Recycling of Almond By-Products for Intestinal Inflammation: Improvement of Physical-Chemical, Technological and Biological Characteristics of a Dried Almond Skins Extract
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Acetonic Almond Skins Extract (ASE) Production and YIELD of the Process (Y)
2.3. Almond Skins Extract (ASE) Technological Characterization
2.4. Formulation Studies
2.5. Complex Characterization
2.5.1. Liophylization Process Yield (LPY), Actual Extract Content (AEC) and Inclusion Efficiency (IE)
2.5.2. Absorption Spectra
2.5.3. Differential Scanning Calorimetry (DSC)
2.5.4. Morphology
2.5.5. Solubility Studies: Complex Water Solubility (WS) and Dissolution Rate
2.6. Biological Activity
2.6.1. Cell Culture
2.6.2. Cell Treatment
2.6.3. Antiproliferative Activity Evaluation
2.6.4. Tumor Necrosis Factor (TNF-α) Evaluation
2.6.5. Evaluation of COX-2 and iNOS Expression by Cytofluorimetry
2.6.6. Intracellular Reactive Oxygen Species (ROS) Release Evaluation
2.7. Data Analysis
3. Results and Discussion
3.1. Technological Data
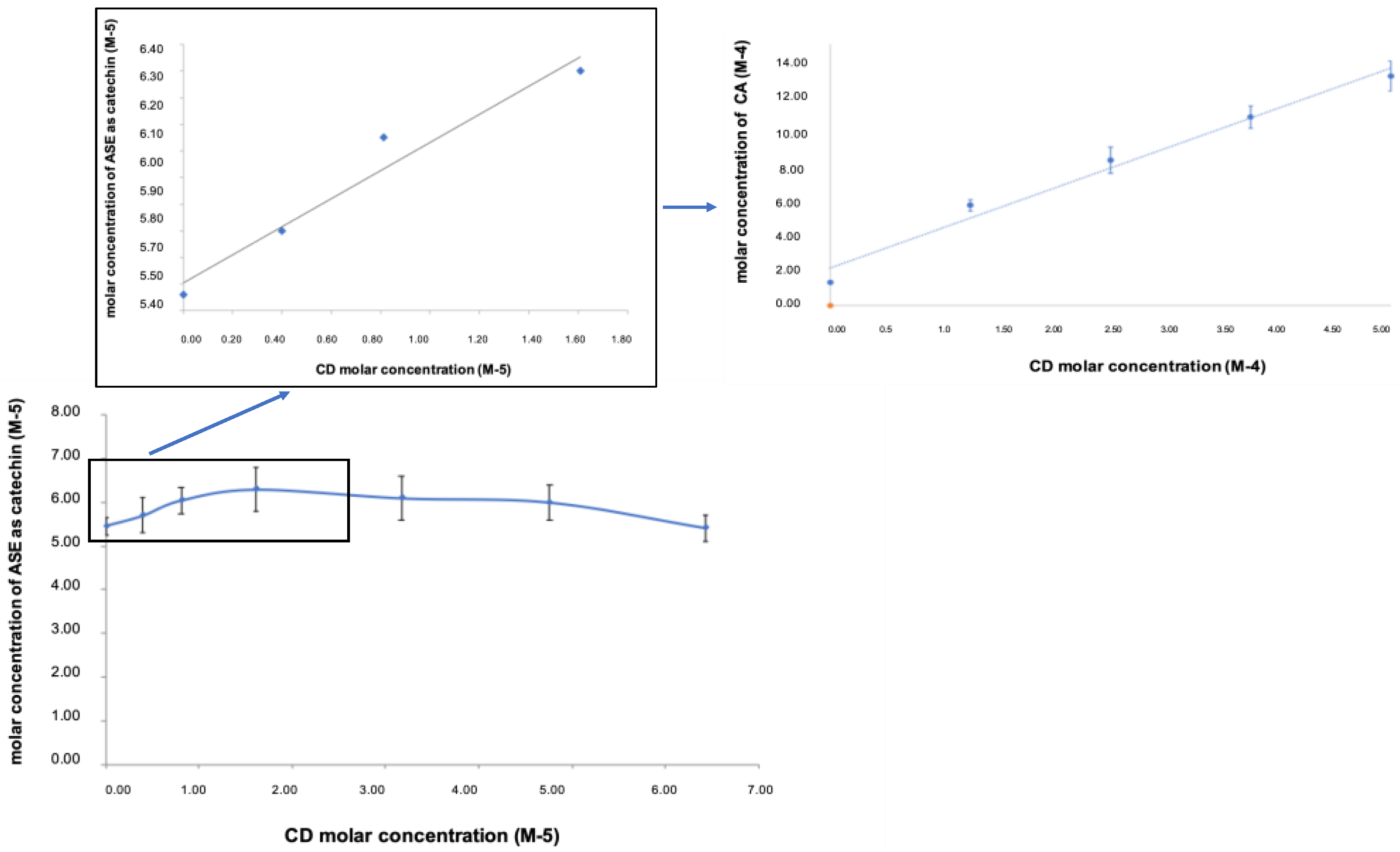
