Abstract
Pregnancy is associated with physiological changes that may affect drug pharmacokinetics (PKs). The aim of this study was to establish a maternal–fetal physiologically based pharmacokinetic (PBPK) model of oxcarbazepine (OXC) and its active metabolite, 10,11-dihydro-10-hydroxy-carbazepine (MHD), to (1) assess differences in pregnancy, (2) predict changes in PK target parameters of these molecules following the current dosing regimen, (3) assess predicted concentrations of these molecules in the umbilical vein at delivery, and (4) compare different methods for estimating drug placental penetration. Predictions using the pregnancy PBPK model of OXC resulted in maternal concentrations within a 2-fold error, and extrapolation of the model to early-stage pregnancies indicated that changes in median PK parameters remained above target thresholds, requiring increased frequency of monitoring. The dosing simulation results suggested dose adjustment in the last two trimesters. We generally recommend that women administer ≥ 1.5× their baseline dose of OXC during their second and third trimesters. Test methods for predicting placental transfer showed varying performance, with the in vitro method showing the highest predictive accuracy. Exposure to MHD in maternal and fetal venous blood was similar. Overall, the above-mentioned models can enhance understanding of the maternal–fetal PK behavior of drugs, ultimately informing drug-treatment decisions for pregnant women and their fetuses.
1. Introduction
Oxcarbazepine (OXC) is approved for use alone or in combination with other antiepileptic drugs (AEDs) for primary generalized tonic double and partial seizures with or without secondary generalized seizures. Its chemical structure is similar to that of carbamazepine, but its metabolism is different. Carbamazepine is mainly metabolized by liver CYP3A4 to the active metabolite carbamazepine-10,11-epoxide, which is then excreted by the kidney through hydration [1]. OXC is rapidly absorbed and metabolized as its main clinical active metabolite, 10,11-dihydro-10-hydroxycarbazepine (monohydroxy derivative, MHD), through aldehyde ketone reductase (AKR1C1-4). Oral OXC formulations have high bioavailability (>95%). Peak concentration is obtained within 1–3 h after a single dose, whereas MHD peak concentration occurs within 4–12 h. At steady state, MHD peaks 2–4 h after drug administration. Approximately 40% of the MHD is bound to plasma proteins [2,3].
Clinically significant changes in the pharmacokinetics (PKs) of AEDs may complicate antiepileptic treatment in pregnant women [4,5]. Lower ratios to target concentration are associated with the worsening of seizures. Early therapeutic drug monitoring (TDM) and dose adjustment may help avoid increased seizure frequencies [6]. According to a review and analysis on the monitoring of therapeutic drugs before and after pregnancy in women with epilepsy by Arfman et al. [7], the trough concentration of AEDs should be monitored twice before pregnancy as a reference concentration. In the presence of risk factors for convulsions, dose adjustments during pregnancy based on TDM should be considered if AED concentrations are reduced by 15–25% from preconception reference concentrations, and dose adjustment is recommended if the change exceeds 25%. A recent retrospective analysis found significant changes in the PKs of multiple newer AEDs in women with epilepsy, underscoring the need for TDM during pregnancy [8]. TDM is encouraged at least once every three months prior to pregnancy, especially in women with poorly controlled seizures, and AED doses should be titrated to compensate for any changes in drug clearance [9,10].
Adequate control of seizures in pregnant women with epilepsy is critical because frequent and prolonged maternal seizures can have deleterious consequences, including miscarriage, fetal intracranial hemorrhage, and preterm birth. However, the teratogenicity of some older generation AEDs (such as phenytoin sodium, valproate sodium, etc.) and the potential induced pharmacokinetic changes in AEDs make the treatment of epilepsy during pregnancy complex [10]. The ideal effect of AEDs is to stably control epileptic seizures in the mother without adverse effects on the fetus. However, prenatal exposure to AEDs has been associated with a greater risk of major congenital malformations [11,12]. The risk of major congenital malformations is influenced not only by the type of AED but also by other variables, such as the dose. For example, a significant risk of malformation was observed with valproic acid and phenobarbital at all doses studied, with carbamazepine at doses greater than 400 mg/d, and with lamotrigine monotherapy at doses less than 300 mg/d [13]. In addition, exposure to antiepileptic drugs during pregnancy and lactation may also affect the development of children’s nervous systems. It is suggested that pregnant women should consider counseling to adjust to the safest treatment scheme [14].
The factors influencing pregnancy-induced pharmacokinetic changes when using AEDs include protein-binding rate, volume, and blood flow. A growing number of researchers have speculated that the decrease in plasma concentrations of the active metabolite of OXC during pregnancy may be due to changes in glucuronyltransferase activity [3,4,5,6,7,8,9,10]. Both OXC and MHD have significant placental transport [1], which may lead to excessive fetal plasma concentrations and result in serious adverse effects on the fetus. Limited data suggest that the administration of OXC during pregnancy can cause serious birth defects. The most common congenital malformations related to maternal OXC treatment include ventricular septal defect, cleft palate with cleft lip, and Down syndrome [10]. According to the North American AED Pregnancy Registry, women exposed to OXC during pregnancy had a relative risk of 1.6 (90% confidence interval (CI): 0.46–5.7) [15] for their child having congenital malformations compared with women not exposed to any AEDs during pregnancy. However, due to the small number of the study population, the teratogenic mechanism of OXC remains unclear, with fetal toxicity studies lacking. Therefore, a PK study of OXC in both the mother and fetus during pregnancy would have great reference value for fetal toxicity guidance and OXC dose adjustment.
Physiologically based pharmacokinetic (PBPK) modeling is a promising approach to overcome the limitations of traditional studies in analyzing the PKs of xenobiotics in special populations where clinical trials are difficult to conduct, such as pregnant women and their fetuses [16]. The purpose of our study was to characterize the maternal–fetal PKs of OXC during pregnancy and to provide recommendations for OXC dose adjustment.
2. Materials and Methods
2.1. Software, Clinical Data, and General Workflow
Open Systems Pharmacology Suite version 11.0 suite, including PK-Sim® and MoBi® (https://github.com/Open-Systems-Pharmacology, accessed on 25 May 2022), was used to perform the modeling work in pregnant women. The reported plasma time concentration data were digitized using GetData Graph Digitizer 2.26. Plots were created, and statistical analysis was performed using OriginPro® (OriginLab, Northampton, MA, USA).
Detailed MHD clinical and TDM data in the First Affiliated Hospital of Fujian Medical University from January 2015 to May 2022 were obtained. The inclusion criteria were: (1) age ≥16 years, (2) epilepsy diagnosis that met the criteria published by the International League Against Epilepsy2014, (3) availability of at least one blood MHD concentration and corresponding oral daily dose,and (4) follow-up of every 3 months. After screening, a total of 121 patients met the requirements, including 54 women of gestational age.
