Valencene, Nootkatone and Their Liposomal Nanoformulations as Potential Inhibitors of NorA, Tet(K), MsrA, and MepA Efflux Pumps in Staphylococcus aureus Strains
Abstract
1. Introduction
2. Materials and Methods
2.1. Drugs and Reagents Used in the Synthesis of Liposomes
2.2. Drugs and Reagents Used in Microbiological Tests
2.3. Synthesis of Liposomes
2.4. Bacterial Strains
2.5. Antibacterial Activity Analysis
2.6. Efflux Pump Inhibition Assessment by MIC Reduction
2.7. Efflux Pump Inhibition Evaluation through EtBr Fluorescence Emission
2.8. Statistical Analysis
3. Results and Discussion
3.1. Physical-Chemical Profile of Liposomes
3.2. Assessment of the Antibacterial Activity of Sesquiterpenes and Nanoformulations
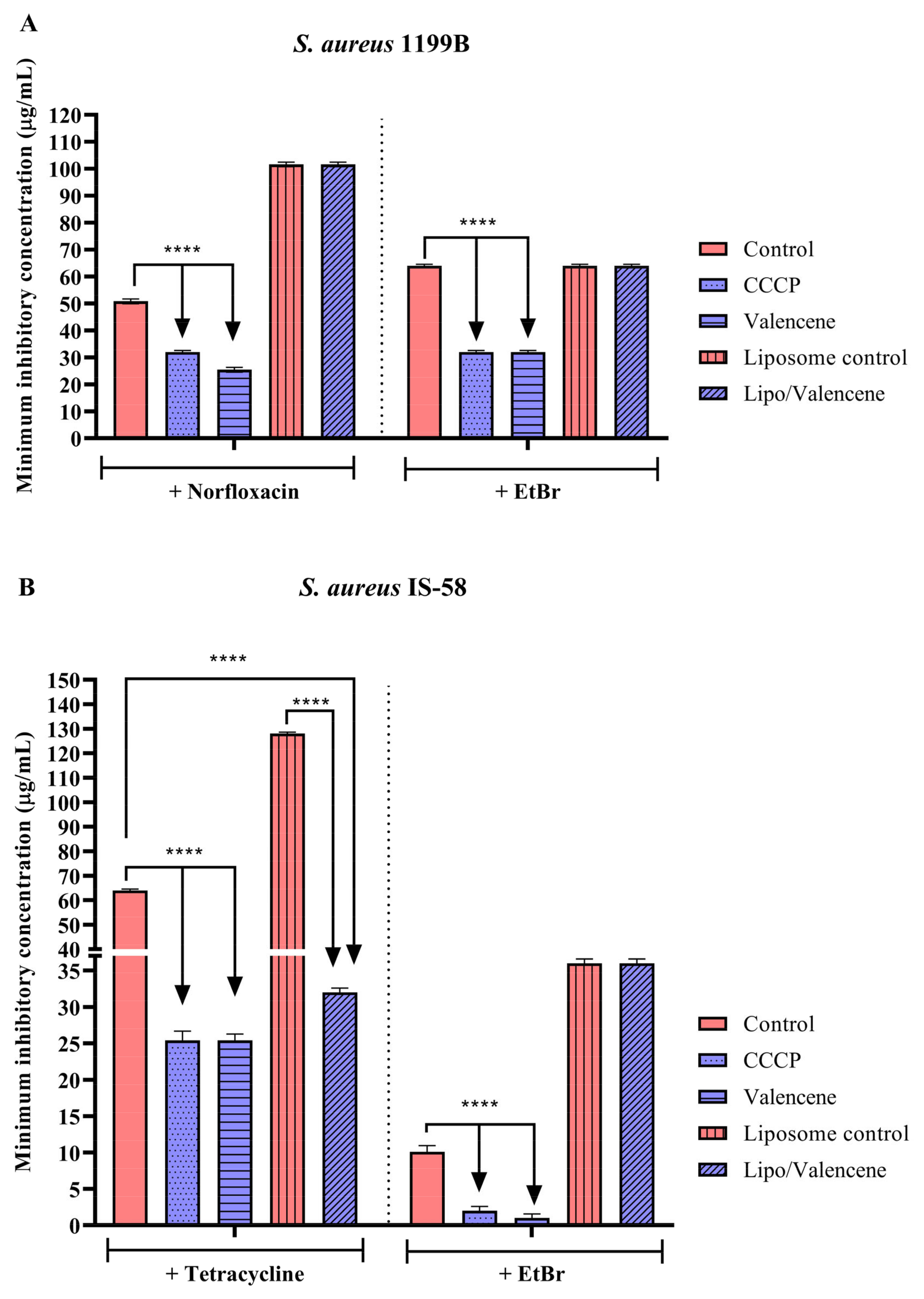
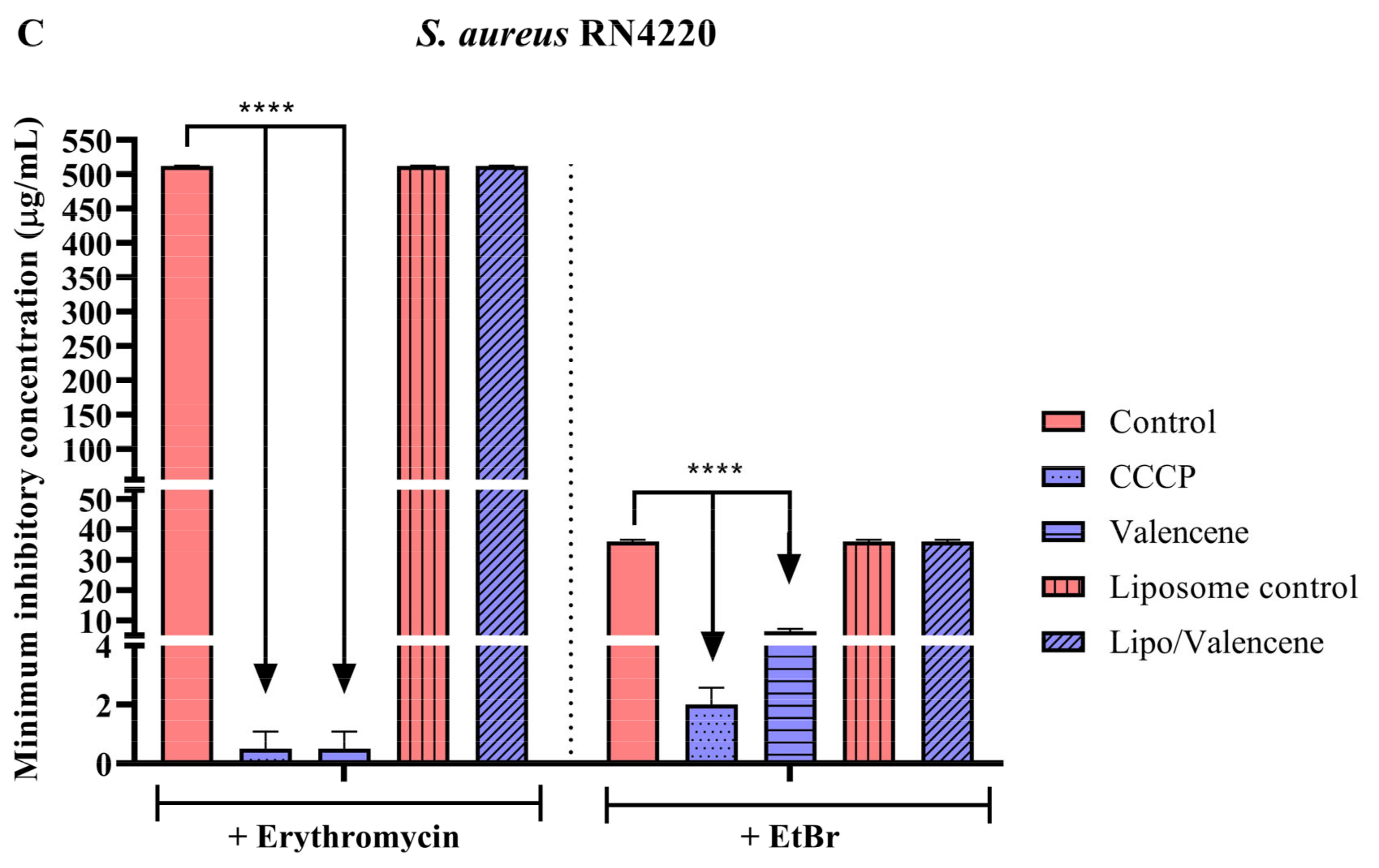
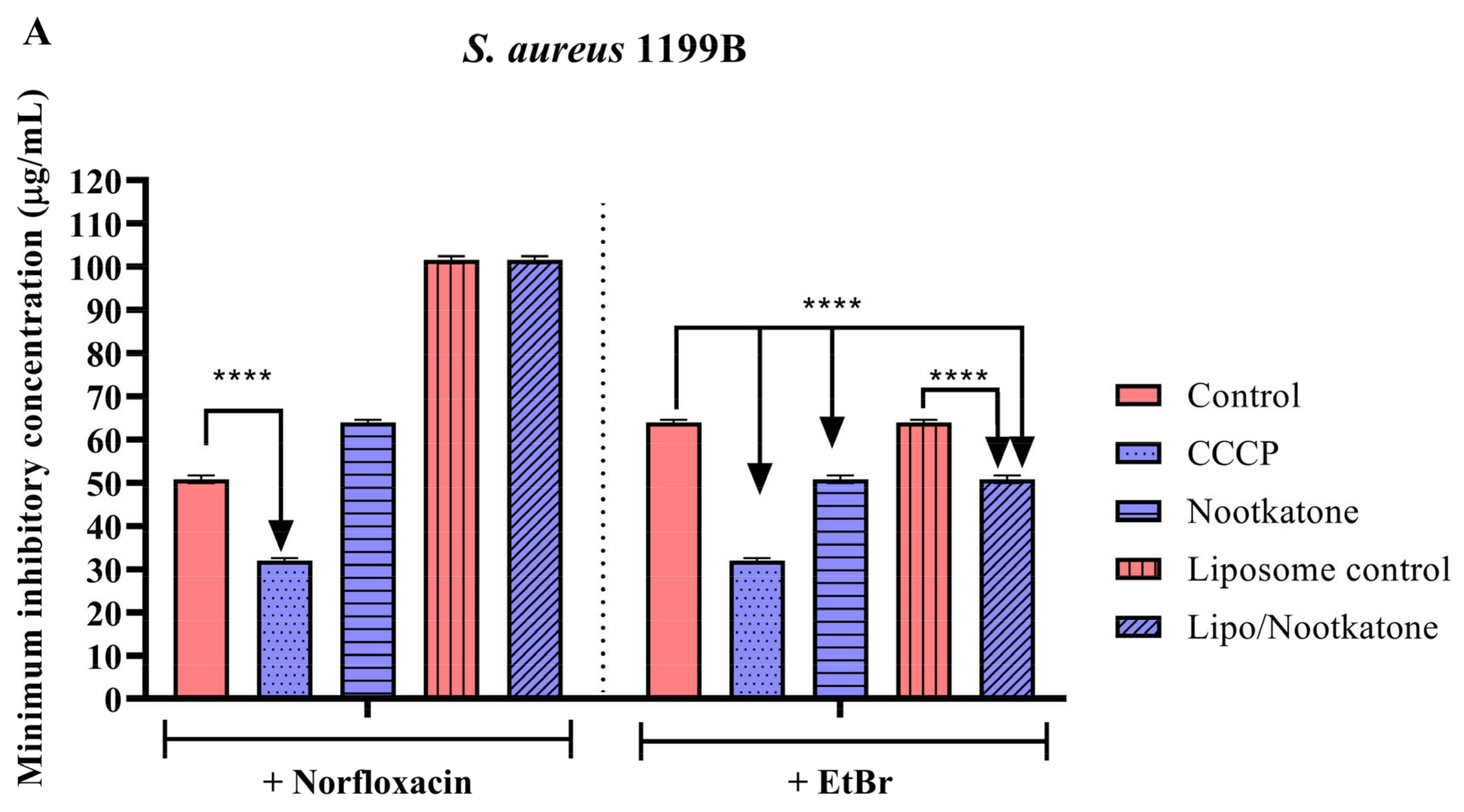
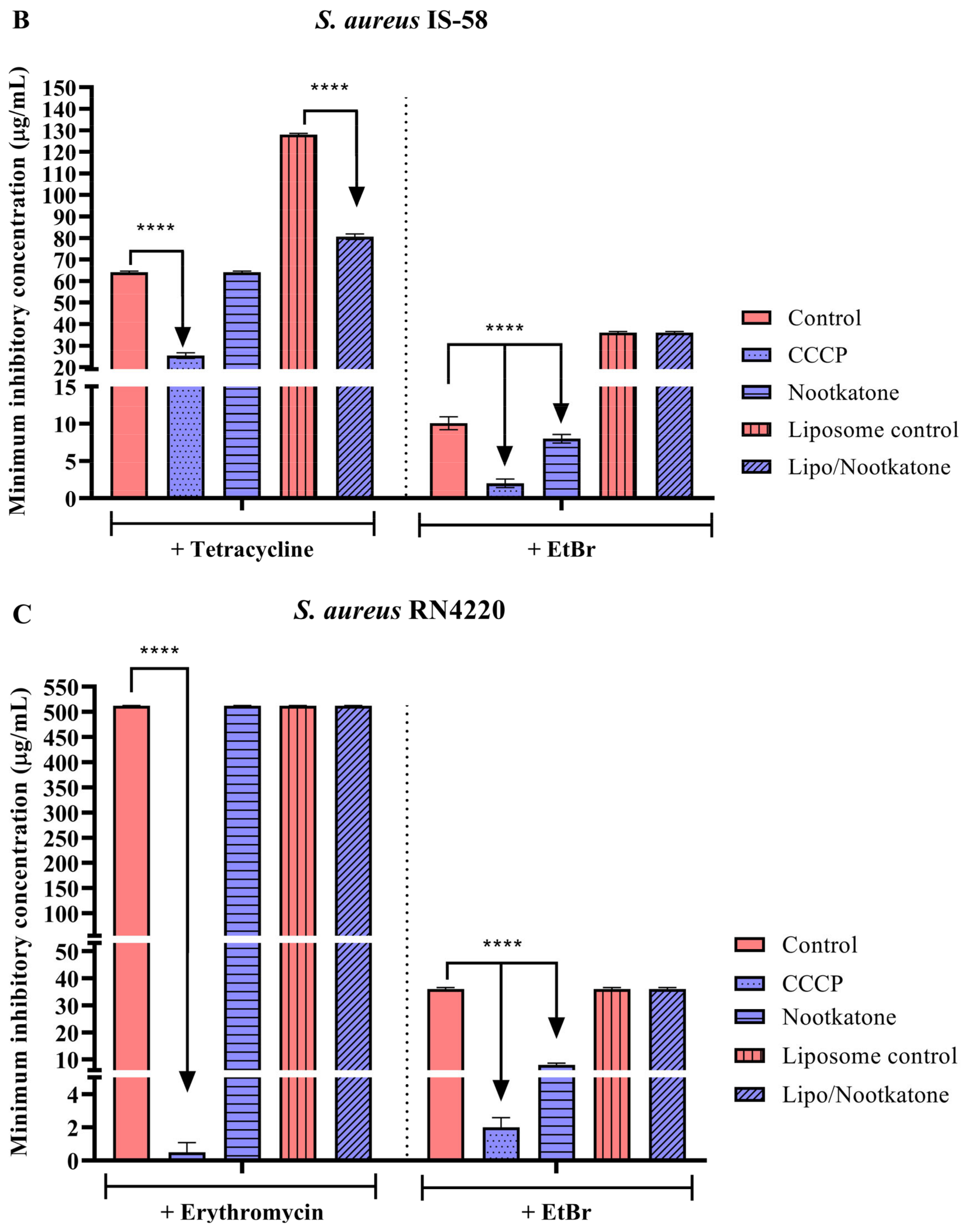
3.3. Effects of Sesquiterpenes on Antibiotic MIC against Efflux Pump-Bearing Strains
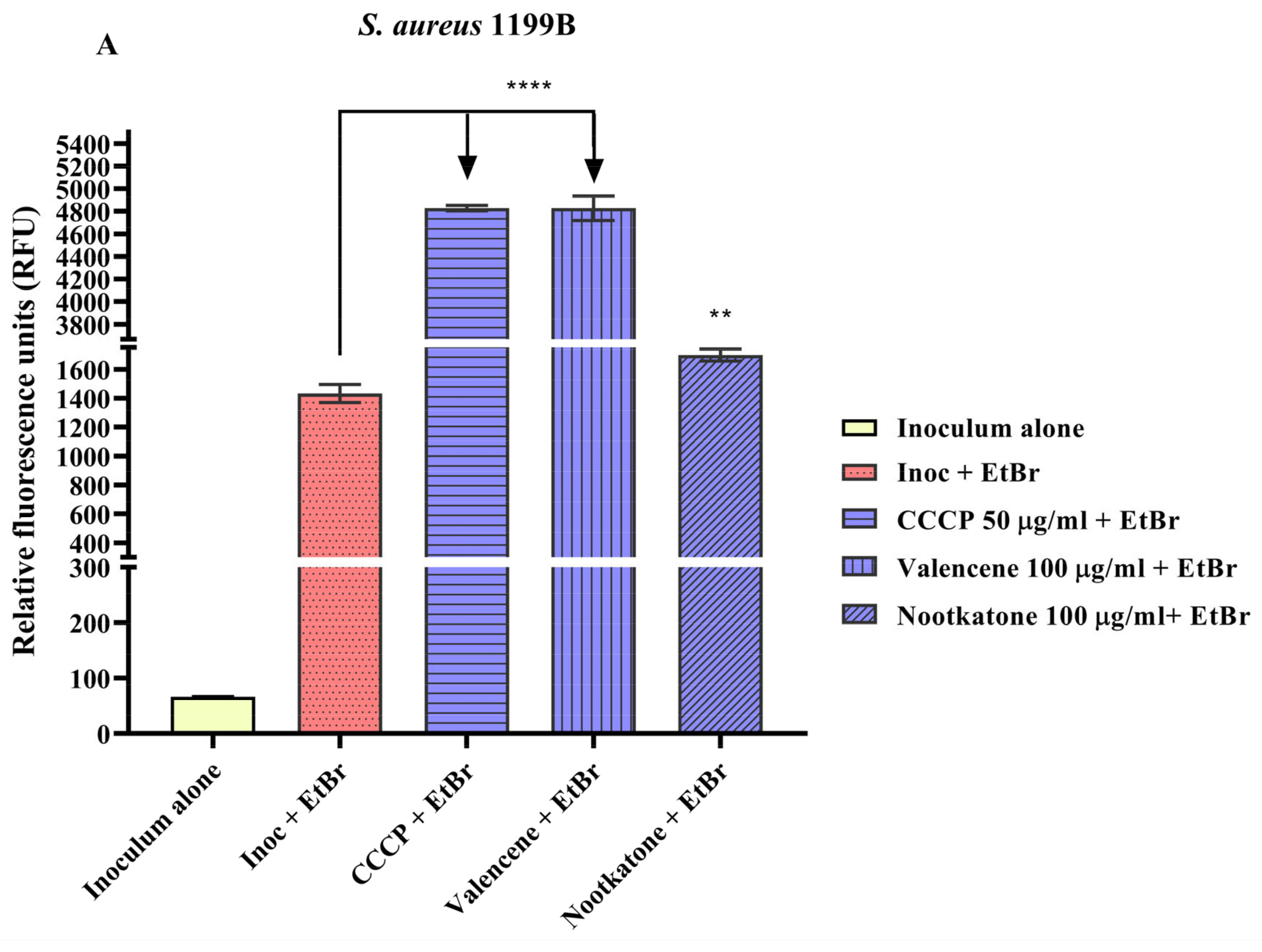
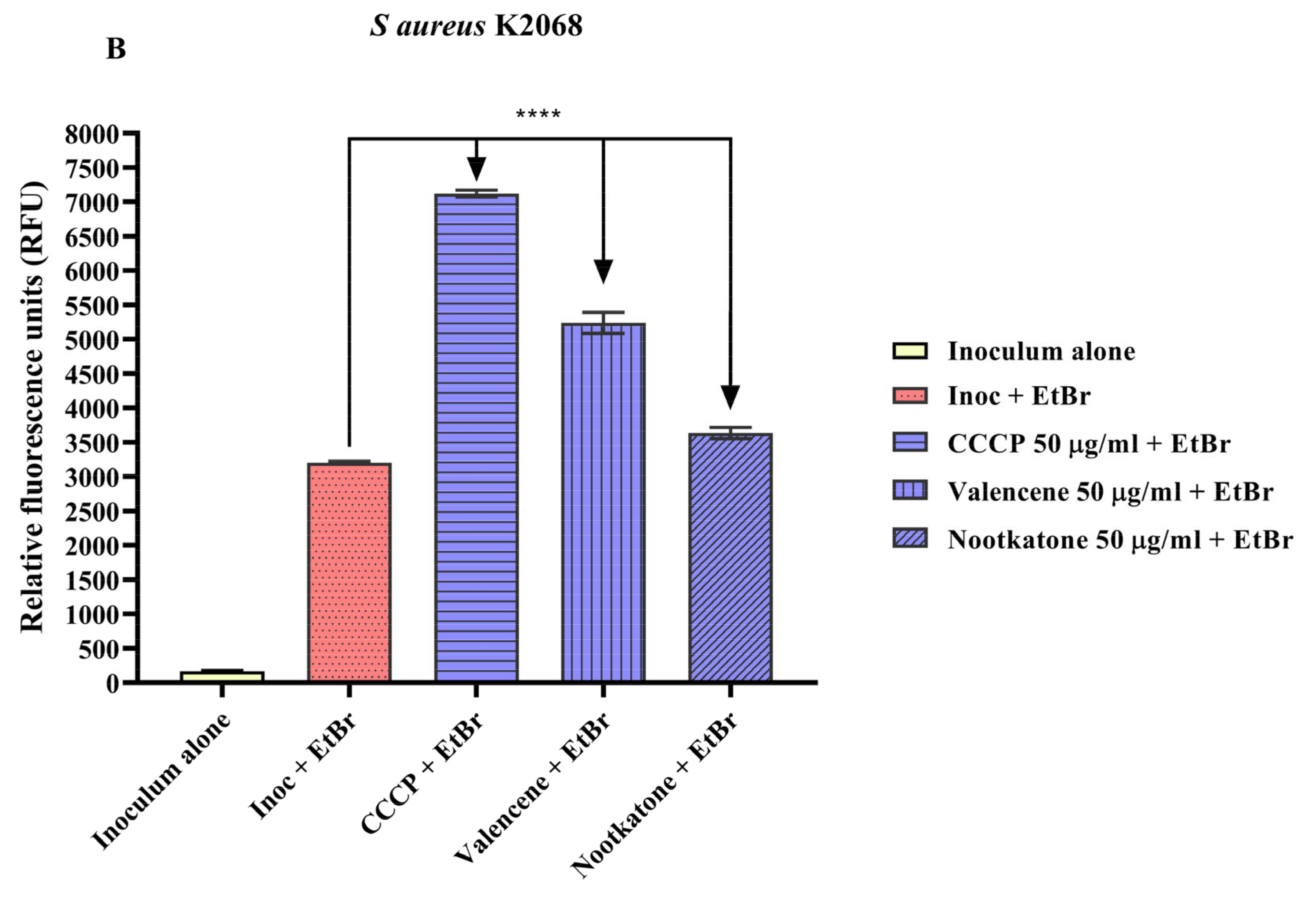
3.4. Efflux Pump Inhibition through EtBr Fluorescence Emission
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lim, C.; Takahashi, E.; Hongsuwan, M.; Wuthiekanun, V.; Thamlikitkul, V.; Hinjoy, S.; Day, N.P.J.; Peacock, S.J.; Limmathurotsakul, D. Epidemiology and Burden of Multidrug-Resistant Bacterial Infection in a Developing Country. eLife 2016, 5, 18082. [Google Scholar] [CrossRef] [PubMed]
- Santoro, A.; Franceschini, E.; Meschiari, M.; Menozzi, M.; Zona, S.; Venturelli, C.; Digaetano, M.; Rogati, C.; Guaraldi, G.; Paul, M.; et al. Epidemiology and Risk Factors Associated with Mortality in Consecutive Patients with Bacterial Bloodstream Infection: Impact of MDR and XDR Bacteria. Open Forum. Infect. Dis. 2020, 7, ofaa461. [Google Scholar] [CrossRef] [PubMed]
- David, M.Z.; Daum, R.S. Treatment of Staphylococcus aureus Infections. Curr. Top. Microbiol. Immunol. 2017, 409, 325–383. [Google Scholar] [CrossRef] [PubMed]
- Hay, R.J.; Morris-Jones, R. Bacterial Infections. In Rook’s Textbook of Dermatology, 9th ed.; Library, W.O., Ed.; Oxford University Press (OUP): Oxford, UK, 2016. [Google Scholar]
- Guo, Y.; Song, G.; Sun, M.; Wang, J.; Wang, Y. Prevalence and Therapies of Antibiotic-Resistance in Staphylococcus aureus. Front. Cell Infect. Microbiol. 2020, 10, 511382. [Google Scholar] [CrossRef] [PubMed]
