Biodegradable Electrospun Nonwovens Releasing Propolis as a Promising Dressing Material for Burn Wound Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Monomers and Initiator
2.2. Copolymerization Procedure
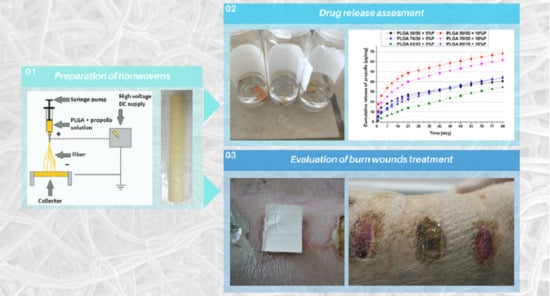
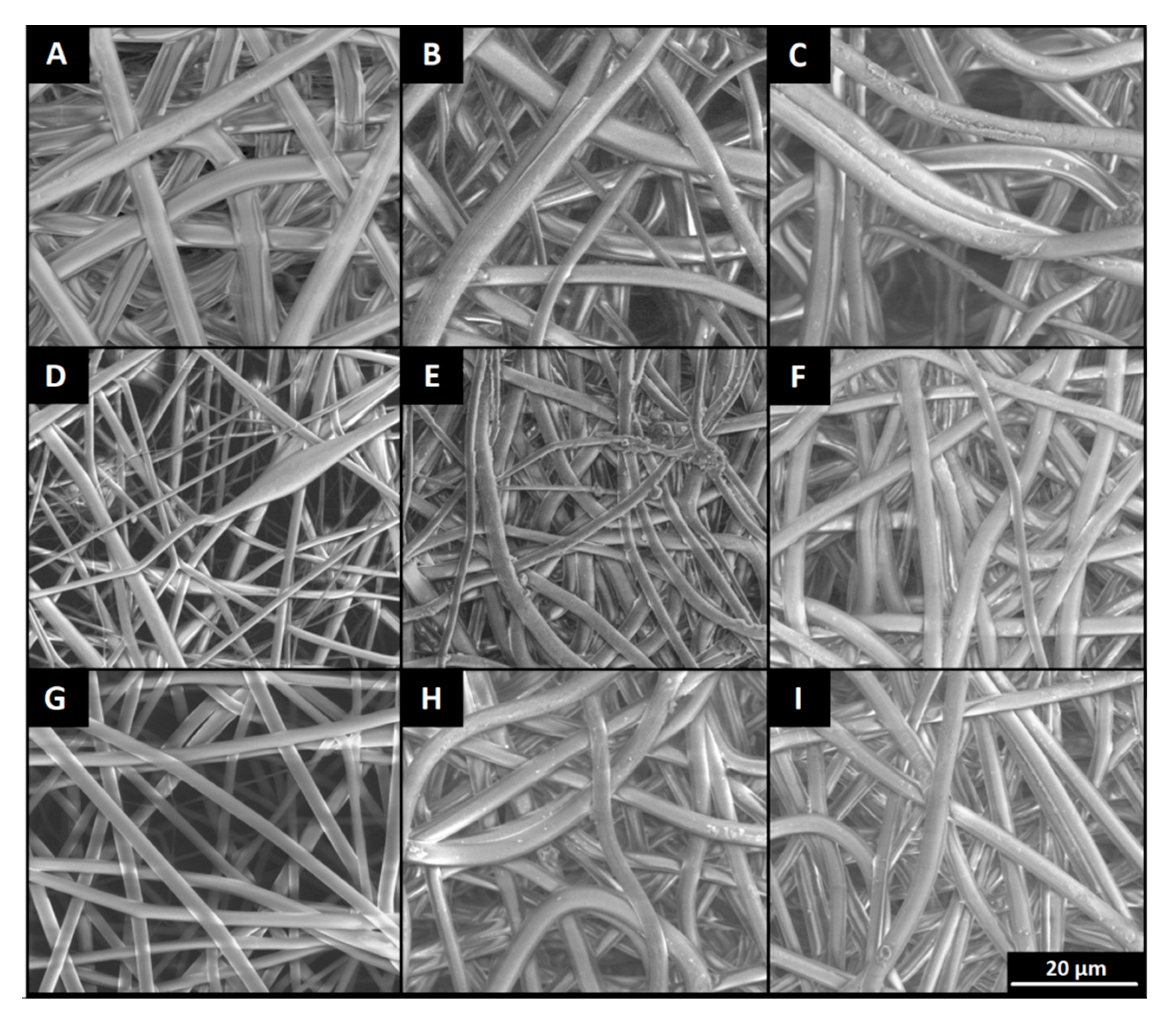
2.3. Preparation of Polymer Nonwovens by Electrospinning
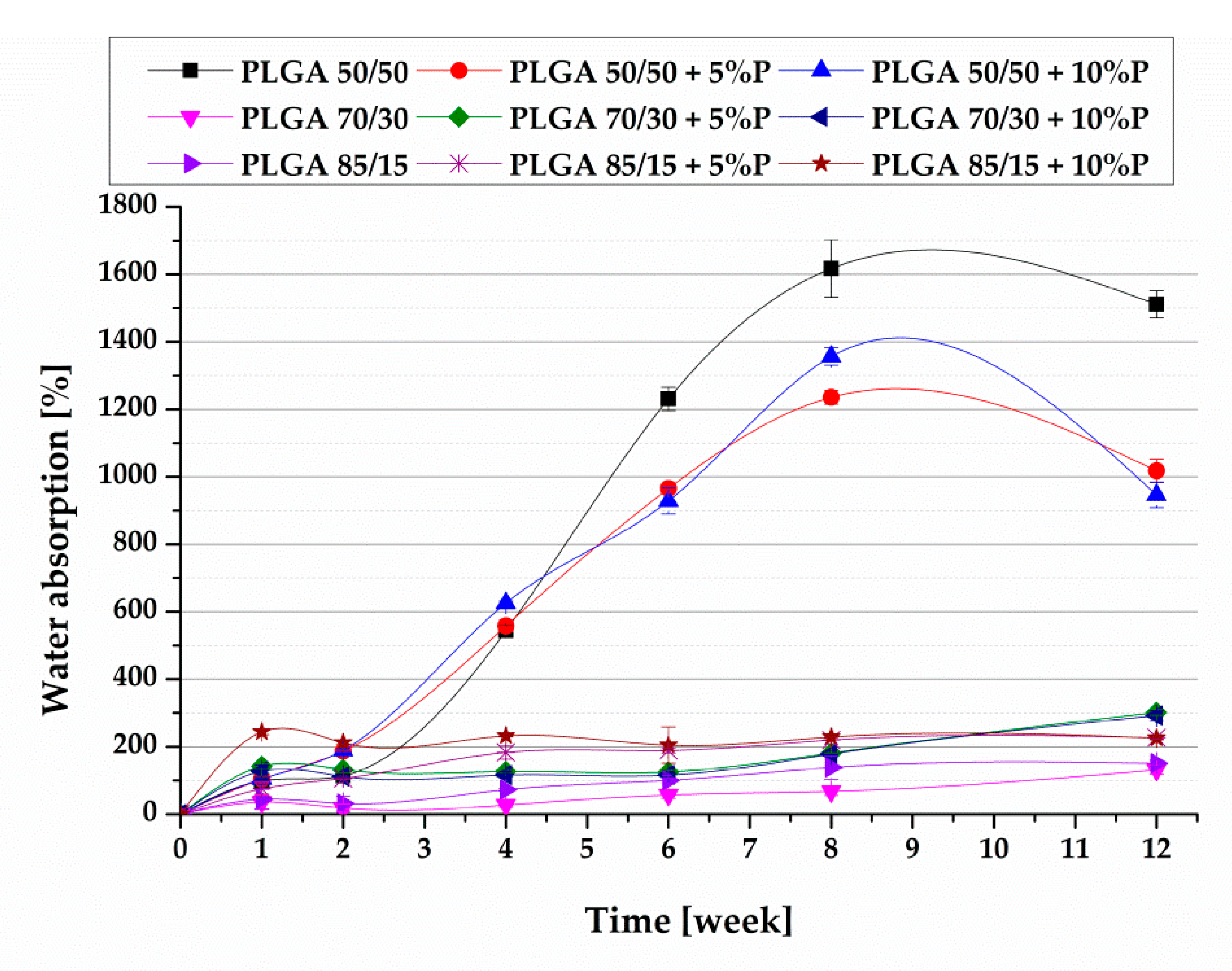
2.4. In Vitro Degradation
2.5. In Vitro Drug Release
2.6. Characterizations and Analysis
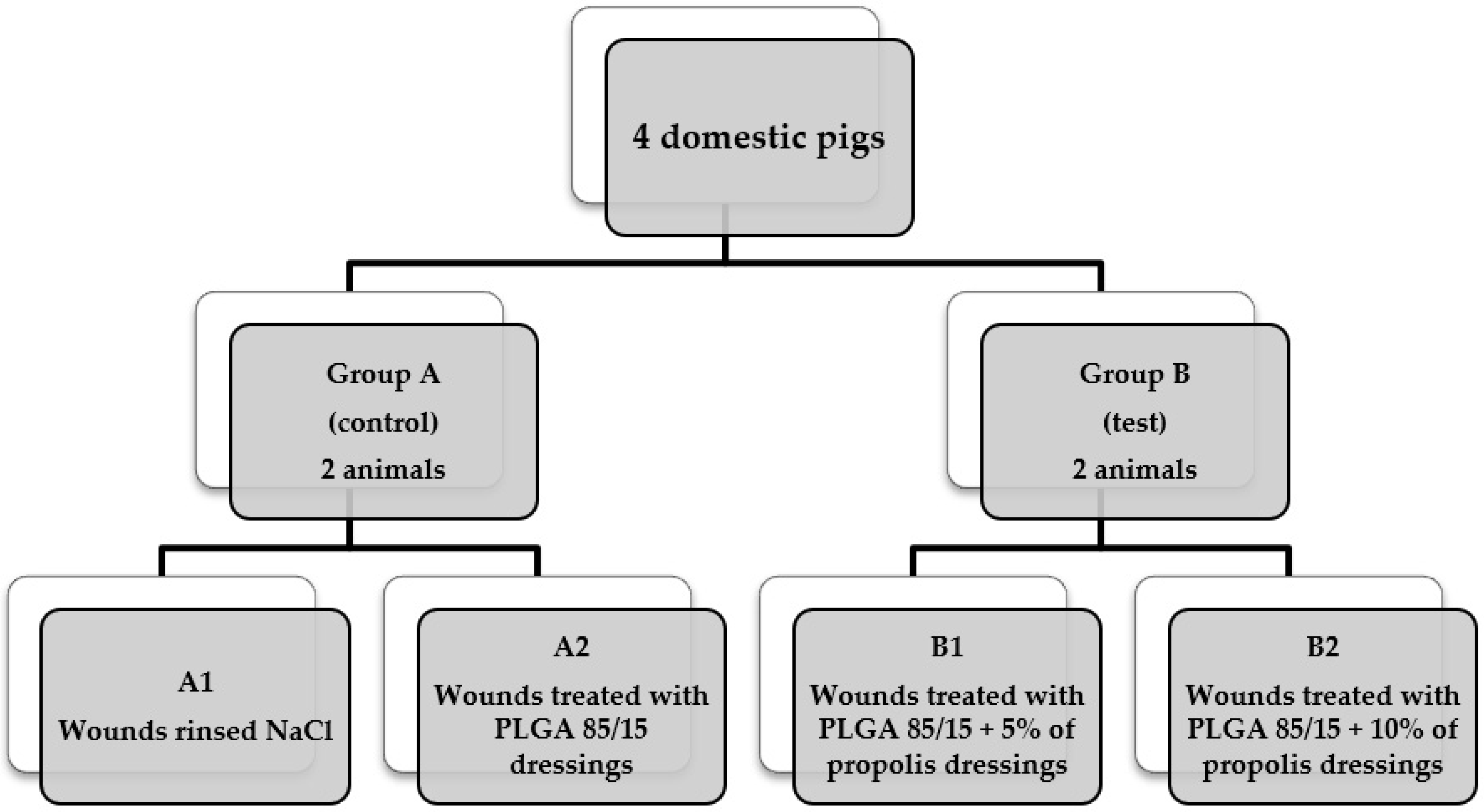
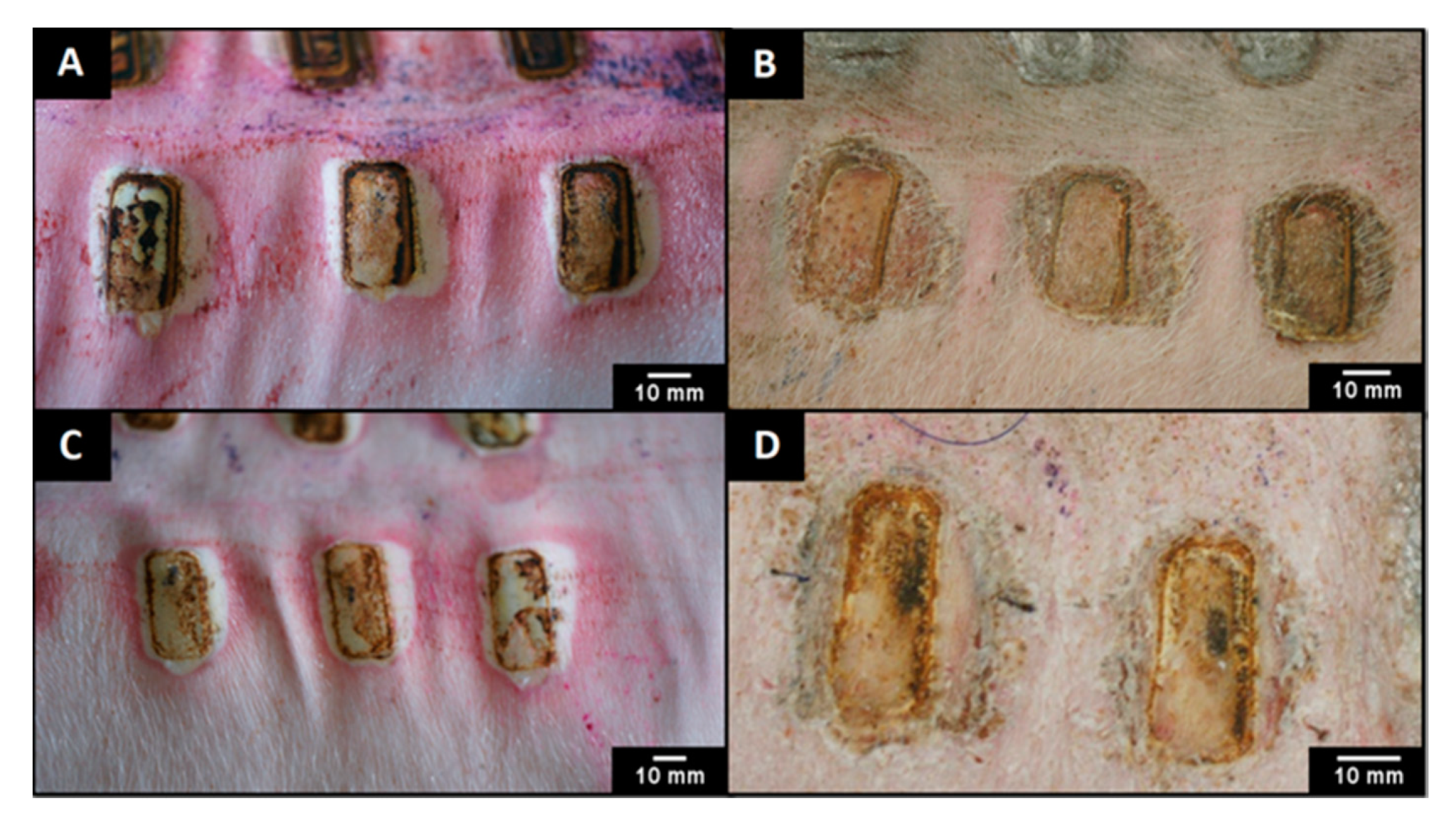
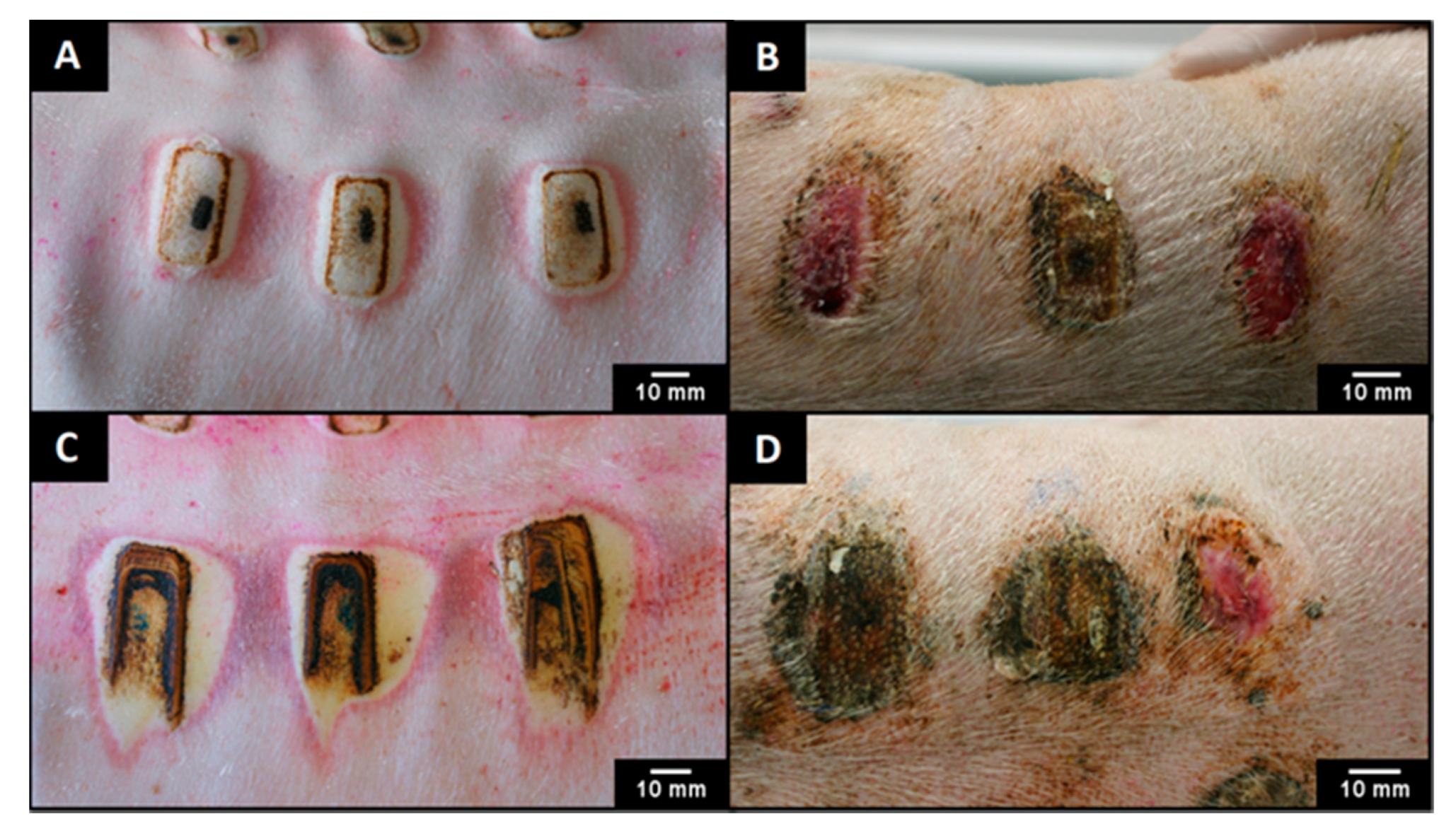
2.7. In Vivo Assessment
3. Results
3.1. In Vitro Degradation
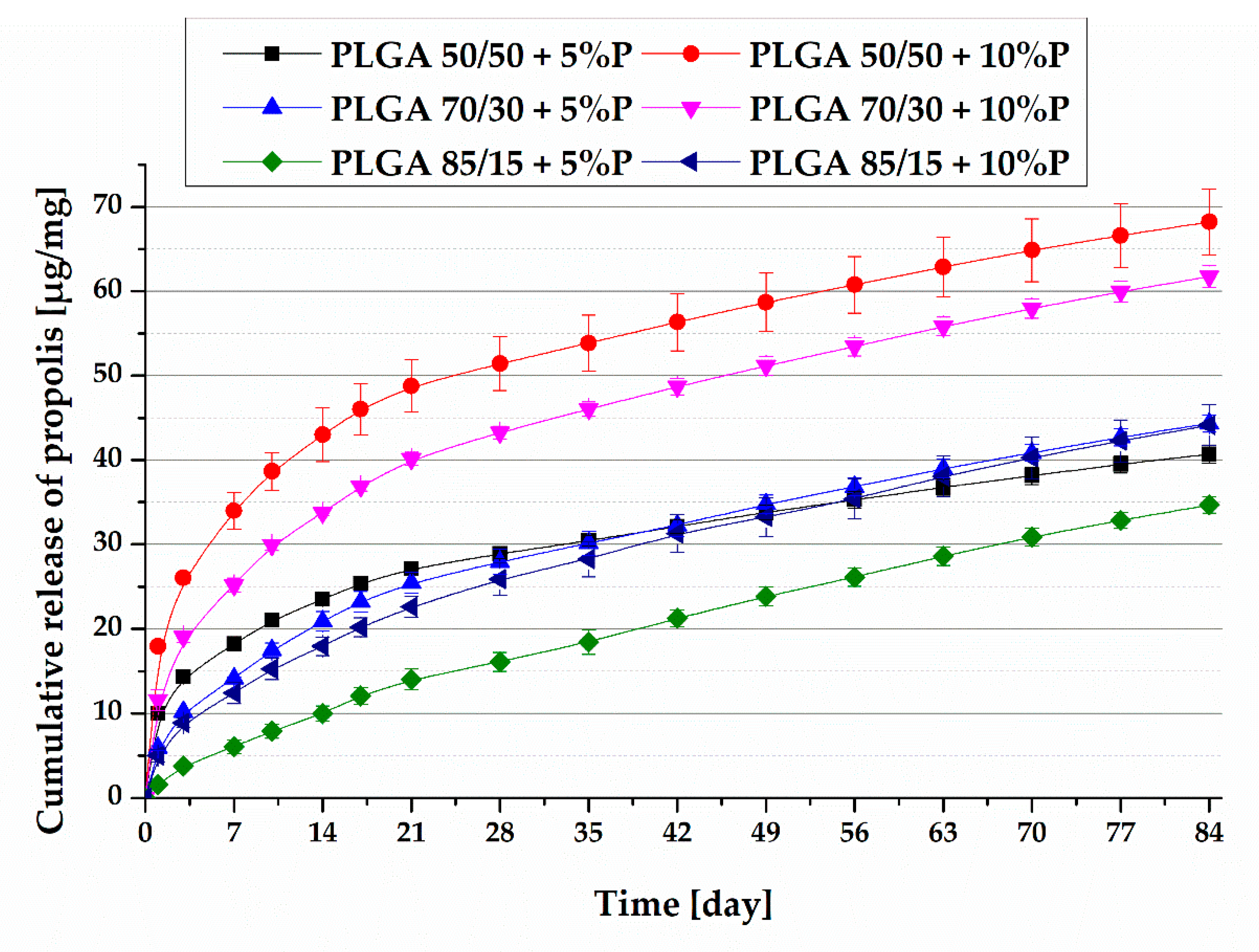
3.2. Drug Release
3.3. In Vivo Assessment
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Conflicts of Interest
References
- Rieger, K.A.; Birch, N.P.; Schiffman, J.D. Designing electrospun nanofiber mats to promote wound healing—A review. J. Mater. Chem. B 2013, 1, 4531–4541. [Google Scholar] [CrossRef] [PubMed]
- Boateng, J.S.; Matthews, K.H.; Stevens, H.N.E.; Eccleston, G.M. Wound healing dressings and drug delivery dystems: A review. J. Pharm. Sci. 2008, 97, 2892–2923. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, P.; Rezaeian, I.; Ranaei-Siadat, S.O.; Jafari, S.H.; Supaphol, P. A review on wound dressings with an emphasis on electrospun nanofibrous polymeric bandages. Polym. Adv. Technol. 2010, 21, 77–95. [Google Scholar] [CrossRef]
- Lee, J.W.; Song, K.Y. Evaluation of a polyurethane foam dressing impregnated with 3% povidone-iodine (Betafoam) in a rat wound model. Ann. Surg. Treat. Res. 2018, 94, 1–7. [Google Scholar] [CrossRef]
