Abstract
A series of new hybrid derivatives 1a–c, 2a–c, 3a–c, 4a–c, 5a–c, inspired by nature, were synthesized and studied as multifunctional agents for the treatment of Alzheimer’s disease (AD). These compounds were designed to merge together the trifluoromethyl benzyloxyaminic bioactive moiety, previously identified, with different acids available in nature. The ability of the synthesized compounds to chelate biometals, such as Cu2+, Zn2+ and Fe2+, was studied by UV–Vis spectrometer, and through a preliminary screening their antioxidant activity was evaluated by DPPH. Then, selected compounds were tested by in vitro ABTS free radical method and ex vivo rat brain TBARS assay. Compounds 2a–c, combining the strongest antioxidant and biometal chelators activities, were studied for their ability to contrast Aβ1-40 fibrillization process. Finally, starting from the promising profile obtained for compound 2a, we evaluated if it could be able to induce a positive cross-interaction between transthyretin (TTR) and Aβ in presence and in absence of Cu2+.
1. Introduction
Alzheimer’s disease (AD) is a neurodegenerative disorder that leads to progressive loss of memory, decline in language skills and in other cognitive functions that certainly induce a downfall in patients’ ability to live in society [1]. Much experimental evidence has suggested that AD is a multifactorial illness characterized by a detrimental reduction of acetylcholine levels combined with high oxidative stress (OS) and dyshomeostasis of biometals [2,3,4]. Metal ions such as Cu, Fe and Zn are fundamental for several physiological functions, especially in the central nervous system; however, studies report that their dysregulation is involved in AD [5,6,7]. A high concentration of Cu, Fe and Zn was found in the brain of AD patients (300–400 μM) [8].
The main hallmarks of AD are the extracellular senile plaques, formed by β–amyloid (Aβ), and the intracellular neurofibrillary tangles composed by hyperphosphorylated τ–protein [9,10,11].
Usually, amyloid aggregates are characterized not only by a single type of amyloid protein, but by different proteins. It has been reported that, in amyloid plaque, Aβ is just the major amyloid protein found with respect to the more than a hundred proteins present [12]. This aspect is related to the cross-interaction that can occur among the proteins, and it can have negative or positive effects [13,14,15]. The negative protein–protein cross-interaction promotes the aggregation, increases the toxicity of aggregates and inhibits the degradation of aggregates (Aβ and τ–protein) [16,17,18], while the positive protein–protein cross-interaction contrasts the formation of amyloid oligomers or amyloid fibrils, reduces the toxicity of aggregates, and promotes the degradation and the dissociation of aggregates [19]. It is possible that an intrinsically amyloidogenic protein can be able to inhibit the amyloid fibril formation of another amyloidogenic protein, and, at the same time, it is able to reduce the toxicity of the aggregate. This is the effect that transthyretin (TTR) has when it binds Aβ in the brain [20,21,22,23,24].
TTR is a plasma homotetrameric protein involved in the transport of the thyroxine (T4) and the retinol, through retinol-binding protein, in blood and cerebrospinal fluid (CSF) [25]. The T4 directly binds TTR occupying a channel, characterized by two hydrophobic binding pockets (T4-BPs), that crosses along the entire tetramer. Each T4-BP is composed by three pairs of sub-sites named halogen binding pockets (HBPs) because of the iodine atoms of the endogen ligand T4 accommodate these hydrophobic depressions [26]. In plasma, the amount of T4 bound to TTR is between 10–15%, and the majority of T4-BPs are empty and available to bind other molecules [25]. In elderly people, wild type TTR (wt-TTR) can lose its tetrameric structure and this triggers the fibril formation [27,28,29]. The stabilization of the TTR tetramer by small molecules able to bind the T4-BP is one of the therapeutic strategies used to contrast the TTR fibril formation [30,31,32,33]. TTR, as well as Aβ, is sensitive to reactive oxygen species (ROS) due to the presence on each monomer of TTR of one Cys10 solvent exposed that can be easily oxidized inducing the destabilization of the tetrameric structure [34,35]. In contrast with the intrinsic amyloidogenic nature of wt-TTR, several studies have reported that under physiological conditions, TTR binds Aβ and inhibits the formation of fibril aggregates in the brain [36,37,38,39,40,41].
The approved drugs available for the treatment of AD include two disease-modifying monoclonal antibodies directed against the aggregated forms of Aβ (Aducanumab and Lecanemab), and four symptomatic treatments (donepezil, galantamine, rivastigmine, memantine), alone or in combination [42,43]. However, due to the multifactorial aspect of AD, none of these treatments is curative, especially for the loss of efficacy over time, and the lack of an efficient blood-brain barrier passage. Therefore, alternative therapeutic approaches such as the use of a multifunctional compounds that simultaneously act by inhibiting Aβ aggregation, contrasting ROS, chelating biometals and inducing positive protein–protein cross interaction are currently a fertile ground for innovative research [44,45,46].
Natural bioactive compounds extracted from plants and organisms, commonly named nutraceuticals, have been used in traditional medicine, handing over their use from one generation to another [47,48,49]. The multifunctional character of natural products has drawn the attention of the researchers because these molecules, and their derivatives, are able to simultaneously act on several targets of AD. Recently, many natural products are placed under investigation in pre-clinical and clinical trials in the treatment of AD [50,51,52].
The synthesis of bifunctional conjugates of natural products represents one of the growing areas in modern drug discovery. With this aim, several natural-products-based hybrid compounds have been developed as new agents to contrast AD progression [53,54,55]. In a previous work, we synthesized ferulic acid (FA) hybrids as potential multifunctional compounds suitable in the context of multifactorial diseases like AD. Among the FA hybrids, we have identified the good ability of the trifluoromethyl benzyloxyamidic moiety to reduce the Aβ1-40 fibrillization especially in presence of Cu2+ [56]. In this context, the conjugation of natural acids with the trifluoromethyl benzyloxyaminic bioactive group has led to a series of new benzyloxyamidic derivatives 1a–c, 2a–c, 3a–c, 4a–c, 5a–c, which were characterized and studied as potential multifunctional agents for the treatment of AD, Figure 1.
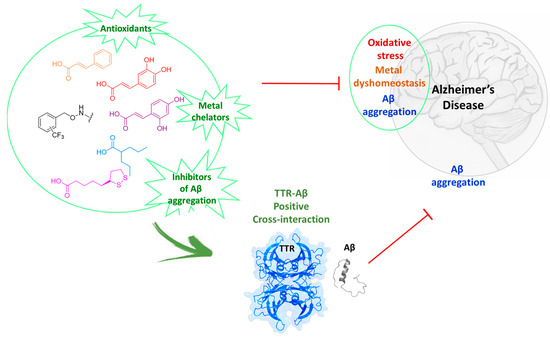
Figure 1.
Overview of natural-products-based hybrid compounds as potential agents against AD.
The new hybrid compounds were designed merging together the previously identified trifluoromethyl benzyloxyaminic moiety, with different acids. i.e., cinnamic acid (1a–c), caffeic acid (2a–c), umbellic acid (3a–c), valproic acid (4a–c), and lipoic acid (5a–c). The cinnamic acid, caffeic acid and umbellic acid were selected in order to investigate the influence of the absence or the presence of more than one hydroxyl group on the new hybrids compare to the FA ones, while valproic acid and lipoic acid were chosen for their known properties to contrast the AD progression [57,58].
The ability of the new synthesized compounds 1–5 to chelate biometals, such as Cu2+, Zn2+ and Fe2+, was studied by UV–Vis spectrometer. Then, the antioxidant activity was investigated by in vitro DPPH assay and ex vivo TBARS colorimetric test in rat brains. Combining the results, compounds 2a–c, the strongest antioxidant and biometal chelators, were selected for studying their ability to contrast Aβ aggregation. Finally, we have also investigated whether the compounds 2a–c participate to the positive cross-interaction between TTR and Aβ in presence and in absence of Cu2+.
2. Materials and Methods
2.1. Chemistry
Analytical grade reagents and solvents were bought from Sigma-Aldrich (St. Louis, MO, USA) and were used without any purification. Each chemical reactions were followed by Thin Layer Chromatography (TLC) on 0.25 mm aluminum plates, pre-coated with silica gel and containing a fluorescent indicator (Merck Silica Gel 60 F254, Darmstadt, Germany). The UV lamp (254 nm) was used to visualize the spots on TLC. The organic solutions were dehydrated using Na2SO4, then the evaporation was performed under vacuum conditions in a rotating evaporator. The crude products were purified by flash column chromatography (Kieselgel 40, 0.040−0.063 mm, Merck, Darmstadt, Germany) or using ISOLUTE Flash Si II column cartridges (Biotage, Uppsala, Sweden).
The structural characterization of compounds and their purity were performed by the determination of melting points, by NMR and Mass Spectrometry techniques. Melting points (m.p.) were measured with a Leica Galen III microscope, and 1H and 13C NMR spectra were recorded using a Bruker Ultrashield™ 400 MHz (Fällander, Switzerland), at 25 °C. Chemical shifts (δ) are reported in ppm and coupling constant values (J) are in hertz (Hz). Signals in NMR spectra are indicated by the following abbreviations: s = singlet, d = doublet, m = multiplet, dd = doublet of doublet, q = quartet, sex = sextet, sept = septet, bs (broad signal). For derivatives 1a–c, 4a–c and 5a–c, the high-resolution mass spectra were obtained using a TOF LCT Premier apparatus (Waters) with an electrospray ionization source (Agilet Technologies, Santa Clara, CA, USA). While, for compounds 2a–c and 3a–c the ESI-MS spectra were recorded by direct injection at 5 (positive) and 7 (negative) μL min−1 flow rate in an Orbitrap high-resolution mass spectrometer (Thermo, San Jose, CA, USA), equipped with HESI source.
General Procedure for the Synthesis of Benzyloxyamidic Derivatives (1a–c, 2a–c, 3a–c, 4a–c, 5a–c)
A solution of commercially available acids 7–11, (1 eq) in anhydrous DMF (2 mL) under inert nitrogen atmosphere, was added by hydroxybenzotriazole (HOBt) (1.2 eq), N-methylmorpholine (3 eq), the opportune benzylhydroxylamine hydrochloride 6a–c (3.1 eq) and N-(3-dimethylaminopropyl)-N’-ethylcarbodiimide hydrochloride (EDCI) (1.4 eq) [56]. The mixture obtained was stirred at room temperature (r.t.) and monitored by TLC. Then, the solution was extracted with AcOEt and washed with H2O. The organic portion was dried, filtered, and evaporated to furnish the crude derivatives that was purified by trituration or ISOLUTE Flash Si II column cartridges afforded the desired hybrid compounds 1a–c, 2a–c, 3a–c, 4a–c, 5a–c. The 1H-NMR, 13C-NMR and ESI-MS spectra of all compounds are available in supplementary materials (Spectras S1–S45).
N-((2-(trifluoromethyl)benzyl)oxy)cinnamamide (1a) The crude product was triturated with hexane furnishing compound 1a as a white solid. Yield: 47%; m.p.: 91–93 °C. 1H-NMR (400 MHz, CD3OD) δ: 7.82–7.80 (m, 1H, Ar); 7.73 (d, J = 8.0 Hz, 1H, Ar); 7.68 (t, J = 7.6 Hz, 1H, Ar); 7.62 (d, J = 15.7 Hz, 1H, Ar-CH=CH); 7.56–7.53 (m, 3H, Ar); 7.41–7.38 (m, 3H, Ar); 6.42 (d, J = 15.7 Hz, 1H, Ar-CH=CH); 5.14 (s, 2H, O-CH2). 13C NMR (100 MHz, CD3OD) δ: 166.3; 142.8; 135.9; 135.4; 133.4; 132.5; 131.1; 129.9; 129.5; 128.9; 126.9; 125.7 (q, 1JC-F = 272.0 Hz); 117.8; 75.0. m/z ESI-MS: [M + H]+ 322.11.