- Huemer, M.; Mairpady Shambat, S.; Brugger, S.D.; Zinkernagel, A.S. Antibiotic Resistance and Persistence-Implications for Human Health and Treatment Perspectives. EMBO Rep. 2020, 21, e51034. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Tintino, C.D.d.M.; Muniz, D.F.; dos Santos Barbosa, C.R.; Silva Pereira, R.L.; Begnini, I.M.; Rebelo, R.A.; da Silva, L.E.; Mireski, S.L.; Nasato, M.C.; Lacowicz Krautler, M.I.; et al. NorA, Tet(K), MepA, and MsrA Efflux Pumps in Staphylococcus aureus, Their Inhibitors and 1,8-Naphthyridine Sulfonamides. Curr. Pharm. Des. 2022, 29, 323–355. [Google Scholar] [CrossRef]
- Reygaert, W.C. An Overview of the Antimicrobial Resistance Mechanisms of Bacteria. AIMS Microbiol. 2018, 4, 482–501. [Google Scholar] [CrossRef]
- Cascioferro, S.; Parrino, B.; Carbone, D.; Pecoraro, C.; Diana, P. Novel Strategies in the War against Antibiotic Resistance. Future Med. Chem. 2021, 13, 529–531. [Google Scholar] [CrossRef]
- Tintino, S.R.; Oliveira-Tintino, C.D.M.; Campina, F.F.; Weslley Limaverde, P.; Pereira, P.S.; Siqueira-Junior, J.P.; Coutinho, H.D.M.; Quintans-Júnior, L.J.; da Silva, T.G.; Leal-Balbino, T.C.; et al. Vitamin K Enhances the Effect of Antibiotics Inhibiting the Efflux Pumps of Staphylococcus Aureus Strains. Med. Chem. Res. 2018, 27, 261–267. [Google Scholar] [CrossRef]
- Parrino, B.; Carbone, D.; Cirrincione, G.; Diana, P.; Cascioferro, S. Inhibitors of Antibiotic Resistance Mechanisms: Clinical Applications and Future Perspectives. Future Med. Chem. 2020, 12, 357–359. [Google Scholar] [CrossRef]
- Dias, K.J.S.D.O.; Miranda, G.M.; Bessa, J.R.; De Araújo, A.C.J.; Freitas, P.R.; De Almeida, R.S.; Paulo, C.L.R.; Neto, J.B.D.A.; Coutinho, H.D.M.; Ribeiro-Filho, J. Terpenes as Bacterial Efflux Pump Inhibitors: A Systematic Review. Front. Pharmacol. 2022, 13, 953982. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Chen, Q.; Liu, Y.; Zhou, X.; Wang, X. Isolation and Biological Activities of Decanal, Linalool, Valencene, and Octanal from Sweet Orange Oil. J. Food Sci. 2012, 77, C1156–C1161. [Google Scholar] [CrossRef] [PubMed]
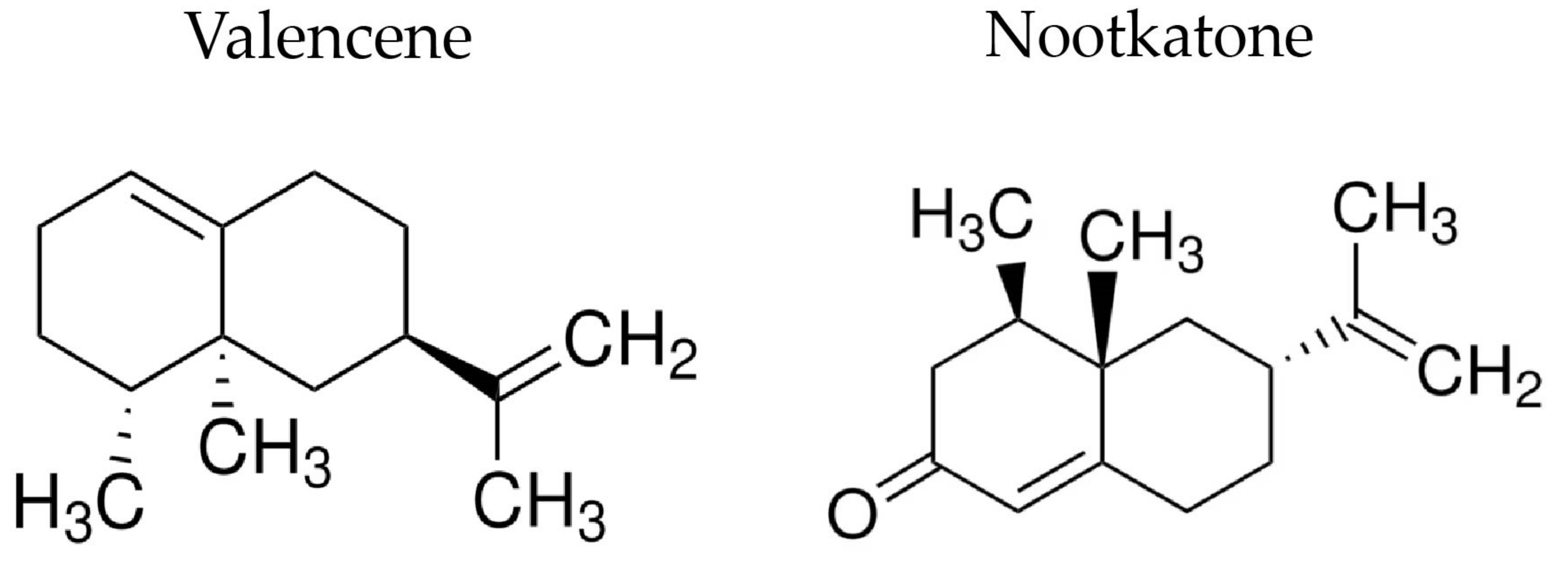