- Souza, S.O.L.; Cotrim, M.A.P.; Oréfice, R.L.; Carvalho, S.G.; Dutra, J.A.P.; de Paula Careta, F.; Resende, J.A.; Villanova, J.C.O.; Lee, J.W.; Song, K.Y. Electrospun poly(ε-caprolactone) matrices containing silver sulfadiazine complexed with β-cyclodextrin as a new pharmaceutical dosage form to wound healing: Preliminary physicochemical and biological evaluation. J. Mater. Sci. Mater. Med. 2018, 29, 67. [Google Scholar] [CrossRef]
- Gao, D.; Zhou, X.; Gao, Z.H.; Shi, X.; Wang, Z.; Wang, Y.; Zhang, P. Preparation and characterization of silver sulfadiazine–loaded polyvinyl alcohol hydrogels as an antibacterial wound dressing. J. Pharm. Sci. 2018, 107, 2377–2384. [Google Scholar] [CrossRef]
- Rembe, J.D.; Fromm-Dornieden, C.; Böhm, J.; Stuermer, E.K. Influence of human acute wound fluid on the antibacterial efficacy of different antiseptic polyurethane foam dressings: An in vitro analysis. Wound Repair Regen. 2018, 26, 27–35. [Google Scholar] [CrossRef]
- Schmidt, K.; Estes, C.; McLaren, A.; Spangehl, M.J. Chlorhexidine antiseptic irrigation eradicates staphylococcus epidermidis from biofilm: An in vitro study. Clin. Orthop. Relat. Res. 2018, 476, 648–653. [Google Scholar] [CrossRef]
- Canpolat, I.; Başa, A. Wound healing and current treatment techniques. Agric. Vet. Sci. 2017, 1, 180–184. [Google Scholar]
- Jawień, A.; Bartoszewicz, M.; Przondo-Mordarska, A.; Szewczyk, M.; Kaszuba, A.; Urbanek, T.; Staszkiewicz, W.; Sopata, M.; Kucharzewski, M.; Korzon-Burakowska, A.; et al. Wytyczne postępowania miejscowego i ogólnego w ranach objętych procesem infekcji. Leczenie Ran 2012, 9, 59–75. [Google Scholar]