3.1.1. Pre-Formulation and Formulation Studies
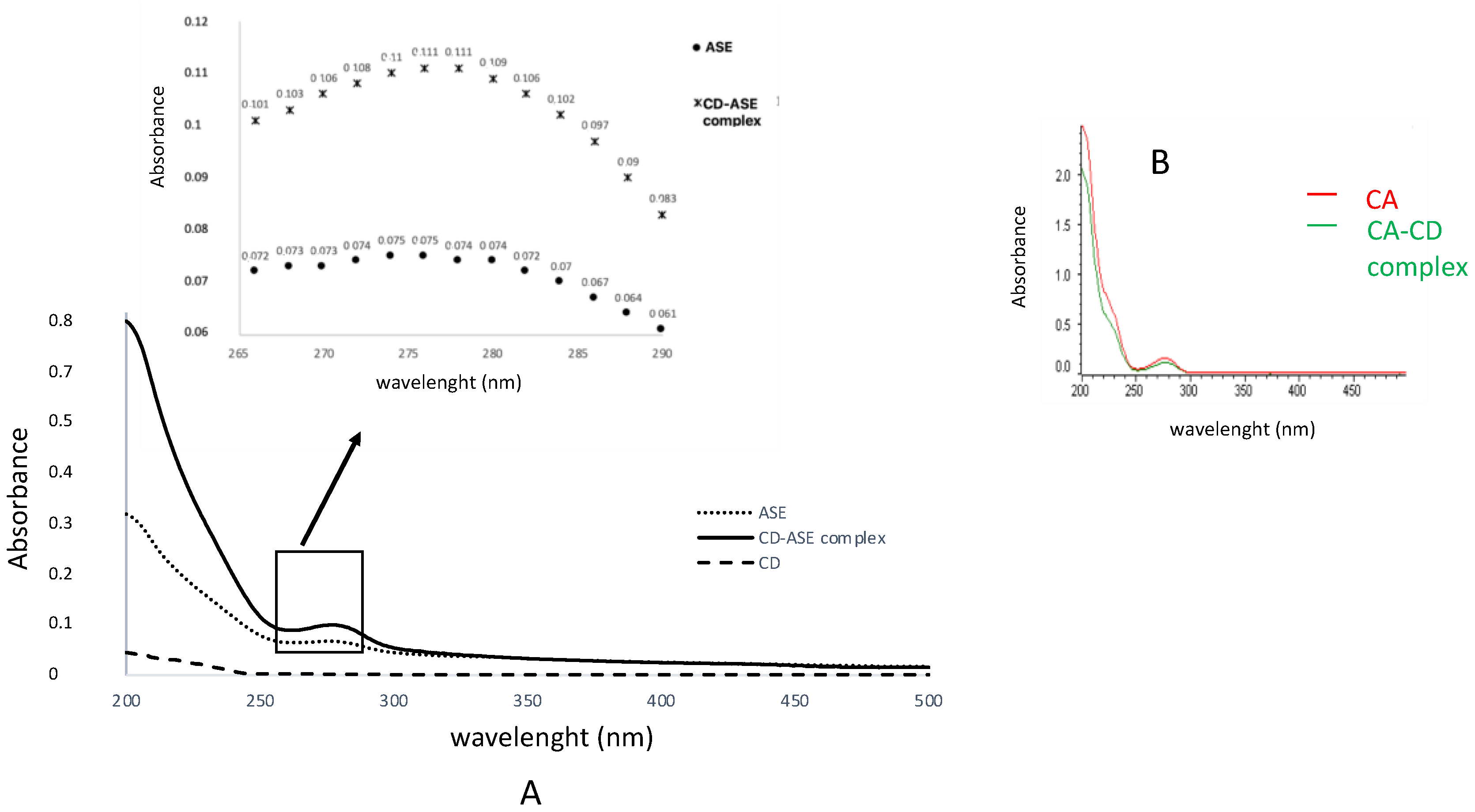
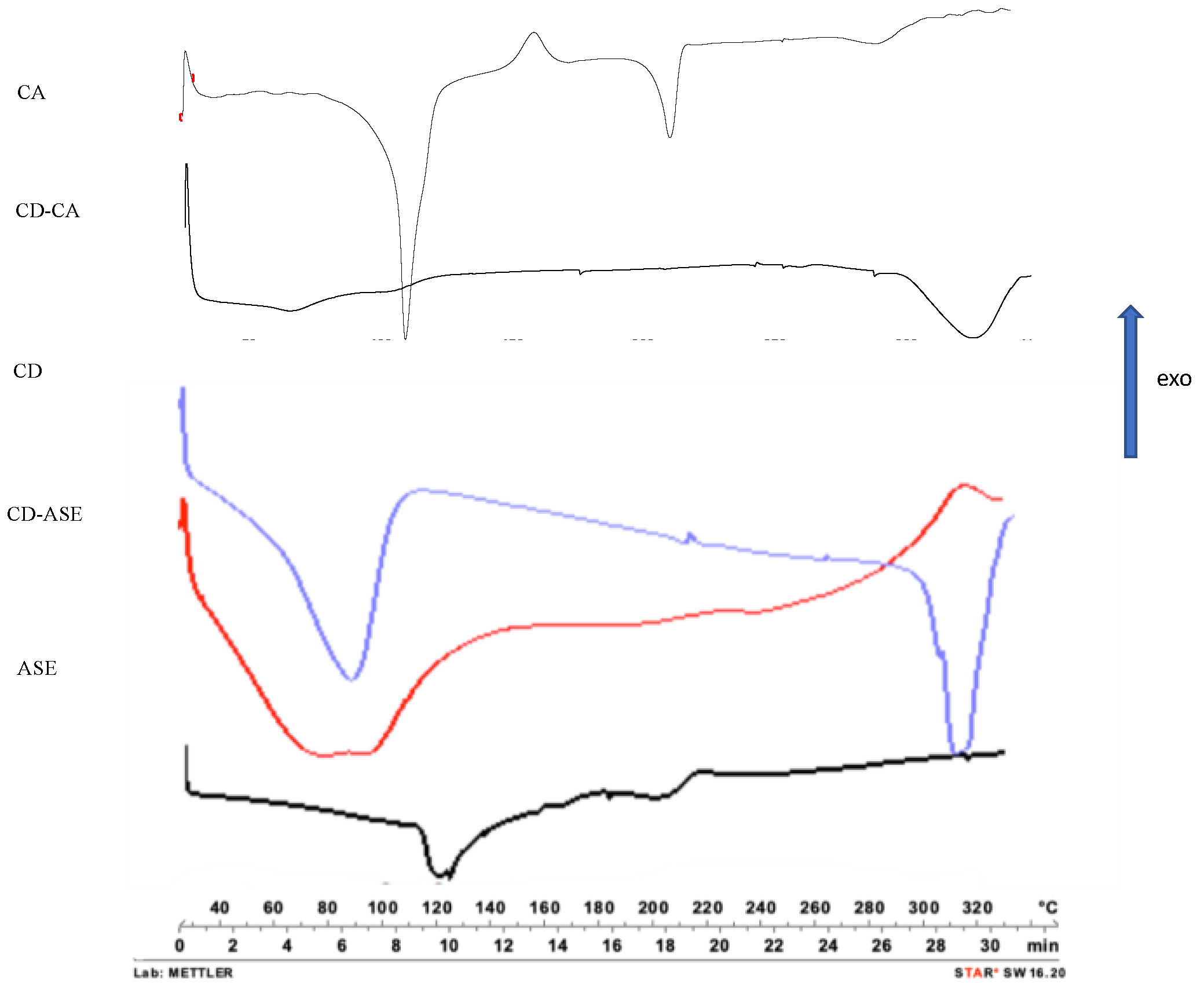
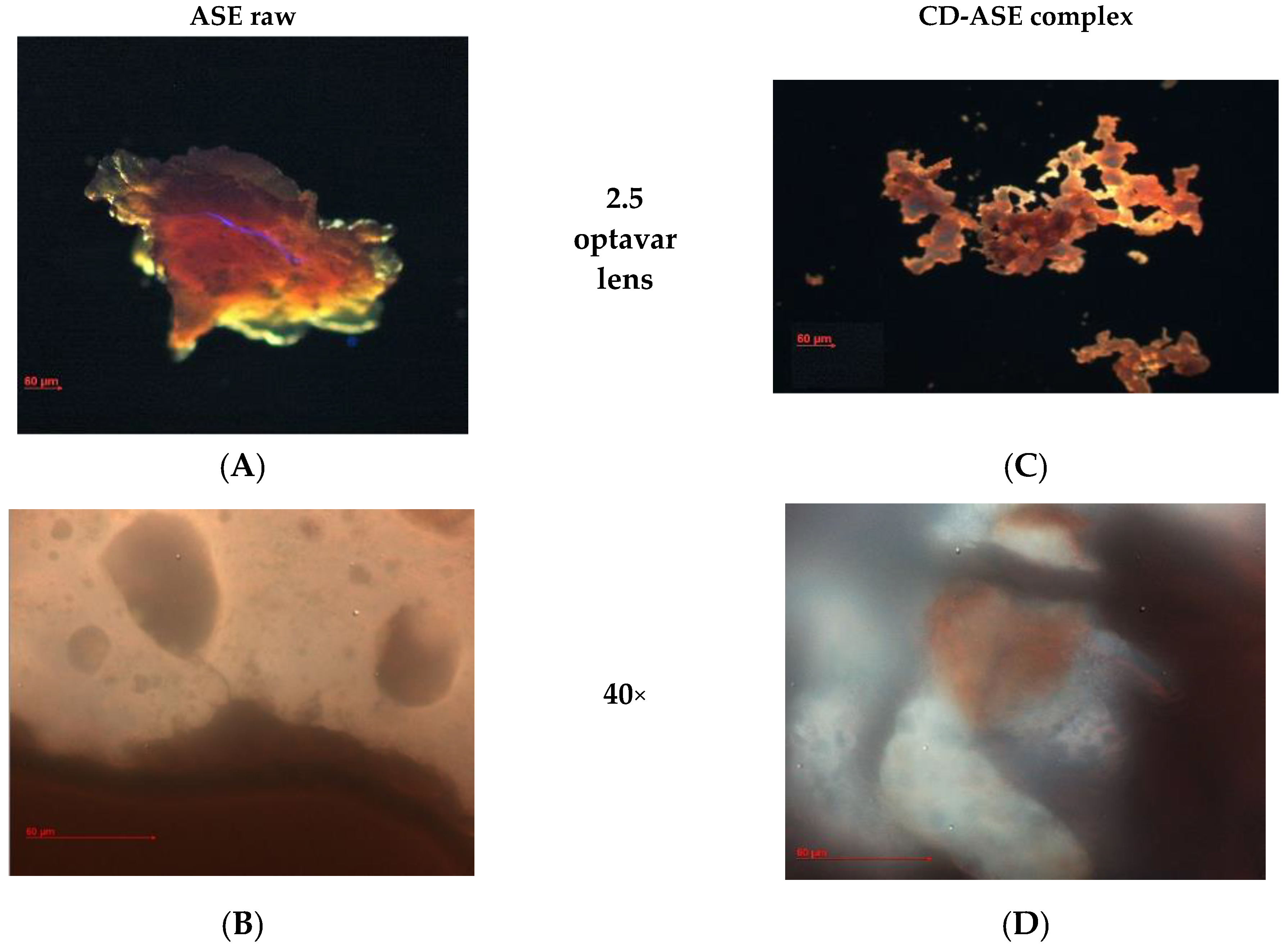
3.1.2. Complex Characterization
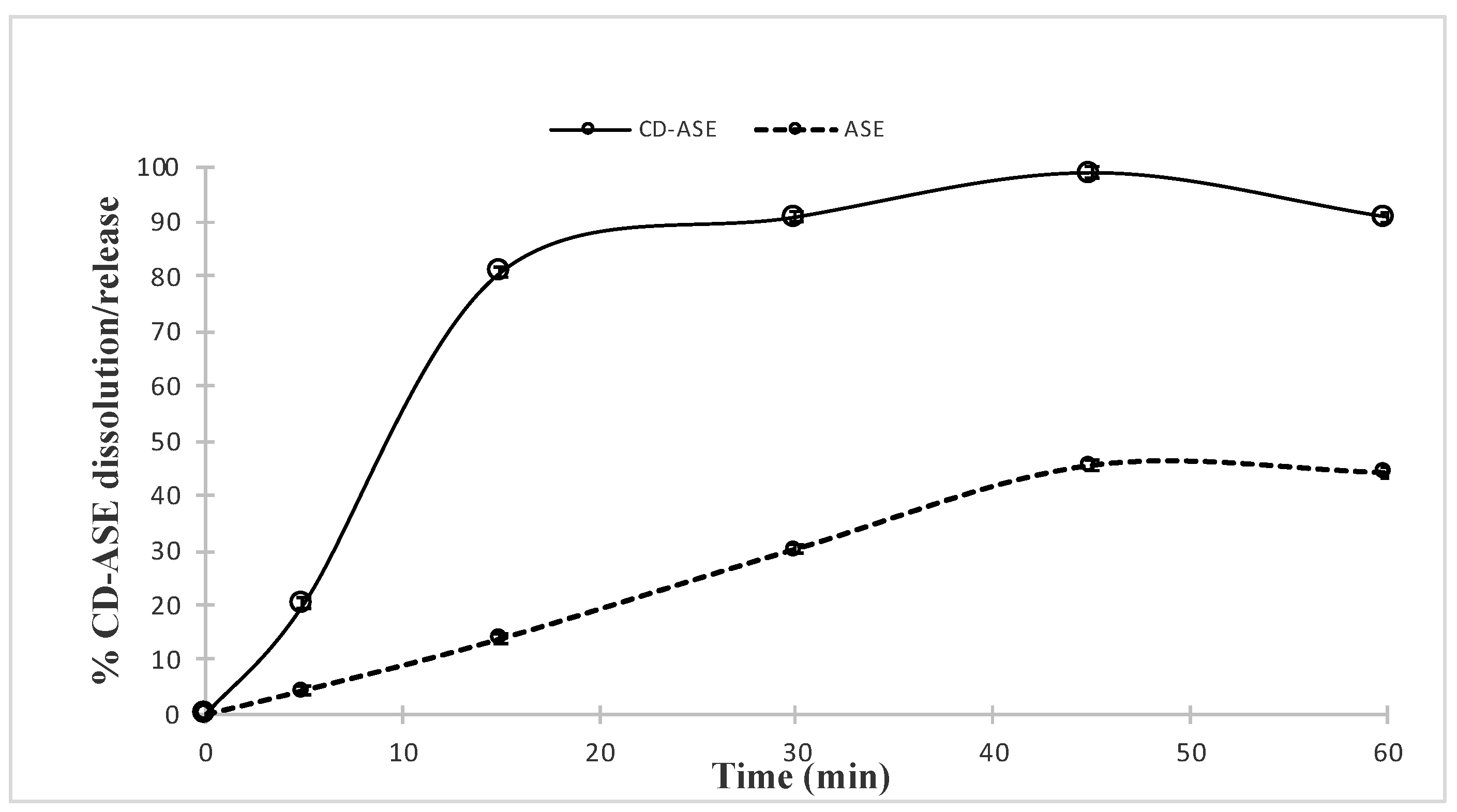
3.1.3. CD-ASE Solubility Studies
3.2. Biological Activity
3.2.1. ASE, and CD-ASE Did Not Affect IEC-6 Viability
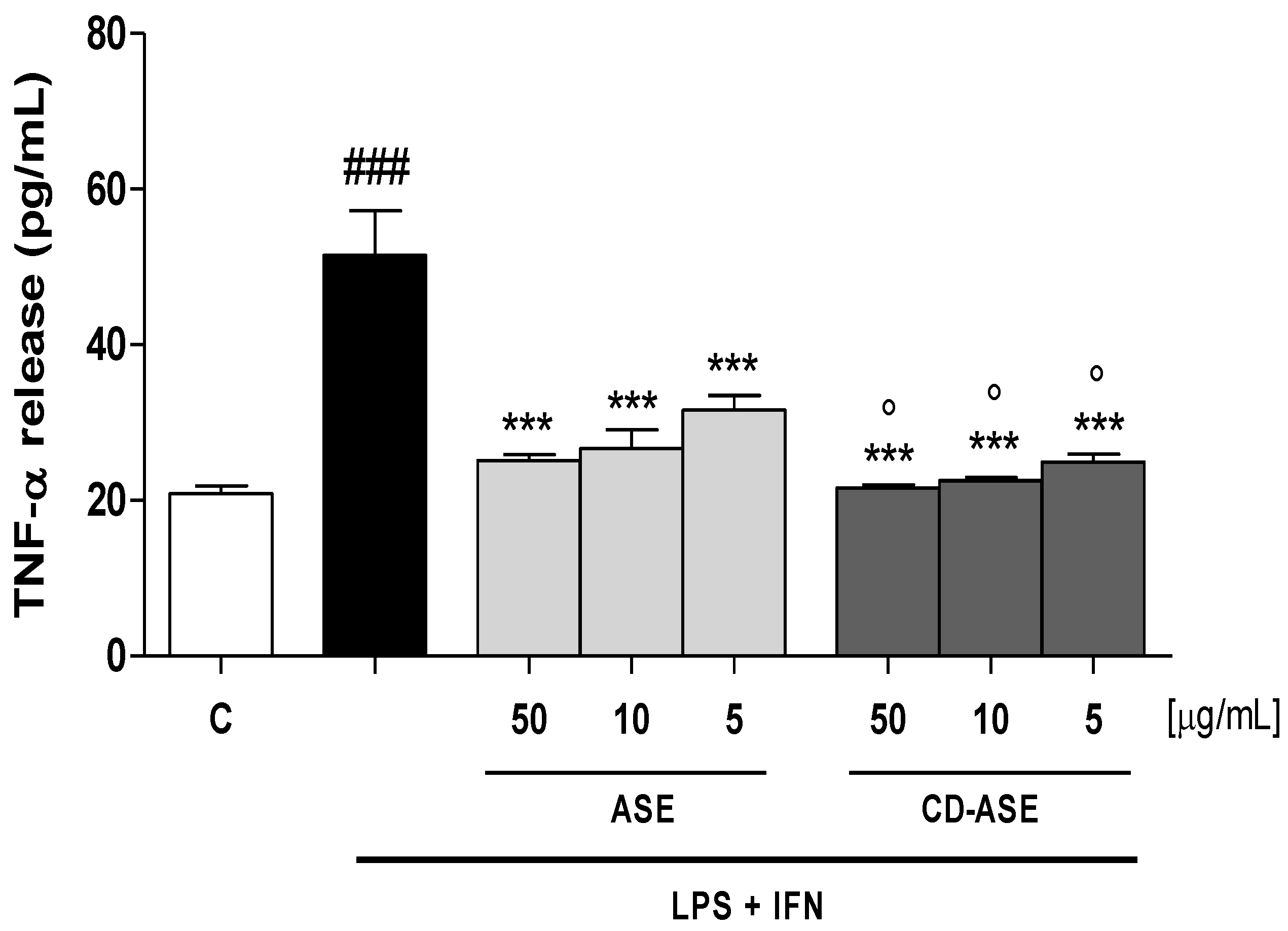
3.2.2. ASE and CD-ASE Reduced TNF-α Release in LPS + IFN-Stimulated IEC-6 Cells
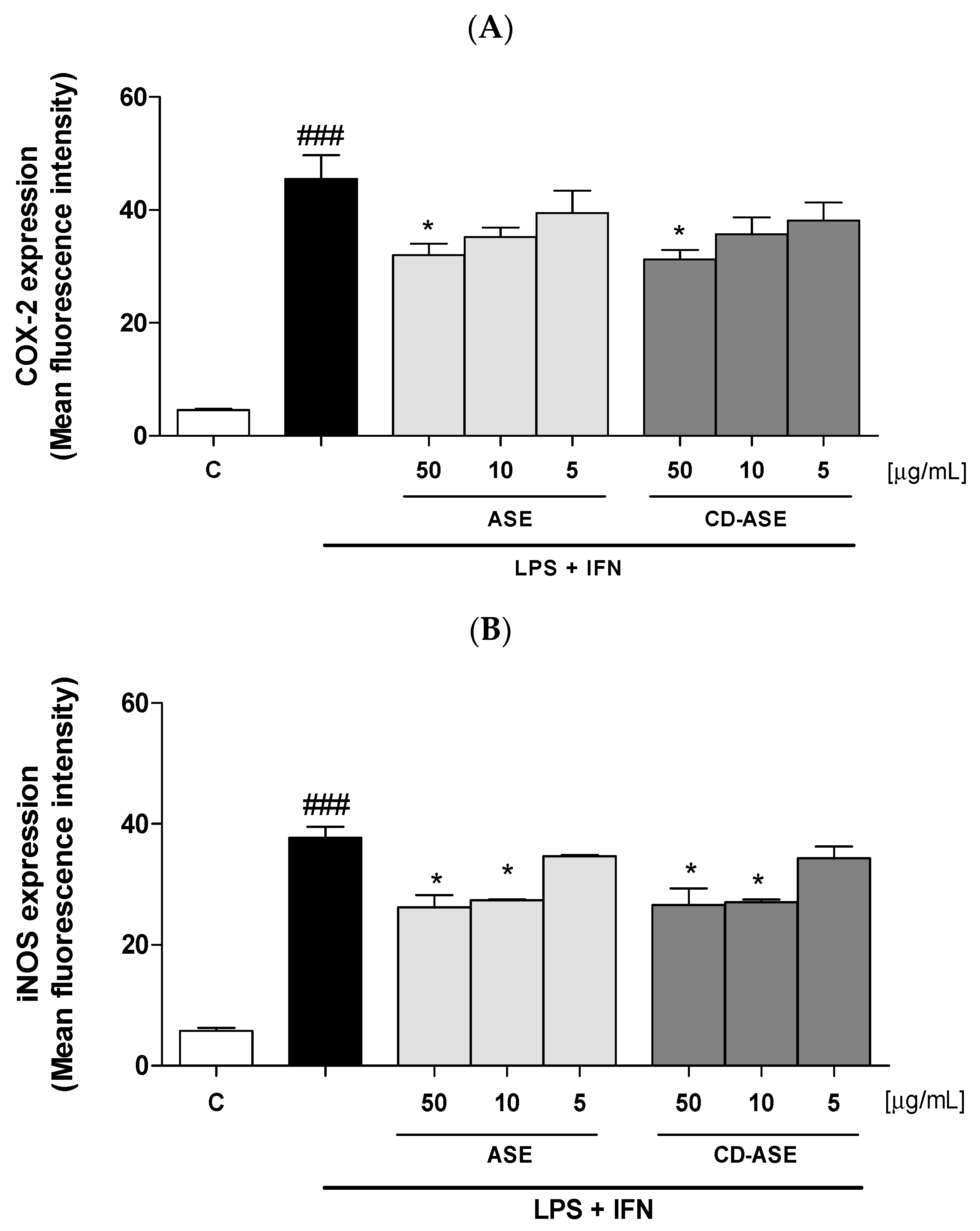
3.2.3. ASE and CD-ASE Inhibited COX-2 and iNOS Expression in LPS + IFN-Stimulated IEC-6 Cells
