Molecular Dynamics Simulation of Drug Solubilization Behavior in Surfactant and Cosolvent Injections
Abstract
1. Introduction
2. Modeling and Methods
2.1. Formulation Information
2.2. Simulation Details
3. Analyses and Results
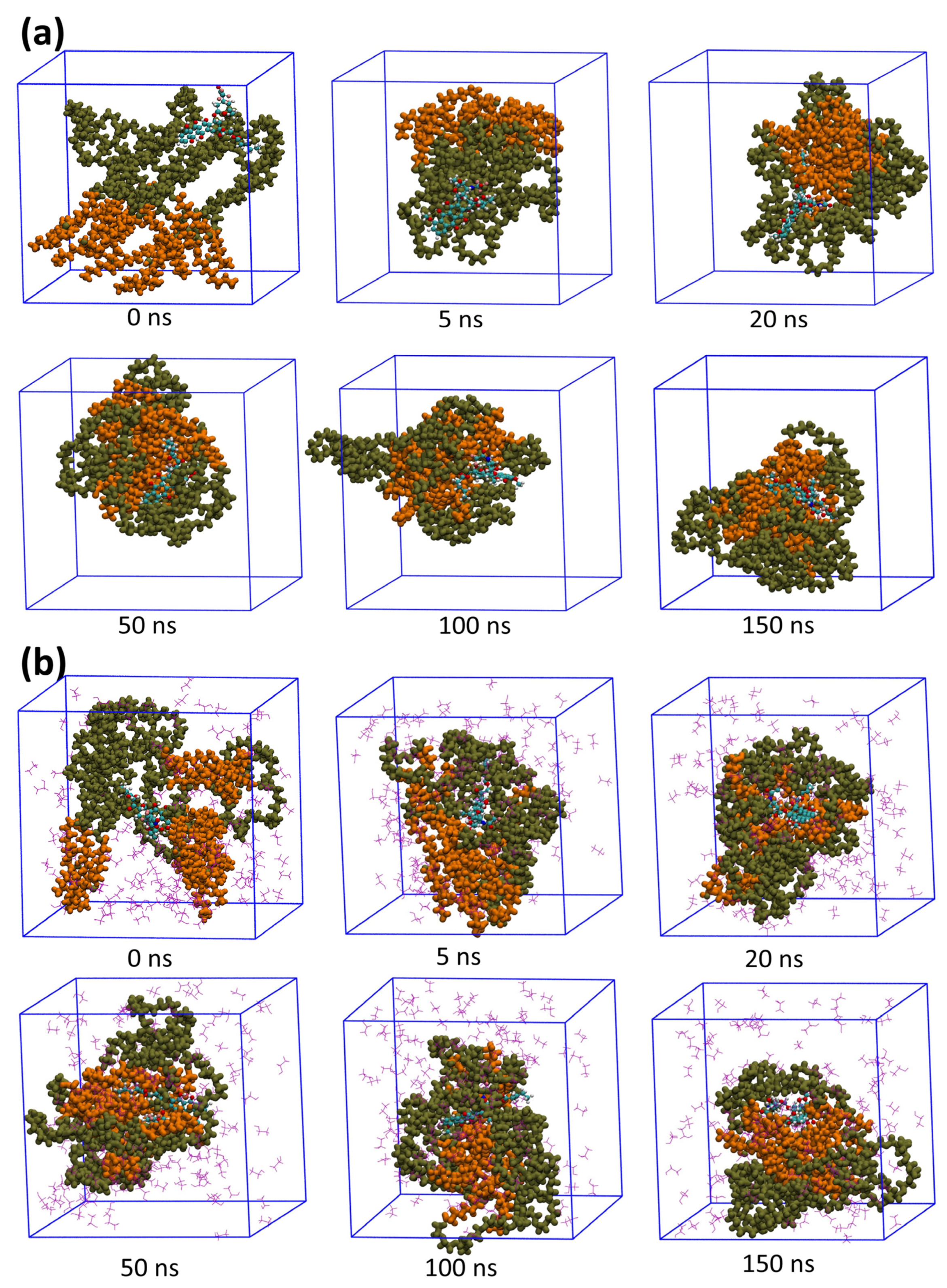
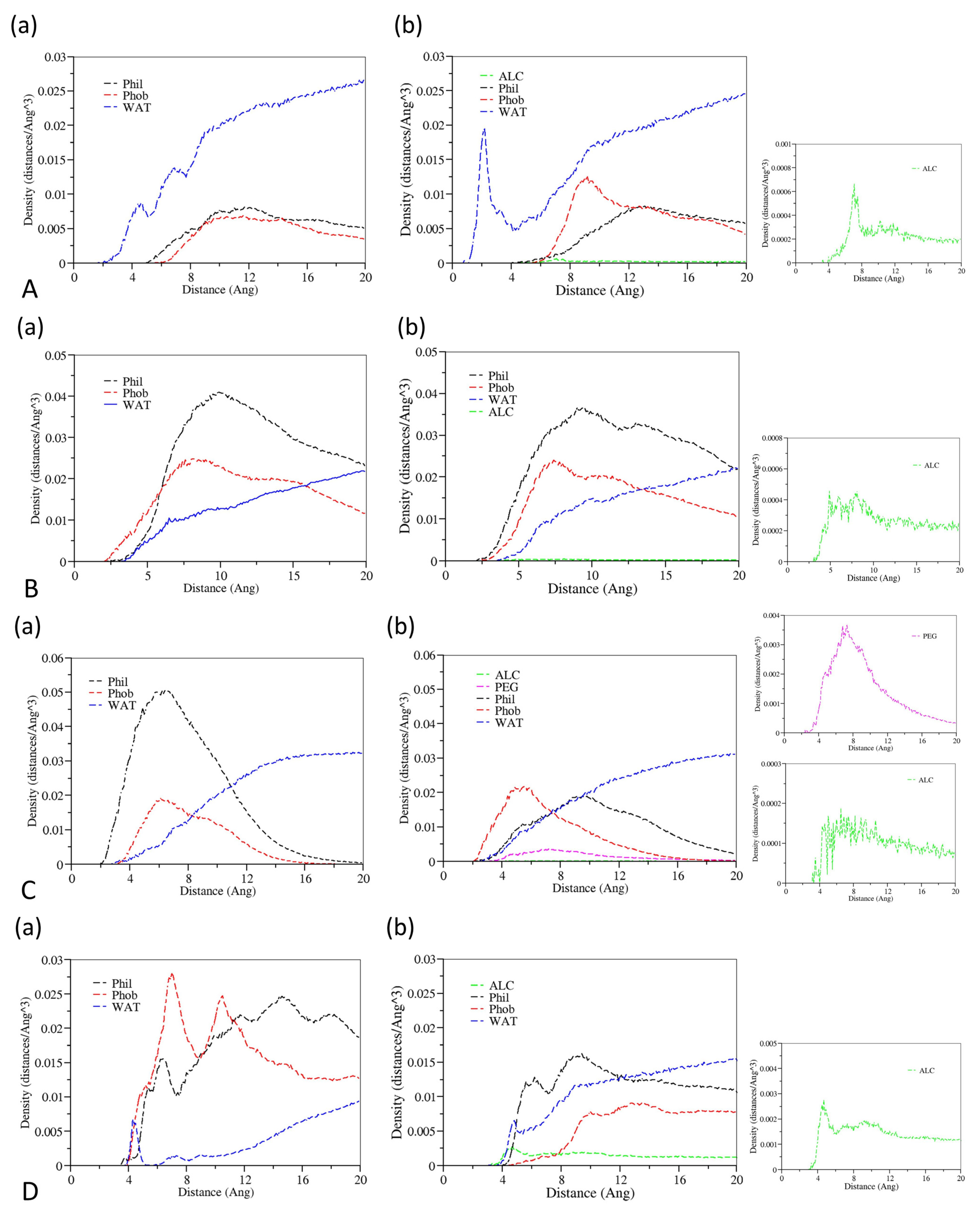
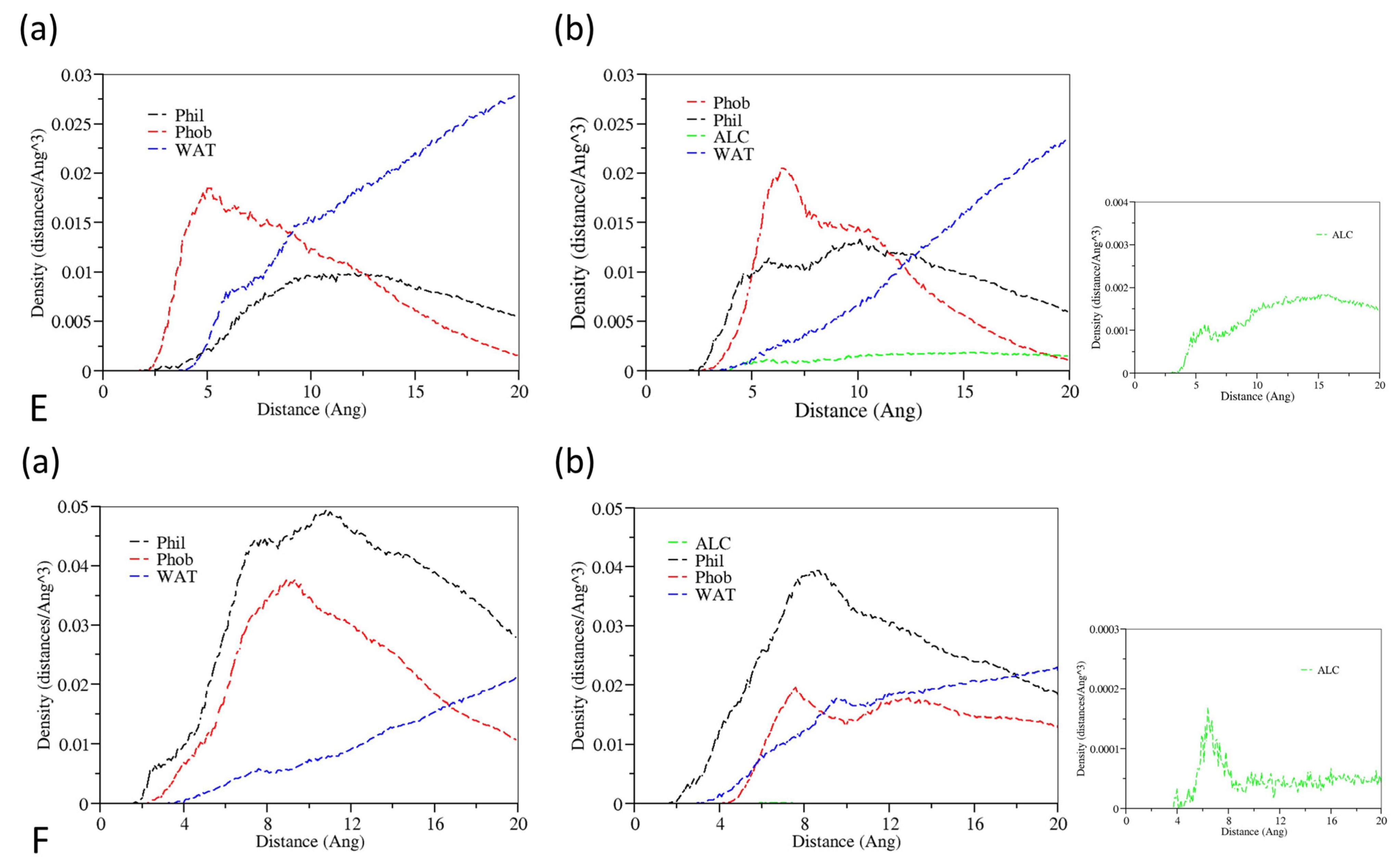
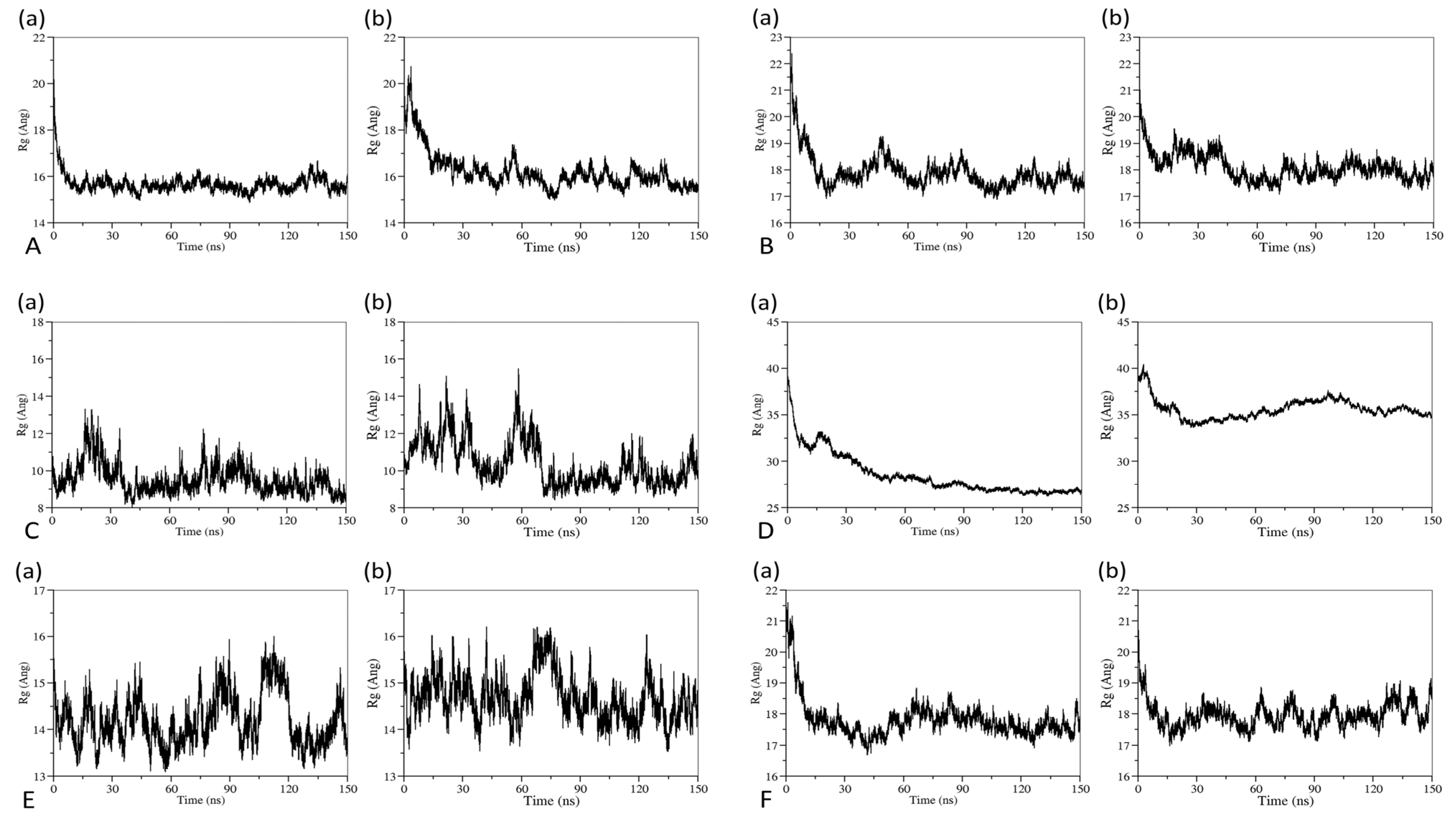
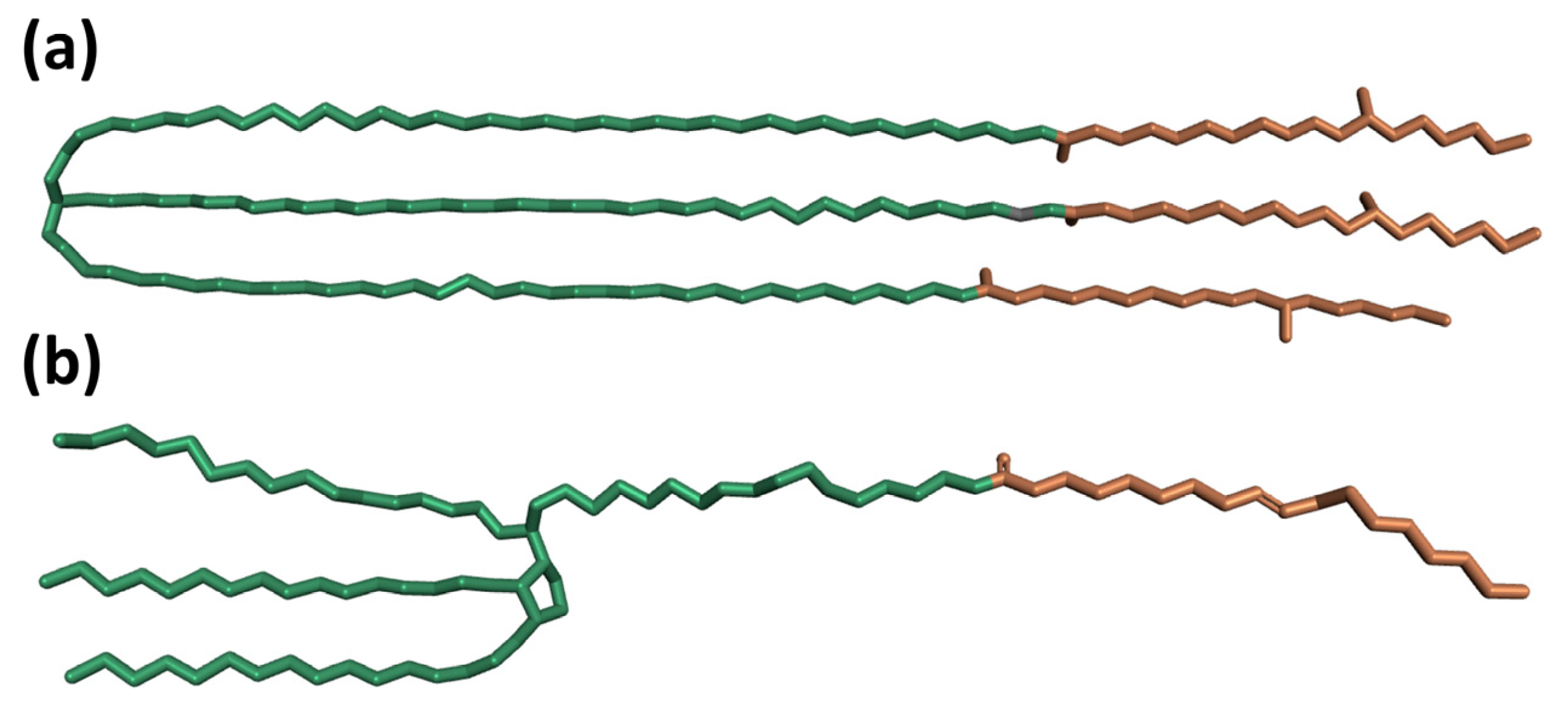
3.1. Aggregation Processes of Micelles
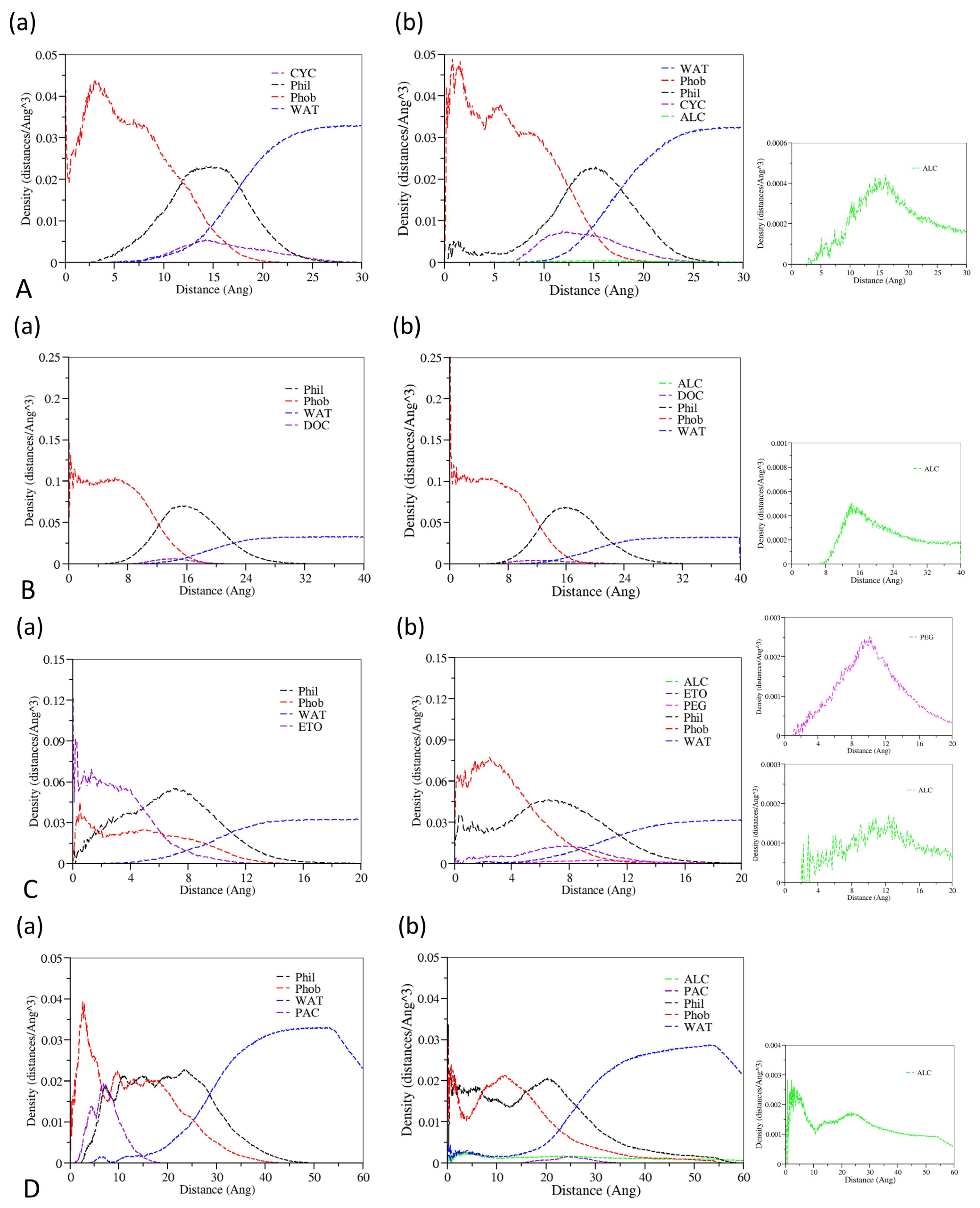
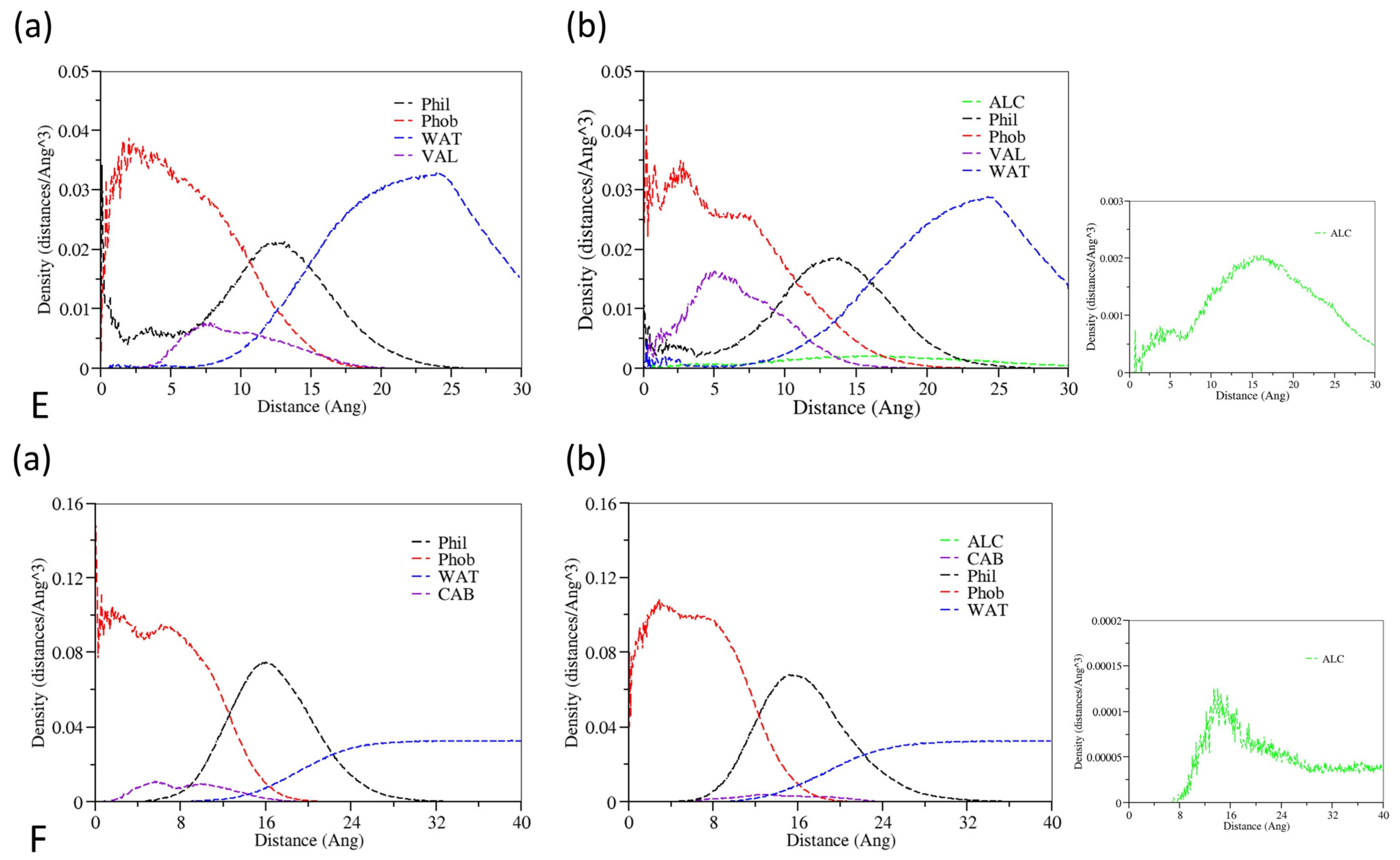
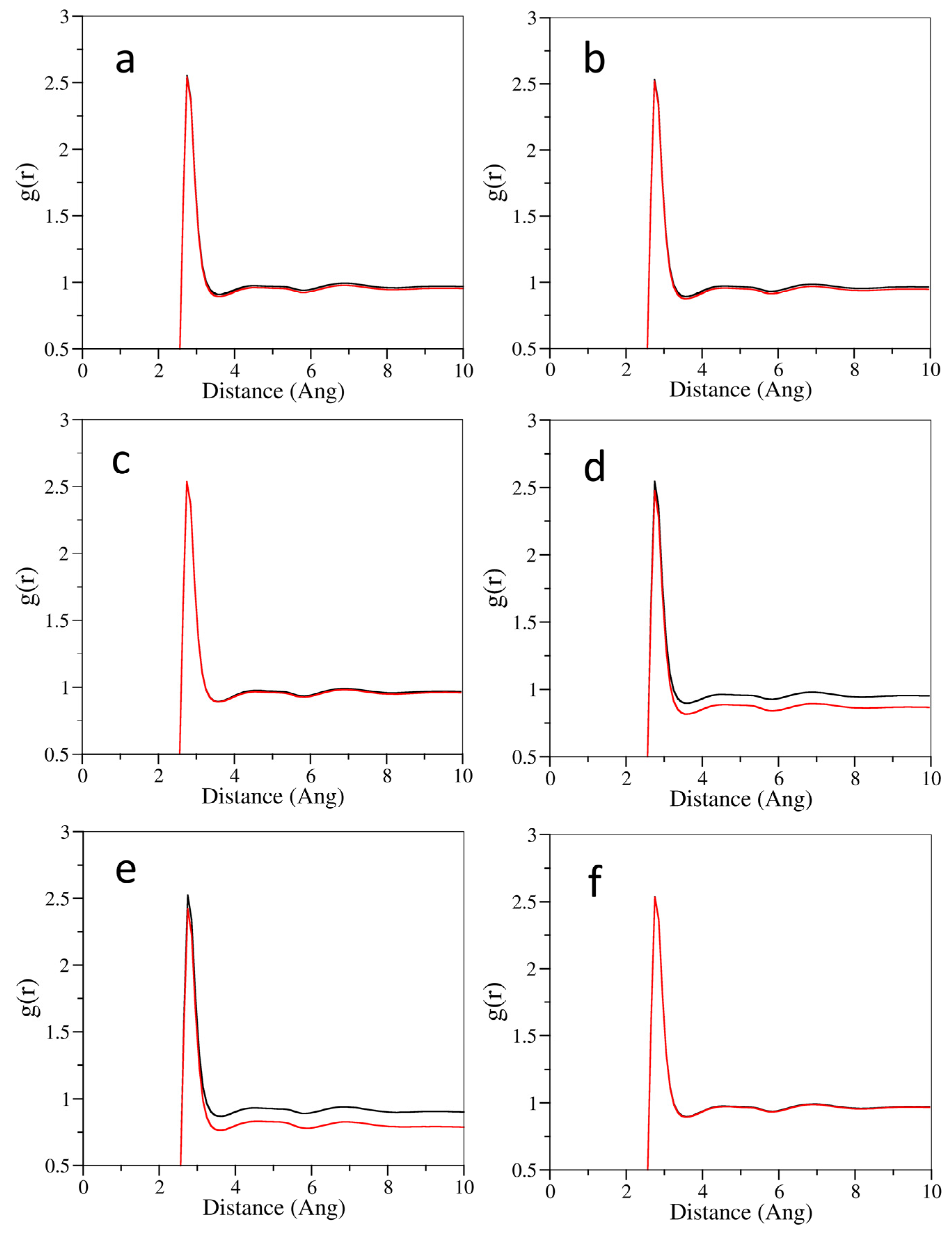
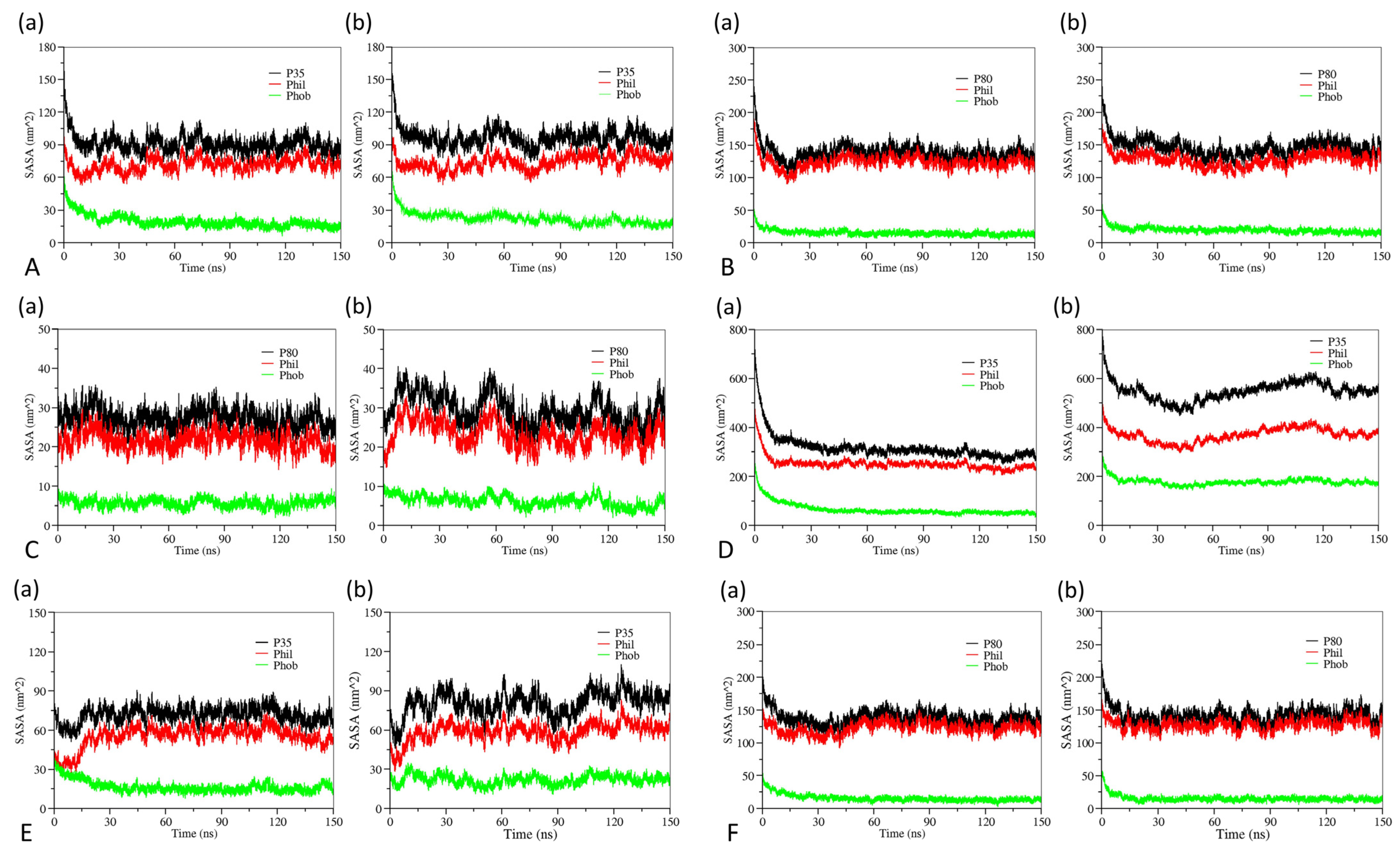
3.2. The Effect of Cosolvent on the Properties of Bulk Solvent
3.3. The Effect of Cosolvent on Micellar Formation
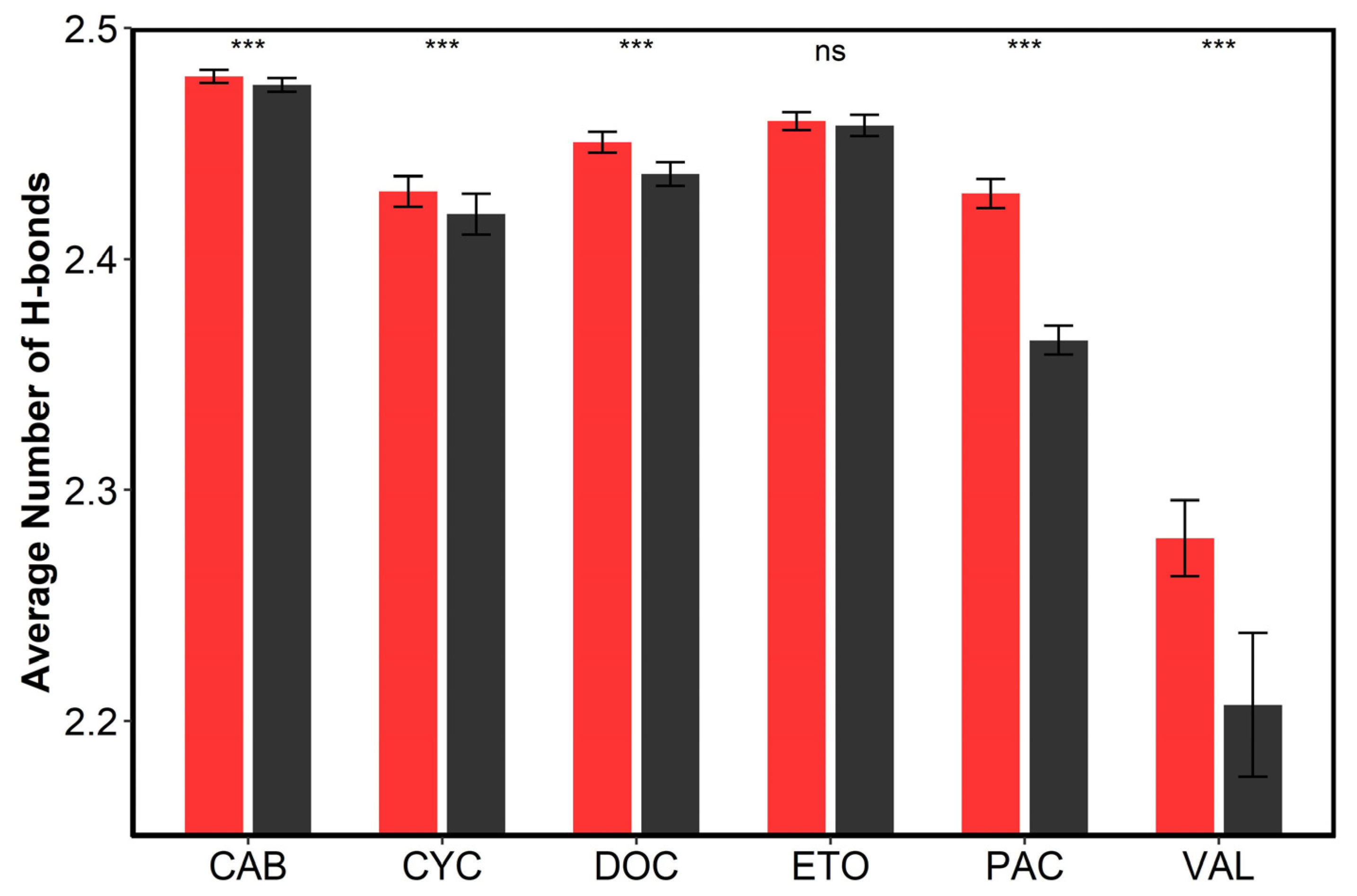