- Dissemond, J.; Augustin, M.; Eming, S.A.; Goerge, T.; Horn, T.; Karrer, S.; Schumann, H.; Stücker, M. Modern wound care—Practical aspects of non-interventional topical treatment of patients with chronic wounds. J. Ger. Soc. Dermatol. 2014, 12, 541–554. [Google Scholar] [CrossRef] [PubMed]
- Sousa, M.P.; Neto, A.I.; Correia, T.R.; Miguel, S.P.; Matsusaki, M.; Correia, I.J.; Mano, J.F. Bioinspired multilayer membranes as potential adhesive patches for skin wound healing. Biomater. Sci. 2018, 6, 1962–1975. [Google Scholar] [CrossRef] [PubMed]
- Chew, S.Y.; Wen, Y.; Dzenis, Y.; Leong, K.W. The role of electrospinning in the emerging field of nanomedicine. Curr. Pharm. Des. 2006, 12, 4751–4770. [Google Scholar] [CrossRef] [PubMed]
- Adomavičiūtė, E.; Pupkevičiūtė, S.; Juškaitė, V.; Žilius, M.; Stanys, S.; Pavilonis, A.; Briedis, V. Formation and investigation of electrospun PLA materials with propolis extracts and silver nanoparticles for biomedical applications. J. Nanomater. 2017, 2017, 8612819. [Google Scholar] [CrossRef]
- Wang, J.; Planz, V.; Vukosavljevic, B.; Windbergs, M. Multifunctional electrospun nanofibers for wound application—Novel insights into the control of drug release and antimicrobial activity. Eur. J. Pharm. Biopharm. 2018, 129, 175–183. [Google Scholar] [CrossRef]
- Stojkovska, J.; Djurdjevic, Z.; Jancic, I.; Bufan, B.; Milenkovic, M.; Jankovic, R.; Miskovic-Stankovic, V.; Obradovic, B. Comparative in vivo evaluation of novel formulations based on alginate and silver nanoparticles for wound treatments. J. Biomater. Appl. 2018, 32, 1197–1211. [Google Scholar] [CrossRef]
- Hu, X.; Liu, S.; Zhou, G.; Huang, Y.; Xie, Z.; Jing, X. Electrospinning of polymeric nanofibers for drug delivery applications. J. Control. Release 2014, 185, 12–21. [Google Scholar] [CrossRef]