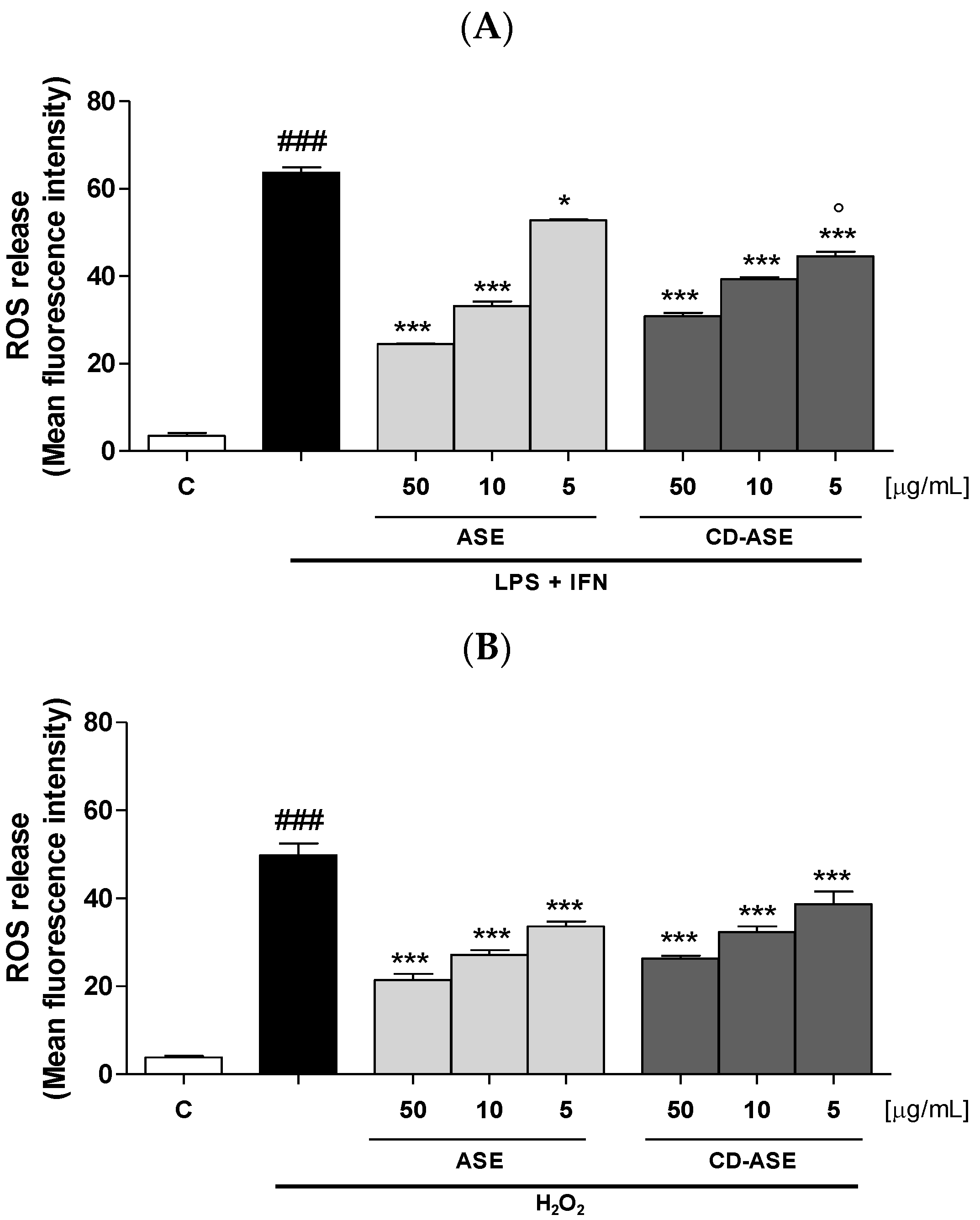
3.2.4. ASE and CD-ASE Reduced Intracellular ROS Release in IEC-6 Cells
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Food and Drug Administration (FDA). Qualified Health Claims: Letter of Enforcement Discretion-Nuts and Coronary Heart Disease; Docket 02P-0505; Food and Drug Administration: Washington, DC, USA, 2003. Available online: http://wayback.archive-it.org/7993/20171114183724/https://www.fda.gov/Food/IngredientsPackagingLabeling/LabelingNutrition/ucm072926.htm (accessed on 28 May 2020).
