Image-Based Artificial Intelligence Methods for Product Control of Tablet Coating Quality
Abstract
1. Introduction
2. Material and Methods
2.1. Materials
2.2. Methods
Scanning
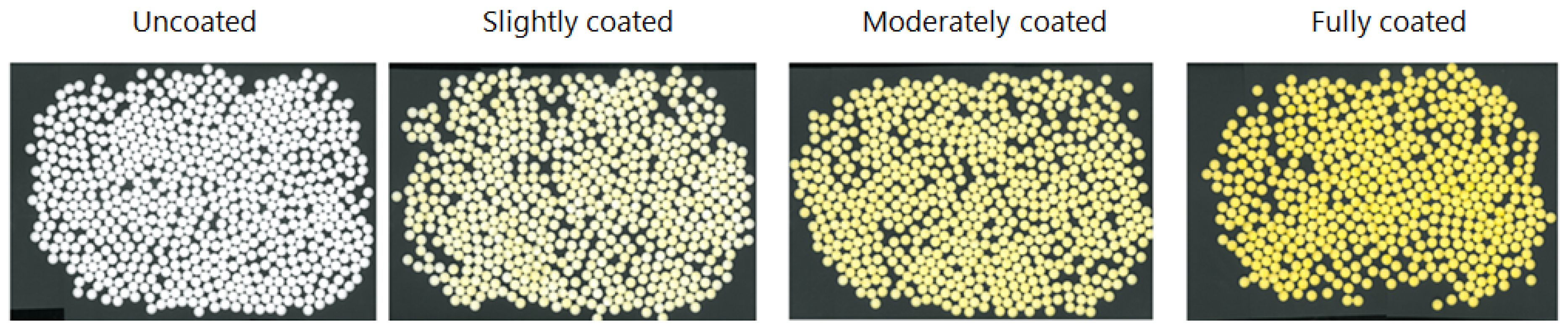
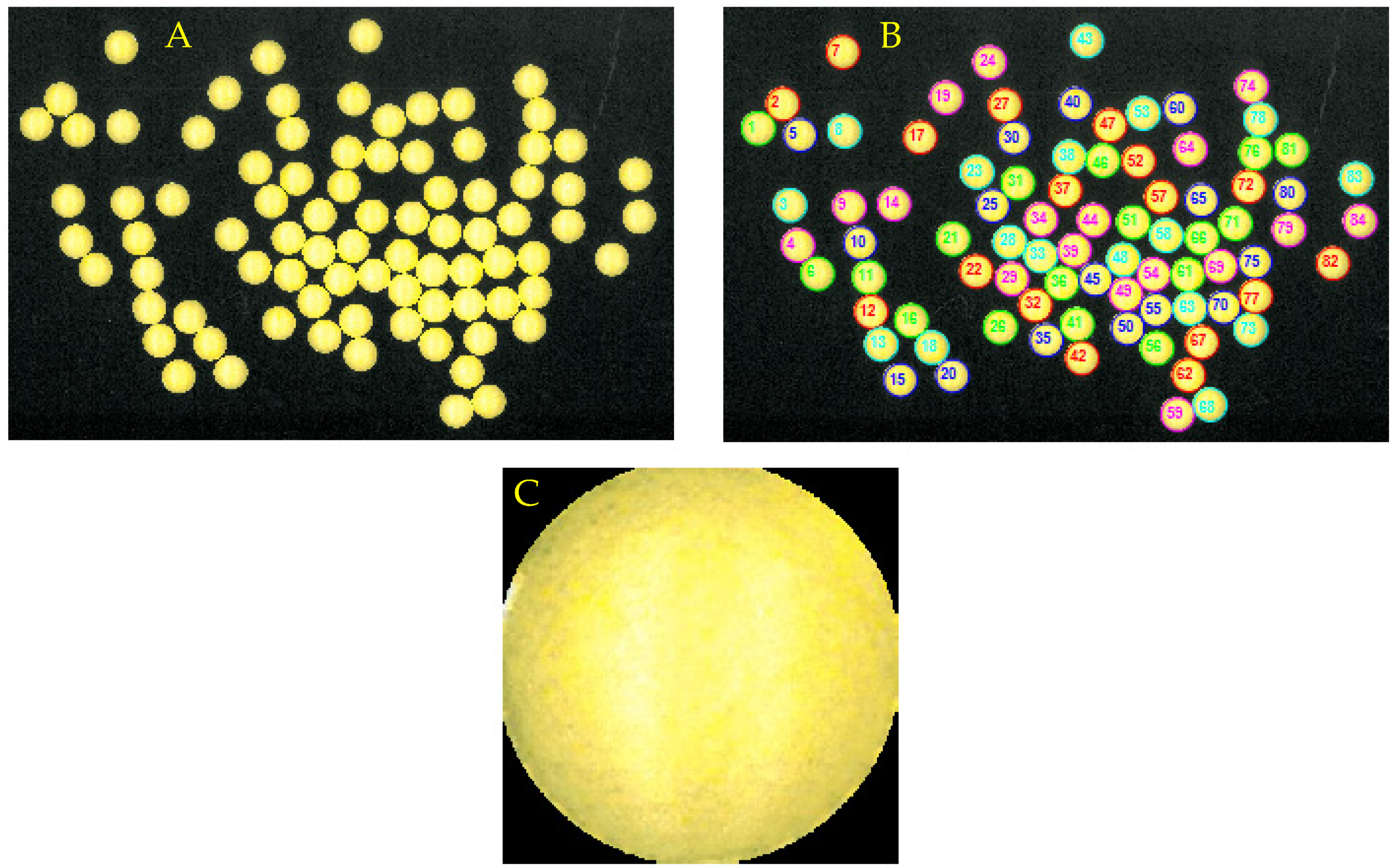
3. Segmentation and Computational Analysis of Tablets
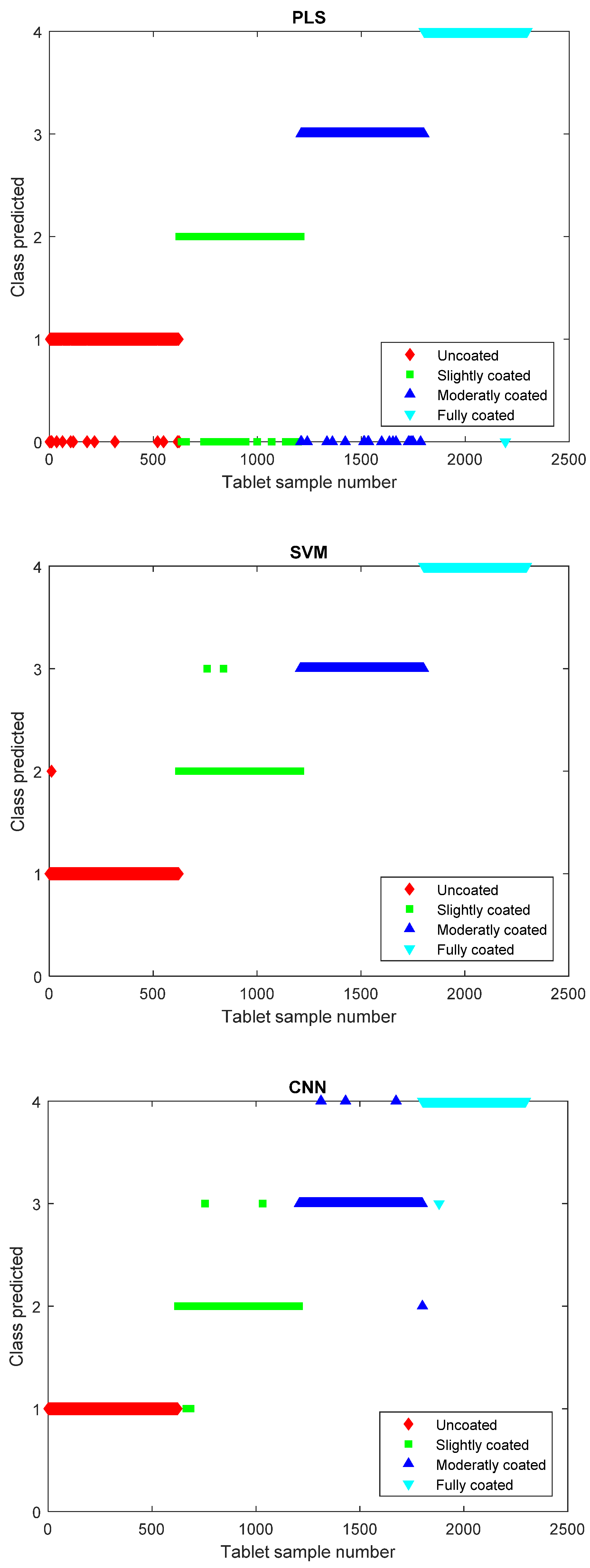
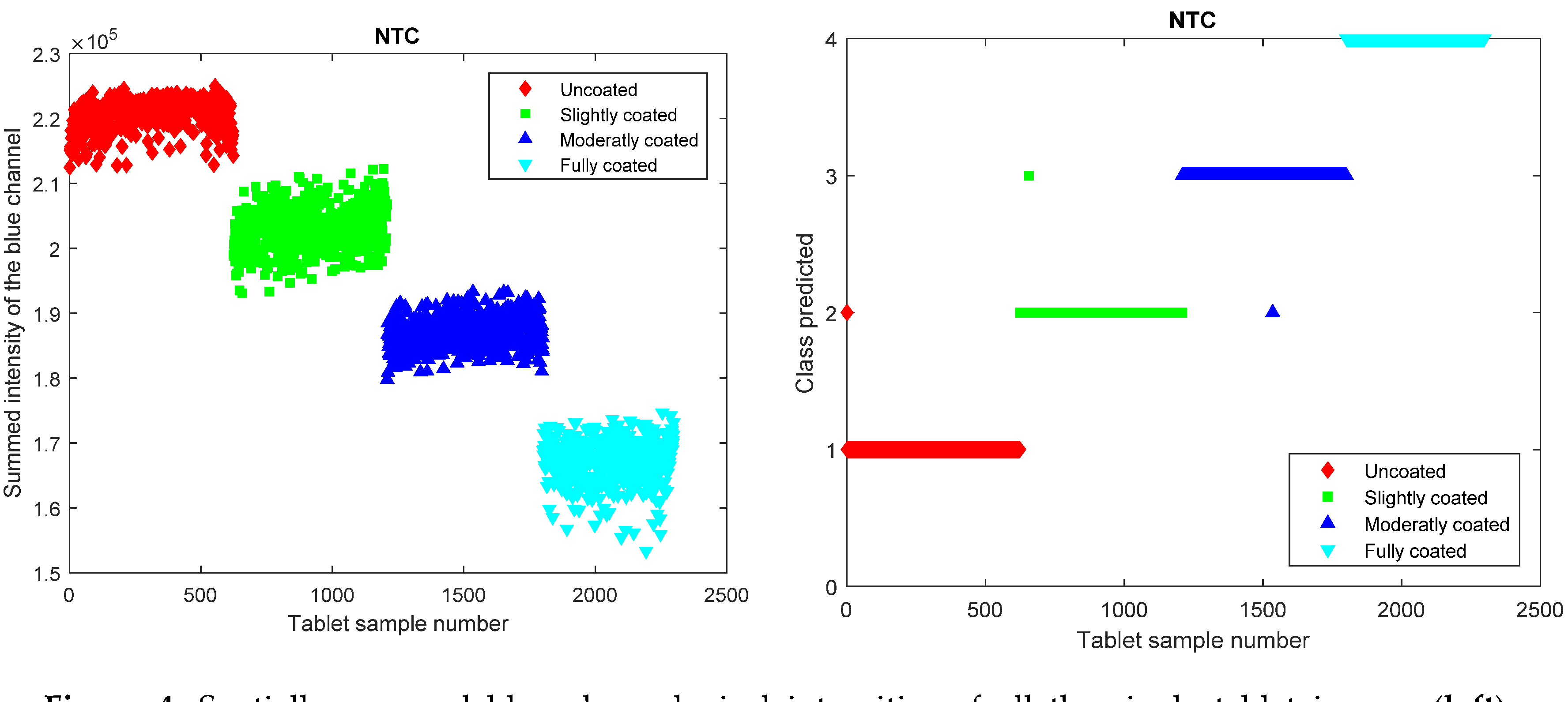
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Patel, S.; Nail, S.L.; Pikal, M.J.; Geidobler, R.; Winter, G.; Hawe, A.; Davagnino, J.; Gupta, S.R. Lyophilized Drug Product Cake Appearance: What Is Acceptable? J. Pharm. Sci. 2017, 106, 1706–1721. [Google Scholar] [CrossRef] [PubMed]
- Laitinen, N.; Antikainen, O.; Rantanen, J.; Yliruusi, J. New Perspectives for Visual Characterization of Pharmaceutical Solids. J. Pharm. Sci. 2004, 93, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Trnka, H.; Wu, J.X.; Van De Weert, M.; Grohganz, H.; Rantanen, J. Fuzzy Logic-Based Expert System for Evaluating Cake Quality of Freeze-Dried Formulations. J. Pharm. Sci. 2013, 102, 4364–4374. [Google Scholar] [CrossRef]
- Brock, D.; Zeitler, J.A.; Funke, A.; Knop, K.; Kleinebudde, P. A comparison of quality control methods for active coating processes. Int. J. Pharm. 2012, 439, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Banker, G.S. Film Coating Theory and Practice. J. Pharm. Sci. 1966, 55, 81–89. [Google Scholar] [CrossRef]
- Hattori, Y.; Sugata, M.; Kamata, H.; Nagata, M.; Nagato, T.; Hasegawa, K.; Otsuka, M. Real-time monitoring of the tablet-coating process by near-infrared spectroscopy—Effects of coating polymer concentrations on pharmaceutical properties of tablets. J. Drug Deliv. Sci. Technol. 2018, 46, 111–121. [Google Scholar] [CrossRef]
