Physicochemical Properties and Hematocompatibility of Layered Double Hydroxide-Based Anticancer Drug Methotrexate Delivery System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Pristine LDH and MTX-LDH
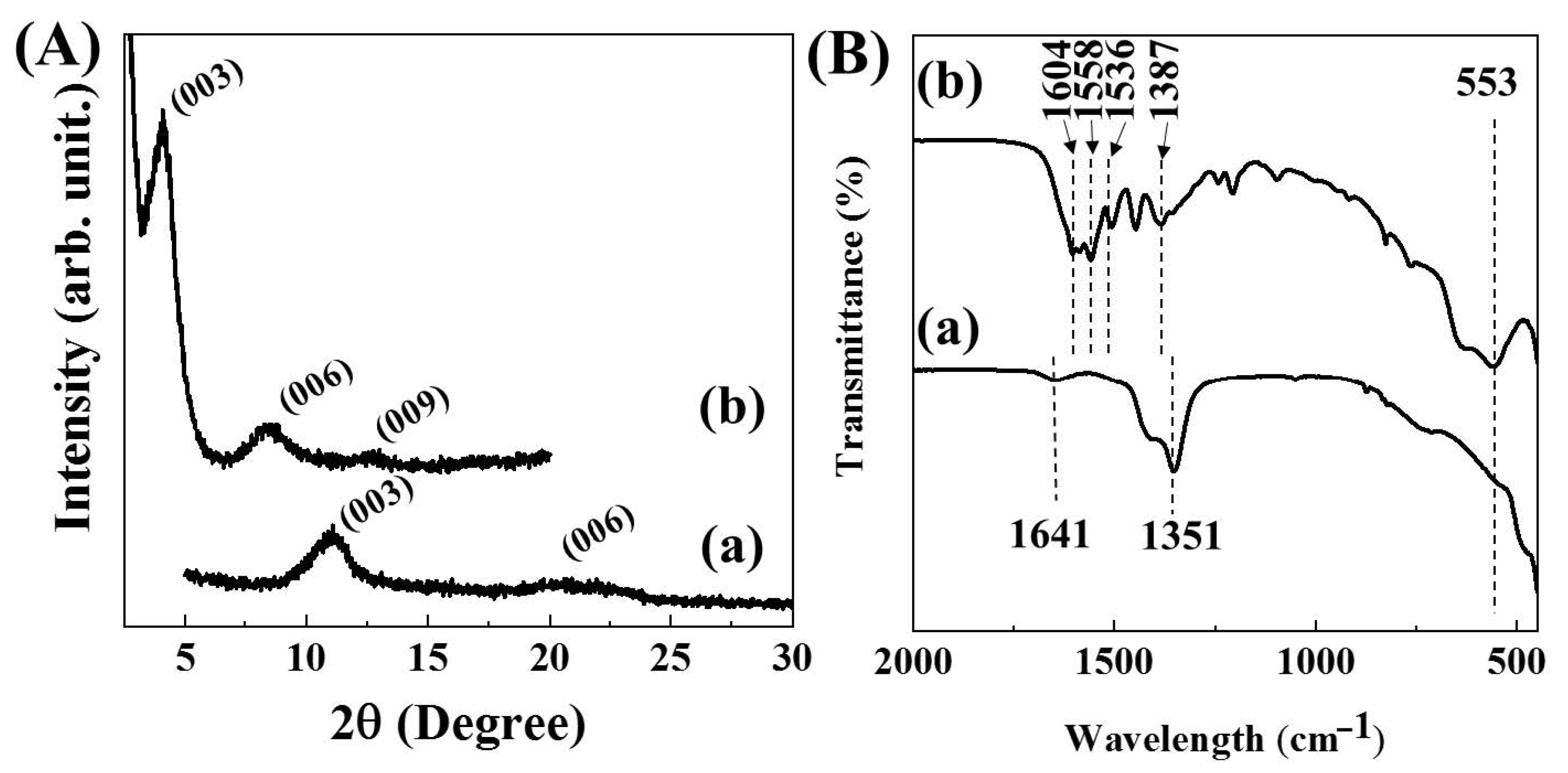
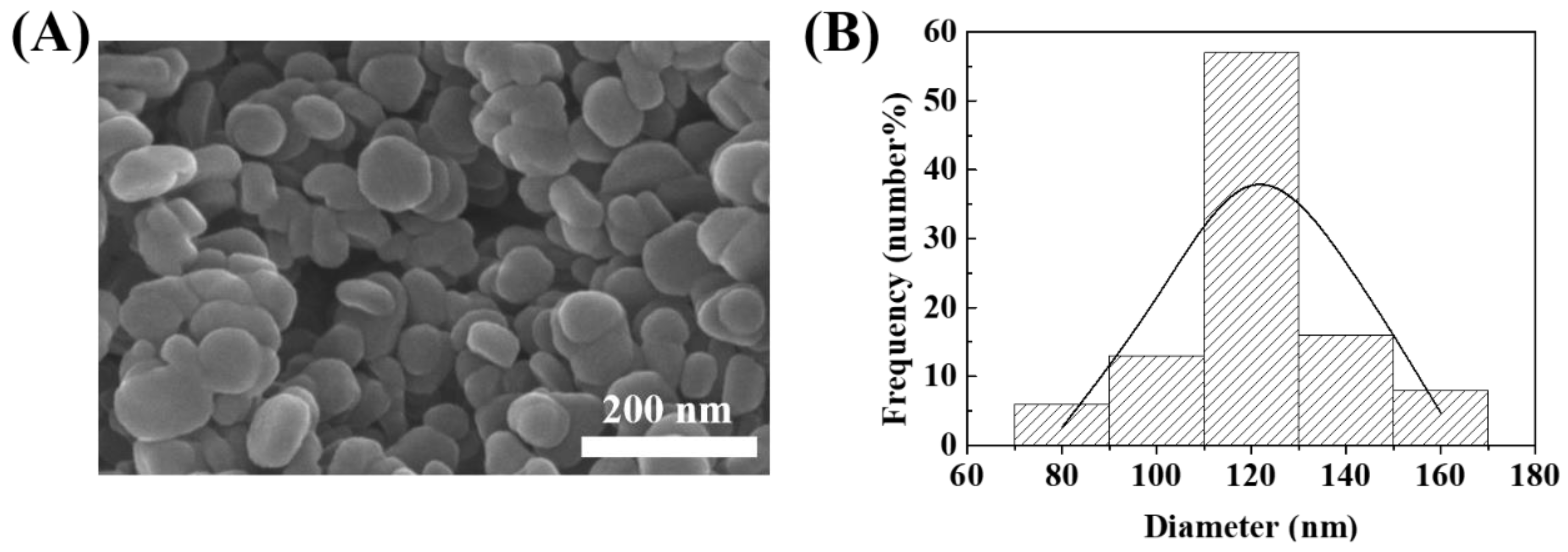
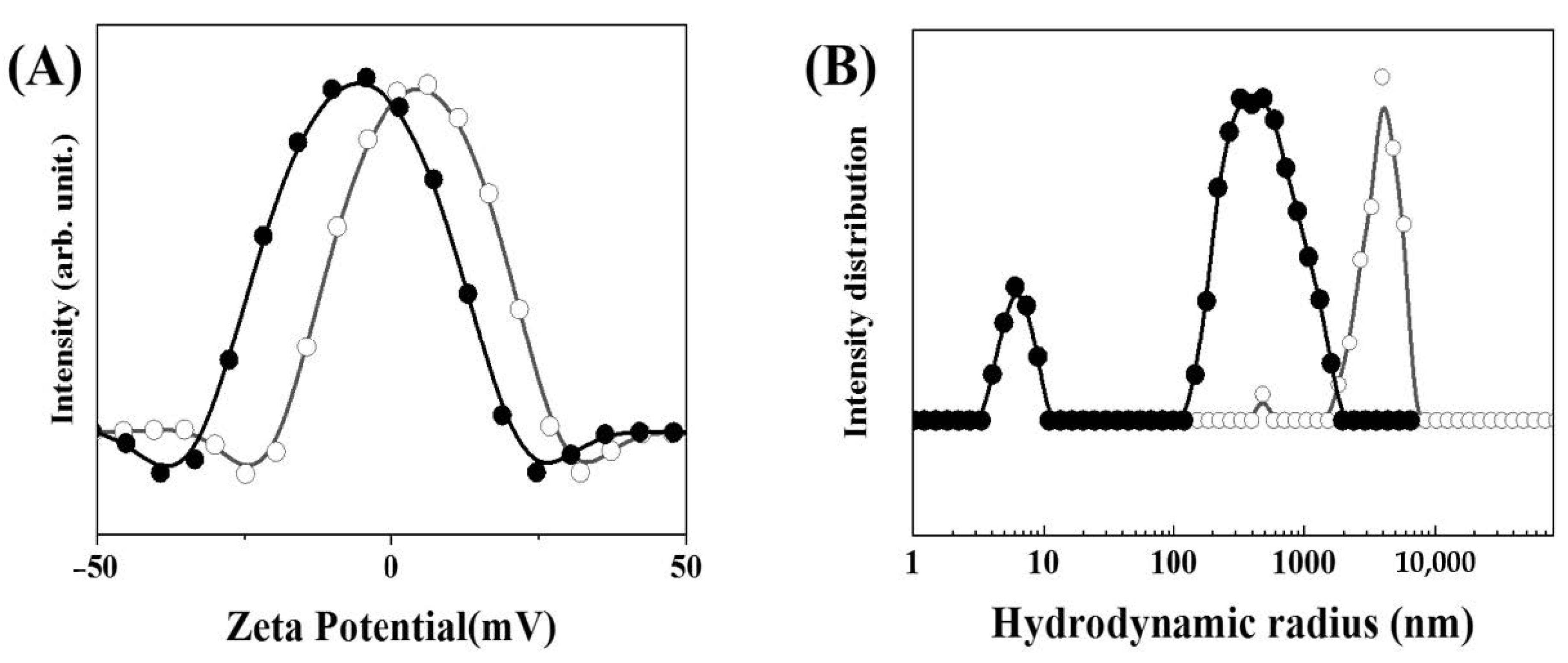
2.3. Characterization of MTX-LDH
2.4. In Vitro Biological Assay: Plasma Protein Fluorescence Quenching
2.5. In Vivo Biological Assay: Hematocompatibility in Mouse Model
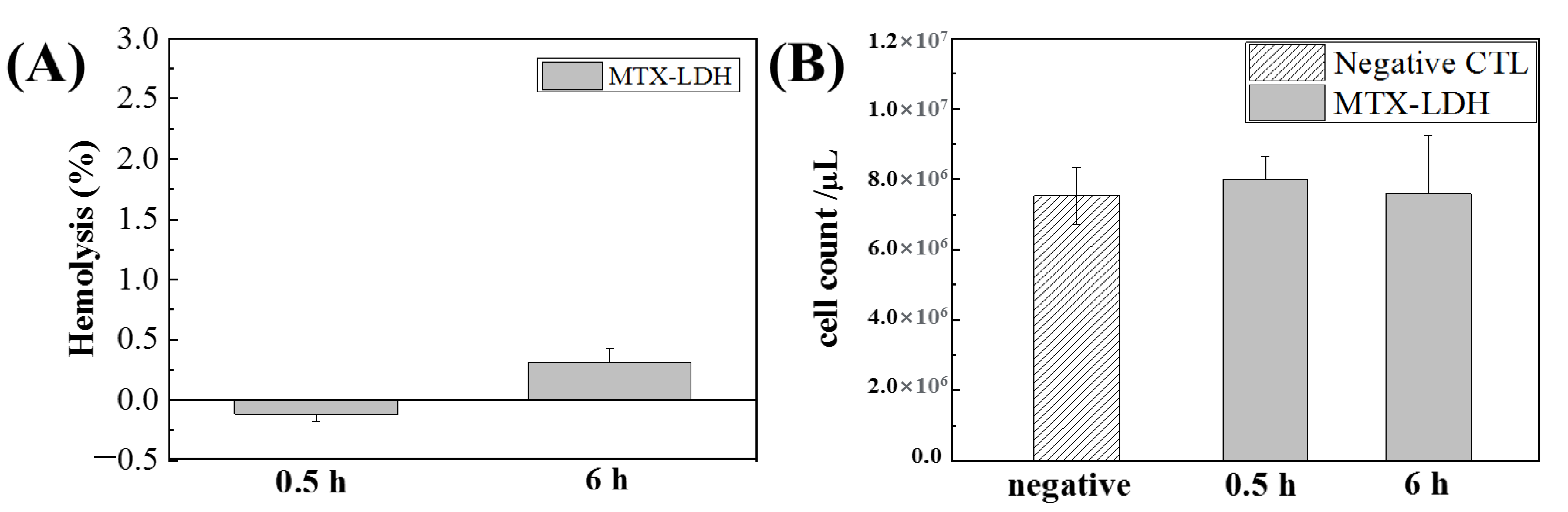
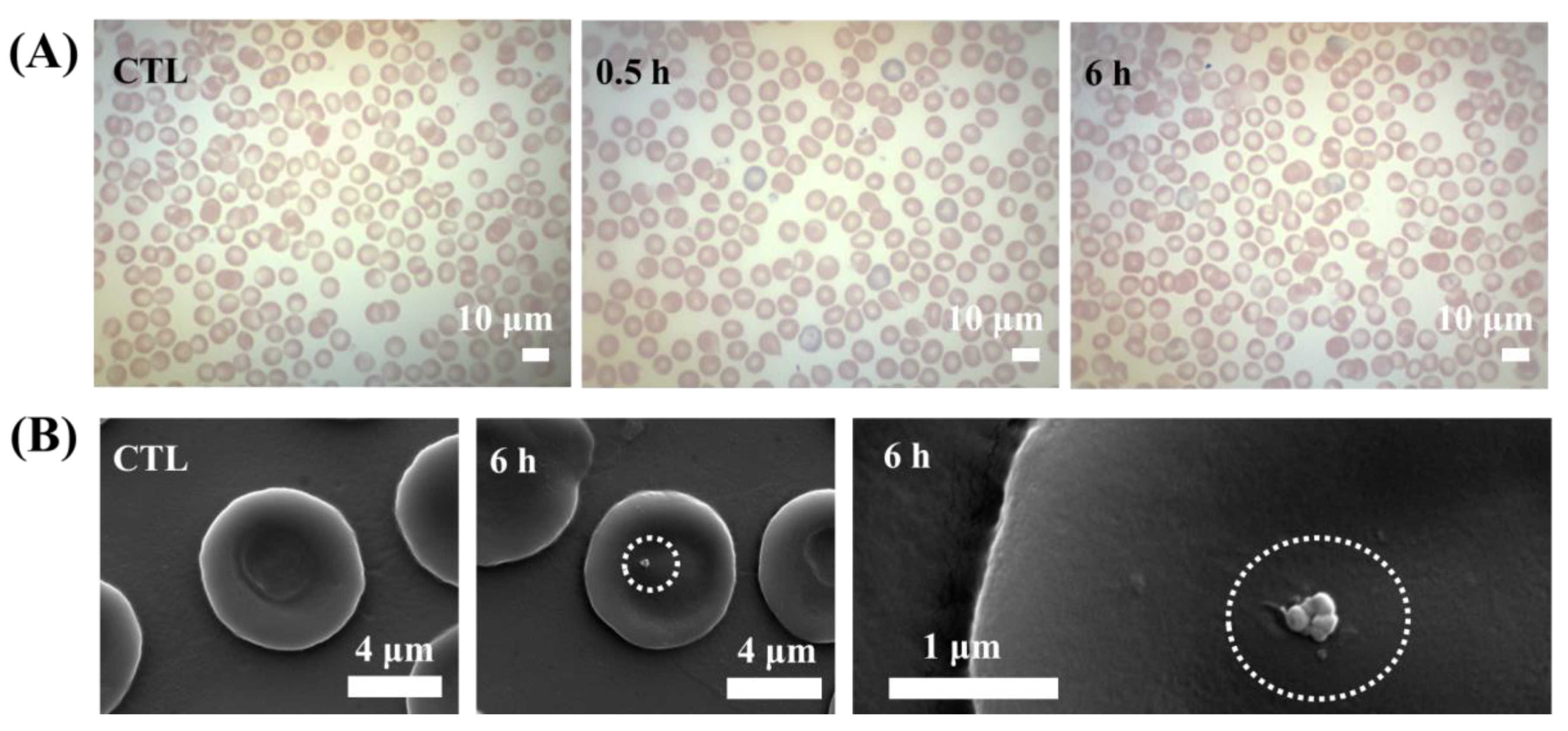
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Webster, T.J. Nanomedicine: What’s in a definition? Int. J. Nanomed. 2006, 1, 115–116. [Google Scholar] [CrossRef] [PubMed]
- Deb, S.; Ghosh, K.; Shetty, S.D. Nanoimaging in cardiovascular diseases: Current state of the art. Indian J. Med. Res. 2015, 141, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Lee, G.J.; Choi, A.-J.; Kim, T.-H.; Kim, T.-i.; Oh, J.-M. Layered Double Hydroxide Nanomaterials Encapsulating Angelica gigas Nakai Extract for Potential Anticancer Nanomedicine. Front. Pharmacol. 2018, 9, 723. [Google Scholar] [CrossRef] [PubMed]
- Ko, W.C.; Kim, M.-S.; Kwon, Y.J.; Jeong, J.; Kim, W.R.; Choi, H.; Park, J.K.; Jeong, Y.K. Two-dimensional semiconducting covalent organic nanosheets for highly sensitive and stable NO2 sensing under humid conditions. J. Mater. Chem. A 2020, 8, 19246–19253. [Google Scholar] [CrossRef]
- Oh, J.-M.; Choi, S.-J.; Kim, S.-T.; Choy, J.-H. Cellular Uptake Mechanism of an Inorganic Nanovehicle and Its Drug Conjugates: Enhanced Efficacy Due To Clathrin-Mediated Endocytosis. Bioconjugate Chem. 2006, 17, 1411–1417. [Google Scholar] [CrossRef]
- Angelova, A.; Garamus, V.M.; Angelov, B.; Tian, Z.; Li, Y.; Zou, A. Advances in structural design of lipid-based nanoparticle carriers for delivery of macromolecular drugs, phytochemicals and anti-tumor agents. Adv. Colloid Interface Sci. 2017, 249, 331–345. [Google Scholar] [CrossRef]
- Angelova, A.; Drechsler, M.; Garamus, V.M.; Angelov, B. Pep-Lipid Cubosomes and Vesicles Compartmentalized by Micelles from Self-Assembly of Multiple Neuroprotective Building Blocks Including a Large Peptide Hormone PACAP-DHA. ChemNanoMat 2019, 5, 1381–1389. [Google Scholar] [CrossRef]