N-((3-(trifluoromethyl)benzyl)oxy)cinnamamide (1b) The crude product was triturated with hexane furnishing compound 1b as a white solid. Yield: 47%; m.p.: 91–93 °C. 1H-NMR (400 MHz, CD3OD) δ: 7.80 (s, 1H, Ar); 7.72 (d, J = 7.6 Hz, 1H, Ar); 7.68–7.63 (m, 2H, Ar); 7.61–7.57 (m, 2H, Ar); 7.56–7.53 (m, 2H, Ar); 7.42–7.38 (m, 3H, Ar); 6.41 (d, J = 16.0 Hz, 1H, Ar-CH=CH); 5.01 (s, 2H, O-CH2). 13C NMR (100 MHz, CD3OD) δ: 166.3; 142.8; 138.4; 135.9; 133.8; 131.8 (q, 2JC-F = 32.0 Hz); 131.1; 130.3; 129.4; 128.9; 126.7; 126.3; 125.5 (q, 1JC-F = 271.0 Hz); 117.9; 78.2. m/z ESI-MS: [M + H]+ 322.11.
N-((4(trifluoromethyl)benzyl)oxy)cinnamamide (1c) The crude product was triturated with hexane furnishing compound 1c as a white solid. Yield: 46.2%. Mp: 148–150 °C.
1H NMR (400 Hz, CD3OD): 7.71–7.64 (m, 4H, Ar); 7.57 (d, J = 16.0 Hz, 1H, Ar-CH=CH); 7.55–7.53 (m, 2H, Ar); 7.42–7.35 (m, 3H, Ar); 6.41 (d, J = 16.0 Hz, 1H, Ar-CH=CH); 5.02 (s, 2H, O-CH2). 13C NMR (100 MHz, CD3OD) δ: 160.1; 136.6; 135.4; 129.8; 125.4 (q, 2JC-F = 32.2 Hz); 124.9; 124.3; 123.8; 122.7; 120.2; 119.4 (q, 1JC-F = 271.3 Hz); 111.7; 72.0. m/z ESI-MS: [M + H]+ 322.11.
(E)-3-(3,4-dihydroxyphenyl)-N-((2-(trifluoromethyl)benzyl)oxy)acrylamide (2a) The crude product was purified by flash chromatography (CHCl3/MeOH 60:1) furnishing compound 2a as a white solid. Yield: 37%; m.p.: 80–82 °C. 1H-NMR (400 MHz, CD3OD) δ: 7.80 (d, J = 7.6 Hz, 1H, Ar); 7.73 (d, J = 7.6 Hz, 1H, Ar); 7.68 (t, J = 7.6 Hz, 1H, Ar); 7.54 (t, J = 7.6 Hz, 1H, Ar); 7.48 (d, J = 15.5 Hz, 1H, Ar-CH=CH); 6.99 (d, J = 1.9 Hz, 1H, Ar); 6.90 (dd, J1 = 8.1 Hz, J2 = 1.9 Hz, 1H, Ar); 6.76 (d, J = 8.1 Hz, 1H, Ar); 6.16 (d, J = 15.5 Hz, 1H, Ar-CH=CH); 5.12 (s, 2H, O-CH2). 13C NMR (100 MHz, CD3CD) δ: 167.2; 149.1; 146.7; 143.4; 135.5; 133.4; 132.5; 129.9; 128.0; 127.1; 126.9; 124.4; 122.3; 116.4; 115.1; 114.2; 75.0. m/z ESI-MS: [M + H]+ 354.09.
(E)-3-(3,4-dihydroxyphenyl)-N-((3-(trifluoromethyl)benzyl)oxy)acrylamide (2b) The crude product was purified by ISOLUTE (CHCl3/MeOH 60:1) furnishing compound 2b as a white solid. Yield: 27%; m.p.: 110–112 °C. 1H-NMR (400 MHz, CD3OD) δ: 7.78 (s, 1H, Ar); 7.72–7.70 (m, 1H, Ar); 7.67–7.65 (m, 1H, Ar); 7.60–7.56 (m, 1H, Ar); 7.47 (d, J = 15.6 Hz, 1H, Ar-CH=CH); 6.99 (d, J = 1.6 Hz, 1H, Ar); 6.90 (dd, J1 = 8.0 Hz, J2 = 1.6 Hz, 1H, Ar); 6.76 (d, J = 8.0 Hz, 1H, Ar); 6.17 (d, J = 15.6 Hz, 1H, Ar-CH=CH); 4.98 (s, 2H, O-CH2). 13C NMR (100 MHz, CD3OD) δ: 167.2; 149.1; 146.8; 143.4; 138.5; 133.8; 131.8 (q, 2JC-F = 32.0 Hz); 130.3; 128.0; 126.8; 126.3; 125.6 (q, 1JC-F = 271.4 Hz); 122.3; 116.5; 115.1; 114.2; 78.3. m/z ESI-MS: [M + H]+ 354.09.
(E)-4-(3,4-dihydroxyphenyl)-N-((2-(trifluoromethyl)benzyl)oxy)acrylamide (2c) The crude product was purified by ISOLUTE (CHCl3/MeOH 80:1) furnishing compound 2c as a white solid. Yield: 17%; m.p.: 188–190 °C. 1H-NMR (400 MHz, CD3OD) δ: 7.70 (d, J = 8.4 Hz, 2H, Ar); 7.65 (d, J = 8.4 Hz, 2H, Ar); 7.48 (d, 1H, J = 15.6 Hz, Ar-CH=CH); 6.99 (d, J = 2.0 Hz, 1H, Ar); 6.90 (dd, J1 = 8.0 Hz, J2 = 2.0 Hz, 1H, Ar); 6.76 (d, J = 8.0 Hz, 1H, Ar); 6.16 (d, J = 15.6 Hz, 1H, Ar-CH=CH); 5.00 (s, 2H, O-CH2). 13C NMR (100 MHz, CD3OD) δ: 167.0; 148.9; 146.6; 143.2; 141.4; 131.4 (q, 2JC-F = 32.4 Hz); 130.3; 127.7; 126.2; 125.44 (q, 1JC-F = 271.3 Hz); 122.1; 116.2; 114.8; 113.9; 79.3. m/z ESI-MS: [M + H]+ 354.09.
(E)-3-(2,4-dihydroxyphenyl)-N-((2-(trifluoromethyl)benzyl)oxy)acrylamide (3a) The crude product was purified by ISOLUTE (CHCl3/MeOH 80:1) furnishing compound 3a as a white solid. Yield: 20%; m.p.: 165–167 °C. 1H-NMR (400 MHz, CD3OD) δ: 7.83–7.79 (m, 2H, Ar); 7.73 (d, J = 7.6 Hz, 1H, Ar); 7.68 (t, J = 7.6 Hz, 1H, Ar); 7.55 (t, J = 7.6 Hz, 1H, Ar); 7.27–7.25 (m, 1H, Ar); 6.40 (d, J = 16.4 Hz, 1H, Ar-CH=CH); 6.31–6.29 (m, 2H, Ar); 5.12 (s, 2H, O-CH2). 13C NMR (100 MHz, CD3OD) δ: 168.3; 161.8; 159.9; 139.4; 135.5; 133.4; 132.4; 131.6; 129.7; 129.4; 126.9; 125.7 (q, 1JC-F = 273.1 Hz); 115.1; 113.6; 108.6; 103.5; 75.0. m/z ESI-MS: [M + H]+ 354.09.
(E)-3-(2,4-dihydroxyphenyl)-N-((3-(trifluoromethyl)benzyl)oxy)acrylamide (3b) The crude product was purified by ISOLUTE (CHCl3/MeOH 80:1) furnishing compound 3b as a white solid. Yield: 24%; m.p.: 157–158 °C. 1H-NMR (400 MHz, CD3OD) δ: 7.81–7.77 (m, 2H, Ar); 7.72 (d, J = 7.6 Hz, 1H, Ar); 7.66 (d, J = 7.6 Hz, 1H, Ar); 7.58 (t, J = 7.6 Hz, 1H, Ar); 7.26–7.24 (m, 2H, Ar); 6.40 (d, J = 16.0 Hz, 1H, Ar-CH=CH); 6.30–6.29 (m, 2H, Ar); 4.98 (s, 2H, O-CH2). 13C NMR (100 MHz, CD3OD) δ: 166.9; 160.4; 158.5; 138.1; 132.4; 130.9; 130.6; 130.3; 128.9; 125.3; 124.8; 122.9; 113.7; 112.3; 107.2; 102.2; 76.9. m/z ESI-MS: [M + H]+ 354.09.
(E)-3-(2,4-dihydroxyphenyl)-N-((4-(trifluoromethyl)benzyl)oxy)acrylamide (3c) The crude product was purified by ISOLUTE (CHCl3/MeOH 80:1) furnishing compound 3c as a white solid. Yield: 28%; m.p.: 176–178 °C. 1H-NMR (400 MHz, CD3OD) δ: 7.81 (d, J = 16.0 Hz, 1H, Ar); 7.73–7.66 (m, 4H, Ar); 7.28–7.26 (m, 1H, Ar); 6.41 (d, J = 16.0 Hz, 1H, Ar-CH=CH); 6.33–6.31 (m, 2H, Ar); 5.01 (s, 2H, O-CH2). 13C NMR (100 MHz, CD3OD) δ: 168.3; 161.8; 159.9; 141.7; 139.4; 131.7; 131,4; 130.5; 126.3; 125.7 (q, 1JC-F = 271.2 Hz); 115.1; 113.6; 108.6; 103.5; 78.2. m/z ESI-MS: [M + H]+ 354.09.
2-propyl-N-((2-(trifluoromethyl)benzyl)oxy)pentanamide (4a) The crude product was purified by ISOLUTE (hexane/AcOEt 15:1) furnishing compound 4a as a white solid. Yield: 34%; m.p.: 73–75 °C. 1H-NMR (400 MHz, CD3OD) δ: 7.80 (d, J = 8.0 Hz, 1H, Ar); 7.71 (d, J = 8.0 Hz, 1H, Ar); 7.66 (t, J = 8.0 Hz, 1H, Ar); 7.52 (t, J = 8.0 Hz, 1H, Ar); 5.07 (s, 2H, O-CH2); 2.02 (sept, J = 4.8 Hz, 1H, CHCO); 1.59–1.50 (m, 2H, CH-CH2); 1.38–1.25 (m, 2H, CH-CH2); 1.24–1.18 (m, 4H, 2 CH2-CH2); 0.88 (t, 6H, J= 7.2 Hz, 2 CH3). 13C NMR (100 MHz, CD3OD) δ: 175.7; 135.6; 133.4; 132.1; 129.7; 129.3; 126.8; 125.7 (q, 1JC-F = 272.9 Hz); 74.8; 44.3; 35.0; 21.6; 14.3. m/z ESI-MS: [M + H]+ 318.17.
2-propyl-N-((3-(trifluoromethyl)benzyl)oxy)pentanamide (4b) The crude product was purified by ISOLUTE (hexane/AcOEt 15:1) furnishing compound 4b as a white solid. Yield: 30%; m.p.: 74–76 °C. 1H-NMR (400 MHz, CD3OD) δ: 7.74 (s, 1H, Ar); 7.70 (d, J = 7.6 Hz, 1H, Ar); 7.65 (d, J = 7.6 Hz, 1H, Ar); 7.57 (t, J = 7.6 Hz, 1H, Ar); 4.93 (s, 2H, O-CH2); 1.97 (sept, J = 4.8 Hz, 1H, CHCO); 1.56–1.47 (m, 2H, CH-CH2); 1.35–1.29 (m, 2H, CH-CH2); 1.19 (sex, J = 7.2 Hz, 4H, 2 CH2-CH2); 0.86 (t, J = 7.2 Hz, 6H, 2 CH3). 13C NMR (100 MHz, CD3OD) δ: 175.5; 138.5; 133.9; 131.7 (q, 2JC-F = 32.0 Hz); 130.3; 126.9; 126.3; 125.6 (q, 1JC-F = 271.4 Hz); 77.9; 44.4; 36.0; 21.6; 14.2. m/z ESI-MS: [M + H]+ 318.17.
