Cathepsin B-Responsive Liposomes for Controlled Anticancer Drug Delivery in Hep G2 Cells
Abstract
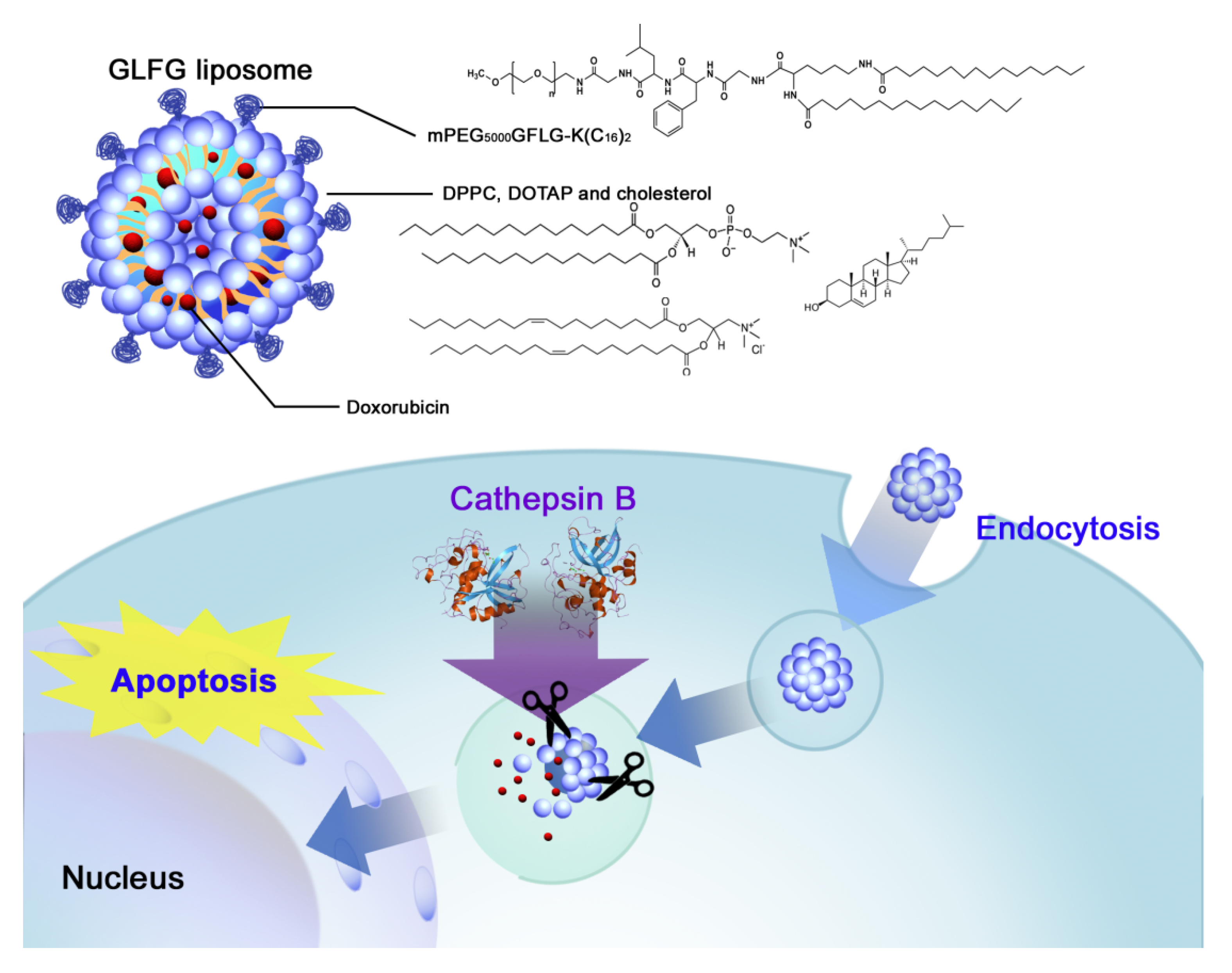
1. Introduction
2. Materials and Methods
2.1. Materials
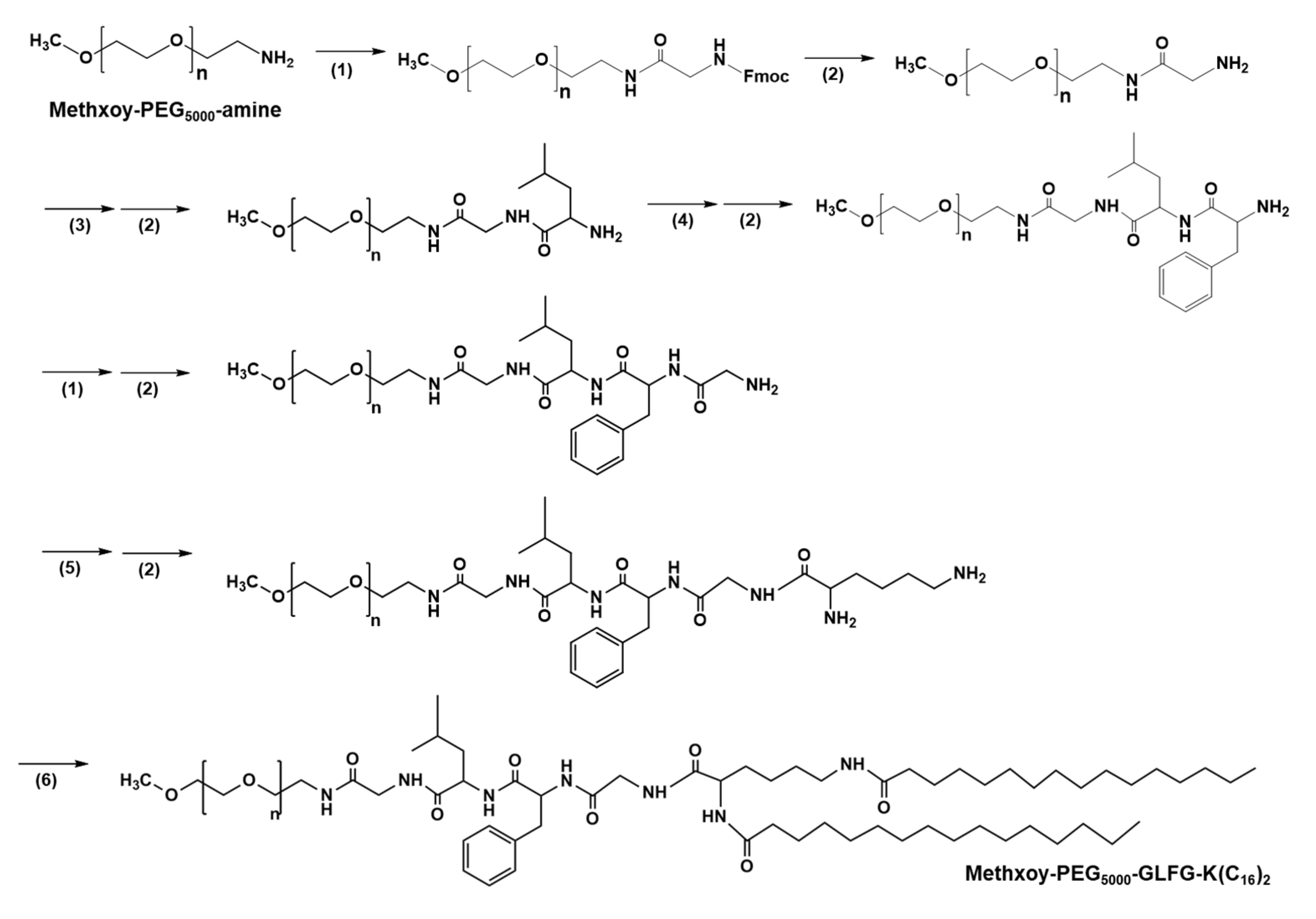
2.2. Synthesis PEG-GLFG-K(C16)2 (PEG-GLFG)
2.3. Formation of GLFG Liposomes and Doxorubicin (Dox) Encapsulation
2.4. Zeta Potential and Dynamic Light Scattering (DLS)
2.5. Field Emission-Scanning Electron Microscopy (FE-SEM)
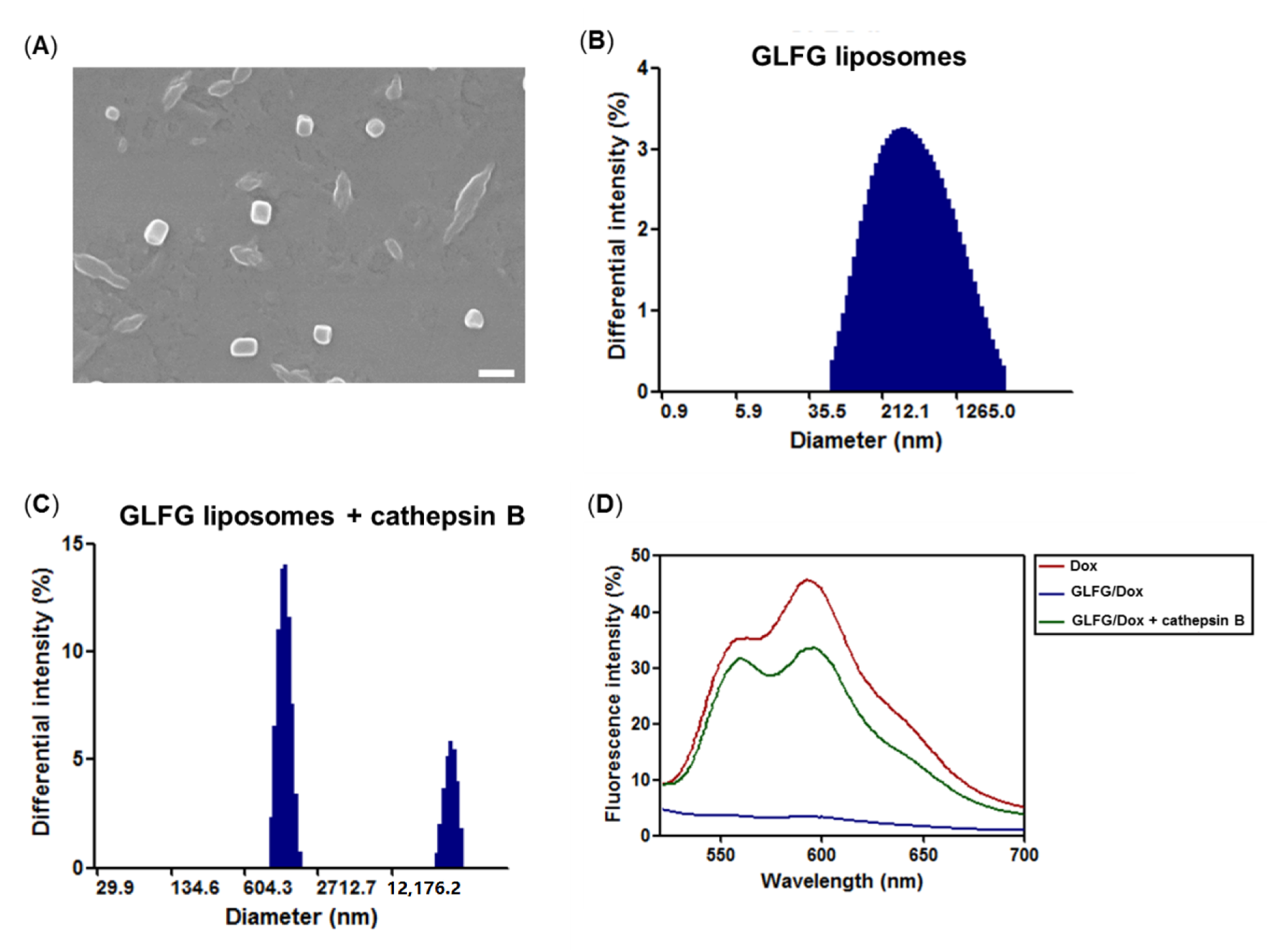
2.6. GLFG/Dox Liposomes Degradation Test by Cathepsin B
2.7. Cell Culture and Cytotoxicity Assay
2.8. Confocal Microscopy
2.9. Zebrafish In Vivo
3. Results and Discussion
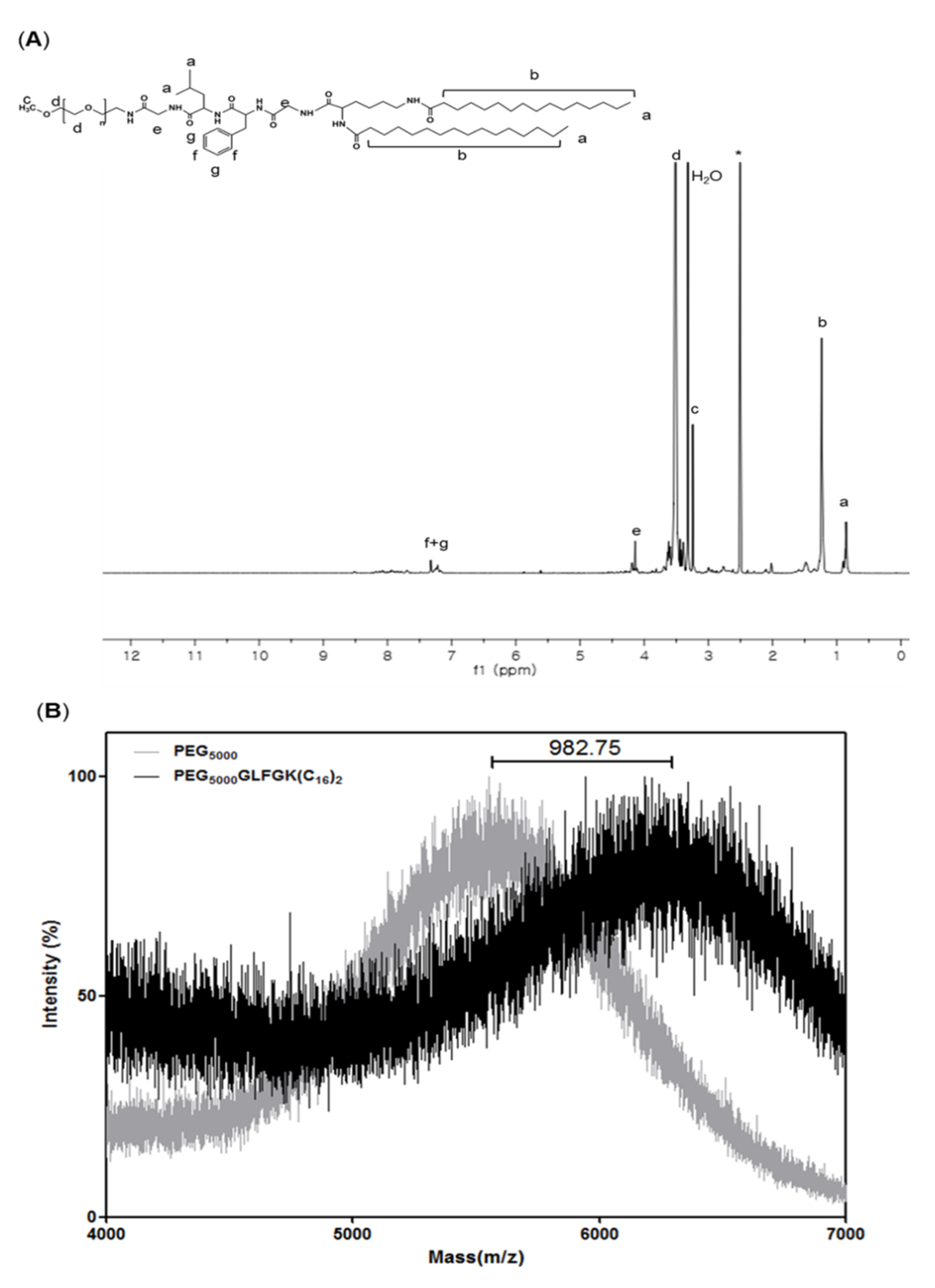
3.1. Characterizations of PEG-GLFG Lipid
3.2. Characterization of GLFG Liposomes
3.3. Cytotoxicity Assay of GLFG Lipid and Liposomes in Hep G2 Cells
3.4. Anticancer Effect and Internalization of GLFG/Dox in Hep G2 Cells
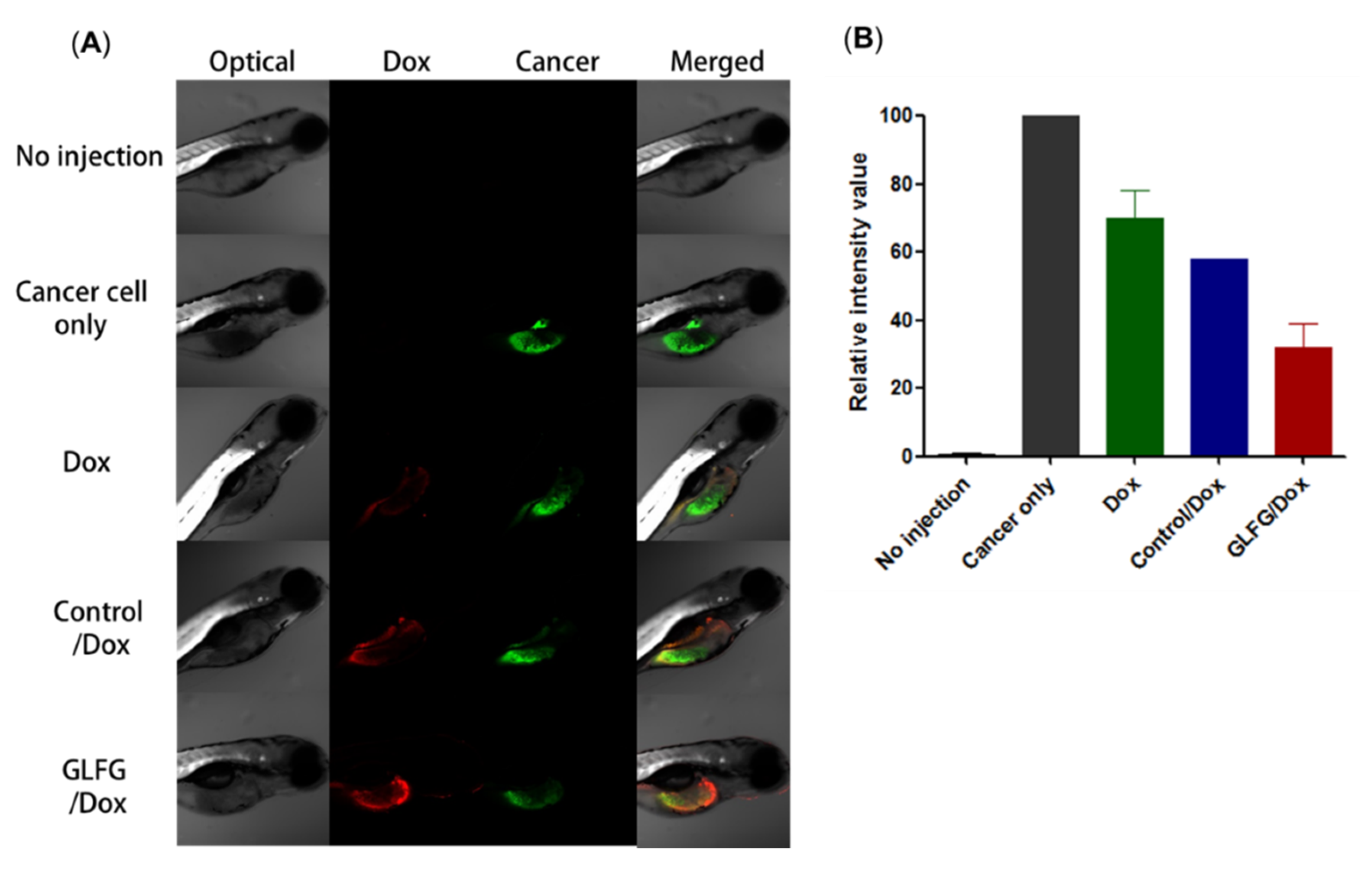
3.5. Analysis of Anticancer Effects of GLFG/Dox Using a Cancer Injection Model in Zebrafish In Vivo