- Rakotoarisoa, M.; Angelov, B.; Espinoza, S.; Khakurel, K.; Bizien, T.; Angelova, A. Cubic Liquid Crystalline Nanostructures Involving Catalase and Curcumin: BioSAXS Study and Catalase Peroxidatic Function after Cubosomal Nanoparticle Treatment of Differentiated SH-SY5Y Cells. Molecules 2019, 24, 3058. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Shi, L.; Wu, C.; Su, Y.; Qian, J.; Deng, H.; Zhu, X. Construction of a Supramolecular Drug–Drug Delivery System for Non-Small-Cell Lung Cancer Therapy. ACS Appl. Mater. Interfaces 2017, 9, 29505–29514. [Google Scholar] [CrossRef]
- Gil, E.S.; Hudson, S.M. Stimuli-reponsive polymers and their bioconjugates. Prog. Polym. Sci. 2004, 29, 1173–1222. [Google Scholar] [CrossRef]
- Jung, S.-Y.; Gwak, G.-H.; Park, J.K.; Oh, J.-M. Finely crafted quasi-core–shell gadolinium/layered double hydroxide hybrids for switching on/off bimodal CT/MRI contrasting nanodiagnostic platforms. RSC Adv. 2020, 10, 5838–5844. [Google Scholar] [CrossRef]
- Vaccari, A. Preparation and catalytic properties of cationic and anionic clays. Catal. Today 1998, 41, 53–71. [Google Scholar] [CrossRef]
- Hwang, S.H.; Han, Y.-S.; Choy, J.-H. Intercalation of functional organic molecules with pharmaceutical, cosmeceutical and nutraceutical functions into layered double hydroxides and zinc basic salts. Bull. Korean Chem. Soc. 2001, 22, 1019–1022. [Google Scholar] [CrossRef] [Green Version]
- Shin, J.; Choi, C.-J.; Kim, T.-H.; Oh, J.-M. Phase Transformation from Brucite to Highly Crystalline Layered Double Hydroxide through a Combined Dissolution–Reprecipitation and Substitution Mechanism. Cryst. Growth Des. 2018, 18, 5398–5405. [Google Scholar] [CrossRef] [Green Version]
- Choy, J.-H.; Kim, Y.-K.; Son, Y.-H.; Choy, Y.B.; Oh, J.-M.; Jung, H.; Hwang, S.-J. Nanohybrids of edible dyes intercalated in ZnAl layered double hydroxides. J. Phys. Chem. Solids 2008, 69, 1547–1551. [Google Scholar] [CrossRef]
