Stabilization by Nano Spray Dryer of Pioglitazone Polymeric Nanosystems: Development, In Vivo, Ex Vivo and Synchrotron Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Strategy
- i.
- Develop and select the most suitable PGZ-PLGA-PEG NP colloidal system based on physicochemical properties.
- ii.
- Dry the selected nanosystem with spray dryer Büchi Nano B-90 under different conditions and select the most suitable formulation in terms of physicochemical properties, in vitro release, and ex vivo corneal and scleral permeation in comparison to the colloidal system without drying.
- iii.
- iv.
- Small-angle X-ray scattering (SAXS) analysis in the corneal tissue.
2.3. Development and Preparation of PGZ-NPs
2.4. Spray Drying of PGZ-NPs
2.5. Physicochemical Characterization
2.6. Water Content
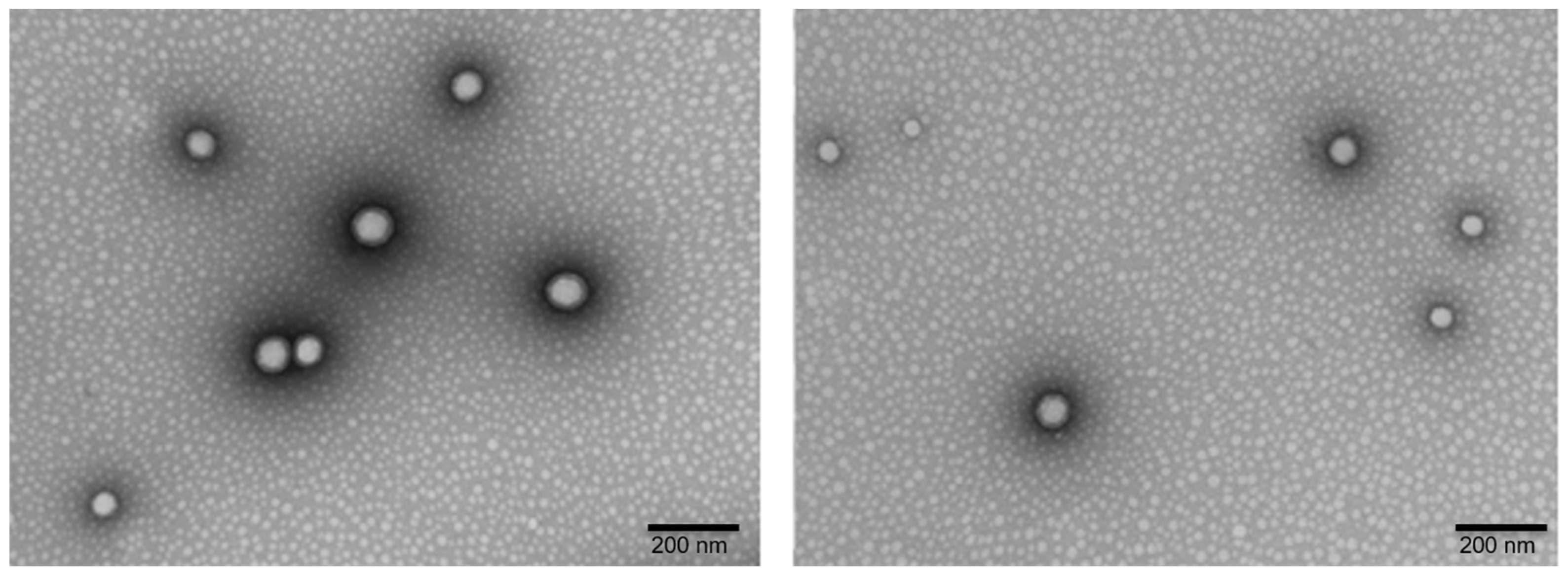
2.7. Microscopy Analysis (TEM and SEM)
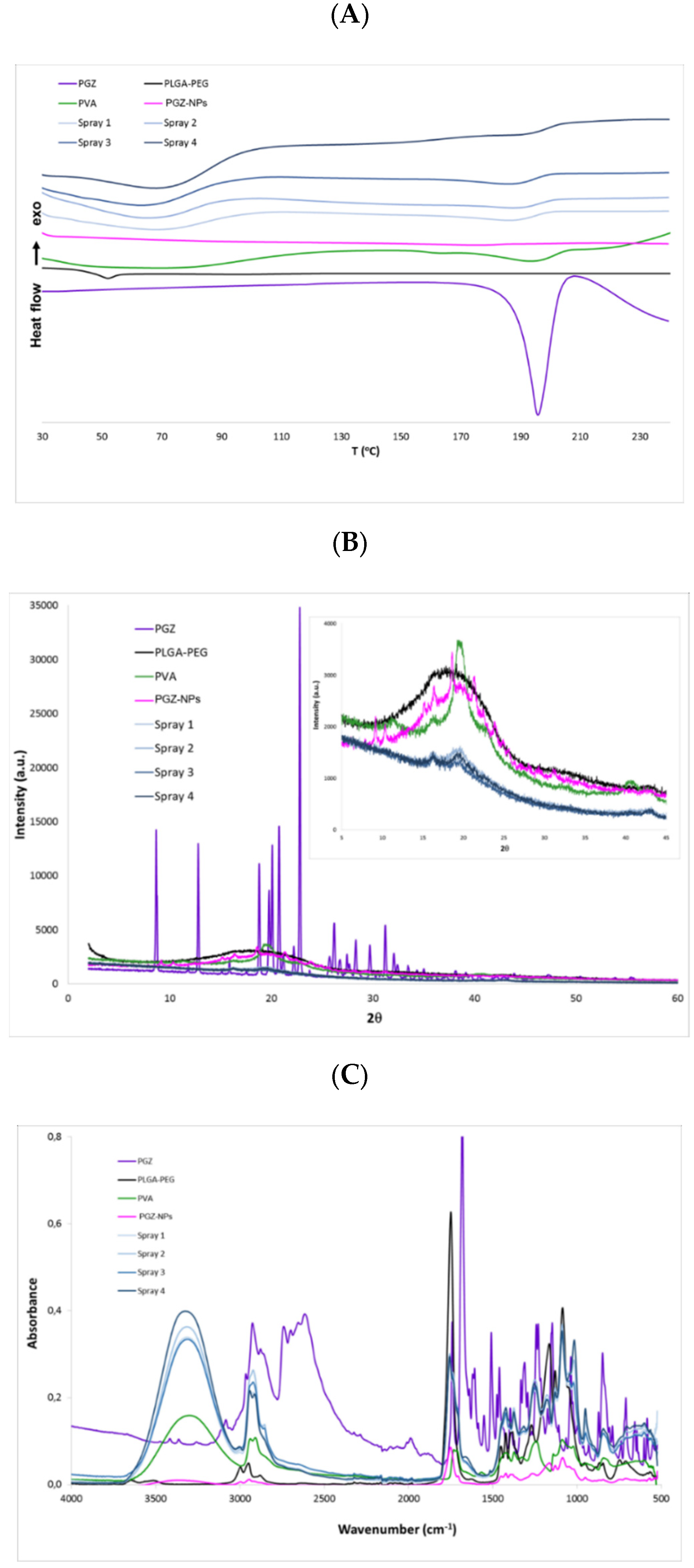
2.8. Interactions Studies: DSC, X-ray Spectroscopy, and FTIR
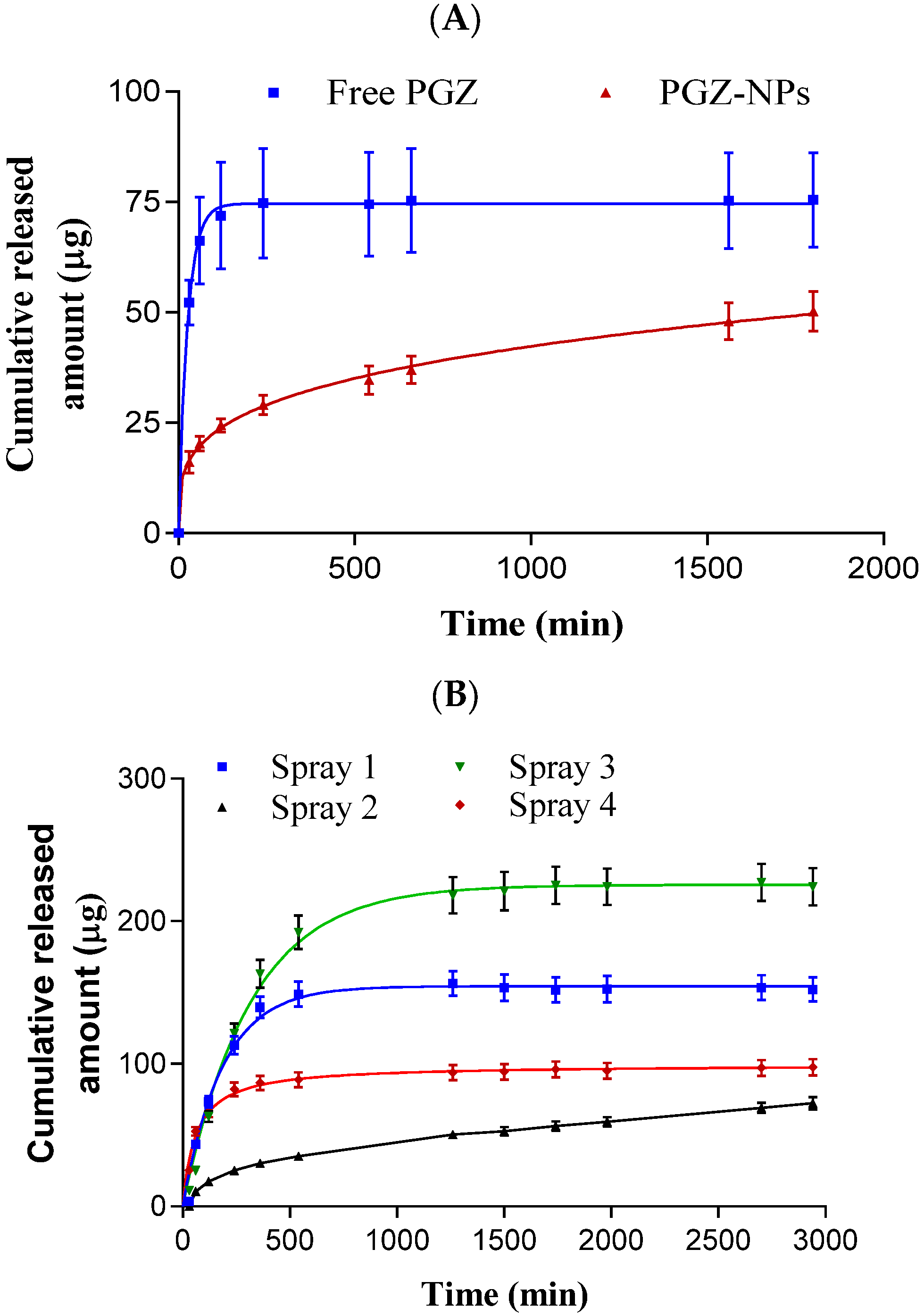
2.9. Release Profile of PGZ-NPs
2.10. Corneal and Scleral Permeation Studies
2.11. Ocular Permeation Parameters
2.12. In Vivo Study
2.13. Small-Angle X-ray Scattering (SAXS) Data Collection and Analysis
2.14. Stability Studies
2.15. Statistical Analysis of Synchrotron Data
3. Results
3.1. Development and Characterization
3.2. Water Content
3.3. Interaction Studies for PGZ-NPs and Their Components Using the Nanoparticle-Based Dry Powder
3.4. Release Profile
3.5. Ex Vivo Permeation Studies
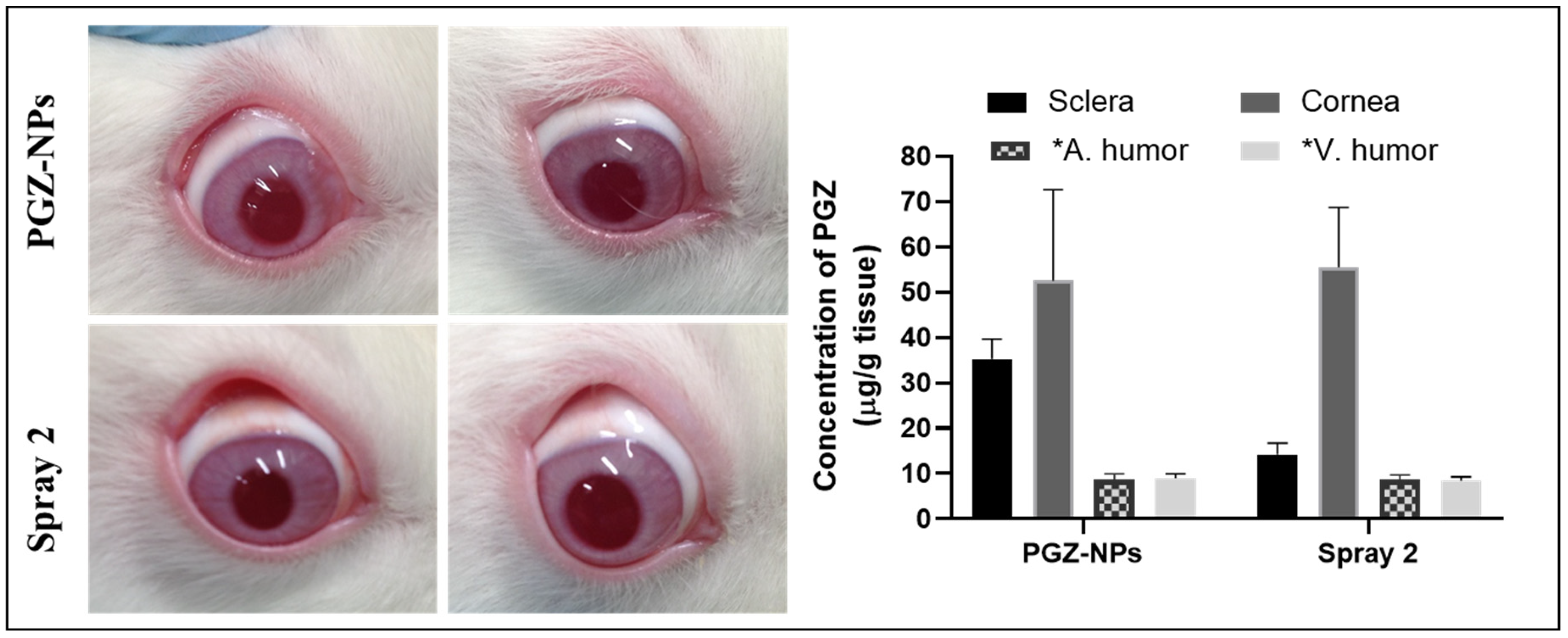
3.6. In Vivo Studies and X-ray Synchrotron Corneal Analysis
3.7. Stability Studies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hans, M.; Lowman, A. A dual-responsive mesoporous silica nanoparticle for tumor-triggered targeting drug deliverys for drug delivery and targeting. Curr. Opin. Solid State Mater. Sci. 2002, 6, 319–327. [Google Scholar] [CrossRef]
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf. B Biointerfaces 2010, 75, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 1–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacob, J.; Haponiuk, J.; Thomas, S.; Gopi, S. Biopolymer based nanomaterials in drug delivery systems: A review. Mater. Today Chem. 2018, 9, 43–55. [Google Scholar] [CrossRef]