- Dantas, L.B.R.; Alcântara, I.S.; Júnior, C.P.S.; de Oliveira, M.R.C.; Martins, A.O.B.P.B.; Dantas, T.M.; Ribeiro-Filho, J.; Coutinho, H.D.M.; Passos, F.R.S.; Quintans-Júnior, L.J.; et al. In Vivo and in Silico Anti-Inflammatory Properties of the Sesquiterpene Valencene. Biomed. Pharmacother. 2022, 153, 113478. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.K.; Gairola, S.; Kundu, S.; Doye, P.; Syed, A.M.; Ram, C.; Kulhari, U.; Kumar, N.; Murty, U.S.; Sahu, B.D. Biological Activities, Pharmacokinetics and Toxicity of Nootkatone: A Review. Mini-Reviews Med. Chem. 2022, 22, 2244–2259. [Google Scholar] [CrossRef]
- Yamaguchi, T. Antibacterial Properties of Nootkatone against Gram-Positive Bacteria. Nat. Prod. Commun. 2019, 14, 1934578X19859999. [Google Scholar] [CrossRef]
- Farha, A.K.; Yang, Q.Q.; Kim, G.; Zhang, D.; Mavumengwana, V.; Habimana, O.; Li, H.-B.; Corke, H.; Gan, R.Y. Inhibition of Multidrug-Resistant Foodborne Staphylococcus aureus Biofilms by a Natural Terpenoid (+)-Nootkatone and Related Molecular Mechanism. Food Control 2020, 112, 107154. [Google Scholar] [CrossRef]
- Gonzalez Gomez, A.; Hosseinidoust, Z. Liposomes for Antibiotic Encapsulation and Delivery. ACS Infect. Dis. 2020, 6, 896–908. [Google Scholar] [CrossRef]
- Colletier, J.P.; Chaize, B.; Winterhalter, M.; Fournier, D. Protein Encapsulation in Liposomes: Efficiency Depends on Interactions between Protein and Phospholipid Bilayer. BMC Biotechnol. 2002, 2, 9. [Google Scholar] [CrossRef]
- Liu, G.; Hou, S.; Tong, P.; Li, J. Liposomes: Preparation, Characteristics, and Application Strategies in Analytical Chemistry. Crit. Rev. Anal. Chem. 2020, 52, 392–412. [Google Scholar] [CrossRef]
- Barros, N.B.; Migliaccio, V.; Facundo, V.A.; Ciancaglini, P.; Stábeli, R.G.; Nicolete, R.; Silva-Jardim, I. Liposomal-Lupane System as Alternative Chemotherapy against Cutaneous Leishmaniasis: Macrophage as Target Cell. Exp. Parasitol. 2013, 135, 337–343. [Google Scholar] [CrossRef][Green Version]
- CLSI M100ED29E; Performance Standards for Antimicrobial Susceptibility Testing: 29th Informational Supplement 20. CLSI: Wayne, PA, USA, 2019; ISBN 9781684400324.
- Oliveira-Tintino, C.D.d.M.; Tintino, S.R.; Muniz, D.F.; Barbosa, C.R.d.S.; Pereira, R.L.S.; Begnini, I.M.; Rebelo, R.A.; da Silva, L.E.; Mireski, S.L.; Nasato, M.C.; et al. Do 1,8-Naphthyridine Sulfonamides Possess an Inhibitory Action against Tet(K) and MsrA Efflux Pumps in Multiresistant Staphylococcus aureus Strains? Microb. Pathog. 2020, 147, 104268. [Google Scholar] [CrossRef] [PubMed]
