Bee-Derived Products: Chemical Composition and Applications in Skin Tissue Engineering
Abstract
:1. Introduction
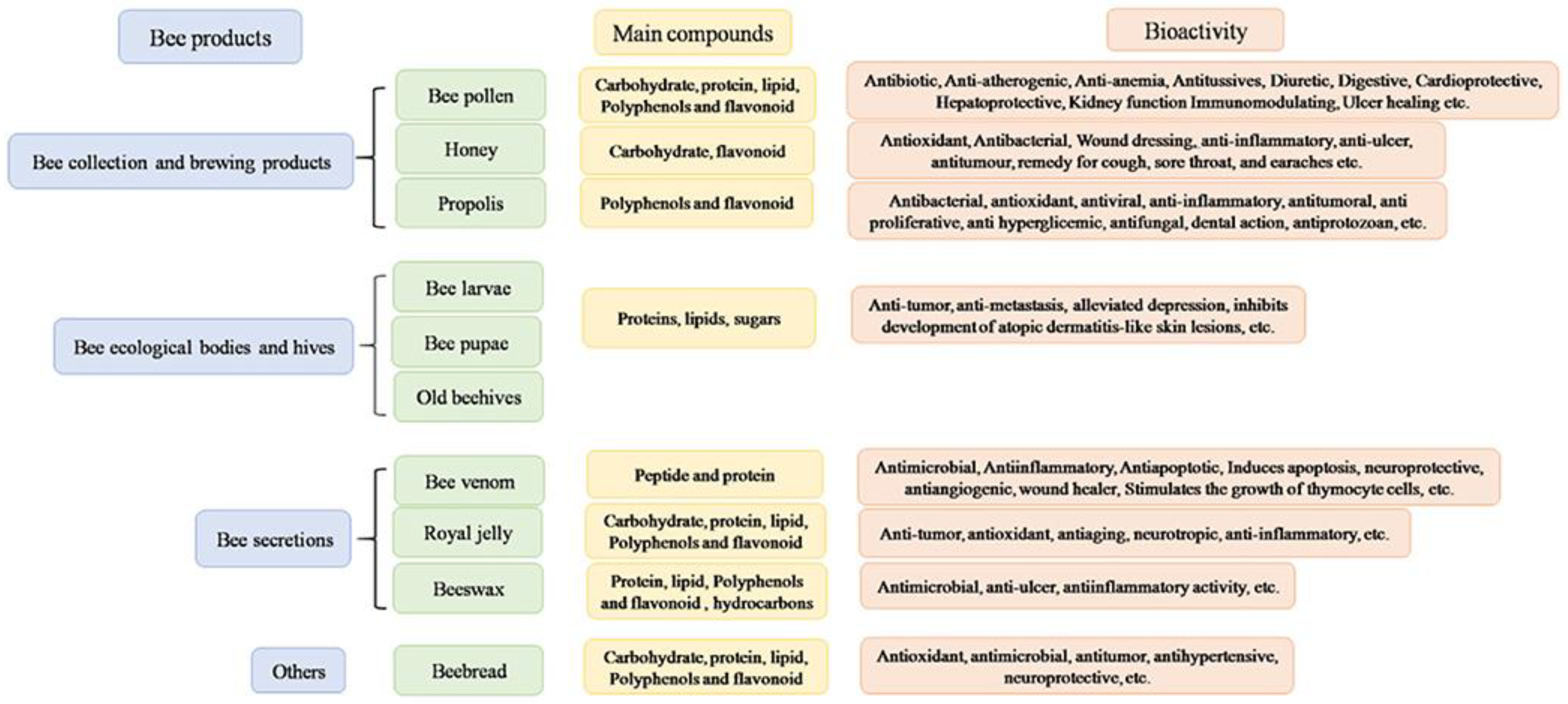
2. Bee-Derived Products: Chemical Composition and Biological Properties
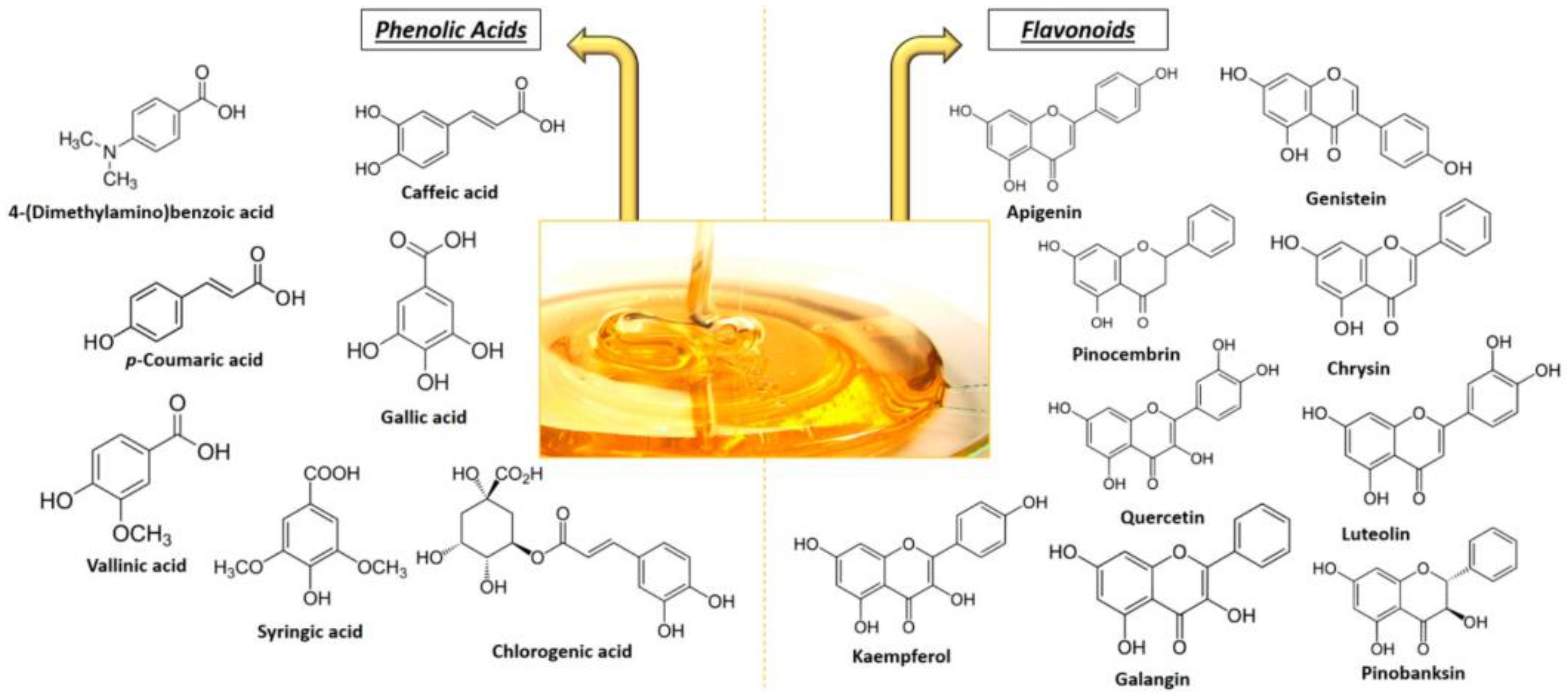
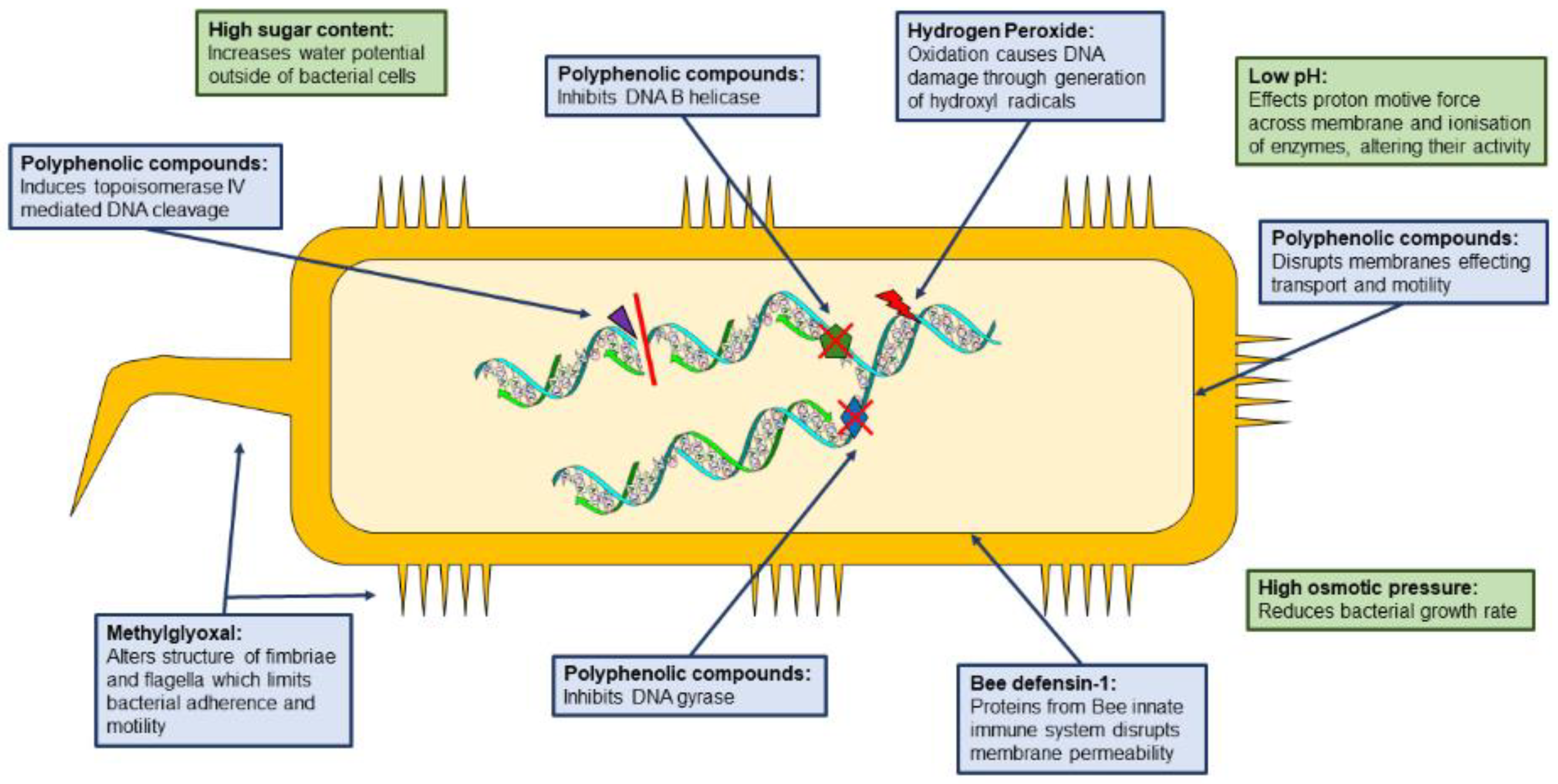
2.1. Honey
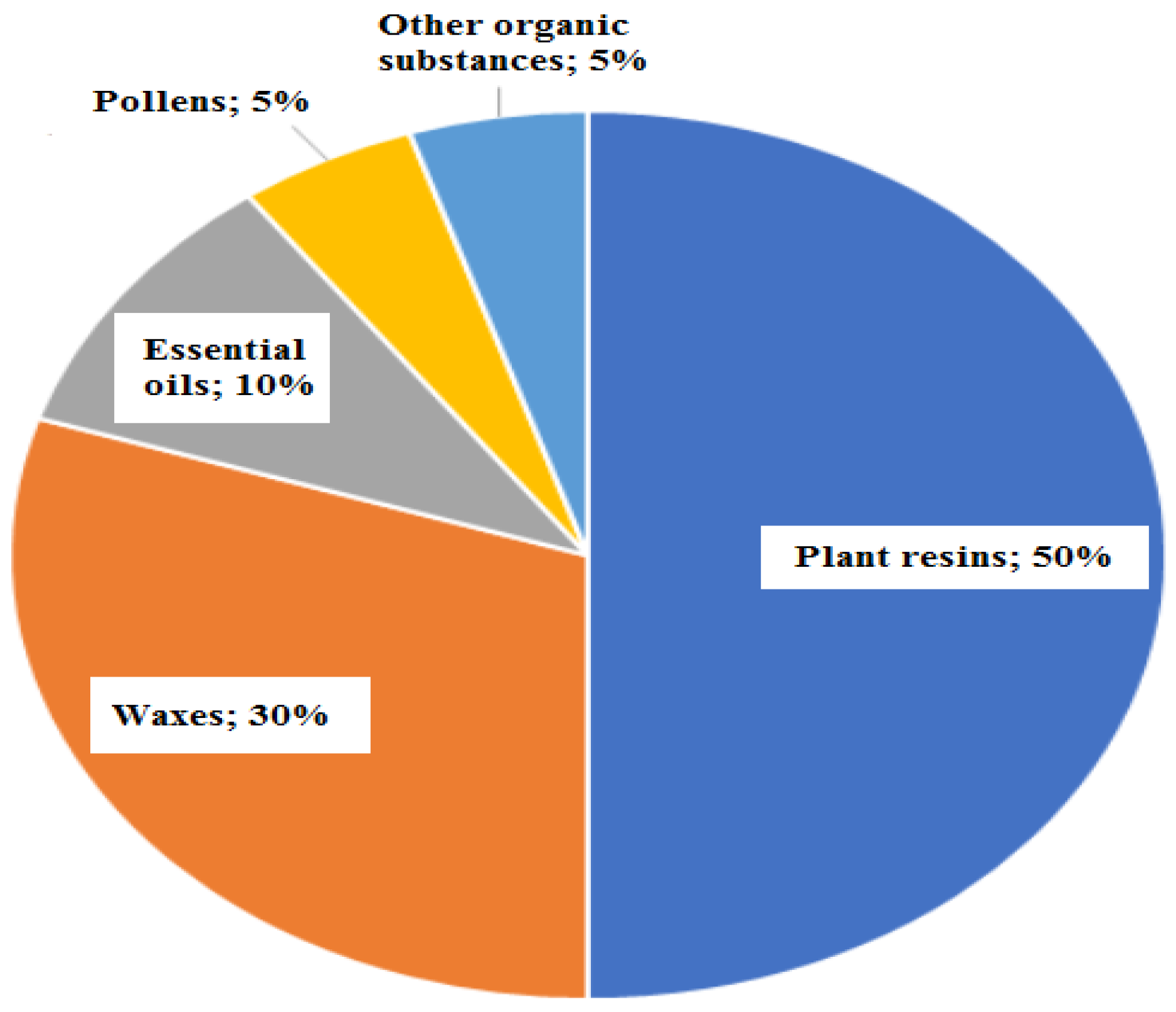
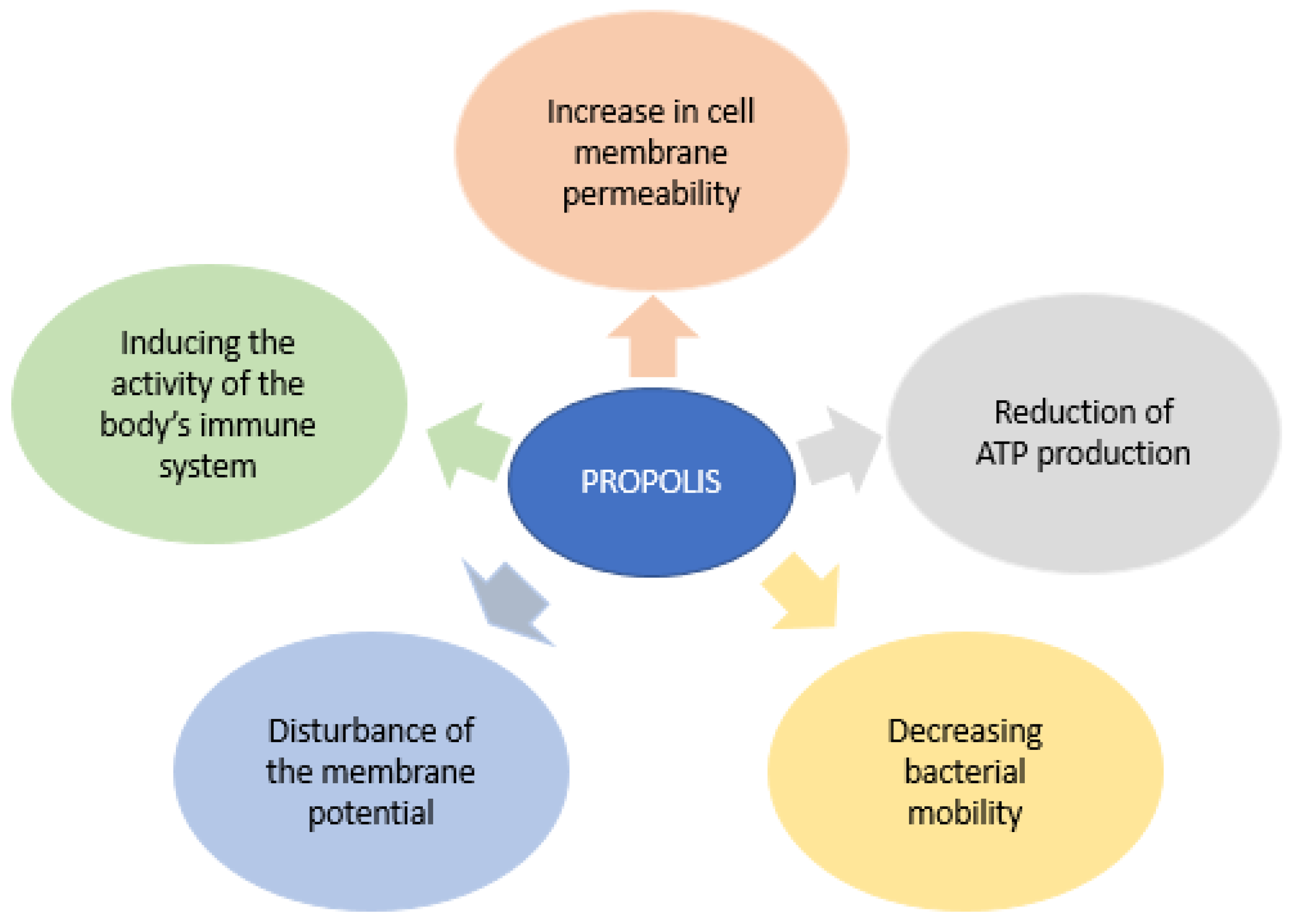
2.2. Propolis
| Propolis Type | Origin | Major Constituents |
|---|---|---|
| Poplar | Europe, North America, and the nontropical regions of Asia | Flavonoids and phenolic acid esters (flavones, quercetin derivates, pinocembrin derivates, and daidzein) |
| Red propolis | Cuba, Mexico, Brazil | Derivatives of p-coumaric acids, artepillin C, different caffeoylquinic acids, and lower amounts of flavonoids |
| Pacific | Taiwan, Japan | C-prenyl-flavanones |
| Mediterranean propolis | Greece, Malta, Crete, southern Italy | Diterpenes |
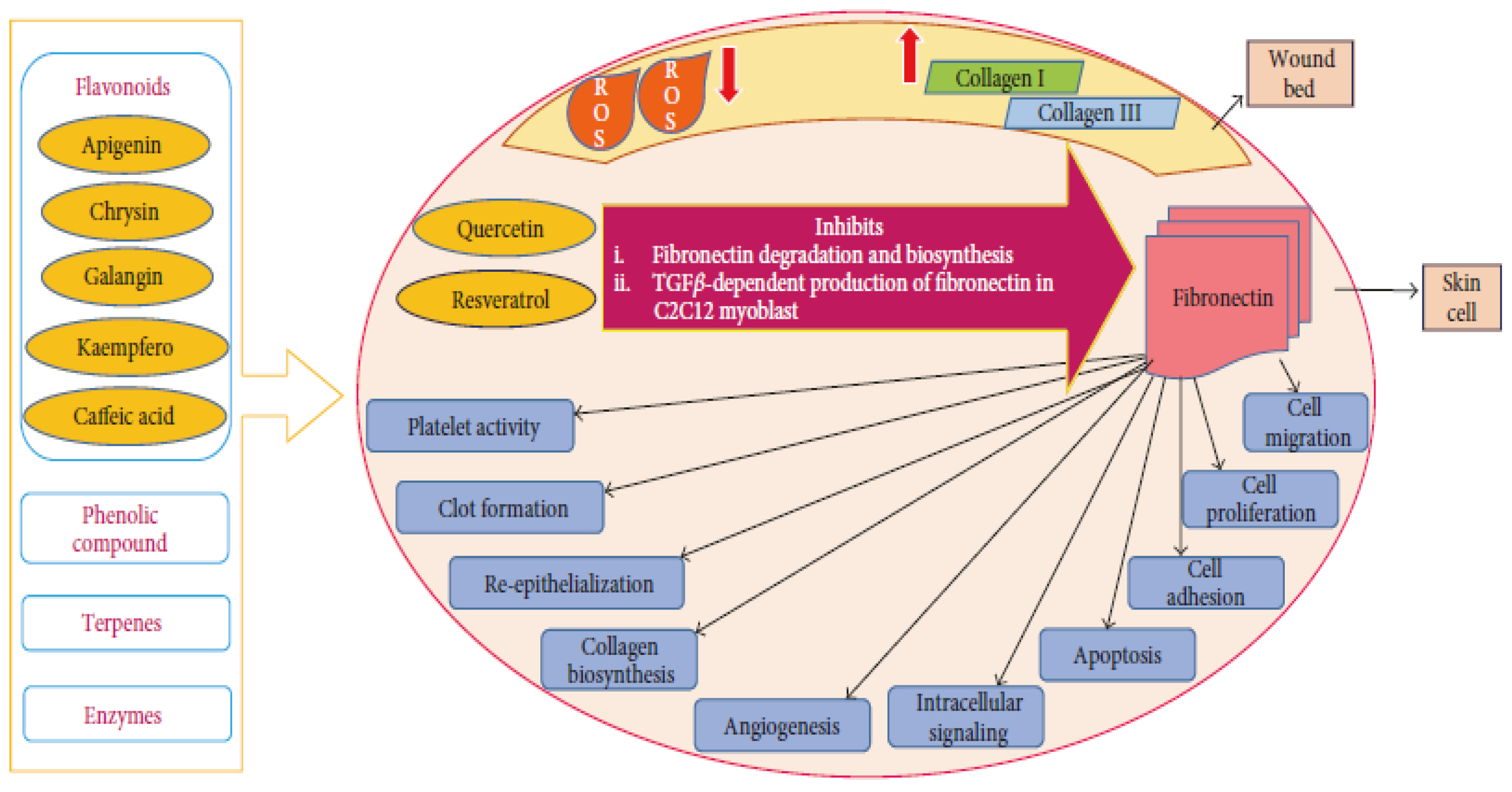
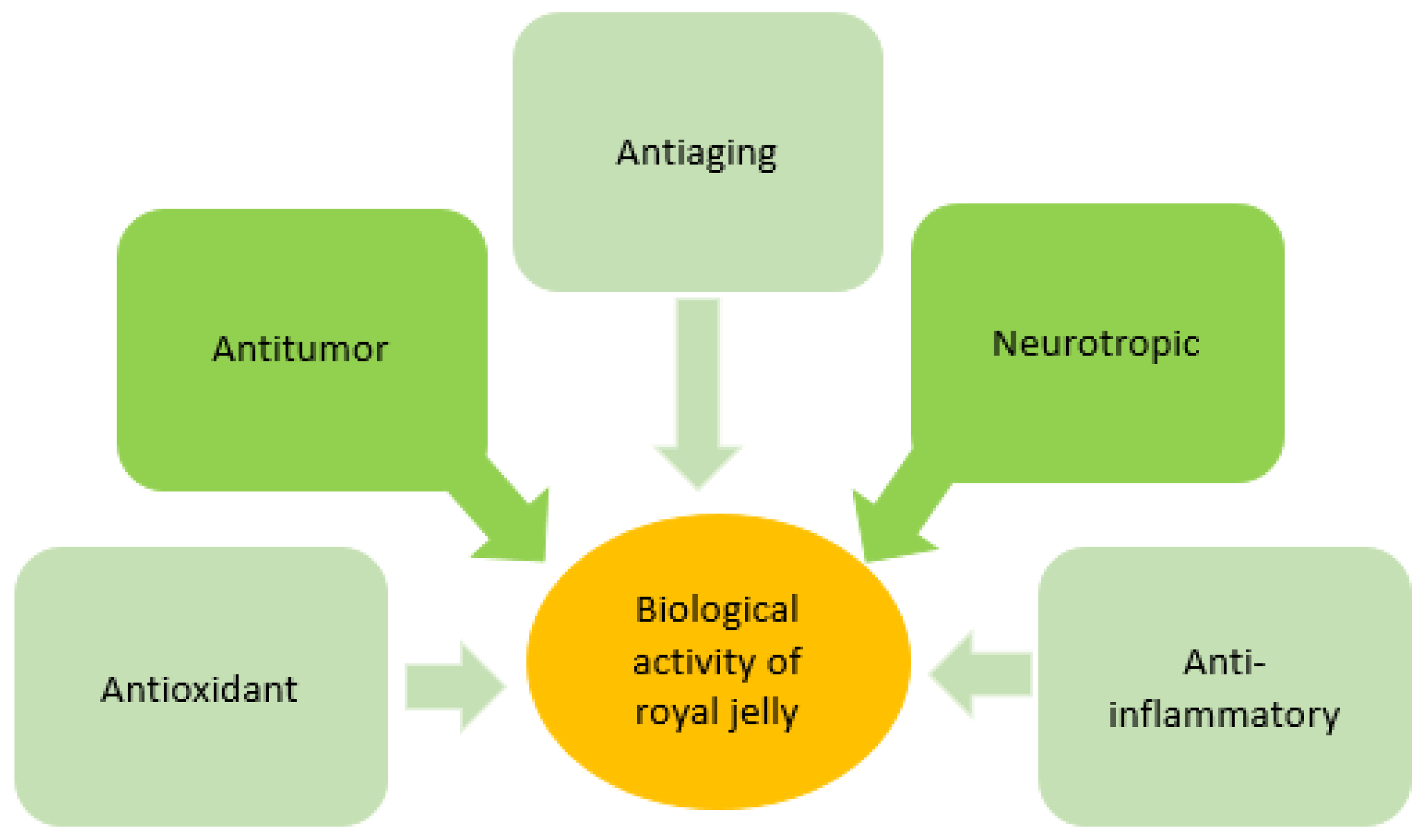
2.3. Royal Jelly
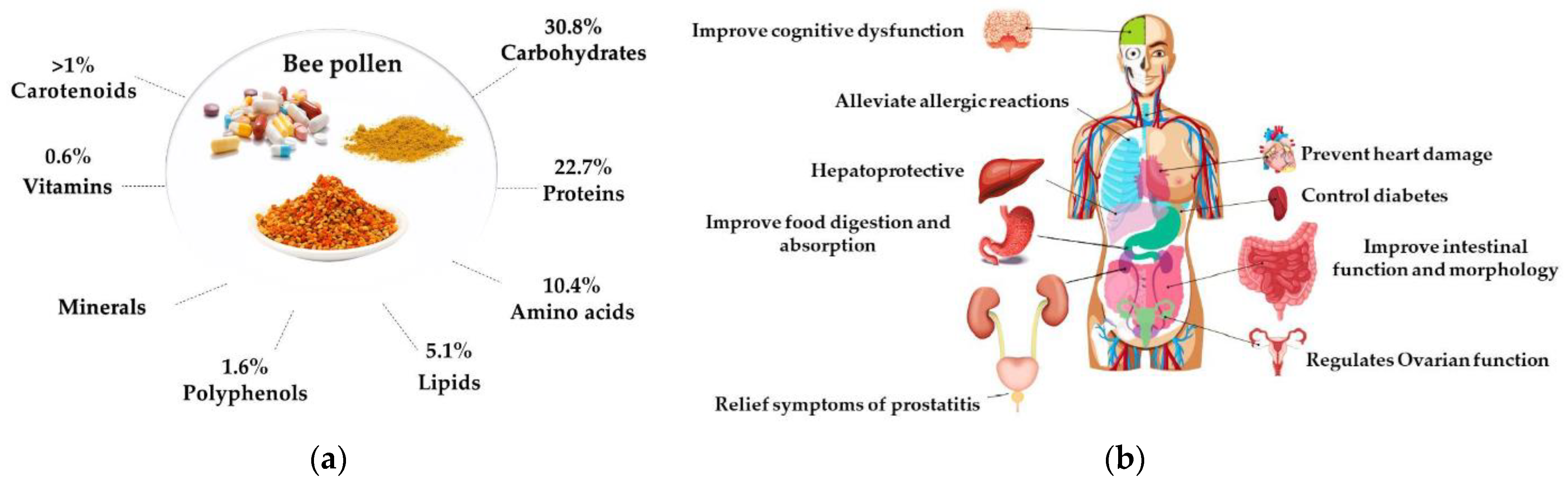
2.4. Bee Pollen
2.5. Beeswax
2.6. Bee Venom
3. Skin Regeneration Applications
3.1. Formulations Based on Honey
3.2. Formulations Based on Propolis
3.3. Formulations Based on Royal Jelly
3.4. Formulations Based on Bee Pollen
3.5. Formulations Based on Beeswax
3.6. Formulations Based on Bee Venom
4. Remarks and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ozhathil, D.K.; Wolf, S.E. Prevention and treatment of burn wound infections: The role of topical antimicrobials. Expert Rev. Anti-Infect. Ther. 2022, 1–16. [Google Scholar] [CrossRef]
- Pavel, T.I.; Chircov, C.; Rădulescu, M.; Grumezescu, A.M. Regenerative wound dressings for skin cancer. Cancers 2020, 12, 2954. [Google Scholar] [CrossRef]
- Tottoli, E.M.; Dorati, R.; Genta, I.; Chiesa, E.; Pisani, S.; Conti, B. Skin Wound Healing Process and New Emerging Technologies for Skin Wound Care and Regeneration. Pharmaceutics 2020, 12, 735. [Google Scholar] [CrossRef]
- Warby, R.; Maani, C.V. Burn Classification; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Stoica, A.E.; Chircov, C.; Grumezescu, A.M. Hydrogel Dressings for the Treatment of Burn Wounds: An Up-To-Date Overview. Materials 2020, 13, 2853. [Google Scholar] [CrossRef]
- Niculescu, A.G.; Grumezescu, A.M. An Up-to-Date Review of Biomaterials Application in Wound Management. Polymers 2022, 14, 421. [Google Scholar] [CrossRef]
- Xu, J.; Su, M.; Jin, Z.; Zhou, W.; Sun, Y.; Jin, Y.; Shi, Z. Effects of Natural Brown Cotton Bleached Gauze on Wound Healing. Materials 2022, 15, 2070. [Google Scholar] [CrossRef] [PubMed]
- Oba, J.; Okabe, M.; Yoshida, T.; Soko, C.; Fathy, M.; Amano, K.; Kobashi, D.; Wakasugi, M.; Okudera, H. Hyperdry human amniotic membrane application as a wound dressing for a full-thickness skin excision after a third-degree burn injury. Burn. Trauma 2020, 8, tkaa014. [Google Scholar] [CrossRef] [PubMed]
- Barski, D.; Gerullis, H.; Ecke, T.; Varga, G.; Boros, M.; Pintelon, I.; Timmermans, J.-P.; Otto, T. Human amniotic membrane dressing for the treatment of an infected wound due to an entero-cutaneous fistula: Case report. Int. J. Surg. Case Rep. 2018, 51, 11–13. [Google Scholar] [CrossRef]
- Cui, R.; Zhang, L.; Ou, R.; Xu, Y.; Xu, L.; Zhan, X.-Y.; Li, D. Polysaccharide-Based Hydrogels for Wound Dressing: Design Considerations and Clinical Applications. Front. Bioeng. Biotechnol. 2022, 10, 845735. [Google Scholar] [CrossRef]
- Debele, T.A.; Su, W.-P. Polysaccharide and protein-based functional wound dressing materials and applications. Int. J. Polym. Mater. Polym. Biomater. 2022, 71, 87–108. [Google Scholar] [CrossRef]
- Rivero, G.; Meuter, M.; Pepe, A.; Guevara, M.G.; Boccaccini, A.R.; Abraham, G.A. Nanofibrous membranes as smart wound dressings that release antibiotics when an injury is infected. Colloids Surf. A Physicochem. Eng. Asp. 2020, 587, 124313. [Google Scholar] [CrossRef]
- Schulte-Werning, L.V.; Murugaiah, A.; Singh, B.; Johannessen, M.; Engstad, R.E.; Škalko-Basnet, N.; Holsæter, A.M. Multifunctional Nanofibrous Dressing with Antimicrobial and Anti-Inflammatory Properties Prepared by Needle-Free Electrospinning. Pharmaceutics 2021, 13, 1527. [Google Scholar] [CrossRef]
- Hussain, Z.; Thu, H.E.; Rawas-Qalaji, M.; Naseem, M.; Khan, S.; Sohail, M. Recent developments and advanced strategies for promoting burn wound healing. J. Drug Deliv. Sci. Technol. 2022, 68, 103092. [Google Scholar] [CrossRef]
- Shalaby, M.A.; Anwar, M.M.; Saeed, H. Nanomaterials for application in wound Healing: Current state-of-the-art and future perspectives. J. Polym. Res. 2022, 29, 91. [Google Scholar] [CrossRef]
- Neacsu, I.A.; Melente, A.E.; Holban, A.M.; Ficai, A.; Ditu, L.M.; Kamerzan, C.M.; Tihauan, B.M.; Nicoara, A.I.; Bezirtzoglou, E.; Chifiriuc, M.C.; et al. Novel hydrogels based on collagen and ZnO nanoparticles with antibacterial activity for improved wound dressings. Rom. Biotechnol. Lett. 2019, 24, 317–323. [Google Scholar] [CrossRef]