- De Jong, W.H.; Borm, P.J.A. Drug delivery and nanoparticles: Applications and hazards. Int. J. Nanomed. 2008, 3, 133–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, Z.; Gao, S.; Cui, X.; Sun, D.; Zhao, K. Adjuvants and delivery systems based on polymeric nanoparticles for mucosal vaccines. Int. J. Pharm. 2019, 572, 118731. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-T.; Lee, J.-Y.; Kim, D.-D.; Yoon, I.-S.; Cho, H.-J. Recent Progress in the Development of Poly (lactic-co-glycolic acid)-Based Nanostructures for Cancer Imaging and Therapy. Pharmaceutics 2019, 11, 280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagaraju, G.P.; Srivani, G.; Dariya, B.; Chalikonda, G.; Farran, B.; Behera, S.K.; Alam, A.; Kamal, M.A. Nanoparticles guided drug delivery and imaging in gastric cancer. Semin. Cancer Biol. 2020, 69, 69–76. [Google Scholar] [CrossRef]
- Su, S.; Kang, P.M. Systemic Review of Biodegradable Nanomaterials in Nanomedicine. Nanomaterials 2020, 10, 656. [Google Scholar] [CrossRef] [Green Version]
- Tornesello, A.L.; Tagliamonte, M.; Tornesello, M.L.; Buonaguro, F.M.; Buonaguro, L. Nanoparticles to Improve the E ffi cacy of Peptide-Based Cancer Vaccines. Cancers 2020, 12, 1049. [Google Scholar] [CrossRef]
- Mir, M.; Ahmed, N.; Rehman, A.U. Recent applications of PLGA based nanostructures in drug delivery. Colloids Surfaces B Biointerfaces 2017, 159, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Préat, V. PLGA-based nanoparticles: An overview of biomedical applications. J. Control. Release 2012, 161, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Liang, W.; Xiao, X.; Qian, Y. Nanotechnology, an alternative with promising prospects and advantages for the treatment of cardiovascular diseases. Int. J. Nanomed. 2018, 13, 7349–7362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rezvantalab, S.; Drude, N.; Moraveji, M.K.; Güvener, N.; Koons, E.K.; Shi, Y.; Lammers, T.; Kiessling, F. PLGA-Based Nanoparticles in Cancer Treatment. Front. Pharmacol. 2018, 9, 1260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Q.; Ensign, L.M.; Boylan, N.J.; Schön, A.; Gong, X.; Yang, J.-C.; Lamb, N.W.; Cai, S.; Yu, T.; Freire, E.; et al. Impact of Surface Polyethylene Glycol (PEG) Density on Biodegradable Nanoparticle Transport in Mucus ex Vivo and Distribution in Vivo. ACS Nano 2015, 9, 9217–9227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gref, R.; Domb, A.J.; Quellec, P.; Blunk, T.; Müller, R.; Verbavatz, J.; Langer, R. The controlled intravenous delivery of drugs using PEG-coated sterically stabilized nanospheres. Adv. Drug Deliv. Rev. 2012, 64, 316–326. [Google Scholar] [CrossRef]
- Owens, D.E.; Peppas, N.A. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int. J. Pharm. 2006, 307, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, E.; Franchini, M.C. Biodegradable PLGA-b-PEG polymeric nanoparticles: Synthesis, properties, and nanomedical applications as drug delivery system. J. Nanoparticle Res. 2012, 14, 1–17. [Google Scholar] [CrossRef]