Figure S1 depicts the 27-compartment pregnancy model structure and general modeling workflow. As a first step, we constructed an adult PBPK model of OXC using the default 18-compartment model structure designed for small molecules [17].The model was validated using PK data in multiple settings, including hospital TDM data. Clinical PK data for model development and validation were identified and divided into test and validation sets (Table 1). The pregnancy intervals of nine pregnancy types and pregnancy-related anatomical/physiological changes were added through MoBi®. After the parameters, such as pregnancy-related protein binding, were modified, the model was transferred back to PK-Sim® to be included in the pregnant population and modified into the corresponding gestational age parameters. The obtained PK data were used for verification, and the non-pregnant adult PBPK model was extrapolated to the pregnant population [8,9,10,11,12,13,14,15,16,17,18]. In vitro human placental perfusion data were incorporated into the pPBPK (pregnancy PBPK) model to estimate fetal PK. Placenta, fetal blood, tissues, and amniotic fluid chambers were added as models. The parameters of placental metastasis were estimated by different subarea models of perfusion data.

Table 1.
List of reported clinical studies that were used for modeling adults who are healthy.
2.2. Development of a Non-Pregnant Population Model
The population PBPK model of non-women incorporated the PK-Sim® standard model structure containing 18 organs and tissues as described previously [25,26,27,28,29]. The physical, chemical, and biochemical parameters of the OXC model are shown in Table 2. First, the basic parameters of OXC, such as its molecular weight, lipophilicity, protein-binding rate, and solubility, were input into the model. The Weibull 50% dissolution time (in minutes) was generated by internal optimization of the software, and the tissue plasma partition coefficient was calculated using the Berezhkovskiy method [30,31]. OXC is rapidly and almost completely converted into a pharmacologically active metabolite by aldehyde ketone reductases. MHD is an active substance that plays a major pharmacological role in OXC activity [27,28,29,30]. In addition, MHD is metabolized through glucuronidation, a process mainly involving the enzymes UGT1A9 and UGT2B7 [32]; a small portion of MHD is also oxidized to the 10,11-dihydroxy derivative that is devoid of pharmacological activity [32]. Herein, we used the Michaelis–Menten kinetics of substrate consumption to characterize MHD metabolism. UGT1A9 accounts for 40–45% of the total metabolism, and UGT2B7 accounts for 15–20%. UGT1A4 contributes only a small and negligible fraction. The metabolic fractions of UGT1A9 and UGT2B7 were previously reported to be 0.45 and 0.2, respectively [32]. The Michaelis–Menten constant (Km) was obtained from the literature, while the kcat value, a turnover number described as the maximum reaction rate (Vmax) per unit of the enzyme, was determined by fitting it to metabolic fractions.

Table 2.
Summary of input data for the physiologically based pharmacokinetic models for oxcarbazepine and its metabolites, as implemented in the PK-Sim template.
2.3. Development of Pregnancy Population PBPK Models
In PK-Sim® using clinical PK data search and TDM data collected from hospitals, the PBPK model of non-pregnant people was established and validated. Then it was transferred to MoBi® to add pregnancy model structure and finally returned to PK-Sim®; the simulation was carried out in the pregnant population [17,25,26,27,28,29]. Changes in anatomical/physiological parameters associated with pregnancy have been fully described in previous studies [1]. At this stage, except for fraction unbound (Fu) and enzyme clearance, the other parameters basically remained unchanged. Fu (Equations (1) and (2)) in a specific week after fertilization was adjusted according to the initial value and the change in plasma albumin level with the duration of pregnancy [7,25,28].
where KA is the equilibrium association constant, P is the drug concentration bound to protein (i.e., albumin), and X is the number of weeks after fertilization.
P = 14.7exp(−0.0454X) + 31.7
In women who are not pregnant, KA can be estimated using Equation (1) [16,17]. Assuming that KA and the number of binding sites are not affected by pregnancy, can be extended to women who are pregnant through Equation (2). Pregnancy-specific concentrations of albumin or α-1-acid glycoprotein (AAG) were used [16,17]. Neither OXC nor MHD is bound to AAG; however, MHD is bound to serum albumin.
2.4. Dose Simulations Based on the Pregnancy Model
The TDM range for MHD is 3–35 mg/L [33]. As a single-drug treatment, the maintenance-dose range of OXC is 600–2400 mg/d, and efficacy is realized in most patients on a daily dose of 900 mg/d [34,35,36,37]. We used the validated PBPK model for the simulation of dose optimization. According to the fertilization week range, three virtual pregnancy groups were created, namely the first three months of pregnancy (1–11 weeks), the second trimester (12–26 weeks), and the third trimester (27–38 weeks), with people who were not pregnant (20–40 years old) as the reference population. Each virtual population contained 1000 individuals [25].This model was used to predict the steady-state PK of OXC at different doses in women who were not pregnant and those who were pregnant, a scheme that was adjusted according to the dosage before pregnancy.
2.5. Parameterization of Placental Transfer
We used three different modalities to assess the two unknown parameters (Dpl: passive fusion clearance through the placenta, Kf,m: partition between fetal and maternal compartments) of placental transfer [18].
- (1)
- In vitro cotyledon perfusion experiment
For estimates of transplacental transfer parameters, drug transfer across the placenta was modeled as a cotyledon divided into maternal and fetal compartments (Figure S5). Models of OXC and MHD transplacental transfer were investigated, namely simple diffusion, linear transfer, saturation transfer, and addition of placental elimination rate or tissue protein binding [36]. Briefly, we built a four-compartment model in MoBi® with a slight modification of the ordinary equation system and used it to describe observational data,as reported in previous studies [38,39,40]. We assumed that there was no metabolism of OXC in the placenta. The time-dependent change in the molar drug amount in the four compartments, viz. maternal pool, maternal cotyledon, fetal cotyledon, and fetal pool (N{m, mp, fp, f}), was described using the following system of ordinary differential equations:
Maternal reservoir
Maternal cotyledon
Fetal cotyledon
Fetal reservoir
where C represents the concentration (mg/L), Q represents the flow rate (L/h), and V represents the volume (L). The subscripts m, f, and p denote the mother, fetus, and placenta, respectively. KPpl is the placental partition coefficient, Dcot is the diffusion parameter (L/h), and kPE is the placental elimination parameter (h−1). KPpl, Dcot, and kPE were estimated.
