Formulation, Optimization and Evaluation of Luteolin-Loaded Topical Nanoparticulate Delivery System for the Skin Cancer
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials
2.2. Optimization
2.3. Formulation of Luteolin Nanovesicles (LT-NVs)
2.4. Vesicle Characterization
2.5. Encapsulation Efficiency (EE)
2.6. Formulation of Luteolin Nanovesicles Based Gel (LT-NVoptG)
2.7. LT-NVoptG Characterization
2.8. Drug Release
2.9. Permeation Study
2.10. Antioxidant Assessment
2.11. Antimicrobial Activity
2.12. Cytotoxicity Study
2.13. Irritation Study
2.14. Statistical Analysis
3. Results and Discussion
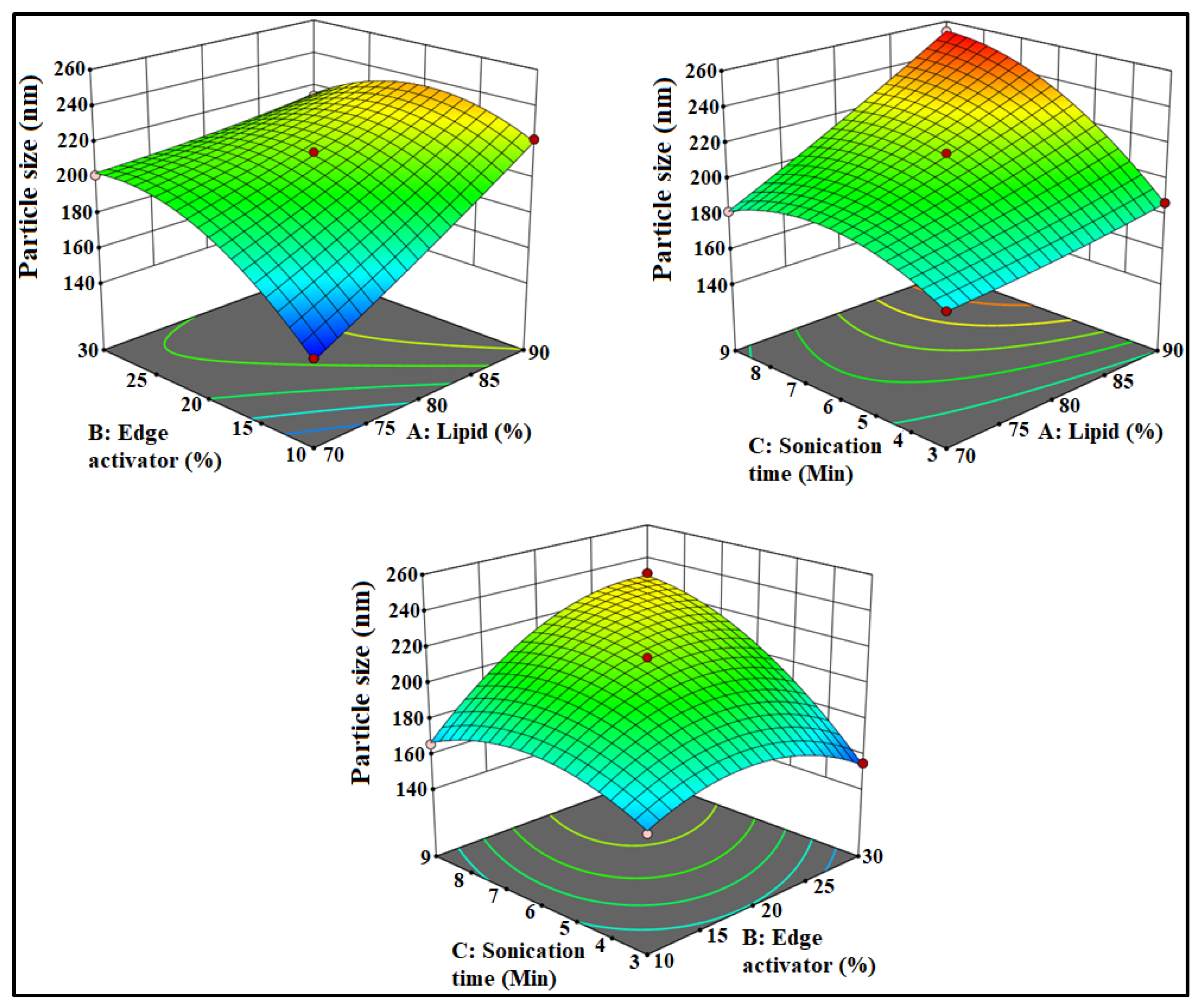
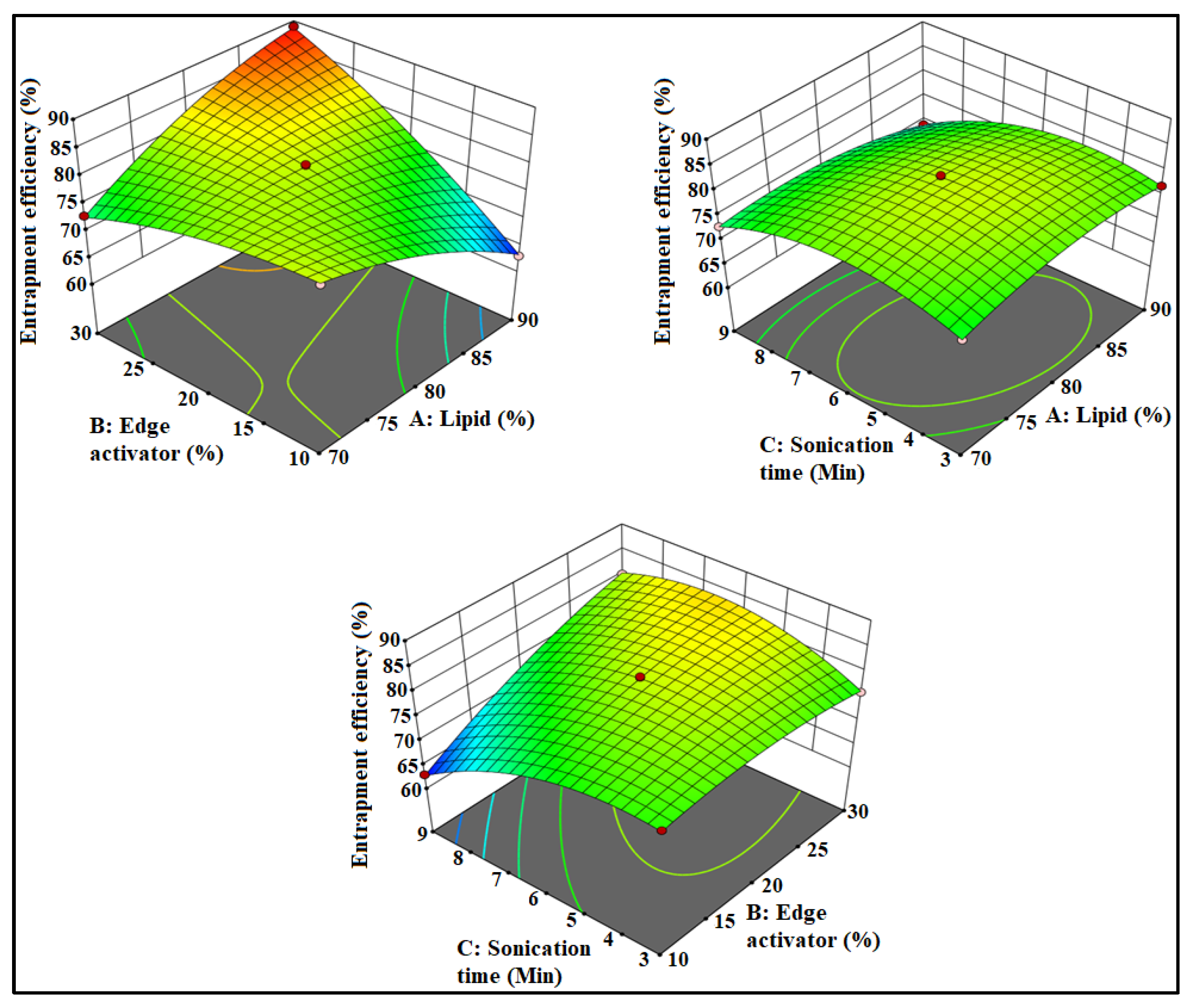
3.1. Optimization
3.2. Effect of Formulation Factors on PS
3.3. Effect of Formulation Factors on EE
3.4. Point Prediction
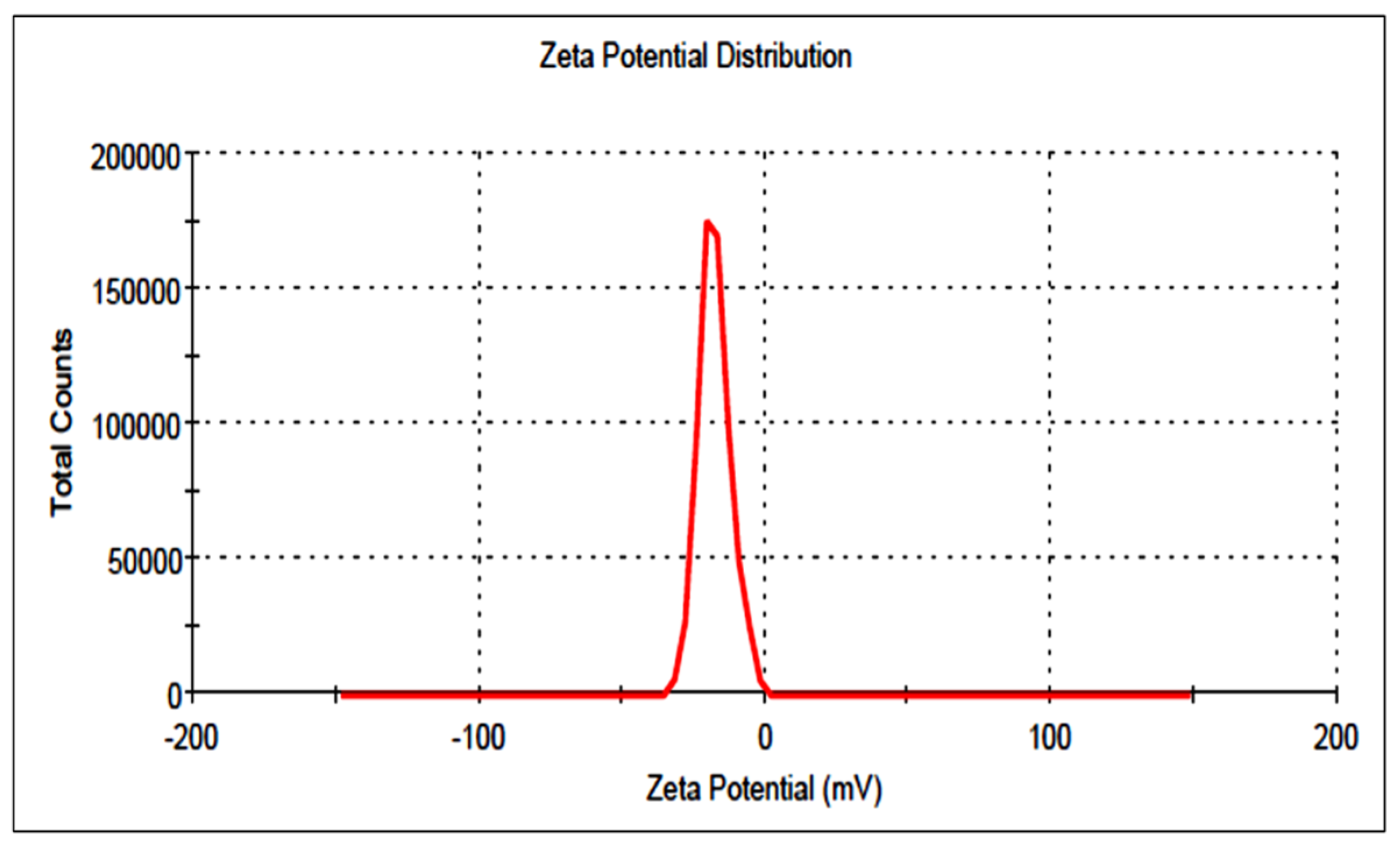
3.5. Particle Size and Surface Charge
3.6. Formulation of Gel
3.7. Characterization of Gel
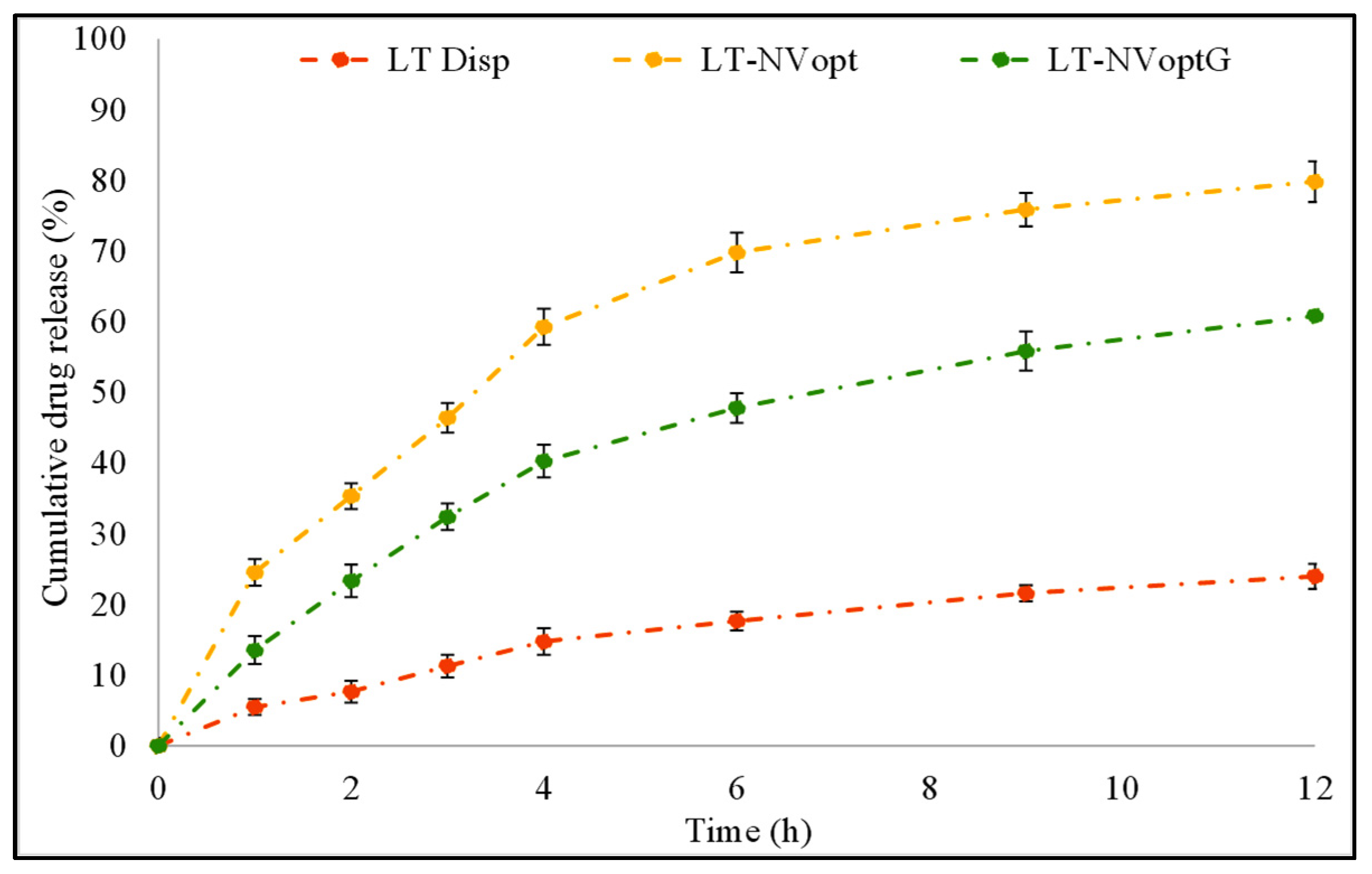
3.8. Drug Release
3.9. Permeation Study
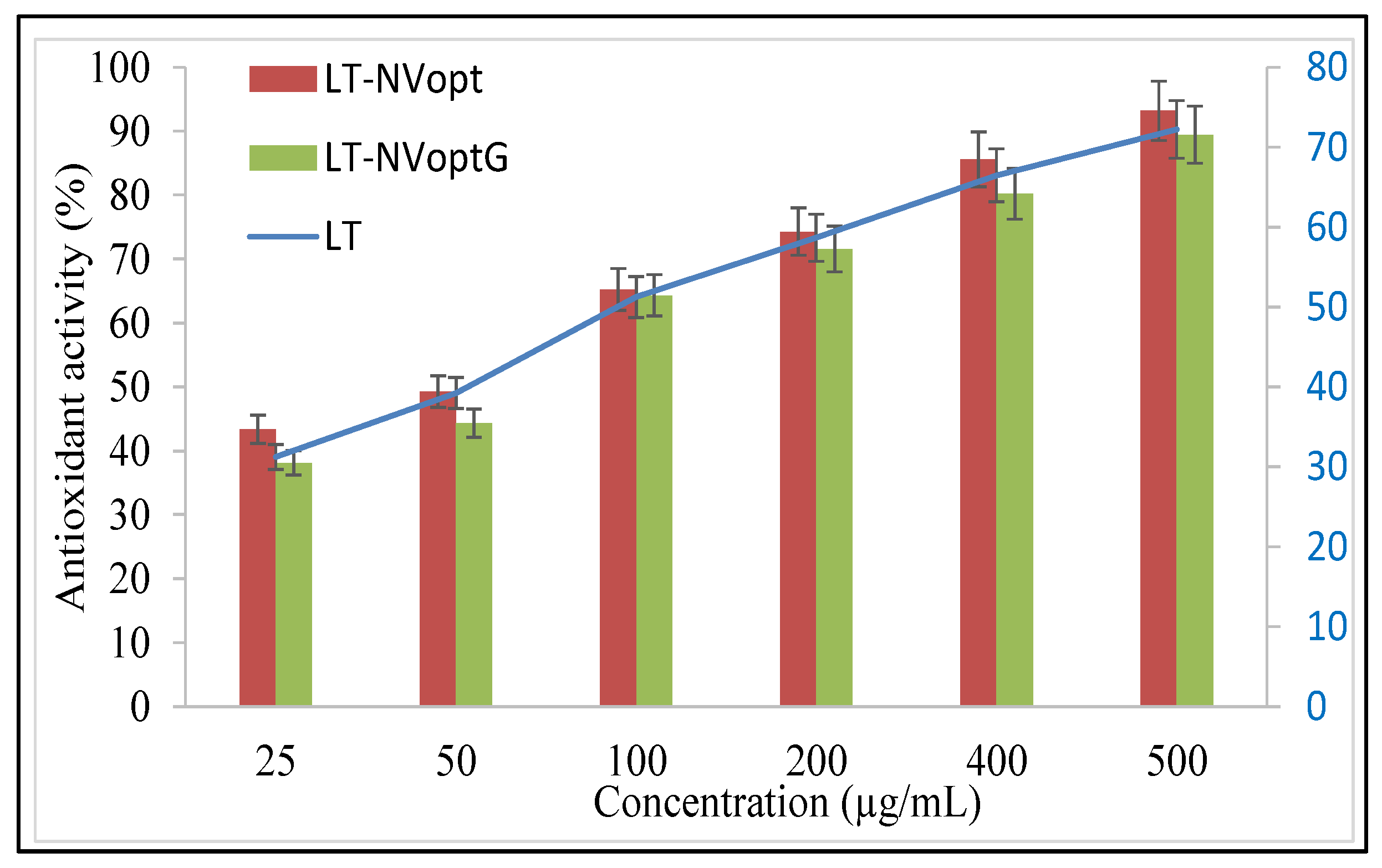
3.10. Antioxidant Activity
3.11. Antibacterial Activity
3.12. Cell Viability
3.13. Irritation Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Imran, M.; Rauf, A.; Abu-Izneid, T.; Nadeem, M.; Ali, M.; Khan, I.A.; Imran, A.; Orhan, I.E.; Rizwan, M.; Atif, M.; et al. Luteolin, a flavonoid, as an anticancer agent: A review. Biomed. Pharmacother. 2019, 112, 108612. [Google Scholar] [CrossRef]
- Naves, L.B.; Dhand, C.; Venugopal, J.R. Nanotechnology for the treatment of melanoma skin cancer. Prog. Biomater. 2017, 6, 13–26. [Google Scholar] [CrossRef] [Green Version]
- Calienni, M.N.; Temprana, C.F.; Prieto, M.J. Nano-formulation for topical treatment of precancerous lesions: Skin penetration, in vitro, and in vivo toxicological evaluation. Drug Deliv. Transl. Res. 2018, 8, 496–514. [Google Scholar] [CrossRef] [Green Version]
- Kryczyk-Poprawa, A.; Kwiecień, A.; Opoka, W. Photostability of Topical Agents Applied to the Skin: A Review. Pharmaceutics 2020, 12, 10. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Shi, R.; Wang, X.H.; Shen, H. Luteolin, a flavonoid with potentials for cancer prevention and therapy. Curr. Cancer Drug Targets 2008, 8, 634–646. [Google Scholar] [CrossRef]