- Radulescu, M.; Ficai, D.; Oprea, O.; Ficai, A.; Andronescu, E.; Holban, A.M. Antimicrobial Chitosan based Formulations with Impact on Different Biomedical Applications. Curr. Pharm. Biotechnol. 2015, 16, 128–136. [Google Scholar] [CrossRef]
- Neacsu, I.A.; Leau, S.A.; Marin, S.; Holban, A.M.; Vasile, B.S.; Nicoara, A.I.; Ene, V.L.; Bleotu, C.; Kaya, M.G.A.; Ficai, A. Collagen-Carboxymethylcellulose Biocomposite Wound-Dressings with Antimicrobial Activity. Materials 2021, 14, 1153. [Google Scholar] [CrossRef]
- Paduraru, A.; Ghitulica, C.; Trusca, R.; Surdu, V.A.; Neacsu, I.A.; Holban, A.M.; Birca, A.C.; Iordache, F.; Vasile, B.S. Antimicrobial Wound Dressings as Potential Materials for Skin Tissue Regeneration. Materials 2019, 12, 1859. [Google Scholar] [CrossRef] [Green Version]
- Radulescu, D.-M.; Neacsu, I.A.; Grumezescu, A.-M.; Andronescu, E. New Insights of Scaffolds Based on Hydrogels in Tissue Engineering. Polymers 2022, 14, 799. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.F. Chapter 36—Hydrogels in Regenerative Medicine. In Principles of Regenerative Medicine, 3rd ed.; Atala, A., Lanza, R., Mikos, A.G., Nerem, R., Eds.; Academic Press: Boston, MA, USA, 2019; pp. 627–650. [Google Scholar]
- Negut, I.; Grumezescu, V.; Grumezescu, A.M. Treatment Strategies for Infected Wounds. Molecules 2018, 23, 2392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, X.; Dong, Y.; Gu, C.; Zhang, X.; Ma, H. Processing Technologies for Bee Products: An Overview of Recent Developments and Perspectives. Front. Nutr. 2021, 8, 7181. [Google Scholar] [CrossRef]
- Mello, B.C.B.S.; Petrus, J.C.C.; Hubinger, M.D. Concentration of flavonoids and phenolic compounds in aqueous and ethanolic propolis extracts through nanofiltration. J. Food Eng. 2010, 96, 533–539. [Google Scholar] [CrossRef]
- Pellati, F.; Prencipe, F.P.; Bertelli, D.; Benvenuti, S. An efficient chemical analysis of phenolic acids and flavonoids in raw propolis by microwave-assisted extraction combined with high-performance liquid chromatography using the fused-core technology. J. Pharm. Biomed. Anal. 2013, 81, 126–132. [Google Scholar] [CrossRef]
- Ciulu, M.; Spano, N.; Pilo, M.I.; Sanna, G. Recent Advances in the Analysis of Phenolic Compounds in Unifloral Honeys. Molecules 2016, 21, 451. [Google Scholar] [CrossRef]
- Pyrzynska, K.; Biesaga, M. Analysis of phenolic acids and flavonoids in honey. TrAC Trends Anal. Chem. 2009, 28, 893–902. [Google Scholar] [CrossRef]
- López-Gutiérrez, N.; Aguilera-Luiz, M.d.M.; Romero-González, R.; Vidal, J.L.M.; Garrido Frenich, A. Fast analysis of polyphenols in royal jelly products using automated TurboFlow™-liquid chromatography–Orbitrap high resolution mass spectrometry. J. Chromatogr. B 2014, 973, 17–28. [Google Scholar] [CrossRef]
- Sawicki, T.; Starowicz, M.; Kłębukowska, L.; Hanus, P. The Profile of Polyphenolic Compounds, Contents of Total Phenolics and Flavonoids, and Antioxidant and Antimicrobial Properties of Bee Products. Molecules 2022, 27, 1301. [Google Scholar] [CrossRef]
- Minden-Birkenmaier, B.A.; Bowlin, G.L. Honey-Based Templates in Wound Healing and Tissue Engineering. Bioengineering 2018, 5, 46. [Google Scholar] [CrossRef] [Green Version]
- Meo, S.A.; Al-Asiri, S.A.; Mahesar, A.L.; Ansari, M.J. Role of honey in modern medicine. Saudi J. Biol. Sci. 2017, 24, 975–978. [Google Scholar] [CrossRef] [PubMed]
- Albaridi, N.A. Antibacterial Potency of Honey. Int. J. Microbiol. 2019, 2019, 2464507. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, E.I.; Rao, S.; Madathil, L.; Hegde, S.K.; Baliga-Rao, M.P.; George, T.; Baliga, M.S. Honey in oral health and care: A mini review. J. Oral Biosci. 2019, 61, 32–36. [Google Scholar] [CrossRef]
- Cianciosi, D.; Forbes-Hernandez, T.Y.; Afrin, S.; Gasparrini, M.; Reboredo-Rodriguez, P.; Manna, P.P.; Zhang, J.; Bravo Lamas, L.; Martinez Florez, S.; Agudo Toyos, P.; et al. Phenolic Compounds in Honey and Their Associated Health Benefits: A Review. Molecules 2018, 23, 2322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, H.T.L.; Panyoyai, N.; Kasapis, S.; Pang, E.; Mantri, N. Honey and Its Role in Relieving Multiple Facets of Atherosclerosis. Nutrients 2019, 11, 167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habryka, C.; Socha, R.; Juszczak, L. The Effect of Enriching Honey with Propolis on the Antioxidant Activity, Sensory Characteristics, and Quality Parameters. Molecules 2020, 25, 1176. [Google Scholar] [CrossRef] [Green Version]
- Al-Ghamdi, A.A.; Ansari, M.J. Biological and therapeutic roles of Saudi Arabian honey: A comparative review. J. King Saud Univ.-Sci. 2021, 33, 101329. [Google Scholar] [CrossRef]
- Nolan, V.C.; Harrison, J.; Cox, J.A.G. Dissecting the Antimicrobial Composition of Honey. Antibiotics 2019, 8, 251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almasaudi, S. The antibacterial activities of honey. Saudi J. Biol. Sci. 2021, 28, 2188–2196. [Google Scholar] [CrossRef]
