Transferrin-Functionalized Liposomes for the Delivery of Gallic Acid: A Therapeutic Approach for Alzheimer’s Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of GA-Loaded Liposomes
2.2.1. Lipid Film Hydration
2.2.2. Reverse-Phase Evaporation
2.2.3. Ethanol Injection
2.2.4. Ethanol Permeabilization
2.3. Determination of Entrapment Efficiency and Loading Capacity
2.4. Functionalization of Liposomes with Transferrin
2.5. Determination of Transferrin Conjugation Efficiency
2.6. Physicochemical Characterization of Liposomes
2.6.1. Dynamic Light Scattering
2.6.2. Electrophoretic Light Scattering
2.6.3. Attenuated Total Reflection-Fourier Transform Infrared Spectroscopy
2.7. Stability of Liposomes at Storage Conditions
2.8. In Vitro Release of GA from Transferrin-Functionalized Liposomes
2.9. Anti-Amyloidogenic Activity of GA-Loaded Transferrin-Functionalized Liposomes
2.9.1. Preparation of Aβ Stock Solutions
2.9.2. Thioflavin T Fluorescence Assay
2.9.3. Kinetic Model of Aβ Fibril Formation
2.9.4. Transmission Electron Microscopy
2.10. Statistical Analysis
3. Results and Discussion
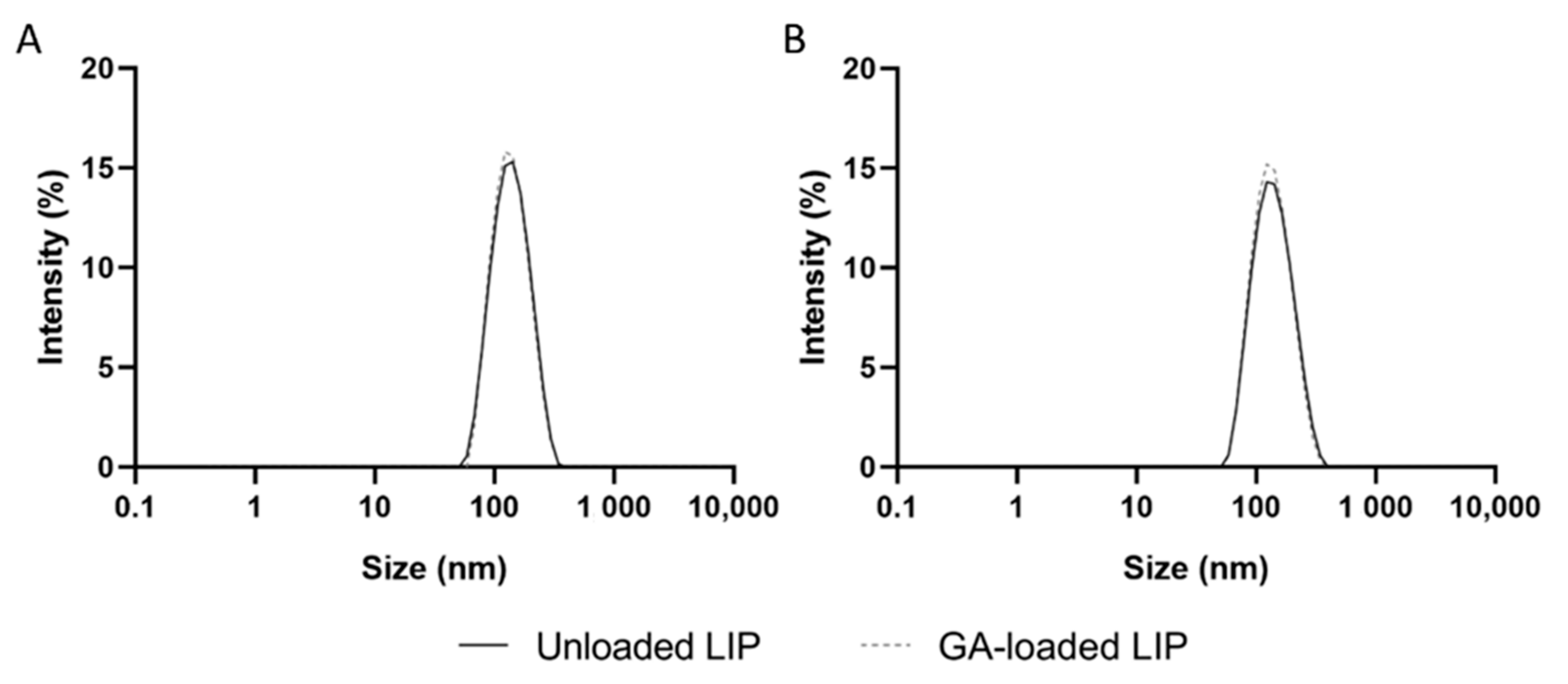
3.1. Physicochemical Characterization of Unloaded and GA-Loaded Liposomes
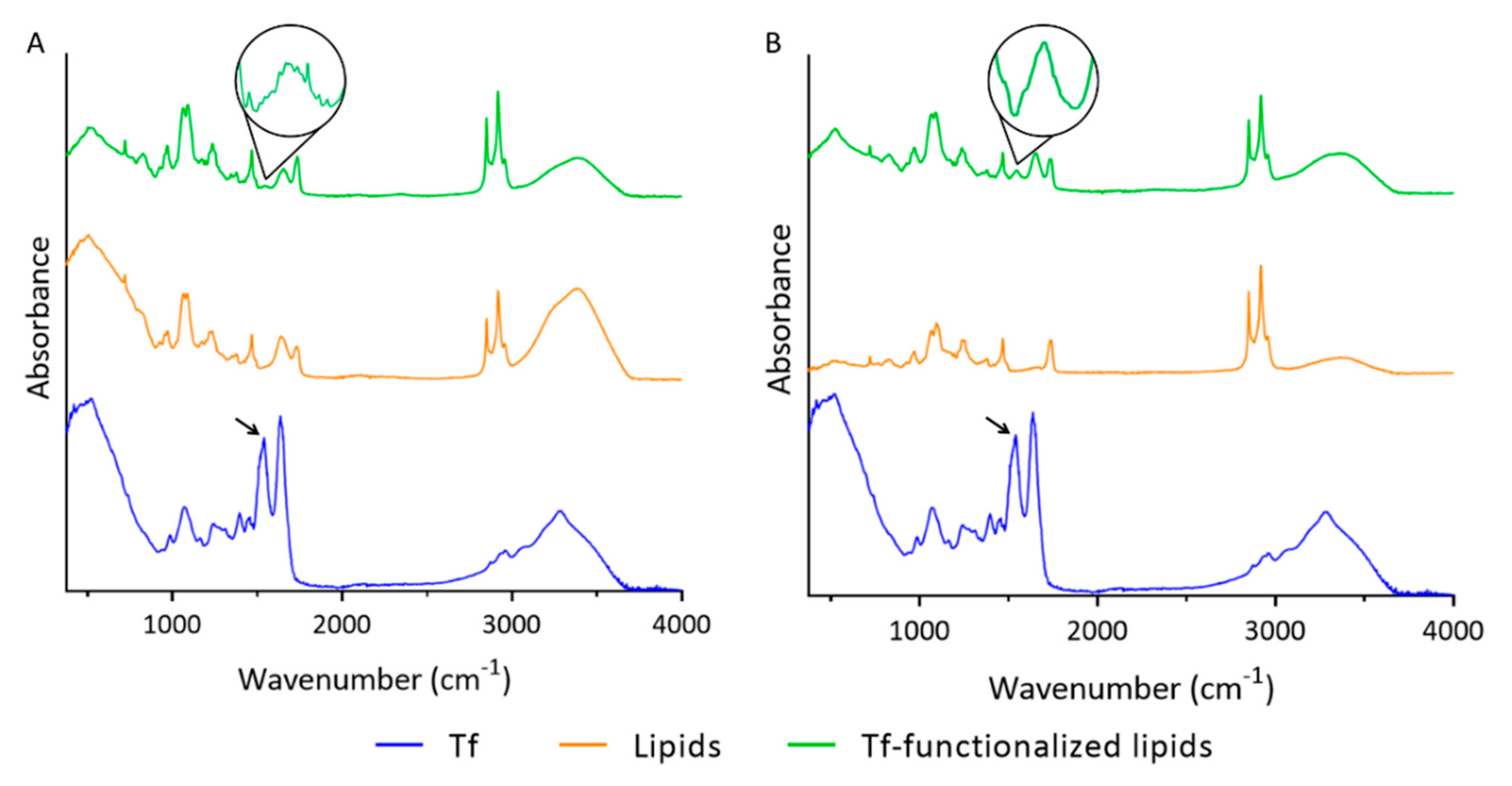
3.2. Functionalization of Liposomes with Tf
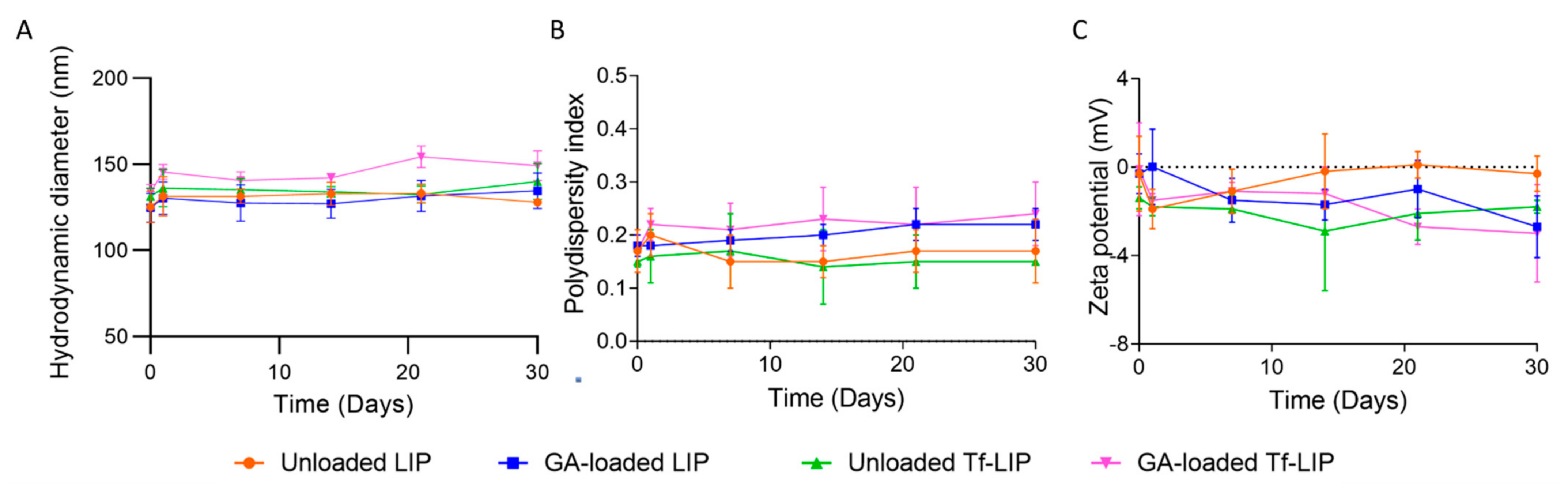
3.3. Physical Stability of Liposomes at Storage Conditions
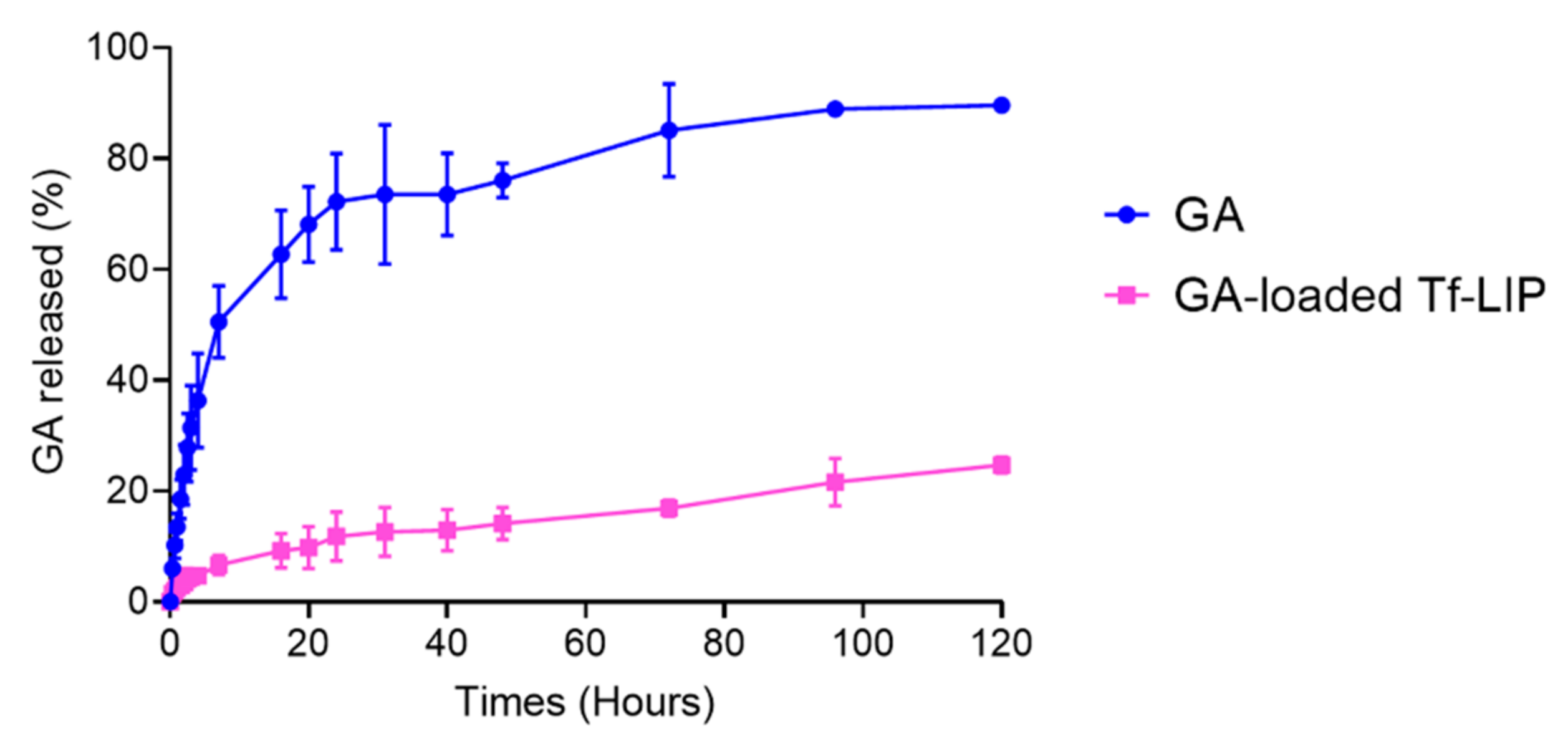
3.4. In Vitro Release of GA from Tf-Functionalized Liposomes
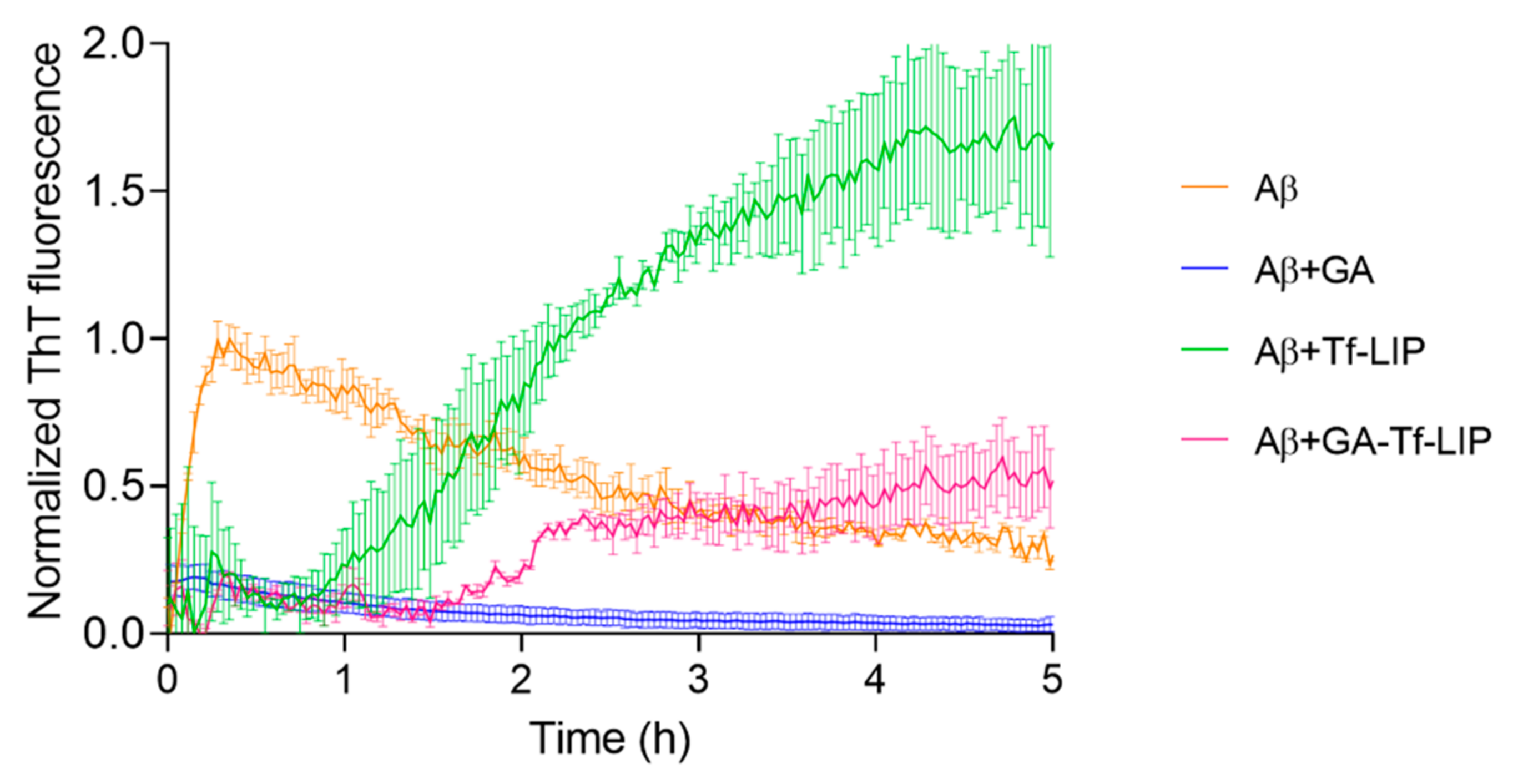
3.5. Anti-Amyloidogenic Activity of GA-Loaded Liposomes Functionalized with Transferrin
3.5.1. Inhibition of Aβ Fibril Formation
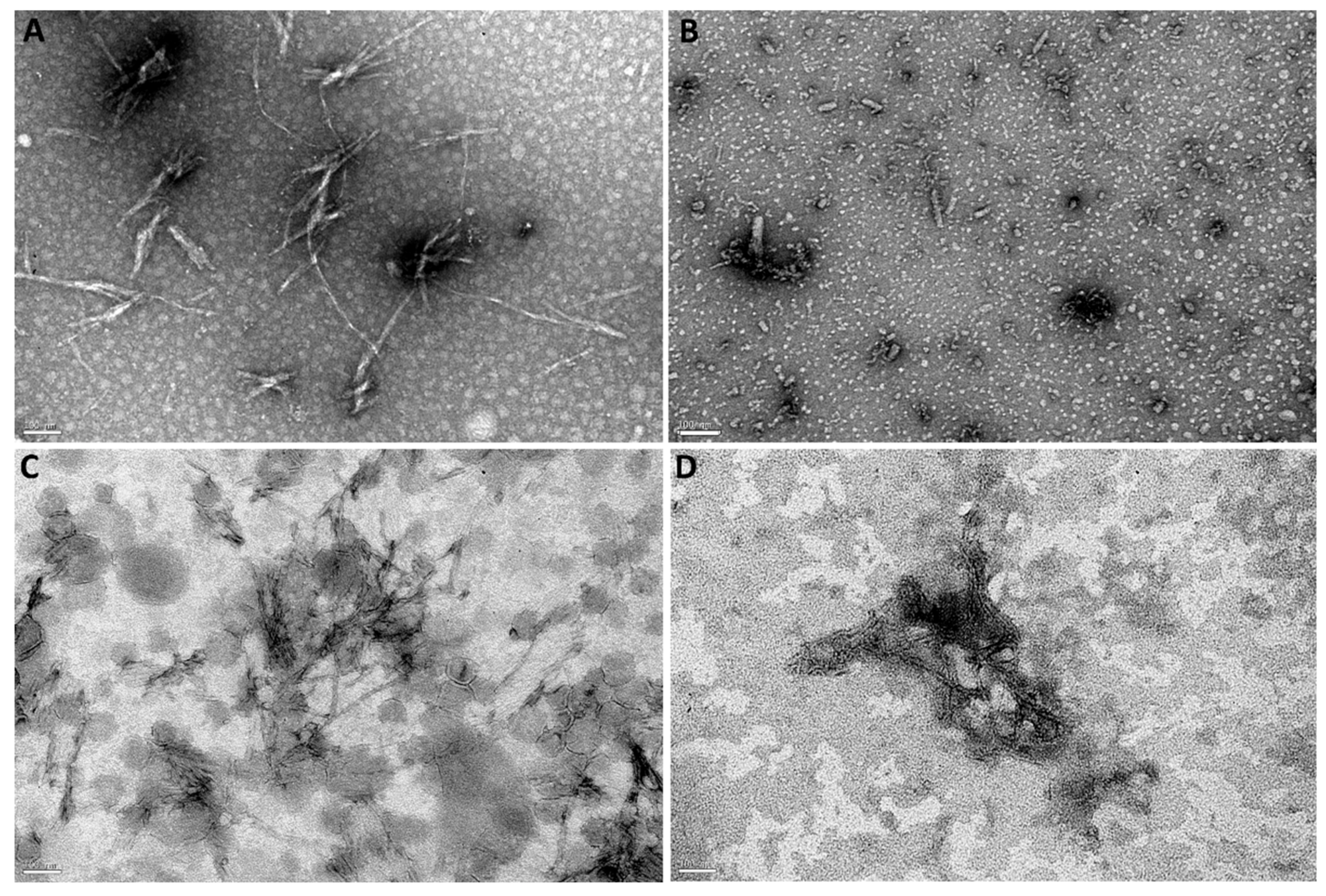
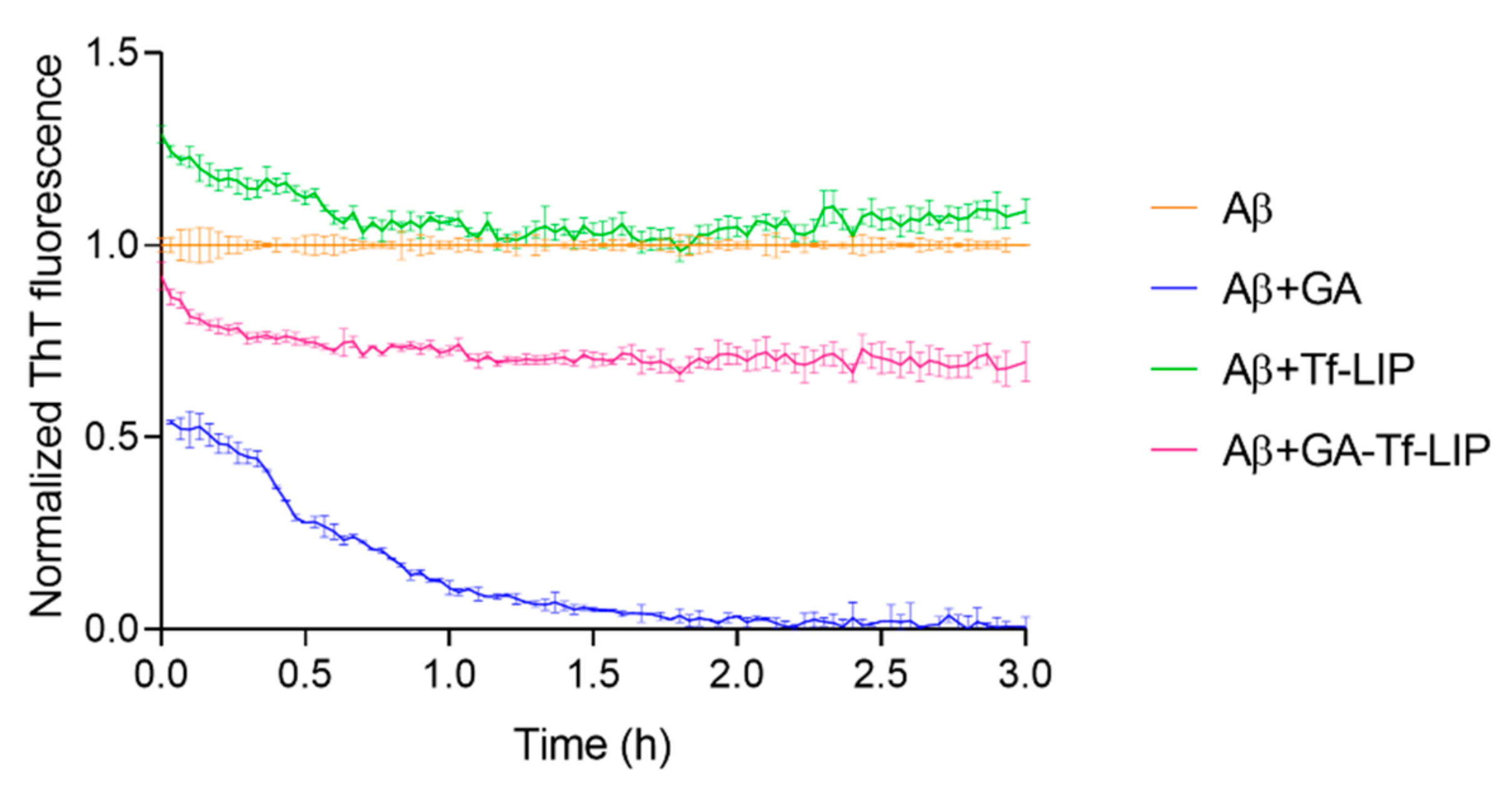
3.5.2. Disaggregation of Mature Aβ Fibrils
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Rodrigues, B.d.S.; Kanekiyo, T.; Singh, J. Nerve Growth Factor Gene Delivery across the Blood–Brain Barrier to Reduce Beta Amyloid Accumulation in AD Mice. Mol. Pharm. 2020, 17, 2054–2063. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos Rodrigues, B.; Kanekiyo, T.; Singh, J. ApoE-2 brain-targeted gene therapy through transferrin and penetratin tagged liposomal nanoparticles. Pharm. Res. 2019, 36, 161. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Zheng, Y.; Liu, X.; Fang, W.; Chen, X.; Liao, W.; Jing, X.; Lei, M.; Tao, E.; Ma, Q. Curcumin-loaded PLGA-PEG nanoparticles conjugated with B6 peptide for potential use in Alzheimer’s disease. Drug Deliv. 2018, 25, 1091–1102. [Google Scholar] [CrossRef] [PubMed]
- Shabani, S.; Rabiei, Z.; Amini-Khoei, H. Exploring the multifaceted neuroprotective actions of gallic acid: A review. Int. J. Food Prop. 2020, 23, 736–752. [Google Scholar] [CrossRef]
- Ramalho, M.J.; Coelho, M.A.N.; Pereira, M.C. Chapter 18—Nanocarriers for the delivery of temozolomide in the treatment of glioblastoma: A review. In Design and Development of New Nanocarriers; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2018; pp. 687–722. [Google Scholar]
- Loureiro, J.A.; Ramalho, M.J.; Pereira, M.d.C. Immuno-nanocarriers for brain delivery: Limitations from in vitro to preclinical and clinical studies. Nanomedicine 2020, 15, 543–545. [Google Scholar] [CrossRef]
- Yang, X.; Li, X.; Liu, L.; Chen, Y.-H.; You, Y.; Gao, Y.; Liu, Y.-Y.; Yang, L.; Tong, K.; Chen, D.-S. Transferrin-Pep63-liposomes accelerate the clearance of Aβ and rescue impaired synaptic plasticity in early Alzheimer’s disease models. Cell Death Discov. 2021, 7, 1–13. [Google Scholar] [CrossRef]
- Daraee, H.; Etemadi, A.; Kouhi, M.; Alimirzalu, S.; Akbarzadeh, A. Application of liposomes in medicine and drug delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 381–391. [Google Scholar] [CrossRef]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and Challenges of Liposome Assisted Drug Delivery. Front Pharm. 2015, 6, 286. [Google Scholar] [CrossRef]
- Kadry, H.; Noorani, B.; Cucullo, L. A blood–brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS 2020, 17, 69. [Google Scholar] [CrossRef]
- Tashima, T. Smart strategies for therapeutic agent delivery into brain across the blood–brain barrier using receptor-mediated transcytosis. Chem. Pharm. Bull. 2020, 68, 316–325. [Google Scholar] [CrossRef]