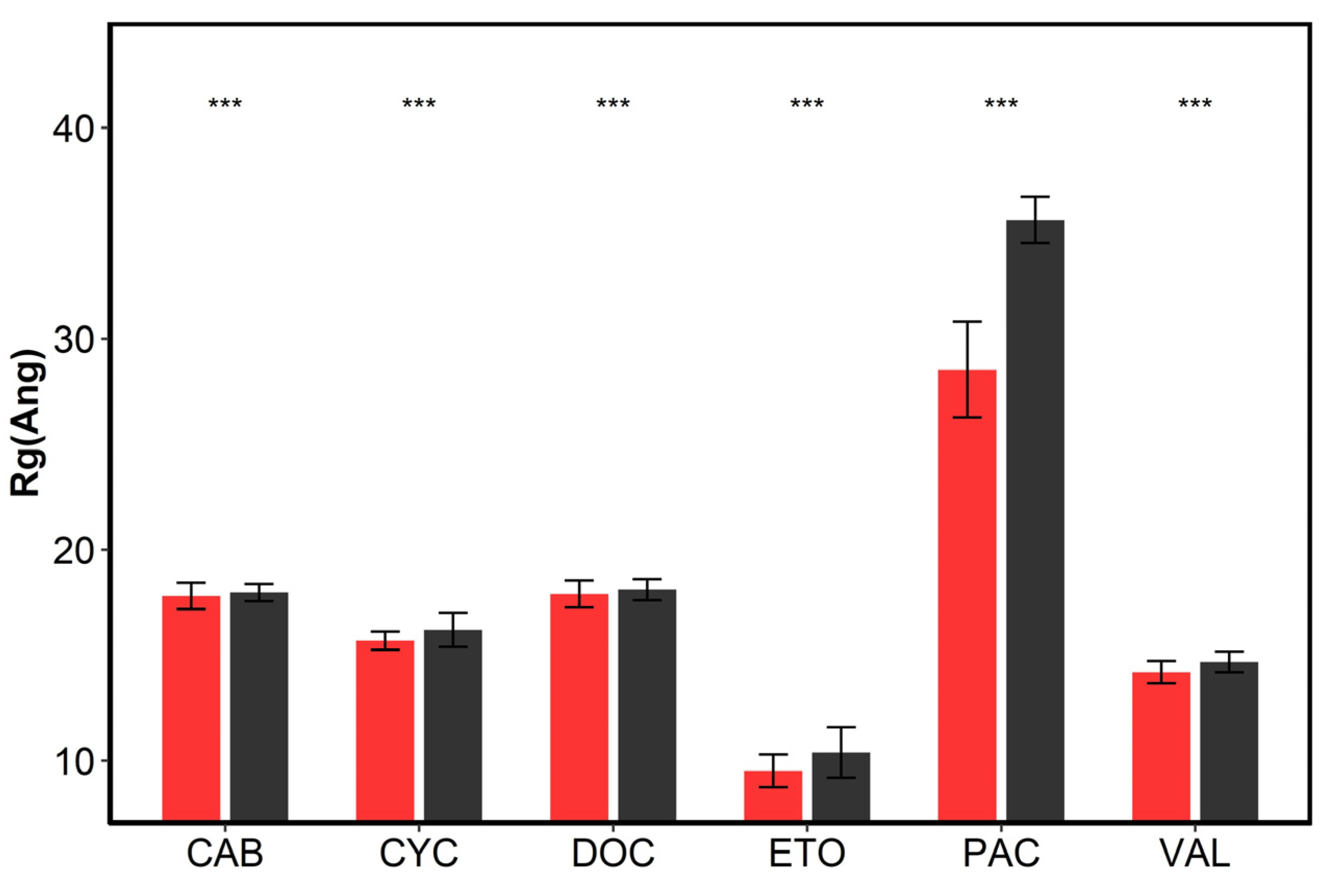
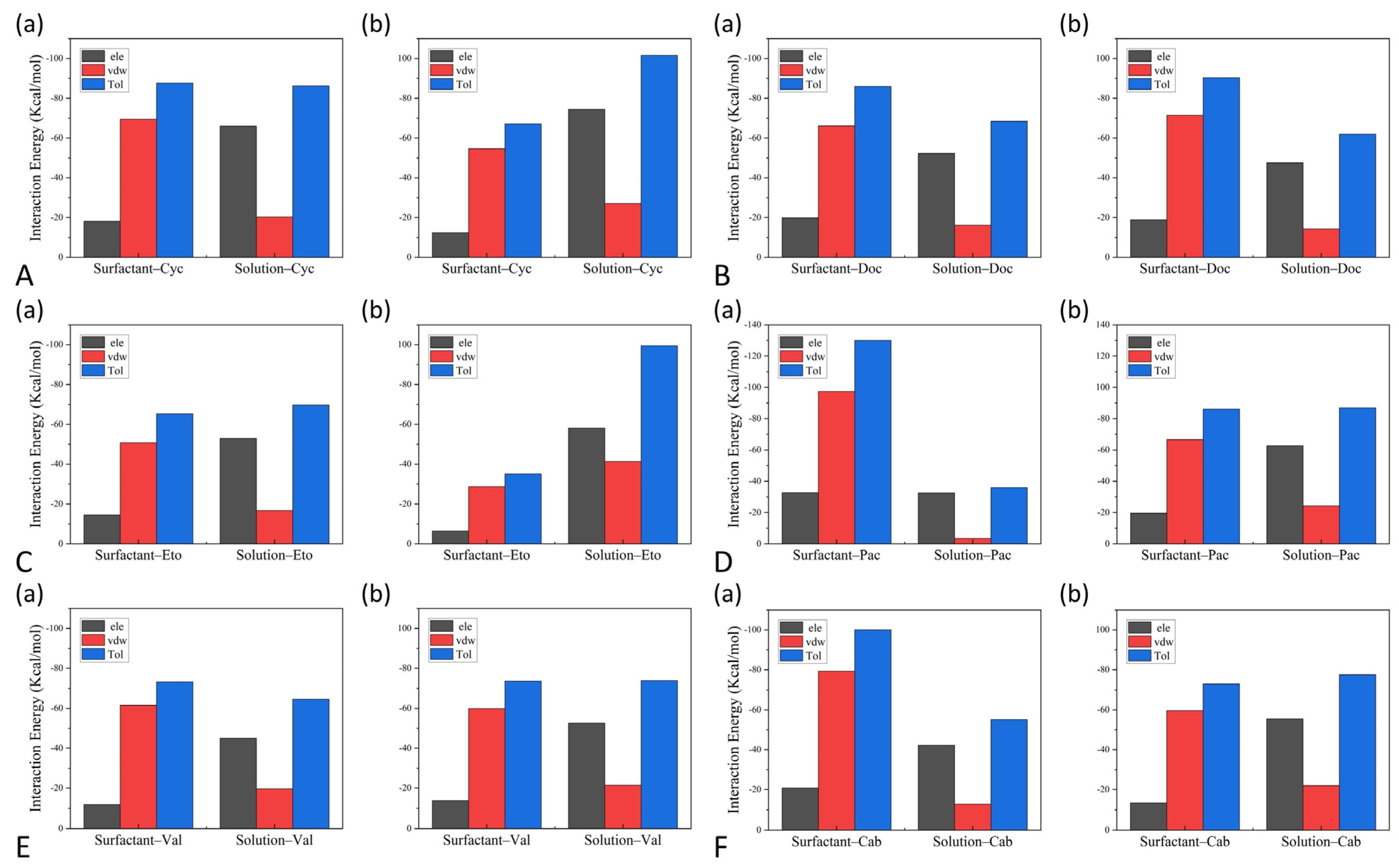
3.4. The Interactions between Drugs and Excipients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van Hoogevest, P.; Liu, X.; Fahr, A. Drug delivery strategies for poorly water-soluble drugs: The industrial perspective. Expert Opin. Drug Deliv. 2011, 8, 1481–1500. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Porter, W.; Merdan, T.; Li, L.C. Recent advances in intravenous delivery of poorly water-soluble compounds. Expert Opin. Drug Deliv. 2009, 6, 1261–1282. [Google Scholar] [CrossRef]
- Jain, S.; Patel, N.; Lin, S. Solubility and dissolution enhancement strategies: Current understanding and recent trends. Drug Dev. Ind. Pharm. 2015, 41, 875–887. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Huang, L. Nanoparticles containing insoluble drug for cancer therapy. Biotechnol. Adv. 2014, 32, 778–788. [Google Scholar] [CrossRef] [PubMed]