- Martinotti, S.; Ranzato, E. Honey, Wound Repair and Regenerative Medicine. J. Funct. Biomater. 2018, 9, 34. [Google Scholar] [CrossRef] [Green Version]
- Iftikhar, A.; Nausheen, R.; Mukhtar, I.; Iqbal, R.K.; Raza, A.; Yasin, A.; Anwar, H. The regenerative potential of honey: A comprehensive literature review. J. Apic. Res. 2022, 1–16. [Google Scholar] [CrossRef]
- Khan, S.U.; Anjum, S.I.; Rahman, K.; Ansari, M.J.; Khan, W.U.; Kamal, S.; Khattak, B.; Muhammad, A.; Khan, H.U. Honey: Single food stuff comprises many drugs. Saudi J. Biol. Sci. 2018, 25, 320–325. [Google Scholar] [CrossRef]
- Da Silva, B.; Caon, T.; Mohr, E.T.B.; Biluca, F.C.; Gonzaga, L.V.; Fett, R.; Dalmarco, E.M.; Costa, A.C.O. Phenolic profile and in vitro anti-inflammatory activity of Mimosa scabrella Bentham honeydew honey in RAW 264.7 murine macrophages. J. Food Biochem. 2022, 46, e14076. [Google Scholar] [CrossRef] [PubMed]
- Osés, S.M.; Cantero, L.; Puertas, G.; Fernández-Muiño, M.Á.; Sancho, M.T. Antioxidant, antimicrobial and anti-inflammatory activities of ling-heather honey powder obtained by different methods with several carriers. LWT 2022, 159, 113235. [Google Scholar] [CrossRef]
- De-Melo, A.; Almeida-Muradian, L.; Sancho, M.; Pascual Maté, A. Composition and properties of Apis mellifera honey: A review. J. Apic. Res. 2017, 57, 1–33. [Google Scholar] [CrossRef]
- Vică, M.L.; Glevitzky, M.; Tit, D.M.; Behl, T.; Heghedűş-Mîndru, R.C.; Zaha, D.C.; Ursu, F.; Popa, M.; Glevitzky, I.; Bungău, S. The antimicrobial activity of honey and propolis extracts from the central region of Romania. Food Biosci. 2021, 41, 101014. [Google Scholar] [CrossRef]
- Kavanagh, S.; Gunnoo, J.; Marques Passos, T.; Stout, J.C.; White, B. Physicochemical properties and phenolic content of honey from different floral origins and from rural versus urban landscapes. Food Chem. 2019, 272, 66–75. [Google Scholar] [CrossRef]
- Ahmed, S.; Sulaiman, S.A.; Baig, A.A.; Ibrahim, M.; Liaqat, S.; Fatima, S.; Jabeen, S.; Shamim, N.; Othman, N.H. Honey as a Potential Natural Antioxidant Medicine: An Insight into Its Molecular Mechanisms of Action. Oxid. Med. Cell. Longev. 2018, 2018, 8367846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zabaiou, N.; Fouache, A.; Trousson, A.; Baron, S.; Zellagui, A.; Lahouel, M.; Lobaccaro, J.A. Biological properties of propolis extracts: Something new from an ancient product. Chem. Phys. Lipids 2017, 207, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Oryan, A.; Alemzadeh, E.; Moshiri, A. Potential role of propolis in wound healing: Biological properties and therapeutic activities. Biomed. Pharm. 2018, 98, 469–483. [Google Scholar] [CrossRef] [PubMed]
- Alvear, M.; Santos, E.; Cabezas, F.; Pérez-Sanmartín, A.; Lespinasse, M.; Veloz, J. Geographic Area of Collection Determines the Chemical Composition and Antimicrobial Potential of Three Extracts of Chilean Propolis. Plants 2021, 10, 1543. [Google Scholar] [CrossRef] [PubMed]
- Przybyłek, I.; Karpiński, T.M. Antibacterial Properties of Propolis. Molecules 2019, 24, 2047. [Google Scholar] [CrossRef] [Green Version]
- Soós, Á.; Bódi, É.; Várallyay, S.; Molnár, S.; Kovács, B. Element composition of propolis tinctures prepared from Hungarian raw propolis. LWT 2022, 154, 112762. [Google Scholar] [CrossRef]
- Nalbantsoy, A.; Sarıkahya, N.B.; Özverel, C.S.; Barlas, A.B.; Kırcı, D.; Akgün, İ.H.; Yalçın, T.; Düven, G.; Kışla, D.; Demirci, B.; et al. Chemical composition and biological activities of Cypriot propolis. J. Apic. Res. 2022, 61, 233–245. [Google Scholar] [CrossRef]
- Mutlu, C.; Özer-Atakoğlu, Ö.; Erbaş, M.; Yalçın, M.G. Advances in the Elemental Composition Analysis of Propolis Samples from Different Regions of Turkey by X-ray Fluorescence Spectrometry. Biol. Trace Elem. Res. 2022, 1–9. [Google Scholar] [CrossRef]
- Rufatto, L.C.; dos Santos, D.A.; Marinho, F.; Henriques, J.A.P.; Roesch Ely, M.; Moura, S. Red propolis: Chemical composition and pharmacological activity. Asian Pac. J. Trop. Biomed. 2017, 7, 591–598. [Google Scholar] [CrossRef]
- Alsayed, M.F.S.; Hashem, A.; Al-Hazzani, A.A.; Abd_Allah, E.F. Biological control of yeast contamination of industrial foods by propolis. Saudi J. Biol. Sci. 2020, 27, 935–946. [Google Scholar] [CrossRef] [PubMed]
- Toreti, V.C.; Sato, H.H.; Pastore, G.M.; Park, Y.K. Recent progress of propolis for its biological and chemical compositions and its botanical origin. Evid.-Based Complementary Altern. Med. Ecam. 2013, 2013, 697390. [Google Scholar] [CrossRef]
- Galeotti, F.; Maccari, F.; Fachini, A.; Volpi, N. Chemical Composition and Antioxidant Activity of Propolis Prepared in Different Forms and in Different Solvents Useful for Finished Products. Foods 2018, 7, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Šuran, J.; Cepanec, I.; Mašek, T.; Radić, B.; Radić, S.; Tlak Gajger, I.; Vlainić, J. Propolis Extract and Its Bioactive Compounds—From Traditional to Modern Extraction Technologies. Molecules 2021, 26, 2930. [Google Scholar] [CrossRef]
