Novel Nanoparticles Based on N,O-Carboxymethyl Chitosan-Dopamine Amide Conjugate for Nose-to-Brain Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Quantitative Determination of Dopamine and N,O-Carboxymethylchitosan-DA Amide Conjugate (N,O-CMCS-DA)
2.3. Preparation of N,O-CMCS- or N,O-CMCS-DA-Loaded NPs (N,O-CMCS- or N,O-CMCS-DA-NPs) and Corresponding FITC Labelled NPs (FITC-N,O-CMCS or FITC-N,O-CMCS-DA NPs)
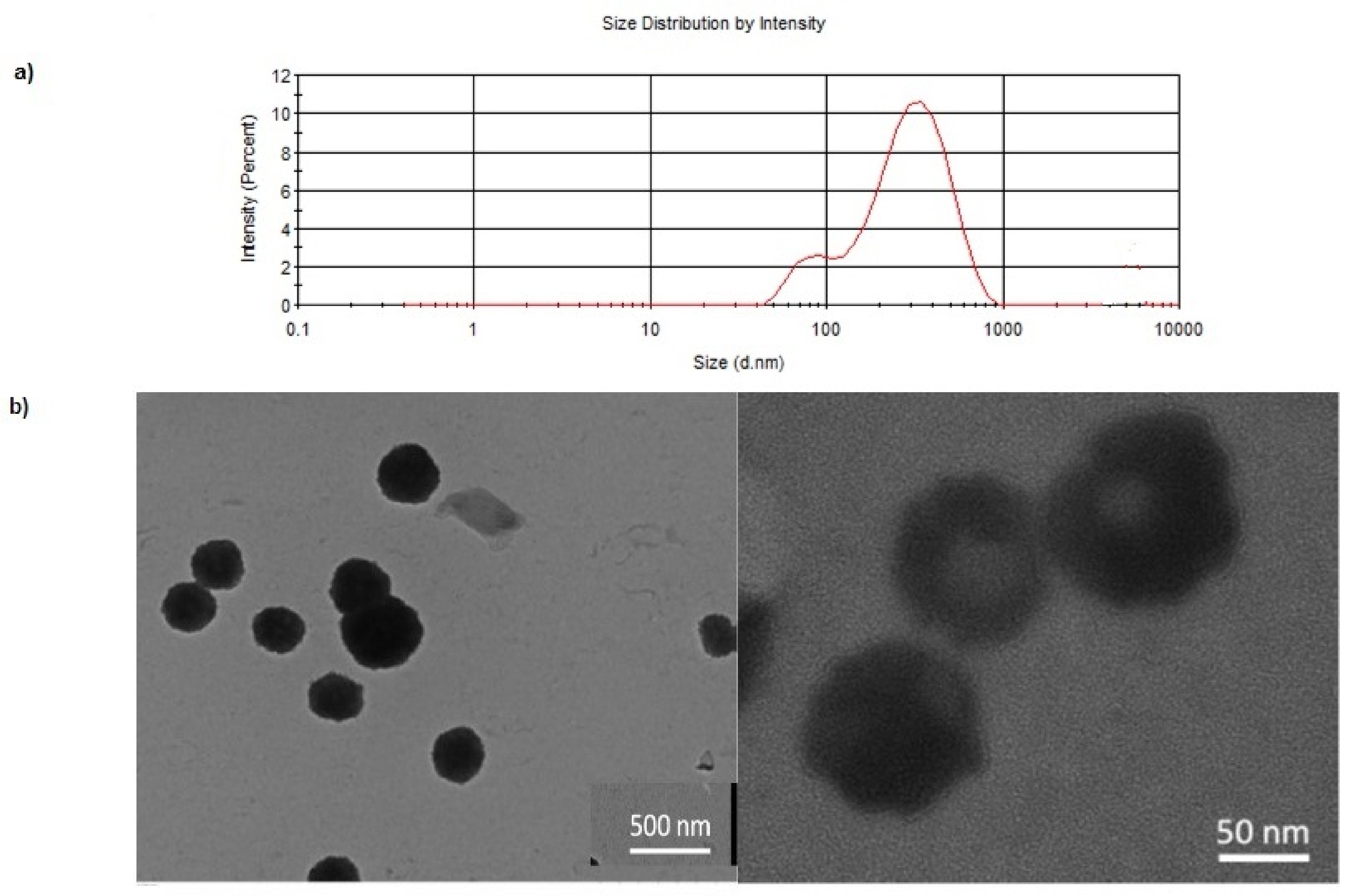
2.4. Physicochemical Characterization of Nanoparticles Prepared
2.5. Solid State Studies
2.5.1. Fourier Transform Infrared (FT-IR) Spectroscopy
2.5.2. Differential Scanning Calorimetry (DSC)
2.5.3. Thermogravimetric Analysis (TGA)
2.5.4. X-ray Photoelectron Spectroscopy (XPS)
2.6. Physical Stability of N,O-CMCS-DA NPs on Storage
2.7. In Vitro Release in Simulated Nasal Fluid/Mucin
2.8. Cytotoxicity Studies with Olfactory Ensheathing Cells (OECs)
2.9. Flow Cytometry
2.10. Epifluorescence Microscopy
2.11. Statistics
3. Results
3.1. Formulation and Characterization of DA/CSNPs
3.2. Solid State Studies
3.3. Physical Stability of N,O-CMCS-DA NPs on Storage
3.4. In Vitro Release in Simulated Nasal Fluid
3.5. Cytotoxicity Studies in OECs
3.6. Uptake Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, J.; Rudrapal, M.; Bhat, E.A.; Ali, A.; Alaidarous, M.; Alshehri, B.; Banwas, S.; Ismail, R.; Egbuna, C. Perspective Insights to Bio-Nanomaterials for the Treatment of Neurological Disorders. Front. Bioeng. Biotechnol. 2021, 9, 724158. [Google Scholar] [CrossRef]
- Saraiva, C.; Praca, C.; Ferreira, R.; Santos, T.; Ferreira, L.; Bernardino, L. Nanoparticle-mediated brain drug delivery: Overcoming blood-brain barrier to treat neurodegenerative diseases. J. Control. Release 2016, 235, 34–47. [Google Scholar] [CrossRef] [Green Version]
- Abbott, N.J.; Chugani, D.C.; Zaharchuk, G.; Rosen, B.R.; Lo, E.H. Delivery of imaging agents into brain. Adv. Drug Deliv. Rev. 1999, 37, 253–277. [Google Scholar] [CrossRef]
- Kanwar, J.R.; Sriramoju, B.; Kanwar, R.K. Neurological disorders and therapeutics targeted to surmount the blood-brain barrier. Int. J. Nanomed. 2012, 7, 3259–3278. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, M.; Saraf, S.; Saraf, S.; Antimisiaris, S.G.; Chougule, M.B.; Shoyele, S.A.; Alexander, A. Nose-to-brain drug delivery: An update on clinical challenges and progress towards approval of anti-Alzheimer drugs. J. Control. Release 2018, 281, 139–177. [Google Scholar] [CrossRef]
