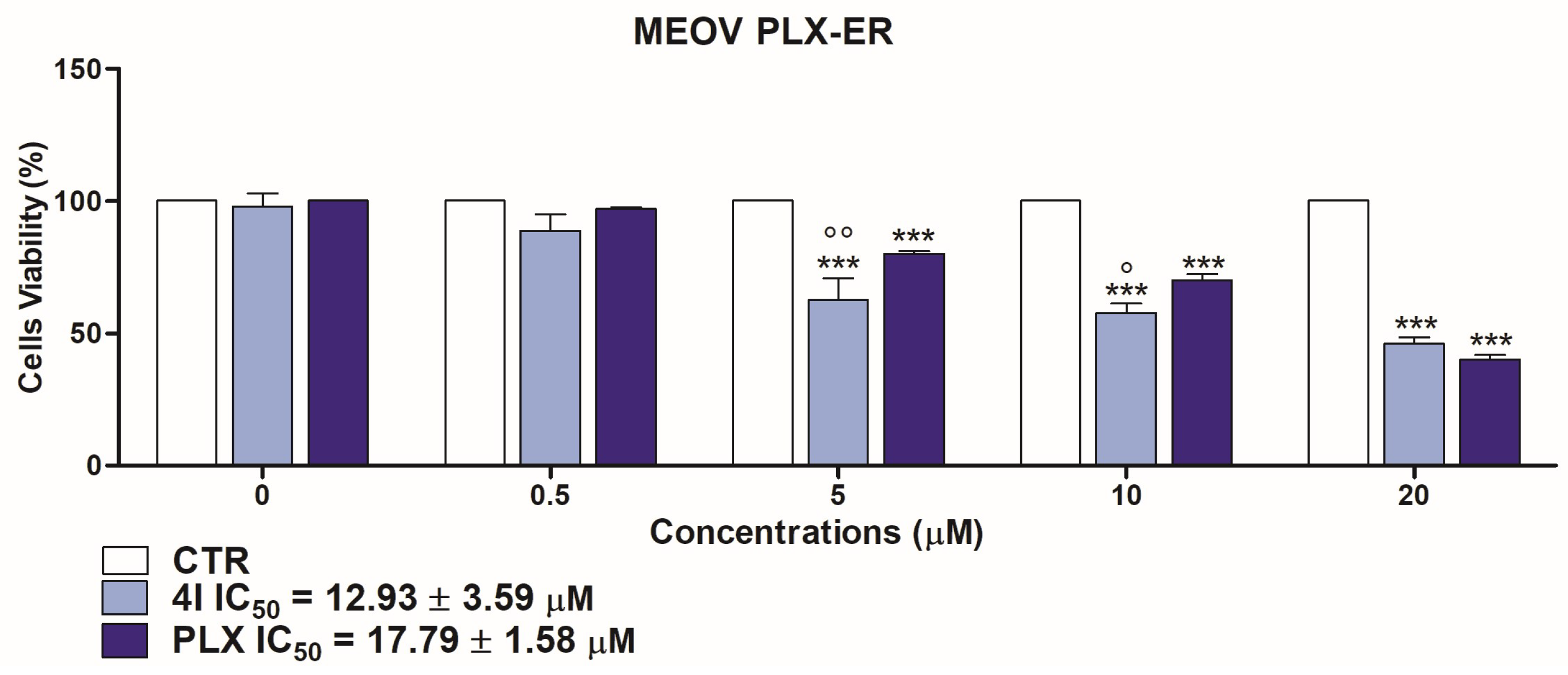
3.2.1. Cytotoxic Effects of 4I and 4G on MM Cells
3.2.2. Dose-Dependent Reactive Oxygen Species (ROS) Production in MEOV NT and MEOV PLXR Cells
3.2.3. Correlation between H2O2 Production and Cytotoxicity of 4I
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Linares, M.A.; Zakaria, A.; Nizran, P. Skin Cancer. Prim. Care Clin. Off. Pract. 2015, 42, 645–659. [Google Scholar] [CrossRef]
- Ciążyńska, M.; Kamińska-Winciorek, G.; Lange, D.; Lewandowski, B.; Reich, A.; Sławińska, M.; Pabianek, M.; Szczepaniak, K.; Hankiewicz, A.; Ułańska, M.; et al. The Incidence and Clinical Analysis of Non-Melanoma Skin Cancer. Sci. Rep. 2021, 11, 4337. [Google Scholar] [CrossRef]
- Siegal, R.; Miller, K.; Jemal, A. Cancer statistics, 2012. CA A Cancer J. Clin. 2014, 64, 9–29. [Google Scholar] [CrossRef]
- Switzer, B.; Puzanov, I.; Skitzki, J.J.; Hamad, L.; Ernstoff, M.S. Managing Metastatic Melanoma in 2022: A Clinical Review. JCO Oncol. Pract. 2022, 18, 335–351. [Google Scholar] [CrossRef]
- Gershenwald, J.E.; Guy, J.; Gery, P. Stemming the Rising Incidence of Melanoma: Calling Prevention to Action. JNCI J. Natl. Cancer Inst. 2015, 108, djv381. [Google Scholar] [CrossRef]
- Zhu, Z.; Liu, W.; Gotlieb, V. The Rapidly Evolving Therapies for Advanced Melanoma—Towards Immunotherapy, Molecular Targeted Therapy, and Beyond. Crit. Rev. Oncol./Hematol. 2016, 99, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Gordon, R. Skin Cancer: An Overview of Epidemiology and Risk Factors. Semin. Oncol. Nurs. 2013, 29, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Nazir, S.; Khan, M.U.A.; Al-Arjan, W.S.; Razak, S.I.A.; Javed, A.; Kadir, M.R.A. Nanocomposite Hydrogels for Melanoma Skin Cancer Care and Treatment: In-Vitro Drug Delivery, Drug Release Kinetics and Anti-Cancer Activities. Arab. J. Chem. 2021, 14, 103120. [Google Scholar] [CrossRef]
- Saginala, K.; Barsouk, A.; Aluru, J.S.; Rawla, P.; Barsouk, A. Epidemiology of Melanoma. Med. Sci. 2021, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- NHI. Melanoma Treatment (PDQ®)–Patient Version. Available online: https://www.cancer.gov/types/skin/patient/melanoma-treatment-pdq (accessed on 19 September 2023).
- Haider, T.; Pandey, V.; Banjare, N.; Gupta, P.N.; Soni, V. Drug Resistance in Cancer: Mechanisms and Tackling Strategies. Pharmacol. Rep. 2020, 72, 1125–1151. [Google Scholar] [CrossRef]