- Martinotti, S.; Ranzato, E. Propolis: A new frontier for wound healing? Burn. Trauma 2015, 3, 9. [Google Scholar] [CrossRef] [Green Version]
- Sforcin, J.M. Biological Properties and Therapeutic Applications of Propolis. Phytother. Res. 2016, 30, 894–905. [Google Scholar] [CrossRef]
- Anjum, S.I.; Ullah, A.; Khan, K.A.; Attaullah, M.; Khan, H.; Ali, H.; Bashir, M.A.; Tahir, M.; Ansari, M.J.; Ghramh, H.A.; et al. Composition and functional properties of propolis (bee glue): A review. Saudi J. Biol. Sci. 2019, 26, 1695–1703. [Google Scholar] [CrossRef] [PubMed]
- Freitas, A.S.; Cunha, A.; Oliveira, R.; Almeida-Aguiar, C. Propolis antibacterial and antioxidant synergisms with gentamicin and honey. J. Appl. Microbiol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Aljadaan, S.A.N.; Elias, R.S.; Al-Anssari, R.A. Investigation of the Antioxidant and Antibacterial Activity of Novel Quercetin Derivatives. Biointerface Res. Appl. Chem. 2020, 10, 7329–7336. [Google Scholar] [CrossRef]
- Selamoglu, Z.; Sevindik, M.; Bal, C.; Ozaltun, B.; Sen, I.; Pasdaran, A. Antioxidant, antimicrobial and DNA protection activities of phenolic content of Tricholoma virgatum (Fr.) P.Kumm. Biointerface Res. Appl. Chem. 2020, 10, 5500–5506. [Google Scholar] [CrossRef]
- Uslu, M.E.; Mele, A.; Bayraktar, O. Evaluation of the hemostatic activity of Equisetum arvense extract: The role of varying phenolic composition and antioxidant activity due to different extraction conditions. Biointerface Res. Appl. Chem. 2019, 9, 4157–4163. [Google Scholar]
- Cao, X.-P.; Chen, Y.-F.; Zhang, J.-L.; You, M.-M.; Wang, K.; Hu, F.-L. Mechanisms underlying the wound healing potential of propolis based on its in vitro antioxidant activity. Phytomedicine 2017, 34, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Pasupuleti, V.R.; Sammugam, L.; Ramesh, N.; Gan, S.H. Honey, Propolis, and Royal Jelly: A Comprehensive Review of Their Biological Actions and Health Benefits. Oxid. Med. Cell. Longev. 2017, 2017, 1259510. [Google Scholar] [CrossRef]
- Braakhuis, A. Evidence on the Health Benefits of Supplemental Propolis. Nutrients 2019, 11, 2705. [Google Scholar] [CrossRef] [Green Version]
- Franchin, M.; Freires, I.A.; Lazarini, J.G.; Nani, B.D.; da Cunha, M.G.; Colón, D.F.; de Alencar, S.M.; Rosalen, P.L. The use of Brazilian propolis for discovery and development of novel anti-inflammatory drugs. Eur. J. Med. Chem. 2018, 153, 49–55. [Google Scholar] [CrossRef]
- Hassan, A.A.-m.; Elenany, Y.E.; Nassrallah, A.; Cheng, W.; Abd El-Maksoud, A.A. Royal jelly improves the physicochemical properties and biological activities of fermented milk with enhanced probiotic viability. LWT 2022, 155, 112912. [Google Scholar] [CrossRef]
- Mullen, A. Ancient residues, dietary clues. Nat. Food 2021, 2, 317. [Google Scholar] [CrossRef]
- Ahmad, S.; Campos, M.G.; Fratini, F.; Altaye, S.Z.; Li, J. New Insights into the Biological and Pharmaceutical Properties of Royal Jelly. Int. J. Mol. Sci. 2020, 21, 382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramanathan, A.; Nair, A.; Sugunan, V. A review on Royal Jelly proteins and peptides. J. Funct. Foods 2018, 44, 255–264. [Google Scholar] [CrossRef]
- Fratini, F.; Cilia, G.; Mancini, S.; Felicioli, A. Royal Jelly: An ancient remedy with remarkable antibacterial properties. Microbiol. Res. 2016, 192, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Kocot, J.; Kielczykowska, M.; Luchowska-Kocot, D.; Kurzepa, J.; Musik, I. Antioxidant Potential of Propolis, Bee Pollen, and Royal Jelly: Possible Medical Application. Oxid. Med. Cell. Longev. 2018, 2018, 7074209. [Google Scholar] [CrossRef]
- Kim, B.Y.; Lee, K.S.; Jung, B.; Choi, Y.S.; Kim, H.K.; Yoon, H.J.; Gui, Z.-Z.; Lee, J.; Jin, B.R. Honeybee (Apis cerana) major royal jelly protein 4 exhibits antimicrobial activity. J. Asia-Pac. Entomol. 2019, 22, 175–182. [Google Scholar] [CrossRef]
- Maleki, V.; Jafari-Vayghan, H.; Saleh-Ghadimi, S.; Adibian, M.; Kheirouri, S.; Alizadeh, M. Effects of Royal jelly on metabolic variables in diabetes mellitus: A systematic review. Complementary Ther. Med. 2019, 43, 20–27. [Google Scholar] [CrossRef]
- Khazaei, M.; Ansarian, A.; Ghanbari, E. New Findings on Biological Actions and Clinical Applications of Royal Jelly: A Review. J. Diet. Suppl. 2018, 15, 757–775. [Google Scholar] [CrossRef] [PubMed]
- Alreshoodi, F.; Sultanbawa, Y. Antimicrobial Activity of Royal Jelly. Anti-Infect. Agents 2015, 13, 50–59. [Google Scholar] [CrossRef]
- Cornara, L.; Biagi, M.; Xiao, J.; Burlando, B. Therapeutic Properties of Bioactive Compounds from Different Honeybee Products. Front. Pharmacol. 2017, 8, 412. [Google Scholar] [CrossRef]