- Savjani, K.T.; Gajjar, A.K.; Savjani, J.K. Drug solubility: Importance and enhancement techniques. ISRN Pharm. 2012, 2012, 195727. [Google Scholar] [CrossRef]
- Rodriguez-Aller, M.; Guillarme, D.; Veuthey, J.-L.; Gurny, R. Strategies for formulating and delivering poorly water-soluble drugs. J. Drug Deliv. Sci. Technol. 2015, 30, 342–351. [Google Scholar] [CrossRef]
- Kalepu, S.; Nekkanti, V. Insoluble drug delivery strategies: Review of recent advances and business prospects. Acta Pharm. Sin. B 2015, 5, 442–453. [Google Scholar] [CrossRef]
- Kawabata, Y.; Wada, K.; Nakatani, M.; Yamada, S.; Onoue, S. Formulation design for poorly water-soluble drugs based on biopharmaceutics classification system: Basic approaches and practical applications. Int. J. Pharm. 2011, 420, 1–10. [Google Scholar] [CrossRef]
- Fahr, A.; Liu, X. Drug delivery strategies for poorly water-soluble drugs. Expert Opin. Drug Deliv. 2007, 4, 403–416. [Google Scholar] [CrossRef]
- Miyako, Y.; Khalef, N.; Matsuzaki, K.; Pinal, R. Solubility enhancement of hydrophobic compounds by cosolvents: Role of solute hydrophobicity on the solubilization effect. Int. J. Pharm. 2010, 393, 48–54. [Google Scholar] [CrossRef]
- Jouyban, A. Review of the cosolvency models for predicting solubility of drugs in water-cosolvent mixtures. J. Pharm. Pharm. Sci. 2008, 11, 32–58. [Google Scholar] [CrossRef] [PubMed]
- Millard, J.W.; Alvarez-Nunez, F.A.; Yalkowsky, S.H. Solubilization by cosolvents. Establishing useful constants for the log-linear model. Int. J. Pharm. 2002, 245, 153–166. [Google Scholar] [CrossRef]
