Abstract
Faecalibacterium duncaniae is an intestinal commensal bacterium proposed as a next-generation probiotic due to its promising outcomes in the treatment and prevention of several human diseases, which demonstrate its multiple contributions to the host’s health. However, its strict anaerobic nature has created several hurdles in the development of functional foods, nutraceuticals, and biotherapeutic products. Herein, we explored freeze-dried formulations containing prebiotics, cryoprotectants, and antioxidant agents as a technological strategy to enhance the viability of F. duncaniae DSM 17677 upon aerobic storage and gastrointestinal tract conditions. Our results indicate that freeze-dried F. duncaniae in a matrix containing inulin, sucrose, cysteine, and riboflavin survived at levels higher than 106 CFU/g and around 105 CFU/g after 1 and 4 days of aerobic storage at room temperature, respectively. Thus, the freeze-dried formulation with inulin, sucrose, cysteine, and riboflavin presents as a protective strategy to improve F. duncaniae viability under aerobic environments. Nevertheless, incorporation of a suitable coating aimed at protecting F. duncaniae against the detrimental gastrointestinal passage effects is urgently required, given its high susceptibility to extreme acidic pH values and bile.
1. Introduction
In the last decades, live bacterial cells have been widely exploited, either as therapeutic agents or as carriers to deliver drugs, presenting great outcomes in the treatment of several human diseases [1,2]. Specifically, the use of live beneficial microbes, termed as probiotics, in dietary supplements, functional foods, or pharmabiotic forms, constitutes one of the most successful approaches to achieving health benefits and improving people’s welfare and quality of life [3]. Moreover, the growing knowledge regarding the key role of gut microbiota in human health has increased interest in using intestinal commensal bacteria, as well as the traditionally used lactobacilli and bifidobacteria species, as probiotics [4]. Among those, Faecalibacterium duncaniae (formerly designated Faecalibacterium prausnitzii) is one of the most promising candidates proposed as a next-generation probiotic, given its great potential to treat and prevent various inflammatory diseases [4,5,6]. More specifically, oral administration of F. duncaniae DSM 17677 or its supernatant displayed protective effects in the colitis mice model [7]. Furthermore, analysis of the phenotypic and genotypic antimicrobial resistance profile of the F. duncaniae DSM 17677 strain showed that it has a low risk of carrying acquired antimicrobial resistance. This finding is a valuable contribution for the establishment of this strain as safe for human and animal consumption, and, consequently, the finding increases the likelihood of its approval to be applied as a food or feed additive [8]. Despite the promising outcomes, the strict anaerobic nature of F. duncaniae has created serious technological obstacles to its cultivation and handling, consequently hampering its application in the food and pharmaceutical industries [9,10]. Envisaging its use as a probiotic, effective delivery strategies must be developed to ensure that this bacterium is maintained at high viability levels during the production process, distribution chain, and shelf-life. In addition, they must also guarantee its survival after ingestion throughout the gastrointestinal tract (GIT) passage in order to ultimately reach the colon in the appropriate amounts that are known to exert the intended positive effects on the host [9].
Encapsulation techniques are gathering the attention of industries and the scientific community as a strategy to ensure the high stability/viability of probiotic strains, which is defined as the cellular entrapment/coating within a material or mixture of materials [11,12,13]. Among the several methods that may be used to encapsulate bioactive compounds, including probiotics, drying techniques are frequently preferred because the drying process reduces the formulation’s water content, contributing to a higher stability over time [13,14,15,16]. Freeze-drying is one of the most popular drying techniques for long-term probiotic preservation [14,17].
Freeze-drying, also known as lyophilization, involves three main steps: (1) freezing of the cell culture; (2) primary drying, in which the frozen water is removed by sublimation under vacuum; and (3) secondary drying, in which the unfrozen water is removed by desorption [14]. As freeze-drying conditions are milder than those of other drying techniques, such as spray-drying, probiotic cultures dried by this technique frequently display higher survival rates [18,19]. However, freeze-drying is an expensive and time-consuming batch process, and the final product is often compact and hard, which requires an additional step to obtain individual powder particles [14,19].
During the freezing-drying process and subsequent storage, microbial cells are exposed to harsh conditions, including mechanical, osmotic, and oxidative stressors. The effect of these stressors can be minimized by the incorporation of cryoprotectants (e.g., inulin, sucrose, and trehalose) and antioxidant agents (e.g., cysteine and riboflavin) [19,20]. For instance, Khan and coworkers freeze-dried the strict anaerobic F. duncaniae using a formulation containing inulin, cysteine, and riboflavin and obtained around 70% survival upon 24 h of storage with ambient air [10]. Using the work of Khan et al. as a starting point, the present study aimed to explore freeze-dried formulations, combining different protective agents to improve the viability of F. duncaniae DSM 17677 during aerobic storage and when exposed to GIT conditions. Thus, first, we evaluated the viability of F. duncaniae DSM 17677 free cells when exposed to an aerobic atmosphere and to GIT conditions, specifically acidic pH values (3 and 5) and bile. Then, the impact of the freeze-drying process, using different combinations of protective agents, on F. duncaniae viability during aerobic storage at room temperature was evaluated. Finally, the protective effect of a selected freeze-dried formulation on F. duncaniae viability when exposed to acidic pH and bile was assessed.