- Lin, Y.; Shao, Q.; Zhang, M.; Lu, C.; Fleming, J.; Su, S. Royal jelly-derived proteins enhance proliferation and migration of human epidermal keratinocytes in an in vitro scratch wound model. BMC Complementary Altern. Med. 2019, 19, 175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bucekova, M.; Sojka, M.; Valachova, I.; Martinotti, S.; Ranzato, E.; Szep, Z.; Majtan, V.; Klaudiny, J.; Majtan, J. Bee-derived antibacterial peptide, defensin-1, promotes wound re-epithelialisation in vitro and in vivo. Sci. Rep. 2017, 7, 7340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurek-Górecka, A.; Górecki, M.; Rzepecka-Stojko, A.; Balwierz, R.; Stojko, J. Bee Products in Dermatology and Skin Care. Molecules 2020, 25, 556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maghsoudlou, A.; Sadeghi, A.; Mohebodini, H.; Toldrá, F. Royal Jelly: Chemistry, Storage and Bioactivities. J. Apic. Sci. 2019, 63, 17–40. [Google Scholar] [CrossRef] [Green Version]
- Maqsoudlou, A.; Mahoonak, A.S.; Mora, L.; Mohebodini, H.; Toldrá, F.; Ghorbani, M. Peptide identification in alcalase hydrolysated pollen and comparison of its bioactivity with royal jelly. Food Res. Int. Ott. Ont. 2019, 116, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Jung, C. Changes in nutritional composition from bee pollen to pollen patty used in bumblebee rearing. J. Asia-Pac. Entomol. 2020, 23, 701–708. [Google Scholar] [CrossRef]
- Mosić, M.; Trifković, J.; Vovk, I.; Gašić, U.; Tešić, Ž.; Šikoparija, B.; Milojković-Opsenica, D. Phenolic Composition Influences the Health-Promoting Potential of Bee-Pollen. Biomolecules 2019, 9, 783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karabagias, I.; Karabagias, V.; Gatzias, I.; Riganakos, K. Bio-Functional Properties of Bee Pollen: The Case of “Bee Pollen Yoghurt”. Coatings 2018, 8, 423. [Google Scholar] [CrossRef] [Green Version]
- Adaškevičiūtė, V.; Kaškonienė, V.; Kaškonas, P.; Barčauskaitė, K.; Maruška, A. Comparison of Physicochemical Properties of Bee Pollen with Other Bee Products. Biomolecules 2019, 9, 819. [Google Scholar] [CrossRef] [Green Version]
- Khalifa, S.A.M.; Elashal, M.H.; Yosri, N.; Du, M.; Musharraf, S.G.; Nahar, L.; Sarker, S.D.; Guo, Z.; Cao, W.; Zou, X.; et al. Bee Pollen: Current Status and Therapeutic Potential. Nutrients 2021, 13, 1876. [Google Scholar] [CrossRef]
- Duan, H.; Dong, Z.; Li, H.; Li, W.R.; Shi, S.X.; Wang, Q.; Cao, W.G.; Fang, X.M.; Fang, A.D.; Zhai, K.F. Quality evaluation of bee pollens by chromatographic fingerprint and simultaneous determination of its major bioactive components. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2019, 134, 110831. [Google Scholar] [CrossRef]
- Castagna, A.; Benelli, G.; Conte, G.; Sgherri, C.; Signorini, F.; Nicolella, C.; Ranieri, A.; Canale, A. Drying Techniques and Storage: Do They Affect the Nutritional Value of Bee-Collected Pollen? Molecules 2020, 25, 4925. [Google Scholar] [CrossRef] [PubMed]
- Thakur, M.; Nanda, V. Composition and functionality of bee pollen: A review. Trends Food Sci. Technol. 2020, 98, 82–106. [Google Scholar] [CrossRef]
- Kieliszek, M.; Piwowarek, K.; Kot, A.; Błażejak, S.; Chlebowska-Śmigiel, A.; Wolska, I. Pollen and bee bread as new health-oriented products: A review. Trends Food Sci. Technol. 2018, 71, 170–180. [Google Scholar] [CrossRef]
- Sforcin, J.; Bankova, V.; Kuropatnicki, A. Medical Benefits of Honeybee Products. Evid.-Based Complementary Altern. Med. 2017, 2017, 2702106. [Google Scholar] [CrossRef]
- Rzepecka-Stojko, A.; Stojko, J.; Kurek-Górecka, A.; Górecki, M.; Kabała-Dzik, A.; Kubina, R.; Moździerz, A.; Buszman, E. Polyphenols from Bee Pollen: Structure, Absorption, Metabolism and Biological Activity. Molecules 2015, 20, 21732–21749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Didaras, N.A.; Karatasou, K.; Dimitriou, T.G.; Amoutzias, G.D.; Mossialos, D. Antimicrobial Activity of Bee-Collected Pollen and Beebread: State of the Art and Future Perspectives. Antibiotics 2020, 9, 811. [Google Scholar] [CrossRef] [PubMed]
- Mărgăoan, R.; Stranț, M.; Varadi, A.; Topal, E.; Yücel, B.; Cornea-Cipcigan, M.; Campos, M.G.; Vodnar, D.C. Bee Collected Pollen and Bee Bread: Bioactive Constituents and Health Benefits. Antioxidants 2019, 8, 568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denisow, B.; Denisow-Pietrzyk, M. Biological and therapeutic properties of bee pollen: A review. J. Sci. Food Agric. 2016, 96, 4303–4309. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Lari, Q.H.; Khan, M. Therapeutic Uses of Mom Zard (Beeswax) in Unani System of Medicine—A Review. J. Anal. Pharm. Res. 2016, 3, 44. [Google Scholar] [CrossRef] [Green Version]
- Lima, W.G.; Brito, J.C.M.; da Cruz Nizer, W.S. Bee products as a source of promising therapeutic and chemoprophylaxis strategies against COVID-19 (SARS-CoV-2). Phytother. Res. PTR 2020, 35, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Hortal, M.D.; Orantes-Bermejo, F.J.; Sánchez-González, C.; Varela-López, A.; Giampieri, F.; Torres Fernández-Piñar, C.; Serra-Bonvehí, J.; Forbes-Hernández, T.Y.; Reboredo-Rodríguez, P.; Llopis, J.; et al. Industrial-Scale Decontamination Procedure Effects on the Content of Acaricides, Heavy Metals and Antioxidant Capacity of Beeswax. Molecules 2019, 24, 1518. [Google Scholar] [CrossRef] [Green Version]
