Anti-Cocaine IgA Rather Than IgG Mediates Vaccine Protection from Cocaine Use
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Quantitative Antibody Measurement
2.3. Urine Cocaine Metabolites
2.4. Data Analyses
3. Results
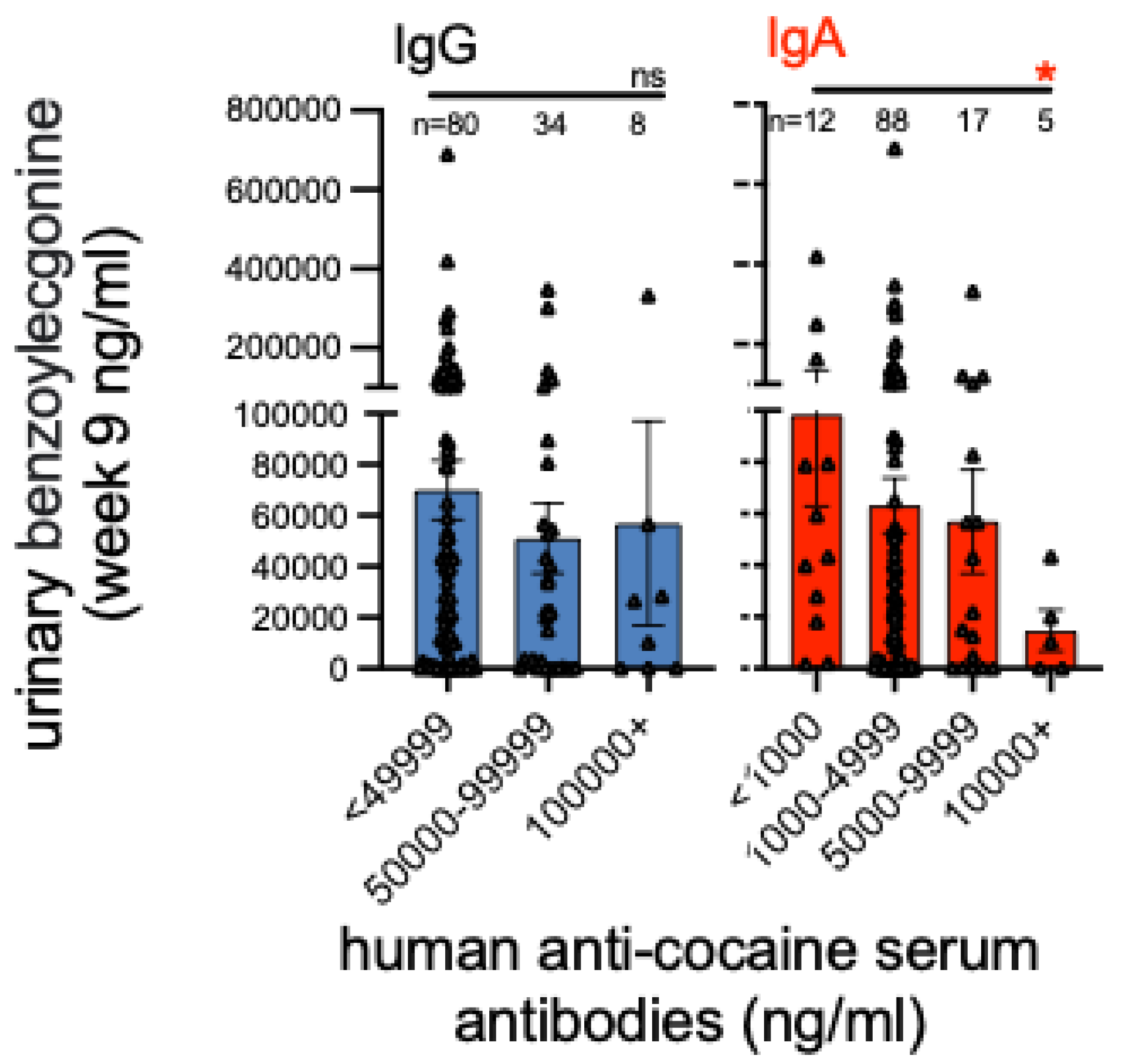
IgG and IgA Antibody Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McLellan, A.T.; Lewis, D.C.; O’Brien, C.P.; Kleber, H.D. Drug dependence, a chronic medical illness: Implications for treatment, insurance, and outcomes evaluation. JAMA 2000, 284, 1689–1695. [Google Scholar] [CrossRef] [PubMed]
- Trends & Statistics: Overdose Death Rates. NIDA. Available online: https://nida.nih.gov/research-topics/trends-statistics/overdose-death-rates (accessed on 2 September 2022).