2-propyl-N-((4-(trifluoromethyl)benzyl)oxy)pentanamide (4c) The crude product was purified by ISOLUTE (hexane/AcOEt 15:1) furnishing compound 4c as a white solid. Yield: 23%; m.p.: 78–80 °C. 1H-NMR (400 MHz, CD3OD) δ: 7.69–7.67 (m, 2H, Ar); 7.64–7.61 (m, 2H, Ar); 4.93 (s, 2H, O-CH2); 1.96 (sept, J = 4.9 Hz, 1H, CHCO); 1.56–1.47 (m, 2H, CH-CH2); 1.36–1.29 (m, 2H, CH-CH2); 1.19 (sex, J = 7.2 Hz, 4H, 2 CH2-CH2); 0.85 (t, J = 7.2 Hz, 6H, 2 CH3). 13C NMR (100 MHz, CD3OD) δ: 175.2; 141.4; 131.3 (q, 2JC-F = 32.3 Hz); 130.3; 126.0; 125.41 (q, 1JC-F = 271.1 Hz); 77.6; 44.2; 35.8; 21.4; 14.0. m/z ESI-MS: [M + H]+ 318.17.
(R)-5-(1,2-dithiolan-3-yl)-N-((2-(trifluoromethyl)benzyl)oxy)pentanamide (5a) The crude product was purified by ISOLUTE (hexane/AcOEt in gradient 10:1, 5:1, 4:1 and 2:1) furnishing compound 5a, vitreous solid. Yield: 39%. 1H-NMR (400 MHz, CDCl3) δ: 8.32 (bs, 1H, NHCO); 7.69–7.63 (m, 2H, Ar); 7.56–7.53 (m, 1H, Ar); 7.43–7.41 (m, 1H, Ar); 5.07 (s, 2H, O-CH2); 3.54–3.49 (m, 1H, CHS); 3.17–3.04 (m, 2H, CH2-S); 2.45–2.37 (m, 1H, H-CH-CH2S); 2.07 (bs, 2H, CH2CO); 1.90–1.82 (m, 1H, H-CH-CH2S); 1.70–1.55 (m, 4H, 2 CH2), 1.43–134 (m, 2H, CH2). 13C NMR (100 MHz, CDCl3) δ: 170.6; 132.1; 131.1; 128.5; 126.0; 125.4; 122.7; 74.2; 56.3; 40.2; 38.4; 34.5; 32.7; 28.7; 24.9. m/z ESI-MS: [M + H]+ 380.10.
(R)-5-(1,2-dithiolan-3-yl)-N-((3-(trifluoromethyl)benzyl)oxy)pentanamide (5b) The crude product was purified by ISOLUTE (hexane/AcOEt in gradient 20:1, 10:1, 5:1, 2:1) furnishing compound 5b, vitreous solid. Yield: 29%. %. 1H-NMR (400 MHz, CD3OD) δ: 7.76 (s, 1H, Ar); 7.73–7.65 (m, 2H, Ar); 7.59–7.54 (m, 1H, Ar); 4.93 (s, 2H, O-CH2); 3.58–3.51 (m, 1H, CH-S); 3.20–3.06 (m, 2H, CH2-S); 2.44 (sex, J = 6.4 Hz, 1H, H-CH-CH2S); 2.07 (t, J = 7.3 Hz, 2H, CH2CO); 1.86 (sex, J= 6.4 Hz, 1H, H-CH-CH2S); 1.71–1.55 (m, 4H, 2 CH2); 1.46–128 (m, 2H, CH2). 13C NMR (100 MHz, CD3OD) δ: 172.8; 138.4; 133.8, 131.7 (q, 2JC-F = 32.0 Hz); 130.3; 126.8; 126.3; 125.6 (q, 1JC-F = 271.4 Hz); 78.0; 57.4; 41.3; 39.3; 35.6; 33.5; 29.7; 26.3. m/z ESI-MS: [M + H]+ 380.10.
(R)-5-(1,2-dithiolan-3-yl)-N-((4-(trifluoromethyl)benzyl)oxy)pentanamide (5c) The crude product was purified by ISOLUTE (hexane/AcOEt in gradient, 10:1, 5:1, 3:1) furnishing compound 5c, vitreous solid. Yield: 19%. 1H-NMR (400 MHz, CD3OD) δ: 7.70–7.68 (m, 2H, Ar); 7.63–7.61 (m, 2H, Ar); 4.93 (s, 2H, O-CH2); 3.57–3.53 (m, 1H, CHS); 3.19–3.06 (m, 2H, CH2-S); 2.47 (sex, J = 6.4 Hz, 1H, H-CH-CH2S); 2.08 (t, J = 7.3 Hz, 2H, CH2CO); 1.86 (sex, J = 6.4 Hz, 1H, H-CH-CH2S); 1.71–1.55 (m, 4H, 2 CH2); 1.46–128 (m, 2H, CH2). 13C NMR (100 MHz, CD3OD) δ: 172.9; 141.6; 131.6 (q, 2JC-F = 32.2 Hz); 130.6; 126.4; 125.6 (q, 1JC-F = 271.0 Hz); 77.9; 57.5; 41.3; 39.4; 35.6; 33.5; 29.7, 26.3. m/z ESI-MS: [M + H]+ 380.10.
2.2. UV–Vis Metal Binding Studies
The synthesized compounds were tested for their metal chelating effect through spectroscopic measurements [59,60]. The UV–Vis absorbance of compounds was recorded in the absence and presence of metal ions using a UV–Vis spectrometer (a SPECTROstarNano (220–1000 nm), Ortenberg, Germany. The experiment was performed in 96-well plates and each experiment was made in triplicates. A fixed amount of investigated ligand (20 μM) solubilized in MeOH was mixed with increasing concentration of Cu2+ (CuCl2), Fe2+ (FeSO4) and Zn2+ (ZnCl2) from 0 to 80 μM and incubated for 30 min at room temperature (r.t.). The absorbance change was monitored at wavelengths ranging from 220 to 700 nm. The stoichiometry of studied compounds for Cu2+ and Fe2+ complexes was determined by employing the mole ratio method.
Due to a non-measurable UV absorbance for compounds 4a and 5c, a different protocol using pyrocatechol violet (PV) was applied [61]. The compounds (200 µM) incubated for 10 min with CuSO4 (200 µM), after 200 µM PV was added and the plate was incubated at 25 °C for 20 min. The absorption spectra were recorded. The same protocol was used for Fe2+ (FeSO4) in presence of ferrozine.
2.3. Investigation of Antioxidant Capacity
2.3.1. DPPH Radical Scavenging Assay
The DPPH assay was carried out in a 96-microwell plate according to a previously described protocol [62]. In brief, DPPH solution (60 µM in MeOH) was added to studied compounds or MeOH as the control. The 96-microwell plate was incubated (in the dark, at r.t. for 1 h) and the absorbance values were read at 517 nm by a SPECTROstarNano (220–1000 nm) UV–Vis spectrophotometer, Ortenberg, Germany. During the first screening, all compounds were tested ad 100 µM, then for compounds 2a–c the EC50 (effective concentration), the concentration of substrate that causes a 50% reduction in the DPPH colour, was calculated.
All experiments were made in triplicate. The percentage of the antioxidant activity (%AA) of the compounds was determined according to Equation (1):
%AA = ((AbsDPPH − Abssample)/AbsDPPH) × 100
AbsDPPH is the absorbance of DPPH solution, and Abssample is the absorbance of DPPH solution containing the tested compound.
The DPPH radical scavenging ability of the most active compounds 2a–c was expressed as Trolox equivalent antioxidant capacity (TEAC) value by using Trolox standard regression curve [63]. Each concentration was tested in triplicate and the EC50 value was reported as the mean ± the standard error of the mean (SEM) of three independent experiments.
2.3.2. ABTS Assay
The ABTS assay was performed according to the methods reported in literature [64,65]. Briefly, 7 mM of ABTS was mixed with 2.45 mM of K2S2O8 solution, ratio 1:1, in 10 mM phosphate buffer (pH 7.4). The mixture was incubated in a dark box at r.t. for 12 h, then the stock solution was diluted until the absorbance was 0.70 ± 0.02 at 734 nm.
In a 96-well plate, 190 µL ABTS working solution and 10 µL sample solution (compounds 2a–c, 3a–c at concentration of 100 μM) were mixed and the absorbance was measured (734 nm) using a SPECTROstarNano (220–1000 nm) UV–Vis spectrophotometer, Ortenberg, Germany. Then, for compounds 2a–c the EC50 was calculated and the values were expressed in TEAC equivalents [66,67]. Each concentration was tested in triplicate and the EC50 was reported as the mean ± SEM of three independent experiments.
2.3.3. Thiobarbituric Acid Reagent Substance (TBARS) Assay
The TBARS assay was carried on in accordance with a previously published protocol [62]. Briefly, the rat brain, freshly isolated, was homogenized in 10% (w/v) phosphate buffer, pH 7.4. The stock solutions of compounds 2a–c and 3a–c (10 mM in DMSO) were prepared and then diluted at three different concentrations (1 mM, 100 µM, and 10 µM) with phosphate buffer pH 7.4. The experiment was performed in a falcon tube where the rat brain homogenate (100 µL) was added to FeCl3 (20 µM) and ascorbic acid (100 µM) with or without test compounds. The final volume of 1 mL volume was achieved with phosphate buffer at pH 7.4. All samples were heated at 37 °C and were stirred for 30 min. After this period, thiobarbituric acid (1% w/v in 0.05 N NaOH) and 25% v/v HCl were added and the falcon tubes were boiled for 10 min. Then, they were cooled in an ice-cold water bath. Finally, the extraction was executed using n-butanol (3 mL) and the tubes were centrifuged at 2000 g for 10 min. The organic portion was transferred into 96-well plates and the absorbance, at 532 nm, was recorded using a SPECTROstarNano (220–1000 nm) UV–Vis spectrophotometer, Ortenberg, Germany. The TBARS was reported as nmoles of malondialdehyde (MDA)/10 mg of rat brain tissue, by interpolation with the standard curve of 1,1,3,3-tetramethoxypropane. Data represent the mean ± SEM of the triplicate.
2.4. ANS Competitive Binding Assay
The binding of ANS (8-anilino-1-naphthalenesulfonic acid), Sigma-Aldrich (St. Louis, MO, USA) and its displacement by the three selected compounds 2a–c were studied using Human Plasma TTR (Merck Millipore, Molsheim, France) according to the protocol previously described [68,69]. Briefly, TTR (8 µM) was incubated with ANS (16 µM) at r.t. for 15 min in 96-well plates. Then, compounds 2a–c at concentrations of 100 µM were added. After 10 min, the fluorescent emission spectra (400–540 nm) were recorded exciting at 280 nm using Molecular Devices SpectraMax Gemini XPS plate reader, (San Jose, CA, USA). For compounds 2a, the IC50 was calculated and value was expressed as the mean ± SEM of the triplicate. All experiments were made in triplicate and, the fluorescence increases of ANS bound to TTR solution compared to its control without TTR, and TTR alone, in phosphate buffer, were used as a control.
2.5. Thioflavin T (ThT) Fluorescence Assay
Aβ1-40 was obtained from Bachem (Bubendorf, Switzerland). To remove preformed aggregates, it was dissolved during 15–20 min at r.t. in 1% NH4OH to give a concentration of 2 mg/mL, followed by immediate lyophilisation. The Aβ1-40 peptide was then dissolved in an aqueous 1% ammonia solution to a concentration of 1 mM and then, just prior to use, was diluted to 0.2 mM with 10 mM Tris-HCl, 100 mM NaCl buffer (pH 7.4). Compounds (2a–c) were dissolved in DMSO (4.9 mM), while TTR in phosphate buffer 20 mM buffer (pH 7.4) at 1 mM concentration. ThT fluorescence was measured to evaluate the development of Aβ1-40 fibrils over time using a fluorescence plate reader (Vantastar, BMG lab-tech, Ortenberg, Germany) with standard 96-well black microliter plates. The ThT fluorescence intensity was recorded by using excitation/emission settings at 440−15 nm/480−15nm for 21 h and applying a double orbital shaking of 10 s before the first cycle. Experiments were triggered by adding Aβ1-40 achieving a final concentration of 10 μM into a mixture containing 40 μM ThT in 10 mM Tris-HCl, 100 mM NaCl buffer (pH 7.4) with and without the studied compounds (10 μM) at 25 °C, and with and without CuCl2 (10 µM). When TTR was assessed in the presence of compound 2a, the ratio between TTR and 2a was adjusted to 1:2 (TTR:2a) in order to let the compound interacting with both hydrophobic pockets. A time of 3 h of incubation was employed before adding the solution into the well. The F reduction is depicted as the intensity of experimental fluorescence plateau observed with the studied compound relative to the value obtained without the compound and is quantified as the following percentage: ((F Aβ1-40+compound − F Aβ1-40)/(F Aβ1-40)) × 100. The data of fluorescence intensity come from at least two independent experiments, where each condition has been performed in duplicate, in the case of the presence of TTR (10 µM), or triplicate, in the case of the absence of TTR (10 µM), respectively. The values are given as averaged percentage ± SEM.