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct. Target. Ther. 2018, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- Thorn, C.F.; Oshiro, C.; Marsh, S.; Hernandez-Boussard, T.; McLeod, H.; Klein, T.E.; Altman, R.B. Doxorubicin pathways: Pharmacodynamics and adverse effects. Pharmacogenet. Genomics 2011, 21, 440–446. [Google Scholar] [CrossRef]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef]
- Clarke, S.J.; Rivory, L.P. Clinical Pharmacokinetics of Docetaxel. Clin. Pharmacokinet. 1999, 36, 99–114. [Google Scholar] [PubMed]
- Tran, S.; DeGiovanni, P.-J.; Piel, B.; Rai, P. Cancer nanomedicine: A review of recent success in drug delivery. Clin. Transl. Med. 2017, 6, 44. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V. Multifunctional, stimuli-sensitive nanoparticulate systems for drug delivery. Nat. Rev. Drug Discov. 2014, 13, 813–827. [Google Scholar] [CrossRef]
- Wolfram, J.; Ferrari, M. Clinical cancer nanomedicine. Nano Today 2019, 25, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Van Der Meel, R.; Sulheim, E.; Shi, Y.; Kiessling, F.; Mulder, W.J.M.; Lammers, T. Smart cancer nanomedicine. Nat. Nanotechnol. 2019, 14, 1007–1017. [Google Scholar] [CrossRef]