- Maciejowska, J.; Kasperczyk, J.; Dobrzyñski, P.; Bero, M. The influence of chain microstructure on hydrolytic degradation of glycolide/lactide copolymers used in drug delivery systems. J. Control Release 2006, 116, e6–e8. [Google Scholar] [CrossRef]
- Fredenberg, S.; Wahlgren, M.; Reslow, M.; Axelsson, A. The mechanisms of drug release in poly(lactic-co-glycolic acid)-based drug delivery systems—A review. Int. J. Pharm. 2011, 415, 34–52. [Google Scholar] [CrossRef]
- Kapoor, D.N.; Bhatia, A.; Kaur, R.; Sharma, R.; Kaur, G.; Dhawan, S. PLGA: A unique polymer for drug delivery. Ther. Deliv. 2015, 6, 41–58. [Google Scholar] [CrossRef]
- Arenbergerova, M.; Arenberger, P.; Bednar, M.; Kubat, P.; Mosinger, J. Light-activated nanofibre textiles exert antibacterial effects in the setting of chronic wound healing. Exp. Dermatol. 2012, 21, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Kędzia, B.; Hołderna-Kędzia, E. Chemical composition of propolis in nowadays researches. Herba Pol. 1991, 38, 95–107. [Google Scholar]
- Huang, S.; Zhang, C.P.; Wang, K.; Li, G.Q.; Hu, F.L. Recent advances in the chemical composition of propolis. Molecules 2014, 19, 19610–19632. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.L. Biological activity of bee propolis In health and disease. Asian Pac. J. Cancer Prev. 2006, 7, 22–31. [Google Scholar]
- De Castro, S.L. Propolis: Biological and pharmacological activities. Arbs Ann. Rev. Biomed. Sci. 2001, 3, 49–83. [Google Scholar]
- Olczyk, P.; Wisowski, G.; Komosinska-Vassev, K.; Stojko, J.; Klimek, K.; Olczyk, M.; Kozma, E.M. Propolis modifies collagen types I and III accumulation in the matrix of burnt tissue. Evid. Based. Complement. Altern. Med. 2013, 2013, 423809. [Google Scholar] [CrossRef]
