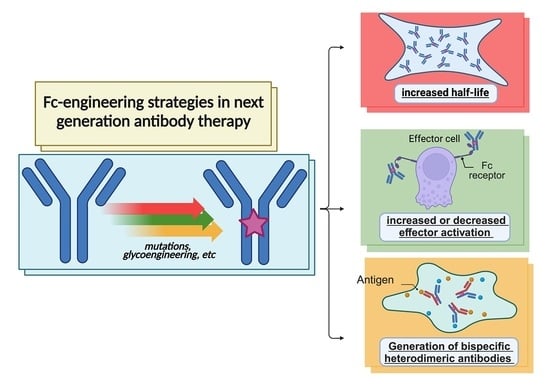
Fc-Engineered Therapeutic Antibodies: Recent Advances and Future Directions
Abstract
1. Antibodies—Magic Bullets in Therapy
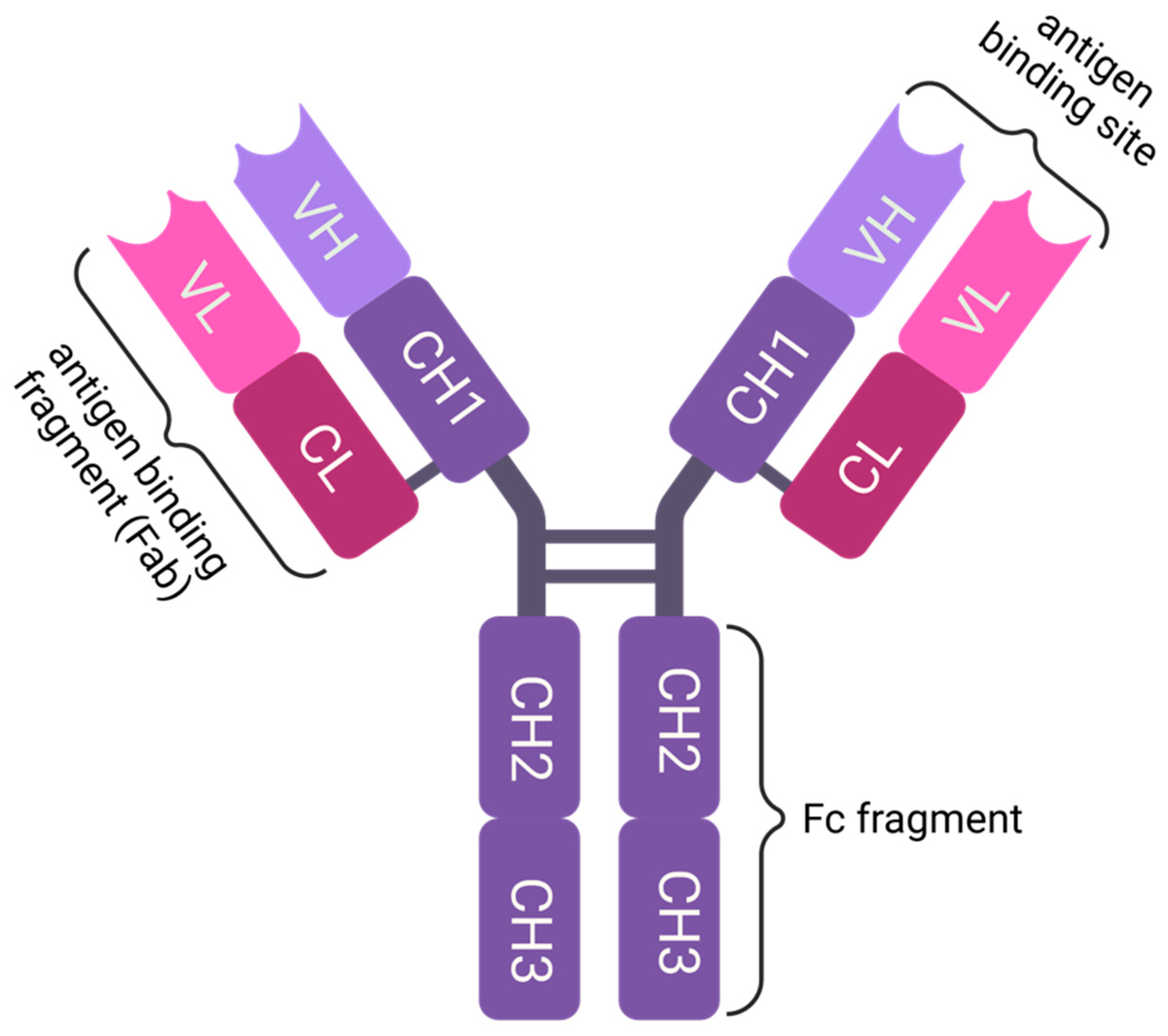
1.1. Molecular Structure of Immunoglobulin G (IgG)
1.2. Therapeutic Antibodies—How It All Started
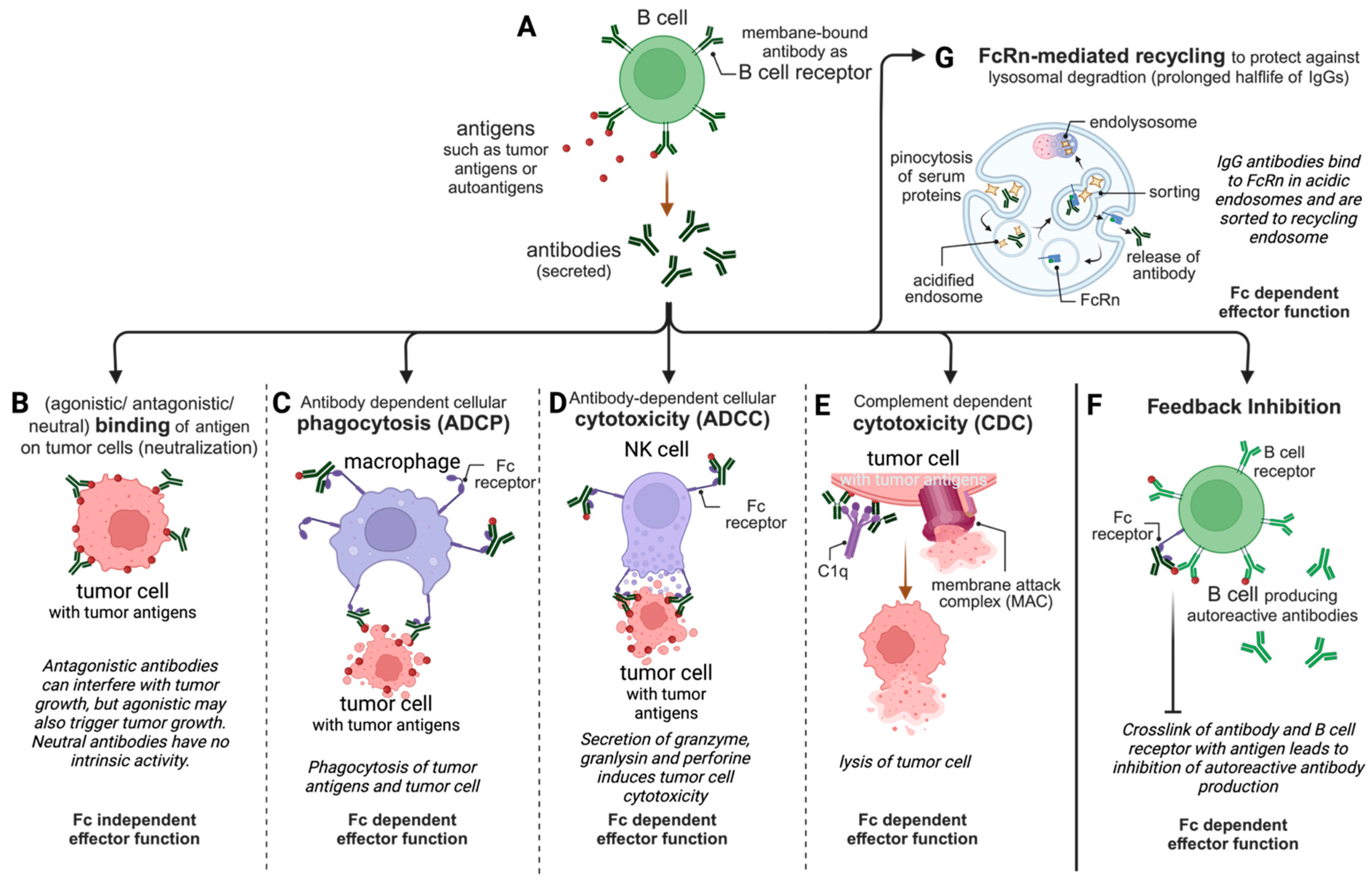
2. IgG Effector Functions—The Gunpowder of Magic Bullets
2.1. Fc-Independent Binding/Neutralization
2.2. Fc-Dependent Antibody-Dependent Cell-Mediated Phagocytosis (ADCP)
2.3. Fc-Dependent Antibody-Dependent Cell-Mediated Cytotoxicity (ADCC)
2.4. Fc-Dependent Complement-Dependent Cytotoxicity (CDC)
2.5. Fc-Dependent Inhibitory Effects
2.6. Fc-Dependent FcRn-Mediated Transport of IgGs Resulting in a Prolonged Serum Half-Life and Mucosal Immunity
3. Specific Fc-Based Mutations and Fc Glycoengineering to Improve Clinical Outcomes
3.1. The Impact of Glycosylation and Aglycosylation
3.2. Reduced Fucosylation and Afucosylation for Improved ADCC
3.3. Improving ADCC with the Introduction of Mutations
3.4. Improving CDC with the Introduction of Mutations
3.4.1. Hexabodies to Improve CDC Induction by Stabilizing IgG Hexamers
3.4.2. Other Mutations to Improve C1q Binding
3.5. Modulating Feedback Inhibition
3.5.1. Reducing FcγRIIB Affinity to Prevent Inhibitory Effects in Tumor Therapy
3.5.2. Increasing FcγRIIB Affinity for Agonistic Tumor Immune Therapy
3.5.3. Increasing FcγRIIB Affinity for the Therapy of Allergic Diseases
3.6. Reducing and Disabling the Fc-Mediated Effector Functions
4. Fc Engineering to Alter Half-Life
4.1. Modified Fc–FcRn Interactions and Their Clinical Implications
4.2. Half-Life-Extended Therapeutic Antibodies
4.3. Antibodies That Block FcRn Binding to Wash out Autoreactive Endogenous IgGs
5. Fc Engineering to Achieve Bispecific Heterodimeric IgG-Based Antibodies
5.1. Bispecific Antibody Designs for a Heterodimeric Assembly during Expression
5.2. Bispecific Antibodies with Manufacturing-Specific Steps for Heterodimerization
6. Conclusions and Outlook
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADCC | antibody-dependent cellular cytotoxicity |
| ADCP | antibody-dependent cellular phagocytosis |
| Ang-2 | angiopoietin-2 |
| ART-Ig | asymmetric reengineering technology-immunoglobulin |
| BiTE | bispecific T-cell engager |
| C1q | complement component 1q |
| CD | cluster of differentiation |
| CDC | complement-dependent cytotoxicity |
| CDR | complementarity-determining region |
| CH | constant region of heavy chain |
| CHO | Chinese hamster ovarian |
| CTLA-4 | cytotoxic T-lymphocyte-associated protein-4 |
| Fab | fragment antigen binding |
| Fc | fragment crystallizable |
| Fcγ | fragment crystallizable gamma |
| FcRn | neonatal Fc receptor |
| FcγR | Fc gamma receptor |
| FIXa | activated coagulation factor IXa |
| FUT | fucosyltransferase |
| FVIII | coagulation factor VIII |
| FX | coagulation factor X |
| FXa | activated coagulation factor X |
| GlcNAc | N-acetylglucosamine |
| GnTIII | N-acetylglucosaminyltransferase III |
| HAMA | human anti-mouse antibody |
| HER2 | human epidermal growth factor receptor-2 |
| HIV | human immunodeficiency virus |
| IgG | immunoglobulin G |
| IL | interleukin |
| ITAM | tyrosine-based activation motif |
| ITIM | immunoreceptor tyrosine-based inhibitory motif |
| IVIg | intravenous immunoglobulin G |
| κ | kappa light chain |
| kDa | kilodalton (a unit of molecular weight) |
| KiH | “knob-into-holes” design |
| mAbs | monoclonal antibodies |
| MAC | membrane attack complex |
| MHC | major histocompatibility complex 4 |
| NET | neutrophil extracellular trap |
| NK cells | natural killer cells |
| PD-1 | programmed cell death protein-1 |
| PD-L1 | programmed cell death ligand 1 |
| scFv | single-chain variable fragment |
| sdAb | single-domain antibody |
| SEED | strand-exchange engineered domain |
| TNFα | tumor necrosis factor-alpha |
| VEGF | vascular endothelial growth factor |
| VH | variable region of heavy chain |
References
- Lu, R.M.; Hwang, Y.C.; Liu, I.J.; Lee, C.C.; Tsai, H.Z.; Li, H.J.; Wu, H.C. Development of therapeutic antibodies for the treatment of diseases. J. Biomed. Sci. 2020, 27, 1. [Google Scholar] [CrossRef] [PubMed]