- Davis, M.; Walker, G. Recent strategies in spray drying for the enhanced bioavailability of poorly water-soluble drugs. J. Control. Release 2018, 269, 110–127. [Google Scholar] [CrossRef] [Green Version]
- Fonte, P.; Reis, S.; Sarmento, B. Facts and evidences on the lyophilization of polymeric nanoparticles for drug delivery. J. Control. Release 2016, 225, 75–86. [Google Scholar] [CrossRef]
- Ziaee, A.; Albadarin, A.; Padrela, L.; Femmer, T.; O’Reilly, E.; Walker, G. Spray drying of pharmaceuticals and biopharmaceuticals: Critical parameters and experimental process optimization approaches. Eur. J. Pharm. Sci. 2018, 127, 300–318. [Google Scholar] [CrossRef]
- Magri, G.; Franzé, S.; Musazzi, U.M.; Selmin, F.; Cilurzo, F. Maltodextrins as drying auxiliary agent for the preparation of easily resuspendable nanoparticles. J. Drug Deliv. Sci. Technol. 2019, 50, 181–187. [Google Scholar] [CrossRef]
- Ribeiro, R.F.; Motta, M.H.; Härter, A.P.G.; Flores, F.C.; Beck, R.; Schaffazick, S.R.; Silva, C.D.B.D. Spray-dried powders improve the controlled release of antifungal tioconazole-loaded polymeric nanocapsules compared to with lyophilized products. Mater. Sci. Eng. C 2016, 59, 875–884. [Google Scholar] [CrossRef]
- Kamiya, S.; Nakashima, K. Physicochemical interaction mechanism between nanoparticles and tetrasaccharides (stachyose) during freeze-drying. Drug Dev. Ind. Pharm. 2017, 43, 2026–2031. [Google Scholar] [CrossRef]
- Pandey, P.; Dua, K.; Dureja, H. Erlotinib loaded chitosan nanoparticles: Formulation, physicochemical characterization and cytotoxic potential. Int. J. Biol. Macromol. 2019, 139, 1304–1316. [Google Scholar] [CrossRef]
- Ding, S.; Serra, C.A.; Anton, N.; Yu, W.; Vandamme, T.F. Production of dry-state ketoprofen-encapsulated PMMA NPs by coupling micromixer-assisted nanoprecipitation and spray drying. Int. J. Pharm. 2018, 558, 1–8. [Google Scholar] [CrossRef]
- Booysen, L.; Kalombo, L.; Brooks, E.; Hansen, R.; Gilliland, J.; Gruppo, V.; Lungenhofer, P.; Semete-Makokotlela, B.; Swai, H.; Kotze, A.; et al. In vivo/in vitro pharmacokinetic and pharmacodynamic study of spray-dried poly-(dl-lactic-co-glycolic) acid nanoparticles encapsulating rifampicin and isoniazid. Int. J. Pharm. 2013, 444, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Abdelwahed, W.; Degobert, G.; Stainmesse, S.; Fessi, H. Freeze-drying of nanoparticles: Formulation, process and storage considerations. Adv. Drug Deliv. Rev. 2006, 58, 1688–1713. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ma, Y.; Gu, Y.; Liu, Y.; Zhao, J.; Yan, B.; Wang, Y. Cryoprotectant choice and analyses of freeze-drying drug suspension of nanoparticles with functional stabilisers. J. Microencapsul. 