- Daraee, H.; Eatemadi, A.; Kouhi, M.; Alimirzalu, S.; Akbarzadeh, A. Application of liposomes in medicine and drug delivery. Artif. Cells Nanomed. Biotechnol. 2014, 44, 381–391. [Google Scholar] [CrossRef]
- Pattni, B.S.; Chupin, V.V.; Torchilin, V. New Developments in Liposomal Drug Delivery. Chem. Rev. 2015, 115, 10938–10966. [Google Scholar] [CrossRef]
- Li, Q.; Li, W.; Di, H.; Luo, L.; Zhu, C.; Yang, J.; Yin, X.; Yin, H.; Gao, J.; Du, Y.-Z.; et al. A photosensitive liposome with NIR light triggered doxorubicin release as a combined photodynamic-chemo therapy system. J. Control. Release 2018, 277, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Chang, M.; Fan, Y.; Shi, Y.; Lin, G. NGR-modified pH-sensitive liposomes for controlled release and tumor target delivery of docetaxel. Colloids Surf. B 2017, 160, 395–405. [Google Scholar] [CrossRef]
- Kaplan, L.D.; Deitcher, S.R.; Silverman, J.A.; Morgan, G. Phase II Study of Vincristine Sulfate Liposome Injection (Marqibo) and Rituximab for Patients With Relapsed and Refractory Diffuse Large B-Cell Lymphoma or Mantle Cell Lymphoma in Need of Palliative Therapy. Clin. Lymphoma Myeloma Leuk. 2014, 14, 37–42. [Google Scholar] [CrossRef]
