Instillation of Ophthalmic Formulation Containing Nilvadipine Nanocrystals Attenuates Lens Opacification in Shumiya Cataract Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Chemicals
2.3. Preparation of NIL-NP Dispersions
2.4. Measurement of NIL
2.5. Characteristics of Ophthalmic Formulations Containing NIL
2.6. Solubility of NIL in the Ophthalmic Formulations
2.7. Dispersibility of NIL in the Ophthalmic Formulations
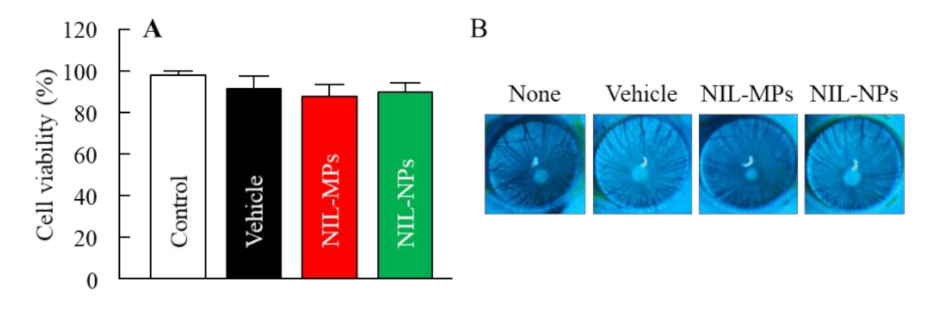
2.8. Corneal Stimulation of NIL Ophthalmic Formulations in Cultured Human Corneal Epithelial Cells
2.9. Corneal Toxicity of NIL Ophthalmic Formulations in the Rats
2.10. Measurement of NIL Content in the Lenses
2.11. Scheimpflug Slit Images in the SCR
2.12. Evaluation of Cataract-Related Factors
2.13. Measurement of Blood Pressure (BP)
2.14. Statistical Analysis
3. Results
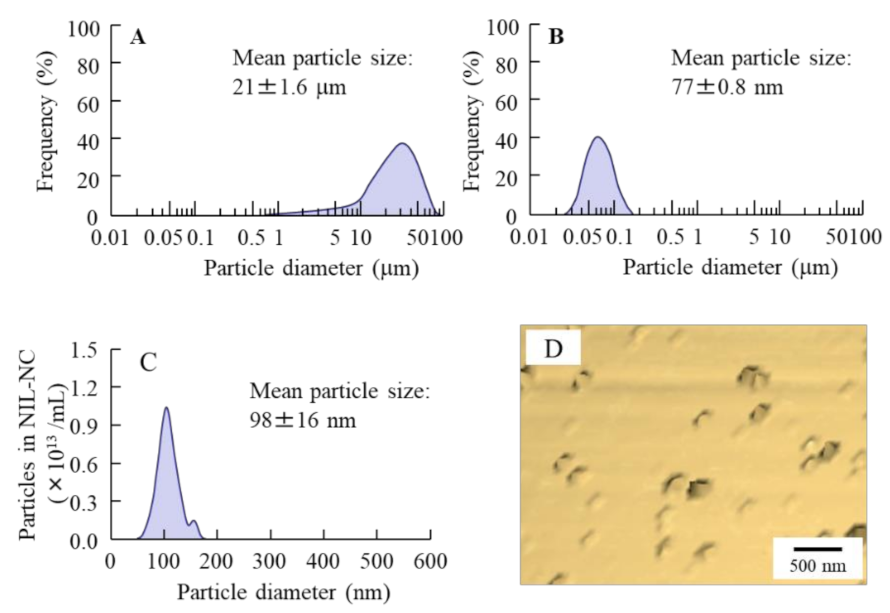
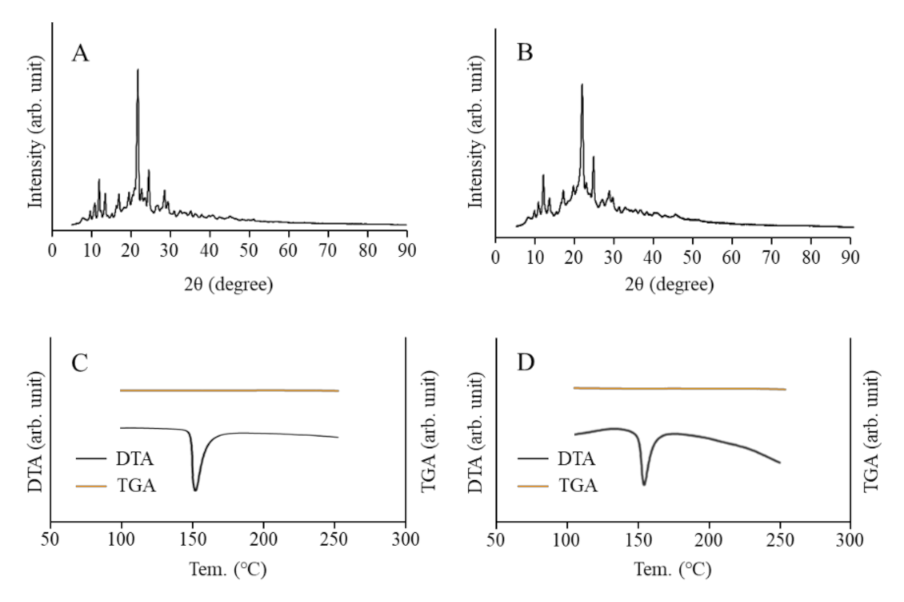
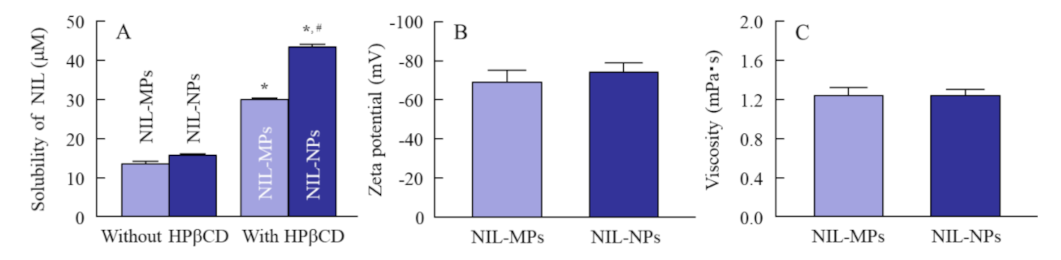
3.1. Physical Properties of NIL-NP Dispersions
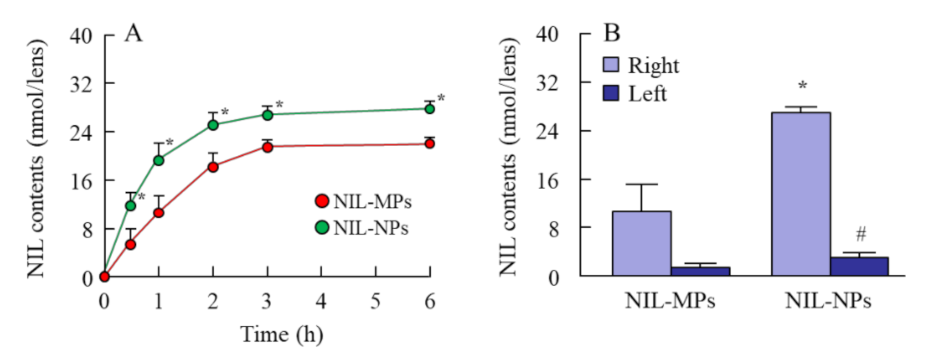
3.2. Drug Delivery to Lens by the Instillation of NIL-NP Dispersions
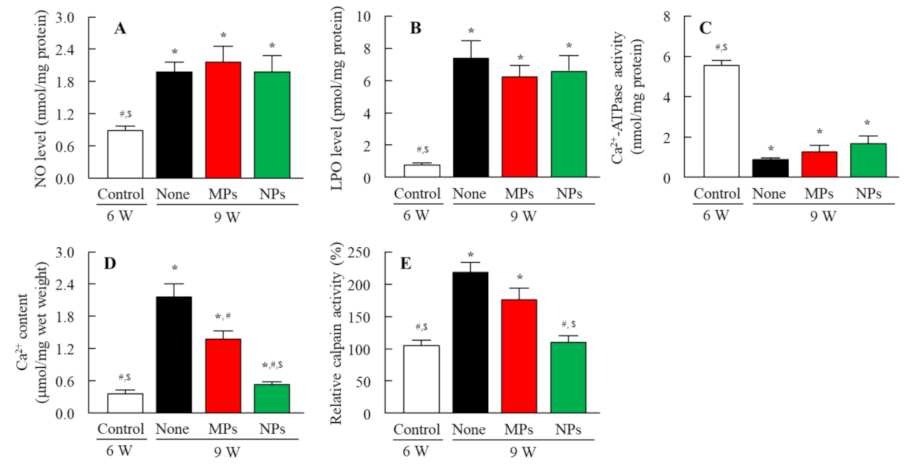
3.3. Delay Effect of NIL-NPs on the Onset of Lens Opacification in the SCR
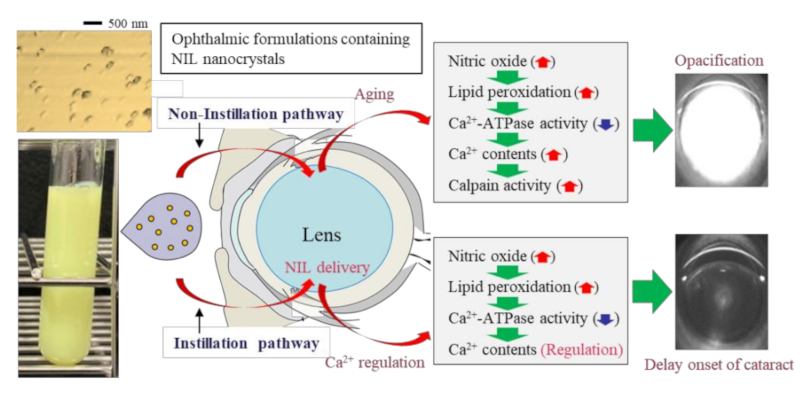
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Vision 2020 the Right to Sight. Global Initiative for the Elimination of Avoidable Blindness. Available online: https://www.who.int/blindness/Vision2020_report.pdf (accessed on 1 November 2021).
- Garland, D. Role of site-specific, metal-catalyzed oxidation in lens aging and cataract: A hypothesis. Exp. Eye Res. 1990, 50, 677–682. [Google Scholar] [CrossRef]
- Rasi, V.; Costantini, S.; Moramarco, A.; Giordano, R.; Giustolisi, R.; Gabrieli, C.B. Inorganic element concentrations in cataractous human lenses. Ann. Ophthalmol. 1992, 24, 459–464. [Google Scholar]
- Ye, J.; Zadunaisky, J. Study of the Ca2+/Na+ exchange mechanism in vesicles isolated from apical membranes of lens epithelium of spiny dogfish (Squalus acanthias) and bovine eye. Exp. Eye Res. 1992, 55, 243–250. [Google Scholar] [CrossRef]
- Cekic, O.; Bardak, Y. Lenticular calcium, magnesium, and iron levels in diabetic rats and verapamil effect. Ophthalmic. Res. 1998, 30, 107–112. [Google Scholar] [CrossRef]