- Martin, K.R. Targeting apoptosis with dietary bioactive agents. Exp. Biol. Med. 2006, 231, 117–129. [Google Scholar] [CrossRef] [Green Version]
- Tai, Z.; Lin, Y.; He, Y.; Huang, J.; Guo, J.; Yang, L.; Zhang, G.; Wang, F. Luteolin sensitizes the antiproliferative effect of interferon α/β by activation of Janus kinase/ signal transducer and activator of transcription pathway signaling through protein kinase A-mediated inhibition of protein tyrosine phosphatase SHP-2 in cancer cells. Cell. Signal. 2014, 26, 619–628. [Google Scholar]
- Wu, C.; Xu, Q.; Chen, X.; Liu, J. Delivery luteolin with folacin-modified nanoparticle for glioma therapy. Int. J. Nanomed. 2019, 14, 7515–7531. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Zhong, C.; Zu, Y.; Zhao, X.; Deng, Y.; Wu, W.; Sun, X.; Wang, L.; Wu, M. Preparation and characterization of luteolin nanoparticles for enhance bioavailability and inhibit liver microsomal peroxidation in rats. J. Funct. Foods 2019, 55, 57–64. [Google Scholar] [CrossRef]
- Shinde, P.; Agraval, H.; Singh, A.; Yadav, U.C.S.; Kumar, U. Synthesis of luteolin loaded zein nanoparticles for targeted cancer therapy improving bioavailability and efficacy. J. Drug Deliv. Sci. Technol. 2019, 52, 369–378. [Google Scholar] [CrossRef]
- Gilani, S.J.; Bin-Jumah, M.; Rizwanullah, M.; Imam, S.S.; Imtiyaz, K.; Alshehri, S.; Alam, M.R.M. Chitosan Coated Luteolin Nanostructured Lipid Carriers: Optimization, In Vitro-Ex Vivo Assessments and Cytotoxicity Study in Breast Cancer Cells. Coatings 2021, 11, 158. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Feng, W.; Yuan, Q.; Qi, X.; Chen, S.; Yao, P.; Dai, Q.; Xia, P.; Zhang, D.; et al. Folic acid-modified ROS-responsive nanoparticles encapsulating luteolin for targeted breast cancer treatment. Drug Deliv. 2021, 28, 1695–1708. [Google Scholar] [CrossRef]
- Tawornchat, P.; Pattarakankul, T.; Palaga, T.; Intasanta, V.; Wanichwecharungruang, S. Polymerized Luteolin Nanoparticles: Synthesis, Structure Elucidation, and Anti-Inflammatory Activity. ACS Omega 2021, 6, 2846–2855. [Google Scholar] [CrossRef]