2. Materials and Methods
2.1. Bacterial Strain and Growth Conditions
Faecalibacterium duncaniae DSM 17677 strain (Leibniz Institute DSMZ-German Collection of Microorganisms and Cell Cultures, Braunschweig, Germany) was used in this study. It was initially cultured according to the recommended conditions proposed by DSMZ [21]. For long term storage, this strain was kept frozen at −80 °C in sBHI broth [Brain Heart Infusion medium (37 g/L; VWR International, Leuven, Belgium) supplemented with yeast extract (5 g/L; VWR International), hemin (5 mg/L; Alfa Aesar, Kandel, Germany), vitamin K1 (5 µL/L; Sigma-Aldrich Co., St. Louis, MO, USA), and L-cysteine (2 g/L; Alfa Aesar)], as previously used by Maier et al. [22], with 20% (v/v) of glycerol (Fisher Scientific, Loughborough, UK). For each assay, a F. duncaniae glycerol stock was thawed and grown in sBHI broth for 16 h at 37 °C under anaerobic conditions (85% N2, 5% H2, and 10% CO2) achieved in an anaerobic incubator (Whitley A35 HEPA anaerobic workstation, Bingley, UK). Afterwards, the previously grown culture was transferred to sBHI broth (in a proportion of 1:100), and this bacterial suspension was anaerobically incubated during 10 h at 37°C for the following experiments.
2.2. Aerobic Environments Tolerance of Free Cells
The tolerance of F. duncaniae DSM 17677 free cells (non-formulated nor freeze-dried cells) to an aerobic atmosphere was measured through two approaches, namely exposing both (i) sBHI agar plates inoculated with F. duncaniae (cell concentration of 107 CFU/mL) and (ii) 15 mL centrifuge tubes containing a bacterial suspension of F. duncaniae in sBHI broth (cell concentration of 107 CFU/mL) for 1, 2, 3, and 5 min at ambient air (without agitation). After exposure, the plates and tubes were placed inside the anaerobic chamber. The viability of F. duncaniae in the plates was assessed directly by incubating the plates anaerobically at 37 °C for 48 h. For the bacterial suspensions, the viability at each sampling timepoint was assessed via colony-forming units (CFU) enumeration by plating 10 μL of decimal dilutions on sBHI agar plates, which were then anaerobically incubated at 37 °C for 48 h. The appropriate growth controls were prepared without exposure to the aerobic atmosphere and processed as mentioned for each procedure. All assays were repeated independently at least twice, and CFU plating was performed in triplicate.
2.3. Acid and Bile Susceptibility of Free Cells
For the acidic pH susceptibility experiments, hydrochloric acid at 6 M was added to 15 mL centrifuge tubes containing grown F. duncaniae cultures in sBHI broth (in a concentration of 107 CFU/mL) in order to reach pH values of 3 and 5. After 1 and 2 h of exposure to acidic pH, F. duncaniae CFU enumeration was performed as described previously.
For the bile susceptibility assays, bile solution at 5% (m/v) was prepared by dissolving 0.5 g of bile extract porcine (Sigma) in 10 mL of sterile deionized water. As bile solubilization requires exhaustive mixing [23], the bile solution was subsequently placed in an orbital shaker (Bench Top Shaking Incubator, Wiggen Hauser, Berlin, Germany) at 37 °C and 200 rpm for 30 min. Afterwards, the bile solution was added to 15 mL centrifuge tubes containing a bacterial suspension of F. duncaniae in sBHI broth (concentration of 107 CFU/mL) to reach a final bile concentration of 0.1% (m/v), 0.25% (m/v), and 0.5% (m/v). Cultures were incubated at 37 °C for 3 h under anaerobic conditions, with CFU enumeration being performed every hour, as described previously.
For both tests, growth controls were included without hydrochloric acid and bile solution, respectively. All assays were repeated independently at least twice and included two replicates for each pH and bile concentration tested, in which CFU plating was performed in triplicate.
2.4. Formulation Procedure
The formulation procedure was based on the previous work by Khan et al. [10] with some modifications. Briefly, broth cultures of F. duncaniae (in a concentration of 107 CFU/mL) were transferred to 50 mL centrifuge tubes and centrifuged at 4470× g for 5 min at 4 °C. Bacterial pellets were then washed in phosphate buffer saline (PBS, VWR Chemicals, Aurora, OH, USA) and re-centrifuged in the same conditions to obtain a cell-washed pellet. Bacterial pellets from 35 mL of F. duncaniae broth cultures were re-suspended in one of the following, supplemented with 200 µL of 16.5 mM riboflavin (Sigma; solution prepared in PBS):
- 400 µL of a solution containing inulin [5% (m/v), Orafti Beneo, Mannheim, Germany], trehalose dihydrate [5% (m/v), Sigma], and 0.2% (m/v) cysteine prepared in PBS (ITCR);
- 400 µL of a solution containing inulin [5% (m/v)], sucrose [2.5% (m/v)], trehalose dihydrate [2.5% (m/v)], and 0.2% (m/v) cysteine prepared in PBS (ISTCR);
- 400 µL of a solution containing inulin [5% (m/v)], sucrose [5% (m/v), Sigma], and 0.2% (m/v) cysteine prepared in PBS (ISCR);
- 400 µL of a solution containing inulin [10% (m/v)] with 0.2% (m/v) cysteine prepared in PBS (ICR).