- Dilsiz, N.; Olcucu, A.; Atas, M. Determination of calcium, sodium, potassium and magnesium concentrations in human senile cataractous lenses. Cell Biochem. Funct. 2000, 18, 259–262. [Google Scholar] [CrossRef]
- Rhodes, J.D.; Sanderson, J. The mechanisms of calcium homeostasis and signalling in the lens. Exp. Eye Res. 2009, 88, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Duncan, G.; Collison, D.J. Calcium signalling in ocular tissues: Functional activity of G-protein and tyrosine-kinase coupled receptors. Exp. Eye Res. 2002, 75, 377–389. [Google Scholar] [CrossRef]
- Shearer, T.R.; Ma, H.; Fukiage, C.; Azuma, M. Selenite nuclear cataract: Review of the model. Mol. Vis. 1997, 23, 8–17. [Google Scholar]
- Sanderson, J.; Marcantonio, J.M.; Duncan, G. A human lens model of cortical cataract: Ca2+-induced protein loss, vimentin cleavage and opacification. Investig. Ophthalmol. Vis. Sci. 2000, 41, 2255–2261. [Google Scholar]
- Truscott, R.J.; Marcantonio, J.M.; Tomlinson, J.; Duncan, G. Calcium-induced opacification and proteolysis in the intact rat lens. Investig. Ophthalmol. Vis. Sci. 1990, 31, 2405–2411. [Google Scholar]
- Nakamura, Y.; Fukiage, C.; Shih, M.; Ma, H.; David, L.L.; Azuma, M.; Shearer, T.R. Contribution of calpain Lp82-induced proteolysis to experimental cataractogenesis in mice. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1460–1466. [Google Scholar]
- Ohtsuka, M.; Ono, T.; Hiroi, J.; Esumi, K.; Kikuchi, H.; Kumada, S. Comparison of the Cardiovascular Effect of FR34235, a New Dihydropyridine, with Other Calcium Antagonists. J. Cardiovasc. Pharmacol. 1983, 5, 1074–1082. [Google Scholar] [CrossRef] [PubMed]
- Maddala, R.; Nagendran, T.; De Ridder, G.G.; Schey, K.L.; Rao, P.V. L-Type Calcium Channels Play a Critical Role in Maintaining Lens Transparency by Regulating Phosphorylation of Aquaporin-0 and Myosin Light Chain and Expression of Connexins. PLoS ONE 2013, 8, e64676. [Google Scholar] [CrossRef]
- Urtti, A. Challenges and obstacles of ocular pharmacokinetics and drug delivery. Adv. Drug Deliv. Rev. 2006, 58, 1131–1135. [Google Scholar] [CrossRef]
- Mannermaa, E.; Vellonen, K.-S.; Urtti, A. Drug transport in corneal epithelium and blood–retina barrier: Emerging role of transporters in ocular pharmacokinetics. Adv. Drug Deliv. Rev. 2006, 58, 1136–1163. [Google Scholar] [CrossRef]