- Jangdey, M.S.; Gupta, A.; Saraf, S.; Saraf, S. Development and optimization of apigenin-loaded transfersomal system for skin cancer delivery: In vitro evaluation. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1452–1462. [Google Scholar] [CrossRef] [Green Version]
- Cristiano, M.C.; Froiio, F.; Spaccapelo, R.; Mancuso, A.; Nisticò, S.P.; Udongo, B.P.; Fresta, M.; Paolino, D. Sulforaphane-Loaded Ultradeformable Vesicles as A Potential Natural Nanomedicine for the Treatment of Skin Cancer Diseases. Pharmaceutics 2020, 12, 6. [Google Scholar] [CrossRef] [Green Version]
- Ramezani, V.; Honarvar, M.; Seyedabadi, M.; Karimollah, A.; Ranjbar, A.M.; Hashemi, M. Formulation and optimization of transfersome containing minoxidil and caffeine. J. Drug Deliv. Sci. Technol. 2018, 44, 129–135. [Google Scholar] [CrossRef]
- Omar, M.M.; Hasan, O.A.; El Sisi, A.M. Preparation and optimization of lidocaine transferosomal gel containing permeation enhancers: A promising approach for enhancement of skin permeation. Int. J. Nanomed. 2019, 14, 1551–1562. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Shamim, M.A.; Shahid, A.; Yeung, S.; Andresen, B.T.; Wang, J.; Nekkanti, V.; Meyskens, F.L., Jr.; Kelly, K.M.; Huang, Y. Topical Delivery of Carvedilol Loaded Nano-Transfersomes for Skin Cancer Chemoprevention. Pharmaceutics 2020, 12, 1151. [Google Scholar] [CrossRef]
- Elsayed, M.M.; Abdallah, O.Y.; Naggar, V.F.; Khalafallah, N.M. Deformable liposomes and ethosomes as carriers for skin delivery of ketotifen. Die Pharm. 2007, 62, 133–137. [Google Scholar]
- Lalotra, A.S.; Singh, V.; Khurana, B.; Agrawal, S.; Shrestha, S.; Arora, D.A. Comprehensive Review on Nanotechnology-Based Innovations in Topical Drug Delivery for Treatment of Skin Cancer. Curr. Pharm. Des. 2020, 26, 5720–5731. [Google Scholar] [CrossRef]
- El Zaafarany, G.M.; Awad, G.A.; Holayel, S.M.; Mortada, N.D. Role of edge activators and surface charge in developing ultradeformable vesicles with enhanced skin delivery. Int. J. Pharm. 2010, 397, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, J.; Qiao, H.; Liu, H.; Ni, J.; Zhang, W.; Shi, Y. Formulation optimization of dihydroartemisinin nanostructured lipid carrier using response surface methodology. Powder Technol. 2010, 197, 120–128. [Google Scholar] [CrossRef]