2.6. Statistical Analysis
The statistical analysis was performed using the GraphPad Prism software, version 8.0 (GraphPad Software Inc., San Diego, CA, USA). Data were presented as the mean ± standard error (SEM) of at least two independent experiments.
3. Results
3.1. Chemistry
The studied trifluoromethyl benzyloxyamidic compounds 1a–c, 2a–c, 3a–c, 4a–c, 5a–c (Figure S1) were synthesized according to the synthetic procedure reported in Scheme 1, following the method previously described [56]. The O-arylmethylhydroxylamine hydrochloride 6a–c were prepared applying the synthetic route already described [70,71]. Briefly, the derivatives 6a–c were obtained by reaction between the appropriate benzyl bromide (2–CF3, 3–CF3 and 4–CF3 substituted) and the N-hydroxyphalimide. Then, the deprotection of the phtalimido group was carried on by ammonia solution 7 N in MeOH and the compounds 6a–c were precipitated as hydrochloride salts. The coupling reaction of the free amino group of derivatives 6a–c with the appropriate natural acids 7–11 (7: cinnamic acid; 8: caffeic acid; 9: umbellic acid; 10: valproic acid; 11: lipoic acid commercially available) was carried out in anhydrous DMF and under inert argon atmosphere, in the presence of the carboxyl activating agent N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDCI), hydroxybenzotriazole (HOBt) and N-methylmorfoline to give the final products 1a–c, 2a–c, 3a–c, 4a–c, 5a–c as white solids.
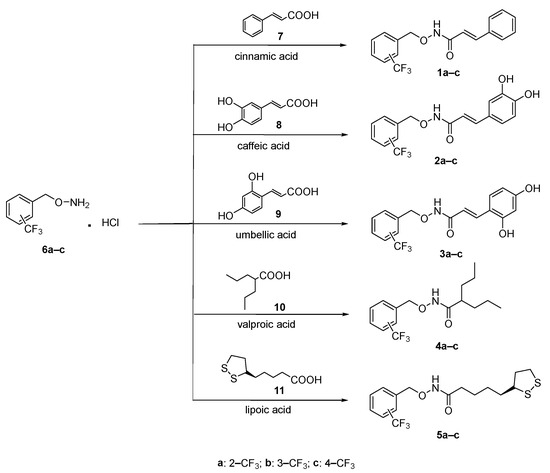
Scheme 1.
General procedure for the synthesis of compounds 1a–c, 2a–c, 3a–c, 4a–c, 5a–c. Reaction conditions: EDCI, HOBt, N-methylmorpholine, anhydrous DMF, r.t., 24 h.
For compounds 1a–c, 2a–c, 3a–c, only one of the two possible E/Z configurational isomers was obtained. The E configuration was attributed by 1H-NMR spectra. The characteristics proton Ar-CH=CH gave ppm values between 7.62–7.47 according to data reported in literature [56,70].
3.2. Metal Chelating Properties
The ability of the synthesized compounds 1a–c, 2a–c, 3a–c, 4a–c, 5a–c to chelate biometals, such as Zn2+, Fe2+ and Cu2+, was studied by UV–Vis spectrometry. The first screening was executed by comparing the spectra of the o-CF3 substituted of each triad (1a, 2a, 3a, 4a, 5a at 20 μM) without metal, with those, of the same molecules, in the presence of Zn2+, Fe2+ and Cu2+ (80 µM) [59]. Compounds 1a, 2a, 3a showed a significant shift in UV spectrum in the presence of Cu2+, while only compounds 2a displayed a weak change when Fe2+ was added. No detectable shift for all investigated compounds was recorded in the presence of Zn2+. A representative spectra obtained during the first screening is reported for derivatives 2a, Figure 2.
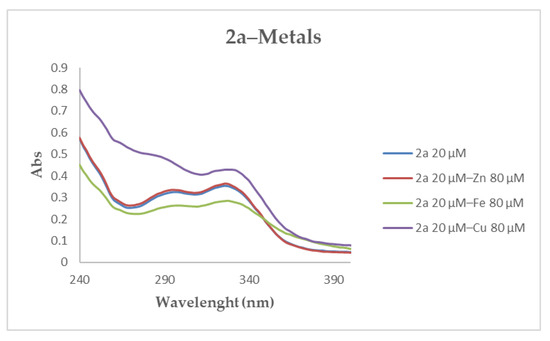
Figure 2.
UV spectra of compound 2a alone and in presence of Zn2+, Fe2+ and Cu2+ in MeOH.
Due to the absence of a remarkable absorbance in the UV spectra of compounds 4a and 5a (Figure S2), their ability to chelate biometals was studied using the colorimetric methods that employs ferrozine and PV (pyrocatechol violet) reagents for Fe2+ and Cu2+, respectively. No interaction between 4a and 5a was recorded in presence of Fe2+, while a slight shift appeared with Cu2+ (Figure S3).
Starting from these preliminary results, the derivatives 1a, 2a–c, and 3a were selected to quantify their ability to chelate Cu2+ using molar ratio method. Therefore, the concentration of the ligands (1a, 2a, 3a) was the same (20 µM) in each experiment while the concentration of the Cu2+ was increased from 0 µM to 80 µM as shown in Figure 3A–C.
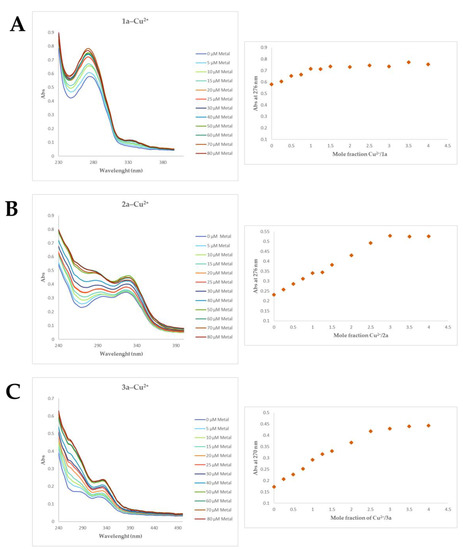
Figure 3.
UV spectra of selected compounds and the stoichiometry of the complex [Ligand–Cu2+]. (A) 1a–cu2+; (B) 2a–cu2+ and (C) 3a–cu2+.
The spectra of compound 1a in the presence of increased concentration of Cu2+ displayed a shift in absorbance from 0.577 to 0.755 at 276 nm. The molar fraction analysis suggested that, under the experimental conditions, the stoichiometry for the Cu2+–1a complex was 1.0, indicating a complex with 1:1 [Cu2+–1a] molar ratio, Figure 3A. Compounds 2a–c and 3a, in the presence of increasing concentration of Cu2+, showed a remarkable shift up in absorbance of UV spectra from 260 to 360 nm, Figure 3B,C and Figures S4 and S5. Regarding the compounds 2a–c and 3a, the stoichiometry for the [Cu2+–ligand] complex is around 2, suggesting a complex with 2:1 [Cu2+–ligand] molar ratio.
During the first screening with Fe2+ (80 µM), compound 2a showed a detectable shift down in absorbance at two different wavelengths: 294 and 330 nm, Figure 4. In order to determine the stoichiometry of the complex 2a–Fe2+, a series of solutions where the concentration of 2a was maintained the same while the amount of Fe2+ was increased from 0 to 30 µM (plateaus), were tested. As reported in Figure 4, plotting the absorbance changes at 330 nm the molar fraction found is 1, indicating a stoichiometry 1:1 of compound 2a.
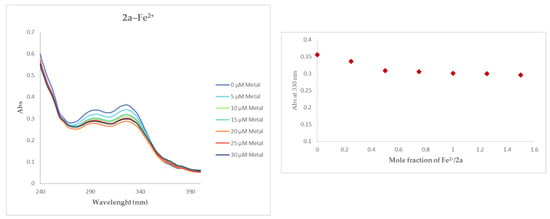
Figure 4.
UV spectrum of compound 2a–Fe2+and the stoichiometry of the complex.
3.3. Antioxidant Properties of Synthesized Compounds
3.3.1. DPPH Assay
The %AA of all newly synthesized compounds (1a–c, 2a–c, 3a–c, 4a–c, 5a–c) was determined using the DPPH assay. The first screening was performed by testing all derivatives at 100 µM, and compounds 2a–c, with catechol, gave high %AA (2a 95%; 2b 96%; 2c 96%), compound 3a–c gave a %AA around 60 (3a 59%; 3b 60%; 2c 58%) while no antioxidant activity was detected for compounds 1a–c, 4a–c, 5a–c (%AA 0). Starting from these results, molecules 2a–c were studied in order to quantify the EC50 and the values were then expressed as TEAC, Table 1. The low EC50 values between 6 and 8.9 µM indicate that compounds 2a–c possess a greater free radical scavenging activity than the caffeic acid (EC50 13.3 µM) [72].

Table 1.
Half maximal effective concentration EC50, Trolox equivalent antioxidant capacity (TEAC).
3.3.2. ABTS Assay
The synthesized compounds 2a–c, 3a–c were selected for a first screening at 100 µM. At this concentration, resorcinol derivatives showed good radical scavenger activity (3a 68%, 3b 67%, 3c 65%).
Then, considering the elevate antioxidant activity found for compounds 2a–c (2a 99.1%, 2b 98.8% and 2c 97.3%) they were tested at different concentrations in order to determinate the EC50. The EC50 was expressed in TEAC values, as reported in Table 2. Compounds 2a (EC50 9.9 µM) and 2b (EC50 13.4 µM) showed an EC50 value comparable to caffeic acid (10.9 µM [72]) while derivative 2c displayed an EC50 of 22.95 µM, slightly higher than parent natural acid.

Table 2.
ABTS assay: EC50 of compounds 2a–c and its expression in TEAC.
3.3.3. TBARS Production in the Rat Brain
The antioxidant properties of derivatives 2a–c and 3a–c were studied ex vivo on rat brain. The hydroxyl radical-dependent lipid peroxidation induced in rat brain homogenate by the oxidant system Fe3+/ascorbic acid, that initiates the Fenton reaction, was investigated in absence and in presence of selected compounds Figure 5. Compounds 2a–c and 3a–c were tested at three different concentrations (1 μM, 10 μM and 100 μM) and the result was expressed in nmoles of MDA in 10 mg of rat brain tissue. A slight antioxidant activity was recorded when 3a–c were tested at 100 μM, while the antioxidant effect became weaker at 10 μM and it is almost lost at 1 μM. In contrast, compounds 2a–c showed a good antioxidant effect at 100 μM. In particular, derivatives 2a–b kept a relevant antioxidant activity at 10 μM and 2b maintained its profile also at 1 μM.
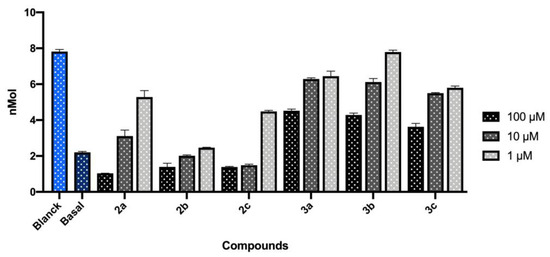
Figure 5.
Effect of compounds 2a–c and 3a–c on the TBARS production in the rat brain. In light blue blank and in blue basal brain activity. Data represent the mean ± SEM of the triplicate.
3.4. Displacement of ANS
The ability of selected compounds 2a–c to bind the TTR-BP was evaluated by the ANS displacement binding test. The derivatives showed a good interaction with TTR at 100 µM, in particular compound 2a displaces ANS of 71%, Figure 6, with an IC50 = 31.9 µM (±0.1 SEM).