2018, 35, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Yacasi, G.R.R.; Campmany, A.C.C.; Gras, M.A.E.; García, M.E.; López, M.L.G. Freeze drying optimization of polymeric nanoparticles for ocular flurbiprofen delivery: Effect of protectant agents and critical process parameters on long-term stability. Drug Dev. Ind. Pharm. 2017, 43, 637–651. [Google Scholar] [CrossRef] [PubMed]
- Arpagaus, C. PLA/PLGA nanoparticles prepared by nano spray drying. J. Pharm. Investig. 2019, 49, 405–426. [Google Scholar] [CrossRef] [Green Version]
- Arpagaus, C.; Collenberg, A.; Rütti, D.; Assadpour, E.; Jafari, S.M. Nano spray drying for encapsulation of pharmaceuticals. Int. J. Pharm. 2018, 546, 194–214. [Google Scholar] [CrossRef]
- Gharsallaoui, A.; Roudaut, G.; Chambin, O.; Voilley, A.; Saurel, R. Applications of spray-drying in microencapsulation of food ingredients: An overview. Food Res. Int. 2007, 40, 1107–1121. [Google Scholar] [CrossRef]
- Sosnik, A.; Seremeta, K. Advantages and challenges of the spray-drying technology for the production of pure drug particles and drug-loaded polymeric carriers. Adv. Colloid Interface Sci. 2015, 223, 40–54. [Google Scholar] [CrossRef]
- Salama, A.H. Spray drying as an advantageous strategy for enhancing pharmaceuticals bioavailability. Drug Deliv. Transl. Res. 2019, 10, 1–12. [Google Scholar] [CrossRef]
- Singh, A.; Mooter, G.V.D. Spray drying formulation of amorphous solid dispersions. Adv. Drug Deliv. Rev. 2015, 100, 27–50. [Google Scholar] [CrossRef]
- De Mohac, L.M.; Raimi-Abraham, B.; Caruana, R.; Gaetano, G.; Licciardi, M. Multicomponent solid dispersion a new generation of solid dispersion produced by spray-drying. J. Drug Deliv. Sci. Technol. 2020, 57, 101750. [Google Scholar] [CrossRef]
- Bachu, R.D.; Chowdhury, P.; Al-Saedi, Z.H.F.; Karla, P.K.; Boddu, S.H.S. Ocular Drug Delivery Barriers—Role of Nanocarriers in the Treatment of Anterior Segment Ocular Diseases. Pharmaceutics 2018, 10, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghafoorianfar, S.; Ghorani-Azam, A.; Mohajeri, S.A.; Farzin, D. Efficiency of nanoparticles for treatment of ocular infections: Systematic literature review. J. Drug Deliv. Sci. Technol. 2020, 57, 101765. [Google Scholar] [CrossRef]
- Gonzalez-Pizarro, R.; Silva-Abreu, M.; Calpena, A.C.; Egea, M.A.; Espina, M.; García, M.L. Development of fluorometholone-loaded PLGA nanoparticles for treatment of inflammatory disorders of anterior and posterior segments of the eye. Int. J. Pharm. 2018, 547, 338–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, D.; Li, Q.; Sun, Y.; Guo, J.; Zhao, Q.; Yin, X.; Wei, H.; Wu, S.; Bi, H. Evaluation of controlled-release triamcinolone acetonide-loaded mPEG-PLGA nanoparticles in treating experimental autoimmune uveitis. Nanotechnology 2019, 30, 165702. [Google Scholar] [CrossRef] [PubMed]