- Blair, J.M.A.; Piddock, L.J.V. How to Measure Export via Bacterial Multidrug Resistance Efflux Pumps. MBio 2016, 7, e00840-16. [Google Scholar] [CrossRef]
- Pal, S.; Misra, A.; Banerjee, S.; Dam, B. Adaptation of Ethidium Bromide Fluorescence Assay to Monitor Activity of Efflux Pumps in Bacterial Pure Cultures or Mixed Population from Environmental Samples. J. King Saud Univ. Sci. 2020, 32, 939–945. [Google Scholar] [CrossRef]
- Santos Da Silva, L.Y.; Cicera, L.; Roque, P.; Felismino Moura, T.; Alves, D.S.; Torres Pessoa, R.; Moura Araújo, I.; Datiane, C.; Oliveira-Tintino, M.; Relison Tintino, S.; et al. Antibacterial Activity of the Essential Oil of Piper tuberculatum Jacq. Fruits against Multidrug-Resistant Strains: Inhibition of Efflux Pumps and β-Lactamase. Plants 2023, 12, 2377. [Google Scholar] [CrossRef] [PubMed]
- Caster, J.M.; Yu, S.K.; Patel, A.N.; Newman, N.J.; Lee, Z.J.; Warner, S.B.; Wagner, K.T.; Roche, K.C.; Tian, X.; Min, Y.; et al. Effect of Particle Size on the Biodistribution, Toxicity, and Efficacy of Drug-Loaded Polymeric Nanoparticles in Chemoradiotherapy. Nanomedicine 2017, 13, 1673. [Google Scholar] [CrossRef]
- Moazeni, M.; Kelidari, H.R.; Saeedi, M.; Morteza-Semnani, K.; Nabili, M.; Gohar, A.A.; Akbari, J.; Lotfali, E.; Nokhodchi, A. Time to Overcome Fluconazole Resistant Candida Isolates: Solid Lipid Nanoparticles as a Novel Antifungal Drug Delivery System. Colloids Surf. B Biointerfaces 2016, 142, 400–407. [Google Scholar] [CrossRef]
- Li, C.; Li, X.; Li, S.; Weng, Y.; Wang, K.; Zhang, T.; Chen, S.; Lu, X.; Jiang, Y.; Xu, J.; et al. Development and Validation of a Method for Determination of Encapsulation Efficiency of CPT-11/DSPE-MPEG2000 Nanoparticles. Med. Chem. Open Access J. 2016, 6, 345–348. [Google Scholar] [CrossRef]
- Honary, S.; Zahir, F. Effect of Zeta Potential on the Properties of Nano-Drug Delivery Systems—A Review (Part 2). Trop. J. Pharm. Res. 2013, 12, 265–273. [Google Scholar] [CrossRef]