- Kaunitz, J.D. Development of Monoclonal Antibodies: The Dawn of mAb Rule. Dig. Dis. Sci. 2017, 62, 831–832. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.K. The history of monoclonal antibody development-Progress, remaining challenges and future innovations. Ann. Med. Surg. 2014, 3, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.; Weaver, C.; Berg, L.; Barton, G. Janeway’s Immunobiology, 10th ed.; W.W. Norton & Company: New York, NY, USA, 2022. [Google Scholar]
- Milling, S. Using monoclonal antibodies to investigate molecular immunology: There’s more to know! Immunology 2019, 157, 281–282. [Google Scholar] [CrossRef] [PubMed]
- Vidarsson, G.; Dekkers, G.; Rispens, T. IgG subclasses and allotypes: From structure to effector functions. Front. Immunol. 2014, 5, 520. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Stacey, H.D.; D’Agostino, M.R.; Tugg, Y.; Marzok, A.; Miller, M.S. Beyond neutralization: Fc-dependent antibody effector functions in SARS-CoV-2 infection. Nat. Rev. Immunol. 2023, 23, 381–396. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, S.H. Remembering Emil von Behring: From Tetanus Treatment to Antibody Cooperation with Phagocytes. mBio 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Tabll, A.; Abbas, A.T.; El-Kafrawy, S.; Wahid, A. Monoclonal antibodies: Principles and applications of immmunodiagnosis and immunotherapy for hepatitis C virus. World J. Hepatol. 2015, 7, 2369–2383. [Google Scholar] [CrossRef]
- Knop, S.; Hebart, H.; Gscheidle, H.; Holler, E.; Kolb, H.J.; Niederwieser, D.; Einsele, H. OKT3 muromonab as second-line and subsequent treatment in recipients of stem cell allografts with steroid-resistant acute graft-versus-host disease. Bone Marrow Transpl. 2005, 36, 831–837. [Google Scholar] [CrossRef]
- Salles, G.; Barrett, M.; Foa, R.; Maurer, J.; O’Brien, S.; Valente, N.; Wenger, M.; Maloney, D.G. Rituximab in B-Cell Hematologic Malignancies: A Review of 20 Years of Clinical Experience. Adv. Ther. 2017, 34, 2232–2273. [Google Scholar] [CrossRef]
- Tangri, S.; Mothe, B.R.; Eisenbraun, J.; Sidney, J.; Southwood, S.; Briggs, K.; Zinckgraf, J.; Bilsel, P.; Newman, M.; Chesnut, R.; et al. Rationally engineered therapeutic proteins with reduced immunogenicity. J. Immunol. 2005, 174, 3187–3196. [Google Scholar] [CrossRef] [PubMed]
- Clark, M. Antibody humanization: A case of the ‘Emperor’s new clothes’? Immunol. Today 2000, 21, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Maadi, H.; Soheilifar, M.H.; Choi, W.S.; Moshtaghian, A.; Wang, Z. Trastuzumab Mechanism of Action; 20 Years of Research to Unravel a Dilemma. Cancers 2021, 13, 3540. [Google Scholar] [CrossRef]
- Townsend, C.M.; Nguyen, T.M.; Cepek, J.; Abbass, M.; Parker, C.E.; MacDonald, J.K.; Khanna, R.; Jairath, V.; Feagan, B.G. Adalimumab for maintenance of remission in Crohn’s disease. Cochrane Database Syst. Rev. 2020, 5, CD012877. [Google Scholar] [CrossRef] [PubMed]
- Frenzel, A.; Schirrmann, T.; Hust, M. Phage display-derived human antibodies in clinical development and therapy. MAbs 2016, 8, 1177–1194. [Google Scholar] [CrossRef] [PubMed]
- Brüggemann, M.; Taussig, M.J. Production of human antibody repertoires in transgenic mice. Curr. Opin. Biotechnol. 1997, 8, 455–458. [Google Scholar] [CrossRef] [PubMed]
- Pelaia, C.; Calabrese, C.; Terracciano, R.; de Blasio, F.; Vatrella, A.; Pelaia, G. Omalizumab, the first available antibody for biological treatment of severe asthma: More than a decade of real-life effectiveness. Ther. Adv. Respir. Dis. 2018, 12, 1753466618810192. [Google Scholar] [CrossRef] [PubMed]
- Majumder, J.; Minko, T. Recent Developments on Therapeutic and Diagnostic Approaches for COVID-19. AAPS J. 2021, 23, 14. [Google Scholar] [CrossRef]
- Bruhns, P.; Jonsson, F. Mouse and human FcR effector functions. Immunol. Rev. 2015, 268, 25–51. [Google Scholar] [CrossRef]
- Li, X.; Ptacek, T.S.; Brown, E.E.; Edberg, J.C. Fcgamma receptors: Structure, function and role as genetic risk factors in SLE. Genes Immun. 2009, 10, 380–389. [Google Scholar] [CrossRef]
- Nimmerjahn, F.; Ravetch, J.V. Fcgamma receptors as regulators of immune responses. Nat. Rev. Immunol. 2008, 8, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Antibody Society. Antibody Therapeutics Approved or in Regulatory Review in the EU or US. Available online: https://www.antibodysociety.org/resources/approved-antibodies/ (accessed on 7 August 2023).