- Imam, S.S.; Ahad, A.; Aqil, M.; Akhtar, M.; Sultana, Y.; Ali, A. Formulation by design based risperidone nano soft lipid vesicle as a new strategy for enhanced transdermal drug delivery: In-vitro characterization, and in-vivo appraisal. Mater. Sci. Eng. C 2017, 75, 1198–1205. [Google Scholar] [CrossRef] [PubMed]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joseph, E.; Singhvi, G. Chapter 4—Multifunctional nanocrystals for cancer therapy: A potential nanocarrier. In Nanomaterials for Drug Delivery and Therapy; William Andrew Publishing: Norwich, NY, USA, 2019; pp. 91–116. [Google Scholar]
- Abou Samra, M.M.; Salama, A.H.; Awad, G.E.A.; Mansy, S.S. Formulation and Evaluation of Novel Hybridized Nanovesicles for Enhancing Buccal Delivery of Ciclopirox Olamine. AAPS PharmSciTech 2020, 21, 283. [Google Scholar] [CrossRef]
- Yang, X.; Trinh, H.M.; Agrahari, V.; Sheng, Y.; Pal, D.; Mitra, A.K. Nanoparticle-Based Topical Ophthalmic Gel Formulation for Sustained Release of Hydrocortisone Butyrate. AAPS PharmSciTech 2016, 17, 294–306. [Google Scholar] [CrossRef] [Green Version]
- Nautiyal, U.; Mohammed, J.; Kazmi, I. Preparation and Evaluation of Antifungal Micro-Emulsion/Gel using reduce Dose of Silver, Supported by Ciprofloxacin. Int. Pharm. Sci. 2012, 2, 72–87. [Google Scholar]
- Barot, B.S.; Parejiya, P.B.; Patel, H.K.; Gohel, H.C.; Shelat, P.K. Microemulsion-based gel of terbinafine for the treatment of onychomycosis: Optimization of formulation using D-optimal design. AAPS PharmSciTech 2012, 13, 184–192. [Google Scholar] [CrossRef] [Green Version]
- Gaba, B.; Fazil, M.; Khan, S.; Ali, A.; Baboota, S.; Ali, J. Nanostructured lipid carrier system for topical delivery of terbinafine hydrochloride. Bull. Fac. Pharm. Cairo Univ. 2015, 53, 147–159. [Google Scholar] [CrossRef] [Green Version]
- Byeon, J.C.; Lee, S.E.; Kim, T.H.; Ahn, J.B.; Kim, D.H.; Choi, J.S.; Park, J.S. Design of novel proliposome formulation for antioxidant peptide, glutathione with enhanced oral bioavailability and stability. Drug Deliv. 