- Hagigit, T.; Abdulrazik, M.; Orucov, F.; Valamanesh, F.; Lambert, M.; Lambert, G.; Behar-Cohen, F.; Benita, S. Topical and intravitreous administration of cationic nanoemulsions to deliver antisense oligonucleotides directed towards VEGF KDR receptors to the eye. J. Control. Release 2010, 145, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Gan, Y.; Zhu, C.; Zhang, X.; Zhu, J. Novel microemulsion in situ electrolyte-triggered gelling system for ophthalmic delivery of lipophilic cyclosporine A: In vitro and in vivo results. Int. J. Pharm. 2009, 365, 143–149. [Google Scholar] [CrossRef]
- Sun, D.; Maeno, H.; Gujrati, M.; Schur, R.; Maeda, A.; Maeda, T.; Palczewski, K.; Lu, Z.-R. Self-Assembly of a Multifunctional Lipid with Core-Shell Dendrimer DNA Nanoparticles Enhanced Efficient Gene Delivery at Low Charge Ratios into RPE Cells. Macromol. Biosci. 2015, 15, 1663–1672. [Google Scholar] [CrossRef]
- Hironaka, K.; Inokuchi, Y.; Fujisawa, T.; Shimazaki, H.; Akane, M.; Tozuka, Y.; Tsuruma, K.; Shimazawa, M.; Hara, H.; Takeuchi, H. Edaravone-loaded liposomes for retinal protection against oxidative stress-induced retinal damage. Eur. J. Pharm. Biopharm. 2011, 79, 119–125. [Google Scholar] [CrossRef]
- Shen, J.; Wang, Y.; Ping, Q.; Xiao, Y.; Huang, X. Mucoadhesive effect of thiolated PEG stearate and its modified NLC for ocular drug delivery. J. Control. Release 2009, 137, 217–223. [Google Scholar] [CrossRef]
- Otake, H.; Goto, R.; Ogata, F.; Isaka, T.; Kawasaki, N.; Kobayakawa, S.; Matsunaga, T.; Nagai, N. Fixed-Combination Eye Drops Based on Fluorometholone Nanoparticles and Bromfenac/Levofloxacin Solution Improve Drug Corneal Penetration. Int. J. Nanomed. 2021, 16, 5343–5356. [Google Scholar] [CrossRef]
- Nagai, N.; Isaka, T.; Deguchi, S.; Minami, M.; Yamaguchi, M.; Otake, H.; Okamoto, N.; Nakazawa, Y. In Situ Gelling Systems Using Pluronic F127 Enhance Corneal Permeability of Indomethacin Nanocrystals. Int. J. Mol. Sci. 2020, 21, 7083. [Google Scholar] [CrossRef]
- Nagai, N.; Ogata, F.; Ishii, M.; Fukuoka, Y.; Otake, H.; Nakazawa, Y.; Kawasaki, N. Involvement of Endocytosis in the Transdermal Penetration Mechanism of Ketoprofen Nanoparticles. Int. J. Mol. Sci. 2018, 19, 2138. [Google Scholar] [CrossRef]