- Yin, T.; Yang, L.; Liu, Y.; Zhou, X.; Sun, J.; Liu, J. Sialic acid (SA)-modified selenium nanoparticles coated with a high blood–brain barrier permeability peptide-B6 peptide for potential use in Alzheimer’s disease. Acta Biomater. 2015, 25, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Zaki, A.A.; Hui, J.Z.; Muzykantov, V.R.; Tsourkas, A. Multifunctional Nanoparticles: Cost Versus Benefit of Adding Targeting and Imaging Capabilities. Science 2012, 338, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Ying, X.; Wen, H.; Lu, W.-L.; Du, J.; Guo, J.; Tian, W.; Men, Y.; Zhang, Y.; Li, R.-J.; Yang, T.-Y. Dual-targeting daunorubicin liposomes improve the therapeutic efficacy of brain glioma in animals. J. Control. Release 2010, 141, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Lopalco, A.; Cutrignelli, A.; Denora, N.; Lopedota, A.; Franco, M.; Laquintana, V. Transferrin functionalized liposomes loading dopamine HCl: Development and permeability studies across an in vitro model of human blood–brain barrier. Nanomaterials 2018, 8, 178. [Google Scholar] [CrossRef]
- Kong, L.; Li, X.-T.; Ni, Y.-N.; Xiao, H.-H.; Yao, Y.-J.; Wang, Y.-Y.; Ju, R.-J.; Li, H.-Y.; Liu, J.-J.; Fu, M.; et al. Transferrin-Modified Osthole PEGylated Liposomes Travel the Blood-Brain Barrier and Mitigate Alzheimer’s Disease-Related Pathology in APP/PS-1 Mice. Int. J. Nanomed. 2020, 15, 2841–2858. [Google Scholar] [CrossRef]
- Chen, Z.-L.; Huang, M.; Wang, X.-R.; Fu, J.; Han, M.; Shen, Y.-Q.; Xia, Z.; Gao, J.-Q. Transferrin-modified liposome promotes α-mangostin to penetrate the blood–brain barrier. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Papadia, K.; Giannou, A.D.; Markoutsa, E.; Bigot, C.; Vanhoute, G.; Mourtas, S.; Van der Linded, A.; Stathopoulos, G.T.; Antimisiaris, S.G. Multifunctional LUV liposomes decorated for BBB and amyloid targeting-B. In Vivo brain targeting potential in wild-type and APP/PS1 mice. Eur. J. Pharm. Sci. 2017, 102, 180–187. [Google Scholar] [CrossRef]
- Kuo, Y.-C.; Chou, P.-R. Neuroprotection against degeneration of SK-N-MC cells using neuron growth factor-encapsulated liposomes with surface cereport and transferrin. J. Pharm. Sci. 2014, 103, 2484–2497. [Google Scholar] [CrossRef]
- Neves, A.; van der Putten, L.; Queiroz, J.; Pinheiro, M.; Reis, S. Transferrin-functionalized lipid nanoparticles for curcumin brain delivery. J. Biotechnol. 2021, 331, 108–117. [Google Scholar] [CrossRef]