- Smith, M.C.; Crist, R.M.; Clogston, J.D.; McNeil, S.E. Zeta Potential: A Case Study of Cationic, Anionic, and Neutral Liposomes. Anal. Bioanal. Chem. 2017, 409, 5779–5787. [Google Scholar] [CrossRef]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of Nanoparticle Delivery to Tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- González-Vega, J.G.; García-Ramos, J.C.; Chavez-Santoscoy, R.A.; Castillo-Quiñones, J.E.; Arellano-Garcia, M.E.; Toledano-Magaña, Y. Lung Models to Evaluate Silver Nanoparticles’ Toxicity and Their Impact on Human Health. Nanomaterials 2022, 12, 2316. [Google Scholar] [CrossRef]
- White, D.C.; Frerman, F.E. Extraction, Characterization, and Cellular Localization of the Lipids of Staphylococcus aureus. J. Bacteriol. 1967, 94, 1854–1867. [Google Scholar] [CrossRef] [PubMed]
- Oku, Y.; Kurokawa, K.; Ichihashi, N.; Sekimizu, K. Characterization of the Staphylococcus aureus MprF Gene, Involved in Lysinylation of Phosphatidylglycerol. Microbiology 2004, 150, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Sohlenkamp, C.; Geiger, O. Bacterial Membrane Lipids: Diversity in Structures and Pathways. FEMS Microbiol. Rev. 2016, 40, 133–159. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, S.; Slavetinsky, C.J.; Peschel, A. Synthesis and Function of Phospholipids in Staphylococcus Aureus. Int. J. Med. Microbiol. 2015, 305, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Kaatz, G.W.; Moudgal, V.V.; Seo, S.M.; Kristiansen, J.E. Phenothiazines and Thioxanthenes Inhibit Multidrug Efflux Pump Activity in Staphylococcus Aureus. Antimicrob. Agents Chemother. 2003, 47, 719–726. [Google Scholar] [CrossRef]
- Schindler, B.D.; Jacinto, P.; Kaatz, G.W. Inhibition of Drug Efflux Pumps in Staphylococcus aureus: Current Status of Potentiating Existing Antibiotics. Future Microbiol. 2013, 8, 491–507. [Google Scholar] [CrossRef]
- DeMarco, C.E.; Cushing, L.A.; Frempong-Manso, E.; Seo, S.M.; Jaravaza, T.A.A.; Kaatz, G.W. Efflux-Related Resistance to Norfloxacin, Dyes, and Biocides in Bloodstream Isolates of Staphylococcus aureus. Antimicrob. Agents Chemother. 2007, 51, 3235–3239. [Google Scholar] [CrossRef]
- Costa, S.; Falcão, C.; Viveiros, M.; MacHado, D.; Martins, M.; Melo-Cristino, J.; Amaral, L.; Couto, I. Exploring the Contribution of Efflux on the Resistance to Fluoroquinolones in Clinical Isolates of Staphylococcus aureus. BMC Microbiol. 2011, 11, 241. [Google Scholar] [CrossRef]