Estimation of in vitro “transdermal leaf transfer parameters” (Dcot, Kf,m) was achieved in the fetal–maternal PBPK model after scaling Dcotto Dpl using Equation (7) [18,38]:
where Dpl (mL/min) and Dcot (mL/min) represent transplacental clearance and transembryonic passive fusion clearance, respectively, and Vpl (mL) and Vcot (mL) represent placental and cotyledon volumes, respectively.
- (2)
- Two other methods
Specifically, Dpl was estimated according to the method proposed by Zhang et al. [41], where Dpl was estimated from the permeability of the Caco2 cell line. Another approach was implemented by default in the Open Systems Pharmacology software, estimating permeability across organ membranes from physicochemical descriptors of lipophilicity and molecular weight.
2.6. Evaluation of the PBPK Models
The accuracy of the models was verified using published clinical data. The simulation conditions of the models were consistent with the research conditions reported in the clinical trials. The observed in vivo concentration below the lower limit of quantification (LLOQ) was considered “half LLOQ”. Model validation included the fold errors of the blood concentration–time curve and PK parameters (including AUC and Cmax). The fold error of each PK parameter was calculated as follows:
where Predicted represents the predicted model value and Observed represents the measured clinical value or observed value. If the fold error was between 0.5 and 2.0, the prediction of the model was considered acceptable [39,42]. Other visual prediction checks included goodness of fit plots in which individual in vivo concentration values at each time point were converted to geometric mean values [28].
3. Results
3.1. OXC Model Development and Verification
The measured data [19,20,21,22,23,24,43] of single-dose and multi-dose oral OXC in people who are healthy were fitted with the results predicted by the PBPK model. The dose scheme and demographic characteristics of these adults are shown in Table 2. As depicted in Figure 1, most of the measured data points of blood drug concentration are distributed on both sides of the blood drug concentration–time curve predicted by the PBPK model, and the predicted and actual values fit well. As shown in Table 3, the PK parameters predicted by the model, including AUC, Cmax, and Tmax, are basically consistent with the measured values. According to the collected clinical data, as shown in the goodness of fit diagram (Figure 1J), more than 95% of the predicted drug concentrations are within the error range of the measured values.
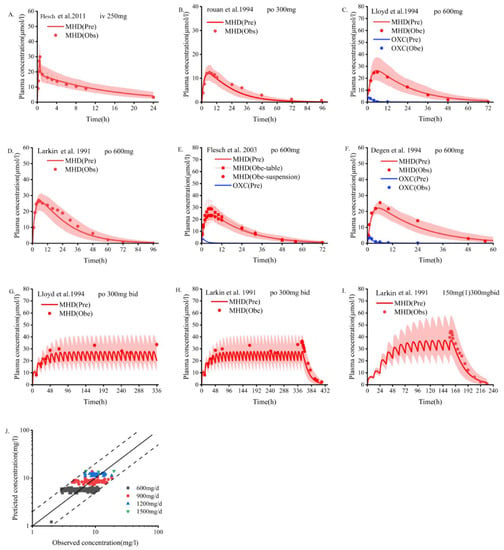
Figure 1.
Population PBPK simulations (A–I) predicting median plasma concentrations of OXC in the non-pregnant population are depicted as dark lines, with shaded areas representing the 5th to 95th predicted range. Solid points are mean plasma concentrations extracted from clinical studies. Blue indicates oxcarbazepine; red indicates MHD. (A) Intravenous MHD; (B–I) Oral OXC; (J) Goodness-of-fit plots predicted by the OXC plasma concentration model. Different colors represent data from different doses. PBPK, physiologically based pharmacokinetic; OXC, oxcarbazepine; MHD, 10,11-dihydro-10-hydroxy-carbazepine [19,20,22,23,24,43].

Table 3.
Observed and simulated pharmacokinetics parameters of OXC following oral administration of different dosing regimens in adults who were healthy.
3.2. PK Prediction in the Pregnant Population
Steady-state PK simulations were performed during the first (6 weeks), second (20 weeks), and third (34 weeks) trimesters and compared to those of baseline levels [25]. The predicted values of the normalized concentrations of total and unbound OXC doses during pregnancy were close to the measured values, with errors ranging from 0.8 to 1.2 (Table 4). UGT2B7 and AKR1C enzyme activities remained unchanged [44,45], and UGT1A9 was upregulated [45], but the overall change was not statistically significant. The simulated dose scheme and demographic characteristics of the women who were pregnant are shown in Table S1. The model was validated using clinically measured data [8,46]. The PBPK model accurately predicted the steady-state PK of maternal plasma OXC and MHD during pregnancy (Figure 2 and Figures S2–S4).The average plasma concentration at the 300 mg/d dose fluctuated but basically remained stable; however, it was not guaranteed that the lowest effective therapeutic concentration could be reached at any time during the dosing interval in the third trimester (18.4 μmol/L). Throughout pregnancy, the steady-state Cmin, Cmax, AUCτ-ss, and t1/2 of OXC and MHD varied slightly (varied by no more than 50%) (Table 5). The model predicts that the average steady-state trough concentration of MHD decreases by 15.9%, 34.9%, and 41.5% in the first, second, and third trimesters (1st trimester, 2nd trimester, and 3rd trimester), respectively (Table 5). This predicted decline is slightly higher than the observed decline calculated from the TDM data (Figure 2d).

Table 4.
Total and unbound oxcarbazepine dose-normalized concentrations during pregnancy period.
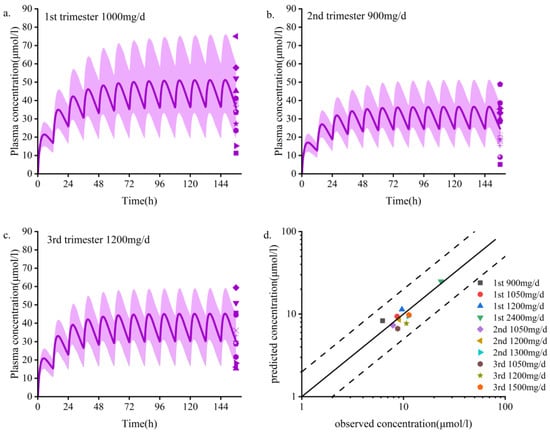
Figure 2.
Population PBPK simulations (a–d) predicting median plasma concentrations of OXC in the pregnant population are shown as dark lines, with shaded areas representing the 5th to 95th predicted range. (a–c) First, second, and third trimester periods; d. Goodness-of-fit plots predicted using the MHD plasma concentration model for women who are pregnant. Different colors represent data from different doses. PBPK, physiologically based pharmacokinetic; MHD, 10,11-dihydro-10-hydroxy-carbazepine. Data sources: Yin et al. [8] and Christensen et al. [46].