- Vasilev, K.; Chen, H.; Murray, P.; Mantovani, D. The Potential of Nanomaterials for Drug Delivery, Cell Tracking, and Regenerative Medicine 2014. J. Nanomater. 2015, 2015, 869308. [Google Scholar] [CrossRef] [Green Version]
- Kang, H.; Kim, H.-J.; Yang, J.-H.; Kim, T.-H.; Choi, G.; Paek, S.-M.; Choi, A.-J.; Choy, J.-H.; Oh, J.-M. Intracrystalline structure and release pattern of ferulic acid intercalated into layered double hydroxide through various synthesis routes. Appl. Clay Sci. 2015, 112–113, 32–39. [Google Scholar] [CrossRef]
- Ko, S.J.; Yamaguchi, T.; Salles, F.; Oh, J.M. Systematic utilization of layered double hydroxide nanosheets for effective removal of methyl orange from an aqueous system by π-π stacking-induced nanoconfinement. J. Environ. Manag. 2021, 277, 111455. [Google Scholar] [CrossRef]
- Oh, J.-M.; Hwang, S.-H.; Choy, J.-H. The effect of synthetic conditions on tailoring the size of hydrotalcite particles. Solid State Ion. 2002, 151, 285–291. [Google Scholar] [CrossRef]
- Jung, S.-Y.; Kim, B.-K.; Hirata, S.; Inada, M.; Oh, J.-M. Particle size effect of layered double hydroxide on the porosity of calcined metal oxide. Appl. Clay Sci. 2020, 195, 105701. [Google Scholar] [CrossRef]
- Choy, J.-H.; Kwak, S.-Y.; Jeong, Y.-J.; Park, J.-S. Inorganic Layered Double Hydroxides as Nonviral Vectors. Angew. Chem. Int. Ed. 2000, 39, 4041–4045. [Google Scholar] [CrossRef]
- Kim, J.Y.; Choi, S.J.; Oh, J.M.; Park, T.; Choy, J.H. Anticancer drug-inorganic nanohybrid and its cellular interaction. J. Nanosci. Nanotechnol. 2007, 7, 3700–3705. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.-M.; Choi, S.-J.; Lee, G.-E.; Han, S.-H.; Choy, J.-H. Inorganic Drug-Delivery Nanovehicle Conjugated with Cancer-Cell-Specific Ligand. Adv. Funct. Mater. 2009, 19, 1617–1624. [Google Scholar] [CrossRef]
- Zuo, H.; Chen, W.; Cooper, H.M.; Xu, Z.P. A Facile Way of Modifying Layered Double Hydroxide Nanoparticles with Targeting Ligand-Conjugated Albumin for Enhanced Delivery to Brain Tumour Cells. ACS Appl. Mater. Interfaces 2017, 9, 20444–20453. [Google Scholar] [CrossRef]
- Yoon, Y.-s.; Lee, B.-I.; Lee, K.S.; Im, G.H.; Byeon, S.-H.; Lee, J.H.; Lee, I.S. Surface Modification of Exfoliated Layered Gadolinium Hydroxide for the Development of Multimodal Contrast Agents for MRI and Fluorescence Imaging. Adv. Funct. Mater. 2009, 19, 3375–3380. [Google Scholar] [CrossRef]