- Amin, M.; Putra, N.; Kosasih, E.A.; Prawiro, E.; Luanto, R.A.; Mahlia, T.M.I. Thermal properties of beeswax/graphene phase change material as energy storage for building applications. Appl. Therm. Eng. 2017, 112, 273–280. [Google Scholar] [CrossRef]
- Szulc, J.; Machnowski, W.; Kowalska, S.; Jachowicz, A.; Ruman, T.; Steglińska, A.; Gutarowska, B. Beeswax-Modified Textiles: Method of Preparation and Assessment of Antimicrobial Properties. Polymers 2020, 12, 344. [Google Scholar] [CrossRef] [Green Version]
- Bayir, Y.; Un, H.; Ugan, R.; Akpinar, E.; Calik, I.; Halici, Z. The effects of Beeswax, Olive oil and Butter impregnated bandage on burn wound healing. Burns 2019, 45, 1410–1417. [Google Scholar] [CrossRef]
- Bogdanov, S. Beeswax: Production, Properties, Composition, Control. In Beeswax Book; Bee World: London, UK, 2016. [Google Scholar]
- Karabey, T.; KaragÖZoĞLu, Ş. Using Beeswax, Olive Oil and Centaury Oil for Pressure Ulcers. Turk. Klin. J. Case Rep. 2019, 27, 153–156. [Google Scholar] [CrossRef]
- Zhang, Y.; Simpson, B.K.; Dumont, M.-J. Effect of beeswax and carnauba wax addition on properties of gelatin films: A comparative study. Food Biosci. 2018, 26, 88–95. [Google Scholar] [CrossRef]
- Fratini, F.; Cilia, G.; Turchi, B.; Felicioli, A. Beeswax: A minireview of its antimicrobial activity and its application in medicine. Asian Pac. J. Trop. Med. 2016, 9, 839–843. [Google Scholar] [CrossRef]
- Nader, R.A.; Mackieh, R.; Wehbe, R.; El Obeid, D.; Sabatier, J.M.; Fajloun, Z. Beehive Products as Antibacterial Agents: A Review. Antibiotics 2021, 10, 717. [Google Scholar] [CrossRef] [PubMed]
- Sakka, A.E.; Abdulrhman, M.; Shehata, I.H. Comparison between topical application of Honey, Bees wax and Olive Oil Propolis extract and Nystatin for treatment of Diaper Dermatitis in Infants. Int. J. Pediatrics Child Health 2013, 1, 39–42. [Google Scholar]
- Kim, D.-H.; Lee, H.-W.; Park, H.-W.; Lee, H.-W.; Chun, K.-H. Bee venom inhibits the proliferation and migration of cervical-cancer cells in an HPV E6/E7-dependent manner. BMB Rep. 2020, 53, 419–424. [Google Scholar] [CrossRef]
- Sİg, A.K.; Güney, M.; Özlem, Ö.Z.; Hüseyin, Ş.A.N. Bee venom: A medical perspective. Turk. J. Clin. Lab. 2019, 10, 414–421. [Google Scholar] [CrossRef]
- Wehbe, R.; Frangieh, J.; Rima, M.; El Obeid, D.; Sabatier, J.-M.; Fajloun, Z. Bee Venom: Overview of Main Compounds and Bioactivities for Therapeutic Interests. Molecules 2019, 24, 2997. [Google Scholar] [CrossRef] [Green Version]
- Lin, T.-Y.; Hsieh, C.-L. Clinical Applications of Bee Venom Acupoint Injection. Toxins 2020, 12, 618. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; Kim, K.H. Clinical Effectiveness and Adverse Events of Bee Venom Therapy: A Systematic Review of Randomized Controlled Trials. Toxins 2020, 12, 558. [Google Scholar] [CrossRef] [PubMed]
- Carpena, M.; Nuñez-Estevez, B.; Soria-Lopez, A.; Simal-Gandara, J. Bee Venom: An Updating Review of Its Bioactive Molecules and Its Health Applications. Nutrients 2020, 12, 3360. [Google Scholar] [CrossRef]
- Kurek-Górecka, A.; Komosinska-Vassev, K.; Rzepecka-Stojko, A.; Olczyk, P. Bee Venom in Wound Healing. Molecules 2021, 26, 148. [Google Scholar] [CrossRef]
- Weis, W.A.; Ripari, N.; Conte, F.L.; Honorio, M.d.S.; Sartori, A.A.; Matucci, R.H.; Sforcin, J.M. An overview about apitherapy and its clinical applications. Phytomedicine Plus 2022, 2, 100239. [Google Scholar] [CrossRef]
- El-Ashram, S.; El-Samad, L.M.; Basha, A.A.; El Wakil, A. Naturally-derived targeted therapy for wound healing: Beyond classical strategies. Pharmacol. Res. 2021, 170, 105749. [Google Scholar] [CrossRef]
- El-Kased, R.F.; Amer, R.I.; Attia, D.; Elmazar, M.M. Honey-based hydrogel: In vitro and comparative In vivo evaluation for burn wound healing. Sci. Rep. 2017, 7, 9692. [Google Scholar] [CrossRef] [Green Version]
- Movaffagh, J.; Fazly Bazzaz, B.S.; Yazdi, A.T.; Sajadi-Tabassi, A.; Azizzadeh, M.; Najafi, E.; Amiri, N.; Taghanaki, H.B.; Ebrahimzadeh, M.H.; Moradi, A. Wound Healing and Antimicrobial Effects of Chitosan-hydrogel/Honey Compounds in a Rat Full-thickness Wound Model. Wounds A Compend. Clin. Res. Pract. 2019, 31, 228–235. [Google Scholar]
- Combarros-Fuertes, P.; Estevinho, L.M.; Teixeira-Santos, R.; Rodrigues, A.G.; Pina-Vaz, C.; Fresno, J.M.; Tornadijo, M.E. Antibacterial Action Mechanisms of Honey: Physiological Effects of Avocado, Chestnut, and Polyfloral Honey upon Staphylococcus aureus and Escherichia coli. Molecules 2020, 25, 1252. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, R.; Cooper, R. Improving antibiotic activity against wound pathogens with manuka honey in vitro. PLoS ONE 2012, 7, e45600. [Google Scholar] [CrossRef] [Green Version]