- Jhajj, H.S.; Lwo, T.S.; Yao, E.L.; Tessier, P.M. Unlocking the potential of agonist antibodies for treating cancer using antibody engineering. Trends Mol. Med. 2023, 29, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Attarwala, H. TGN1412: From Discovery to Disaster. J. Young Pharm. 2010, 2, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.L.; Suscovich, T.J.; Fortune, S.M.; Alter, G. Beyond binding: Antibody effector functions in infectious diseases. Nat. Rev. Immunol. 2018, 18, 46–61. [Google Scholar] [CrossRef] [PubMed]
- Florek, K.; Mutschler, J.; McLean, H.Q.; King, J.P.; Flannery, B.; Belongia, E.A.; Friedrich, T.C. Antibody-dependent cell-mediated cytotoxicity antibody responses to inactivated and live-attenuated influenza vaccination in children during 2014–15. Vaccine 2020, 38, 2088–2094. [Google Scholar] [CrossRef] [PubMed]
- Bergtold, A.; Desai, D.D.; Gavhane, A.; Clynes, R. Cell surface recycling of internalized antigen permits dendritic cell priming of B cells. Immunity 2005, 23, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Mellor, J.D.; Brown, M.P.; Irving, H.R.; Zalcberg, J.R.; Dobrovic, A. A critical review of the role of Fc gamma receptor polymorphisms in the response to monoclonal antibodies in cancer. J. Hematol. Oncol. 2013, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Gul, N.; van Egmond, M. Antibody-Dependent Phagocytosis of Tumor Cells by Macrophages: A Potent Effector Mechanism of Monoclonal Antibody Therapy of Cancer. Cancer Res. 2015, 75, 5008–5013. [Google Scholar] [CrossRef]
- Zohar, T.; Loos, C.; Fischinger, S.; Atyeo, C.; Wang, C.; Slein, M.D.; Burke, J.; Yu, J.; Feldman, J.; Hauser, B.M.; et al. Compromised Humoral Functional Evolution Tracks with SARS-CoV-2 Mortality. Cell 2020, 183, 1508–1519.e1512. [Google Scholar] [CrossRef]
- Bournazos, S.; Wang, T.T.; Dahan, R.; Maamary, J.; Ravetch, J.V. Signaling by Antibodies: Recent Progress. Annu. Rev. Immunol. 2017, 35, 285–311. [Google Scholar] [CrossRef]
- Diniz, F.; Coelho, P.; Duarte, H.O.; Sarmento, B.; Reis, C.A.; Gomes, J. Glycans as Targets for Drug Delivery in Cancer. Cancers 2022, 14, 911. [Google Scholar] [CrossRef] [PubMed]
- Boune, S.; Hu, P.; Epstein, A.L.; Khawli, L.A. Principles of N-Linked Glycosylation Variations of IgG-Based Therapeutics: Pharmacokinetic and Functional Considerations. Antibodies 2020, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Aloulou, M.; Ben Mkaddem, S.; Biarnes-Pelicot, M.; Boussetta, T.; Souchet, H.; Rossato, E.; Benhamou, M.; Crestani, B.; Zhu, Z.; Blank, U.; et al. IgG1 and IVIg induce inhibitory ITAM signaling through FcgammaRIII controlling inflammatory responses. Blood 2012, 119, 3084–3096. [Google Scholar] [CrossRef] [PubMed]
- Anania, J.C.; Chenoweth, A.M.; Wines, B.D.; Hogarth, P.M. The Human FcgammaRII (CD32) Family of Leukocyte FcR in Health and Disease. Front. Immunol. 2019, 10, 464. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, J. Current status and future directions of cancer immunotherapy. J. Cancer 2018, 9, 1773–1781. [Google Scholar] [CrossRef] [PubMed]
- Diebolder, C.A.; Beurskens, F.J.; de Jong, R.N.; Koning, R.I.; Strumane, K.; Lindorfer, M.A.; Voorhorst, M.; Ugurlar, D.; Rosati, S.; Heck, A.J.; et al. Complement is activated by IgG hexamers assembled at the cell surface. Science 2014, 343, 1260–1263. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yang, C.; Jin, X.; Du, Q.; Wu, H.; Dall’Acqua, W.; Mazor, Y. Regulation of antibody-mediated complement-dependent cytotoxicity by modulating the intrinsic affinity and binding valency of IgG for target antigen. MAbs 2020, 12, 1690959. [Google Scholar] [CrossRef] [PubMed]
- Reis, E.S.; Mastellos, D.C.; Ricklin, D.; Mantovani, A.; Lambris, J.D. Complement in cancer: Untangling an intricate relationship. Nat. Rev. Immunol. 2018, 18, 5–18. [Google Scholar] [CrossRef]
- Wang, J.; Li, Z.; Xu, L.; Yang, H.; Liu, W. Transmembrane domain dependent inhibitory function of FcgammaRIIB. Protein. Cell 2018, 9, 1004–1012. [Google Scholar] [CrossRef]
- Smith, K.G.; Clatworthy, M.R. FcgammaRIIB in autoimmunity and infection: Evolutionary and therapeutic implications. Nat. Rev. Immunol. 2010, 10, 328–343. [Google Scholar] [CrossRef]
- Lim, S.H.; Vaughan, A.T.; Ashton-Key, M.; Williams, E.L.; Dixon, S.V.; Chan, H.T.; Beers, S.A.; French, R.R.; Cox, K.L.; Davies, A.J.; et al. Fc gamma receptor IIb on target B cells promotes rituximab internalization and reduces clinical efficacy. Blood 2011, 118, 2530–2540. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Oldham, R.J.; Teal, E.; Beers, S.A.; Cragg, M.S. Fc-Engineering for Modulated Effector Functions-Improving Antibodies for Cancer Treatment. Antibodies 2020, 9, 64. [Google Scholar] [CrossRef]
- Gable, K.L.; Guptill, J.T. Antagonism of the Neonatal Fc Receptor as an Emerging Treatment for Myasthenia Gravis. Front. Immunol. 2019, 10, 3052. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Krippendorff, B.F.; Shah, D.K. Influence of Molecular size on the clearance of antibody fragments. Pharm. Res. 2017, 34, 2131–2141. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, M.; Bonagura, V.R.; Morrison, S.L.; Bjorkman, P.J. Analysis of the pH dependence of the neonatal Fc receptor/immunoglobulin G interaction using antibody and receptor variants. Biochemistry 1995, 34, 14649–14657. [Google Scholar] [CrossRef] [PubMed]
- Martin, W.L.; West, A.P., Jr.; Gan, L.; Bjorkman, P.J. Crystal structure at 2.8 A of an FcRn/heterodimeric Fc complex: Mechanism of pH-dependent binding. Mol. Cell 2001, 7, 867–877. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.J. Pharmacokinetic models for FcRn-mediated IgG disposition. J. Biomed. Biotechnol. 2012, 2012, 282989. [Google Scholar] [CrossRef] [PubMed]
- Arnold, J.N.; Wormald, M.R.; Sim, R.B.; Rudd, P.M.; Dwek, R.A. The impact of glycosylation on the biological function and structure of human immunoglobulins. Annu. Rev. Immunol. 2007, 25, 21–50. [Google Scholar] [CrossRef]
- Liu, L. Antibody glycosylation and its impact on the pharmacokinetics and pharmacodynamics of monoclonal antibodies and Fc-fusion proteins. J. Pharm. Sci. 2015, 104, 1866–1884. [Google Scholar] [CrossRef]
- Lund, J.; Tanaka, T.; Takahashi, N.; Sarmay, G.; Arata, Y.; Jefferis, R. A protein structural change in aglycosylated IgG3 correlates with loss of huFc gamma R1 and huFc gamma R111 binding and/or activation. Mol. Immunol. 1990, 27, 1145–1153. [Google Scholar] [CrossRef]
- Tao, M.H.; Morrison, S.L. Studies of aglycosylated chimeric mouse-human IgG. Role of carbohydrate in the structure and effector functions mediated by the human IgG constant region. J. Immunol. 1989, 143, 2595–2601. [Google Scholar] [CrossRef] [PubMed]
- Dashivets, T.; Thomann, M.; Rueger, P.; Knaupp, A.; Buchner, J.; Schlothauer, T. Multi-Angle Effector Function Analysis of Human Monoclonal IgG Glycovariants. PLoS ONE 2015, 10, e0143520. [Google Scholar] [CrossRef] [PubMed]