Table 5.
Mean steady-state trough concentration of oxcarbazepine predicted by the PBPK model and reported TDM data-based regression curve.
3.3. Dose Simulation in Pregnancy
The main PK parameter data for different doses during pregnancy are shown in Table 6. According to the simulation results, the monitoring frequency using TDM can be increased in the first trimester, and the drug concentration can be adjusted according to the baseline dose in the second and third trimesters. The PBPK model showed increased dose adjustment in the last two trimesters. We generally recommend that women take at least 1.5 times their baseline dose of OXC during the second and third trimesters. If efficacy is still not achieved at 2400 mg/d, continuing to increase the dose is not recommended because most patients cannot tolerate higher daily doses, and no systematic studies have been conducted on daily doses greater than 2400 mg. In those situations, it is necessary to consider changing the medicine or combining medications. As depicted in Figure 3 and Figure 4, a dose of 300 mg/d administered during pregnancy may be lower than the minimum effective dose and will lack efficacy. The suggested minimum dose during pregnancy is 600 mg/d.

Table 6.
Changes in main pharmacokinetic parameters in women during different pregnancy periods compared with those of women of gestational age.
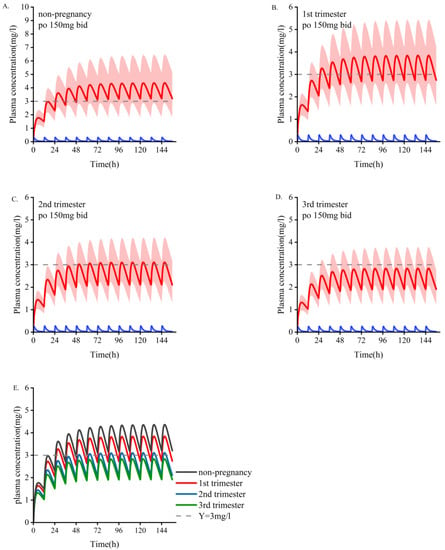
Figure 3.
Simulate the pharmacokinetics of oral oxcarbazepine 150 mg twice daily in women who are pregnant and those who are nonpregnant. Blue represents oxcarbazepine; red indicates the metabolites of MHD. (A) The average age is 30 years old. (B–D) Women in the early, middle, and late stages of pregnancy. (E) Comparison of plasma concentrations of the active part of oxcarbazepine under the above four administration conditions. The mean concentration curve is represented by a dark line, and the shaded area represents the standard deviation. The dotted line indicates the lower limit of the recommended treatment range of oxcarbazepine (3 mg/L).
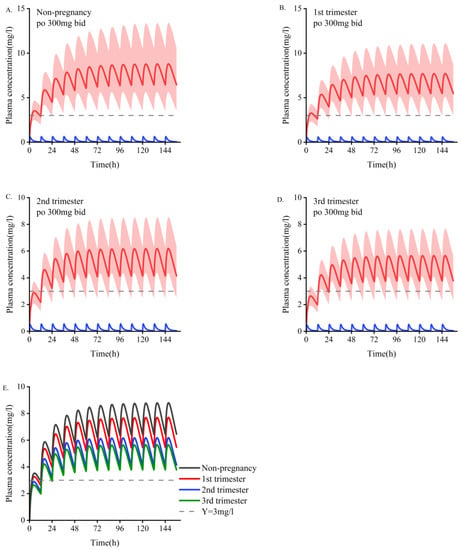
Figure 4.
Simulate the pharmacokinetics of oral oxcarbazepine 300 mg twice daily in women who are pregnant and those who are nonpregnant. Blue represents oxcarbazepine; red indicates the metabolites of MHD. (A). Non-pregnant population with an average age of 30 years. (B–D). Women in the first, second, and third trimesters of pregnancy. (E). Comparison of plasma concentrations of the active fraction of oxcarbazepine under the above four administration conditions. The average concentration curve is displayed as dark lines, and the shaded areas indicate the standard deviation. The dotted line indicates the lower limit of the recommended treatment range of oxcarbazepine (3 mg/L).
3.4. Comparison of Different Estimation Methods of Placental Permeability
The results of different methods for evaluating the placental permeability of MHD are as follows: (a) in vitro cotyledon perfusion method [1], 129.62 mL/min (the ratio of cotyledon permeability to placental permeability according to Equation (2)); (b) the method suggested by Zhang et al. [48], 140 mL/min; (c) Open Systems Pharmacology software default method, 199.42 mL/min. Figure 5 compares the simulated concentrations in an in vitro human perfusion model. The parameter estimates are summarized in Table 7. In order to illustrate the effect of these differences on predicted fetal concentrations, the umbilical vein PK profile and observational data are presented in Figure 6. As shown in Table 8, the results of the in vitro cotyledon perfusion experiment are within the acceptable range, and the placental transport parameters obtained by this method seem to be more suitable for the molecules investigated herein. The results showed that fetal umbilical vein blood concentration during pregnancy was similar to that of the maternal vein during delivery, and the average umbilical vein concentration predicted by the ex vivo cotyledon perfusion approach was 20.64 μmol/L.
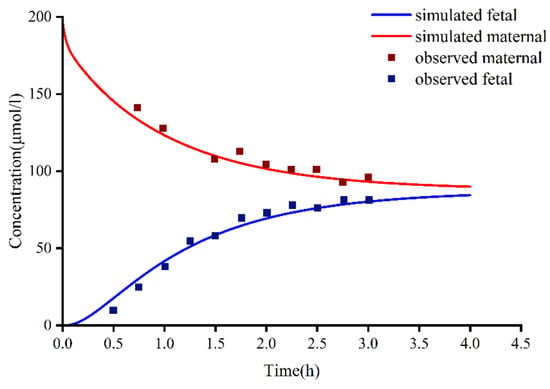
Figure 5.
In vitro cotyledon perfusion experiments to observe fetal and maternal oxcarbazepine concentrations compared to fetal and maternal-simulated MHD concentrations. MHD, 10,11-dihydro-10-hydroxy-carbazepine.

Table 7.
Values of placental transfer parameters calculated using three different methods, as implemented in a pharmacokinetic model based on fetal–maternal physiology.
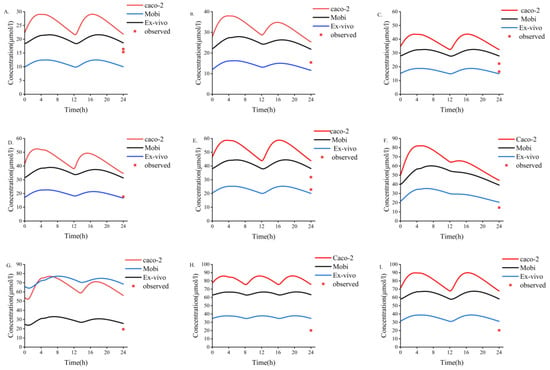
Figure 6.