- Möltgen, C.-V.; Puchert, T.; Menezes, J.C.; Lochmann, D.; Reich, G. A novel in-line NIR spectroscopy application for the monitoring of tablet film coating in an industrial scale process. Talanta 2012, 92, 26–37. [Google Scholar] [CrossRef]
- Korasa, K.; Hudovornik, G.; Vrečer, F. Applicability of near-infrared spectroscopy in the monitoring of film coating and curing process of the prolonged release coated pellets. Eur. J. Pharm. Sci. 2016, 93, 484–492. [Google Scholar] [CrossRef]
- Sibanc, R.; Luštrik, M.; Dreu, R. Analysis of pellet coating uniformity using a computer scanner. Int. J. Pharm. 2017, 533, 377–382. [Google Scholar] [CrossRef]
- Ho, L.; Müller, R.; Gordon, K.C.; Kleinebudde, P.; Pepper, M.; Rades, T.; Shen, Y.; Taday, P.F.; Zeitler, J.A. Applications of terahertz pulsed imaging to sustained-release tablet film coating quality assessment and dissolution performance. J. Control. Release 2008, 127, 79–87. [Google Scholar] [CrossRef]
- Barimani, S.; Kleinebudde, P. Evaluation of in-line Raman data for end-point determination of a coating process: Comparison of Science–Based Calibration, PLS-regression and univariate data analysis. Eur. J. Pharm. Biopharm. 2017, 119, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Muller, J.; Knop, K.; Thies, J.; Uerpmann, C.; Kleinebudde, P. Feasibility of Raman spectroscopy as PAT tool in active coating. Drug Dev. Ind. Pharm. 2010, 36, 234–243. [Google Scholar] [CrossRef]