- Bremer, P.T.; Janda, K.D. Conjugate Vaccine Immunotherapy for Substance Use Disorder. Pharmacol. Rev. 2017, 69, 298–315. [Google Scholar] [CrossRef] [PubMed]
- Hatsukami, D.K.; Jorenby, D.E.; Gonzales, D.; Rigotti, N.A.; Glover, E.D.; Oncken, C.A.; Tashkin, D.P.; Reus, V.I.; Akhavain, R.C.; Fahim, R.E.F.; et al. Immunogenicity and smoking-cessation out-comes for a novel nicotine immunotherapeutic. Clin. Pharmacol. Ther. 2011, 89, 392–399. [Google Scholar] [CrossRef]
- Fahim, R.E.; Kessler, P.D.; Fuller, S.A.; Kalnik, M.W. Nicotine vaccines. CNS Neurol. Disord. Drug Targets 2011, 10, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Martell, B.A.; Orson, F.M.; Poling, J.; Mitchell, E.; Rossen, R.D.; Gardner, T.; Kosten, T. Cocaine vaccine for the treatment of cocaine dependence in methadone-maintained patients: A randomized, double-blind, placebo-controlled efficacy trial. Arch. Gen. Psychiatry 2009, 66, 1116–1123. [Google Scholar] [CrossRef]
- Kosten, T.R.; Domingo, C.B.; Shorter, D.; Orson, F.; Green, C.; Somoza, E.; Sekerka, R.; Levin, F.R.; Mariani, J.J.; Sitzer, M.; et al. Vaccine for cocaine dependence: A randomized double-blind pla-cebo-controlled efficacy trial. Drug Alcohol Depend. 2014, 140, 42–47. [Google Scholar] [CrossRef]
- Stone, A.E.; Scheuermann, S.E.; Haile, C.N.; Cuny, G.D.; Velasquez, M.L.; Linhuber, J.P.; Duddupudi, A.L.; Vigliaturo, J.R.; Pravetoni, M.; Kosten, T.A.; et al. Fentanyl conjugate vaccine by injected or mucosal delivery with dmLT or LTA1 adjuvants implicates IgA in protection from drug challenge. NPJ Vaccines 2021, 6, 69. [Google Scholar] [CrossRef]
- Clements, J.D.; Norton, E.B. The Mucosal Vaccine Adjuvant LT(R192G/L211A) or dmLT. mSphere 2018, 3, e00215–e00218. [Google Scholar] [CrossRef]
- Valli, E.; Harriett, A.J.; Nowakowska, M.K.; Baudier, R.L.; Provosty, W.B.; McSween, Z.; Lawson, L.B.; Nakanishi, Y.; Norton, E.B. LTA1 is a safe, intranasal enterotoxin-based adjuvant that improves vaccine protection against influenza in young, old and B-cell-depleted (muMT) mice. Sci. Rep. 2019, 9, 15128. [Google Scholar] [CrossRef]
- El-Kamary, S.S.; Cohen, M.A.; Bourgeois, A.L.; Verg, L.V.D.; Bauers, N.; Reymann, M.; Pasetti, M.F.; Chen, W.H. Safety and immunogenicity of a single oral dose of recombinant double mutant heat-labile toxin derived from enterotoxigenic Escherichia coli. Clin. Vaccine Immunol. 2013, 20, 1764–1770. [Google Scholar] [CrossRef]
- Lundgren, A.; Bourgeois, L.; Carlin, N.; Clements, J.; Gustafsson, B.; Hartford, M.; Holmgren, J.; Petzold, M.; Walker, R.; Svennerholm, A.; et al. Safety and immunogenicity of an improved oral in-activated multivalent enterotoxigenic Escherichia coli (ETEC) vaccine administered alone and to-gether with dmLT adjuvant in a double-blind, randomized, placebo-controlled Phase I study. Vaccine 2014, 32, 7077–7084. [Google Scholar] [CrossRef] [PubMed]
- Harro, C.; Bourgeois, A.L.; Sack, D.; Walker, R.; DeNearing, B.; Brubaker, J.; Maier, N.; Fix, A.; Dally, L.; Chakraborty, S.; et al. Live attenuated enterotoxigenic Escherichia coli (ETEC) vaccine with dmLT adjuvant protects human volunteers against virulent experimental ETEC challenge. Vaccine 2019, 37, 1978–1986. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, D.I.; Pasetti, M.F.; Brady, R.; Buskirk, A.D.; Wahid, R.; Dickey, M.; Cohen, M.; Baughman, H.; El-Khorazaty, J.; Maier, N.; et al. A Phase 1 dose escalating study of double mutant heat-labile toxin LTR192G/L211A (dmLT) from Enterotoxigenic Escherichia coli (ETEC) by sublingual or oral immunization. Vaccine 2019, 37, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Tennant, S.M.; Teele, A.D.; Pasetti, M.F. Highlights of the 8th International Conference on Vaccines for Enteric Diseases: The Scottish Encounter To Defeat Diarrheal Diseases. Clin. Vaccine Immunol. 2016, 23, 272–281. [Google Scholar] [CrossRef]