Three different methods used to predict fetal umbilical vein blood concentration during delivery. (A–I) Simulation of fetal umbilical vein blood concentration of MHD after oral administration of different doses of OXC. (A) 300 mg bid dose; (B) 300 mg qd +450 mg qd dose; (C) 450 mg bid dose; (D) 450 mg qd +600 mg qd dose; (E) 600 mg bid dose; (F) 300 mg qd +1200 mg qd dose; (G) 600 mg qd +900 mg qd dose; (H) 600 mg tid dose; (I) 900 mg bid dose. bid, twice daily; tid, thrice daily; qd, once daily. MHD, 10,11-dihydro-10-hydroxy-carbazepine; OXC, oxcarbazepine.

Table 8.
Cmin ratio obtained after simulation during pregnancy at steady state (unit: μmol/L).
Oxcarbazepine was simulated in 100 pregnant women (300 mg, bid). The results showed that the mean steady-state concentration of MHD (Css,avg) was 6.04 mg/L in non-pregnant women, while Css,avg were 4.92, 3.68, and 3.56 mg/L in 1st trimester, 2nd trimester, and 3rd trimester, respectively. The ratio of fetal umbilical venous blood concentration to maternal venous blood concentration (Cf/Cm) in 1st trimester is 0.89, and 0.87 and 0.94 in 2nd trimester and 3rd trimesters, respectively.
4. Discussion
PBPK models are increasingly being used in clinical settings for predicting PK in special populations. In this study, we (1) initially established a PBPK model for MHD in adults, (2) developed a PBPK model for women who are pregnant based on the dosing recommendations of OXC in those with epilepsy (Table 6), and (3) established different methods to predict placental transport permeability for comparative evaluation.
Based on previous PK studies, it is speculated that the increased intrinsic clearance of MHD throughout pregnancy may be due to increased glucuronidation of MHD, increased renal excretion, decreased absorption/intake of OXC, decreased binding of MHD to proteins, and increased volume of distribution [6,49]. OXC is almost completely absorbed and rapidly metabolized to MHD after oral administration, with little change during pregnancy. Changes in protein binding may have had an effect, but the magnitude of the change was not as high as expected due to the low protein binding of the drug itself [2]. The volume of distribution typically increases during pregnancy, but since the mean concentration of steady-state MHD is independent of the volume of distribution, this is not expected to affect the concentration of MHD [20]. Combined with the above analyses, increased glucuronidation and increased renal excretion of MHD are the most likely major contributing factors.
This study investigated the PK of OXC in women who are pregnant and the findings provide a reference for drug adjustment during pregnancy. To the best of our knowledge, this is the first pregnancy model of OXC. According to the model that was established, we postulate that one of the main reasons for the changes in OXC PK may be that the enzyme expression of UGT1A9 changes with pregnancy (Table S2) [21]. However, further clinical trials are needed to confirm our speculations. While the overall magnitude of the simulated change is acceptable (<50%), steady-state plasma concentrations may drop below therapeutically effective concentrations (Figure 4).
We used our PBPK model to predict PK during pregnancy. The simulation results showed that with an increase in the gestational period, both the Cmin and AUC of OXC decreased to varying degrees (Table 5). The PK was not significantly different between the non-pregnant and pregnant populations in the first trimester, but it was significantly affected in the second and third trimesters (Figure 2). This increases the risk of seizure frequency, as similarly reported in previous studies [7,9]. Since the therapeutic effect of OXC is related to its concentration, dose adjustments can also be made based on the preconception baseline dose (Table 5 summarizes the adjustment strategy).
We used three methods to predict the unknown parameters Dcot and Kf,m during placental transport and applied them to the PBPK model to simulate fetal umbilical venous blood concentrations at delivery (Figure 6). The results showed that the average umbilical vein concentration predicted by the in vitro method was 20.64 μmol/L, and the average concentration of the measured data was 18.94 μmol/L [49]. Experimental simulations of in vitro cotyledon perfusion may be more suitable for predicting the maternal–fetal PK of OXC than the other two methods. However, more complete study data are still needed for verification and analysis. Based on previous studies, fetal umbilical venous blood concentrations were similar to maternal venous concentrations at delivery, with a Cf/Cm ratio of 0.61–0.92 [49,50]. The ratio of simulated fetal umbilical venous blood concentration to maternal venous blood concentration (Cf/Cm) determined using the Caco-2, MoBi, and in vitro methods was 1.31, 1.16, and 0.62, respectively. Regardless of the method, all values above indicate that OXC and its active metabolites have good placental permeability, and the higher lipid solubility of MHD may explain why the concentration of OXC in fetal circulation reaches that in maternal circulation earlier than the concentration of MHD does. However, the placental transfer of OXC requires more in-depth research.
As shown in Figure 6, the results obtained by three different methods do not seem perfect. There are many reasons for this. First, it is reported that the human placenta may express a small amount of glucuronidase, which metabolizes OXC into MHD in the placenta [1,51]. However, for the lack of the amount data on glucuronidase in the placenta, the placental metabolic effect could not be included in the model at present. One study indicated that the metabolism of OXC to MHD in the placenta had only a trace level [51], which indicated that such an effect might be little. Second, the UGT1A9 gene polymorphism factor was not included in the model, also for the lack of expression data in the placenta and fetus of different UGT1A9 genotypes during pregnancy. Third, the transporter factor was not included in the model. Both oxcarbazepine and MHD are substrates of P-gp transporters [52], which indicate that P-gp might mediate placental exocytosis to a certain extent, although many studies indicated that oxcarbazepine and its active metabolite were mainly passively transported on the placenta due to their high-fat solubility [1,49].
Our study has some limitations, and further research is needed to improve the model. First, there were little clinical validation data for the pregnancy model, and our final model validation was insufficient. Second, the change in AKR1C1-4 enzyme activity remained unclear, which may have affected the simulation results. Third, OXC and MHD are both substrates of P-gp. The effect of P-gp was not included in our model. However, due to the high-fat solubility, the attribution of P-gp on the transportation of OXC and MHD in the placenta might be little. Fourth, the prediction of fetal exposure was not perfect, and more placental transport and metabolism data are needed to improve the model. The observation data of the umbilical vein during fetal delivery only used steady-state concentration, and complete concentration–time profile data for verification were unavailable. Despite these limitations, the model could predict maternal and fetal PK of OXC monotherapy and guide epilepsy patients regarding the use of OXC.