- Bourganis, V.; Kammona, O.; Alexopoulos, A.; Kiparissides, C. Recent advances in carrier mediated nose-to-brain delivery of pharmaceutics. Eur. J. Pharm. Biopharm. 2018, 128, 337–362. [Google Scholar] [CrossRef]
- Samaridou, E.; Alonso, M.J. Nose-to-brain peptide delivery—The potential of nanotechnology. Bioorg. Med. Chem. 2018, 26, 2888–2905. [Google Scholar] [CrossRef]
- Ganger, S.; Schindowski, K. Tailoring Formulations for Intranasal Nose-to-Brain Delivery: A Review on Architecture, Physico-Chemical Characteristics and Mucociliary Clearance of the Nasal Olfactory Mucosa. Pharmaceutics 2018, 10, 116. [Google Scholar] [CrossRef] [Green Version]
- Musani, I.E.; Chandan, N.V. A comparison of the sedative effect of oral versus nasal midazolam combined with nitrous oxide in uncooperative children. Eur. Arch. Paediatr. Dent. 2015, 16, 417–424. [Google Scholar] [CrossRef]
- Kaur, G.; Arora, M.; Ravi Kumar, M.N.V. Oral Drug Delivery Technologies-A Decade of Developments. J. Pharmacol. Exp. Ther. 2019, 370, 529–543. [Google Scholar] [CrossRef]
- Trapani, G.; Franco, M.; Trapani, A.; Lopedota, A.; Latrofa, A.; Gallucci, E.; Micelli, S.; Liso, G. Frog intestinal sac: A new in vitro method for the assessment of intestinal permeability. J. Pharm. Sci. 2004, 93, 2909–2919. [Google Scholar] [CrossRef]
- Mistry, A.; Stolnik, S.; Illum, L. Nanoparticles for direct nose-to-brain delivery of drugs. Int. J. Pharm. 2009, 379, 146–157. [Google Scholar] [CrossRef]
- Re, F.; Gregori, M.; Masserini, M. Nanotechnology for neurodegenerative disorders. Maturitas 2012, 73, 45–51. [Google Scholar] [CrossRef]
- Hawthorne, G.H.; Bernuci, M.P.; Bortolanza, M.; Tumas, V.; Issy, A.C.; Del-Bel, E. Nanomedicine to Overcome Current Parkinson’s Treatment Liabilities: A Systematic Review. Neurotox. Res. 2016, 30, 715–729. [Google Scholar] [CrossRef]
- Islam, S.U.; Shehzad, A.; Ahmed, M.B.; Lee, Y.S. Intranasal Delivery of Nanoformulations: A Potential Way of Treatment for Neurological Disorders. Molecules 2020, 25, 1929. [Google Scholar] [CrossRef] [Green Version]
- Reeve, A.; Simcox, E.; Turnbull, D. Ageing and Parkinson’s disease: Why is advancing age the biggest risk factor? Ageing Res. Rev. 2014, 14, 19–30. [Google Scholar] [CrossRef]
- Rodriguez-Nogales, C.; Garbayo, E.; Carmona-Abellan, M.M.; Luquin, M.R.; Blanco-Prieto, M.J. Brain aging and Parkinson’s disease: New therapeutic approaches using drug delivery systems. Maturitas 2016, 84, 25–31. [Google Scholar] [CrossRef]
- Mirzaei, S.; Kulkarni, K.; Zhou, K.; Crack, P.J.; Aguilar, M.I.; Finkelstein, D.I.; Forsythe, J.S. Biomaterial Strategies for Restorative Therapies in Parkinson’s Disease. ACS Chem. Neurosci. 2021, 12, 4224–4235. [Google Scholar] [CrossRef]