- Strickley, R.G. Solubilizing Excipients in Oral and Injectable Formulations. Pharm. Res. 2004, 21, 201–230. [Google Scholar] [CrossRef] [PubMed]
- Williams, H.D.; Trevaskis, N.L.; Charman, S.A.; Shanker, R.M.; Charman, W.N.; Pouton, C.W.; Porter, C.J.H. Strategies to Address Low Drug Solubility in Discovery and Development. Pharmacol. Rev. 2013, 65, 315–499. [Google Scholar] [CrossRef] [PubMed]
- Mallick, S.; Pattnaik, S.; Swain, K.; De, P.K. Current perspectives of solubilization: Potential for improved bioavailability. Drug Dev. Ind. Pharm. 2007, 33, 865. [Google Scholar] [CrossRef]
- Vinarov, Z.; Katev, V.; Radeva, D.; Tcholakova, S.; Denkov, N.D. Micellar solubilization of poorly water-soluble drugs: Effect of surfactant and solubilizate molecular structure. Drug Dev. Ind. Pharm. 2017, 44, 677–686. [Google Scholar] [CrossRef]
- Torchilin, V.P. Micellar nanocarriers: Pharmaceutical perspectives. Pharm. Res. 2007, 24, 1–16. [Google Scholar] [CrossRef]
- Rangel-Yagui, C.O.; Pessoa Jr, A.; Tavares, L.C. Micellar solubilization of drugs. J. Pharm. Pharm. Sci. 2005, 8, 147–163. [Google Scholar]
- Akers, M.J. Excipient-drug interactions in parenteral formulations. J. Pharm. Sci. 2002, 91, 2283–2300. [Google Scholar] [CrossRef]
- Zana, R. Aqueous surfactant-alcohol systems: A review. Adv. Colloid Interface Sci. 1995, 57, 1–64. [Google Scholar] [CrossRef]
- Neoral, P. Pr SANDIMMUNE® IV (Cyclosporine for Injection). Available online: https://pdf.hres.ca/dpd_pm/00028894.PDF (accessed on 25 July 2022).