- Koudelka, S.; Turánek, J. Liposomal paclitaxel formulations. J. Control. Release 2012, 163, 322–334. [Google Scholar] [CrossRef]
- Gondi, C.S.; Rao, J.S. Cathepsin B as a cancer target. Expert Opin. Ther. Targets 2013, 17, 281–291. [Google Scholar] [CrossRef]
- Song, S.J.; Lee, S.; Lee, Y.; Choi, J.S. Enzyme-responsive destabilization of stabilized plasmid-lipid nanoparticles as an efficient gene delivery. Eur. J. Pharm. Sci. 2016, 91, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Son, S.J.; Song, S.J.; Ha, T.H.; Choi, J.S. Polyamidoamine (PAMAM) Dendrimers Modified with Cathepsin-B Cleavable Oligopeptides for Enhanced Gene Delivery. Polymers 2017, 9, 224. [Google Scholar] [CrossRef]
- Ta, T.; Porter, T.M. Thermosensitive liposomes for localized delivery and triggered release of chemotherapy. J. Control. Release 2013, 169, 112–125. [Google Scholar] [CrossRef]
- Stewart, M.P.; Lorenz, A.; Dahlman, J.; Sahay, G. Challenges in carrier-mediated intracellular delivery: Moving beyond endosomal barriers. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 8, 465–478. [Google Scholar] [CrossRef]
- Campbell, R.B.; Balasubramanian, S.V.; Straubinger, R.M. Phospholipid-cationic lipid interactions: Influences on membrane and vesicle properties. Biochim. Et Biophys. Acta (BBA)-Bioenerg. 2001, 1512, 27–39. [Google Scholar] [CrossRef]