- Borrok, M.J.; Jung, S.T.; Kang, T.H.; Monzingo, A.F.; Georgiou, G. Revisiting the role of glycosylation in the structure of human IgG Fc. ACS Chem. Biol. 2012, 7, 1596–1602. [Google Scholar] [CrossRef] [PubMed]
- Davies, A.M.; Jefferis, R.; Sutton, B.J. Crystal structure of deglycosylated human IgG4-Fc. Mol. Immunol. 2014, 62, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Subedi, G.P.; Barb, A.W. The Structural Role of Antibody N-Glycosylation in Receptor Interactions. Structure 2015, 23, 1573–1583. [Google Scholar] [CrossRef] [PubMed]
- Saunders, K.O. Conceptual Approaches to Modulating Antibody Effector Functions and Circulation Half-Life. Front. Immunol. 2019, 10, 1296. [Google Scholar] [CrossRef] [PubMed]
- Liu, L. Pharmacokinetics of monoclonal antibodies and Fc-fusion proteins. Protein Cell 2018, 9, 15–32. [Google Scholar] [CrossRef]
- Li, M.; Zhao, R.; Chen, J.; Tian, W.; Xia, C.; Liu, X.; Li, Y.; Li, S.; Sun, H.; Shen, T.; et al. Next generation of anti-PD-L1 Atezolizumab with enhanced anti-tumor efficacy in vivo. Sci. Rep. 2021, 11, 5774. [Google Scholar] [CrossRef]
- Pucic, M.; Knezevic, A.; Vidic, J.; Adamczyk, B.; Novokmet, M.; Polasek, O.; Gornik, O.; Supraha-Goreta, S.; Wormald, M.R.; Redzic, I.; et al. High throughput isolation and glycosylation analysis of IgG-variability and heritability of the IgG glycome in three isolated human populations. Mol. Cell Proteom. 2011, 10, M111.010090. [Google Scholar] [CrossRef]
- Ferrara, C.; Stuart, F.; Sondermann, P.; Brunker, P.; Umana, P. The carbohydrate at FcgammaRIIIa Asn-162. An element required for high affinity binding to non-fucosylated IgG glycoforms. J. Biol. Chem. 2006, 281, 5032–5036. [Google Scholar] [CrossRef]
- Jefferis, R. Glycosylation of antibody therapeutics: Optimisation for purpose. Methods Mol. Biol. 2009, 483, 223–238. [Google Scholar] [CrossRef] [PubMed]
- Umana, P.; Jean-Mairet, J.; Moudry, R.; Amstutz, H.; Bailey, J.E. Engineered glycoforms of an antineuroblastoma IgG1 with optimized antibody-dependent cellular cytotoxic activity. Nat. Biotechnol. 1999, 17, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Kellner, C.; Otte, A.; Cappuzzello, E.; Klausz, K.; Peipp, M. Modulating Cytotoxic Effector Functions by Fc Engineering to Improve Cancer Therapy. Transfus. Med. Hemother. 2017, 44, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, T. Engineered therapeutic antibodies with enhanced effector functions: Clinical application of the Potelligent(R) Technology. Korean J. Hematol. 2011, 46, 148–150. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lifely, M.R.; Hale, C.; Boyce, S.; Keen, M.J.; Phillips, J. Glycosylation and biological activity of CAMPATH-1H expressed in different cell lines and grown under different culture conditions. Glycobiology 1995, 5, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Dekkers, G.; Treffers, L.; Plomp, R.; Bentlage, A.E.H.; de Boer, M.; Koeleman, C.A.M.; Lissenberg-Thunnissen, S.N.; Visser, R.; Brouwer, M.; Mok, J.Y.; et al. Decoding the Human Immunoglobulin G-Glycan Repertoire Reveals a Spectrum of Fc-Receptor- and Complement-Mediated-Effector Activities. Front. Immunol. 2017, 8, 877. [Google Scholar] [CrossRef]
- Lippold, S.; Nicolardi, S.; Dominguez-Vega, E.; Heidenreich, A.K.; Vidarsson, G.; Reusch, D.; Haberger, M.; Wuhrer, M.; Falck, D. Glycoform-resolved FcɣRIIIa affinity chromatography-mass spectrometry. MAbs 2019, 11, 1191–1196. [Google Scholar] [CrossRef]
- Peschke, B.; Keller, C.W.; Weber, P.; Quast, I.; Lunemann, J.D. Fc-Galactosylation of Human Immunoglobulin Gamma Isotypes Improves C1q Binding and Enhances Complement-Dependent Cytotoxicity. Front. Immunol. 2017, 8, 646. [Google Scholar] [CrossRef]
- Vattepu, R.; Sneed, S.L.; Anthony, R.M. Sialylation as an Important Regulator of Antibody Function. Front. Immunol. 2022, 13, 818736. [Google Scholar] [CrossRef]
- Shields, R.L.; Namenuk, A.K.; Hong, K.; Meng, Y.G.; Rae, J.; Briggs, J.; Xie, D.; Lai, J.; Stadlen, A.; Li, B.; et al. High resolution mapping of the binding site on human IgG1 for Fc gamma RI, Fc gamma RII, Fc gamma RIII, and FcRn and design of IgG1 variants with improved binding to the Fc gamma R. J. Biol. Chem. 2001, 276, 6591–6604. [Google Scholar] [CrossRef]
- Lazar, G.A.; Dang, W.; Karki, S.; Vafa, O.; Peng, J.S.; Hyun, L.; Chan, C.; Chung, H.S.; Eivazi, A.; Yoder, S.C.; et al. Engineered antibody Fc variants with enhanced effector function. Proc. Natl. Acad. Sci. USA 2006, 103, 4005–4010. [Google Scholar] [CrossRef] [PubMed]
- Nedved, A.; Maddocks, K.; Nowakowski, G.S. Clinical Treatment Guidelines for Tafasitamab Plus Lenalidomide in Patients with Relapsed or Refractory Diffuse Large B-Cell Lymphoma. Oncologist 2023, 28, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Danilov, A.V.; Spurgeon, S.E.; Siddiqi, T.; Quinson, A.M.; Maier, D.; Smith, D.; Brown, J.R. A phase Ib, open label, dose escalation trial of the anti-CD37 monoclonal antibody, BI 836826, in combination with ibrutinib in patients with relapsed/refractory chronic lymphocytic leukemia. Investig. New Drugs 2021, 39, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.A.; Keremane, S.R.; Vielmetter, J.; Bjorkman, P.J. Structural characterization of GASDALIE Fc bound to the activating Fc receptor FcgammaRIIIa. J. Struct. Biol. 2016, 194, 78–89. [Google Scholar] [CrossRef] [PubMed]
- DiLillo, D.J.; Ravetch, J.V. Differential Fc-Receptor Engagement Drives an Anti-tumor Vaccinal Effect. Cell 2015, 161, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Subudhi, S.K.; Blando, J.; Vence, L.; Wargo, J.; Allison, J.P.; Ribas, A.; Sharma, P. Anti-CTLA-4 Immunotherapy Does Not Deplete FOXP3(+) Regulatory T Cells (Tregs) in Human Cancers-Response. Clin. Cancer Res. 2019, 25, 3469–3470. [Google Scholar] [CrossRef] [PubMed]
- O’Malley, D.M.; Neffa, M.; Monk, B.J.; Melkadze, T.; Huang, M.; Kryzhanivska, A.; Bulat, I.; Meniawy, T.M.; Bagameri, A.; Wang, E.W.; et al. Dual PD-1 and CTLA-4 Checkpoint Blockade Using Balstilimab and Zalifrelimab Combination as Second-Line Treatment for Advanced Cervical Cancer: An Open-Label Phase II Study. J. Clin. Oncol. 2022, 40, 762–771. [Google Scholar] [CrossRef]
- Moore, G.L.; Chen, H.; Karki, S.; Lazar, G.A. Engineered Fc variant antibodies with enhanced ability to recruit complement and mediate effector functions. MAbs 2010, 2, 181–189. [Google Scholar] [CrossRef]
- de Jong, R.N.; Beurskens, F.J.; Verploegen, S.; Strumane, K.; van Kampen, M.D.; Voorhorst, M.; Horstman, W.; Engelberts, P.J.; Oostindie, S.C.; Wang, G.; et al. A Novel Platform for the Potentiation of Therapeutic Antibodies Based on Antigen-Dependent Formation of IgG Hexamers at the Cell Surface. PLoS Biol. 2016, 14, e1002344. [Google Scholar] [CrossRef]
- Genmab. Antibody Technology Plattforms: Hexabody. Available online: https://www.genmab.com/research-innovation/antibody-technology-platforms/ (accessed on 8 August 2023).