- Güven, U.M.; Yenilmez, E. Olopatadine hydrochloride loaded Kollidon® SR nanoparticles for ocular delivery: Nanosuspension formulation and in vitro–in vivo evaluation. J. Drug Deliv. Sci. Technol. 2019, 51, 506–512. [Google Scholar] [CrossRef]
- Sah, A.K.; Suresh, P.K.; Verma, V.K. PLGA nanoparticles for ocular delivery of loteprednol etabonate: A corneal penetration study. Artif. Cells Nanomed. Biotechnol. 2016, 45, 1156–1164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cañadas, C.; Alvarado, H.; Calpena, A.C.; Silva, A.M.; Souto, E.B.; García, M.L.; Abrego, G. In vitro, ex vivo and in vivo characterization of PLGA nanoparticles loading pranoprofen for ocular administration. Int. J. Pharm. 2016, 511, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Abrego, G.; Alvarado, H.; Souto, E.B.; Guevara, B.; Bellowa, L.H.; Parra, A.; Calpena, A.C.; Garcia, M.L. Biopharmaceutical profile of pranoprofen-loaded PLGA nanoparticles containing hydrogels for ocular administration. Eur. J. Pharm. Biopharm. 2015, 95, 261–270. [Google Scholar] [CrossRef] [Green Version]
- Silva-Abreu, M.; Espinoza, L.C.; Rodríguez-Lagunas, M.J.; Fabrega, M.J.; Espina, M.; García, M.L.; Calpena, A.C. Human Skin Permeation Studies with PPARγ Agonist to Improve Its Permeability and Efficacy in Inflammatory Processes. Int. J. Mol. Sci. 2017, 18, 2548. [Google Scholar] [CrossRef] [Green Version]
- Kanemaru, M.; Asai, J.; Jo, J.-I.; Arita, T.; Kawai-Ohnishi, M.; Tsutsumi, M.; Wada, M.; Tabata, Y.; Katoh, N. Nanoparticle-mediated local delivery of pioglitazone attenuates bleomycin-induced skin fibrosis. J. Dermatol. Sci. 2019, 93, 41–49. [Google Scholar] [CrossRef] [Green Version]
- Silva-Abreu, M.; Gonzalez-Pizarro, R.; Espinoza, L.C.; Rodríguez-Lagunas, M.J.; Espina, M.; García, M.L.; Calpena, A.C. Thiazolidinedione as an alternative to facilitate oral administration in geriatric patients with Alzheimer’s disease. Eur. J. Pharm. Sci. 2019, 129, 173–180. [Google Scholar] [CrossRef]
- Tokutome, M.; Matoba, T.; Nakano, Y.; Okahara, A.; Fujiwara, M.; Koga, J.-I.; Nakano, K.; Tsutsui, H.; Egashira, K. Peroxisome proliferator-activated receptor-gamma targeting nanomedicine promotes cardiac healing after acute myocardial infarction by skewing monocyte/macrophage polarization in preclinical animal models. Cardiovasc. Res. 2018, 115, 419–431. [Google Scholar] [CrossRef]