- Conese, M.; Cassano, R.; Gavini, E.; Trapani, G.; Rassu, G.; Sanna, E.; Di Gioia, S.; Trapani, A. Harnessing Stem Cells and Neurotrophic Factors with Novel Technologies in the Treatment of Parkinson’s Disease. Curr. Stem Cell Res. Ther. 2019, 14, 549–569. [Google Scholar] [CrossRef]
- Pillay, S.; Pillay, V.; Choonara, Y.E.; Naidoo, D.; Khan, R.A.; du Toit, L.C.; Ndesendo, V.M.; Modi, G.; Danckwerts, M.P.; Iyuke, S.E. Design, biometric simulation and optimization of a nano-enabled scaffold device for enhanced delivery of dopamine to the brain. Int. J. Pharm. 2009, 382, 277–290. [Google Scholar] [CrossRef]
- Trapani, A.; De Giglio, E.; Cafagna, D.; Denora, N.; Agrimi, G.; Cassano, T.; Gaetani, S.; Cuomo, V.; Trapani, G. Characterization and evaluation of chitosan nanoparticles for dopamine brain delivery. Int. J. Pharm. 2011, 419, 296–307. [Google Scholar] [CrossRef]
- De Giglio, E.; Trapani, A.; Cafagna, D.; Sabbatini, L.; Cometa, S. Dopamine-loaded chitosan nanoparticles: Formulation and analytical characterization. Anal. Bioanal. Chem. 2011, 400, 1997–2002. [Google Scholar] [CrossRef]
- Md, S.; Khan, R.A.; Mustafa, G.; Chuttani, K.; Baboota, S.; Sahni, J.K.; Ali, J. Bromocriptine loaded chitosan nanoparticles intended for direct nose to brain delivery: Pharmacodynamic, pharmacokinetic and scintigraphy study in mice model. Eur. J. Pharm. Sci. 2013, 48, 393–405. [Google Scholar] [CrossRef]
- Rashed, E.R.; Abd El-Rehim, H.A.; El-Ghazaly, M.A. Potential efficacy of dopamine loaded-PVP/PAA nanogel in experimental models of Parkinsonism: Possible disease modifying activity. J. Biomed. Mater. Res. A 2015, 103, 1713–1720. [Google Scholar] [CrossRef]
- Pahuja, R.; Seth, K.; Shukla, A.; Shukla, R.K.; Bhatnagar, P.; Chauhan, L.K.; Saxena, P.N.; Arun, J.; Chaudhari, B.P.; Patel, D.K.; et al. Trans-blood brain barrier delivery of dopamine-loaded nanoparticles reverses functional deficits in parkinsonian rats. ACS Nano 2015, 9, 4850–4871. [Google Scholar] [CrossRef]
- Monge-Fuentes, V.; Biolchi Mayer, A.; Lima, M.R.; Geraldes, L.R.; Zanotto, L.N.; Moreira, K.G.; Martins, O.P.; Piva, H.L.; Felipe, M.S.S.; Amaral, A.C.; et al. Dopamine-loaded nanoparticle systems circumvent the blood-brain barrier restoring motor function in mouse model for Parkinson’s Disease. Sci. Rep. 2021, 11, 15185. [Google Scholar] [CrossRef]
- Ekladious, I.; Colson, Y.L.; Grinstaff, M.W. Polymer-drug conjugate therapeutics: Advances, insights and prospects. Nat. Rev. Drug Discov. 2019, 18, 273–294. [Google Scholar] [CrossRef]