- van der Horst, H.J.; Gelderloos, A.T.; Chamuleau, M.E.D.; Breij, E.C.W.; Zweegman, S.; Nijhof, I.S.; Overdijk, M.B.; Mutis, T. Potent preclinical activity of HexaBody-DR5/DR5 in relapsed and/or refractory multiple myeloma. Blood Adv. 2021, 5, 2165–2172. [Google Scholar] [CrossRef]
- Idusogie, E.E.; Wong, P.Y.; Presta, L.G.; Gazzano-Santoro, H.; Totpal, K.; Ultsch, M.; Mulkerrin, M.G. Engineered antibodies with increased activity to recruit complement. J. Immunol. 2001, 166, 2571–2575. [Google Scholar] [CrossRef] [PubMed]
- Natsume, A.; In, M.; Takamura, H.; Nakagawa, T.; Shimizu, Y.; Kitajima, K.; Wakitani, M.; Ohta, S.; Satoh, M.; Shitara, K.; et al. Engineered antibodies of IgG1/IgG3 mixed isotype with enhanced cytotoxic activities. Cancer Res. 2008, 68, 3863–3872. [Google Scholar] [CrossRef]
- Nur Husna, S.M.; Wong, K.K. Margetuximab and trastuzumab deruxtecan: New generation of anti-HER2 immunotherapeutic agents for breast cancer. Mol. Immunol. 2022, 152, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Alasmari, M.M. A Review of Margetuximab-Based Therapies in Patients with HER2-Positive Metastatic Breast Cancer. Cancers 2022, 15, 38. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Goldberg, M.V.; Chiu, M.L. Fc Engineering Approaches to Enhance the Agonism and Effector Functions of an Anti-OX40 Antibody. J. Biol. Chem. 2016, 291, 27134–27146. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Li, F.; Wu, Y.; Cheng, C.; Han, P.; Wang, J.; Yang, X. Optimization of 4-1BB antibody for cancer immunotherapy by balancing agonistic strength with FcgammaR affinity. Nat. Commun. 2019, 10, 2141. [Google Scholar] [CrossRef] [PubMed]
- Daud, A.; Albany, C.; Velcheti, V.; Hauke, R.J.; Ahnert, J.R.; Karp, D.D.; Tsimberidou, A.M.; Cohen, J.W.; Schmidt, E.V.; Wang, J.; et al. First-in-human, phase 1a dose finding of LVGN6051 CD137/4-1BB agonistic antibody with or without pembrolizumab in patients with advanced solid tumors. J. Clin. Oncol. 2023, 41, 2525. [Google Scholar] [CrossRef]
- Fu, S.; Harb, W.A.; Patel, S.P.; Lu, C.; Halperin, D.M.; Hsu, Y.H.; Shi, N.; Yamamura, Y.; Tang, T.; Jiang, L.; et al. Early safety and efficacy from a phase I open-label clinical trial of CD137(4-1BB) agonistic antibody LVGN6051 as monotherapy and in combination with pembrolizumab. J. Clin. Oncol. 2021, 39, 2521. [Google Scholar] [CrossRef]
- Claus, C.; Ferrara-Koller, C.; Klein, C. The emerging landscape of novel 4-1BB (CD137) agonistic drugs for cancer immunotherapy. MAbs 2023, 15, 2167189. [Google Scholar] [CrossRef]
- Chu, S.Y.; Vostiar, I.; Karki, S.; Moore, G.L.; Lazar, G.A.; Pong, E.; Joyce, P.F.; Szymkowski, D.E.; Desjarlais, J.R. Inhibition of B cell receptor-mediated activation of primary human B cells by coengagement of CD19 and FcgammaRIIb with Fc-engineered antibodies. Mol. Immunol. 2008, 45, 3926–3933. [Google Scholar] [CrossRef]
- Guntern, P.; Eggel, A. Past, present, and future of anti-IgE biologics. Allergy 2020, 75, 2491–2502. [Google Scholar] [CrossRef] [PubMed]
- Pharmaceutical Business Review. Aimmune Secures Licence to Xencor’s XmA 7195 to Develop Food Allergy Treatments. Available online: https://pharmaceutical-business-review.com/news/aimmune-xencor-food-allergy/ (accessed on 10 August 2023).
- Xu, D.; Alegre, M.L.; Varga, S.S.; Rothermel, A.L.; Collins, A.M.; Pulito, V.L.; Hanna, L.S.; Dolan, K.P.; Parren, P.W.; Bluestone, J.A.; et al. In vitro characterization of five humanized OKT3 effector function variant antibodies. Cell Immunol. 2000, 200, 16–26. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Spevigo EPAR Product Information-Annex. Available online: https://www.ema.europa.eu/en/documents/product-information/spevigo-epar-product-information_en.pdf (accessed on 8 August 2023).
- Herold, K.C.; Bundy, B.N.; Long, S.A.; Bluestone, J.A.; DiMeglio, L.A.; Dufort, M.J.; Gitelman, S.E.; Gottlieb, P.A.; Krischer, J.P.; Linsley, P.S.; et al. An Anti-CD3 Antibody, Teplizumab, in Relatives at Risk for Type 1 Diabetes. N. Engl. J. Med. 2019, 381, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Schlothauer, T.; Herter, S.; Koller, C.F.; Grau-Richards, S.; Steinhart, V.; Spick, C.; Kubbies, M.; Klein, C.; Umana, P.; Mossner, E. Novel human IgG1 and IgG4 Fc-engineered antibodies with completely abolished immune effector functions. Protein Eng. Des. Sel. 2016, 29, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Kaplon, H.; Crescioli, S.; Chenoweth, A.; Visweswaraiah, J.; Reichert, J.M. Antibodies to watch in 2023. MAbs 2023, 15, 2153410. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Imfinzi EPAR Product Information-Annex. Available online: https://www.ema.europa.eu/en/documents/product-information/imfinzi-epar-product-information_en.pdf (accessed on 8 August 2023).
- Xencor. A Deep Pipeline of Xmab Antibody Drug Candidates. Available online: https://xencor.com/pipeline/ (accessed on 10 August 2023).