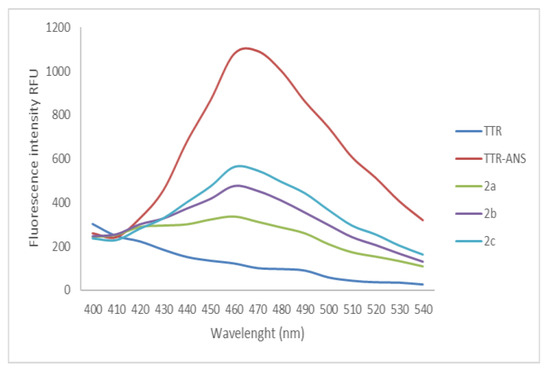
Figure 6.
ANS displacement binding assay for compounds 2a–c at tested at concentration of 100 µM.
4. ThT Test Fluorescence Spectroscopy Assay
According to the results obtained above, we selected compounds 2a–c, the best antioxidant and chelating agents, to evenly assess their ability to reduce the fibril formation of Aβ1-40 peptide. The ThT assay was performed both in absence or in presence of Cu2+ and of TTR. We tested the effect of Cu2+ at stoichiometric concentration (10 µM) on the Aβ1-40 aggregation and we observed an increase of ThT fluorescence intensity over time, compared to Aβ1-40 in the absence of Cu2+ (Figure S6), which is consistent with the literature data [73].
In our conditions, Aβ1-40 showed a typical kinetics curve in which the primary and secondary nucleation steps are bypassed and the dominant mechanism responsible for the consumption of monomeric Aβ1-40 is the elongation of the preformed fibrils, as previously observed by Chiti et al. [21]. Indeed, the amyloid fibril formation was very rapid and without a detectable lag phase, as expected due to the presence of seed fibrils. Under these conditions and without Cu2+, compound 2a was the best compound, having a discrete inhibitory activity at 1:1 ratio (Figure 7 and Figure S7). Its activity resulted to be increased when it was assessed in the presence of Cu2+ where compound 2a showed a completely inhibition of the fibril formation catalysed by the Cu2+ ions, (Figure 7 and Figure S8).
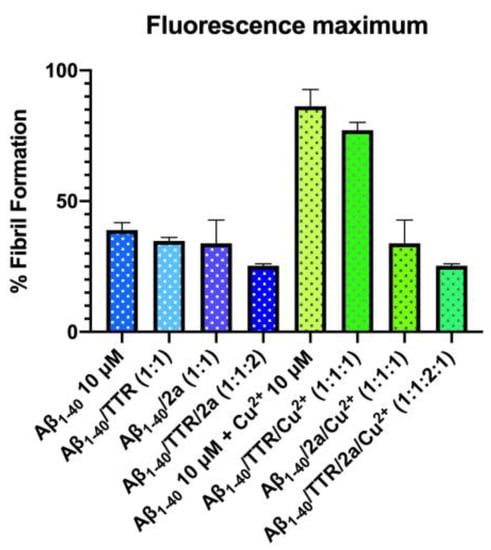
Figure 7.
Percentage of Aβ1-40 fibril formation assessed by ThT fluorescence intensity in the absence and presence of 10 µM of Cu2+, with and without 2a (1:1 ratio) and with or without TTR (10 µM) pre-incubated or not with 2a (20 µM). The values are given as averaged relative fluorescence percentages (mean ± SEM) from triplicates (without TTR) or duplicates (with TTR) of at least two independent experiments.
We then evaluated the effect of TTR under the experimental conditions without Cu2+ (Aβ1-40/TTR, ratio 1:1, Figure 7) and we found a result comparable to the one previously reported by Chiti et al. [21]. In fact, TTR was found not to significantly affect the aggregation kinetics, confirming that Aβ1-40 fibril elongation is not influenced by the presence of tetrameric TTR (Figure S9). A slight positive effect was, however, observed when the tetrameric TTR was previously incubated with compound 2a (1:2 ratio, TTR/2a, Figure 7 and Figure S9). The contribution of TTR without ligand was more visible in the presence of Cu2+ ions (1:1:1 ratio, Aβ1-40/TTR/Cu2+, Figure 7 and Figure S10). This effect was even more remarkable when the TTR was pre-incubated with compound 2a (Figure 7 and Figure S10).
5. Discussion
In the context of a multifactorial neurodegenerative disorder such as AD, we report the synthesis and the characterization of new multifunctional compounds 1a–c, 2a–c, 3a–c, 4a–c, 5a–c that can be considered hybrid structures where a natural acid was covalently conjugate with a promising bioactive moiety (2-(trifluoromethyl)benzyl-hydroxylamine hydrochloride) identified in our precedent work [56]. Considering the results obtained for the ferulic acid (FA) hybrids, that showed a good chelating ability, antioxidant capacity and a moderate activity as inhibitor of Aβ aggregation [56], here we chose cinnamic acid, caffeic acid and umbellic acid in order to investigate the effect of the absence or the presence of more than one hydroxyl group in the new hybrids compared to the previous ones [52,53]. In addition, valproic acid and lipoic acid were selected for their known properties to contrast the AD progression [57,58].
Evidence suggests that the dysregulation of biometals in the brain plays a relevant role in the pathogenesis of AD. In fact, the biometals, such as zinc, iron and copper, are essential for neuronal activity, but at the same time, the loss of metal ion homeostasis is associated with neurodegenerative disorder onset [74,75].
Zn2+ possesses high positive charge density due to its closed shell d10 diamagnetic electron configuration. This characteristic makes Zn2+ able to bind nucleic acids and proteins, and it is a co-factor of more than 300 enzymes [76]. In addition to zinc, iron and copper are transition metals belonging to the fourth period. In the body, iron exists in two main oxidation states II (d6 configuration) and III (d5). Iron forms coordination compounds, especially with ligands containing hydroxide, phenolate and carboxylate. Fe2+ prefers nitrogen ligands, such as amines and imidazole, due to lower charge density. Cu2+ has a d9 electron configuration often characterized by a tetragonal distortion of the coordination geometry due to the Jahn–Teller effect [77]. Cu2+ coordinates with molecules containing oxygen and nitrogen atoms such as amines, amides and heteroaromatics (pyridine, pyridazine and imidazole) [78].
All synthesized derivatives possess a common heteroatomic oxyamidic motive (O–NH–CO-) that potentially can chelate metals, Figure 8. In addition, compounds 2a–c, 3a–c are characterized by a second aromatic portion substituted with hydroxyl groups in catecholic or resorcinol position, commonly known to be able to chelate biometals [2,79], Figure 8.
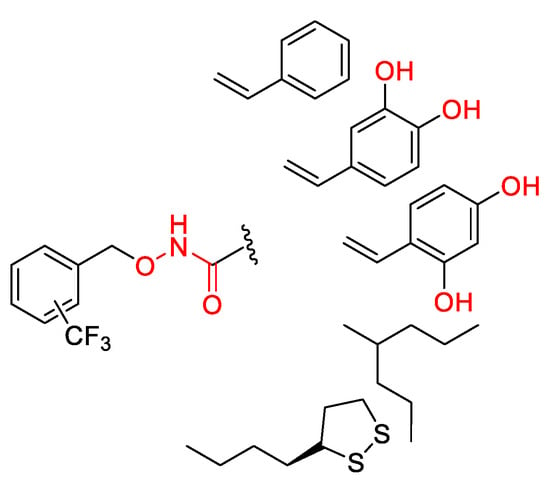
Figure 8.
In red the chemical portions of studied compounds that have been proved to chelate metals.
The ability of the compounds 1a–c, 2a–c, 3a–c, 4a–c, 5a–c to chelate biometals such as Cu2+, Fe2+ and Zn2+ were studied by UV–Vis spectrometer. Studies suggested that phenols substituted in otho- para- or meta- para- positions, potentially can chelate metals as well as molecules which contain the O–NH–CO- motive [78,80]. Unfortunately, despite the presence of different potential Zn2+ chelating groups, under the experimental condition applied, none of the hybrids were able to bind Zn2+. This highlights that the oxyamidic motive is not appropriate to chelate Zn2+, while, regarding the inefficacy of the catechol group, the result agrees with data published where the feeble or the absence of interaction with Zn2+ has been attributed to the high instability of catechol [81].
According to the data reporting in the literature that clearly attributes the metal chelating ability of polyphenols to the presence of ortho-dihydroxy polyphenols (catechol), derivative 2a (Figure 9) chelated Fe2+ [79].
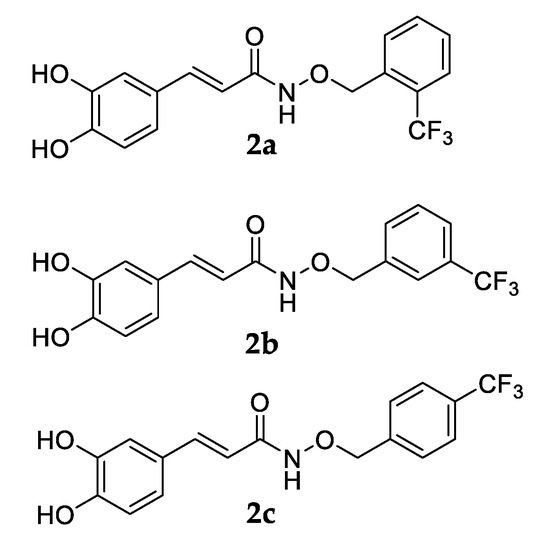
Figure 9.
Chemical structures of compounds 2a–c.
Due to the similarity among 2a–c (Figure 9), which differ only for the –CF3 group position, we can deduce that the catecholic derivatives 2b–c can bind Fe2+ in ratio 1:1.
As mentioned before, the chemical features for a good Cu2+ chelator are the polyhydroxylated moieties, carbonyl and amidic groups [82]. As expected, due to the presence of the linker, all compounds bind Cu2+ and their chelating profiles become better for 2a–c, 3a–c where the additional –OH groups, in catecholic or resorcinol position, increase the stoichiometry of the complex [Ligand–Cu2+] from 1:1 to 1:2 [83]. This new series of hybrid compounds maintains the metal chelation properties of the FA hybrids previously studied [56].
There is a strict connection between the dysregulation of biometals and the oxidative stress that they can induce, favoring the neuronal degeneration. Thus, the results achieved by the metal chelation analysis prompted us to screen 1a–c, 2a–c, 3a–c, 4a–c, 5a–c with the DPPH assay [62]. Considering the good %AA obtained, for the catecholic derivatives 2a–c we calculated the EC50 values (6.0–8.9 μM) confirming that they act as good antioxidant agents stronger than the parent FA hybrids and the caffeic acid [56,72]. Starting from this result, we selected compounds 2a–c and 3a–c (resorcinol derivatives) in order to investigate their radical scavenger activity also by in vitro ABTS assay and ex vivo rat brain using the TBARS assay [62,65]. In line with what is already published, the resorcinol derivatives showed lower antioxidant power compare to catecholic analogues [84,85]. The data obtained from both tests agreed with that of DPPH ones, confirming that, among the synthesized compounds, catecholic derivatives 2a–c have the best antioxidant profile. This evidence suggests that when the chain motive O–NH–CO- is connected to a catecholic group, the resulting molecules act as strong radical scavenger agents. Moreover, the position of the -CF3 (in -ortho, -meta, -para) has a slight effect in the antioxidant power, especially for the ABTS assay. This is probably related to a different conjugation on the aromatic ring. Comparing this result, together with the good Cu2+ and Fe2+ chelating activity that they showed, catecholic derivatives 2a–c were selected to investigate their effect on the kinetic of Aβ aggregation in presence and in absence of Cu2+ and TTR.