- Gulati, O.P.; Berry Ottaway, P. Legislation relating to nutraceuticals in the European Union with a particular focus on botanical-sourced products. Toxicology 2006, 221, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Prgomet, I.; Gonçalves, B.; Domínguez-Perles, R.; Pascual-Seva, N.; Barros, A.I. Valorization Challenges to Almond Residues: Phytochemical Composition and Functional Application. Molecules 2017, 22, 1774. [Google Scholar] [CrossRef] [PubMed]
- Garrido, I.; Monagas, M.; Gómez-Cordovés, C.; Bartolomé, B. Polyphenols and Antioxidant Properties of Almond Skins: Influence of Industrial Processing. J. Food Sci. 2008, 73, C106–C115. [Google Scholar] [CrossRef]
- Monagas, M.; Garrido, I.; Lebrón-Aguilar, R.; Bartolomé, B.; Gómez-Cordovés, C. Almond (Prunus dulcis (Mill.) D.A. Webb) Skins as a Potential Source of Bioactive Polyphenol. J. Agric. Food Chem. 2007, 55, 8498–8507. [Google Scholar] [CrossRef]
- Heather, J. Almond Skins as a Natural Antioxidant. All Theses 2007, 163. Available online: https://tigerprints.clemson.edu/all_theses/163 (accessed on 27 April 2020).