- Choy, J.-H.; Jung, J.-S.; Oh, J.-M.; Park, M.; Jeong, J.; Kang, Y.-K.; Han, O.-J. Layered double hydroxide as an efficient drug reservoir for folate derivatives. Biomaterials 2004, 25, 3059–3064. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, M.; Dasgupta, S.; Soundrapandian, C.; Chakraborty, J.; Ghosh, S.; Mitra, M.K.; Basu, D. Methotrexate intercalated ZnAl-layered double hydroxide. J. Solid State Chem. 2011, 184, 2439–2445. [Google Scholar] [CrossRef]
- Alexa, I.F.; Pastravanu, C.G.; Ignat, M.; Popovici, E. A comparative study on long-term MTX controlled release from intercalated nanocomposites for nanomedicine applications. Colloids Surf. B Biointerfaces 2013, 106, 135–139. [Google Scholar] [CrossRef]
- Oh, J.-M.; Park, M.; Kim, S.-T.; Jung, J.-Y.; Kang, Y.-G.; Choy, J.-H. Efficient delivery of anticancer drug MTX through MTX-LDH nanohybrid system. J. Phys. Chem. Solids 2006, 67, 1024–1027. [Google Scholar] [CrossRef]
- Oh, J.M.; Park, C.B.; Choy, J.H. Intracellular drug delivery of layered double hydroxide nanoparticles. J. Nanosci. Nanotechnol. 2011, 11, 1632–1635. [Google Scholar] [CrossRef]
- Kim, T.-H.; Lee, G.J.; Kang, J.-H.; Kim, H.-J.; Kim, T.-i.; Oh, J.-M. Anticancer Drug-Incorporated Layered Double Hydroxide Nanohybrids and Their Enhanced Anticancer Therapeutic Efficacy in Combination Cancer Treatment. BioMed Res. Int. 2014, 2014, 193401. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.-J.; Choi, G.E.; Oh, J.-M.; Oh, Y.-J.; Park, M.-C.; Choy, J.-H. Anticancer drug encapsulated in inorganic lattice can overcome drug resistance. J. Mater. Chem. 2010, 20, 9463–9469. [Google Scholar] [CrossRef]
- Tian, D.Y.; Wang, W.Y.; Li, S.P.; Li, X.D.; Sha, Z.L. A novel platform designed by Au core/inorganic shell structure conjugated onto MTX/LDH for chemo-photothermal therapy. Int. J. Pharm. 2016, 505, 96–106. [Google Scholar] [CrossRef]
- Soo-Jin, C.; Jae-Min, O.; Hae-Eun, C.; Seung-Hee, H.; In-Hoo, K.; Jin-Ho, C. In Vivo Anticancer Activity of Methotrexate-loaded Layered Double Hydroxide Nanoparticles. Curr. Pharm. Des. 2013, 19, 7196–7202. [Google Scholar] [CrossRef]