All solutions were sterilized by filtration using a cellulose acetate membrane filter (Sartorius, Goettingen, Germany) before their addition to bacterial pellets. Next, all the formulations incorporating F. duncaniae were homogenized and then frozen at −80 °C overnight. Frozen samples were freeze-dried for 24 h using a freeze drier (LyoQuest, Telstar, Barcelona, Spain) and stored inside a desiccator at room temperature (around 21 °C) until further analysis.
It should be noted that the bacterial cultivation, formulation procedure, and testing of tolerance to GIT conditions were conducted anaerobically, while freeze-drying and storage were performed aerobically. For each formulation and time point, two replicates were used and inoculated in triplicate in sBHI agar plates in order to characterize their impact on the viability and stability of F. duncaniae during aerobic storage and when exposed to acidic pH values and bile as described below.
2.5. Viability and Stability of Freeze-Dried Formulations during Aerobic Storage
For viability and stability assays, freeze-dried formulations incorporating F. duncaniae were exposed to atmospheric air at ambient temperature for 0 and 24 h. In addition, the ISCR formulation was selected to test the stability of F. duncaniae during a more prolonged aerobic storage period, with sampling points at 0, 24, 48, 72, and 96 h. For each sampling point, the formulations were placed back in the anaerobic chamber, rehydrated in sBHI, and then ten-fold serial dilutions were performed with PBS. Fifty µL of each dilution were plated in triplicate on sBHI agar plates that were incubated anaerobically at 37 °C for 48 h. After incubation, CFU numbers were determined, and the results were expressed as log CFU per gram (log CFU/g) for each freeze-dried formulation.
2.6. Viability of a Selected Freeze-Dried Formulation after Exposure to Acidic pH and Bile
The acid and bile tolerance assays were performed for the ISCR freeze-dried formulation immediately after the freeze-drying procedure (i.e., at day 0 of aerobic storage). In the acid tolerance assays, the freeze-dried formulation was initially rehydrated with 2 mL of sBHI under anaerobic conditions. Then, the pH of the rehydrated formulations was adjusted to values of 3 and 5 with 2 M HCl and incubated at 37 °C under anaerobic conditions. In addition, a growth control (without addition of HCl) was included. The number of viable cells (log CFU/g) was determined at 0 and after 2 h of exposure to the acidic pH.
The bile tolerance of the freeze-dried formulation was tested by adding bile solution to 1.8 mL of the rehydrated formulation in sBHI to reach the final bile concentrations of 0.25% (m/v) and 0.5% (m/v). Moreover, a growth control (without bile) was included. Then, aliquots were taken at 0 and after 3 h of bile exposure at 37 °C under anaerobic conditions, and cell viability (log CFU/g) was determined.
2.7. Statistical Analysis
Data were expressed as the mean ± standard deviation (SD) of replicates. Results from exposure to oxygen, acid pH values and bile, and aerobic storage were analyzed using the Wilcoxon signed rank test, as the data did not follow a normal distribution according to the Shapiro–Wilk test. All tests were performed with a significance level of 5% (p value < 0.05) using GraphPad Prism software version 5.0 (GraphPad Software, San Diego, CA, USA).
3. Results and Discussion
3.1. Oxygen Sensitivity of F. duncaniae DSM 17677 Free Cells
After the exposure of sBHI agar plates inoculated with the F. duncaniae DSM 17677 strain to ambient air, no viable cells could be detected after just 1 min of exposure. In contrast, F. duncaniae bacterial suspensions within 15 mL centrifuge tubes maintained their viability during 5 min of oxygen exposure (see Table 1). We hypothesize that the disparity of results may be explained by the difference between the surface areas exposed to the aerobic atmosphere. A thin layer of bacterial suspension spread over a wide surface area was exposed to oxygen in the inoculated plates, whereas in the approach involving the exposure of bacterial suspension within 15 mL centrifuge tubes, only the very top layer of the bacterial suspension was exposed to the aerobic atmosphere. Indeed, the diffusion coefficient for oxygen into water is very small, leading to a low permeation into the liquid culture media. In a static culture, the diffusion of oxygen into the medium only occurs at the very top surface of the liquid that is directly exposed to the atmosphere; therefore, everything below approximately 1 mm is considered to be growing under anaerobic conditions [24]. Duncan and colleagues were pioneers in demonstrating the strict anaerobic nature of F. duncaniae DSM 17677 (=A2-165). Their group reported that air ambient exposure of inoculated plates for more than 2 min was enough to prevent subsequent anaerobic growth [5]. The present findings are consistent with those reported by Duncan et al. and provide additional insight when evaluating F. duncaniae viability in broth cultures after aerobic exposure.