5. Conclusions
We successfully developed and validated a maternal–fetal physiological PK model for OXC. The dosing simulation results suggest that the OXC dose in the final two trimesters needs to be adjusted. We generally recommend that women take at least 1.5 times their baseline dose of OXC during the second and third trimesters. In addition, we successfully predicted that MHD exposure in maternal venous blood is similar to that in fetal venous umbilical cord blood. These models can enhance the understanding of the maternal–fetal PK behavior of drugs and may be useful tools for generating MHD PK predictions and supporting dose adjustment or other relevant decision-making scenarios in the clinical setting.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pharmaceutics14112367/s1, Figure S1: Structure of the pregnancy PBPK model and fetal-maternal physiologically based pharmacokinetic (f-m PBPK) model; Figure S2: Schematic representation of the ex vivo cotyledon perfusion model; Figure S3: Median plasma concentration of the population taking different doses of OXC in the first trimester of pregnancy predicted by population PBPK simulation (A–D) is shown as a dark line, and the shaded area represents the 5th to 95th prediction range. OXC, oxcarbazepine; Figure S4: Median plasma concentration of the population taking different doses of OXC in the second trimester of pregnancy predicted by population PBPK simulation (A–D) is shown as a dark line, and the shaded area represents the 5th to 95th prediction range; Figure S5: Median plasma concentration of the population taking different doses of OXC in the third trimester of pregnancy predicted by population PBPK simulation (A–C) is shown as a dark line, and the shaded area represents the 5th to 95th prediction range; Table S1: List of reported clinical studies in pregnancy that were used for modeling; Table S2: The expression concentrations of UGT1A9 and UGT2B7 during different periods, across organs and tissues (mainly), and the difference in renal blood flow between nonpregnant and pregnant women. Ref [53] has cited in Supplementary Materials.
Author Contributions
Conceptualization, C.L.; Formal analysis, L.H. and J.C.; Methodology, L.H., M.K., W.W. and R.L.; Software, G.G.; Supervision, P.H.; Writing—original draft, L.H. and M.K.; Writing—review & editing, C.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Science and Technology Department of Fujian Province, the grant number is 2020Y9009.
Institutional Review Board Statement
Due to the retrospective inclusion of patients with existing data, the ethical review and approval of this study were abandoned.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pienimäki, P.; Lampela, E.; Hakkola, J.; Arvela, P.; Raunio, H.; Vähäkangas, K. Pharmacokinetics of oxcarbazepine and car-bamazepine in human placenta. Epilepsia 1997, 38, 309–316. [Google Scholar] [CrossRef] [PubMed]
- May, T.W.; Korn-Merker, E.; Rambeck, B. Clinical Pharmacokinetics of Oxcarbazepine. Clin. Pharmacokinet. 2003, 42, 1023–1042. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.; Yoon, S.; Kim, T.-J.; Lee, S.; Yu, K.-S.; Jang, I.-J.; Chu, K.; Lee, S.K. Population pharmacokinetic model development and its relationship with adverse events of oxcarbazepine in adult patients with epilepsy. Sci. Rep. 2021, 11, 658–666. [Google Scholar] [CrossRef]
- Sabers, A.; Petrenaite, V. Pharmacokinetics of antiepileptic drugs in pregnancy. Expert Rev. Clin. Pharmacol. 2008, 1, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Mazzucchelli, I.; Onat, F.Y.; Ozkara, C.; Atakli, D.; Specchio, L.M.; La Neve, A.; Gatti, G.; Perucca, E. Changes in the Disposition of Oxcarbazepine and Its Metabolites during Pregnancy and the Puerperium. Epilepsia 2006, 47, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Voinescu, P.E.; Park, S.; Chen, L.Q.; Stowe, Z.N.; Newport, D.J.; Ritchie, J.C.; Pennell, P.B. Antiepileptic drug clearances during pregnancy and clinical implications for women with epilepsy. Neurology 2018, 91, e1228–e1236. [Google Scholar] [CrossRef]
- Arfman, I.J.; der Heijden, E.A.W.-V.; ter Horst, P.G.J.; Lambrechts, D.A.; Wegner, I.; Touw, D.J. Therapeutic Drug Monitoring of Antiepileptic Drugs in Women with Epilepsy before, During, and after Pregnancy. Clin. Pharmacokinet. 2020, 59, 427–445. [Google Scholar] [CrossRef]
- Yin, X.; Liu, Y.; Guo, Y.; Zhao, L.; Li, G.; Tan, X. Pharmacokinetic changes for newer antiepileptic drugs and seizure control during pregnancy. CNS Neurosci. Ther. 2022, 28, 658–666. [Google Scholar] [CrossRef]
- The EURAP Study Group. Seizure control and treatment in pregnancy: Observations from the EURAP Epilepsy Pregnancy Registry. Neurology 2005, 66, 354–360. [Google Scholar] [CrossRef]
- Montouris, G. Safety of the newer antiepileptic drug oxcarbazepine during pregnancy. Curr. Med. Res. Opin. 2005, 21, 693–701. [Google Scholar] [CrossRef]
- Perucca, E. Birth defects after prenatal exposure to antiepileptic drugs. Lancet Neurol. 2005, 4, 781–786. [Google Scholar] [CrossRef]
- Blotière, P.-O.; Raguideau, F.; Weill, A.; Elefant, E.; Perthus, I.; Goulet, V.; Rouget, F.; Zureik, M.; Coste, J.; Dray-Spira, R. Risks of 23 specific malformations associated with prenatal exposure to 10 antiepileptic drugs. Neurology 2019, 93, e167–e180. [Google Scholar] [CrossRef] [PubMed]