- Qadri, F.; Akhtar, M.; Bhuiyan, T.R.; Chowdhury, M.; Ahmed, T.; Rafique, T.; Khan, M.; Rahman, S.; Khanam, F.; Lundgren, A.; et al. Safety and immunogenicity of the oral, inactivated, enterotoxigenic Escherichia coli vaccine ETVAX in Bangladeshi children and infants: A double-blind, randomised, placebo-controlled phase 1/2 trial. Lancet Infect. Dis. 2020, 20, 208–219. [Google Scholar] [CrossRef]
- Lee, T.; Gutierrez, R.L.; Maciel, M.; Poole, S.; Testa, K.J.; Trop, S.; Duplessis, C.; Lane, A.; Riddle, M.S.; Hamer, M.; et al. Safety and immunogenicity of intramuscularly administered CS6 subunit vaccine with a modified heat-labile enterotoxin from enterotoxigenic Escherichia coli. Vaccine 2021, 39, 5548–5556. [Google Scholar] [CrossRef]
- Alving, C.R.; Matyas, G.R.; Torres, O.; Jalah, R.; Zoltan, B. Adjuvants for vaccines to drugs of abuse and addiction. Vaccine 2014, 32, 5382–5389. [Google Scholar] [CrossRef]
- Haile, C.N.; Baker, M.D.; Sanchez, S.A.; Lopez-Arteaga, C.A.; Duppupudi, A.L.; Cuny, G.D.; Norton, E.B.; Kosten, T.R.; Kosten, T.A. An immunconjugate vaccine alters distribution and reduces the an-tinociceptive, behavioral and physiological effects of fentanyl in male and female rats. Pharmaceutics 2022, 14, 2290. [Google Scholar] [CrossRef]
- Fitzpatrick, Z.; Frazer, G.; Ferro, A.; Clare, S.; Bouladoux, N.; Ferdinand, J.; Tuong, Z.K.; Negro-Demontel, M.L.; Kumar, N.; Suchanek, O.; et al. Gut-educated IgA plasma cells defend the menin-geal venous sinuses. Nature 2020, 587, 472–476. [Google Scholar] [CrossRef]
- Haney, M.; Gunderson, E.W.; Jiang, H.; Collins, E.D.; Foltin, R.W. Cocaine-specific antibodies blunt the subjective effects of smoked cocaine in humans. Biol. Psychiatry 2010, 67, 59–65. [Google Scholar] [CrossRef]
- Paula, S.; Tabet, M.R.; Farr, C.D.; Norman, A.B.; Ball, W.J., Jr. Three-dimensional quantitative structure-activity relationship modeling of cocaine binding by a novel human monoclonal antibody. J. Med. Chem. 2004, 47, 133–142. [Google Scholar] [CrossRef]
- Norman, A.B.; Tabet, M.R.; Norman, M.K.; Buesing, W.R.; Pesce, A.J.; Ball, W.J. A chimeric hu-man/murine anti-cocaine monoclonal antibody inhibits the distribution of cocaine to the brain in mice. J. Pharmacol. Exp. Ther. 2007, 320, 145–153. [Google Scholar] [CrossRef]
- John, A.L.S.; Choi, H.W.; Walker, Q.D.; Blough, B.; Kuhn, C.M.; Abraham, S.N.; Staats, H.F. Novel mucosal adjuvant, mastoparan-7, improves cocaine vaccine efficacy. NPJ Vaccines 2020, 5, 12. [Google Scholar] [CrossRef]
- Sindic, C.J.; Delacroix, D.L.; Vaerman, J.P.; Laterre, E.C.; Masson, P.L. Study of IgA in the cerebro-spinal fluid of neurological patients with special reference to size, subclass and local production. J. Neuroimmunol. 1984, 7, 65–75. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosten, T.R.; Haile, C.N.; Domingo, C.B.; Norton, E.B. Anti-Cocaine IgA Rather Than IgG Mediates Vaccine Protection from Cocaine Use. Pharmaceutics 2022, 14, 2368. https://doi.org/10.3390/pharmaceutics14112368
Kosten TR, Haile CN, Domingo CB, Norton EB. Anti-Cocaine IgA Rather Than IgG Mediates Vaccine Protection from Cocaine Use. Pharmaceutics. 2022; 14(11):2368. https://doi.org/10.3390/pharmaceutics14112368
Chicago/Turabian StyleKosten, Thomas R., Colin N. Haile, Coreen B. Domingo, and Elizabeth B. Norton. 2022. "Anti-Cocaine IgA Rather Than IgG Mediates Vaccine Protection from Cocaine Use" Pharmaceutics 14, no. 11: 2368. https://doi.org/10.3390/pharmaceutics14112368
APA StyleKosten, T. R., Haile, C. N., Domingo, C. B., & Norton, E. B. (2022). Anti-Cocaine IgA Rather Than IgG Mediates Vaccine Protection from Cocaine Use. Pharmaceutics, 14(11), 2368. https://doi.org/10.3390/pharmaceutics14112368