- Katiyar, R.S.; Jha, P.K. Molecular simulations in drug delivery: Opportunities and challenges. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2018, 8, e1358. [Google Scholar] [CrossRef]
- Hossain, S.; Kabedev, A.; Parrow, A.; Bergström, C.A.; Larsson, P. Molecular simulation as a computational pharmaceutics tool to predict drug solubility, solubilization processes and partitioning. Eur. J. Pharm. Biopharm. 2019, 137, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Maleki, R.; Afrouzi, H.H.; Hosseini, M.; Toghraie, D.; Rostami, S. Molecular dynamics simulation of Doxorubicin loading with N-isopropyl acrylamide carbon nanotube in a drug delivery system. Comput. Methods Programs Biomed. 2020, 184, 105303. [Google Scholar] [CrossRef] [PubMed]
- Rezaeisadat, M.; Bordbar, A.-K.; Omidyan, R. Molecular dynamics simulation study of curcumin interaction with nano-micelle of PNIPAAm-b-PEG co-polymer as a smart efficient drug delivery system. J. Mol. Liq. 2021, 332, 115862. [Google Scholar] [CrossRef]
- Khezri, A.; Karimi, A.; Yazdian, F.; Jokar, M.; Mofradnia, S.R.; Rashedi, H.; Tavakoli, Z. Molecular dynamic of curcumin/chitosan interaction using a computational molecular approach: Emphasis on biofilm reduction. Int. J. Biol. Macromol. 2018, 114, 972–978. [Google Scholar] [CrossRef]
- Amani, A.; York, P.; de Waard, H.; Anwar, J. Molecular dynamics simulation of a polysorbate 80 micelle in water. Soft Matter 2011, 7, 2900–2908. [Google Scholar] [CrossRef]
- Suys, E.J.A.; Warren, D.B.; Pham, A.C.; Nowell, C.J.; Clulow, A.J.; Benameur, H.; Porter, C.J.H.; Pouton, C.W.; Chalmers, D.K. A Nonionic Polyethylene Oxide (PEO) Surfactant Model: Experimental and Molecular Dynamics Studies of Kolliphor EL. J. Pharm. Sci. 2019, 108, 193–204. [Google Scholar] [CrossRef]
- Salomon-Ferrer, R.; Case, D.A.; Walker, R.C. An overview of the Amber biomolecular simulation package. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2013, 3, 198–210. [Google Scholar] [CrossRef]
- Mark, P.; Nilsson, L. Structure and dynamics of the TIP3P, SPC, and SPC/E water models at 298 K. J. Phys. Chem. A 2001, 105, 9954–9960. [Google Scholar] [CrossRef]
- Andersen, H.C. Rattle: A “velocity” version of the shake algorithm for molecular dynamics calculations. J. Comput. Phys. 1983, 52, 24–34. [Google Scholar] [CrossRef]
- Nayak, A.K.; Panigrahi, P.P. Solubility enhancement of etoricoxib by cosolvency approach. Int. Sch. Res. Not. 2012, 2012, 820653. [Google Scholar]
- Sobisch, T.; Wüstneck, R. The influence of alcohols of different alkyl chain length on the solubilization of solutions containing polyoxyethylene p-tert-octylphenyl ether. Colloids Surf. 1992, 62, 187–198. [Google Scholar] [CrossRef]
- Alexandridis, P.; Yang, L. SANS Investigation of Polyether Block Copolymer Micelle Structure in Mixed Solvents of Water and Formamide, Ethanol, or Glycerol. Macromolecules 2000, 33, 5574–5587. [Google Scholar] [CrossRef]
- Huang, J.B.; Mao, M.; Zhu, B.Y. The surface physico-chemical properties of surfactants in ethanol–water mixtures. Colloids Surf. A Physicochem. Eng. Asp. 1999, 155, 339–348. [Google Scholar] [CrossRef]
- Sarkar, B.; Ravi, V.; Alexandridis, P. Micellization of amphiphilic block copolymers in binary and ternary solvent mixtures. J. Colloid Interface Sci. 2013, 390, 137–146. [Google Scholar]
- Das, S.; Mondal, S.; Ghosh, S. Physicochemical Studies on the Micellization of Cationic, Anionic, and Nonionic Surfactants in Water–Polar Organic Solvent Mixtures. J. Chem. Eng. Data 2013, 58, 2586–2595. [Google Scholar] [CrossRef]
- Sundar, S.; Sandilya, A.A.; Priya, M.H. Unraveling the influence of osmolytes on water hydrogen-bond network: From local structure to graph theory analysis. J. Chem. Inf. Model. 2021, 61, 3927–3944. [Google Scholar] [CrossRef]
- Huang, L.; Nishinari, K. Interaction between poly (ethylene glycol) and water as studied by differential scanning calorimetry. J. Polym. Sci. Part B Polym. Phys. 2001, 39, 496–506. [Google Scholar] [CrossRef]
- Richmond, T.J. Solvent accessible surface area and excluded volume in proteins: Analytical equations for overlapping spheres and implications for the hydrophobic effect. J. Mol. Biol. 1984, 178, 63–89. [Google Scholar] [CrossRef]