- Wöckel, A.; Wolters, R.; Wiegel, T.; Novopashenny, I.; Janni, W.; Kreienberg, R.; Wischnewsky, M.; Schwentner, L. The Impact of Adjuvant Radiotherapy on the Survival of Primary Breast Cancer Patients: A Retrospective Multicenter Cohort Study of 8935 Subjects†. Ann. Oncol. 2014, 25, 628–632. [Google Scholar] [CrossRef] [PubMed]
- Valizadeh, A.; Khaleghi, A.A.; Alipanah, H.; Zarenezhad, E.; Osanloo, M. Anticarcinogenic Effect of Chitosan Nanoparticles Containing Syzygium Aromaticum Essential Oil or Eugenol Toward Breast and Skin Cancer Cell Lines. BioNanoScience 2021, 11, 678–686. [Google Scholar] [CrossRef]
- Huang, X.; He, Y.; Zhang, M.; Lu, Z.; Zhang, T.; Wang, B. GPP-TSAIII Nanocomposite Hydrogel-Based Photothermal Ablation Facilitates Melanoma Therapy. Expert Opin. Drug Deliv. 2023, 1–19. [Google Scholar] [CrossRef]
- Bollag, G.; Hirth, P.; Tsai, J.; Zhang, J.; Ibrahim, P.N.; Cho, H.; Spevak, W.; Zhang, C.; Zhang, Y.; Habets, G.; et al. Clinical Efficacy of a RAF Inhibitor Needs Broad Target Blockade in BRAF-Mutant Melanoma. Nature 2010, 467, 596–599. [Google Scholar] [CrossRef]
- Hallmeyer, S.; Gonzalez, R.; Lawson, D.H.; Cranmer, L.D.; Linette, G.P.; Puzanov, I.; Taback, B.; Cowey, C.L.; Ribas, A.; Daniels, G.A.; et al. Vemurafenib Treatment for Patients with Locally Advanced, Unresectable Stage IIIC or Metastatic Melanoma and Activating Exon 15 BRAF Mutations Other than V600E. Melanoma Res. 2017, 27, 585–590. [Google Scholar] [CrossRef]
- Yu, S.; Sun, H.; Li, Y.; Wei, S.; Xu, J.; Liu, J. Hydrogels as Promising Platforms for Engineered Living Bacteria-Mediated Therapeutic Systems. Mater. Today Bio 2022, 16, 100435. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.U.A.; Raza, M.A.; Razak, S.I.A.; Abdul Kadir, M.R.; Haider, A.; Shah, S.A.; Mohd Yusof, A.H.; Haider, S.; Shakir, I.; Aftab, S. Novel Functional Antimicrobial and Biocompatible Arabinoxylan/Guar Gum Hydrogel for Skin Wound Dressing Applications. J. Tissue Eng. Regen. Med. 2020, 14, 1488–1501. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.U.A.; Iqbal, I.; Ansari, M.N.M.; Razak, S.I.A.; Raza, M.A.; Sajjad, A.; Jabeen, F.; Riduan Mohamad, M.; Jusoh, N. Development of Antibacterial, Degradable and PH-Responsive Chitosan/Guar Gum/Polyvinyl Alcohol Blended Hydrogels for Wound Dressing. Molecules 2021, 26, 5937. [Google Scholar] [CrossRef]