- Kristiansen, J.E.; Thomsen, V.F.; Martins, A.; Viveiros, M.; Amaral, L. Non-Antibiotics Reverse Resistance of Bacteria to Antibiotics. In Vivo 2010, 24, 751–754. [Google Scholar]
- Patel, D.; Kosmidis, C.; Seo, S.M.; Kaatz, G.W. Ethidium Bromide MIC Screening for Enhanced Efflux Pump Gene Expression or Efflux Activity in Staphylococcus aureus. Antimicrob. Agents Chemother. 2010, 54, 5070–5073. [Google Scholar] [CrossRef] [PubMed]
- Piddock, L.J.V. Clinically Relevant Chromosomally Encoded Multidrug Resistance Efflux Pumps in Bacteria. Clin. Microbiol. Rev. 2006, 19, 382–402. [Google Scholar] [CrossRef] [PubMed]
- El-Baky, R.M.A.; Sandle, T.; John, J.; Abuo-Rahma, G.E.D.A.; Hetta, H.F. A Novel Mechanism of Action of Ketoconazole: Inhibition of the Nora Efflux Pump System and Biofilm Formation in Multidrug-Resistant Staphylococcus aureus. Infect. Drug Resist. 2019, ume 12, 1703–1718. [Google Scholar] [CrossRef]
- Ramalhete, C.; Spengler, G.; Martins, A.; Martins, M.; Viveiros, M.; Mulhovo, S.; Ferreira, M.J.U.; Amaral, L. Inhibition of Efflux Pumps in Meticillin-Resistant Staphylococcus aureus and Enterococcus faecalis Resistant Strains by Triterpenoids from Momordica balsamina. Int. J. Antimicrob. Agents 2011, 37, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, S.; Oluwatuyi, M.; Kaatz, G.W. A Novel Inhibitor of Multidrug Efflux Pumps in Staphylococcus aureus. J. Antimicrob. Chemother. 2003, 51, 13–17. [Google Scholar] [CrossRef]
- Markham, P.N.; Westhaus, E.; Klyachko, K.; Johnson, M.E.; Neyfakh, A.A. Multiple Novel Inhibitors of the NorA Multidrug Transporter of Staphylococcus aureus. Antimicrob. Agents Chemother. 1999, 43, 2404–2408. [Google Scholar] [CrossRef]
- Khokra, S.; Prakash, O.; Jain, S.; Aneja, K.; Dhingra, Y. Essential Oil Composition and Antibacterial Studies of Vitex Negundo Linn. Extracts. Indian J. Pharm. Sci. 2008, 70, 522. [Google Scholar] [CrossRef]
- Oliveira, A.P.; França, H.S.; Kuster, R.M.; Teixeira, L.A.; Rocha, L.M. Chemical Composition and Antibacterial Activity of Brazilian Propolis Essential Oil. J. Venom. Anim. Toxins Incl. Trop. Dis. 2010, 16, 121–130. [Google Scholar] [CrossRef]
- Zahid, N.; Ali Khan, S.; Al-Hamaid, F.M.; Maroof Shah, M.; Al Farraj, D.A.; Elshikh, M.S.; Ahmad, R.; Ahmad Alghmdi, M.; Mehmood Abbasi, A. In Vitro and in Silico Validation of Antibacterial Potential of Pinus roxburghii and Cedrus deodara Leaves’ Extract against Human Pathogenic Bacteria. J. King Saud Univ. Sci. 2023, 35, 102518. [Google Scholar] [CrossRef]