Table 1.
Viability of F. duncaniae DSM 17677 free cells when exposed to ambient air. Data represent the mean values of log CFU/mL ± standard deviation (SD) obtained in the exposure of inoculated plates and after plating of bacterial suspension within 15 mL centrifuge tubes exposed for different time periods.
3.2. Acid and Bile Sensitivity of F. duncaniae DSM 17677 Free Cells
Faecalibacterium duncaniae free cells’ viability under low pH values and in the presence of bile was evaluated in order to assess the two main stressors encountered by F. duncaniae during gastrointestinal transit. As presented in Table 2, losses in bacterial viability higher than 4 log CFU/mL (to levels below the limit of detection) were observed just after exposure to pH 3 for 1 h. In contrast, after 1 and 2 h exposure to pH 5, F. duncaniae viability only underwent slight fluctuations (p > 0.05; see Table 2). Furthermore, F. duncaniae DSM 17677 free cells demonstrated a high sensitivity to bile, since cultivable cell numbers were lower than the limit of detection of the CFU enumeration technique for all times of exposure, independently of the bile concentrations (0.1%, 0.25%, and 0.5%) tested. In addition, our results are in accordance with previous reports in terms of pH values tolerated by the Faecalibacterium species and its sensitivity to bile. Indeed, several Faecalibacterium strains have been described as being able to grow at pH values ranging between 5.0 and 6.7, while the absence of bacterial growth was found at pH values lower than 4.5 and in the presence of bile at 0.1%, 0.25%, and 0.5% [25,26,27]. However, it should be noted that these previous studies evaluated bacterial growth through measurements of optical density, which is a simple, inexpensive, and quick technique, albeit less accurate because it just estimates viability. The absence of a direct correlation between CFU counts and optical density measurements in Faecalibacterium cultures has been previously reported [28]. Taking this into account, our results further substantiate this knowledge, as the evaluation of the extent of the effects of exposure to acid pH (pH 3 and pH 5) and bile on the F. duncaniae free cells viability uses the CFU enumeration technique, which provides the determination of viable and cultivable cells. Additionally, our findings highlight the need to develop a suitable delivery system for F. duncaniae, given its high susceptibility to oxygen and gastrointestinal conditions.

Table 2.
Viability of F. duncaniae DSM 17677 free cells when exposed to acidic pH. Data represent the mean values of log CFU/mL ± standard deviation (SD) obtained in growth control and after exposure to pH 3 and pH 5 for 1 and 2 h.
3.3. Aerobic Exposure of F. duncaniae Freeze-Dried Formulations at Room Temperature
Freeze-drying is a technique often used for probiotic preservation, allowing cost-effective delivery and management. However, during the freeze-drying procedure and subsequent storage, probiotic strains are subjected to stress conditions that may impair their viability and functionality [19,20]. Therefore, in order to maintain the viability of probiotic strains, exploitation of stabilizing strategies provided by cryoprotectant, prebiotic, and antioxidant compounds during freeze-drying and storage is a crucial and challenging task in the development of probiotic formulations. This rationale was taken as a starting point for the development of four preservation matrices in this study. Several studies support the use of inulin, trehalose, and sucrose as prebiotic/cryopreserving agents able to act as nutritional substrates and protecting agents during the freeze-drying procedure [20,29,30], and the use of cysteine and riboflavin as antioxidant and redox mediators [10,31]. Thus, four combinations of these agents were tested. As can be seen in Figure 1, all formulations provided protection during freeze-drying and subsequent aerobic storage at room temperature, but at different magnitudes. ITCR, ISTCR, and ISCR formulations offered higher protection during freeze-drying, maintaining F. duncaniae viability around 107 CFU/g. In contrast, the ICR formulation presented the lowest viability for F. duncaniae (below 105 CFU/g) in the post freeze-drying period. Although there was no statistically significant difference (p > 0.05) in cell viability found in each freeze-dried formulation when comparing timepoint 0 h with 24 h, Figure 1 shows that ITCR offered the highest stabilization effect (without loss in F. duncaniae viability, comparing the mean log CFU/g values between 0 and 24 h), followed by ISCR and ICR with viability reductions of 0.41 and 0.44 log CFU/g, respectively, when comparing mean log CFU/g values of timepoint 0 h with 24 h. In contrast, the ISTCR formulation exerted the lowest stabilization effect during 1 day of aerobic storage, with a viability reduction of 0.73 log CFU/g when comparing mean values of log CFU/g at 0 h versus 24 h.
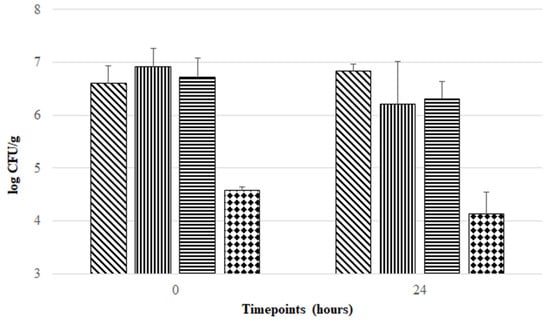
Figure 1.