- Uziel, D.; Rozental, R. Neurologic birth defects after prenatal exposure to antiepileptic drugs. Epilepsia 2008, 49, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Veroniki, A.A.; Rios, P.; Cogo, E.; Straus, S.E.; Finkelstein, Y.; Kealey, R.; Reynen, E.; Soobiah, C.; Thavorn, K.; Hutton, B.; et al. Comparative safety of antiepileptic drugs for neurological development in children exposed during pregnancy and breast feeding: A systematic review and network meta-analysis. BMJ Open 2017, 7, e017248. [Google Scholar] [CrossRef] [PubMed]
- Tomson, T.; Battino, D.; Bonizzoni, E.; Craig, J.; Lindhout, D.; Perucca, E.; Sabers, A.; Thomas, S.V.; Vajda, F.; Faravelli, F.; et al. Comparative risk of major congenital malformations with eight different antiepileptic drugs: A prospective cohort study of the EURAP registry. Lancet Neurol. 2018, 17, 530–538. [Google Scholar] [CrossRef]
- Kapraun, D.F.; Wambaugh, J.F.; Setzer, R.W.; Judson, R.S. Empirical models for anatomical and physiological changes in a human mother and fetus during pregnancy and gestation. PLoS ONE 2019, 14, e0215906. [Google Scholar] [CrossRef]
- Dallmann, A.; Solodenko, J.; Ince, I.; Eissing, T. Applied Concepts in PBPK Modeling: How to Extend an Open Systems Pharmacology Model to the Special Population of Pregnant Women. CPT Pharmacometrics Syst. Pharmacol. 2018, 7, 419–431. [Google Scholar] [CrossRef]
- Mian, P.; Allegaert, K.; Conings, S.; Annaert, P.; Tibboel, D.; Pfister, M.; van Calsteren, K.; Anker, J.N.V.D.; Dallmann, A. Integration of Placental Transfer in a Fetal–Maternal Physiologically Based Pharmacokinetic Model to Characterize Acetaminophen Exposure and Metabolic Clearance in the Fetus. Clin. Pharmacokinet. 2020, 59, 911–925. [Google Scholar] [CrossRef]
- Flesch, G.; Czendlik, C.; Renard, D.; Lloyd, P. Pharmacokinetics of the Monohydroxy Derivative of Oxcarbazepine and Its Enantiomers after a Single Intravenous Dose Given as Racemate Compared with a Single Oral Dose of Oxcarbazepine. Drug Metab. Dispos. 2011, 39, 1103–1110. [Google Scholar] [CrossRef]
- Lloyd, P.; Flesch, G.; Dieterle, W. Clinical Pharmacology and Pharmacokinetics of Oxcarbazepine. Epilepsia 1994, 35, S10–S13. [Google Scholar] [CrossRef]
- Van Heiningen, P.N.M.; Eve, M.D.; Oosterhuis, B.; Jonkman, J.H.G.; De Bruin, H.; Hulsman, J.A.R.J.; Richens, A.; Jensen, P.K. The influence of age on the pharmacokinetics of the antiepileptic agent oxcarbazepine. Clin. Pharmacol. Ther. 1991, 50, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Flesch, G.; Tudor, D.; Denouel, J.; Bonner, J.; Camisasca, R. Assessment of the bioequivalence of two oxcarbazepine oral suspensions versus a film-coated tablet in healthy subjects. Int. J. Clin. Pharmacol. Ther. 2003, 41, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Larkin, J.; McKee, P.; Forrest, G.; Beastall, G.; Park, B.; Lowrie, J.; Lloyd, P.; Brodie, M. Lack of enzyme induction with oxcarbazepine (600 mg daily) in healthy subjects. Br. J. Clin. Pharmacol. 1991, 31, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Rouan, M.C.; Lecaillon, J.B.; Godbillon, J.; Menard, F.; Darragon, T.; Meyer, P.; Kourilsky, O.; Hillion, D.; Aldigier, J.C.; Jungers, P. The effect of renal impairment on the pharmacokinetics of oxcarbazepine and its metabolites. Eur. J. Clin. Pharmacol. 1994, 47, 161–167. [Google Scholar] [CrossRef]
- Zheng, L.; Yang, H.; Dallmann, A.; Jiang, X.; Wang, L.; Hu, W. Physiologically Based Pharmacokinetic Modeling in Pregnant Women Suggests Minor Decrease in Maternal Exposure to Olanzapine. Front. Pharmacol. 2022, 12, 793346. [Google Scholar] [CrossRef]
- Sinha, J.; Karatza, E.; Gonzalez, D. Physiologically-Based Pharmacokinetic Modeling of Oxcarbazepine and Levetiracetam During Adjunctive Antiepileptic Therapy in Children and Adolescents. CPT Pharmacomet. Syst. Pharmacol. 2021, 11, 225–239. [Google Scholar] [CrossRef]
- Zheng, L.; Tang, S.; Tang, R.; Xu, M.; Jiang, X.; Wang, L. Dose Adjustment of Quetiapine and Aripiprazole for Pregnant Women Using Physiologically Based Pharmacokinetic Modeling and Simulation. Clin. Pharmacokinet. 2020, 60, 623–635. [Google Scholar] [CrossRef]
- Dallmann, A.; Ince, I.; Solodenko, J.; Meyer, M.; Willmann, S.; Eissing, T.; Hempel, G. Physiologically Based Pharmacokinetic Modeling of Renally Cleared Drugs in Pregnant Women. Clin. Pharmacokinet. 2017, 56, 1525–1541. [Google Scholar] [CrossRef]
- Dallmann, A.; Ince, I.; Meyer, M.; Willmann, S.; Eissing, T.; Hempel, G. Gestation-Specific Changes in the Anatomy and Physiology of Healthy Pregnant Women: An Extended Repository of Model Parameters for Physiologically Based Pharmacokinetic Modeling in Pregnancy. Clin. Pharmacokinet. 2017, 56, 1303–1330. [Google Scholar] [CrossRef]
- Berezhkovskiy, L.M. A Valid Equation for the Well-Stirred Perfusion Limited Physiologically Based Pharmacokinetic Model that Consistently Accounts for the Blood–Tissue Drug Distribution in the Organ and the Corresponding Valid Equation for the Steady State Volume of Distribution. J. Pharm. Sci. 2010, 99, 475–485. [Google Scholar] [CrossRef]
- Berezhkovskiy, L.M. Exploration of PBPK Model—Calculation of Drug Time Course in Tissue Using IV Bolus Drug Plasma Concentration-Time Profile and the Physiological Parameters of the Organ. J. Pharm. Sci. 2016, 105, 2453–2458. [Google Scholar] [CrossRef] [PubMed]
- Malátková, P.; Havlíková, L.; Wsól, V. The role of carbonyl reducing enzymes in oxcarbazepine in vitro metabolism in man. Chem. Interact. 2014, 220, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Patsalos, P.N.; Berry, D.J.; Bourgeois, B.F.D.; Cloyd, J.C.; Glauser, T.A.; Johannessen, S.I.; Leppik, I.E.; Tomson, T.; Perucca, E. Antiepileptic drugs—Best practice guidelines for therapeutic drug monitoring: A position paper by the subcommission on therapeutic drug monitoring, ILAE Commission on Therapeutic Strategies. Epilepsia 2008, 49, 1239–1276. [Google Scholar] [CrossRef] [PubMed]