- Choi, G.; Kwon, O.-J.; Oh, Y.; Yun, C.-O.; Choy, J.-H. Inorganic Nanovehicle Targets Tumor in an Orthotopic Breast Cancer Model. Sci. Rep. 2014, 4, 4430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ritz, S.; Schöttler, S.; Kotman, N.; Baier, G.; Musyanovych, A.; Kuharev, J.; Landfester, K.; Schild, H.; Jahn, O.; Tenzer, S.; et al. Protein Corona of Nanoparticles: Distinct Proteins Regulate the Cellular Uptake. Biomacromolecules 2015, 16, 1311–1321. [Google Scholar] [CrossRef]
- Satzer, P.; Svec, F.; Sekot, G.; Jungbauer, A. Protein adsorption onto nanoparticles induces conformational changes: Particle size dependency, kinetics, and mechanisms. Eng. Life Sci. 2016, 16, 238–246. [Google Scholar] [CrossRef] [Green Version]
- Dobrovolskaia, M.A.; Clogston, J.D.; Neun, B.W.; Hall, J.B.; Patri, A.K.; McNeil, S.E. Method for analysis of nanoparticle hemolytic properties in vitro. Nano Lett. 2008, 8, 2180–2187. [Google Scholar] [CrossRef]
- Huang, H.; Lai, W.; Cui, M.; Liang, L.; Lin, Y.; Fang, Q.; Liu, Y.; Xie, L. An Evaluation of Blood Compatibility of Silver Nanoparticles. Sci. Rep. 2016, 6, 25518. [Google Scholar] [CrossRef] [Green Version]
- Wadhwa, R.; Aggarwal, T.; Thapliyal, N.; Kumar, A.; Priya; Yadav, P.; Kumari, V.; Reddy, B.S.C.; Chandra, P.; Maurya, P.K. Red blood cells as an efficient in vitro model for evaluating the efficacy of metallic nanoparticles. 3 Biotech 2019, 9, 279. [Google Scholar] [CrossRef]
- Avsievich, T.; Popov, A.; Bykov, A.; Meglinski, I. Mutual interaction of red blood cells influenced by nanoparticles. Sci. Rep. 2019, 9, 5147. [Google Scholar] [CrossRef]
- Yiying, B.; Kim, K.; Thien, N.; Kim, I.; Bae, O.-N.; Lim, K.-M.; Chung, J.-H. Silver nanoparticles promote procoagulant activity of red blood cells: A potential risk of thrombosis in susceptible population. Part. Fiber Toxicol. 2019. [Google Scholar] [CrossRef]