Evolution of viability of F. duncaniae DSM 17677 (mean values in log CFU/g) in different freeze-dried formulations (diagonal lines: ITCR; vertical lines: ISTCR; horizontal lines: ISCR; and diamond pattern: ICR) during aerobic storage at room temperature for 24 h. Error bars represent standard deviation of the mean.
To the best of our knowledge, only one previous study assessed the stabilizing effect of prebiotic, cryoprotectant, and antioxidant agents in enhancing F. duncaniae viability during freeze-drying and subsequent aerobic storage. Khan and colleagues were pioneers in this matter when they demonstrated that F. duncaniae DSM 17677 freeze-dried in a matrix composed of 10% (m/v) inulin, 0.2% (m/v) cysteine, and 16.5 mM riboflavin (corresponding to the ICR formulation tested in the present work) was able to survive ambient air exposure for 24 h with a percentage of survival around 70% [10]. However, these researchers did not refer to F. duncaniae viability in terms of CFU/g (nor did they assess viability beyond 24 h). Nevertheless, it has been reported that probiotic bacteria must be present at minimum concentrations of 106–107 CFU/g or CFU/mL in order to exert their positive effects [32,33]. Considering this requirement, our results suggest that the formulation proposed by Khan and colleagues (ICR formulation) does not ensure this minimum level of probiotic bacteria that should be present in probiotic products.
After the initial screening, the ISCR formulation was selected to test the stability of F. duncaniae DSM 17677 during a more prolonged aerobic storage period, i.e., 96 h (4 days) at room temperature. This formulation was chosen because enabling high F. duncaniae viability and stability during 24 h of aerobic storage (with levels between 106–107 CFU/g) is economically more viable in comparison with the other formulations tested. As can be observed in Figure 2, a downward trend in the viability of F. duncaniae DSM 17677 was found throughout aerobic storage from 0 to 72 h, with a reduction of around 2.5 log cycles, reaching a mean log CFU/g of 4.62 at the timepoint of 72 h. After 96 h of aerobic exposure, viability appeared to reach a stabilizing effect, as it was maintained at levels of around 105 CFU/g. Thus, the ISCR formulation containing inulin [5% (m/v)], sucrose [5% (m/v)], cysteine [0.2% (m/v)], and riboflavin (16.5 mM) appears to be a promising solution to enhance the survival of this strict anaerobic bacterium in aerobic environments and simultaneously protect against detrimental conditions underlying the freeze-drying procedure.
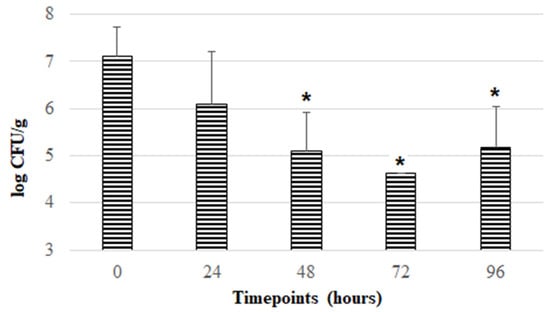
Figure 2.
Evolution of viability of F. duncaniae DSM 17677 (mean values in log CFU/g) incorporated in freeze-dried formulation containing inulin, sucrose, cysteine, and riboflavin during aerobic storage at room temperature throughout 96 h. Error bars represent standard deviation of the mean. Bars marked with * represent statistically significant differences (p < 0.05) in comparison to data reported at 0 h.
3.4. Exposure of F. duncaniae Freeze-Dried in Inulin, Sucrose, Cysteine and Riboflavin Matrix to Acidic pH Values and Bile
It has been indicated that probiotic strains must survive manufacture, storage, and, after consumption, the passage through the harsh GIT conditions in order to reach the colon in adequate viable cell numbers [9]. In the present work, the ISCR formulation was exposed to two of the main stressors of the digestive tract: acidic pH (pH 3 and pH 5) and bile (at 0.25% and 0.5%). As presented in Table 3, F. duncaniae viability decreased to undetectable levels in the freeze-dried formulation after exposure to pH 3 for 2 h or to bile concentrations of 0.25% (m/v) and 0.5% (m/v) for 3 h. However, freeze-dried bacteria when subjected to pH 5 for 2 h showed a lower reduction of viability. These results demonstrate that the ISCR formulation does not offer protection to F. duncaniae when exposed to pH 3 and bile concentrations of 0.25 and 0.5% (m/v), similar to what was verified for the free cells. In alignment with our findings, recently Raise and colleagues used one Faecalibacterium isolate (named F. prausnitzii CNCM I-4573) and demonstrated that both free and freeze-dried cells—the latter in a matrix containing sucrose (0.49 M) and cysteine (5 mM)—had a full viability loss after contact with simulated gastric fluid, containing pepsin (3 g/L) at pH 1.8, and the simulated distal jejunum buffer, containing pancreatin (5 g/L) and bile salts (3 g/L) at pH 6.8 [34]. Together, these findings corroborate that F. duncaniae is highly sensitive to acidic pH (equal to or lower than 3) and bile, suggesting the urgent need to develop suitable coatings to protect this bacterium from harsh GIT conditions, mainly extreme acidic pH and bile, in a similar way to previous studies involving other anaerobic beneficial bacteria [11,30,35].