- Alfei, S.; Zorzoli, A.; Marimpietri, D.; Zuccari, G.; Russo, E.; Caviglia, D.; Schito, A.M. A Self-Forming Hydrogel from a Bactericidal Copolymer: Synthesis, Characterization, Biological Evaluations and Perspective Applications. Int. J. Mol. Sci. 2022, 23, 15092. [Google Scholar] [CrossRef]
- Khan, M.U.A.; Razak, S.I.A.; Haider, S.; Mannan, H.A.; Hussain, J.; Hasan, A. Sodium Alginate-f-GO Composite Hydrogels for Tissue Regeneration and Antitumor Applications. Int. J. Biol. Macromol. 2022, 208, 475–485. [Google Scholar] [CrossRef]
- Alfei, S.; Zuccari, G.; Russo, E.; Villa, C.; Brullo, C. Hydrogel Formulations of Antibacterial Pyrazoles Using a Synthesized Polystyrene-Based Cationic Resin as a Gelling Agent. Int. J. Mol. Sci. 2023, 24, 1109. [Google Scholar] [CrossRef]
- Schito, A.M.; Caviglia, D.; Piatti, G.; Alfei, S. A Highly Efficient Polystyrene-Based Cationic Resin to Reduce Bacterial Contaminations in Water. Polymers 2022, 14, 4690. [Google Scholar] [CrossRef] [PubMed]
- Brullo, C.; Rapetti, F.; Alfei, S.; Maric, I.; Rizzelli, F.; Mapelli, M.; Rosano, C.; Viale, M.; Bruno, O. Discovery of New Antiproliferative Imidazopyrazole Acylhydrazones Able to Interact with Microtubule Systems. ChemMedChem 2020, 15, 961–969. [Google Scholar] [CrossRef]
- Garbarino, O.; Valenti, G.E.; Monteleone, L.; Pietra, G.; Mingari, M.C.; Benzi, A.; Bruzzone, S.; Ravera, S.; Leardi, R.; Farinini, E.; et al. PLX4032 resistance of patient-derived melanoma cells: Crucial role of oxidative metabolism. Front. Oncol. 2023, 13, 1210130. [Google Scholar] [CrossRef] [PubMed]
- Arshady, R.; Atherton, E.; Clive, D.L.J.; Sheppard, R.C. Peptide Synthesis. Part 1. Preparation and Use of Polar Supports Based on Poly(Dimethylacrylamide). J. Chem. Soc. Perkin Trans. 1 1981, 529–537. [Google Scholar] [CrossRef]
- Alfei, S.; Marengo, B.; Valenti, G.E.; Domenicotti, C. Synthesis of Polystyrene-Based Cationic Nanomaterials with Pro-Oxidant Cytotoxic Activity on Etoposide-Resistant Neuroblastoma Cells. Nanomaterials 2021, 11, 977. [Google Scholar] [CrossRef]
- Alfei, S.; Caviglia, D.; Piatti, G.; Zuccari, G.; Schito, A.M. Synthesis, Characterization and Broad-Spectrum Bactericidal Effects of Ammonium Methyl and Ammonium Ethyl Styrene-Based Nanoparticles. Nanomaterials 2022, 12, 2743. [Google Scholar] [CrossRef]