2019, 26, 216–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saleem, M.N.; Idris, M. Formulation Design and Development of a Unani Transdermal Patch for Antiemetic Therapy and Its Pharmaceutical Evaluation. Scientifica 2016, 2016, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunzi-Rapp, K.; Ruck, A.; Kaufmann, R. Characterization of the chick chorioallantoic membrane model as a short-term in vivo system for human skin. Arch. Dermatol. Res. 1999, 291, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Tay, S.L.M.; Heng, P.W.S.; Chan, L.W. An investigation of the chick chorioallantoic membrane as an alternative model to various biological tissues for permeation studies. J. Pharm. Pharmacol. 2011, 63, 1283–1289. [Google Scholar] [CrossRef] [PubMed]
- Haigh, J.M.; Smith, E.W. The selection and use of natural and synthetic membranes for in vitro diffusion experiments. Eur. J. Pharm. Sci. 1994, 2, 311–330. [Google Scholar] [CrossRef]
- Caddeo, C.; Pucci, L.; Gabriele, M.; Carbone, C.; Fernàndez-Busquetsd, X.; Valenti, D.; Pons, R.; Vassallo, A.; Fadda, A.M.; Manconi, M. Stability, biocompatibility and antioxidant activity of PEG-modified liposomes containing resveratrol. Int. J. Pharm. 2018, 538, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Alshehri, S.; Imam, S.S.; Hussain, A.; Altamimi, M.A. Formulation of Piperine Ternary Inclusion Complex Using β CD and HPMC: Physicochemical characterization, molecular docking and antimicrobial testing. Processes 2020, 8, 1450. [Google Scholar] [CrossRef]
- Hafeez, A.; Kazmi, I. Dacarbazine nanoparticle topical delivery system for the treatment of melanoma. Sci. Rep. 2017, 7, 16517. [Google Scholar] [CrossRef] [Green Version]
- Mehling, A.; Kleber, M.; Hensen, H. Comparative studies on the ocular and dermal irritation potential of surfactants. Food Chem. Toxicol. 2007, 45, 747–758. [Google Scholar] [CrossRef]
- Irimia, T.; Dinu-Pîrvu, C.E.; Ghica, M.V.; Lupuleasa, D.; Muntean, D.L.; Udeanu, D.I.; Popa, L. Chitosan-Based In Situ Gels for Ocular Delivery of Therapeutics: A State-of-the-Art Review. Mar. Drugs 2018, 16, 373. [Google Scholar] [CrossRef] [Green Version]
- Zafar, A.; Alruwaili, N.K.; Imam, S.S.; Alotaibi, N.H.; Alharbi, K.S.; Afzal, M.; Ali, R.; Alshehri, S.; Alzarea, S.I.; Elmowafy, M.; et al. Bioactive Apigenin loaded oral nano bilosomes: Formulation optimization to preclinical assessment. Saudi Pharm. J. 2021, 29, 269–279. [Google Scholar] [CrossRef]