- Mandracchia, D.; Trapani, A.; Perteghella, S.; Sorrenti, M.; Catenacci, L.; Torre, M.L.; Trapani, G.; Tripodo, G. pH-sensitive inulin-based nanomicelles for intestinal site-specific and controlled release of celecoxib. Carbohydr. Polym. 2018, 181, 570–578. [Google Scholar] [CrossRef]
- Cassano, R.; Trapani, A.; Di Gioia, M.L.; Mandracchia, D.; Pellitteri, R.; Tripodo, G.; Trombino, S.; Di Gioia, S.; Conese, M. Synthesis and characterization of novel chitosan-dopamine or chitosan-tyrosine conjugates for potential nose-to-brain delivery. Int. J. Pharm. 2020, 589, 119829. [Google Scholar] [CrossRef]
- Di Gioia, S.; Trapani, A.; Cassano, R.; Di Gioia, M.L.; Trombino, S.; Cellamare, S.; Bolognino, I.; Hossain, M.N.; Sanna, E.; Trapani, G.; et al. Nose-to-brain delivery: A comparative study between carboxymethyl chitosan based conjugates of dopamine. Int. J. Pharm. 2021, 599, 120453. [Google Scholar] [CrossRef]
- Garcia-Pardo, J.; Novio, F.; Nador, F.; Cavaliere, I.; Suarez-Garcia, S.; Lope-Piedrafita, S.; Candiota, A.P.; Romero-Gimenez, J.; Rodriguez-Galvan, B.; Bove, J.; et al. Bioinspired Theranostic Coordination Polymer Nanoparticles for Intranasal Dopamine Replacement in Parkinson’s Disease. ACS Nano 2021, 15, 8592–8609. [Google Scholar] [CrossRef]
- Tripodo, G.; Trapani, A.; Rosato, A.; Di Franco, C.; Tamma, R.; Trapani, G.; Ribatti, D.; Mandracchia, D. Hydrogels for biomedical applications from glycol chitosan and PEG diglycidyl ether exhibit pro-angiogenic and antibacterial activity. Carbohydr. Polym. 2018, 198, 124–130. [Google Scholar] [CrossRef]
- Mandracchia, D.; Trapani, A.; Tripodo, G.; Perrone, M.G.; Giammona, G.; Trapani, G.; Colabufo, N.A. In vitro evaluation of glycol chitosan based formulations as oral delivery systems for efflux pump inhibition. Carbohydr. Polym. 2017, 166, 73–82. [Google Scholar] [CrossRef]
- Trapani, A.; Laquintana, V.; Lopedota, A.; Franco, M.; Latrofa, A.; Talani, G.; Sanna, E.; Trapani, G.; Liso, G. Evaluation of new propofol aqueous solutions for intravenous anesthesia. Int. J. Pharm. 2004, 278, 91–98. [Google Scholar] [CrossRef]
- Carta, F.; Vullo, D.; Maresca, A.; Scozzafava, A.; Supuran, C.T. Mono-/dihydroxybenzoic acid esters and phenol pyridinium derivatives as inhibitors of the mammalian carbonic anhydrase isoforms I, II, VII, IX, XII and XIV. Bioorg. Med. Chem. 2013, 21, 1564–1569. [Google Scholar] [CrossRef] [Green Version]
- Musumeci, T.; Ventura, C.A.; Giannone, I.; Ruozi, B.; Montenegro, L.; Pignatello, R.; Puglisi, G. PLA/PLGA nanoparticles for sustained release of docetaxel. Int. J. Pharm. 2006, 325, 172–179. [Google Scholar] [CrossRef]