- ’s-Gravenmade, E.J.; Wemes, J.C.; Dankert, J. Quantitative Measurements of the Diffusion in Vitro of Some Aldehydes in Root Canals of Human Teeth. Oral Surg. Oral Med. Oral Pathol. 1981, 52, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Shoukat, H.; Pervaiz, F.; Khan, M.; Rehman, S.; Akram, F.; Abid, U.; Noreen, S.; Nadeem, M.; Qaiser, R.; Ahmad, R.; et al. Development of β-Cyclodextrin/Polyvinypyrrolidone-Co-Poly (2-Acrylamide-2-Methylpropane Sulphonic Acid) Hybrid Nanogels as Nano-Drug Delivery Carriers to Enhance the Solubility of Rosuvastatin: An in Vitro and in Vivo Evaluation. PLoS ONE 2022, 17, e0263026. [Google Scholar] [CrossRef]
- Khan, K.U.; Akhtar, N.; Minhas, M.U. Poloxamer-407-Co-Poly (2-Acrylamido-2-Methylpropane Sulfonic Acid) Cross-Linked Nanogels for Solubility Enhancement of Olanzapine: Synthesis, Characterization, and Toxicity Evaluation. AAPS PharmSciTech 2020, 21, 141. [Google Scholar] [CrossRef] [PubMed]
- Colla, R.; Izzotti, A.; De Ciucis, C.; Fenoglio, D.; Ravera, S.; Speciale, A.; Ricciarelli, R.; Furfaro, A.L.; Pulliero, A.; Passalacqua, M.; et al. Glutathione-Mediated Antioxidant Response and Aerobic Metabolism: Two Crucial Factors Involved in Determining the Multi-Drug Resistance of High-Risk Neuroblastoma. Oncotarget 2016, 7, 70715–70737. [Google Scholar] [CrossRef]
- Alfei, S.; Grasso, F.; Orlandi, V.; Russo, E.; Boggia, R.; Zuccari, G. Cationic Polystyrene-Based Hydrogels as Efficient Adsorbents to Remove Methyl Orange and Fluorescein Dye Pollutants from Industrial Wastewater. Int. J. Mol. Sci. 2023, 24, 2948. [Google Scholar] [CrossRef] [PubMed]
- Hoare, T.R.; Kohane, D.S. Hydrogels in Drug Delivery: Progress and Challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef]
- Cheng, H.; Shi, Z.; Yue, K.; Huang, X.; Xu, Y.; Gao, C.; Yao, Z.; Zhang, Y.S.; Wang, J. Sprayable Hydrogel Dressing Accelerates Wound Healing with Combined Reactive Oxygen Species-Scavenging and Antibacterial Abilities. Acta Biomater. 2021, 124, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, H.; Guo, J.; Li, L.; Xi, X.; Yu, Y. Biocompatible supramolecular pseudorotaxane hydrogels for controllable release of doxorubicin in ovarian cancer SKOV-3 cells. RSC Adv. 2020, 10, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Alfei, S.; Oliveri, P.; Malegori, C. Assessment of the Efficiency of a Nanospherical Gallic Acid Dendrimer for Long-Term Preservation of Essential Oils: An Integrated Chemometric-Assisted FTIR Study. Chem. Sel. 2019, 4, 8891–8901. [Google Scholar] [CrossRef]