- Korasa, K.; Vrečer, F. Overview of PAT process analysers applicable in monitoring of film coating unit operations for manufacturing of solid oral dosage forms. Eur. J. Pharm. Sci. 2018, 111, 278–292. [Google Scholar] [CrossRef] [PubMed]
- Barimani, S.; Kleinebudde, P. Monitoring of tablet coating processes with colored coatings. Talanta 2018, 178, 686–697. [Google Scholar] [CrossRef] [PubMed]
- Muller, J.; Brock, D.; Knop, K.; Zeitler, J.A.; Kleinebudde, P.; Zeitler, J.A. Prediction of dissolution time and coating thickness of sustained release formulations using Raman spectroscopy and terahertz pulsed imaging. Eur. J. Pharm. Biopharm. 2012, 80, 690–697. [Google Scholar] [CrossRef]
- Romero-Torres, S.; Pérez-Ramos, J.D.; Morris, K.R.; Grant, E.R. Raman spectroscopic measurement of tablet-to-tablet coating variability. J. Pharm. Biomed. Anal. 2005, 38, 270–274. [Google Scholar] [CrossRef]
- Riolo, D.; Piazza, A.; Cottini, C.; Serafini, M.; Lutero, E.; Cuoghi, E.; Gasparini, L.; Botturi, D.; Marino, I.G.; Aliatis, I.; et al. Raman spectroscopy as a PAT for pharmaceutical blending: Advantages and disadvantages. J. Pharm. Biomed. Anal. 2018, 149, 329–334. [Google Scholar] [CrossRef]
- Gowen, A.A.; O’Donnell, C.; Cullen, P.J.; Bell, S. Recent applications of Chemical Imaging to pharmaceutical process monitoring and quality control. Eur. J. Pharm. Biopharm. 2008, 69, 10–22. [Google Scholar] [CrossRef]
- Amigo, J.M. Practical issues of hyperspectral imaging analysis of solid dosage forms. Anal. Bioanal. Chem. 2010, 398, 93–109. [Google Scholar] [CrossRef]