- Kawakami, K.; Oda, N.; Miyoshi, K.; Funaki, T.; Ida, Y. Solubilization behavior of a poorly soluble drug under combined use of surfactants and cosolvents. Eur. J. Pharm. Sci. 2006, 28, 7–14. [Google Scholar] [CrossRef]
- Kawakami, K.; Miyoshi, K.; Ida, Y. Solubilization behavior of poorly soluble drugs with combined use of Gelucire 44/14 and cosolvent. J. Pharm. Sci. 2004, 93, 1471–1479. [Google Scholar] [CrossRef] [PubMed]
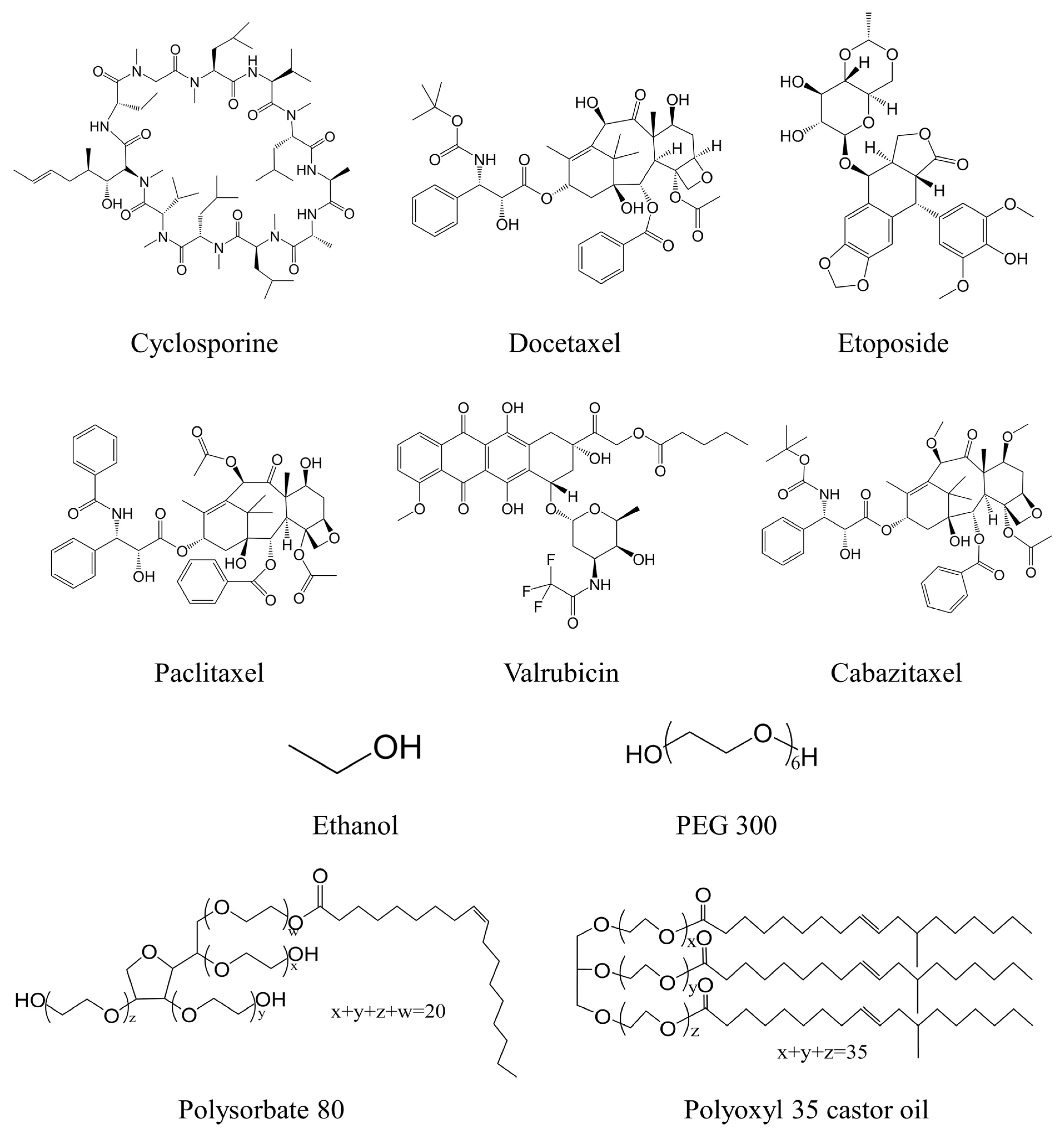

| Brand Name/Generic Name | Formulation (Each mL) | Clinical Use |
|---|---|---|
| Sandimmune/Cyclosporine | Cyclosporine 50 mg Polyoxyl 35 castor oil 650 mg Ethanol 32.9% (v/v) | 20 mL solution diluted with 100 mL Sodium Chloride or Dextrose injection |
| Taxotere/Docetaxel | Docetaxel 20 mg Polysorbate 80 540 mg Ethanol 395 mg | Diluted with Sodium Chloride or Dextrose injection to 0.3–0.74 mg/mL |
| Toposar/Etoposide | Etoposide 20 mg Citric acid 2 mg Polysorbate 80 80 mg Polyethylene glycol 300 650 mg Ethanol 262 mg | Diluted with Dextrose or Sodium Chloride Injection to 0.2–0.4 mg/mL |
| Taxol/Paclitaxel | Paclitaxel 6 mg Polyoxyl 35 castor oil 527 mg Ethanol 49.7% (v/v) Citric acid 2 mg | Diluted with Sodium Chloride or Dextrose injection to 0.3–1.2 mg/mL |
| Valstar/Valrubicin | Valrubicin 40 mg Polyoxyl 35 castor oil 50% v/v Ethanol 50% v/v | 20 mL Valstar diluted with 55 mL Sodium Chloride Injection |
| Jevtana/Cabazitaxel | Cabazitaxel 40 mg Polysorbate 80 1040 mg | Diluted with 5.7 mL 13% (w/w) ethanol solution, followed by dilution of sodium chloride or dextrose solution |
| System | NO. of Molecule(s) | Cdrug 3 (mg/mL) | Cdrug 4 (mg/mL) | |||
|---|---|---|---|---|---|---|
| Drug | Surfactant | Cosolvent | Water | |||
| Cyc 1 | 1 | 6 | 137 | 25,502 | 0.5–2.5 | 2.45 |
| Doc 1 | 1 | 17 | 346 | 60,026 | 0.3–0.74 | 0.70 |
| Eto 1 | 1 | 2 | 68/167 5 | 80,258 | 0.2–0.4 | 0.40 |
| Pac 1 | 1 | 30 | 1228 | 33,933 | 0.3–1.2 | 1.12 |
| Val 1 | 1 | 4 | 157 | 2917 | 10.67 | 10.04 |
| Cab 1 | 1 | 17 | 183 | 160,932 | 0.1–0.26 | 0.26 |
| Cyc 2 | 1 | 6 | - | 25,927 | - | 2.45 |
| Doc 2 | 1 | 17 | - | 61,091 | - | 0.70 |
| Eto 2 | 1 | 2 | - | 81,794 | - | 0.40 |
| Pac 2 | 1 | 30 | - | 37,622 | - | 1.12 |
| Val 2 | 1 | 4 | - | 3388 | - | 10.04 |
| Cab 2 | 1 | 17 | - | 161,486 | - | 0.26 |
| Complex | Cyc 1 | Cyc 2 | Doc 1 | Doc 2 | Eto 1 | Eto 2 |
| I1:I2:I3 | 1.37:1.26:1 | 1.43:1.26:1 | 1.34:1.18:1 | 1.30:1.18:1 | 1.76:1.51:1 | 1.81:1.55:1 |
| e | 0.69 | 0.71 | 0.67 | 0.64 | 0.82 | 0.83 |
| Complex | Pac 1 | Pac2 | Val 1 | Val 2 | Cab 1 | Cab 2 |
| I1:I2:I3 | 1.68:1.49:1 | 3.62:3.52:1 | 1.59:1.34:1 | 1.49:1.33:1 | 1.28:1.17:1 | 1.46:1.28:1 |
| e | 0.80 | 0.96 | 0.78 | 0.74 | 0.63 | 0.73 |
| System | Cyc | Cyc-co | Doc | Doc-co | Eto | Eto-co |
| SASA(nm2) 1 | 90.25 | 95.36 | 135.89 | 147.02 | 26.23 | 27.12 |
| SASA(phob) 2 | 16.22 | 17.88 | 12.92 | 16.13 | 5.76 | 4.80 |
| SASA(phil) 3 | 74.03 | 77.48 | 122.97 | 130.89 | 20.47 | 22.32 |
| hydrophobic % 4 | 17.97 | 18.75 | 9.51 | 10.97 | 21.97 | 17.69 |
| System | Pac | Pac-co | Val | Val-co | Cab | Cab-co |
| SASA(nm2) 1 | 284.55 | 574.53 | 69.21 | 90.31 | 135.24 | 143.44 |
| SASA(phob) 2 | 51.29 | 176.93 | 14.96 | 23.95 | 12.73 | 13.81 |
| SASA(phil) 3 | 233.26 | 397.60 | 54.25 | 66.36 | 122.51 | 129.63 |
| hydrophobic% 4 | 18.02 | 30.80 | 21.62 | 26.52 | 9.41 | 9.63 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, M.; Zheng, W.; Wang, N.; Gao, H.; Ouyang, D.; Huang, Z. Molecular Dynamics Simulation of Drug Solubilization Behavior in Surfactant and Cosolvent Injections. Pharmaceutics 2022, 14, 2366. https://doi.org/10.3390/pharmaceutics14112366
He M, Zheng W, Wang N, Gao H, Ouyang D, Huang Z. Molecular Dynamics Simulation of Drug Solubilization Behavior in Surfactant and Cosolvent Injections. Pharmaceutics. 2022; 14(11):2366. https://doi.org/10.3390/pharmaceutics14112366
Chicago/Turabian StyleHe, Meiqi, Wenwen Zheng, Nannan Wang, Hanlu Gao, Defang Ouyang, and Zunnan Huang. 2022. "Molecular Dynamics Simulation of Drug Solubilization Behavior in Surfactant and Cosolvent Injections" Pharmaceutics 14, no. 11: 2366. https://doi.org/10.3390/pharmaceutics14112366
APA StyleHe, M., Zheng, W., Wang, N., Gao, H., Ouyang, D., & Huang, Z. (2022). Molecular Dynamics Simulation of Drug Solubilization Behavior in Surfactant and Cosolvent Injections. Pharmaceutics, 14(11), 2366. https://doi.org/10.3390/pharmaceutics14112366