- Soni, G.; Yadav, K.S. High Encapsulation Efficiency of Poloxamer-Based Injectable Thermoresponsive Hydrogels of Etoposide. Pharm. Dev. Technol. 2014, 19, 651–661. [Google Scholar] [CrossRef]
- Wang, J.; Liu, C.; Shuai, Y.; Cui, X.; Nie, L. Controlled release of anticancer drug using graphene oxide as a drug-binding effector in konjac glucomannan/sodium alginate hydrogels. Colloids Surfaces. B Biointerfaces 2014, 113, 223–229. [Google Scholar] [CrossRef]
- Pourtalebi, J.L.; Rothammer, M.; Fuhrmann, G. Polysaccharide hydrogel platforms as suitable carriers of liposomes and extracellular vesicles for dermal applications. Adv. Drug Deliv. Rev. 2023, 200, 115028. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Hao, R.; Ren, X.; Yu, L.; Yang, H.; Yu, H. PEG/Lecithin–Liquid-Crystalline Composite Hydrogels for Quasi-Zero-Order Combined Release of Hydrophilic and Lipophilic Drugs. RSC Adv. 2013, 3, 22927–22930. [Google Scholar] [CrossRef]
- Mircioiu, C.; Voicu, V.; Anuta, V.; Tudose, A.; Celia, C.; Paolino, D.; Fresta, M.; Sandulovici, R.; Mircioiu, I. Mathematical Modelling of Release Kinetics from Supramolecular Drug Delivery Systems. Pharmaceutics 2019, 11, 140. [Google Scholar] [CrossRef]
- Bruschi, M.L. (Ed.) 5-Mathematical Models of Drug Release. In Strategies to Modify the Drug Release from Pharmaceutical Systems; Woodhead Publishing: Sawston, UK, 2015; pp. 63–86. ISBN 978-0-08-100092-2. [Google Scholar]
- Wu, I.Y.; Bala, S.; Škalko-Basnet, N.; di Cagno, M.P. Interpreting non-linear drug diffusion data: Utilizing Korsmeyer-Peppas model to study drug release from liposomes. Eur. J. Pharm. Sci. 2019, 138, 105026. [Google Scholar] [CrossRef]
- Talevi, A.; Ruiz, M.E. Korsmeyer-Peppas, Peppas-Sahlin, and Brazel-Peppas: Models of Drug Release. In The ADME Encyclopedia: A Comprehensive Guide on Biopharmacy and Pharmacokinetics; Springer International Publishing: Cham, Switzerland, 2021; pp. 1–9. ISBN 978-3-030-51519-5. [Google Scholar]
- Heredia, N.S.; Vizuete, K.; Flores-Calero, M.; Pazmiño, V., K.; Pilaquinga, F.; Kumar, B.; Debut, A. Comparative Statistical Analysis of the Release Kinetics Models for Nanoprecipitated Drug Delivery Systems Based on Poly(Lactic-Co-Glycolic Acid). PLoS ONE 2022, 17, e0264825. [Google Scholar] [CrossRef]
- Abasalizadeh, F.; Moghaddam, S.V.; Alizadeh, E.; Akbari, E.; Kashani, E.; Fazljou, S.M.; Torbati, M.; Akbarzadeh, A. Alginate-based hydrogels as drug delivery vehicles in cancer treatment and their applications in wound dressing and 3D bioprinting. J. Biol. Eng. 2020, 14, 8. [Google Scholar] [CrossRef]