- Palou, A.; Cruz, J.; Blanco, M.; Tomàs, J.; Ríos, J.D.L.; Alcalá, M. Determination of drug, excipients and coating distribution in pharmaceutical tablets using NIR-CI. J. Pharm. Anal. 2011, 2, 90–97. [Google Scholar] [CrossRef]
- Boehling, P.; Toschkoff, G.; Just, S.; Knop, K.; Kleinebudde, P.; Funke, A.; Rehbaum, H.; Rajniak, P.; Khinast, J. Simulation of a tablet coating process at different scales using DEM. Eur. J. Pharm. Sci. 2016, 93, 74–83. [Google Scholar] [CrossRef]
- Suzzi, D.; Toschkoff, G.; Radl, S.; Machold, D.; Fraser, S.D.; Glasser, B.J.; Khinast, J. DEM simulation of continuous tablet coating: Effects of tablet shape and fill level on inter-tablet coating variability. Chem. Eng. Sci. 2012, 69, 107–121. [Google Scholar] [CrossRef]
- Niblett, D.; Porter, S.; Reynolds, G.K.; Morgan, T.; Greenamoyer, J.; Hach, R.; Sido, S.; Karan, K.; Gabbott, I. Development and evaluation of a dimensionless mechanistic pan coating model for the prediction of coated tablet appearance. Int. J. Pharm. 2017, 528, 180–201. [Google Scholar] [CrossRef] [PubMed]
- Román-Ospino, A.; Singh, R.; Ierapetritou, M.; Ramachandran, R.; Méndez, R.; Ortega-Zuñiga, C.; Muzzio, F.J.; Romañach, R.J. Near infrared spectroscopic calibration models for real time monitoring of powder density. Int. J. Pharm. 2016, 512, 61–74. [Google Scholar] [CrossRef] [PubMed]
- García-Muñoz, S.; Gierer, D.S. Coating uniformity assessment for colored immediate release tablets using multivariate image analysis. Int. J. Pharm. 2010, 395, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Foody, G.M.; Mathur, A. A relative evaluation of multiclass image classification by support vector machines. IEEE Trans. Geosci. Remote Sens. 2004, 42, 1335–1343. [Google Scholar] [CrossRef]