- Tsai, L.-W.; Lin, Y.-C.; Perevedentseva, E.; Lugovtsov, A.; Priezzhev, A.; Cheng, C.-L. Nanodiamonds for Medical Applications: Interaction with Blood in Vitro and in Vivo. Int. J. Mol. Sci. 2016, 17, 1111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, G.; Kim, S.Y.; Oh, J.-M.; Choy, J.-H. Drug-Ceramic 2-Dimensional Nanoassemblies for Drug Delivery System in Physiological Condition. J. Am. Ceram. Soc. 2012, 95, 2758–2765. [Google Scholar] [CrossRef]
- Hill, A.V. The Combinations of Haemoglobin with Oxygen and Carbon Monoxide, and the effects of Acid and Carbon Dioxide. Biochem. J. 1921, 15, 577–586. [Google Scholar] [CrossRef] [Green Version]
- Hill, A.V. The Combinations of Haemoglobin with Oxygen and with Carbon Monoxide. I. Biochem. J. 1913, 7, 471–480. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Jiang, K.; Qian, Y.; Han, H.; Qiao, P.; Zhang, H. Effect of organically intercalation modified layered double hydroxides-graphene oxide hybrids on flame retardancy of thermoplastic polyurethane nanocomposites. J. Therm. Anal. Calorim. 2020, 142, 723–733. [Google Scholar] [CrossRef]
- Shabanian, M.; Hajibeygi, M.; Raeisi, A. 2—FTIR characterization of layered double hydroxides and modified layered double hydroxides. In Layered Double Hydroxide Polymer Nanocomposites; Thomas, S., Daniel, S., Eds.; Woodhead Publishing: Sawston, UK, 2020; pp. 77–101. [Google Scholar] [CrossRef]
- Cai, J.; Heng, H.-M.; Hu, X.-P.; Xu, Q.-K.; Miao, F. A facile method for the preparation of novel fire-retardant layered double hydroxide and its application as nanofiller in UP. Polym. Degrad. Stab. 2016, 126, 47–57. [Google Scholar] [CrossRef]
- Hashad, R.A.; Ishak, R.A.; Geneidi, A.S.; Mansour, S. Methotrexate loading in chitosan nanoparticles at a novel pH: Response surface modeling, optimization and characterization. Int. J. Biol. Macromol. 2016, 91, 630–639. [Google Scholar] [CrossRef]
- Choy, J.-H.; Park, J.-S.; Kwak, S.-Y.; Jeong, Y.-J.; Han, Y.-S. Layered Double Hydroxide as Gene Reservoir. Mol. Cryst. Liquid Cryst. 2000, 341, 425–429. [Google Scholar] [CrossRef]
- Lu, Y.; Miller, J.D. Carboxyl Stretching Vibrations of Spontaneously Adsorbed and LB-Transferred Calcium Carboxylates as Determined by FTIR Internal Reflection Spectroscopy. J. Colloid Interface Sci. 2002, 256, 41–52. [Google Scholar] [CrossRef]