- Jojo, G.M.; Kuppusamy, G.; De, A.; Karri, V.V.S.N.R. Formulation and optimization of intranasal nanolipid carriers of pioglitazone for the repurposing in Alzheimer’s disease using Box-Behnken design. Drug Dev. Ind. Pharm. 2019, 45, 1061–1072. [Google Scholar] [CrossRef]
- Okunuki, Y.; Usui, Y.; Nakagawa, H.; Tajima, K.; Matsuda, R.; Ueda, S.; Hattori, T.; Kezuka, T.; Goto, H. Peroxisome proliferator-activated receptor-γ agonist pioglitazone suppresses experimental autoimmune uveitis. Exp. Eye Res. 2013, 116, 291–297. [Google Scholar] [CrossRef]
- Silva-Abreu, M.; Calpena, A.C.; Espina, M.; Silva, A.M.; Gimeno, A.; Egea, M.A.; García, M.L. Optimization, Biopharmaceutical Profile and Therapeutic Efficacy of Pioglitazone-loaded PLGA-PEG Nanospheres as a Novel Strategy for Ocular Inflammatory Disorders. Pharm. Res. 2018, 35, 11. [Google Scholar] [CrossRef] [Green Version]
- Uchiyama, M.; Shimizu, A.; Masuda, Y.; Nagasaka, S.; Fukuda, Y.; Takahashi, H. An ophthalmic solution of a peroxisome proliferator-activated receptor gamma agonist prevents corneal inflammation in a rat alkali burn model. Mol. Vis. 2013, 19, 2135–2150. [Google Scholar]
- Mehta, M. Biopharmaceutics Classification System (BCS); John Wiley & Sons: New York, NY, USA, 2017; ISBN 978-1-118-47661-1. [Google Scholar]
- Silva-Abreu, M.; Espinoza, L.C.; Halbaut, L.; Espina, M.; García, M.L.; Calpena, A.C. Comparative Study of Ex Vivo Transmucosal Permeation of Pioglitazone Nanoparticles for the Treatment of Alzheimer’s Disease. Polymers 2018, 10, 316. [Google Scholar] [CrossRef] [Green Version]
- Silva-Abreu, M.; Calpena, A.C.; Andrés-Benito, P.; Aso, E.; A Romero, I.; Roig-Carles, D.; Gromnicova, R.; Espina, M.; Ferrer, I.; García, M.L.; et al. PPARγ agonist-loaded PLGA-PEG nanocarriers as a potential treatment for Alzheimer’s disease: In vitro and in vivo studies. Int. J. Nanomed. 2018, 13, 5577–5590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fessi, H.; Puisieux, F.; Devissaguet, J.; Ammoury, N.; Benita, S. Nanocapsule formation by interfacial polymer deposition following solvent displacement. Int. J. Pharm. 1989, 55, R1–R4. [Google Scholar] [CrossRef]
- Li, X.; Anton, N.; Arpagaus, C.; Belleteix, F.; Vandamme, T.F. Nanoparticles by spray drying using innovative new technology: The Büchi Nano Spray Dryer B-90. J. Control. Release 2010, 147, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Heng, D.; Lee, S.H.; Ng, W.K.; Tan, R.B.H. The nano spray dryer B-90. Expert Opin. Drug Deliv. 2011, 8, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Berghof Easy H2O. (Berghof Products+Instruments GmbH). Available online: https://www.lambda-at.com/pdf/easyH2O.pdf (accessed on 5 October 2021).