- Olczyk, P.; Komosinska-Vassev, K.; Winsz-Szczotka, K.; Stojko, J.; Klimek, K.; Kozma, E.M. Propolis induces chondroitin/dermatan sulphate and hyaluronic acid accumulation in the skin of burned wound. Evid.-Based Complement. Altern. Med. 2013, 2013, 290675. [Google Scholar] [CrossRef]
- Olczyk, P.; Komosinska-Vassev, K.; Winsz-Szczotka, K.; Kozma, E.M.; Wisowski, G.; Stojko, J.; Klimek, K.; Olczyk, K. Propolis modulates vitronectin, laminin, and heparan sulfate/heparin expression during experimental burn healing. J. Zhejiang Univ. Sci. B 2012, 13, 932–941. [Google Scholar] [CrossRef]
- Olczyk, P.; Komosinska-Vassev, K.; Wisowski, G.; Mencner, L.; Stojko, J.; Kozma, E.M. Propolis modulates fibronectin expression in the matrix of thermal injury. Biomed Res. Int. 2014, 2014, 748101. [Google Scholar] [CrossRef]
- Oryan, A.; Alemzadeh, E.; Moshiri, A. Potential role of propolis in wound healing: Biological properties and therapeutic activities. Biomed. Pharmacother. 2018, 98, 469–483. [Google Scholar] [CrossRef]
- Ignatova, M.; Rashkov, I.; Manolova, N. Drug-loaded electrospun materials in wound -dressing applications and in local cancer treatment. Expert Opin. Drug Deliv. 2013, 10, 469–483. [Google Scholar] [CrossRef] [PubMed]
- Sarhan, W.A.; Azzazy, H.M. Apitherapeutics and phage-loaded nanofibers as wound dressings with enhanced wound healing and antibacterial activity. Nanomedicine 2017, 12, 2055–2067. [Google Scholar] [CrossRef] [PubMed]
- Kwiecińska-Piróg, J.; Skowron, K.; Śniegowska, A.; Przekwas, J.; Balcerek, M.; Załuski, D.; Gospodarek-Komkowska, E. The impact of ethanol extract of propolis on biofilm forming by Proteus Mirabilis strains isolated from chronic wounds infections. Nat. Prod. Res. 2019, 33, 3293–3297. [Google Scholar] [CrossRef] [PubMed]