- Trapani, A.; Corbo, F.; Agrimi, G.; Ditaranto, N.; Cioffi, N.; Perna, F.; Quivelli, A.; Stefano, E.; Lunetti, P.; Muscella, A.; et al. Oxidized Alginate Dopamine Conjugate: In Vitro Characterization for Nose-to-Brain Delivery Application. Materials 2021, 14, 3495. [Google Scholar] [CrossRef]
- Trapani, A.; Guerra, L.; Corbo, F.; Castellani, S.; Sanna, E.; Capobianco, L.; Monteduro, A.G.; Manno, D.E.; Mandracchia, D.; Di Gioia, S.; et al. Cyto/Biocompatibility of Dopamine Combined with the Antioxidant Grape Seed-Derived Polyphenol Compounds in Solid Lipid Nanoparticles. Molecules 2021, 26, 916. [Google Scholar] [CrossRef]
- Trapani, A.; Catalano, A.; Carocci, A.; Carrieri, A.; Mercurio, A.; Rosato, A.; Mandracchia, D.; Tripodo, G.; Schiavone, B.I.P.; Franchini, C.; et al. Effect of Methyl-beta-Cyclodextrin on the antimicrobial activity of a new series of poorly water-soluble benzothiazoles. Carbohydr. Polym. 2019, 207, 720–728. [Google Scholar] [CrossRef]
- Pellitteri, R.; Spatuzza, M.; Russo, A.; Stanzani, S. Olfactory ensheathing cells exert a trophic effect on the hypothalamic neurons in vitro. Neurosci. Lett. 2007, 417, 24–29. [Google Scholar] [CrossRef]
- Musumeci, T.; Pellitteri, R.; Spatuzza, M.; Puglisi, G. Nose-to-brain delivery: Evaluation of polymeric nanoparticles on olfactory ensheathing cells uptake. J. Pharm. Sci. 2014, 103, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Castellani, S.; Trapani, A.; Spagnoletta, A.; di Toma, L.; Magrone, T.; Di Gioia, S.; Mandracchia, D.; Trapani, G.; Jirillo, E.; Conese, M. Nanoparticle delivery of grape seed-derived proanthocyanidins to airway epithelial cells dampens oxidative stress and inflammation. J. Transl. Med. 2018, 16, 140. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.K.; Prud’homme, R.K. Nanoparticle size distribution quantification from transmission electron microscopy (TEM) of ruthenium tetroxide stained polymeric nanoparticles. J. Colloid Interface Sci. 2021, 604, 208–220. [Google Scholar] [CrossRef] [PubMed]
- Mourya, V.K.; Inamdar, N.N.; Tiwari, A. Carboxymethyl chitosan and its applications. Adv. Mat. Lett. 2010, 1, 11–33. [Google Scholar] [CrossRef]
- Bukzem, A.L.; Signini, R.; Dos Santos, D.M.; Liao, L.M.; Ascheri, D.P. Optimization of carboxymethyl chitosan synthesis using response surface methodology and desirability function. Int. J. Biol. Macromol. 2016, 85, 615–624. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. Dopamine Hydrochloride; National Library of Medicine: Bethesda, MD, USA, 2021.