- AlSawaftah, N.M.; Awad, N.S.; Paul, V.; Kawak, P.S.; Al-Sayah, M.H.; Husseini, G.A. Transferrin-modified liposomes triggered with ultrasound to treat HeLa cells. Sci. Rep. 2021, 11, 1–15. [Google Scholar] [CrossRef]
- Abri, A.; Maleki, M. Isolation and identification of gallic acid from the elaeagnus angustifolia leaves and determination of total phenolic, flavonoids contents and investigation of antioxidant activity. Q. J. Iran. Chem. Commun. 2016, 4, 146–154. [Google Scholar]
- Dejeu, I.L.; Vicaș, L.G.; Jurca, T.; Teușdea, A.C.; Mureșan, M.E.; Fritea, L.; Svera, P.; Gabor, G.A.; Dejeu, G.E.; Maghiar, O.A. Liposomes with caffeic acid: Morphological and structural characterisation, their properties and stability in time. Processes 2021, 9, 912. [Google Scholar] [CrossRef]
- Andrade, S.; Loureiro, J.A.; Pereira, M.C. Influence of in vitro neuronal membranes on the anti-amyloidogenic activity of gallic acid: Implication for the therapy of Alzheimer’s disease. Arch. Biochem. Biophys. 2021, 711, 109022. [Google Scholar] [CrossRef] [PubMed]
- Andrade, S.; Loureiro, J.A.; Pereira, M.C. Caffeic acid for the prevention and treatment of Alzheimer’s disease: The effect of lipid membranes on the inhibition of aggregation and disruption of Aβ fibrils. Int. J. Biol. Macromol. 2021, 190, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Andrade, S.; Loureiro, J.A.; Ramirez, S.; Catumbela, C.S.G.; Soto, C.; Morales, R.; Pereira, M.C. Multi-Dose Intravenous Administration of Neutral and Cationic Liposomes in Mice: An Extensive Toxicity Study. Pharmaceuticals 2022, 15, 761. [Google Scholar] [CrossRef]
- Ceña, V.; Játiva, P. Nanoparticle crossing of blood–brain barrier: A road to new therapeutic approaches to central nervous system diseases. Nanomedicine 2018, 13, 1513–1516. [Google Scholar] [CrossRef]
- Hoshyar, N.; Gray, S.; Han, H.; Bao, G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomed. (Lond) 2016, 11, 673–692. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef]
- Saraiva, C.; Praça, C.; Ferreira, R.; Santos, T.; Ferreira, L.; Bernardino, L. Nanoparticle-mediated brain drug delivery: Overcoming blood–brain barrier to treat neurodegenerative diseases. J. Control. Release 2016, 235, 34–47. [Google Scholar] [CrossRef]