Recently, several studies have reported that there is a positive cross-interaction between TTR and Aβ because TTR participates in the Aβ clearance from the brain to the liver [22,38,86]. Even if the mechanism by which TTR acts as neuroprotective protein is still unknown, experimental evidence suggests that the stability of the TTR protein is essential for the positive cross interaction with Aβ [87,88]. One approach to contribute to the TTR stabilization is the use of small molecules able to bind the T4-BPs, avoiding the first step of the tetramer dissociation. Due the morphology of the T4-BPs, compounds characterized by two aromatic groups connected together and, decorated with acid moieties (-OH, -COOH) and/or halogen atoms, can bind the TTR channel occupying the halogen binding pockets (HPBs) [89]. Looking at the chemical structure of compounds 2a–c that remember the classic motif of a TTR binder, it can be hypothesized that they enter into the T4-BPs [90,91,92]. The ANS displacement assay [69] confirmed that the catecholic derivatives 2a–c, at concentration of 100 μM, are able to interact with T4-BPs (Figure 6) and in particular 2a showed the best profile. Thus, it is plausible thinking that the aromatic rings take place into the hydrophobic depression orienting the –CF3 group towards the HBPs while the catecholic towards the hydrophilic amino acids.
Considering all the results achieved, compounds 2a–c were selected to assess if beyond their ability to chelate biometals, to act as antioxidants and to bind TTR they were also capable to reduce the fibril formation of Aβ1-40 peptide. We decided to work with Aβ1-40 because in a previous study it had been observed that Cu2+ accentuated distinct misfolding of Aβ1-40 and Aβ1–42 peptides, with an acceleration of fibril formation of Aβ1-40 in the presence of Cu2+ which was still visible in the ThT test [73]. Thus, we designed a ThT fluorescence spectroscopy assay which simultaneously allowed to study the effect of the compounds on the fibrillization process of Aβ1-40 in the presence or absence of Cu2+ ions. Moreover, applying the same protocol we can also evaluate if the stabilization of TTR tetramer structure, through the interaction of a small molecule with the hydrophobic pockets, can improve the positive cross-interaction between TTR and Aβ1-40; always in the presence or absence of Cu2+ ions.
As previous mentioned, among the catecholic derivatives, compound 2a showed the best inhibitory profile on Aβ1-40 fibrillization process in the absence of Cu2+. Moreover, a more pronounced effect was observed when the fibril formation was catalysed by the Cu2+ ions confirming the strong chelating property of 2a. When the tetrameric TTR was previously incubated with compound 2a (1:2 ratio, TTR/2a), a slight positive effect was observed, suggesting that the interaction between 2a and TTR tetramer can improve its activity on the Aβ1-40 fibril elongation process (Figure 7 and Figure S9). This result is in agreement with data reported in literature [87,93,94].
Under high metal ion conditions, TTR, in absence of ligand, seems to increase its ability to affect the elongation process of Aβ1-40, thus confirming its higher affinity for Aβ1-40 peptide in the presence of Cu2+ [23] (Figure 7 and Figure S10). In addition, this effect is higher when TTR is pre-incubated with 2a before being added to the solution containing Aβ1-40 and Cu2+ (Aβ1-40/TTR/2a/Cu2+, 1:1:2:1 ratio). The last result suggests that a combination of two different effects, such as metal chelation and TTR tetramer stabilization, has a great impact on the inhibition of Aβ1-40 fibril formation, especially in conditions with high metal ion concentration.
6. Conclusions
Due to the multifactorial character of AD, here we propose a series of hybrid compound natural products based as multifunctional agents. Among the newly synthesized compounds, catecholic derivatives 2a–c showed a promising profile. They are good chelators of Cu2+ and Fe2+, two of the main biometals involved in neurodegeneration, as well as great antioxidants. Compound 2a was found to be the best multifunctional molecule. In fact, in addition to being a good biometals chelator and a potent antioxidant agent, it is also capable of interacting with the TTR-binding pockets, strengthening the positive cross-interaction between TTR and Aβ1-40, especially in the presence of Cu2+.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pharmaceutics15102369/s1, Spectra S1–S45: 1H-NMR, 13C-NMR and mass spectra of compounds 1a–c, 2a–c, 3a–c, 4a–c, 5a–c; Figure S1: Chemical structure of all synthesized compounds. Figure S2: UV Spectra of MeOH, compound 4a and 5a; Figure S3: Colorimetric metal chelation study of compounds 4a and 5a. A Derivatives 4a and 5a in presence of PV and Cu2+; B Derivatives 4a and 5a in presence of Ferrozine and Fe2+; Figure S4: UV Spectra of compound 2c with increased concentration of Cu2+ and molar fraction of the complex; Figure S5: UV Spectra of compound 2c with increased concentration of Cu2+ and molar fraction of the complex; Figure S6: Aggregation kinetics curves of Aβ1-40 (10 µM) in the absence (blue) or presence (grey) of 10 µM of Cu2+; Figure S7: Aggregation kinetics curves of Aβ1-40 (10 µM) in the absence (blue) or presence (orange, grey and yellow, respectively) of compounds 2a–c (10 µM); Figure S8: Aggregation kinetics curves of Aβ1-40 (10 µM) with 10 µM of Cu2+ in the absence (blue) or presence (orange) of compound 2a (10 µM); Figure S9: Aggregation kinetics curves of Aβ1-40 (10 µM) in the presence (orange) or absence (blue) of 10 µM of TTR with (grey) or without (orange) the pre-incubation with compound 2a (20 µM); Figure S10: Aggregation kinetics curves of Aβ1-40 (10 µM) and 10 µM of Cu2+ in the presence (orange) or absence (blue) of 10 µM of TTR with (orange) or without (grey) the pre-incubation with compound 2a (20 µM).
Author Contributions
Conceptualization, L.C., S.N., N.T. and E.O.; methodology, L.C., C.C.; N.T. and L.B.; formal analysis, L.C., N.T., L.B. and C.C.; investigation L.C., N.T., C.F.G., L.B., G.P. and J.K.; data curation, L.C., N.T., L.B. and C.C.; writing—L.C., N.T. and C.C.; writing—review and editing S.N., A.R., C.L.M. and E.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Italian Ministry of Education, University, and Research (MIUR) S.N. and E.O., grant number 2017SNRXH3.
Institutional Review Board Statement
All procedures were performed according to European (EEC Directive 2010/63) and Italian (D.L. 4 March 2014 n. 26) legislation (protocol number DB173.N.IXS, 4 January 2023).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank undergraduate Irene Garfagnini and Elisa Mariotti for their contribution to chemical synthesis. We would like to thank Lara Testai for assistance in setting up TBARS experiment.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| AD | Alzheimer’s disease |
| ANS | 8-anilino-1-naphthalenesulfonic acid |
| Aβ | amyloid β |
| CSF | cerebrospinal fluid |
| DPPH | 2,2-Diphenyl-1-Picrylhydrazyl |
| FA | ferulic acid |
| MDA | malondialdehyde |
| OS | oxidative stress |
| ROS | reactive oxygen species |
| SEM | standard error of the mean |
| T4 | thyroxine |
| T4-BPs | thyroxine binding pockets |
| TBARS | Thiobarbituric Acid Reagent Substance |
| TEAC | Trolox equivalent antioxidant capacity |
| ThT | Thioflavin T |
| TLC | Thin Layer Chromatography |
| TTR | Transthyretin |
| wt-TTR | wild type TTR |
References
- Knopman, D.S.; Amieva, H.; Petersen, R.C.; Chételat, G.; Holtzman, D.M.; Hyman, B.T.; Nixon, R.A.; Jones, D.T. Alzheimer Disease. Nat. Rev. Dis. Primers 2021, 7, 33. [Google Scholar] [CrossRef]
- Aaseth, J.; Skalny, A.V.; Roos, P.M.; Alexander, J.; Aschner, M.; Tinkov, A.A. Copper, Iron, Selenium and Lipo-Glycemic Dysmetabolism in Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 9461. [Google Scholar] [CrossRef] [PubMed]
- Fasae, K.D.; Abolaji, A.O.; Faloye, T.R.; Odunsi, A.Y.; Oyetayo, B.O.; Enya, J.I.; Rotimi, J.A.; Akinyemi, R.O.; Whitworth, A.J.; Aschner, M. Metallobiology and Therapeutic Chelation of Biometals (Copper, Zinc and Iron) in Alzheimer’s Disease: Limitations, and Current and Future Perspectives. J. Trace Elem. Med. Biol. 2021, 67, 126779. [Google Scholar] [CrossRef] [PubMed]
- Maret, W. Metallomics: The Science of Biometals and Biometalloids. In Metallomics: The Science of Biometals; Advances in Experimental Medicine and Biology; Arruda, M.A.Z., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–20. ISBN 978-3-319-90143-5. [Google Scholar]
- Liu, F.; Zhang, Z.; Zhang, L.; Meng, R.; Gao, J.; Jin, M.; Li, M.; Wang, X. Effect of Metal Ions on Alzheimer’s Disease. Brain Behav. 2022, 12, e2527. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Nguyen, M.; Robert, A.; Meunier, B. Metal Ions in Alzheimer’s Disease: A Key Role or Not? Acc. Chem. Res. 2019, 52, 2026–2035. [Google Scholar] [CrossRef]
- Wang, L.; Yin, Y.-L.; Liu, X.-Z.; Shen, P.; Zheng, Y.-G.; Lan, X.-R.; Lu, C.-B.; Wang, J.-Z. Current Understanding of Metal Ions in the Pathogenesis of Alzheimer’s Disease. Transl. Neurodegener. 2020, 9, 10. [Google Scholar] [CrossRef]
- Lovell, M.A.; Robertson, J.D.; Teesdale, W.J.; Campbell, J.L.; Markesbery, W.R. Copper, Iron and Zinc in Alzheimer’s Disease Senile Plaques. J. Neurol. Sci. 1998, 158, 47–52. [Google Scholar] [CrossRef]
- Andreadis, A. Misregulation of Tau Alternative Splicing in Neurodegeneration and Dementia. Prog. Mol. Subcell. Biol. 2006, 44, 89–107. [Google Scholar] [CrossRef]
- Rudinskiy, N.; Fuerer, C.; Demurtas, D.; Zamorano, S.; De Piano, C.; Herrmann, A.G.; Spires-Jones, T.L.; Oeckl, P.; Otto, M.; Frosch, M.P.; et al. Amyloid-Beta Oligomerization Is Associated with the Generation of a Typical Peptide Fragment Fingerprint. Alzheimer’s Dement. 2016, 12, 996–1013. [Google Scholar] [CrossRef]