- Nagai, N.; Minami, M.; Deguchi, S.; Otake, H.; Sasaki, H.; Yamamoto, N. An In Situ Gelling System Based on Methylcellulose and Tranilast Solid Nanoparticles Enhances Ocular Residence Time and Drug Absorption Into the Cornea and Conjunctiva. Front. Bioeng. Biotechnol. 2020, 8, 764. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Ogata, F.; Otake, H.; Nakazawa, Y.; Kawasaki, N. Energy-dependent endocytosis is responsible for drug transcorneal penetration following the instillation of ophthalmic formulations containing indomethacin nanoparticles. Int. J. Nanomed. 2019, 14, 1213–1227. [Google Scholar] [CrossRef]
- Tripathi, B.J.; Tripathi, R.C.; Borisuth, N.S.; Dhaliwal, R.; Dhaliwal, D. Rodent models of congenital and hereditary cataract in man. Lens Eye Toxic. Res. 1991, 8, 373–413. [Google Scholar] [PubMed]
- Nagai, N.; Ito, Y. Excessive hydrogen peroxide enhances the attachment of amyloid β1–42 in the lens epithelium of UPL rats, a hereditary model for cataracts. Toxicology 2014, 315, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Ito, Y. Adverse effects of excessive nitric oxide on cytochrome c oxidase in lenses of hereditary cataract UPL rats. Toxicology 2007, 242, 7–15. [Google Scholar] [CrossRef]
- Shumiya, S. Establishment of the hereditary cataract rat strain (SCR) and genetic analysis. Lab. Anim. Sci. 1995, 45, 671–673. [Google Scholar]
- Inomata, M.; Nomura, K.; Takehana, M.; Saido, T.C.; Kawashima, S.; Shumiya, S. Evidence for the involvement of calpain in cataractogenesis in Shumiya cataract rat (SCR). Biochim. Biophys. Acta Mol. Basis Dis. 1997, 1362, 11–23. [Google Scholar] [CrossRef]
- Nagai, N.; Ito, Y.; Inomata, M.; Shumiya, S.; Tai, H.; Hataguchi, Y.; Nakagawa, K. Delay of Cataract Development in the Shumiya Cataract Rat by the Administration of Drinking Water Containing High Concentration of Magnesium Ion. Biol. Pharm. Bull. 2006, 29, 1234–1238. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nagai, N.; Ito, Y.; Okamoto, N.; Shimomura, Y. A nanoparticle formulation reduces the corneal toxicity of indomethacin eye drops and enhances its corneal permeability. Toxicology 2014, 319, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Deguchi, S.; Ogata, F.; Watanabe, M.; Otake, H.; Yamamoto, N.; Kawasaki, N.; Nagai, N. Nanocrystalline Suspensions of Irbesartan Enhance Oral Bioavailability by Improving Drug Solubility and Leading Endocytosis Uptake into the Intestine. Pharmaceutics 2021, 13, 1404. [Google Scholar] [CrossRef]