- Forest, V.; Pourchez, J. Preferential binding of positive nanoparticles on cell membranes is due to electrostatic interactions: A too simplistic explanation that does not take into account the nanoparticle protein corona. Mater. Sci. Eng. C 2017, 70, 889–896. [Google Scholar] [CrossRef]
- Sharma, G.; Parchur, A.K.; Jagtap, J.M.; Hansen, C.P.; Joshi, A. Chapter 8—Hybrid Nanostructures in Targeted Drug Delivery. In Hybrid Nanostructures for Cancer Theranostics; Ashok Bohara, R., Thorat, N., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 139–158. [Google Scholar]
- Gonzalez Gomez, A.; Syed, S.; Marshall, K.; Hosseinidoust, Z. Liposomal nanovesicles for efficient encapsulation of staphylococcal antibiotics. ACS Omega 2019, 4, 10866–10876. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, M.; Eslamifar, M.; Khezri, K.; Dizaj, S.M. Applications of nanotechnology in drug delivery to the central nervous system. Biomed. Pharmacother. 2019, 111, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Lajoie, J.M.; Shusta, E.V. Targeting Receptor-Mediated Transport for Delivery of Biologics Across the Blood-Brain Barrier. Annu Rev Pharm. 2015, 55, 613–631. [Google Scholar] [CrossRef] [PubMed]
- Pulgar, V.M. Transcytosis to Cross the Blood Brain Barrier, New Advancements and Challenges. Front. Neurosci. 2019, 12, 1019. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, H. Transferrin and transferrin receptors update. Free Radic. Biol. Med. 2019, 133, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Yucel, C.; De Gim, Z.; Yilmaz, S. Development of Cisplatin-loaded liposome and evaluation of transport properties through Caco-2 cell line. Turk. J. Pharm. Sci 2016, 13, 95–108. [Google Scholar] [CrossRef]
- Loureiro, J.A.; Andrade, S.; Duarte, A.; Neves, A.R.; Queiroz, J.F.; Nunes, C.; Sevin, E.; Fenart, L.; Gosselet, F.; Coelho, M.A.N.; et al. Resveratrol and Grape Extract-loaded Solid Lipid Nanoparticles for the Treatment of Alzheimer’s Disease. Molecules 2017, 22, 277. [Google Scholar] [CrossRef]
- Andrade, S.; Ramalho, M.J.; Loureiro, J.A.; Pereira, M.C. Interaction of natural compounds with biomembrane models: A biophysical approach for the Alzheimer’s disease therapy. Colloids Surf. B 2019, 180, 83–92. [Google Scholar] [CrossRef]