- Prasathkumar, M.; Sadhasivam, S. Chitosan/Hyaluronic acid/Alginate and an assorted polymers loaded with honey, plant, and marine compounds for progressive wound healing—Know-how. Int. J. Biol. Macromol. 2021, 186, 656–685. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, C.; Li, C.; Qin, Y.; Wang, Z.; Yang, F.; Li, Z.; Wang, J. A functional chitosan-based hydrogel as a wound dressing and drug delivery system in the treatment of wound healing. RSC Adv. 2018, 8, 7533–7549. [Google Scholar] [CrossRef] [Green Version]
- Rafati, Z.; Sirousazar, M.; Hassan, Z.M.; Kheiri, F. Honey-Loaded Egg White/Poly(vinyl alcohol)/Clay Bionanocomposite Hydrogel Wound Dressings: In Vitro and In Vivo Evaluations. J. Polym. Environ. 2020, 28, 32–46. [Google Scholar] [CrossRef]
- Nezhad-Mokhtari, P.; Javanbakht, S.; Asadi, N.; Ghorbani, M.; Milani, M.; Hanifehpour, Y.; Gholizadeh, P.; Akbarzadeh, A. Recent advances in honey-based hydrogels for wound healing applications: Towards natural therapeutics. J. Drug Deliv. Sci. Technol. 2021, 66, 102789. [Google Scholar] [CrossRef]
- Santos, A.; Moreira, A.; Piler Carvalho, C.; Luchese, R.; Prudencio, E.; McGuinness, G.; Mendes, M.; Oliveira, R. Physically Cross-Linked Gels of PVA with Natural Polymers as Matrices for Manuka Honey Release in Wound-Care Applications. Materials 2019, 12, 559. [Google Scholar] [CrossRef] [Green Version]
- Abraham, S.A.; Yashavanth, G.; Deveswaran, R.; Bharath, S.; Azamathulla, M.; Shanmuganathan, S. Honey based hydrogel as delivery system for wound healing. Mater. Today Proc. 2022, 49, 1709–1718. [Google Scholar] [CrossRef]
- Governa, P.; Cusi, M.G.; Borgonetti, V.; Sforcin, J.M.; Terrosi, C.; Baini, G.; Miraldi, E.; Biagi, M. Beyond the Biological Effect of a Chemically Characterized Poplar Propolis: Antibacterial and Antiviral Activity and Comparison with Flurbiprofen in Cytokines Release by LPS-Stimulated Human Mononuclear Cells. Biomedicines 2019, 7, 73. [Google Scholar] [CrossRef] [Green Version]
- Abdullah, N.A.; Ja’afar, F.; Yasin, H.M.; Taha, H.; Petalcorin, M.I.R.; Mamit, M.H.; Kusrini, E.; Usman, A. Physicochemical analyses, antioxidant, antibacterial, and toxicity of propolis particles produced by stingless bee Heterotrigona itama found in Brunei Darussalam. Heliyon 2019, 5, e02476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afonso, A.; Gonçalves, J.; Luís, Â.; Gallardo, E.; Duarte, A. Evaluation of the In Vitro Wound-Healing Activity and Phytochemical Characterization of Propolis and Honey. Appl. Sci. 2020, 10, 1845. [Google Scholar] [CrossRef] [Green Version]
- Abbaszadeh, A.; Rajabzadeh, A.; Zarei, L. Effect of Chitosan/Propolis Biodegradable Film on Full-Thickness Wound Healing in Rats. Iran. J. Vet. Surg. 2019, 14, 9–17. [Google Scholar] [CrossRef]
- Mendez-Pfeiffer, P.; Juarez, J.; Hernandez, J.; Taboada, P.; Virués, C.; Valencia, D.; Velazquez, C. Nanocarriers as drug delivery systems for propolis: A therapeutic approach. J. Drug Deliv. Sci. Technol. 2021, 65, 102762. [Google Scholar] [CrossRef]
- El-Seedi, H.R.; Eid, N.; Abd El-Wahed, A.A.; Rateb, M.E.; Afifi, H.S.; Algethami, A.F.; Zhao, C.; Al Naggar, Y.; Alsharif, S.M.; Tahir, H.E.; et al. Honey Bee Products: Preclinical and Clinical Studies of Their Anti-inflammatory and Immunomodulatory Properties. Front. Nutr. 2022, 8, 1109. [Google Scholar] [CrossRef]
- Eskandarinia, A.; Kefayat, A.; Agheb, M.; Rafienia, M.; Amini Baghbadorani, M.; Navid, S.; Ebrahimpour, K.; Khodabakhshi, D.; Ghahremani, F. A Novel Bilayer Wound Dressing Composed of a Dense Polyurethane/Propolis Membrane and a Biodegradable Polycaprolactone/Gelatin Nanofibrous Scaffold. Sci. Rep. 2020, 10, 3063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nascimento, A.P.; Moraes, L.A.R.; Ferreira, N.U.; Moreno, G.d.P.; Uahib, F.G.M.; Barizon, E.A.; Berretta, A.A. The Lyophilization Process Maintains the Chemical and Biological Characteristics of Royal Jelly. Evid.-Based Complementary Altern. Med. Ecam 2015, 2015, 825068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, J.; Wang, Z.; Chen, Y.; Cao, J.; Tian, W.; Ma, B.; Dong, Y. Active components and biological functions of royal jelly. J. Funct. Foods 2021, 82, 104514. [Google Scholar] [CrossRef]
- Mierzejewski, M. The antimicrobial effects of royal jelly, propolis and honey against bacteria of clinical significance in comparison to three antibiotics. Coll. Arts Sci. Biol. 2014. [Google Scholar]
- Kaškonienė, V.; Adaškevičiūtė, V.; Kaškonas, P.; Mickienė, R.; Maruška, A. Antimicrobial and antioxidant activities of natural and fermented bee pollen. Food Biosci. 2020, 34, 100532. [Google Scholar] [CrossRef]
- Spulber, R.; Doğaroğlu, M.; Băbeanu, N.; Popa, O. Physicochemical characteristics of fresh bee pollen from different botanical origins. Rom. Biotechnol. Lett. 2018, 23, 13357–13365. [Google Scholar]
- Kacaniova, M.; Vuković, N.; Chlebo, R.; Haščík, P.; Rovná, K.; Cubon, J.; Dzugan, M.; Pasternakiewicz, A. The antimicrobial activity of honey, bee pollen loads and beeswax from Slovakia. Arch. Biol. Sci. 2012, 64, 927–934. [Google Scholar] [CrossRef]