- Minami, M.; Otake, H.; Nakazawa, Y.; Okamoto, N.; Yamamoto, N.; Sasaki, H.; Nagai, N. Balance of Drug Residence and Diffusion in Lacrimal Fluid Determine Ocular Bioavailability in In Situ Gels Incorporating Tranilast Nanoparticles. Pharmaceutics 2021, 13, 1425. [Google Scholar] [CrossRef]
- Le Bourlais, C.; Acar, L.; Zia, H.; Sado, P.A.; Needham, T.; Leverge, R. Ophthalmic drug delivery systems—Recent advances. Prog. Retin. Eye Res. 1998, 17, 33–58. [Google Scholar] [CrossRef]
- Nagai, N.; Ito, Y.; Takeuchi, N. Inhibitive effects of enhanced lipid peroxidation on Ca2+-ATPase in lenses of hereditary cataract ICR/f rats. Toxicology 2008, 247, 139–144. [Google Scholar] [CrossRef]
- Nagai, N.; Ito, Y.; Takeuchi, N.; Usui, S.; Hirano, K. Comparison of the Mechanisms of Cataract Development Involving Differences in Ca2+ Regulation in Lenses among Three Hereditary Cataract Model Rats. Biol. Pharm. Bull. 2008, 31, 1990–1995. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nagai, N.; Umachi, K.; Otake, H.; Oka, M.; Hiramatsu, N.; Sasaki, H.; Yamamoto, N. Ophthalmic in situ Gelling System Containing Lanosterol Nanoparticles Delays Collapse of Lens Structure in Shumiya Cataract Rats. Pharmaceutics 2020, 12, 629. [Google Scholar] [CrossRef]
- Nagai, N.; Yoshioka, C.; Tanino, T.; Ito, Y.; Okamoto, N.; Shimomura, Y. Decrease in Corneal Damage due to Benzalkonium Chloride by the Addition of Mannitol into Timolol Maleate Eye Drops. J. Oleo. Sci. 2015, 64, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Jansen, T.; Xhonneux, B.; Mesens, J.; Borgers, M. Beta-cyclodextrins as vehicles in eye-drop formulations: An evaluation of their effects on rabbit corneal epithelium. Lens Eye Toxic. Res. 1990, 7, 459–468. [Google Scholar] [PubMed]
- Kambhampati, S.P.; Kannan, R.M. Dendrimer Nanoparticles for Ocular Drug Delivery. J. Ocul. Pharmacol. Ther. 2013, 29, 151–165. [Google Scholar] [CrossRef]
- Duncan, G.; Bushell, A. Ion analyses of human cataractous lenses. Exp. Eye Res. 1975, 20, 223–230. [Google Scholar] [CrossRef]
- Spector, A. Oxidative stress-induced cataract: Mechanism of action. FASEB J. 1995, 9, 1173–1182. [Google Scholar] [CrossRef] [PubMed]