- Perteghella, S.; Mandracchia, D.; Torre, M.L.; Tamma, R.; Ribatti, D.; Trapani, A.; Tripodo, G. Anti-angiogenic activity of N,O-carboxymethyl-chitosan surface modified solid lipid nanoparticles for oral delivery of curcumin. J. Drug Deliv. Sci. Technol. 2020, 56, 101494. [Google Scholar] [CrossRef]
- Trapani, A.; Mandracchia, D.; Tripodo, G.; Cometa, S.; Cellamare, S.; De Giglio, E.; Klepetsanis, P.; Antimisiaris, S.G. Protection of dopamine towards autoxidation reaction by encapsulation into non-coated- or chitosan- or thiolated chitosan-coated-liposomes. Colloids Surf. B Biointerfaces 2018, 170, 11–19. [Google Scholar] [CrossRef]
- Tang, S.; Wang, A.; Yan, X.; Chu, L.; Yang, X.; Song, Y.; Sun, K.; Yu, X.; Liu, R.; Wu, Z.; et al. Brain-targeted intranasal delivery of dopamine with borneol and lactoferrin co-modified nanoparticles for treating Parkinson’s disease. Drug Deliv. 2019, 26, 700–707. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Cui, X.; Ke, P.C.; Mortimer, M.; Wang, X.; Bao, L.; Chen, C. Nanomaterials as novel agents for amelioration of Parkinson’s disease. NanoToday 2021, 41, 101328. [Google Scholar] [CrossRef]
- Craparo, E.F.; Musumeci, T.; Bonaccorso, A.; Pellitteri, R.; Romeo, A.; Naletova, I.; Cucci, L.M.; Cavallaro, G.; Satriano, C. mPEG-PLGA Nanoparticles Labelled with Loaded or Conjugated Rhodamine-B for Potential Nose-to-Brain Delivery. Pharmaceutics 2021, 13, 1508. [Google Scholar] [CrossRef]
- Lammari, N.; Louaer, O.; Meniai, A.H.; Elaissari, A. Encapsulation of Essential Oils via Nanoprecipitation Process: Overview, Progress, Challenges and Prospects. Pharmaceutics 2020, 12, 431. [Google Scholar] [CrossRef]
- Chen, X.-G.; Tang, H.-J. Chemical characteristics of O-carboxymethyl chitosans related to the preparation conditions. Carbohydr. Polym. 2003, 53, 355–359. [Google Scholar] [CrossRef]
- Cometa, S.; Bonifacio, M.A.; Trapani, G.; Di Gioia, S.; Dazzi, L.; De Giglio, E.; Trapani, A. In vitro investigations on dopamine loaded Solid Lipid Nanoparticles. J. Pharm. Biomed. Anal. 2020, 185, 113257. [Google Scholar] [CrossRef]
- Lethem, M.I. The role of tracheal bronchial mucus in drug administration to the airway. Adv. Drug Deliv. Rev. 1993, 11, 271–298. [Google Scholar] [CrossRef]
- Palazzo, C.; Trapani, G.; Ponchel, G.; Trapani, A.; Vauthier, C. Mucoadhesive properties of low molecular weight chitosan- or glycol chitosan- and corresponding thiomer-coated poly(isobutylcyanoacrylate) core-shell nanoparticles. Eur. J. Pharm. Biopharm. 2017, 117, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Trapani, A.; Palazzo, C.; Contino, M.; Perrone, M.G.; Cioffi, N.; Ditaranto, N.; Colabufo, N.A.; Conese, M.; Trapani, G.; Puglisi, G. Mucoadhesive properties and interaction with P-glycoprotein (P-gp) of thiolated-chitosans and -glycol chitosans and corresponding parent polymers: A comparative study. Biomacromolecules 2014, 15, 882–893. [Google Scholar] [CrossRef]