- Alfei, S.; Orlandi, V.; Grasso, F.; Boggia, R.; Zuccari, G. Cationic Polystyrene-Based Hydrogels: Low-Cost and Regenerable Adsorbents to Electrostatically Remove Nitrites from Water. Toxics 2023, 11, 312. [Google Scholar] [CrossRef] [PubMed]
- Khatooni, H.; Peighambardoust, S.J.; Foroutan, R.; Mohammadi, R.; Ramavandi, B. Adsorption of methylene blue using sodium carboxymethyl cellulose-g-poly (acrylamide-co-methacrylic acid)/Cloisite 30B nanocomposite hydrogel. J. Polym. Environ. 2023, 31, 297–311. [Google Scholar] [CrossRef]
- Valenti, G.E.; Alfei, S.; Caviglia, D.; Domenicotti, C.; Marengo, B. Antimicrobial Peptides and Cationic Nanoparticles: A Broad-Spectrum Weapon to Fight Multi-Drug Resistance Not Only in Bacteria. Int. J. Mol. Sci. 2022, 23, 6108. [Google Scholar] [CrossRef]
- Zu, G.; Meijer, M.; Mergel, O.; Zhang, H.; van Rijn, P. 3D-Printable Hierarchical Nanogel-GelMA Composite Hydrogel System. Polymers 2021, 13, 2508. [Google Scholar] [CrossRef]
- Stojkov, G.; Niyazov, Z.; Picchioni, F.; Bose, R.K. Relationship between Structure and Rheology of Hydrogels for Various Applications. Gels 2021, 7, 255. [Google Scholar] [CrossRef] [PubMed]
- Zuccari, G.; Baldassari, S.; Alfei, S.; Marengo, B.; Valenti, G.E.; Domenicotti, C.; Ailuno, G.; Villa, C.; Marchitto, L.; Caviglioli, G. D-α-Tocopherol-Based Micelles for Successful Encapsulation of Retinoic Acid. Pharmaceuticals 2021, 14, 212. [Google Scholar] [CrossRef] [PubMed]
- Mezger, T. For Users of Rotational and Oscillatory Rheometers; Vincentz Network: Hannover, Germany, 2020; ISBN 978-3-7486-0370-2. [Google Scholar]
- De Corcuera, J.I.R. Foreword. In Introduction to Food Engineering, 5th ed.; Food Science and Technology International Series; Singh, R.P., Heldman, D.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; p. 160. ISBN 978-0-12-398530-9. [Google Scholar]
- Bair, S. Tribology and Interface Engineering Series; Bair, S., McCabe, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; Volume 54, pp. xvii–xviii. ISBN 1572-3364. [Google Scholar]
- Xie, J.; Jin, Y.-C. Parameter Determination for the Cross Rheology Equation and Its Application to Modeling Non-Newtonian Flows Using the WC-MPS Method. Eng. Appl. Comput. Fluid Mech. 2016, 10, 111–129. [Google Scholar] [CrossRef]
- Altair. Cross Model. Available online: https://2021.help.altair.com/2021/hwdesktop/mfs/mfs.htm?crossmodel.htm (accessed on 14 July 2023).