- Roda, A.R.; Serra-Mir, G.; Montoliu-Gaya, L.; Tiessler, L.; Villegas, S. Amyloid-Beta Peptide and Tau Protein Crosstalk in Alzheimer’s Disease. Neural Regen. Res. 2022, 17, 1666. [Google Scholar] [CrossRef]
- Liao, L.; Cheng, D.; Wang, J.; Duong, D.M.; Losik, T.G.; Gearing, M.; Rees, H.D.; Lah, J.J.; Levey, A.I.; Peng, J. Proteomic Characterization of Postmortem Amyloid Plaques Isolated by Laser Capture Microdissection. J. Biol. Chem. 2004, 279, 37061–37068. [Google Scholar] [CrossRef]
- Biza, K.V.; Nastou, K.C.; Tsiolaki, P.L.; Mastrokalou, C.V.; Hamodrakas, S.J.; Iconomidou, V.A. The Amyloid Interactome: Exploring Protein Aggregation. PLoS ONE 2017, 12, e0173163. [Google Scholar] [CrossRef] [PubMed]
- Thompson, T.B.; Chaggar, P.; Kuhl, E.; Goriely, A.; Alzheimer’s Disease Neuroimaging Initiative. Protein-Protein Interactions in Neurodegenerative Diseases: A Conspiracy Theory. PLoS Comput. Biol. 2020, 16, e1008267. [Google Scholar] [CrossRef] [PubMed]
- Rehman, A.U.; Khurshid, B.; Ali, Y.; Rasheed, S.; Wadood, A.; Ng, H.-L.; Chen, H.-F.; Wei, Z.; Luo, R.; Zhang, J. Computational Approaches for the Design of Modulators Targeting Protein-Protein Interactions. Expert Opin. Drug Discov. 2023, 18, 315–333. [Google Scholar] [CrossRef]
- Clinton, L.K.; Blurton-Jones, M.; Myczek, K.; Trojanowski, J.Q.; LaFerla, F.M. Synergistic Interactions between Aβ, Tau, and α-Synuclein: Acceleration of Neuropathology and Cognitive Decline. J. Neurosci. 2010, 30, 7281–7289. [Google Scholar] [CrossRef]
- Pascoal, T.A.; Mathotaarachchi, S.; Shin, M.; Benedet, A.L.; Mohades, S.; Wang, S.; Beaudry, T.; Kang, M.S.; Soucy, J.-P.; Labbe, A.; et al. Synergistic Interaction between Amyloid and Tau Predicts the Progression to Dementia. Alzheimer’s Dement. 2017, 13, 644–653. [Google Scholar] [CrossRef]
- Busche, M.A.; Hyman, B.T. Synergy between Amyloid-β and Tau in Alzheimer’s Disease. Nat. Neurosci. 2020, 23, 1183–1193. [Google Scholar] [CrossRef] [PubMed]
- Ciccone, L.; Shi, C.; di Lorenzo, D.; Van Baelen, A.-C.; Tonali, N. The Positive Side of the Alzheimer’s Disease Amyloid Cross-Interactions: The Case of the Aβ 1-42 Peptide with Tau, TTR, CysC, and ApoA1. Molecules 2020, 25, 2439. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Murphy, R.M. Characterization of the Interaction of β-Amyloid with Transthyretin Monomers and Tetramers. Biochemistry 2010, 49, 8276–8289. [Google Scholar] [CrossRef]
- Ghadami, S.A.; Chia, S.; Ruggeri, F.S.; Meisl, G.; Bemporad, F.; Habchi, J.; Cascella, R.; Dobson, C.M.; Vendruscolo, M.; Knowles, T.P.J.; et al. Transthyretin Inhibits Primary and Secondary Nucleations of Amyloid-β Peptide Aggregation and Reduces the Toxicity of Its Oligomers. Biomacromolecules 2020, 21, 1112–1125. [Google Scholar] [CrossRef]
- Alemi, M.; Gaiteiro, C.; Ribeiro, C.A.; Santos, L.M.; Gomes, J.R.; Oliveira, S.M.; Couraud, P.-O.; Weksler, B.; Romero, I.; Saraiva, M.J.; et al. Transthyretin Participates in Beta-Amyloid Transport from the Brain to the Liver- Involvement of the Low-Density Lipoprotein Receptor-Related Protein 1? Sci. Rep. 2016, 6, 20164. [Google Scholar] [CrossRef]
- Ciccone, L.; Fruchart-Gaillard, C.; Mourier, G.; Savko, M.; Nencetti, S.; Orlandini, E.; Servent, D.; Stura, E.A.; Shepard, W. Copper Mediated Amyloid-β Binding to Transthyretin. Sci. Rep. 2018, 8, 13744. [Google Scholar] [CrossRef]
- Gimeno, A.; Santos, L.M.; Alemi, M.; Rivas, J.; Blasi, D.; Cotrina, E.Y.; Llop, J.; Valencia, G.; Cardoso, I.; Quintana, J.; et al. Insights on the Interaction between Transthyretin and Aβ in Solution. A Saturation Transfer Difference (STD) NMR Analysis of the Role of Iododiflunisal. J. Med. Chem. 2017, 60, 5749–5758. [Google Scholar] [CrossRef]
- Liz, M.A.; Mar, F.M.; Franquinho, F.; Sousa, M.M. Aboard Transthyretin: From Transport to Cleavage. IUBMB Life 2010, 62, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Blake, C.C.F.; Geisow, M.J.; Oatley, S.J.; Rérat, B.; Rérat, C. Structure of Prealbumin: Secondary, Tertiary and Quaternary Interactions Determined by Fourier Refinement at 1.8 Å. J. Mol. Biol. 1978, 121, 339–356. [Google Scholar] [CrossRef] [PubMed]
- Tanskanen, M.; Peuralinna, T.; Polvikoski, T.; Notkola, I.; Sulkava, R.; Hardy, J.; Singleton, A.; Kiuru-Enari, S.; Paetau, A.; Tienari, P.J.; et al. Senile Systemic Amyloidosis Affects 25% of the Very Aged and Associates with Genetic Variation in Alpha2-macroglobulin and Tau: A Population-based Autopsy Study. Ann. Med. 2008, 40, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Ueda, M. Transthyretin: Its Function and Amyloid Formation. Neurochem. Int. 2022, 155, 105313. [Google Scholar] [CrossRef]
- Yee, A.W.; Aldeghi, M.; Blakeley, M.P.; Ostermann, A.; Mas, P.J.; Moulin, M.; de Sanctis, D.; Bowler, M.W.; Mueller-Dieckmann, C.; Mitchell, E.P.; et al. A Molecular Mechanism for Transthyretin Amyloidogenesis. Nat. Commun. 2019, 10, 925. [Google Scholar] [CrossRef]
- Almeida, M.R.; Gales, L.; Damas, A.M.; Cardoso, I.; Saraiva, M.J. Small Transthyretin (TTR) Ligands as Possible Therapeutic Agents in TTR Amyloidoses. Curr. Drug Targets CNS Neurol. Disord. 2005, 4, 587–596. [Google Scholar] [CrossRef]
- Ciccone, L.; Tonali, N.; Nencetti, S.; Orlandini, E. Natural Compounds as Inhibitors of Transthyretin Amyloidosis and Neuroprotective Agents: Analysis of Structural Data for Future Drug Design. J. Enzym. Inhib. Med. Chem. 2020, 35, 1145–1162. [Google Scholar] [CrossRef]
- Guo, X.; Liu, Z.; Zheng, Y.; Li, Y.; Li, L.; Liu, H.; Chen, Z.; Wu, L. Review on the Structures and Activities of Transthyretin Amyloidogenesis Inhibitors. Drug Des. Dev. Ther. 2020, 14, 1057–1081. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, N.; Saraiva, M.J.; Almeida, M.R. Uncovering the Neuroprotective Mechanisms of Curcumin on Transthyretin Amyloidosis. Int. J. Mol. Sci. 2019, 20, 1287. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.D.; Minamino, N.; Takao, T. Free Thiol of Transthyretin in Human Plasma Most Accessible to Modification/Oxidation. Anal. Chem. 2015, 87, 10785–10791. [Google Scholar] [CrossRef]
- Kingsbury, J.S.; Klimtchuk, E.S.; Théberge, R.; Costello, C.E.; Connors, L.H. Expression, Purification, and in Vitro Cysteine-10 Modification of Native Sequence Recombinant Human Transthyretin. Protein Expr. Purif. 2007, 53, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Vieira, M.; Saraiva, M.J. Transthyretin: A Multifaceted Protein. BioMol. Concepts 2014, 5, 45–54. [Google Scholar] [CrossRef]
- Du, J.; Cho, P.Y.; Yang, D.T.; Murphy, R.M. Identification of Beta-Amyloid-Binding Sites on Transthyretin. Protein Eng. Des. Sel. 2012, 25, 337–345. [Google Scholar] [CrossRef]
- Mangrolia, P.; Murphy, R.M. Retinol-Binding Protein Interferes with Transthyretin-Mediated β-Amyloid Aggregation Inhibition. Biochemistry 2018, 57, 5029–5040. [Google Scholar] [CrossRef]
- Li, X.; Zhang, X.; Ladiwala, A.R.A.; Du, D.; Yadav, J.K.; Tessier, P.M.; Wright, P.E.; Kelly, J.W.; Buxbaum, J.N. Mechanisms of Transthyretin Inhibition of -Amyloid Aggregation In Vitro. J. Neurosci. 2013, 33, 19423–19433. [Google Scholar] [CrossRef]
- Yang, D.T.; Joshi, G.; Cho, P.Y.; Johnson, J.A.; Murphy, R.M. Transthyretin as Both Sensor and Scavenger of Aβ Oligomers. Biochemistry 2013, 52, 2849–2861. [Google Scholar] [CrossRef]
- Gião, T.; Saavedra, J.; Cotrina, E.; Quintana, J.; Llop, J.; Arsequell, G.; Cardoso, I. Undiscovered Roles for Transthyretin: From a Transporter Protein to a New Therapeutic Target for Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 2075. [Google Scholar] [CrossRef]
- Pardo-Moreno, T.; González-Acedo, A.; Rivas-Domínguez, A.; García-Morales, V.; García-Cozar, F.J.; Ramos-Rodríguez, J.J.; Melguizo-Rodríguez, L. Therapeutic Approach to Alzheimer’s Disease: Current Treatments and New Perspectives. Pharmaceutics 2022, 14, 1117. [Google Scholar] [CrossRef]
- Van Dyck, C.H.; Swanson, C.J.; Aisen, P.; Bateman, R.J.; Chen, C.; Gee, M.; Kanekiyo, M.; Li, D.; Reyderman, L.; Cohen, S.; et al. Lecanemab in Early Alzheimer’s Disease. N. Engl. J. Med. 2023, 388, 9–21. [Google Scholar] [CrossRef]
- Bansal, Y.; Silakari, O. Multifunctional Compounds: Smart Molecules for Multifactorial Diseases. Eur. J. Med. Chem. 2014, 76, 31–42. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Wang, Y.; Li, X.; Wang, S.; Wang, Z. Recent Advance on Carbamate-Based Cholinesterase Inhibitors as Potential Multifunctional Agents against Alzheimer’s Disease. Eur. J. Med. Chem. 2022, 240, 114606. [Google Scholar] [CrossRef]
- Santos, M.A.; Chand, K.; Chaves, S. Recent Progress in Multifunctional Metal Chelators as Potential Drugs for Alzheimer’s Disease. Coord. Chem. Rev. 2016, 327–328, 287–303. [Google Scholar] [CrossRef]
- Corzo, L.; Fernández-Novoa, L.; Carrera, I.; Martínez, O.; Rodríguez, S.; Alejo, R.; Cacabelos, R. Nutrition, Health, and Disease: Role of Selected Marine and Vegetal Nutraceuticals. Nutrients 2020, 12, 747. [Google Scholar] [CrossRef]
- Pandey, S.N.; Rangra, N.K.; Singh, S.; Arora, S.; Gupta, V. Evolving Role of Natural Products from Traditional Medicinal Herbs in the Treatment of Alzheimer’s Disease. ACS Chem. Neurosci. 2021, 12, 2718–2728. [Google Scholar] [CrossRef]
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The Traditional Medicine and Modern Medicine from Natural Products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef] [PubMed]
- Andrade, S.; Ramalho, M.J.; Loureiro, J.A. Natural Compounds for Alzheimer’s Disease Therapy: A Systematic Review of Preclinical and Clinical Studies. Int. J. Mol. Sci. 2019, 20, 2313. [Google Scholar] [CrossRef] [PubMed]
- Ciccone, L.; Vandooren, J.; Nencetti, S.; Orlandini, E. Natural Marine and Terrestrial Compounds as Modulators of Matrix Metalloproteinases-2 (MMP-2) and MMP-9 in Alzheimer’s Disease. Pharmaceuticals 2021, 14, 86. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.S.; Jhanji, N. Natural Products as Potential Anti-Alzheimer Agents. Curr. Med. Chem. 2020, 27, 5887–5917. [Google Scholar] [CrossRef]