- Visser, C.C.; Stevanović, S.; Voorwinden, L.H.; Bloois, L.v.; Gaillard, P.J.; Danhof, M.; Crommelin, D.J.A.; Boer, A.G.d. Targeting liposomes with protein drugs to the blood–brain barrier in vitro. Eur. J. Pharm. Sci. 2005, 25, 299–305. [Google Scholar] [CrossRef]
- Piraux, H.; Hai, J.; Gaudisson, T.; Merah, S.; Gazeau, F.; Chahine, J.; Hémadi, M. Transferrin-bearing maghemite nano-constructs for biomedical applications. J. Appl. Phys. 2015, 117, 17A336. [Google Scholar] [CrossRef]
- Doi, Y.; Shimizu, T.; Ishima, Y.; Ishida, T. Long-term storage of PEGylated liposomal oxaliplatin with improved stability and long circulation times in vivo. Int. J. Pharm. 2019, 564, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Salem, H.F.; Kharshoum, R.M.; Mahmoud, M.; Azim, S.A.; Ebeid, E.-Z.M. Development and characterization of a novel nano-liposomal formulation of Alendronate Sodium loaded with biodegradable polymer. Lat. Am. J. Pharm. 2018, 59, 9–20. [Google Scholar]
- Lombardo, D.; Calandra, P.; Caccamo, M.T.; Magazù, S.; Kiselev, M.A.; Lombardo, D.; Calandra, P.; Caccamo, M.; Magazù, S.; Kiselev, M. Colloidal stability of liposomes. AIMS Mater. Sci. 2019, 6, 200–213. [Google Scholar] [CrossRef]
- Smith, M.C.; Crist, R.M.; Clogston, J.D.; McNeil, S.E. Zeta potential: A case study of cationic, anionic, and neutral liposomes. Anal. Bioanal. Chem. 2017, 409, 5779–5787. [Google Scholar] [CrossRef]
- Immordino, M.L.; Dosio, F.; Cattel, L. Stealth liposomes: Review of the basic science, rationale, and clinical applications, existing and potential. Int. J. Nanomed. 2006, 1, 297. [Google Scholar]
- Liu, P.; Chen, G.; Zhang, J. A Review of Liposomes as a Drug Delivery System: Current Status of Approved Products, Regulatory Environments, and Future Perspectives. Molecules 2022, 27, 1372. [Google Scholar] [CrossRef]
- Yadav, D.; Sandeep, K.; Pandey, D.; Dutta, R.K. Liposomes for drug delivery. J. Biotechnol. Biomater. 2017, 7, 276. [Google Scholar] [CrossRef]
- Hua, S. Comparison of in vitro dialysis release methods of loperamide-encapsulated liposomal gel for topical drug delivery. Int. J. Nanomed. 2014, 9, 735–744. [Google Scholar] [CrossRef]