- Challa, D.K.; Velmurugan, R.; Ober, R.J.; Sally Ward, E. FcRn: From molecular interactions to regulation of IgG pharmacokinetics and functions. Curr. Top. Microbiol. Immunol. 2014, 382, 249–272. [Google Scholar] [CrossRef] [PubMed]
- Pyzik, M.; Sand, K.M.K.; Hubbard, J.J.; Andersen, J.T.; Sandlie, I.; Blumberg, R.S. The Neonatal Fc Receptor (FcRn): A Misnomer? Front. Immunol. 2019, 10, 1540. [Google Scholar] [CrossRef]
- Saxena, A.; Wu, D. Advances in Therapeutic Fc Engineering-Modulation of IgG-Associated Effector Functions and Serum Half-life. Front. Immunol. 2016, 7, 580. [Google Scholar] [CrossRef]
- Ramdani, Y.; Lamamy, J.; Watier, H.; Gouilleux-Gruart, V. Monoclonal Antibody Engineering and Design to Modulate FcRn Activities: A Comprehensive Review. Int. J. Mol. Sci. 2022, 23, 9604. [Google Scholar] [CrossRef]
- Dall’Acqua, W.F.; Kiener, P.A.; Wu, H. Properties of human IgG1s engineered for enhanced binding to the neonatal Fc receptor (FcRn). J. Biol. Chem. 2006, 281, 23514–23524. [Google Scholar] [CrossRef]
- Yu, X.Q.; Robbie, G.J.; Wu, Y.; Esser, M.T.; Jensen, K.; Schwartz, H.I.; Bellamy, T.; Hernandez-Illas, M.; Jafri, H.S. Safety, Tolerability, and Pharmacokinetics of MEDI4893, an Investigational, Extended-Half-Life, Anti-Staphylococcus aureus Alpha-Toxin Human Monoclonal Antibody, in Healthy Adults. Antimicrob. Agents Chemother. 2017, 61, e01020-16. [Google Scholar] [CrossRef] [PubMed]
- Robbie, G.J.; Criste, R.; Dall’acqua, W.F.; Jensen, K.; Patel, N.K.; Losonsky, G.A.; Griffin, M.P. A novel investigational Fc-modified humanized monoclonal antibody, motavizumab-YTE, has an extended half-life in healthy adults. Antimicrob. Agents Chemother. 2013, 57, 6147–6153. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Beyfortus EPAR Product Information-Annex. Available online: https://www.ema.europa.eu/en/documents/product-information/beyfortus-epar-product-information_en.pdf (accessed on 8 August 2023).
- Levin, M.J.; Ustianowski, A.; De Wit, S.; Launay, O.; Avila, M.; Templeton, A.; Yuan, Y.; Seegobin, S.; Ellery, A.; Levinson, D.J.; et al. Intramuscular AZD7442 (Tixagevimab-Cilgavimab) for Prevention of COVID-19. N. Engl. J. Med. 2022, 386, 2188–2200. [Google Scholar] [CrossRef] [PubMed]
- Lomakin, N.V.; Bakirov, B.A.; Protsenko, D.N.; Mazurov, V.I.; Musaev, G.H.; Moiseeva, O.M.; Pasechnik, E.S.; Popov, V.V.; Smolyarchuk, E.A.; Gordeev, I.G.; et al. The efficacy and safety of levilimab in severely ill COVID-19 patients not requiring mechanical ventilation: Results of a multicenter randomized double-blind placebo-controlled phase III CORONA clinical study. Inflamm. Res. 2021, 70, 1233–1246. [Google Scholar] [CrossRef] [PubMed]
- Puig, L.; Bakulev, A.L.; Kokhan, M.M.; Samtsov, A.V.; Khairutdinov, V.R.; Morozova, M.A.; Zolkin, N.A.; Kuryshev, I.V.; Petrov, A.N.; Artemeva, A.V.; et al. Efficacy and Safety of Netakimab, A Novel Anti-IL-17 Monoclonal Antibody, in Patients with Moderate to Severe Plaque Psoriasis. Results of A 54-Week Randomized Double-Blind Placebo-Controlled PLANETA Clinical Trial. Dermatol. Ther. 2021, 11, 1319–1332. [Google Scholar] [CrossRef] [PubMed]
- Kostareva, O.; Kolyadenko, I.; Ulitin, A.; Ekimova, V.; Evdokimov, S.; Garber, M.; Tishchenko, S.; Gabdulkhakov, A. Fab Fragment of VHH-Based Antibody Netakimab: Crystal Structure and Modeling Interaction with Cytokine IL-17A. Crystals 2019, 9, 177. [Google Scholar] [CrossRef]
- Zalevsky, J.; Chamberlain, A.K.; Horton, H.M.; Karki, S.; Leung, I.W.; Sproule, T.J.; Lazar, G.A.; Roopenian, D.C.; Desjarlais, J.R. Enhanced antibody half-life improves in vivo activity. Nat. Biotechnol. 2010, 28, 157–159. [Google Scholar] [CrossRef] [PubMed]
- Monnet, C.; Jorieux, S.; Urbain, R.; Fournier, N.; Bouayadi, K.; De Romeuf, C.; Behrens, C.K.; Fontayne, A.; Mondon, P. Selection of IgG Variants with Increased FcRn Binding Using Random and Directed Mutagenesis: Impact on Effector Functions. Front. Immunol. 2015, 6, 39. [Google Scholar] [CrossRef]
- Saunders, K.O.; Pegu, A.; Georgiev, I.S.; Zeng, M.; Joyce, M.G.; Yang, Z.Y.; Ko, S.Y.; Chen, X.; Schmidt, S.D.; Haase, A.T.; et al. Sustained Delivery of a Broadly Neutralizing Antibody in Nonhuman Primates Confers Long-Term Protection against Simian/Human Immunodeficiency Virus Infection. J. Virol. 2015, 89, 5895–5903. [Google Scholar] [CrossRef]
- McKeage, K. Ravulizumab: First Global Approval. Drugs 2019, 79, 347–352. [Google Scholar] [CrossRef]
- European Medicines Agency. Xevudy EPAR Product Information-Annex. Available online: https://www.ema.europa.eu/en/documents/product-information/xevudy-epar-product-information_en.pdf (accessed on 8 August 2023).
- Hoy, S.M. Amubarvimab/Romlusevimab: First Approval. Drugs 2022, 82, 1327–1331. [Google Scholar] [CrossRef] [PubMed]
- Hsu, D.C.; Mellors, J.W.; Vasan, S. Can Broadly Neutralizing HIV-1 Antibodies Help Achieve an ART-Free Remission? Front. Immunol. 2021, 12, 710044. [Google Scholar] [CrossRef] [PubMed]
- Ison, M.G.; Popejoy, M.; Evgeniev, N.; Tzekova, M.; Mahoney, K.; Betancourt, N.; Li, Y.; Gupta, D.; Narayan, K.; Hershberger, E.; et al. Efficacy and Safety of Adintrevimab (ADG20) for the Treatment of High-Risk Ambulatory Patients With Mild or Moderate Coronavirus Disease 2019: Results From a Phase 2/3, Randomized, Placebo-Controlled Trial (STAMP) Conducted During Delta Predominance and Early Emergence of Omicron. Open Forum. Infect Dis. 2023, 10, ofad279. [Google Scholar] [CrossRef] [PubMed]
- Globe Newswire. Invivyd Announces General Alignment with FDA on Pathway to Potential EUA for VYD222 and Anticipated Follow-On Monoclonal Antibody Candidates Designed to Prevent COVID-19. Available online: https://www.globenewswire.com/news-release/2023/06/26/2694298/0/en/Invivyd-Announces-General-Alignment-with-FDA-on-Pathway-to-Potential-EUA-for-VYD222-and-Anticipated-Follow-On-Monoclonal-Antibody-Candidates-Designed-to-Prevent-COVID-19.html (accessed on 8 August 2023).
- European Medicines Agency. Vyvgart EPAR Product Information-Annex. Available online: https://www.ema.europa.eu/en/documents/product-information/vyvgart-epar-product-information_en.pdf (accessed on 8 August 2023).