- Schachter, S.; Vazquez, B.; Fisher, R.; Laxer, K.; Montouris, G.; Combs-Cantrell, D.; Faught, E.; Willmore, L.; Morris, G.; Ojemann, L.; et al. Oxcarbazepine: Double-blind, randomized, placebo-control, monotherapy trial for partial seizures. Neurology 1999, 52, 732. [Google Scholar] [CrossRef]
- Beydoun, A.; Sachdeo, R.; Rosenfeld, W.; Krauss, G.; Sessler, N.; Mesenbrink, P.; Kramer, L.; D’Souza, J. Oxcarbazepine monotherapy for partial-onset seizures. Neurology 2000, 54, 2245–2251. [Google Scholar] [CrossRef]
- Sachdeo, R.; Beydoun, A.; Schachter, S.; Vazquez, B.; Schaul, N.; Mesenbrink, P.; Kramer, L.; D’Souza, J. Oxcarbazepine (Trileptal) as monotherapy in patients with partial seizures. Neurology 2001, 57, 864–871. [Google Scholar] [CrossRef]
- Schmidt, D.; Sachdeo, R. Oxcarbazepine for Treatment of Partial Epilepsy: A Review and Recommendations for Clinical Use. Epilepsy Behav. 2000, 1, 396–405. [Google Scholar] [CrossRef]
- Mendes, M.D.S.; Hirt, D.; Vinot, C.; Valade, E.; Lui, G.; Pressiat, C.; Bouazza, N.; Foissac, F.; Blanche, S.; Lê, M.P.; et al. Prediction of human fetal pharmacokinetics using ex vivo human placenta perfusion studies and physiologically based models. Br. J. Clin. Pharmacol. 2016, 81, 646–657. [Google Scholar] [CrossRef]
- Liu, X.I.; Momper, J.D.; Rakhmanina, N.Y.; Green, D.J.; Burckart, G.J.; Cressey, T.R.; Mirochnick, M.; Best, B.M.; Anker, J.N.V.D.; Dallmann, A. Prediction of Maternal and Fetal Pharmacokinetics of Dolutegravir and Raltegravir Using Physiologically Based Pharmacokinetic Modeling. Clin. Pharmacokinet. 2020, 59, 1433–1450. [Google Scholar] [CrossRef]
- Liu, X.I.; Momper, J.D.; Rakhmanina, N.; Anker, J.N.D.; Green, D.J.; Burckart, G.J.; Best, B.M.; Mirochnick, M.; Capparelli, E.V.; Dallmann, A. Physiologically Based Pharmacokinetic Models to Predict Maternal Pharmacokinetics and Fetal Exposure to Emtricitabine and Acyclovir. J. Clin. Pharmacol. 2019, 60, 240–255. [Google Scholar] [CrossRef]
- Zhang, Z.; Unadkat, J.D. Development of a Novel Maternal-Fetal Physiologically Based Pharmacokinetic Model II: Verification of the Model for Passive Placental Permeability Drugs. Drug Metab. Dispos. 2017, 45, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-M.; Rowland, M. The Role of Physiologically Based Pharmacokinetic Modeling in Regulatory Review. Clin. Pharmacol. Ther. 2012, 91, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Degen, P.H.; Flesch, G.; Cardot, J.-M.; Czendlik, C.; Dieterle, W. The influence of food on the disposition of the antiepileptic oxcarbazepine and its major metabolites in healthy volunteers. Biopharm. Drug Dispos. 1994, 15, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Open-Systems-Pharmacology. Available online: https://github.com/Open-Systems-Pharmacology/OSPSuite.Documentation/blob/master/PK-Sim%20Ontogeny%20Database%20Version%207.3.pdf (accessed on 31 August 2022).
- Lu, Y.; Fang, Y.; Wu, X.; Ma, C.; Wang, Y.; Xu, L. Effects of UGT1A9 genetic polymorphisms on monohydroxylated derivative of oxcarbazepine concentrations and oxcarbazepine monotherapeutic efficacy in Chinese patients with epilepsy. Eur. J. Clin. Pharmacol. 2016, 73, 307–315. [Google Scholar] [CrossRef]
- Christensen, J.; Sabers, A.; Sidenius, P. Oxcarbazepine concentrations during pregnancy: A retrospective study in patients with epilepsy. Neurology 2006, 67, 1497–1499. [Google Scholar] [CrossRef]
- Pennell, P.B.; Karanam, A.; Meador, K.J.; Gerard, E.; Kalayjian, L.; Penovich, P.; Matthews, A.; McElrath, T.M.; Birnbaum, A.K.; Cohen, M.; et al. Antiseizure Medication Concentrations During Pregnancy. JAMA Neurol. 2022, 79, 370. [Google Scholar] [CrossRef]
- Fortuna, A.; Alves, G.; Soares-Da-Silva, P.; Falcão, A. Optimization of a Parallel Artificial Membrane Permeability Assay for the Fast and Simultaneous Prediction of Human Intestinal Absorption and Plasma Protein Binding of Drug Candidates: Application to Dibenz[b,f]azepine-5-Carboxamide Derivatives. J. Pharm. Sci. 2012, 101, 530–540. [Google Scholar] [CrossRef]
- Myllynen, P.; Pienimäki, P.; Jouppila, P.; Vähäkangas, K. Transplacental Passage of Oxcarbazepine and Its Metabolites In Vivo. Epilepsia 2002, 42, 1482–1485. [Google Scholar] [CrossRef]
- Bülau, P.; Paar, W.D.; Von Unruh, G.E. Pharmacokinetics of oxcarbazepine and 10-hydroxy-carbazepine in the newborn child of an oxcarbazepine-treated mother. Eur. J. Clin. Pharmacol. 1988, 34, 311–313. [Google Scholar] [CrossRef]
- Myllynen, P.; Pienimäki, P.; Raunio, H.; Vähäkangas, K. Microsomal metabolism of carbamazepine and oxcarbazepine in liver and placenta. Hum. Exp. Toxicol. 1998, 17, 668–676. [Google Scholar] [CrossRef]
- Antunes, N.D.J.; van Dijkman, S.C.; Lanchote, V.L.; Wichert-Ana, L.; Coelho, E.B.; Junior, V.A.; Takayanagui, O.M.; Tozatto, E.; van Hasselt, J.C.; Della Pasqua, O. Population pharmacokinetics of oxcarbazepine and its metabolite 10-hydroxycarbazepine in healthy subjects. Eur. J. Pharm. Sci. 2017, 109, S116–S123. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Yu, Z.; Xu, Y.; Li, X.; Liu, X.; Liu, D.; Zhou, T. Preliminary Physiologically Based Pharmacokinetic Modeling of Renally Cleared Drugs in Chinese Pregnant Women. Biopharm. Drug Dispos. 2020, 41, 248–267. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).