- Behzadi, S.; Serpooshan, V.; Sakhtianchi, R.; Muller, B.; Landfester, K.; Crespy, D.; Mahmoudi, M. Protein corona change the drug release profile of nanocarriers: The “overlooked” factor at the nanobio interface. Colloids Surf. B Biointerfaces 2014, 123, 143–149. [Google Scholar] [CrossRef]
- Monopoli, M.P.; Walczyk, D.; Campbell, A.; Elia, G.; Lynch, I.; Bombelli, F.B.; Dawson, K.A. Physical-chemical aspects of protein corona: Relevance to in vitro and in vivo biological impacts of nanoparticles. J. Am. Chem. Soc. 2011, 133, 2525–2534. [Google Scholar] [CrossRef]
- Fleischer, C.C.; Payne, C.K. Secondary structure of corona proteins determines the cell surface receptors used by nanoparticles. J. Phys. Chem. B 2014, 118, 14017–14026. [Google Scholar] [CrossRef]
- Lai, W.; Li, D.; Wang, Q.; Nan, X.; Xiang, Z.; Ma, Y.; Liu, Y.; Chen, J.; Tian, J.; Fang, Q. A Protein Corona Adsorbed to a Bacterial Magnetosome Affects Its Cellular Uptake. Int. J. Nanomed. 2020, 15, 1481–1498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corbo, C.; Molinaro, R.; Parodi, A.; Toledano Furman, N.E.; Salvatore, F.; Tasciotti, E. The impact of nanoparticle protein corona on cytotoxicity, immunotoxicity and target drug delivery. Nanomedicine 2016, 11, 81–100. [Google Scholar] [CrossRef] [Green Version]
- Bruckner, M.; Simon, J.; Jiang, S.; Landfester, K.; Mailander, V. Preparation of the protein corona: How washing shapes the proteome and influences cellular uptake of nanocarriers. Acta Biomater. 2020, 114, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Cone, R.A. Barrier properties of mucus. Adv. Drug Deliv. Rev. 2009, 61, 75–85. [Google Scholar] [CrossRef]
- Ji, Y.; Wang, Y.; Shen, D.; Kang, Q.; Chen, L. Mucin corona delays intracellular trafficking and alleviates cytotoxicity of nanoplastic-benzopyrene combined contaminant. J. Hazard. Mater. 2021, 406, 124306. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Liu, D.; Qin, M.; Chen, B.; Song, S.; Dai, W.; Zhang, H.; Wang, X.; Wang, Y.; He, B.; et al. Intestinal Mucin Induces More Endocytosis but Less Transcytosis of Nanoparticles across Enterocytes by Triggering Nanoclustering and Strengthening the Retrograde Pathway. ACS Appl. Mater. Interfaces 2018, 10, 11443–11456. [Google Scholar] [CrossRef]
- Pardeshi, C.V.; Belgamwar, V.S. Direct nose to brain drug delivery via integrated nerve pathways bypassing the blood-brain barrier: An excellent platform for brain targeting. Expert Opin. Drug Deliv. 2013, 10, 957–972. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xiong, G.; Tsang, W.C.; Schätzlein, A.G.; Uchegbu, I.F. Nose-to-Brain Delivery. J. Pharmacol. Exp. Ther. 2019, 370, 593–601. [Google Scholar] [CrossRef] [Green Version]