- Zhu, L.N.; Hou, H.M.; Wang, S.; Zhang, S.; Wang, G.G.; Guo, Z.Y.; Wu, J. FcRn inhibitors: A novel option for the treatment of myasthenia gravis. Neural Regen Res. 2023, 18, 1637–1644. [Google Scholar] [CrossRef] [PubMed]
- Ulrichts, P.; Guglietta, A.; Dreier, T.; van Bragt, T.; Hanssens, V.; Hofman, E.; Vankerckhoven, B.; Verheesen, P.; Ongenae, N.; Lykhopiy, V.; et al. Neonatal Fc receptor antagonist efgartigimod safely and sustainably reduces IgGs in humans. J. Clin. Investig. 2018, 128, 4372–4386. [Google Scholar] [CrossRef] [PubMed]
- Bril, V.; Druzdz, A.; Grosskreutz, J.; Habib, A.A.; Mantegazza, R.; Sacconi, S.; Utsugisawa, K.; Vissing, J.; Vu, T.; Boehnlein, M.; et al. Safety and efficacy of rozanolixizumab in patients with generalised myasthenia gravis (MycarinG): A randomised, double-blind, placebo-controlled, adaptive phase 3 study. Lancet Neurol. 2023, 22, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.; Kiessling, A.; Lledo-Garcia, R.; Dixon, K.L.; Christodoulou, L.; Catley, M.C.; Atherfold, P.; D’Hooghe, L.E.; Finney, H.; Greenslade, K.; et al. Generation and characterization of a high affinity anti-human FcRn antibody, rozanolixizumab, and the effects of different molecular formats on the reduction of plasma IgG concentration. MAbs 2018, 10, 1111–1130. [Google Scholar] [CrossRef]
- UCB. UCB Announces U.S. FDA Approval of RYSTIGGO[®] (Rozanolixizumab-Noli) for the Treatment of Adults with Generalized Myasthenia Gravis. Available online: https://www.ucb.com/stories-media/Press-Releases/article/UCB-announces-US-FDA-approval-of-RYSTIGGOR-rozanolixizumab-noli-for-the-treatment-of-adults-with-generalized-myasthenia-gravis (accessed on 31 July 2023).
- Yap, D.Y.H.; Hai, J.; Lee, P.C.H.; Zhou, X.; Lee, M.; Zhang, Y.; Wang, M.; Chen, X. Safety, tolerability, pharmacokinetics, and pharmacodynamics of HBM9161, a novel FcRn inhibitor, in a phase I study for healthy Chinese volunteers. Clin. Transl. Sci. 2021, 14, 1769–1779. [Google Scholar] [CrossRef]
- Ling, L.E.; Hillson, J.L.; Tiessen, R.G.; Bosje, T.; van Iersel, M.P.; Nix, D.J.; Markowitz, L.; Cilfone, N.A.; Duffner, J.; Streisand, J.B.; et al. M281, an Anti-FcRn Antibody: Pharmacodynamics, Pharmacokinetics, and Safety Across the Full Range of IgG Reduction in a First-in-Human Study. Clin. Pharmacol. Ther. 2019, 105, 1031–1039. [Google Scholar] [CrossRef]
- Blumberg, L.J.; Humphries, J.E.; Lasseter, K.; Blumberg, R.S. SYNT001: A Humanized IgG4 Monoclonal Antibody That Disrupts the Interaction of FcRn and IgG for the Treatment of IgG-Mediated Autoimmune Diseases. Blood 2017, 130, 3483. [Google Scholar]
- Werth, V.P.; Culton, D.A.; Concha, J.S.S.; Graydon, J.S.; Blumberg, L.J.; Okawa, J.; Pyzik, M.; Blumberg, R.S.; Hall, R.P., 3rd. Safety, Tolerability, and Activity of ALXN1830 Targeting the Neonatal Fc Receptor in Chronic Pemphigus. J. Investig. Dermatol. 2021, 141, 2858–2865.e4. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Duan, R.S.; Yang, H.; Li, H.F.; Zou, Z.; Zhang, H.; Zhou, H.; Li, X.L.; Zhou, H.; Jiao, L.; et al. Therapeutic Effects of Batoclimab in Chinese Patients with Generalized Myasthenia Gravis: A Double-Blinded, Randomized, Placebo-Controlled Phase II Study. Neurol. Ther. 2022, 11, 815–834. [Google Scholar] [CrossRef] [PubMed]
- Gera, N. The evolution of bispecific antibodies. Expert Opin. Biol. Ther. 2022, 22, 945–949. [Google Scholar] [CrossRef] [PubMed]
- Rispens, T.; Ooijevaar-de Heer, P.; Bende, O.; Aalberse, R.C. Mechanism of immunoglobulin G4 Fab-arm exchange. J. Am. Chem. Soc. 2011, 133, 10302–10311. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, U.; Kontermann, R.E. The making of bispecific antibodies. MAbs 2017, 9, 182–212. [Google Scholar] [CrossRef] [PubMed]
- Edeline, J.; Houot, R.; Marabelle, A.; Alcantara, M. CAR-T cells and BiTEs in solid tumors: Challenges and perspectives. J. Hematol. Oncol. 2021, 14, 65. [Google Scholar] [CrossRef] [PubMed]
- Campos, C.R.; Kemble, A.M.; Niewoehner, J.; Freskgard, P.O.; Urich, E. Brain Shuttle Neprilysin reduces central Amyloid-beta levels. PLoS ONE 2020, 15, e0229850. [Google Scholar] [CrossRef]
- Alzforum. Trontinemab. Available online: https://www.alzforum.org/therapeutics/trontinemab (accessed on 13 August 2023).
- Rabenhold, M.; Steiniger, F.; Fahr, A.; Kontermann, R.E.; Ruger, R. Bispecific single-chain diabody-immunoliposomes targeting endoglin (CD105) and fibroblast activation protein (FAP) simultaneously. J. Control. Release 2015, 201, 56–67. [Google Scholar] [CrossRef]
- Ridgway, J.B.; Presta, L.G.; Carter, P. ‘Knobs-into-holes’ engineering of antibody CH3 domains for heavy chain heterodimerization. Protein Eng. 1996, 9, 617–621. [Google Scholar] [CrossRef]
- Atwell, S.; Ridgway, J.B.; Wells, J.A.; Carter, P. Stable heterodimers from remodeling the domain interface of a homodimer using a phage display library. J. Mol. Biol. 1997, 270, 26–35. [Google Scholar] [CrossRef]
- Klein, C.; Sustmann, C.; Thomas, M.; Stubenrauch, K.; Croasdale, R.; Schanzer, J.; Brinkmann, U.; Kettenberger, H.; Regula, J.T.; Schaefer, W. Progress in overcoming the chain association issue in bispecific heterodimeric IgG antibodies. MAbs 2012, 4, 653–663. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Columvi EPAR Product Information-Annex. Available online: https://www.ema.europa.eu/en/documents/product-information/columvi-epar-product-information_en.pdf (accessed on 8 August 2023).
- Spiess, C.; Bevers, J., 3rd; Jackman, J.; Chiang, N.; Nakamura, G.; Dillon, M.; Liu, H.; Molina, P.; Elliott, J.M.; Shatz, W.; et al. Development of a human IgG4 bispecific antibody for dual targeting of interleukin-4 (IL-4) and interleukin-13 (IL-13) cytokines. J. Biol. Chem. 2013, 288, 26583–26593. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Aziz, A.A.; Shafi, N.A.; Abbas, T.; Khanani, A.M. Targeting Angiopoietin in Retinal Vascular Diseases: A Literature Review and Summary of Clinical Trials Involving Faricimab. Cells 2020, 9, 869. [Google Scholar] [CrossRef] [PubMed]
- Regula, J.T.; Lundh von Leithner, P.; Foxton, R.; Barathi, V.A.; Cheung, C.M.; Bo Tun, S.B.; Wey, Y.S.; Iwata, D.; Dostalek, M.; Moelleken, J.; et al. Targeting key angiogenic pathways with a bispecific CrossMAb optimized for neovascular eye diseases. EMBO Mol. Med. 2016, 8, 1265–1288. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Vabysmo EPAR Product Information-Annex. Available online: https://www.ema.europa.eu/en/documents/product-information/vabysmo-epar-product-information_en.pdf (accessed on 8 August 2023).