- Shearer, T.R.; David, L.L.; Anderson, R.S.; Azuma, M. Review of selenite cataract. Curr. Eye Res. 1992, 11, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Fukuoka, Y.; Sato, K.; Otake, H.; Taga, A.; Oka, M.; Hiramatsu, N.; Yamamoto, N. The Intravitreal Injection of Lanosterol Nanoparticles Rescues Lens Structure Collapse at an Early Stage in Shumiya Cataract Rats. Int. J. Mol. Sci. 2020, 21, 1048. [Google Scholar] [CrossRef]
- Nagai, N.; Ito, Y.; Takeuchi, N. Effect of Disulfiram Eye Drops on Lipid Peroxide Formation via Excessive Nitric Oxide in Lenses of Hereditary Cataract ICR/f Rats. Biol. Pharm. Bull. 2008, 31, 981–985. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Luo, L.-J.; Nguyen, D.D.; Lai, J.-Y. Dually functional hollow ceria nanoparticle platform for intraocular drug delivery: A push beyond the limits of static and dynamic ocular barriers toward glaucoma therapy. Biomaterials 2020, 243, 119961. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.-J.; Nguyen, D.D.; Lai, J.-Y. Harnessing the tunable cavity of nanoceria for enhancing Y-27632-mediated alleviation of ocular hypertension. Theranostics 2021, 11, 5447–5463. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goto, R.; Yamada, S.; Otake, H.; Nakazawa, Y.; Oka, M.; Yamamoto, N.; Sasaki, H.; Nagai, N. Instillation of Ophthalmic Formulation Containing Nilvadipine Nanocrystals Attenuates Lens Opacification in Shumiya Cataract Rats. Pharmaceutics 2021, 13, 1999. https://doi.org/10.3390/pharmaceutics13121999
Goto R, Yamada S, Otake H, Nakazawa Y, Oka M, Yamamoto N, Sasaki H, Nagai N. Instillation of Ophthalmic Formulation Containing Nilvadipine Nanocrystals Attenuates Lens Opacification in Shumiya Cataract Rats. Pharmaceutics. 2021; 13(12):1999. https://doi.org/10.3390/pharmaceutics13121999
Chicago/Turabian StyleGoto, Ryoka, Shigehiro Yamada, Hiroko Otake, Yosuke Nakazawa, Mikako Oka, Naoki Yamamoto, Hiroshi Sasaki, and Noriaki Nagai. 2021. "Instillation of Ophthalmic Formulation Containing Nilvadipine Nanocrystals Attenuates Lens Opacification in Shumiya Cataract Rats" Pharmaceutics 13, no. 12: 1999. https://doi.org/10.3390/pharmaceutics13121999
APA StyleGoto, R., Yamada, S., Otake, H., Nakazawa, Y., Oka, M., Yamamoto, N., Sasaki, H., & Nagai, N. (2021). Instillation of Ophthalmic Formulation Containing Nilvadipine Nanocrystals Attenuates Lens Opacification in Shumiya Cataract Rats. Pharmaceutics, 13(12), 1999. https://doi.org/10.3390/pharmaceutics13121999