Table 3.
Acid and bile tolerance of F. duncaniae DSM 17677 incorporated in the ISCR freeze-dried formulation containing inulin [5% (m/v)]), sucrose [5% (m/v)]), cysteine [0.2% (m/v)]), and riboflavin (16.5 mM). Results were expressed as mean values in log CFU/g ± standard deviation (SD).
4. Conclusions
The present study provides further robustness and brings new insights regarding knowledge of F. duncaniae DSM 17677 susceptibility towards environmental stresses, including aerobic atmosphere, acidic pH values, and bile. Our data indicated that the tolerance of F. duncaniae DSM 17677 free cells to an aerobic atmosphere was higher when the bacterium was suspended in a liquid medium rather than in inoculated sBHI agar plates, which could be explained by the low oxygen diffusion in broth cultures. Furthermore, we demonstrated that the viability of this bacterial strain was strongly impaired at pH 3 and in the presence of bile. To enhance F. duncaniae viability under an aerobic environment, namely acid pH and bile, a freeze-drying strategy with a combination of different protective agents was explored. Interestingly, our results showed that F. duncaniae freeze-dried in an inulin, sucrose, cysteine, and riboflavin matrix was able to survive at levels around 105 CFU/g after 96 h of aerobic storage at room temperature. However, when this formulation was exposed to bile and acidic pH values, no further protection was granted in comparison to free cells. Therefore, future studies performing some adaptations of the freeze-dried formulation are needed in order to reach F. duncaniae levels higher than 106 CFU/g (corresponding to the minimum level required in probiotic products) during prolonged aerobic storage. Furthermore, additional works aiming to develop an optimal coating to protect the F. duncaniae freeze-dried formulation from harsh GIT conditions are urgently required.
Author Contributions
Conceptualization: D.M., M.D., J.C.B., D.A., J.C.A., A.C.F. and A.M.G.; Methodology: D.M., M.D., J.C.B., D.A., J.C.A., A.C.F. and A.M.G.; Formal analysis, D.M. and M.D.; Investigation: D.M., M.D. and D.A.; Resources: A.C.F. and A.M.G.; Writing-original draft preparation: D.M. and M.D.; Writing-review and editing, D.M., M.D., J.C.B., D.A., J.C.A., A.C.F. and A.M.G.; Supervision: J.C.A., A.C.F. and A.M.G.; Funding acquisition: D.M., A.C.F. and A.M.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by national funds through FCT/MEC (PIDDAC), project references IF/00588/2015, under the Scientific Employment Stimulus-Individual Call (CEEC Individual)-CEECIND/00520/2017/CP1404/CT0001, and by Operational Program Competitiveness and Internationalization in its FEDER component, and by the budget of the Foundation for Science and Tech-nology, I.P. (FCT, IP) in its OE component, project reference POCI-01-0145-FEDER-031400-PTDC/BAA-AGR/31400/2017 and EXPL/BIA-MIC/0258/2021. We would also like to thank the scientific collaboration under the FCT project UIDB/50016/2020.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hosseinidoust, Z.; Mostaghaci, B.; Yasa, O.; Park, B.-W.; Singh, A.V.; Sitti, M. Bioengineered and Biohybrid Bacteria-Based Systems for Drug Delivery. Adv. Drug Deliv. Rev. 2016, 106, 27–44. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Liu, J. Decorated Bacteria and the Application in Drug Delivery. Adv. Drug Deliv. Rev. 2022, 188, 114443. [Google Scholar] [CrossRef] [PubMed]
- Voss, G.B.; Machado, D.; Barbosa, J.C.; Campos, D.A.; Gomes, A.M.; Pintado, M. Interplay between Probiotics and Prebiotics for Human Nutrition and Health. In Probiotics for Human Nutrition in Health and Disease; Elsevier: Amsterdam, The Netherlands, 2022; pp. 231–254. [Google Scholar]
- Almeida, D.; Machado, D.; Andrade, J.C.; Mendo, S.; Gomes, A.M.; Freitas, A.C. Evolving Trends in Next-Generation Probiotics: A 5W1H Perspective. Crit. Rev. Food Sci. Nutr. 2020, 60, 1783–1796. [Google Scholar] [CrossRef] [PubMed]
- Duncan, S.H.; Hold, G.L.; Harmsen, H.J.M.; Stewart, C.S.; Flint, H.J. Growth Requirements and Fermentation Products of Fusobacterium Prausnitzii, and a Proposal to Reclassify It as Faecalibacterium Prausnitzii Gen. Nov., Comb. Nov. Int. J. Syst. Evol. Microbiol. 2002, 52, 2141–2146. [Google Scholar] [CrossRef]