- Kabała-Dzik, A.; Szaflarska-Stojko, E.; Wojtyczka, R.D.; Stojko, A.; Stojko, R.; Pacha, J. Comparative studies on the antimicrobial activity of propolis balm and silver sulphadiazine applied to burn wounds in pigs. Bull. Vet. Inst. Pulawy 2003, 47, 541–545. [Google Scholar]
- Komosinska-Vassev, K.; Olczyk, P.; Kasperczyk, J.; Pilawa, B.; Krzyminiewski, R.; Dobosz, B.; Ramos, P.; Stojko, J.; Stojko, M.; Ivanova, D.; et al. EPR spectroscopic examination of different types of paramagnetic centers in the blood in the course of burn healing. Oxid. Med. Cell. Longev. 2019, 2019. [Google Scholar] [CrossRef]
- Kim, J.I.; Pant, H.R.; Sim, H.-J.J.; Lee, K.M.; Kim, C.S. Electrospun propolis/polyurethane composite nanofibers for biomedical applications. Mater. Sci. Eng. C 2014, 44, 52–57. [Google Scholar] [CrossRef]
- Sutjarittangtham, K.; Sanpa, S.; Tunkasiri, T.; Chantawannakul, P.; Intatha, U.; Eitssayeam, S. Bactericidal effects of propolis/polylactic acid (PLA) nanofibres obtained via electrospinning. J. Apic. Res. 2014, 53, 109–115. [Google Scholar] [CrossRef]
- Dobrzynski, P.; Kasperczyk, J.; Janeczek, H.; Bero, M. Synthesis of biodegradable copolymers with the use of low toxic zirconium compounds. 1. Copolymerization of glycolide with l-lactide initiated by Zr(Acac)4. Macromolecules 2001, 34, 5090–5098. [Google Scholar] [CrossRef]
- Balata, G.; El Nahas, H.M.; Radwan, S. Propolis organogel as a novel topical delivery system for treating wounds. Drug Deliv. 2014, 21, 55–61. [Google Scholar] [CrossRef]
- Zong, X.; Ran, S.; Kim, K.-S.; Fang, D.; Hsiao, B.S.; Chu, B. Structure and morphology changes during in vitro degradation of electrospun poly(glycolide-co-lactide) nanofiber membrane. Biomacromolecules 2003, 4, 416–423. [Google Scholar] [CrossRef]