- Orru, G.; Pettersson-Yeo, W.; Marquand, A.F.; Sartori, G.; Mechelli, A. Using Support Vector Machine to identify imaging biomarkers of neurological and psychiatric disease: A critical review. Neurosci. Biobehav. Rev. 2012, 36, 1140–1152. [Google Scholar] [CrossRef] [PubMed]
- Mehle, A.; Likar, B.; Tomazevic, D. In-line recognition of agglomerated pharmaceutical pellets with density-based clustering and convolutional neural network. IPSJ Trans. Comput. Vis. Appl. 2017, 9, 7. [Google Scholar] [CrossRef][Green Version]
- Kuo, C.-C.J. Understanding convolutional neural networks with a mathematical model. J. Vis. Commun. Image Represent. 2016, 41, 406–413. [Google Scholar] [CrossRef]
- Hearst, M.; Dumais, S.; Platt, J.; Osuna, E.; Scholkopf, B. Support vector machines. IEEE Intell. Syst. 1998, 13, 18–28. [Google Scholar] [CrossRef]
- Noble, W.S. What is a support vector machine? Nat. Biotechnol. 2006, 24, 1565–1567. [Google Scholar] [CrossRef] [PubMed]
- Mathworks. BWboundaries. Available online: https://se.mathworks.com/help/images/ref/bwboundaries.html (accessed on 12 September 2020).
- Mathworks. Deeplearning. Available online: https://se.mathworks.com/help/deeplearning/examples/create-simple-deep-learning-network-for-classification.html;jsessionid=fdc5b0b3ed9f2065a96b7d88650d (accessed on 12 September 2020).
- Wold, S.; Kettaneh-Wold, N.; Skagerberg, B. Nonlinear PLS modeling. Chemom. Intell. Lab. Syst. 1989, 7, 53–65. [Google Scholar] [CrossRef]
- Kim, K.; Lee, J.-M.; Lee, I.-B. A novel multivariate regression approach based on kernel partial least squares with orthogonal signal correction. Chemom. Intell. Lab. Syst. 2005, 79, 22–30. [Google Scholar] [CrossRef]