- Sottile, F.; Massaglia, S.; Peano, C. Ecological and Economic Indicators for the Evaluation of Almond (Prunus dulcis L.) Orchard Renewal in Sicily. Agriculture 2020, 10, 301. [Google Scholar] [CrossRef]
- Loftsson, T.; Jarho, P.; Masson, M.; Jarvinen, T. Cyclodextrins in drug delivery. Expert Opin. Drug Deliv. 2005, 2, 335–351. [Google Scholar] [CrossRef]
- Lauro, M.R.; Crascí, L.; Giannone, V.; Ballistreri, G.; Fabroni, S.; Sansone, F.; Rapisarda, P.; Panico, A.M.; Puglisi, G. An Alginate/Cyclodextrin Spray Drying Matrix to Improve Shelf Life and Antioxidant Efficiency of a Blood Orange By-Product Extract Rich in Polyphenols: MMPs Inhibition and Antiglycation Activity in Dysmetabolic Diseases. Oxidative Med. Cell. Longev. 2017, 2017, 2867630. [Google Scholar] [CrossRef] [PubMed]
- Lauro, M.R.; Crascí, L.; Sansone, F.; Cardile, V.; Panico, A.M.; Puglisi, G. Development and In Vitro Evaluation of an Innovative (Dietary Flavonoid Supplement) on Osteoarthritis Process. Oxidative Med. Cell. Longev. 2017, 2017, 7503240. [Google Scholar] [CrossRef]
- Milbury, P.E.; Chen, C.Y.; Dolnikowski, G.G.; Blumberg, J.B. Determination of Flavonoids and Phenolics and Their Distribution in Almonds. J. Agric. Food Chem. 2006, 54, 5027–5033. [Google Scholar] [CrossRef]
- Ho, S.; Thoo, Y.Y.; Young, D.J.; Siow, L.F. Cyclodextrin encapsulated catechin: Effect of pH, relative humidity and various food models on antioxidant stability. LWT-Food Sci. Technol. 2017, 85, 232–239. [Google Scholar] [CrossRef]
- Ho, S.; Thoo, Y.Y.; Young, D.J.; Siow, L.F. Inclusion complexation of catechin by β-cyclodextrins: Characterization and storage stability. LWT-Food Sci. Technol. 2017, 86, 555–565. [Google Scholar] [CrossRef]
- Ho, S.; Thoo, Y.Y.; Young, D.J.; Siow, L.F. Stability and recovery of cyclodextrin encapsulated catechin in various food matrices. Food Chem. 2019, 275, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Yang, J.; Wang, Q.; Ren, L.; Zhou, J. Physicochemical properties of catechin/β-cyclodextrin inclusion complex obtained via co-precipitation. CyTA J. Food. 2019, 17, 544–551. [Google Scholar] [CrossRef]
- Carneiro, S.B.; Costa Duarte, F.Í.; Heimfarth, L.; de Souza Siqueira Quintans, L.; Quintans-Júnior, L.J.; da Veiga Júnior, V.F.; Neves de Lima, A.A. Cyclodextrin-Drug Inclusion Complexes: In Vivo and In Vitro Approaches. Int. J. Mol. Sci. 2019, 20, 642. [Google Scholar] [CrossRef]
- Crascì, L.; Lauro, M.R.; Puglisi, G.; Panico, A.M. Natural Antioxidant Polyphenols on Inflammation Management: Anti-glycation Activity vs Metalloproteinases Inhibition. Crit. Rev. Food Sci. Nutr. 2018, 58, 893–904. [Google Scholar] [CrossRef] [PubMed]
- Florence, A.T.; Attwood, D. Physicochemical Principles of Pharmacy, 5th ed.; Pharmaceutical Press: London, UK, 2011. [Google Scholar]
- Higuchi, T.K.; Connors, A. Phase-solubility techniques. Adv. Anal. Chem. Instrum. 1965, 4, 117–212. [Google Scholar]
- Rapa, S.F.; Waltenberger, B.; Di Paola, R.; Adesso, S.; Siracusa, R.; Peritore, A.F.; D’Amico, R.; Autore, G.; Cuzzocrea, S.; Stuppner, H.; et al. Plumericin prevents intestinal inflammation and oxidative stress in vitro and in vivo. FASEB J. 2020, 34, 1576–1590. [Google Scholar] [CrossRef]
- Basilicata, M.G.; Pepe, G.; Rapa, S.F.; Merciai, F.; Ostacolo, C.; Manfra, M.; Di Sarno, V.; Autore, G.; De Vita, D.; Marzocco, S.; et al. Anti-inflammatory and antioxidant properties of dehydrated potatoes-derived bioactive compounds in intestinal cells. Int. J. Mol. Sci. 2019, 20, 6087. [Google Scholar] [CrossRef]
- Adesso, S.; Russo, R.; Quaroni, A.; Autore, G.; Marzocco, S. Astragalus membranaceus Extract Attenuates Inflammation and Oxidative Stress in Intestinal Epithelial Cells via NF-κB Activation and Nrf2 Response. Int. J. Mol. Sci. 2018, 19, 800. [Google Scholar] [CrossRef]
- Pepe, G.; Sommella, E.; Manfra, M.; De Nisco, M.; Tenore, G.C.; Scopa, A.; Sofo, A.; Marzocco, S.; Adesso, S.; Novellino, T.; et al. Evaluation of anti-inflammatory activity and fast UHPLC-DAD-IT-TOF profiling of polyphenolic compounds extracted from green lettuce (Lactuca sativa L.; var. Maravilla de Verano). Food Chem. 2015, 167, 153–161. [Google Scholar] [CrossRef]
- Del Regno, M.; Adesso, S.; Popolo, A.; Quaroni, A.; Autore, G.; Severino, L.; Marzocco, S. Nivalenol induces oxidative stress and increases deoxynivalenol pro-oxidant effect in intestinal epithelial cells. Toxicol. Appl. Pharmacol. 2015, 285, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Adesso, S.; Magnus, T.; Cuzzocrea, S.; Campolo, M.; Rissiek, B.; Paciello, O.; Autore, G.; Pinto, A.; Marzocco, S. Indoxyl Sulfate Affects Glial Function Increasing Oxidative Stress and Neuroinflammation in Chronic Kidney Disease: Interaction between Astrocytes and Microglia. Front. Pharmacol. 2017, 8, 370. [Google Scholar] [CrossRef]
- Marzocco, S.; Adesso, S.; Alilou, M.; Stuppner, H.; Schwaiger, S. Anti-Inflammatory and Anti-Oxidant Potential of the Root Extract and Constituents of Doronicum austriacum. Molecules 2017, 22, 1003. [Google Scholar] [CrossRef]
- Adesso, S.; Ruocco, M.; Rapa, S.F.; Dal Piaz, F.; Di Iorio, B.R.; Popolo, A.; Autore, G.; Nishijima, F.; Pinto, A.; Marzocco, S. Effect of Indoxyl Sulfate on the Repair and Intactness of Intestinal Epithelial Cells: Role of Reactive Oxygen Species’ Release. Int. J. Mol. Sci. 2019, 20, 2280. [Google Scholar] [CrossRef] [PubMed]