- Oh, J.-M.; Choi, S.-J.; Lee, G.-E.; Kim, J.-E.; Choy, J.-H. Inorganic Metal Hydroxide Nanoparticles for Targeted Cellular Uptake Through Clathrin-Mediated Endocytosis. Chem. Asian J. 2009, 4, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.J.; Oh, J.M.; Choy, J.H. Safety aspect of inorganic layered nanoparticles: Size-dependency in vitro and in vivo. J. Nanosci. Nanotechnol. 2008, 8, 5297–5301. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Li, W.; Chen, H.; Xiao, F.; Huang, L.; Holm, P.E.; Hansen, H.C.B.; Wang, D. Synergistic effect of humic and fulvic acids on Ni removal by the calcined Mg/Al layered double hydroxide. RSC Adv. 2015, 5, 18866–18874. [Google Scholar] [CrossRef]
- Kim, T.-H.; Hong, I.T.; Oh, J.-M. Size- and surface charge-controlled layered double hydroxides for efficient algal flocculation. Environ. Sci. Nano 2018, 5, 183–190. [Google Scholar] [CrossRef]
- Kim, H.-J.; Lee, S.-B.; Choi, A.-J.; Oh, J.-M. Zingiber officinale Extract (ZOE) Incorporated with Layered Double Hydroxide Hybrid through Reconstruction to Preserve Antioxidant Activity of ZOE against Ultrasound and Microwave Irradiation. Nanomaterials 2019, 9, 1281. [Google Scholar] [CrossRef] [Green Version]
- Jeung, D.-G.; Kim, H.-J.; Oh, J.-M. Incorporation of Glycine max Merrill Extract into Layered Double Hydroxide through Ion-Exchange and Reconstruction. Nanomaterials 2019, 9, 1262. [Google Scholar] [CrossRef] [Green Version]
- Wosikowski, K.; Biedermann, E.; Rattel, B.; Breiter, N.; Jank, P.; Löser, R.; Jansen, G.; Peters, G.J. In vitro and in vivo antitumor activity of methotrexate conjugated to human serum albumin in human cancer cells. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2003, 9, 1917–1926. [Google Scholar]
- Fiehn, C.; Muller-Ladner, U.; Gay, S.; Krienke, S.; Freudenberg-Konrad, S.; Funk, J.; Ho, A.D.; Sinn, H.; Wunder, A. Albumin-coupled methotrexate (MTX-HSA) is a new anti-arthritic drug which acts synergistically to MTX. Rheumatology 2004, 43, 1097–1105. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Wang, X.; Song, H.; Deng, S.; Li, W.; Li, J.; Sun, J. Current multifunctional albumin-based nanoplatforms for cancer multi-mode therapy. Asian J. Pharm. Sci. 2020, 15, 1–12. [Google Scholar] [CrossRef]