- Terasawa, Y.; Hotani, T.; Katayama, Y.; Tachibana, M.; Mizuguchi, H.; Sakurai, F. Activity levels of cathepsins B and L in tumor cells are a biomarker for efficacy of reovirus-mediated tumor cell killing. Cancer Gene Ther. 2015, 22, 188–197. [Google Scholar] [CrossRef]
- Nag, O.K.; Awasthi, V. Surface Engineering of Liposomes for Stealth Behavior. Pharmaceutics 2013, 5, 542–569. [Google Scholar] [CrossRef] [PubMed]
- Garbuzenko, O.; Barenholz, Y.; Priev, A. Effect of grafted PEG on liposome size and on compressibility and packing of lipid bilayer. Chem. Phys. Lipids 2005, 135, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, S.K.; Sreedharan, S.; Singh, H.; Khan, M.; Tiwari, K.; Shiras, A.; Smythe, C.G.W.; Thomas, J.A.; Das, A. Mitochondria Targeting Non-Isocyanate-Based Polyurethane Nanocapsules for Enzyme-Triggered Drug Release. Bioconjugate Chem. 2018, 29, 3532–3543. [Google Scholar] [CrossRef]
- Sheena, T.S.; Balaji, P.; Venkatesan, R.; Akbarsha, M.A.; Jeganathan, K.; Salammal, S.T.; Perumalsamy, B.; Kulandaivel, J. Functional evaluation of doxorubicin decorated polymeric liposomal curcumin: A surface tailored therapeutic platform for combination chemotherapy. New J. Chem. 2018, 42, 16608–16619. [Google Scholar] [CrossRef]
- Dubbelboer, I.R.; Pavlovic, N.; Heindryckx, F.; Sjögren, E.; Lennernas, H. Liver Cancer Cell Lines Treated with Doxorubicin under Normoxia and Hypoxia: Cell Viability and Oncologic Protein Profile. Cancers 2019, 11, 1024. [Google Scholar] [CrossRef] [PubMed]
- Manov, I.; Bashenko, Y.; Eliaz-Wolkowicz, A.; Mizrahi, M.; Liran, O.; Iancu, T.C. High-dose acetaminophen inhibits the lethal effect of doxorubicin in Hep G2 cells: The role of P-glycoprotein and mitogen-activated protein kinase p44/42 pathway. J. Pharmacol. Exp. Ther. 2007, 322, 1013–1022. [Google Scholar] [CrossRef]
- Song, S.J.; Lee, S.; Ryu, K.-S.; Choi, J.S. Amphiphilic Peptide Nanorods Based on Oligo-Phenylalanine as a Biocompatible Drug Carrier. Bioconjugate Chem. 2017, 28, 2266–2276. [Google Scholar] [CrossRef]

| Liposome | PEG-GLFG:DPPC:DOTAP:Chol (Molar Ratio) | Size (nm) a | PDI a | Zeta Potential (mV) a |
|---|---|---|---|---|
| Control | 10:70:10:10 | 199.5 ± 8.7 | 0.3 | 7.2 ± 0.3 |