- van der Merwe, J.; Steenekamp, J.; Steyn, D.; Hamman, J. The Role of Functional Excipients in Solid Oral Dosage Forms to Overcome Poor Drug Dissolution and Bioavailability. Pharmaceutics 2020, 12, 393. [Google Scholar] [CrossRef]
- Charumanee, S.; Titwan, A.; Sirithunyalug, J.; Weiss-Greiler, P.; Wolschann, P.; Viernstein, H.; Okonogi, S. Thermodynamics of the encapsulation by cyclodextrins. J. Chem. Technol. Biotechnol. 2006, 81, 523–529. [Google Scholar] [CrossRef]
- Araujo, R.R.; Teixeira, C.C.C.; Freitas, L.A.P. The preparation of ternary solid dispersions of an herbal drug via spray drying of liquid feed. Dry. Technol. 2010, 28, 412–421. [Google Scholar] [CrossRef]
- Moldoveanu, A.F.; Diculescu, M.; Braticevici, C.F. Cytokines in inflammatory bowel disease. Rom. J. Intern. Med. 2015, 53, 118–127. [Google Scholar] [CrossRef]
- Haimhoffer, Á.; Rusznyák, Á.; Réti-Nagy, K.; Vasvári, G.; Váradi, J.; Vecsernyés, M.; Bácskay, I.; Fehér, P.; Ujhelyi, Z.; Fenyvesi, F. Cyclodextrins in Drug Delivery Systems and Their Effects on Biological Barriers. Sci. Pharm. 2019, 87, 33. [Google Scholar] [CrossRef]
- McCarthy, J.; O’Neill, M.J.; Bourre, L.; Walsh, D.; Quinlan, A.; Hurley, G.; Ogier, J.; Shanahan, F.; Melgar, S.; Darcy, R.; et al. Gene silencing of TNF-alpha in a murine model of acute colitis using a modified cyclodextrin delivery system. J Control Release 2013, 168, 28–34. [Google Scholar] [CrossRef]
- Tanaka, T.; de Azevedo, M.B.; Durán, N.; Alderete, J.B.; Epifano, F.; Genovese, S.; Tanaka, M.; Tanaka, T.; Curini, M. Colorectal cancer chemoprevention by 2 beta-cyclodextrin inclusion compounds of auraptene and 4’-geranyloxyferulic acid. Int. J. Cancer 2010, 126, 830–840. [Google Scholar] [CrossRef] [PubMed]
- Lázaro, C.M.; de Oliveira, C.C.; Gambero, A.; Rocha, T.; Cereda, C.; de Araújo, D.R.; Tofoli, G.R. Evaluation of Budesonide-Hydroxypropyl-β-Cyclodextrin Inclusion Complex in Thermoreversible Gels for Ulcerative Colitis. Dig. Dis. Sci. 2020. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; DuBois, R.N. The Role of COX-2 in Intestinal Inflammation and Colorectal Cancer. Oncogene 2010, 29, 781–788. [Google Scholar] [CrossRef]
- Yasukawa, K.; Tokuda, H.; Tun, X.; Utsumi, H.; Yamada, K. The detrimental effect of nitric oxide on tissue is associated with inflammatory events in the vascular endothelium and neutrophils in mice with dextran sodium sulfate-induced colitis. Free Radic. Res. 2012, 46, 1427–1436. [Google Scholar] [CrossRef]
- Balmus, I.M.; Ciobica, A.; Trifan, A.; Stanciu, C. The implications of oxidative stress and antioxidant therapies in Inflammatory Bowel Disease: Clinical aspects and animal models. Saudi J. Gastroenterol. 2016, 22, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Simpson, D.S.A.; Oliver, P.L. ROS Generation in Microglia: Understanding Oxidative Stress and Inflammation in Neurodegenerative Disease. Antioxidants 2020, 9, 743. [Google Scholar] [CrossRef]
- Amodio, G.; Moltedo, O.; Fasano, D.; Zerillo, L.; Oliveti, M.; Di Pietro, P.; Faraonio, R.; Barone, P.; Pellecchia, M.T.; De Rosa, A.; et al. PERK-Mediated Unfolded Protein Response Activation and Oxidative Stress in PARK20 Fibroblasts. Front. Neurosci. 2019, 13, 673. [Google Scholar] [CrossRef]
- García-Sánchez, A.; Miranda-Díaz, A.G.; Cardona-Muñoz, E.G. The Role of Oxidative Stress in Physiopathology and Pharmacological Treatment with Pro- and Antioxidant Properties in Chronic Diseases. Oxidative Med. Cell. Longev. 2020, 2020, 2082145. [Google Scholar] [CrossRef]
- Ranneh, Y.; Ali, F.; Akim, A.; Hamid, H.A.; Khazaai, H.; Fadel, A. Crosstalk between reactive oxygen species and pro-inflammatory markers in developing various chronic diseases: A review. Appl. Biol. Chem. 2017, 60, 327–338. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lauro, M.R.; Marzocco, S.; Rapa, S.F.; Musumeci, T.; Giannone, V.; Picerno, P.; Aquino, R.P.; Puglisi, G. Recycling of Almond By-Products for Intestinal Inflammation: Improvement of Physical-Chemical, Technological and Biological Characteristics of a Dried Almond Skins Extract. Pharmaceutics 2020, 12, 884. https://doi.org/10.3390/pharmaceutics12090884
Lauro MR, Marzocco S, Rapa SF, Musumeci T, Giannone V, Picerno P, Aquino RP, Puglisi G. Recycling of Almond By-Products for Intestinal Inflammation: Improvement of Physical-Chemical, Technological and Biological Characteristics of a Dried Almond Skins Extract. Pharmaceutics. 2020; 12(9):884. https://doi.org/10.3390/pharmaceutics12090884
Chicago/Turabian StyleLauro, Maria Rosaria, Stefania Marzocco, Shara Francesca Rapa, Teresa Musumeci, Virgilio Giannone, Patrizia Picerno, Rita Patrizia Aquino, and Giovanni Puglisi. 2020. "Recycling of Almond By-Products for Intestinal Inflammation: Improvement of Physical-Chemical, Technological and Biological Characteristics of a Dried Almond Skins Extract" Pharmaceutics 12, no. 9: 884. https://doi.org/10.3390/pharmaceutics12090884
APA StyleLauro, M. R., Marzocco, S., Rapa, S. F., Musumeci, T., Giannone, V., Picerno, P., Aquino, R. P., & Puglisi, G. (2020). Recycling of Almond By-Products for Intestinal Inflammation: Improvement of Physical-Chemical, Technological and Biological Characteristics of a Dried Almond Skins Extract. Pharmaceutics, 12(9), 884. https://doi.org/10.3390/pharmaceutics12090884