- Gümüş, K.; Karaman Özlü, Z. The Effect of a Beeswax, Olive Oil and Alkanna tinctoria (L.) Tausch Mixture on Burn Injuries: An Experimental Study with a Control Group. Complementary Ther. Med. 2017, 34, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Hromiš, N.; Lazić, V.; Sinisa, M.; Vaštag, Ž.; Popović, S.; Suput, D.; Džinić, N.; Velićanski, A.; Popović, L. Optimization of chitosan biofilm properties by addition of caraway essential oil and beeswax. J. Food Eng. 2015, 158, 86–93. [Google Scholar] [CrossRef]
- Moustafa, A.; Atiba, A. The Effectiveness of a Mixture of Honey, Beeswax and Olive Oil in Treatment of Canine Deep Second-Degree Burn. Glob. Vet. 2015, 14, 244–250. [Google Scholar] [CrossRef]
- Ebrahimpour, N.; Mehrabani, M.; Iranpour, M.; Kordestani, Z.; Mehrabani, M.; Nematollahi, M.H.; Asadipour, A.; Raeiszadeh, M.; Mehrbani, M. The efficacy of a traditional medicine preparation on second-degree burn wounds in rats. J. Ethnopharmacol. 2020, 252, 112570. [Google Scholar] [CrossRef]
- El-Wahed, A.A.A.; Khalifa, S.A.M.; Elashal, M.H.; Musharraf, S.G.; Saeed, A.; Khatib, A.; Tahir, H.E.; Zou, X.; Naggar, Y.A.; Mehmood, A.; et al. Cosmetic Applications of Bee Venom. Toxins 2021, 13, 810. [Google Scholar] [CrossRef] [PubMed]
- Memariani, H.; Memariani, M. Anti-fungal properties and mechanisms of melittin. Appl. Microbiol. Biotechnol. 2020, 104, 6513–6526. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Z.; Gao, S.; Zhang, W.; Gong, H.; Xu, K.; Luo, C.; Zhi, W.; Weng, J.; Li, J. Polyvinyl Alcohol/Chitosan Composite Hydrogels with Sustained Release of Traditional Tibetan Medicine for Promoting Chronic Diabetic Wound Healing. Biomater. Sci. 2021, 9, 3821–3829. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Han, S.M.; Park, K.-K. Therapeutic Effects of Apamin as a Bee Venom Component for Non-Neoplastic Disease. Toxins 2020, 12, 195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Chemical Compounds | Pharmacological Activity |
|---|---|
| Acacetin | Anti-inflammatory |
| Apigenin | Anti-inflammatory; Antimicrobial |
| Artepilin C | Antitumor activity; Antioxidative |
| Caffeic acid phenethyl ester | Antitumor activity; Anti-inflammatory |
| Chrysin | Anti-inflammatory; Antibacterial |
| Caffeic acid | Antifungal; Antiviral; Anti-inflammatory |
| Cinnamic acid | Anti-inflammatory |
| Dicaffeoylquinic acid derivatives | Hepatoprotective |
| Ferulic acid, Galangin, Gallic acid | Anti-inflammatory |
| Protocatechuic acid | Anti-inflamatory; Antibacterial |
| Pinocembrin | Antifungal; Antimold; Local anesthesia |
| Propofol | Antioxidative |
| Property | Description of the Biological Activity’s Mechanism |
|---|---|
| Nutritive properties | Presence of carbohydrates, proteins, lipids, exogenous amino acids, unsaturated fatty acids, phytosterols, bioelements, phospholipids, and vitamins. |
| Antioxidative properties | Complexing metals; hydroxyl radicals are eliminated. |
| Cardioprotective properties | Inhibition of blood platelets aggregation and ACE activity are inhibited. |
| Hepatoprotective properties | The activity of detoxifying in industrial poisoning; lipofuscin reduction. |
| Anti-inflammatory properties | Inhibitory activity of NO production and COX-2. |
| Antibacterial properties | Bacteria metabolism is affected in the case of Pseudomonas aeruginosa (P. aeruginosa), Staphylococcus epidermis (S. epidermis), B. cerus, E. coli, S. aureus, Listeria monocytogenes (L. monocytogenes), B. subtilis, and Salmonella enterica |
| Anticarcinogenic properties | Brassica camperstris L. bee pollen extract is responsible for decreasing the expression of antiapoptotic proteins and for caspase-3 enzyme activity increase; 17β-estradiol activity is inhibited by Salix alba L. and Cistus incanus L. bee pollens. |
| Antianaemic properties | Hemoglobin level is increasing while blood platelets number is decreasing. |
| Bone tissue effects | Cystus ladaniferus L. bee pollen is responsible for increasing alkaline phosphatase level, resorption inhibition of the femur, and osteoclastic cell formation. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dumitru, C.D.; Neacsu, I.A.; Grumezescu, A.M.; Andronescu, E. Bee-Derived Products: Chemical Composition and Applications in Skin Tissue Engineering. Pharmaceutics 2022, 14, 750. https://doi.org/10.3390/pharmaceutics14040750
Dumitru CD, Neacsu IA, Grumezescu AM, Andronescu E. Bee-Derived Products: Chemical Composition and Applications in Skin Tissue Engineering. Pharmaceutics. 2022; 14(4):750. https://doi.org/10.3390/pharmaceutics14040750
Chicago/Turabian StyleDumitru, Corina Dana, Ionela Andreea Neacsu, Alexandru Mihai Grumezescu, and Ecaterina Andronescu. 2022. "Bee-Derived Products: Chemical Composition and Applications in Skin Tissue Engineering" Pharmaceutics 14, no. 4: 750. https://doi.org/10.3390/pharmaceutics14040750
APA StyleDumitru, C. D., Neacsu, I. A., Grumezescu, A. M., & Andronescu, E. (2022). Bee-Derived Products: Chemical Composition and Applications in Skin Tissue Engineering. Pharmaceutics, 14(4), 750. https://doi.org/10.3390/pharmaceutics14040750