- Sakamoto, M.; Sakurai, N.; Tanno, H.; Iino, T.; Ohkuma, M.; Endo, A. Genome-Based, Phenotypic and Chemotaxonomic Classification of Faecalibacterium Strains: Proposal of Three Novel Species Faecalibacterium duncaniae sp. nov., Faecalibacterium hattorii sp. nov. and Faecalibacterium gallinarum sp. nov. Int. J. Syst. Evol. Microbiol. 2022, 72, 005379. [Google Scholar] [CrossRef] [PubMed]
- Sokol, H.; Pigneur, B.; Watterlot, L.; Lakhdari, O.; Bermudez-Humaran, L.G.; Gratadoux, J.-J.; Blugeon, S.; Bridonneau, C.; Furet, J.-P.; Corthier, G.; et al. Faecalibacterium prausnitzii Is an Anti-Inflammatory Commensal Bacterium Identified by Gut Microbiota Analysis of Crohn Disease Patients. Proc. Natl. Acad. Sci. USA 2008, 105, 16731–16736. [Google Scholar] [CrossRef]
- Machado, D.; Barbosa, J.C.; Domingos, M.; Almeida, D.; Andrade, J.C.; Freitas, A.C.; Gomes, A.M. Revealing Antimicrobial Resistance Profile of the Novel Probiotic Candidate Faecalibacterium prausnitzii DSM 17677. Int. J. Food Microbiol. 2022, 363, 109501. [Google Scholar] [CrossRef]
- Andrade, J.C.; Almeida, D.; Domingos, M.; Seabra, C.L.; Machado, D.; Freitas, A.C.; Gomes, A.M. Commensal Obligate Anaerobic Bacteria and Health: Production, Storage, and Delivery Strategies. Front. Bioeng. Biotechnol. 2020, 8, 550. [Google Scholar] [CrossRef]
- Khan, M.T.; van Dijl, J.M.; Harmsen, H.J.M. Antioxidants Keep the Potentially Probiotic but Highly Oxygen-Sensitive Human Gut Bacterium Faecalibacterium prausnitzii Alive at Ambient Air. PLoS ONE 2014, 9, e96097. [Google Scholar] [CrossRef]
- Almeida, D.; Machado, D.; Sousa, S.; Seabra, C.L.; Barbosa, J.C.; Andrade, J.C.; Gomes, A.M.; Freitas, A.C. Effect of Emulsification/Internal Gelation-Based Microencapsulation on the Viability of Akkermansia muciniphila upon Prolonged Storage and Simulated Gastrointestinal Passage. Food Hydrocoll. Health 2022, 2, 100084. [Google Scholar] [CrossRef]
- Machado, D.; Almeida, D.; Seabra, C.L.; Andrade, J.C.; Gomes, A.M.; Freitas, A.C. Nanoprobiotics: When Technology Meets Gut Health. In Functional Bionanomaterials; Springer: Cham, Switzerland, 2020; pp. 389–425. [Google Scholar]
- Barbosa, J.; Almeida, D.; Machado, D.; Sousa, S.; Freitas, A.; Andrade, J.; Gomes, A. Spray-Drying Encapsulation of the Live Biotherapeutic Candidate Akkermansia muciniphila DSM 22959 to Survive Aerobic Storage. Pharmaceuticals 2022, 15, 628. [Google Scholar] [CrossRef] [PubMed]
- Broeckx, G.; Vandenheuvel, D.; Claes, I.J.J.; Lebeer, S.; Kiekens, F. Drying Techniques of Probiotic Bacteria as an Important Step towards the Development of Novel Pharmabiotics. Int. J. Pharm. 2016, 505, 303–318. [Google Scholar] [CrossRef] [PubMed]
- Baldelli, A.; Liang, D.Y.; Guo, Y.; Pratap-Singh, A. Effect of the Formulation on Mucoadhesive Spray-Dried Microparticles Containing Iron for Food Fortification. Food Hydrocoll. 2023, 134, 107906. [Google Scholar] [CrossRef]
- Baldelli, A.; Boraey, M.A.; Oguzlu, H.; Cidem, A.; Rodriguez, A.P.; Ong, H.X.; Jiang, F.; Bacca, M.; Thamboo, A.; Traini, D.; et al. Engineered Nasal Dry Powder for the Encapsulation of Bioactive Compounds. Drug Discov. Today 2022, 27, 2300–2308. [Google Scholar] [CrossRef] [PubMed]
- Kiepś, J.; Dembczyński, R. Current Trends in the Production of Probiotic Formulations. Foods 2022, 11, 2330. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, J.; Borges, S.; Amorim, M.; Pereira, M.J.; Oliveira, A.; Pintado, M.E.; Teixeira, P. Comparison of Spray Drying, Freeze Drying and Convective Hot Air Drying for the Production of a Probiotic Orange Powder. J. Funct. Foods 2015, 17, 340–351. [Google Scholar] [CrossRef]
- Torp, A.M.; Bahl, M.I.; Boisen, A.; Licht, T.R. Optimizing Oral Delivery of next Generation Probiotics. Trends Food Sci. Technol. 2022, 119, 101–109. [Google Scholar] [CrossRef]
- Basholli-Salihu, M.; Kryeziu, T.L.; Nebija, D.; Salar-Behzadi, S.; Viernstein, H.; Mueller, M. Prebiotics as Excipients for Enhancement of Stability and Functionality of Bifidobacterium longum ssp. infantis with Potential Application as Symbiotics in Food and Pharmaceuticals. Pharmazie 2019, 74, 326–333. [Google Scholar] [CrossRef]
- Deutsche Sammlung von Mikroorganismen und Zellkulturen (DSMZ). Faecalibacterium Duncaniae 17677. Available online: https://www.dsmz.de/collection/catalogue/details/culture/DSM-17677 (accessed on 13 April 2021).