- Blackwood, K.A.; McKean, R.; Canton, I.; Freeman, C.O.; Franklin, K.L.; Cole, D.; Brook, I.; Farthing, P.; Rimmer, S.; Haycock, J.W.; et al. Development of biodegradable electrospun scaffolds for dermal replacement. Biomaterials 2008, 29, 3091–3104. [Google Scholar] [CrossRef] [PubMed]
- Olczyk, P.; Komosinska-Vassev, K.; Krzyminiewski, R.; Kasperczyk, J.; Ramos, P.; Dobosz, B.; Batoryna, O.; Stojko, J.; Stojko, M.; Ivanova, D.; et al. The estimation of blood paramagnetic center changes during burns management with biodegradable propolis-nanofiber dressing. Oxid. Med. Cell. Longev. 2020, 2020, 3675603. [Google Scholar] [CrossRef] [PubMed]



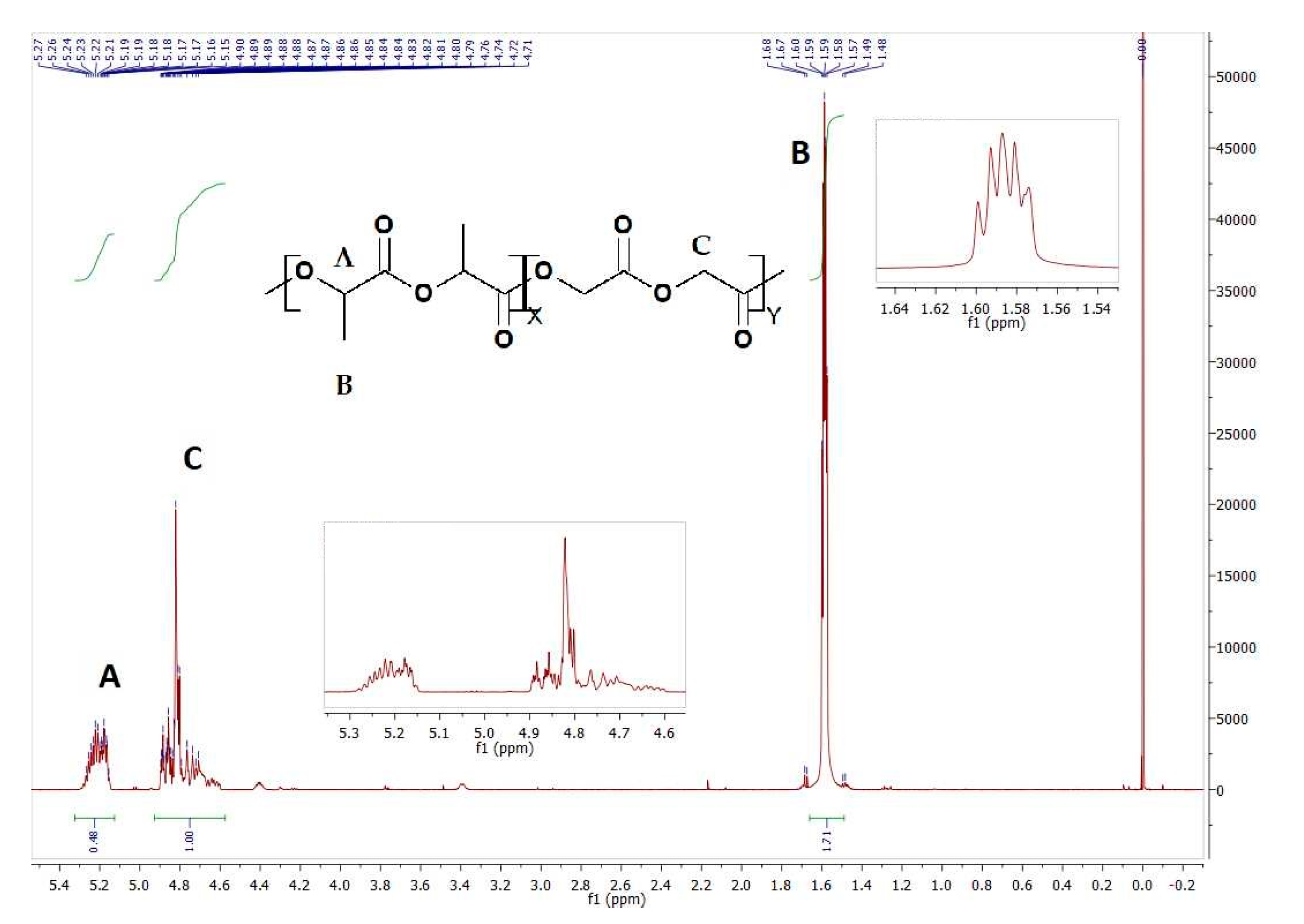
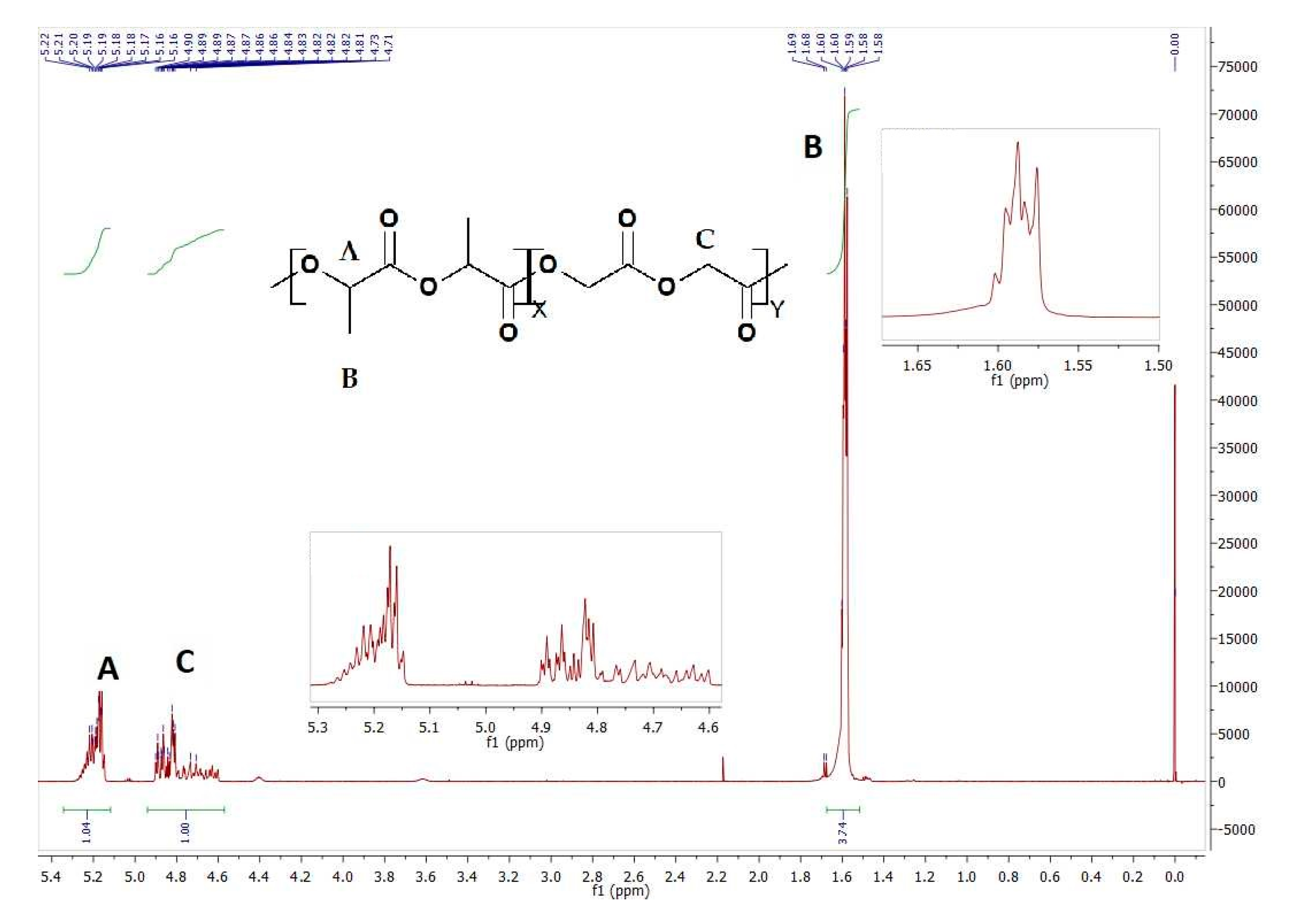
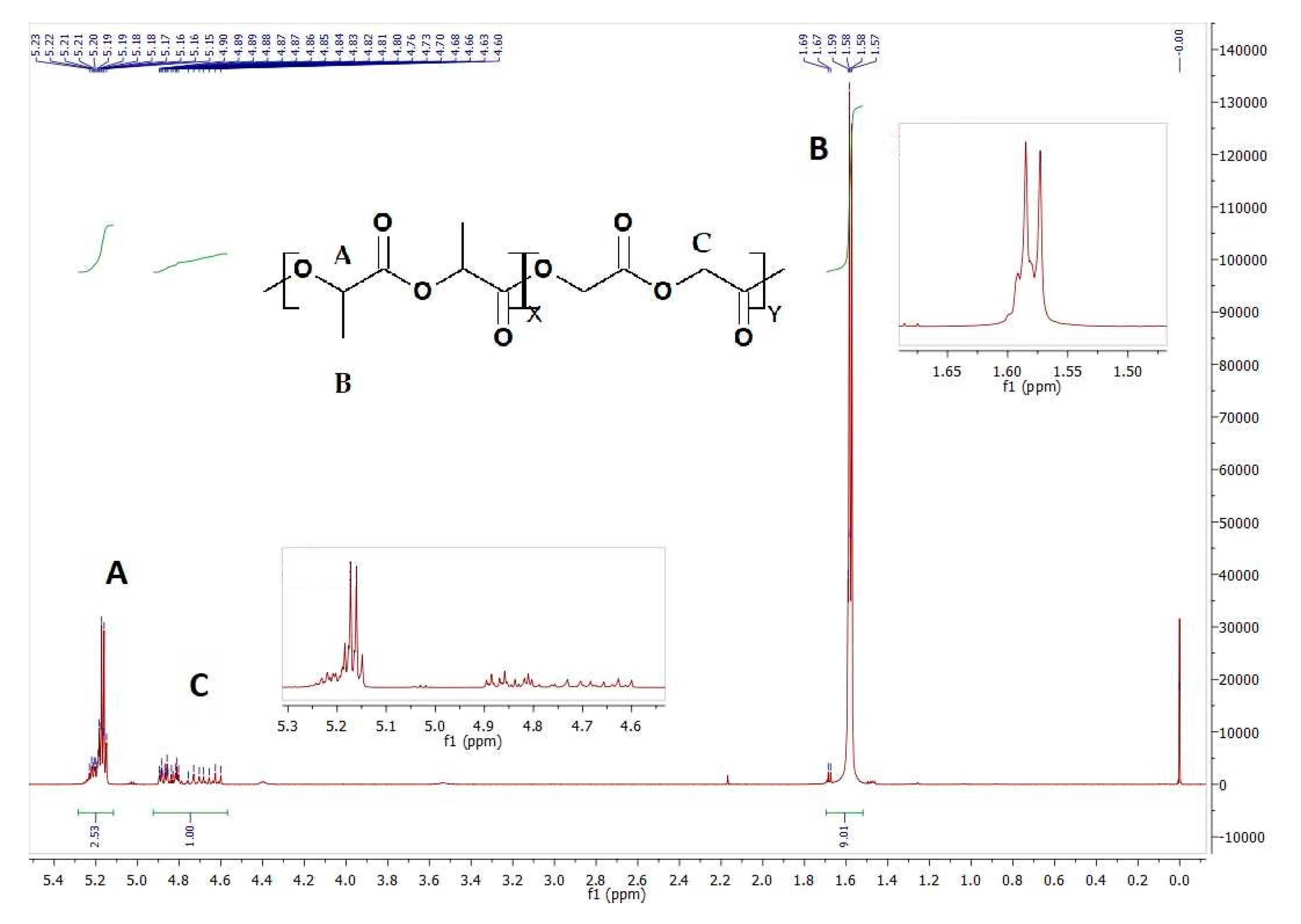
| Sample | Copolymer | Mn [kDa] | Mw [kDa] | D | Tg [°C] |
|---|---|---|---|---|---|
| PLGA 85/15 | (l-LA 83%: GL 17%) | 42.0 | 104.1 | 2.48 | 57 |
| PLGA 70/30 | (l-LA 68%: GL 32%) | 48.9 | 102.0 | 2.1 | 53 |
| PLGA 50/50 | (l-LA 49%: GL 51%) | 31.9 | 78.6 | 2.46 | 48 |
| Content of Propolis | None | 5% | 10% |
|---|---|---|---|
| Unit Ratio | Tg [°C] | ||
| 50/50 | 48 | 47 | 43 |
| 70/30 | 53 | 51 | 50 |
| 85/15 | 57 | 54 | 53 |
| Content of Propolis | None | 5% | 10% | ||||
|---|---|---|---|---|---|---|---|
| Unit Ratio | Degradation Time [weeks] | [l-LA] [%] | [GL] [%] | [l-LA] [%] | [GL] [%] | [l-LA] [%] | [GL] [%] |
| 50/50 | 0 | 49 | 51 | 49 | 51 | 49 | 51 |
| 6 | 55 | 45 | 54 | 46 | 53 | 47 | |
| 12 | 60 | 40 | 57 | 43 | 57 | 43 | |
| 70/30 | 0 | 68 | 32 | 68 | 32 | 68 | 32 |
| 6 | 68 | 32 | 69 | 31 | 69 | 31 | |