- Prasad, R.; Bisht, L.S.; Joshi, D.; Nailwal, M.K.; Melkani, A.B. Chemical Composition and Antibacterial Activity of the Essential Oil from Whole Aerial Parts of Leucas mollissima Wall. Ex Benth. J. Essent. Oil Bear. Plants 2017, 20, 141–147. [Google Scholar] [CrossRef]
- Haznedaroglu, M.Z.; Karabay, N.U.; Zeybek, U. Antibacterial Activity of Salvia Tomentosa Essential Oil. Fitoterapia 2001, 72, 829–831. [Google Scholar] [CrossRef] [PubMed]
- Sultana, R.; Islam, M.D.; Tanjum, F.; Rahman, M.M.; Haque, M.A. Antioxidant, Antibacterial and Antifungal Properties of Black Pepper Essential Oil (Piper nigrum Linn) and Molecular Docking and Pharmacokinetic Studies of Its’ Major Component. Orient. J. Chem. 2022, 38, 1554–1560. [Google Scholar] [CrossRef]
- Li, X.; An, Q.; Qu, S.; Ren, J.-N.; Fan, G.; Zhang, L.-L.; Pan, S.-Y. Differential Proteomic Analysis of Citrus Flavor (+)-Valencene Biotransformation to (+)-Nootkatone by Yarrowia lipolytica. Int. J. Biol. Macromol. 2022, 220, 1031–1048. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Huang, Y.; Shi, B.; Xiang, Z.; Tian, Z.; Huang, M.; Wu, L.; Deng, Z.; Shen, K.; Liu, T. Coupling Cell Growth and Biochemical Pathway Induction in Saccharomyces cerevisiae for Production of (+)-Valencene and Its Chemical Conversion to (+)-Nootkatone. Metab. Eng. 2022, 72, 107–115. [Google Scholar] [CrossRef]
- Elston, A.; Lin, J.; Rouseff, R. Determination of the Role of Valencene in Orange Oil as a Direct Contributor to Aroma Quality. Flavour Fragr. J. 2005, 20, 381–386. [Google Scholar] [CrossRef]
- Gliszczyńska, A.; Łysek, A.; Janeczko, T.; Świtalska, M.; Wietrzyk, J.; Wawrzeńczyk, C. Microbial Transformation of (+)-Nootkatone and the Antiproliferative Activity of Its Metabolites. Bioorg. Med. Chem. 2011, 19, 2464–2469. [Google Scholar] [CrossRef]
| Liposome/Valencene | Liposome/Nootkatone | |
|---|---|---|
| Size | 194.5 nm | 147 nm |
| Intensity | 5 a.u. | 10 a.u. |
| Zeta potential | −11.6 mV | −19.3 mV |
| Concentration | 4.83 × 108 particles/mL | 2.3 × 108 particles/mL |
| Polydispersity index (PDI) | 1.16 | 0.61 |
| Encapsulation efficiency | 72.4% | 76% |
| S. aureus 1199B | S. aureus IS-58 | S. aureus RN4220 | |