- Ma, J.; Mo, Y.; Tang, M.; Shen, J.; Qi, Y.; Zhao, W.; Huang, Y.; Xu, Y.; Qian, C. Bispecific Antibodies: From Research to Clinical Application. Front. Immunol. 2021, 12, 626616. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Leng, E.C.; Gunasekaran, K.; Pentony, M.; Shen, M.; Howard, M.; Stoops, J.; Manchulenko, K.; Razinkov, V.; Liu, H.; et al. A novel antibody engineering strategy for making monovalent bispecific heterodimeric IgG antibodies by electrostatic steering mechanism. J. Biol. Chem. 2015, 290, 7535–7562. [Google Scholar] [CrossRef] [PubMed]
- de Vries Schultink, A.H.M.; Bol, K.; Doornbos, R.P.; Murat, A.; Wasserman, E.; Dorlo, T.P.C.; Schellens, J.H.M.; Beijnen, J.H.; Huitema, A.D.R. Population Pharmacokinetics of MCLA-128, a HER2/HER3 Bispecific Monoclonal Antibody, in Patients with Solid Tumors. Clin. Pharmacokinet. 2020, 59, 875–884. [Google Scholar] [CrossRef]
- Choi, H.J.; Seok, S.H.; Kim, Y.J.; Seo, M.D.; Kim, Y.S. Crystal structures of immunoglobulin Fc heterodimers reveal the molecular basis for heterodimer formation. Mol. Immunol. 2015, 65, 377–383. [Google Scholar] [CrossRef]
- Ha, J.H.; Kim, J.E.; Kim, Y.S. Immunoglobulin Fc Heterodimer Platform Technology: From Design to Applications in Therapeutic Antibodies and Proteins. Front. Immunol. 2016, 7, 394. [Google Scholar] [CrossRef]
- Chen, S.; Li, L.; Zhang, F.; Wang, Y.; Hu, Y.; Zhao, L. Immunoglobulin Gamma-Like Therapeutic Bispecific Antibody Formats for Tumor Therapy. J. Immunol. Res. 2019, 2019, 4516041. [Google Scholar] [CrossRef]
- Morcos, P.N.; Li, J.; Hosseini, I.; Li, C.C. Quantitative Clinical Pharmacology of T-Cell Engaging Bispecifics: Current Perspectives and Opportunities. Clin. Transl. Sci. 2021, 14, 75–85. [Google Scholar] [CrossRef]
- European Medicines Agency. Hemlibra EPAR Product Information-Annex. Available online: https://www.ema.europa.eu/en/documents/product-information/hemlibra-epar-product-information_en.pdf (accessed on 8 August 2023).
- Shiraiwa, H.; Narita, A.; Kamata-Sakurai, M.; Ishiguro, T.; Sano, Y.; Hironiwa, N.; Tsushima, T.; Segawa, H.; Tsunenari, T.; Ikeda, Y.; et al. Engineering a bispecific antibody with a common light chain: Identification and optimization of an anti-CD3 epsilon and anti-GPC3 bispecific antibody, ERY974. Methods 2019, 154, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Kitazawa, T.; Shima, M. Emicizumab, a humanized bispecific antibody to coagulation factors IXa and X with a factor VIIIa-cofactor activity. Int. J. Hematol. 2020, 111, 20–30. [Google Scholar] [CrossRef]
- Davis, J.H.; Aperlo, C.; Li, Y.; Kurosawa, E.; Lan, Y.; Lo, K.M.; Huston, J.S. SEEDbodies: Fusion proteins based on strand-exchange engineered domain (SEED) CH3 heterodimers in an Fc analogue platform for asymmetric binders or immunofusions and bispecific antibodies. Protein Eng. Des. Sel. 2010, 23, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Muda, M.; Gross, A.W.; Dawson, J.P.; He, C.; Kurosawa, E.; Schweickhardt, R.; Dugas, M.; Soloviev, M.; Bernhardt, A.; Fischer, D.; et al. Therapeutic assessment of SEED: A new engineered antibody platform designed to generate mono- and bispecific antibodies. Protein Eng. Des. Sel. 2011, 24, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Labrijn, A.F.; Meesters, J.I.; de Goeij, B.E.; van den Bremer, E.T.; Neijssen, J.; van Kampen, M.D.; Strumane, K.; Verploegen, S.; Kundu, A.; Gramer, M.J.; et al. Efficient generation of stable bispecific IgG1 by controlled Fab-arm exchange. Proc. Natl. Acad. Sci. USA 2013, 110, 5145–5150. [Google Scholar] [CrossRef]
- Thieblemont, C.; Phillips, T.; Ghesquieres, H.; Cheah, C.Y.; Clausen, M.R.; Cunningham, D.; Do, Y.R.; Feldman, T.; Gasiorowski, R.; Jurczak, W.; et al. Epcoritamab, a Novel, Subcutaneous CD3 × CD20 Bispecific T-Cell-Engaging Antibody, in Relapsed or Refractory Large B-Cell Lymphoma: Dose Expansion in a Phase I/II Trial. J. Clin. Oncol. 2023, 41, 2238–2247. [Google Scholar] [CrossRef] [PubMed]
- Lindhofer, H.; Mocikat, R.; Steipe, B.; Thierfelder, S. Preferential species-restricted heavy/light chain pairing in rat/mouse quadromas. Implications for a single-step purification of bispecific antibodies. J. Immunol. 1995, 155, 219–225. [Google Scholar] [CrossRef]
- Cottignies-Calamarte, A.; Tudor, D.; Bomsel, M. Antibody Fc-chimerism and effector functions: When IgG takes advantage of IgA. Front. Immunol. 2023, 14, 1037033. [Google Scholar] [CrossRef]
- Shang, Y.; Liu, T.; Li, J.; Kaweme, N.M.; Wang, X.; Zhou, F. Impact of Treatment Regimens on Antibody Response to the SARS-CoV-2 Coronavirus. Front. Immunol. 2021, 12, 580147. [Google Scholar] [CrossRef]
- Tabarsi, P.; Hashemian, S.M.R.; Bauhofer, A.; Savadkoohi, A.A.; Ghadimi, S.; Haseli, S.; Dastan, F. IgM-enriched immunoglobulin in COVID-19: Case series of 15 severely ill SARS-CoV-2-infected patients. Int. Immunopharmacol 2021, 99, 107998. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, H.; Sun, L. Therapeutic antibodies for COVID-19: Is a new age of IgM, IgA and bispecific antibodies coming? MAbs 2022, 14, 2031483. [Google Scholar] [CrossRef]
- Kuchnio, A.; Yang, D.; Vloemans, N.; Lowenstein, C.; Cornelissen, I.; Amorim, R.; Han, C.; Sukumaran, S.; Janssen, L.; Suls, T.; et al. Characterization of JNJ-80948543, a Novel CD79b × CD20 × CD3 Trispecific T-Cell Redirecting Antibody for the Treatment of B-Cell Non-Hodgkin Lymphoma. Blood 2022, 140, 3105–3106. [Google Scholar] [CrossRef]
- Tapia-Galisteo, A.; Alvarez-Vallina, L.; Sanz, L. Bi- and trispecific immune cell engagers for immunotherapy of hematological malignancies. J. Hematol. Oncol. 2023, 16, 83. [Google Scholar] [CrossRef] [PubMed]
- Green, M.R.; Reville, P.K.; Dai, E.; Sheikh, I.; Deng, Q.; Henderson, J.; Le, C.; Rojas, E.; Okwuchi, C.; Wilson, A.; et al. SAR442257, a CD38/CD28/CD3 trispecific antibody, potentiates CAR T-cell activity against large B-cell lymphoma. Hematol. Oncol. 2023, 41, 275–276. [Google Scholar] [CrossRef]
- Zhou, J.Y.; Oswald, D.M.; Oliva, K.D.; Kreisman, L.S.C.; Cobb, B.A. The Glycoscience of Immunity. Trends Immunol. 2018, 39, 523–535. [Google Scholar] [CrossRef] [PubMed]
- Critcher, M.; O’Leary, T.; Huang, M.L. Glycoengineering: Scratching the surface. Biochem. J. 2021, 478, 703–719. [Google Scholar] [CrossRef]
- Sushant, T.; Onkar, S. Monoclonal Antibodies Market Outlook 2030. Available online: https://www.alliedmarketresearch.com/monoclonal-antibodies-market-A11789 (accessed on 8 August 2023).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdeldaim, D.T.; Schindowski, K. Fc-Engineered Therapeutic Antibodies: Recent Advances and Future Directions. Pharmaceutics 2023, 15, 2402. https://doi.org/10.3390/pharmaceutics15102402
Abdeldaim DT, Schindowski K. Fc-Engineered Therapeutic Antibodies: Recent Advances and Future Directions. Pharmaceutics. 2023; 15(10):2402. https://doi.org/10.3390/pharmaceutics15102402
Chicago/Turabian StyleAbdeldaim, Dalia T., and Katharina Schindowski. 2023. "Fc-Engineered Therapeutic Antibodies: Recent Advances and Future Directions" Pharmaceutics 15, no. 10: 2402. https://doi.org/10.3390/pharmaceutics15102402
APA StyleAbdeldaim, D. T., & Schindowski, K. (2023). Fc-Engineered Therapeutic Antibodies: Recent Advances and Future Directions. Pharmaceutics, 15(10), 2402. https://doi.org/10.3390/pharmaceutics15102402