| GLFG 1 | 1:79:10:10 | 151.7 ± 2.3 | 0.3 | 44.9 ± 1.9 |
| GLFG 5 | 5:75:10:10 | 151.4 ± 1.6 | 0.3 | 25.2 ± 0.2 |
| GLFG 10 | 10:70:10:10 | 334.8 ± 7.0 | 0.3 | 21.0 ± 0.4 |
| GLFG 20 | 20:60:10:10 | 302.4 ± 4.2 | 0.3 | 2.7 ± 1.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Song, S.J.; Lee, J.; Ha, T.H.; Choi, J.S. Cathepsin B-Responsive Liposomes for Controlled Anticancer Drug Delivery in Hep G2 Cells. Pharmaceutics 2020, 12, 876. https://doi.org/10.3390/pharmaceutics12090876
Lee S, Song SJ, Lee J, Ha TH, Choi JS. Cathepsin B-Responsive Liposomes for Controlled Anticancer Drug Delivery in Hep G2 Cells. Pharmaceutics. 2020; 12(9):876. https://doi.org/10.3390/pharmaceutics12090876
Chicago/Turabian StyleLee, Seulgi, Su Jeong Song, Jeil Lee, Tai Hwan Ha, and Joon Sig Choi. 2020. "Cathepsin B-Responsive Liposomes for Controlled Anticancer Drug Delivery in Hep G2 Cells" Pharmaceutics 12, no. 9: 876. https://doi.org/10.3390/pharmaceutics12090876
APA StyleLee, S., Song, S. J., Lee, J., Ha, T. H., & Choi, J. S. (2020). Cathepsin B-Responsive Liposomes for Controlled Anticancer Drug Delivery in Hep G2 Cells. Pharmaceutics, 12(9), 876. https://doi.org/10.3390/pharmaceutics12090876