- Kjeldahl, K.; Bro, R. Some common misunderstandings in chemometrics. J. Chemom. 2010, 24, 558–564. [Google Scholar] [CrossRef]
- Andersen, C.M.; Bro, R. Variable selection in regression-a tutorial. J. Chemom. 2010, 24, 728–737. [Google Scholar] [CrossRef]
| Constituent | %(w/w) |
|---|---|
| Emcompress | 57 |
| Avicel PH102 | 38 |
| Talcum and magnesium stearate (9 + 1) | 5 |
| Constituent | %(w/w) |
|---|---|
| Tartrazine | 0.05 |
| Ponceau-4R | 0.05 |
| Glycerol 85% | 0.53 |
| Water | 99.37 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hirschberg, C.; Edinger, M.; Holmfred, E.; Rantanen, J.; Boetker, J. Image-Based Artificial Intelligence Methods for Product Control of Tablet Coating Quality. Pharmaceutics 2020, 12, 877. https://doi.org/10.3390/pharmaceutics12090877
Hirschberg C, Edinger M, Holmfred E, Rantanen J, Boetker J. Image-Based Artificial Intelligence Methods for Product Control of Tablet Coating Quality. Pharmaceutics. 2020; 12(9):877. https://doi.org/10.3390/pharmaceutics12090877
Chicago/Turabian StyleHirschberg, Cosima, Magnus Edinger, Else Holmfred, Jukka Rantanen, and Johan Boetker. 2020. "Image-Based Artificial Intelligence Methods for Product Control of Tablet Coating Quality" Pharmaceutics 12, no. 9: 877. https://doi.org/10.3390/pharmaceutics12090877
APA StyleHirschberg, C., Edinger, M., Holmfred, E., Rantanen, J., & Boetker, J. (2020). Image-Based Artificial Intelligence Methods for Product Control of Tablet Coating Quality. Pharmaceutics, 12(9), 877. https://doi.org/10.3390/pharmaceutics12090877