- Yamaoka, K.; Nakagawa, T.; Uno, T. Application of Akaike’s information Criterion (AIC) in the evaluation of linear phar-macokinetic equations. J. Pharmacokinet. Biopharm. 1978, 6, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Miralles-Cardiel, E.; Silva-Abreu, M.; Calpena, A.; Casals, I. Development and Validation of an HPLC–MS/MS Method for Pioglitazone from Nanocarriers Quantitation in Ex Vivo and In Vivo Ocular Tissues. Pharmaceutics 2021, 13, 650. [Google Scholar] [CrossRef]
- Abass, A.; Bell, J.S.; Spang, M.T.; Hayes, S.; Meek, K.M.; Boote, C. SAXS4COLL: An integrated software tool for analysing fibrous collagen-based tissues. J. Appl. Crystallogr. 2017, 50, 1235–1240. [Google Scholar] [CrossRef] [Green Version]
- Chaubal, M.V.; Popescu, C. Conversion of Nanosuspensions into Dry Powders by Spray Drying: A Case Study. Pharm. Res. 2008, 25, 2302–2308. [Google Scholar] [CrossRef]
- López, E.S.; Egea, M.; Cano, A.; Espina, M.; Calpena, A.C.; Ettcheto, M.; Camins, A.; Souto, E.; Silva, A.; García, M. PEGylated PLGA nanospheres optimized by design of experiments for ocular administration of dexibuprofen—In vitro, ex vivo and in vivo characterization. Colloids Surfaces B Biointerfaces 2016, 145, 241–250. [Google Scholar] [CrossRef] [Green Version]
- Ghitman, J.; Biru, E.I.; Stan, R.; Iovu, H. Review of hybrid PLGA nanoparticles: Future of smart drug delivery and theranostics medicine. Mater. Des. 2020, 193, 108805. [Google Scholar] [CrossRef]
- Lee, P.W.; Pokorski, J.K. Poly(lactic-co-glycolic acid) devices: Production and applications for sustained protein delivery. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnology 2018, 10, 1516. [Google Scholar] [CrossRef] [PubMed]
- Vega, E.; Egea, M.A.; Calpena, A.C.; Espina, M.; García, M.L. Role of hydroxypropyl-β-cyclodextrin on freeze-dried and gamma-irradiated PLGA and PLGA–PEG diblock copolymer nanospheres for ophthalmic flurbiprofen delivery. Int. J. Nanomed. 2012, 7, 1357–1371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rincón, M.; Calpena, A.C.; Fabrega, M.-J.; Garduño-Ramírez, M.L.; Espina, M.; Rodríguez-Lagunas, M.J.; García, M.L.; Abrego, G. Development of Pranoprofen Loaded Nanostructured Lipid Carriers to Improve Its Release and Therapeutic Efficacy in Skin Inflammatory Disorders. Nanomaterials 2018, 8, 1022. [Google Scholar] [CrossRef] [Green Version]
- Parra, A.; Mallandrich, M.; Clares, B.; Egea, M.A.; Espina, M.; García, M.L.; Calpena, A.C. Bioin-terfaces Design and elaboration of freeze-dried PLGA nanoparticles for the transcorneal permeation of carprofen: Ocular anti-inflammatory applications. Colloids Surf. B 2015, 136, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-López, E.; Esteruelas, G.; Ortiz, A.; Espina, M.; Prat, J.; Muñoz, M.; Cano, A.; Calpena, A.C.; Ettcheto, M.; Camins, A.; et al. Dexibuprofen Biodegradable Nanoparticles: One Step Closer towards a Better Ocular Interaction Study. Nanomaterials 2020, 10, 720. [Google Scholar] [CrossRef] [Green Version]