- Maier, E.; Anderson, R.; Roy, N. Live Faecalibacterium prausnitzii Does Not Enhance Epithelial Barrier Integrity in an Apical Anaerobic Co-Culture Model of the Large Intestine. Nutrients 2017, 9, 1349. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST Static in Vitro Simulation of Gastrointestinal Food Digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Somerville, G.A.; Proctor, R.A. Cultivation Conditions and the Diffusion of Oxygen into Culture Media: The Rationale for the Flask-to-Medium Ratio in Microbiology. BMC Microbiol. 2013, 13, 9. [Google Scholar] [CrossRef] [PubMed]
- Duncan, S.H.; Louis, P.; Thomson, J.M.; Flint, H.J. The Role of PH in Determining the Species Composition of the Human Colonic Microbiota. Environ. Microbiol. 2009, 11, 2112–2122. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Siles, M.; Khan, T.M.; Duncan, S.H.; Harmsen, H.J.M.; Garcia-Gil, L.J.; Flint, H.J. Cultured Representatives of Two Major Phylogroups of Human Colonic Faecalibacterium prausnitzii Can Utilize Pectin, Uronic Acids, and Host-Derived Substrates for Growth. Appl. Environ. Microbiol. 2012, 78, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Foditsch, C.; Santos, T.M.A.; Teixeira, A.G.V.; Pereira, R.V.V.; Dias, J.M.; Gaeta, N.; Bicalho, R.C. Isolation and Characterization of Faecalibacterium prausnitzii from Calves and Piglets. PLoS ONE 2014, 9, e116465. [Google Scholar] [CrossRef] [PubMed]
- Martín, R.; Miquel, S.; Benevides, L.; Bridonneau, C.; Robert, V.; Hudault, S.; Chain, F.; Berteau, O.; Azevedo, V.; Chatel, J.M.; et al. Functional Characterization of Novel Faecalibacterium prausnitzii Strains Isolated from Healthy Volunteers: A Step Forward in the Use of F. prausnitzii as a next-Generation Probiotic. Front. Microbiol. 2017, 8, 1226. [Google Scholar] [CrossRef] [PubMed]
- Bircher, L.; Geirnaert, A.; Hammes, F.; Lacroix, C.; Schwab, C. Effect of Cryopreservation and Lyophilization on Viability and Growth of Strict Anaerobic Human Gut Microbes. Microb. Biotechnol. 2018, 11, 721–733. [Google Scholar] [CrossRef]
- Marcial-Coba, M.S.; Cieplak, T.; Cahú, T.B.; Blennow, A.; Knøchel, S.; Nielsen, D.S. Viability of Microencapsulated Akkermansia muciniphila and Lactobacillus plantarum during Freeze-Drying, Storage and in Vitro Simulated Upper Gastrointestinal Tract Passage. Food Funct. 2018, 9, 5868–5879. [Google Scholar] [CrossRef]
- Khan, M.T.; Duncan, S.H.; Stams, A.J.M.; van Dijl, J.M.; Flint, H.J.; Harmsen, H.J.M. The Gut Anaerobe Faecalibacterium prausnitzii Uses an Extracellular Electron Shuttle to Grow at Oxic-Anoxic Interphases. ISME J. 2012, 6, 1578–1585. [Google Scholar] [CrossRef]
- Marinova, V.Y.; Rasheva, I.K.; Kizheva, Y.K.; Dermenzhieva, Y.D.; Hristova, P.K. Microbiological Quality of Probiotic Dietary Supplements. Biotechnol. Biotechnol. Equip. 2019, 33, 834–841. [Google Scholar] [CrossRef]
- Pupa, P.; Apiwatsiri, P.; Sirichokchatchawan, W.; Pirarat, N.; Muangsin, N.; Shah, A.A.; Prapasarakul, N. The Efficacy of Three Double-Microencapsulation Methods for Preservation of Probiotic Bacteria. Sci. Rep. 2021, 11, 13753. [Google Scholar] [CrossRef]
- Raise, A.; Dupont, S.; Iaconelli, C.; Caliri, C.; Charriau, A.; Gervais, P.; Chambin, O.; Beney, L. Comparison of Two Encapsulation Processes to Protect the Commensal Gut Probiotic Bacterium Faecalibacterium prausnitzii from the Digestive Tract. J. Drug Deliv. Sci. Technol. 2020, 56, 101608. [Google Scholar] [CrossRef]
- Zhang, Z.; Gu, M.; You, X.; Sela, D.A.; Xiao, H.; McClements, D.J. Encapsulation of bifidobacterium in alginate microgels improves viability and targeted gut release. Food Hydrocoll. 2021, 116, 106634. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).