- Patil, P.; Thakur, A.; Sharma, A.; Flora, S.J.S. Natural Products and Their Derivatives as Multifunctional Ligands against Alzheimer’s Disease. Drug Dev. Res. 2020, 81, 165–183. [Google Scholar] [CrossRef]
- Uliassi, E.; Prati, F.; Bongarzone, S.; Bolognesi, M.L. 10—Medicinal Chemistry of Hybrids for Neurodegenerative Diseases. In Design of Hybrid Molecules for Drug Development; Decker, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 259–277. ISBN 978-0-08-101011-2. [Google Scholar]
- Decker, M. Hybrid Molecules Incorporating Natural Products: Applications in Cancer Therapy, Neurodegenerative Disorders and Beyond. Curr. Med. Chem. 2011, 18, 1464–1475. [Google Scholar] [CrossRef] [PubMed]
- Bargagna, B.; Ciccone, L.; Nencetti, S.; Santos, M.A.; Chaves, S.; Camodeca, C.; Orlandini, E. Multifunctional Small Molecules as Potential Anti-Alzheimer’s Disease Agents. Molecules 2021, 26, 6015. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-Z.; Li, X.-J.; Zhang, H.-Y. Valproic Acid as a Promising Agent to Combat Alzheimer’s Disease. Brain Res. Bull. 2010, 81, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Hager, K.; Kenklies, M.; McAfoose, J.; Engel, J.; Münch, G. α-Lipoic Acid as a New Treatment Option for Alzheimer’s Disease—A 48 Months Follow-up Analysis. In Neuropsychiatric Disorders An Integrative Approach; Springer: Cham, Switzerland, 2007; pp. 189–193. [Google Scholar]
- Iraji, A.; Firuzi, O.; Khoshneviszadeh, M.; Tavakkoli, M.; Mahdavi, M.; Nadri, H.; Edraki, N.; Miri, R. Multifunctional Iminochromene-2H-Carboxamide Derivatives Containing Different Aminomethylene Triazole with BACE1 Inhibitory, Neuroprotective and Metal Chelating Properties Targeting Alzheimer’s Disease. Eur. J. Med. Chem. 2017, 141, 690–702. [Google Scholar] [CrossRef]
- Iraji, A.; Firuzi, O.; Khoshneviszadeh, M.; Nadri, H.; Edraki, N.; Miri, R. Synthesis and Structure-Activity Relationship Study of Multi-Target Triazine Derivatives as Innovative Candidates for Treatment of Alzheimer’s Disease. Bioorganic Chem. 2018, 77, 223–235. [Google Scholar] [CrossRef]
- Song, H.; Hwang, Y.J.; Ha, J.W.; Boo, Y.C. Screening of an Epigenetic Drug Library Identifies 4-((Hydroxyamino)Carbonyl)-N-(2-Hydroxyethyl)-N-Phenyl-Benzeneacetamide That Reduces Melanin Synthesis by Inhibiting Tyrosinase Activity Independently of Epigenetic Mechanisms. Int. J. Mol. Sci. 2020, 21, 4589. [Google Scholar] [CrossRef]
- Ciccone, L.; Petrarolo, G.; Barsuglia, F.; Fruchart-Gaillard, C.; Cassar Lajeunesse, E.; Adewumi, A.T.; Soliman, M.E.S.; La Motta, C.; Orlandini, E.; Nencetti, S. Nature-Inspired O-Benzyl Oxime-Based Derivatives as New Dual-Acting Agents Targeting Aldose Reductase and Oxidative Stress. Biomolecules 2022, 12, 448. [Google Scholar] [CrossRef]
- Rumpf, J.; Burger, R.; Schulze, M. Statistical Evaluation of DPPH, ABTS, FRAP, and Folin-Ciocalteu Assays to Assess the Antioxidant Capacity of Lignins. Int. J. Biol. Macromol. 2023, 233, 123470. [Google Scholar] [CrossRef]
- Kim, D.-O.; Chun, O.K.; Kim, Y.J.; Moon, H.-Y.; Lee, C.Y. Quantification of Polyphenolics and Their Antioxidant Capacity in Fresh Plums. J. Agric. Food Chem. 2003, 51, 6509–6515. [Google Scholar] [CrossRef] [PubMed]
- Dudonné, S.; Vitrac, X.; Coutière, P.; Woillez, M.; Mérillon, J.-M. Comparative Study of Antioxidant Properties and Total Phenolic Content of 30 Plant Extracts of Industrial Interest Using DPPH, ABTS, FRAP, SOD, and ORAC Assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Xu, T.; Lu, B.; Liu, R. Guidelines for Antioxidant Assays for Food Components. Food Front. 2020, 1, 60–69. [Google Scholar] [CrossRef]
- Ciccone, L.; Tonali, N.; Fruchart-Gaillard, C.; Barlettani, L.; Rossello, A.; Braca, A.; Orlandini, E.; Nencetti, S. Antioxidant Quercetin 3-O-Glycosylated Plant Flavonols Contribute to Transthyretin Stabilization. Crystals 2022, 12, 638. [Google Scholar] [CrossRef]
- Ciccone, L.; Nencetti, S.; Rossello, A.; Barlettani, L.; Tonali, N.; Nieri, P.; Orlandini, E. Omega-3 PUFAs as a Dietary Supplement in Senile Systemic Amyloidosis. Nutrients 2023, 15, 749. [Google Scholar] [CrossRef]
- Ciccone, L.; Nencetti, S.; Camodeca, C.; Ortore, G.; Cuffaro, D.; Socci, S.; Orlandini, E. Synthesis and Evaluation of Monoaryl Derivatives as Transthyretin Fibril Formation Inhibitors. Pharm. Chem. J. 2022, 56, 38–47. [Google Scholar] [CrossRef]
- Nencetti, S.; La Motta, C.; Rossello, A.; Sartini, S.; Nuti, E.; Ciccone, L.; Orlandini, E. N-(Aroyl)-N-(Arylmethyloxy)-α-Alanines: Selective Inhibitors of Aldose Reductase. Bioorganic Med. Chem. 2017, 25, 3068–3076. [Google Scholar] [CrossRef]
- Spagnol, C.M.; Assis, R.P.; Brunetti, I.L.; Isaac, V.L.B.; Salgado, H.R.N.; Corrêa, M.A. In Vitro Methods to Determine the Antioxidant Activity of Caffeic Acid. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 219, 358–366. [Google Scholar] [CrossRef]
- Matheou, C.J.; Younan, N.D.; Viles, J.H. Cu2+ Accentuates Distinct Misfolding of Aβ(1–40) and Aβ(1–42) Peptides, and Potentiates Membrane Disruption. Biochem. J. 2015, 466, 233–242. [Google Scholar] [CrossRef]
- Huat, T.J.; Camats-Perna, J.; Newcombe, E.A.; Valmas, N.; Kitazawa, M.; Medeiros, R. Metal Toxicity Links to Alzheimer’s Disease and Neuroinflammation. J. Mol. Biol. 2019, 431, 1843–1868. [Google Scholar] [CrossRef] [PubMed]
- Ayton, S.; Lei, P.; Bush, A.I. Biometals and Their Therapeutic Implications in Alzheimer’s Disease. Neurotherapeutics 2015, 12, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Szewczyk, B. Zinc Homeostasis and Neurodegenerative Disorders. Front. Aging Neurosci. 2013, 5, 33. [Google Scholar] [CrossRef] [PubMed]
- La Penna, G.; Minicozzi, V.; Morante, S.; Rossi, G.C.; Stellato, F. A First-Principle Calculation of the XANES Spectrum of Cu2+ in Water. J. Chem. Phys. 2015, 143, 124508. [Google Scholar] [CrossRef]
- Hruby, M.; Martínez, I.I.S.; Stephan, H.; Pouckova, P.; Benes, J.; Stepanek, P. Chelators for Treatment of Iron and Copper Overload: Shift from Low-Molecular-Weight Compounds to Polymers. Polymers 2021, 13, 3969. [Google Scholar] [CrossRef]
- Andjelković, M.; Van Camp, J.; De Meulenaer, B.; Depaemelaere, G.; Socaciu, C.; Verloo, M.; Verhe, R. Iron-Chelation Properties of Phenolic Acids Bearing Catechol and Galloyl Groups. Food Chem. 2006, 98, 23–31. [Google Scholar] [CrossRef]
- Leuci, R.; Brunetti, L.; Laghezza, A.; Loiodice, F.; Tortorella, P.; Piemontese, L. Importance of Biometals as Targets in Medicinal Chemistry: An Overview about the Role of Zinc (II) Chelating Agents. Appl. Sci. 2020, 10, 4118. [Google Scholar] [CrossRef]
- Tauro, M.; Laghezza, A.; Loiodice, F.; Piemontese, L.; Caradonna, A.; Capelli, D.; Montanari, R.; Pochetti, G.; Di Pizio, A.; Agamennone, M.; et al. Catechol-Based Matrix Metalloproteinase Inhibitors with Additional Antioxidative Activity. J. Enzym. Inhib. Med. Chem. 2016, 31, 25–37. [Google Scholar] [CrossRef]
- Lakey-Beitia, J.; Burillo, A.M.; La Penna, G.; Hegde, M.L.; Rao, K.S. Polyphenols as Potential Metal Chelation Compounds Against Alzheimer’s Disease. J. Alzheimer’s Dis. 2021, 82, S335–S357. [Google Scholar] [CrossRef]
- Mao, F.; Yan, J.; Li, J.; Jia, X.; Miao, H.; Sun, Y.; Huang, L.; Li, X. New Multi-Target-Directed Small Molecules against Alzheimer’s Disease: A Combination of Resveratrol and Clioquinol. Org. Biomol. Chem. 2014, 12, 5936–5944. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and Antioxidant Methods: An Updated Overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [PubMed]
- Ilyasov, I.R.; Beloborodov, V.L.; Selivanova, I.A.; Terekhov, R.P. ABTS/PP Decolorization Assay of Antioxidant Capacity Reaction Pathways. Int. J. Mol. Sci. 2020, 21, 1131. [Google Scholar] [CrossRef] [PubMed]
- Garai, K.; Posey, A.E.; Li, X.; Buxbaum, J.N.; Pappu, R.V. Inhibition of Amyloid Beta Fibril Formation by Monomeric Human Transthyretin. Protein Sci. 2018, 27, 1252–1261. [Google Scholar] [CrossRef] [PubMed]
- Alemi, M.; Silva, S.C.; Santana, I.; Cardoso, I. Transthyretin Stability Is Critical in Assisting Beta Amyloid Clearance– Relevance of Transthyretin Stabilization in Alzheimer’s Disease. CNS Neurosci. Ther. 2017, 23, 605–619. [Google Scholar] [CrossRef]
- Saponaro, F.; Kim, J.H.; Chiellini, G. Transthyretin Stabilization: An Emerging Strategy for the Treatment of Alzheimer’s Disease? Int. J. Mol. Sci. 2020, 21, 8672. [Google Scholar] [CrossRef]
- Wojtczak, A.; Cody, V.; Luft, J.R.; Pangborn, W. Structures of Human Transthyretin Complexed with Thyroxine at 2.0 Å Resolution and 3′,5′-Dinitro-N-Acetyl- L -Thyronine at 2.2 Å Resolution. Acta Crystallogr. D Struct. Biol. 1996, 52, 758–765. [Google Scholar] [CrossRef]
- Yokoyama, T.; Mizuguchi, M. Transthyretin Amyloidogenesis Inhibitors: From Discovery to Current Developments. J. Med. Chem. 2020, 63, 14228–14242. [Google Scholar] [CrossRef]
- Connelly, S.; Choi, S.; Johnson, S.M.; Kelly, J.W.; Wilson, I.A. Structure-Based Design of Kinetic Stabilizers That Ameliorate the Transthyretin Amyloidoses. Curr. Opin. Struct. Biol. 2010, 20, 54–62. [Google Scholar] [CrossRef]
- Johnson, S.M.; Wiseman, R.L.; Sekijima, Y.; Green, N.S.; Adamski-Werner, S.L.; Kelly, J.W. Native State Kinetic Stabilization as a Strategy to Ameliorate Protein Misfolding Diseases: A Focus on the Transthyretin Amyloidoses. Acc. Chem. Res. 2005, 38, 911–921. [Google Scholar] [CrossRef]
- Cotrina, E.Y.; Santos, L.M.; Rivas, J.; Blasi, D.; Leite, J.P.; Liz, M.A.; Busquets, M.A.; Planas, A.; Prohens, R.; Gimeno, A.; et al. Targeting Transthyretin in Alzheimer’s Disease: Drug Discovery of Small-Molecule Chaperones as Disease-Modifying Drug Candidates for Alzheimer’s Disease. Eur. J. Med. Chem. 2021, 226, 113847. [Google Scholar] [CrossRef]
- Sinha, A.; Chang, J.C.; Xu, P.; Gindinova, K.; Cho, Y.; Sun, W.; Wu, X.; Li, Y.M.; Greengard, P.; Kelly, J.W.; et al. Brain Permeable Tafamidis Amide Analogs for Stabilizing TTR and Reducing APP Cleavage. ACS Med. Chem. Lett. 2020, 11, 1973–1979. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).