- Abrego, G.; Alvarado, H.L.; Egea, M.A.; Gonzalez-Mira, E.; Calpena, A.C.; Garcia, M.L. Design of Nanosuspensions and Freeze-Dried PLGA Nanoparticles as a Novel Approach for Ophthalmic Delivery of Pranoprofen. J. Pharm. Sci. 2014, 103, 3153–3164. [Google Scholar] [CrossRef] [PubMed]
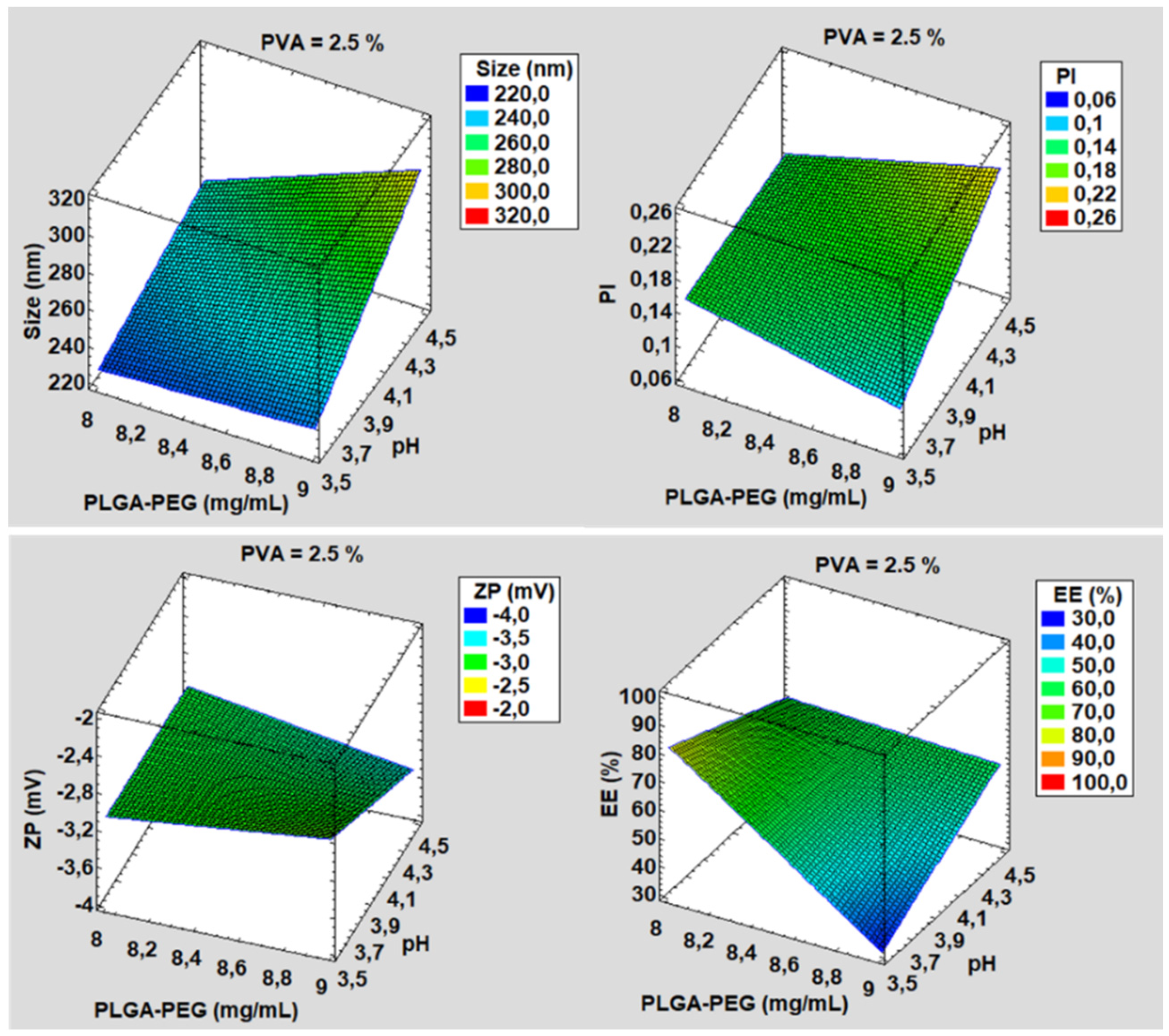




| Formulation | PLGA-PEG (mg/mL) | pH | PVA (%) | Size (nm) | PI | ZP (mV) | EE (%) |
|---|---|---|---|---|---|---|---|
| 1 | 8.0 | 3.5 | 2.0 | 223.90 ± 1.60 | 0.09 ± 0.00 | −2.47 ± 0.08 | 39.45 ± 2.12 |
| 2 | 9.0 | 3.5 | 2.0 | 231.91 ± 0.77 | 0.08 ± 0.01 | −3.30 ± 0.30 | 48.95 ± 1.34 |
| 3 | 8.0 | 4.5 | 2.0 | 242.32 ± 2.85 | 0.16 ± 0.03 | −2.54 ± 0.43 | 79.23 ± 3.21 |
| 4 | 8.0 | 3.5 | 2.5 | 251.42 ± 0.66 | 0.20 ± 0.00 | −3.28 ± 0.38 | 35.74 ± 2.42 |
| 5 | 9.0 | 4.5 | 2.0 | 234.51 ± 0.90 | 0.17 ± 0.01 | −3.42 ± 0.22 | 72.83 ± 1.32 |
| 6 | 9.0 | 3.5 | 2.5 | 302.81 ± 1.94 | 0.23 ± 0.02 | −3.82 ± 0.25 | 49.64 ± 2.21 |
| 7 | 8.0 | 4.5 | 2.5 | 242.52 ± 3.51 | 0.16 ± 0.01 | −2.76 ± 0.50 | 70.01 ± 3.23 |
| 8 | 9.0 | 4.5 | 2.5 | 247.22 ± 2.77 | 0.17 ± 0.03 | −3.34 ± 0.42 | 90.12 ± 1.15 |
| Formulation | Size (nm) | PI | ZP (mV) | EE (%) |
|---|---|---|---|---|
| Spray 1 | 366.61 ± 13.48 | 0.39 ± 0.01 | −6.93 ± 1.04 | 90.05 ± 1.14 |
| Spray 2 | 271.31 ± 2.78 | 0.24 ± 0.00 | −7.57 ± 1.53 | 89.13 ± 2.21 |
| Spray 3 | 328.02 ± 1.34 | 0.29 ± 0.03 | −6.48 ± 0.31 | 90.10 ± 1.54 |
| Spray 4 | 276.64 ± 1.75 | 0.23 ± 0.00 | −9.87 ± 0.48 | 88.92 ± 1.21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva-Abreu, M.; Miralles, E.; Kamma-Lorger, C.S.; Espina, M.; García, M.L.; Calpena, A.C. Stabilization by Nano Spray Dryer of Pioglitazone Polymeric Nanosystems: Development, In Vivo, Ex Vivo and Synchrotron Analysis. Pharmaceutics 2021, 13, 1751. https://doi.org/10.3390/pharmaceutics13111751
Silva-Abreu M, Miralles E, Kamma-Lorger CS, Espina M, García ML, Calpena AC. Stabilization by Nano Spray Dryer of Pioglitazone Polymeric Nanosystems: Development, In Vivo, Ex Vivo and Synchrotron Analysis. Pharmaceutics. 2021; 13(11):1751. https://doi.org/10.3390/pharmaceutics13111751
Chicago/Turabian StyleSilva-Abreu, Marcelle, Esther Miralles, Christina S. Kamma-Lorger, Marta Espina, María Luisa García, and Ana Cristina Calpena. 2021. "Stabilization by Nano Spray Dryer of Pioglitazone Polymeric Nanosystems: Development, In Vivo, Ex Vivo and Synchrotron Analysis" Pharmaceutics 13, no. 11: 1751. https://doi.org/10.3390/pharmaceutics13111751
APA StyleSilva-Abreu, M., Miralles, E., Kamma-Lorger, C. S., Espina, M., García, M. L., & Calpena, A. C. (2021). Stabilization by Nano Spray Dryer of Pioglitazone Polymeric Nanosystems: Development, In Vivo, Ex Vivo and Synchrotron Analysis. Pharmaceutics, 13(11), 1751. https://doi.org/10.3390/pharmaceutics13111751