- He, Y.; Luo, L.; Liang, S.; Long, M.; Xu, H. Influence of probe-sonication process on drug entrapment efficiency of liposomes loaded with a hydrophobic drug. Int. J. Polym. Mater. Polym. Biomater. 2019, 68, 193–197. [Google Scholar] [CrossRef]
- Mavaddati, M.A.; Moztarzadeh, F.; Baghbani, F. Effect of Formulation and Processing Variables on Dexamethasone Entrapment and Release of Niosomes. J. Clust. Sci. 2015, 26, 2065–2078. [Google Scholar] [CrossRef]
- Zhang, S.; Gao, H.; Bao, G. Physical Principles of Nanoparticle Cellular Endocytosis. ACS Nano 2015, 9, 8655–8671. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, S.; Mohamed, M.S.; Raveendran, S.; Rochani, A.K.; Maekawa, T.; Kumar, D.S. Formulation, characterization and evaluation of morusin loaded niosomes for potentiation of anticancer therapy. RSC Adv. 2018, 8, 32621–32636. [Google Scholar] [CrossRef] [Green Version]
- Patel, D.K.; Kesharwani, R.; Kumar, V. Etodolac loaded solid lipid nanoparticle based topical gel for enhanced skin delivery. Biocatal. Agric. Biotechnol. 2020, 29, 101810. [Google Scholar] [CrossRef]
- Riaz, A.; Hendrickx, S.; Elbrink, K.; Caljon, G.; Maes, L.; Ahmed, N.; Kiekens, F.; Khan, G.M. Preparation and Characterization of Nanostructured Lipid Carriers for Improved Topical Drug Delivery: Evaluation in Cutaneous Leishmaniasis and Vaginal Candidiasis Animal Models. AAPS PharmSciTech 2020, 21, 185. [Google Scholar] [CrossRef]
- Koca, H.D.; Doganay, S.; Turgut, A.; Tavman, I.H.; Saidur, R.; Mahbubul, I.M. Effect of particle size on the viscosity of nanofluids: A review. Renew. Sustain. Energy Rev. 2018, 82, 1664–1674. [Google Scholar] [CrossRef] [Green Version]
- Motawea, T.B.; El-Gawad, A. Topical phenytoin nanostructured lipid carriers: Design and development. Drug Dev. Ind. Pharm. 2018, 44, 144–151. [Google Scholar] [CrossRef]
- Uprit, S.; Sahu, R.K.; Roy, A.; Pare, A. Preparation and characterization of minoxidil loaded nanostructured lipid carrier gel for effective treatment of alopecia. Saudi Pharm. J. 2013, 21, 379–385. [Google Scholar] [CrossRef] [Green Version]
- Zeb, A.; Arif, S.T.; Malik, M.; Shah, F.A.; Din, F.U.; Qureshi, O.S.; Lee, E.S.; Lee, G.Y.; Kim, J.K. Potential of nanoparticulate carriers for improved drug delivery via skin. J. Pharm. Investig. 2019, 49, 485–517. [Google Scholar] [CrossRef] [Green Version]