- Botchu, V.S.; Baek, S.W. Flow and dynamic rheological characterization of ethanolamine gel propellant with hybrid gelling agent. Sci. Technol. Energetic Mater. 2015, 76, 62–67. [Google Scholar]
- Gulfam, M.; Jo, S.-H.; Jo, S.-W.; Vu, T.T.; Park, S.-H.; Lim, K.T. Highly Porous and Injectable Hydrogels Derived from Cartilage Acellularized Matrix Exhibit Reduction and NIR Light Dual-Responsive Drug Release Properties for Application in Antitumor Therapy. NPG Asia Mater. 2022, 14, 8. [Google Scholar] [CrossRef]
- Dash, S.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol. Pharm. 2010, 67, 217–223. [Google Scholar]
- Caccavo, D. An overview on the mathematical modeling of hydrogels behavior for drug delivery systems. Int. J. Pharmacol. 2019, 560, 175–190. [Google Scholar] [CrossRef] [PubMed]
- Valenti, G.E.; Tasso, B.; Traverso, N.; Domenicotti, C.; Marengo, B. Glutathione in Cancer Progression and Chemoresistance: An Update. Redox Exp. Med. 2023, 2023, e220023. [Google Scholar] [CrossRef]
- Sies, H.; Belousov, V.V.; Chandel, N.S.; Davies, M.J.; Jones, D.P.; Mann, G.E.; Murphy, M.P.; Yamamoto, M.; Winterbourn, C. Defining Roles of Specific Reactive Oxygen Species (ROS) in Cell Biology and Physiology. Nat. Rev. Mol. Cell Biol. 2022, 23, 499–515. [Google Scholar] [CrossRef] [PubMed]
- Smolyarova, D.D.; Podgorny, O.V.; Bilan, D.S.; Belousov, V.V. A Guide to Genetically Encoded Tools for the Study of H2O2. FEBS J. 2022, 289, 5382–5395. [Google Scholar] [CrossRef]
- Nambiar, N.; Nagireddy, P.K.R.; Pedapati, R.; Kantevari, S.; Lopus, M. Tubulin- and ROS-dependent antiproliferative mechanism of a potent analogue of noscapine, N-propargyl noscapine. Life Sci. 2020, 258, 118238. [Google Scholar] [CrossRef] [PubMed]


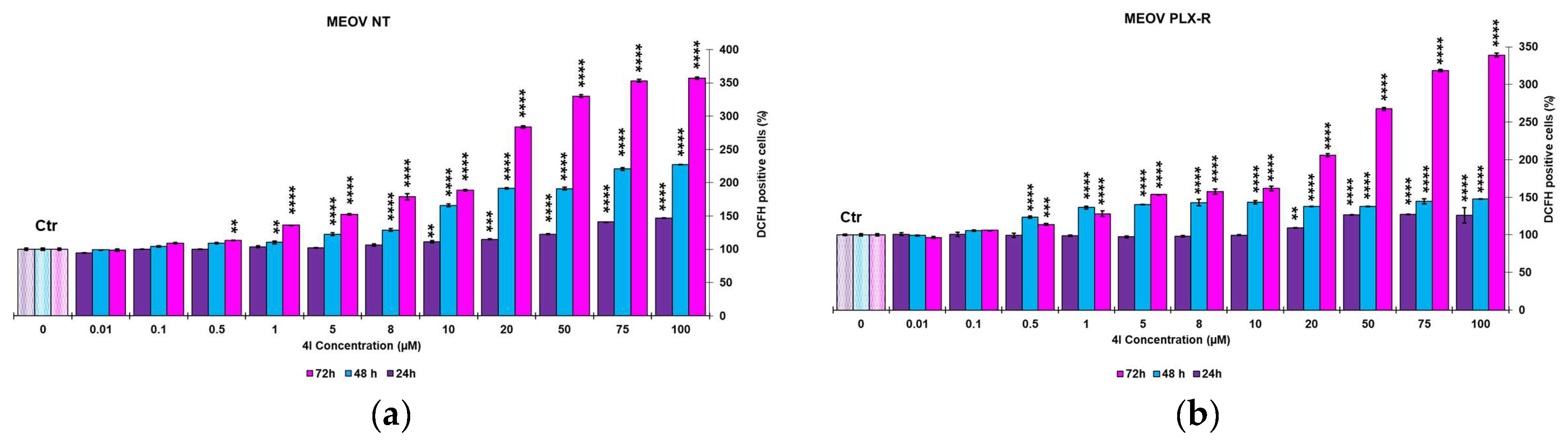
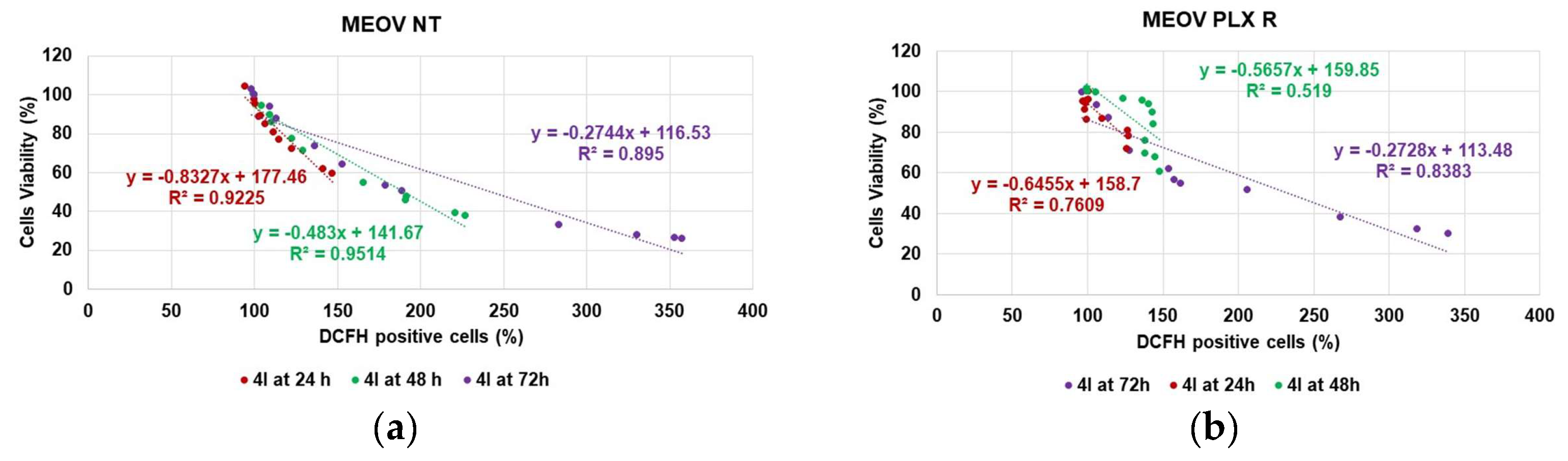
| Times | 4G (μM) | 4I (μM) | ||
|---|---|---|---|---|
| MEOV NT | MEOV PLX-R | MEOV NT | MEOV PLX-R | |
| 24 h | 120.7 ± 0.0 | 224.9 ± 45.5 | 112 ± 9.9 | 162.8 ± 4.9 |
| 48 h | 28.6 ± 0.9 | 128.6 ± 36.6 | 26.3 ± 5.3 | 72.9 ± 10.2 |
| 72 h | 18.5 ± 2.3 | 16.3 ± 0.1 | 10.9 ± 1.7 | 15.8 ± 4.4 |
| Entry | Vi (mL) | Wi (mg) | R4 Vi/Wi (mL/mg) | R4+4I Vi/Wi (mL/mg) | Vf Hydrogel (mL) | H2O (mL) | Dosage (mg/mL) (% wt/v) | EDS (%) | EWC (%) |
|---|---|---|---|---|---|---|---|---|---|
| R4 | 0.69 | 203.1 | 4.50 | 3.81 | 53.3 * (5.3) | 552.2 1 | 84.7 1 | ||