- Miao, Z.L.; Deng, Y.J.; Du, H.Y.; Suo, X.B.; Wang, X.Y.; Wang, X.; Wang, L.; Cui, L.J.; Duan, N. Preparation of a liposomal delivery system and its in vitro release of rapamycin. Exp. Ther. Med. 2015, 9, 941–946. [Google Scholar] [CrossRef]
- Haghiralsadat, F.; Amoabediny, G.; Helder, M.N.; Naderinezhad, S.; Sheikhha, M.H.; Forouzanfar, T.; Zandieh-Doulabi, B. A comprehensive mathematical model of drug release kinetics from nano-liposomes, derived from optimization studies of cationic PEGylated liposomal doxorubicin formulations for drug-gene delivery. Artif. Cells Nanomed. Biotechnol. 2018, 46, 169–177. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, D.; Zhang, X.; Liu, Z.; Dai, K.; Ji, B.; Wang, Q.; Luo, L. Antitumor drug effect of betulinic acid mediated by polyethylene glycol modified liposomes. Mater. Sci. Eng. C 2016, 64, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Ramalho, M.J.; Bravo, M.; Loureiro, J.A.; Lima, J.; Pereira, M.C. Transferrin-modified nanoparticles for targeted delivery of Asiatic acid to glioblastoma cells. Life Sci. 2022, 296, 120435. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, R.G.R.; Granja, A.; Loureiro, J.A.; Pereira, M.C.; Pinheiro, M.; Neves, A.R.; Reis, S. Quercetin lipid nanoparticles functionalized with transferrin for Alzheimer’s disease. Eur. J. Pharm. Sci. 2020, 148, 105314. [Google Scholar] [CrossRef]
- Aubrey, L.D.; Blakeman, B.J.; Lutter, L.; Serpell, C.J.; Tuite, M.F.; Serpell, L.C.; Xue, W.-F. Quantification of amyloid fibril polymorphism by nano-morphometry reveals the individuality of filament assembly. Commun. Chem. 2020, 3, 1–10. [Google Scholar] [CrossRef]


| Technique | Liposomes’ Formulation | Size (nm) | PdI | Zeta Potential (mV) | EE (%) | LC (%) |
|---|---|---|---|---|---|---|
| Lipid film hydration | Unloaded LIP | 117 ± 6 | 0.17 ± 0.03 | −2.3 ± 0.6 | - | - |
| GA-loaded LIP | 112 ± 4 | 0.16 ± 0.02 | −2.2 ± 0.1 | 12 ± 2 | 0.7 ± 0.1 | |
| Reverse-phase evaporation | Unloaded LIP | 129 ± 10 | 0.17 ± 0.04 | −0.7 ± 1.0 | - | - |
| GA-loaded LIP | 126 ± 10 | 0.18 ± 0.02 | −0.4 ± 0.8 | 29 ± 3 | 1.7 ± 0.2 | |
| Ethanol injection | Unloaded LIP | 163 ± 4 | 0.20 ± 0.03 | −1.9 ± 0.5 | - | - |