| 12 | 71 | 29 | 71 | 29 | 71 | 29 | |
| 85/15 | 0 | 83 | 17 | 83 | 17 | 83 | 17 |
| 6 | 83 | 17 | 84 | 16 | 84 | 16 | |
| 12 | 83 | 17 | 84 | 16 | 84 | 16 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stojko, M.; Włodarczyk, J.; Sobota, M.; Karpeta-Jarząbek, P.; Pastusiak, M.; Janeczek, H.; Dobrzyński, P.; Starczynowska, G.; Orchel, A.; Stojko, J.; et al. Biodegradable Electrospun Nonwovens Releasing Propolis as a Promising Dressing Material for Burn Wound Treatment. Pharmaceutics 2020, 12, 883. https://doi.org/10.3390/pharmaceutics12090883
Stojko M, Włodarczyk J, Sobota M, Karpeta-Jarząbek P, Pastusiak M, Janeczek H, Dobrzyński P, Starczynowska G, Orchel A, Stojko J, et al. Biodegradable Electrospun Nonwovens Releasing Propolis as a Promising Dressing Material for Burn Wound Treatment. Pharmaceutics. 2020; 12(9):883. https://doi.org/10.3390/pharmaceutics12090883
Chicago/Turabian StyleStojko, Mateusz, Jakub Włodarczyk, Michał Sobota, Paulina Karpeta-Jarząbek, Małgorzata Pastusiak, Henryk Janeczek, Piotr Dobrzyński, Gabriela Starczynowska, Arkadiusz Orchel, Jerzy Stojko, and et al. 2020. "Biodegradable Electrospun Nonwovens Releasing Propolis as a Promising Dressing Material for Burn Wound Treatment" Pharmaceutics 12, no. 9: 883. https://doi.org/10.3390/pharmaceutics12090883
APA StyleStojko, M., Włodarczyk, J., Sobota, M., Karpeta-Jarząbek, P., Pastusiak, M., Janeczek, H., Dobrzyński, P., Starczynowska, G., Orchel, A., Stojko, J., Batoryna, O., Olczyk, P., Komosińska-Vassev, K., Olczyk, K., & Kasperczyk, J. (2020). Biodegradable Electrospun Nonwovens Releasing Propolis as a Promising Dressing Material for Burn Wound Treatment. Pharmaceutics, 12(9), 883. https://doi.org/10.3390/pharmaceutics12090883