| 4I | 0.1 | 21.4 | 0.69/203.1 | 0.79/224.5 | 6.00 | 5.21 | 43.1 ** (4.3) | 659.5 2 | 86.8 2 |
| Abs | 4I (mg/mL) | 4I (mg) in 82.6 mg * | 4I (mg) in 224.5 mg ** | DL (%) | EE/DLC (%) |
|---|---|---|---|---|---|
| 0.9496 | 0.697 | 6.97 ± 0.19 | 18.9 ± 0. 5 | 8.4 | 88.3 |
| 0.9400 | 0.678 | ||||
| 0.9586 | 0.715 |
| Kinetic Model | R2 of R4HG | R2 of R4HG-4I |
|---|---|---|
| Zero-order | 0.7500 | 0.6239 |
| First-order | 0.8284 | 0.7754 |
| Hixson–Crowel | 0.7500 | 0.6239 |
| Higuchi | 0.8971 | 0.8392 |
| Korsmeyer–Peppas | 0.9266 | 0.8690 |
| R4 | R4HG-4I-D | |
|---|---|---|
| WePSO * | 3.9139 | 0.4286 |
| K2 ** | 0.6574 | 0.7424 |
| Entry | Weight (mg) | HCl 0.1 N (mL) | NH2 (mmol) | mmoles NH2/g | µmoles NH2/g |
|---|---|---|---|---|---|
| R4HG | 50.3 | 0.4 ± 0.01 | 0.04 ± 0.001 | 0.7952 ± 0.0199 | 795.2 ± 10.9 |
| R4HG-4I | 52.8 | 0.4 ± 0.02 | 0.04 ± 0.002 | 0.7576 ± 0.0379 | 757.6 ± 37.9 |
| Entry | n | Yield Stress | |||
|---|---|---|---|---|---|
| R4HG | −1.0005 | 0.1013 | 101.3 | 0.46 | 2.87 |
| R4HG-4I | −1.0594 | 4.2424 | 4242.4 | 42.7 | 25.71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfei, S.; Milanese, M.; Brullo, C.; Valenti, G.E.; Domenicotti, C.; Russo, E.; Marengo, B. Antiproliferative Imidazo-Pyrazole-Based Hydrogel: A Promising Approach for the Development of New Treatments for PLX-Resistant Melanoma. Pharmaceutics 2023, 15, 2425. https://doi.org/10.3390/pharmaceutics15102425
Alfei S, Milanese M, Brullo C, Valenti GE, Domenicotti C, Russo E, Marengo B. Antiproliferative Imidazo-Pyrazole-Based Hydrogel: A Promising Approach for the Development of New Treatments for PLX-Resistant Melanoma. Pharmaceutics. 2023; 15(10):2425. https://doi.org/10.3390/pharmaceutics15102425
Chicago/Turabian StyleAlfei, Silvana, Marco Milanese, Chiara Brullo, Giulia Elda Valenti, Cinzia Domenicotti, Eleonora Russo, and Barbara Marengo. 2023. "Antiproliferative Imidazo-Pyrazole-Based Hydrogel: A Promising Approach for the Development of New Treatments for PLX-Resistant Melanoma" Pharmaceutics 15, no. 10: 2425. https://doi.org/10.3390/pharmaceutics15102425
APA StyleAlfei, S., Milanese, M., Brullo, C., Valenti, G. E., Domenicotti, C., Russo, E., & Marengo, B. (2023). Antiproliferative Imidazo-Pyrazole-Based Hydrogel: A Promising Approach for the Development of New Treatments for PLX-Resistant Melanoma. Pharmaceutics, 15(10), 2425. https://doi.org/10.3390/pharmaceutics15102425