- Dudhipala, N.; Mohammed, R.P.; Youssef, A.A.A.; Banala, N. Effect of lipid and edge activator concentration on development of aceclofenac-loaded transfersomes gel for transdermal application: In vitro and ex-vivo skin permeation. Drug Dev. Ind. Pharm. 2020, 46, 1334–1344. [Google Scholar] [CrossRef] [PubMed]
- Grande, F.; Ragno, G.; Muzzalupo, R.; Occhiuzzi, M.A.; Mazzotta, E.; De Luca, M.; Garofalo, A.; Ioele, G. Gel formulation of Nabumetone and a newly synthesized analog: Microemulsion as a photoprotective topical delivery system. Pharmaceutics 2020, 12, 423. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Liu, Y.; Zhang, Z.; Chen, M.; Zhang, D.; Tian, C.; Liu, M.; Jiang, G. The Antibacterial Activity and Mechanism of Action of Luteolin Against Trueperella pyogenes. Infect. Drug Resist. 2020, 13, 1697–1711. [Google Scholar] [CrossRef]




| Independent Variables | Code | Low (−1) | Medium (0) | High (+1) |
|---|---|---|---|---|
| Phospholipid 90 G (% w/v) | A | 70 | 80 | 90 |
| Edge activator (% w/v) | B | 10 | 20 | 30 |
| Sonication time (min) | C | 3 | 6 | 9 |
| Dependent Variables | ||||
| Particle size (nm) | Y1 | |||
| Encapsulation efficiency (%) | Y2 | |||
| Code | A (%, w/v) | B (%, w/v) | C (min) | Y1 (nm) | Y2 (%) |
|---|---|---|---|---|---|
| 1 | 80.00 | 20.00 | 6.00 | 213.8 ± 1.1 | 81.11 ± 3.3 |
| 2 | 80.00 | 20.00 | 6.00 | 215.1 ± 1.9 | 80.24 ± 3.9 |
| 3 | 90.00 | 10.00 | 6.00 | 222.4 ± 4.3 | 73.56 ± 2.6 |
| 4 | 90.00 | 30.00 | 6.00 | 214.2 ± 1.5 | 89.87 ± 4.1 |
| 5 | 80.00 | 20.00 | 6.00 | 212.6 ± 2.1 | 81.21 ± 3.5 |
| 6 | 70.00 | 10.00 | 6.00 | 148.5 ± 3.7 | 81.11 ± 3.2 |
| 7 | 80.00 | 30.00 | 3.00 | 155.2 ± 2.9 | 76.87 ± 4.1 |
| 8 | 90.00 | 20.00 | 3.00 | 187.2 ± 1.8 | 77.28 ± 4.7 |
| 9 | 90.00 | 20.00 | 9.00 | 254.6 ± 2.3 | 69.11 ± 3.2 |
| 10 | 80.00 | 10.00 | 9.00 | 166.6 ± 4.1 | 63.56 ± 1.9 |
| 11 | 70.00 | 30.00 | 6.00 | 202.4 ± 3.2 | 73.44 ± 2.1 |
| 12 | 80.00 | 30.00 | 9.00 | 231.1 ± 1.5 | 80.12 ± 1.7 |
| 13 | 80.00 | 10.00 | 3.00 | 164.7 ± 1.7 | 77.32 ± 2.3 |
| 14 | 70.00 | 20.00 | 9.00 | 182.4 ± 2.3 | 73.32 ± 3.2 |
| 15 | 70.00 | 20.00 | 3.00 | 173.3 ± 3.5 | 75.65 ± 2.7 |
| Model | R2 | Adjusted R2 | Predicted R2 | SD |