|---|---|---|---|
| Control | 128 µg/mL (Norfloxacin) | 32 µg/mL (Tetracyclin) | 32 µg/mL (Erythromycin) |
| Valencene | 512 µg/mL* | ≥1024 µg/ml | 256 µg/mL * |
| Nootkatone | 406.4 µg/mL * | 101.6 µg/mL * | 203.2 µg/mL * |
| Liposomal Valencene | ≥1024 µg/mL | ≥1024 µg/mL | ≥1024 µg/mL |
| Liposomal Nootkatone | ≥1024 µg/mL | ≥1024 µg/mL | ≥1024 µg/mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira-Tintino, C.D.d.M.; Santana, J.E.G.; Alencar, G.G.; Siqueira, G.M.; Gonçalves, S.A.; Tintino, S.R.; Menezes, I.R.A.d.; Rodrigues, J.P.V.; Gonçalves, V.B.P.; Nicolete, R.; et al. Valencene, Nootkatone and Their Liposomal Nanoformulations as Potential Inhibitors of NorA, Tet(K), MsrA, and MepA Efflux Pumps in Staphylococcus aureus Strains. Pharmaceutics 2023, 15, 2400. https://doi.org/10.3390/pharmaceutics15102400
Oliveira-Tintino CDdM, Santana JEG, Alencar GG, Siqueira GM, Gonçalves SA, Tintino SR, Menezes IRAd, Rodrigues JPV, Gonçalves VBP, Nicolete R, et al. Valencene, Nootkatone and Their Liposomal Nanoformulations as Potential Inhibitors of NorA, Tet(K), MsrA, and MepA Efflux Pumps in Staphylococcus aureus Strains. Pharmaceutics. 2023; 15(10):2400. https://doi.org/10.3390/pharmaceutics15102400
Chicago/Turabian StyleOliveira-Tintino, Cícera Datiane de Morais, Jorge Ederson Gonçalves Santana, Gabriel Gonçalves Alencar, Gustavo Miguel Siqueira, Sheila Alves Gonçalves, Saulo Relison Tintino, Irwin Rose Alencar de Menezes, João Pedro Viana Rodrigues, Vanessa Barbosa Pinheiro Gonçalves, Roberto Nicolete, and et al. 2023. "Valencene, Nootkatone and Their Liposomal Nanoformulations as Potential Inhibitors of NorA, Tet(K), MsrA, and MepA Efflux Pumps in Staphylococcus aureus Strains" Pharmaceutics 15, no. 10: 2400. https://doi.org/10.3390/pharmaceutics15102400
APA StyleOliveira-Tintino, C. D. d. M., Santana, J. E. G., Alencar, G. G., Siqueira, G. M., Gonçalves, S. A., Tintino, S. R., Menezes, I. R. A. d., Rodrigues, J. P. V., Gonçalves, V. B. P., Nicolete, R., Ribeiro-Filho, J., da Silva, T. G., & Coutinho, H. D. M. (2023). Valencene, Nootkatone and Their Liposomal Nanoformulations as Potential Inhibitors of NorA, Tet(K), MsrA, and MepA Efflux Pumps in Staphylococcus aureus Strains. Pharmaceutics, 15(10), 2400. https://doi.org/10.3390/pharmaceutics15102400