- Sonvico, F.; Clementino, A.; Buttini, F.; Colombo, G.; Pescina, S.; Stanisçuaski Guterres, S.; Raffin Pohlmann, A.; Nicoli, S. Surface-Modified Nanocarriers for Nose-to-Brain Delivery: From Bioadhesion to Targeting. Pharmaceutics 2018, 10, 34. [Google Scholar] [CrossRef] [Green Version]
- Maaz, A.; Blagbrough, I.S.; De Bank, P.A. In Vitro Evaluation of Nasal Aerosol Depositions: An Insight for Direct Nose to Brain Drug Delivery. Pharmaceutics 2021, 13, 1079. [Google Scholar] [CrossRef]








| Formulation | Size (nm) | PDI a | Zeta Potential (mV) | E.E. DA (%) | E.E. FITC (%) |
|---|---|---|---|---|---|
| N,O-CMCS NPs | 608 ± 58 | 0.47–0.62 | −9.2 ± 0.7 | - | - |
| N,O-CMCS-DA NPs | 289 ± 50 | 0.48–0.54 | −32.4 ± 1.6 | 94 ± 3 | - |
| FITC-N,O-CMCS NPs | 252 ± 33 | 0.50–0.59 | −20.3 ± 1.0 | - | 99.9 ± 0.0 |
| FITC-N,O-CMCS-DA NPs | 425 ± 28 | 0.34–0.36 | −14.2 ± 1.6 | 89 ± 2 | 97.4 ± 0.7 |
| Sample | Atomic Percentage % | |||||
|---|---|---|---|---|---|---|
| C1s | O1s | N1s | Cl2p | Na1s | Si2p | |
| pure DA•HCl | 71.7 | 15.3 | 6.8 | 6.1 | -- | -- |
| CMCS | 51.5 | 32.3 | 8.8 | 3.8 | 3.6 | -- |
| N,O-CMCS-DA NPs | 74.2 | 22.2 | 0.6 | 0.8 | 0.7 | 1.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trapani, A.; Cometa, S.; De Giglio, E.; Corbo, F.; Cassano, R.; Di Gioia, M.L.; Trombino, S.; Hossain, M.N.; Di Gioia, S.; Trapani, G.; et al. Novel Nanoparticles Based on N,O-Carboxymethyl Chitosan-Dopamine Amide Conjugate for Nose-to-Brain Delivery. Pharmaceutics 2022, 14, 147. https://doi.org/10.3390/pharmaceutics14010147
Trapani A, Cometa S, De Giglio E, Corbo F, Cassano R, Di Gioia ML, Trombino S, Hossain MN, Di Gioia S, Trapani G, et al. Novel Nanoparticles Based on N,O-Carboxymethyl Chitosan-Dopamine Amide Conjugate for Nose-to-Brain Delivery. Pharmaceutics. 2022; 14(1):147. https://doi.org/10.3390/pharmaceutics14010147
Chicago/Turabian StyleTrapani, Adriana, Stefania Cometa, Elvira De Giglio, Filomena Corbo, Roberta Cassano, Maria Luisa Di Gioia, Sonia Trombino, Md Niamat Hossain, Sante Di Gioia, Giuseppe Trapani, and et al. 2022. "Novel Nanoparticles Based on N,O-Carboxymethyl Chitosan-Dopamine Amide Conjugate for Nose-to-Brain Delivery" Pharmaceutics 14, no. 1: 147. https://doi.org/10.3390/pharmaceutics14010147
APA StyleTrapani, A., Cometa, S., De Giglio, E., Corbo, F., Cassano, R., Di Gioia, M. L., Trombino, S., Hossain, M. N., Di Gioia, S., Trapani, G., & Conese, M. (2022). Novel Nanoparticles Based on N,O-Carboxymethyl Chitosan-Dopamine Amide Conjugate for Nose-to-Brain Delivery. Pharmaceutics, 14(1), 147. https://doi.org/10.3390/pharmaceutics14010147