|---|---|---|---|---|
| (Y1) | ||||
| Linear | 0.6021 | 0.4924 | 0.2149 | 21.91 |
| 2F1 | 0.8406 | 0.7210 | 0.4411 | 16.25 |
| Quadratic | 0.9987 | 0.9963 | 0.9835 | 1.88 |
| (Y2) | ||||
| Linear | 0.2857 | 0.0909 | −0.4251 | 6.68 |
| 2F1 | 0.8383 | 0.7170 | 0.5789 | 3.73 |
| Quadratic | 0.9985 | 0.9959 | 0.9876 | 0.45 |
| Test Sample | Egg | Time (min) | Overall Score | |||
|---|---|---|---|---|---|---|
| 0 | 0.5 | 2 | 5 | |||
| LT-NVopG | Egg 1 | 0 | 0 | 0 | 0 | 0.15 |
| Egg 2 | 0 | 0 | 0 | 0 | ||
| Egg 3 | 0 | 0 | 0.2 | 0.4 | ||
| Egg 4 | 0 | 0 | 0 | 0 | ||
| Mean score | 0 | 0 | 0.05 | 0.1 | ||
| SLS, 1% w/v (Positive control) | Egg 1 | 0 | 0.8 | 0.8 | 2 | |
| Egg 2 | 0 | 0.8 | 1.2 | 2 | 2.8 | |
| Egg 3 | 0 | 0.8 | 1.2 | 1 | ||
| Egg 4 | 0 | 0 | 0.8 | 0 | ||
| Mean score | 0 | 0.6 | 1 | 1.2 | ||
| NaCl 0.9% w/v (Negative control) | Egg 1 | 0 | 0 | 0 | 0 | |
| Egg 2 | 0 | 0 | 0 | 0 | 0 | |
| Egg 3 | 0 | 0 | 0 | 0 | ||
| Egg 4 | 0 | 0 | 0 | 0 | ||
| Mean score | 0 | 0 | 0 | 0 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kazmi, I.; Al-Abbasi, F.A.; Nadeem, M.S.; Altayb, H.N.; Alshehri, S.; Imam, S.S. Formulation, Optimization and Evaluation of Luteolin-Loaded Topical Nanoparticulate Delivery System for the Skin Cancer. Pharmaceutics 2021, 13, 1749. https://doi.org/10.3390/pharmaceutics13111749
Kazmi I, Al-Abbasi FA, Nadeem MS, Altayb HN, Alshehri S, Imam SS. Formulation, Optimization and Evaluation of Luteolin-Loaded Topical Nanoparticulate Delivery System for the Skin Cancer. Pharmaceutics. 2021; 13(11):1749. https://doi.org/10.3390/pharmaceutics13111749
Chicago/Turabian StyleKazmi, Imran, Fahad A. Al-Abbasi, Muhammad Shahid Nadeem, Hisham N. Altayb, Sultan Alshehri, and Syed Sarim Imam. 2021. "Formulation, Optimization and Evaluation of Luteolin-Loaded Topical Nanoparticulate Delivery System for the Skin Cancer" Pharmaceutics 13, no. 11: 1749. https://doi.org/10.3390/pharmaceutics13111749
APA StyleKazmi, I., Al-Abbasi, F. A., Nadeem, M. S., Altayb, H. N., Alshehri, S., & Imam, S. S. (2021). Formulation, Optimization and Evaluation of Luteolin-Loaded Topical Nanoparticulate Delivery System for the Skin Cancer. Pharmaceutics, 13(11), 1749. https://doi.org/10.3390/pharmaceutics13111749