| GA-loaded LIP | 150 ± 12 | 0.21 ± 0.07 | −1.2 ± 0.4 | 11 ± 4 | 0.6 ± 0.2 | |
| Ethanol permeabilization | Unloaded LIP | 145 ± 2 | 0.19 ± 0.01 | −0.8 ± 0.4 | - | - |
| GA-loaded LIP | 147 ± 5 | 0.16 ± 0.03 | −1.7 ± 0.1 | 15 ± 4 | 0.9 ± 0.3 |
| Formulation | Size (nm) | PdI | Zeta Potential (mV) | EE (%) | LC (%) | CE (%) |
|---|---|---|---|---|---|---|
| Unloaded LIP | 129 ± 10 | 0.17 ± 0.04 | −0.7 ± 1.0 | - | - | - |
| Unloaded Tf-LIP | 132 ± 4 | 0.15 ± 0.02 | −1.3 ± 0.5 | - | - | 35 ± 3 |
| GA-loaded LIP | 126 ± 10 | 0.18 ± 0.02 | −0.4 ± 0.8 | 29 ± 3 | 1.7 ± 0.2 | - |
| GA-loaded Tf-LIP | 131 ± 9 | 0.16 ± 0.02 | −0.8 ± 1.0 | 31 ± 6 | 1.8 ± 0.4 | 26 ± 3 * |
| Kinetic Parameters | Aβ | Aβ + Tf-LIP | Aβ + GA-Tf-LIP |
|---|---|---|---|
| tlag (h) | 0.015 ± 0.002 | 0.9 ± 0.4 * | 1.4 ± 0.03 * |
| t1/2 (h) | 0.10 ± 0.01 | 2.0 ± 0.5 * | 2.2 ± 0.3 * |
| k (h−1) | 22.7 ± 0.6 | 1.9 ± 0.1 * | 4.9 ± 3.4 * |
| Max. Fluorescence | 0.9 ± 0.1 | 1.7 ± 0.3 * | 0.5 ± 0.2 *,# |
| Aβ Fibril Content (%) | |||
|---|---|---|---|
| Time (h) | Aβ + GA | Aβ + Tf-LIP | Aβ + GA-Tf-LIP |
| 0 | 54 ± 5 * | 129 ± 2 * | 92 ± 4 § |
| 3 | 1 ± 1 *,# | 109 ± 3 # | 70 ± 5 *,#,§ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrade, S.; Loureiro, J.A.; Pereira, M.C. Transferrin-Functionalized Liposomes for the Delivery of Gallic Acid: A Therapeutic Approach for Alzheimer’s Disease. Pharmaceutics 2022, 14, 2163. https://doi.org/10.3390/pharmaceutics14102163
Andrade S, Loureiro JA, Pereira MC. Transferrin-Functionalized Liposomes for the Delivery of Gallic Acid: A Therapeutic Approach for Alzheimer’s Disease. Pharmaceutics. 2022; 14(10):2163. https://doi.org/10.3390/pharmaceutics14102163
Chicago/Turabian StyleAndrade, Stéphanie, Joana A. Loureiro, and Maria C. Pereira. 2022. "Transferrin-Functionalized Liposomes for the Delivery of Gallic Acid: A Therapeutic Approach for Alzheimer’s Disease" Pharmaceutics 14, no. 10: 2163. https://doi.org/10.3390/pharmaceutics14102163
APA StyleAndrade, S., Loureiro, J. A., & Pereira, M. C. (2022). Transferrin-Functionalized Liposomes for the Delivery of Gallic Acid: A Therapeutic Approach for Alzheimer’s Disease. Pharmaceutics, 14(10), 2163. https://doi.org/10.3390/pharmaceutics14102163