- Lacerda, S.H.; Park, J.J.; Meuse, C.; Pristinski, D.; Becker, M.L.; Karim, A.; Douglas, J.F. Interaction of gold nanoparticles with common human blood proteins. ACS Nano 2010, 4, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-M.; Kim, K.-M.; Lee, K.; Kim, Y.S.; Oh, J.-M. Nano–Bio Interaction between Graphite Oxide Nanoparticles and Human Blood Components. Eur. J. Inorg. Chem. 2012, 2012, 5343–5349. [Google Scholar] [CrossRef]
- Kapur, A.; Aldeek, F.; Ji, X.; Safi, M.; Wang, W.; Del Cid, A.; Steinbock, O.; Mattoussi, H. Self-Assembled Gold Nanoparticle–Fluorescent Protein Conjugates as Platforms for Sensing Thiolate Compounds via Modulation of Energy Transfer Quenching. Bioconjugate Chem. 2017, 28, 678–687. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Aphale, A.N.; Macwan, I.G.; Patra, P.K.; Gonzalez, W.G.; Miksovska, J.; Leblanc, R.M. Graphene Oxide as a Quencher for Fluorescent Assay of Amino Acids, Peptides, and Proteins. ACS Appl. Mater. Interfaces 2012, 4, 7069–7075. [Google Scholar] [CrossRef]
- Kim, H.-M.; Kim, K.-M.; Jung, B.C.; Kim, Y.S.; Choy, J.-H.; Oh, J.-M. Hematocompatibility and Interaction of Layered Double Hydroxide Nanomaterials with Plasma Proteins. Sci. Adv. Mater. 2014, 6, 1582–1589. [Google Scholar] [CrossRef]
- Choi, S.-J.; Choy, J.-H. Effect of physico-chemical parameters on the toxicity of inorganic nanoparticles. J. Mater. Chem. 2011, 21, 5547–5554. [Google Scholar] [CrossRef]
- Woolley Iii, P.V.; Sacher, R.A.; Priego, V.M.; Schanfield, M.S.; Bonnem, E.M. Methotrexate-induced immune haemolytic anaemia. Br. J. Haematol. 1983, 54, 543–552. [Google Scholar] [CrossRef]
- Fukuda, T.; Asou, E.; Nogi, K.; Goto, K. Evaluation of mouse red blood cell and platelet counting with an automated hematology analyzer. J. Vet. Med. Sci. 2017, 79, 1707–1711. [Google Scholar] [CrossRef] [Green Version]
- Hoffman, R.; Benz, E.J.; Silberstein, L.E.; Heslop, H.; Weitz, J.; Anastasi, J. Hematology: Diagnosis and Treatment E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Zhao, Z.; Ukidve, A.; Gao, Y.; Kim, J.; Mitragotri, S. Erythrocyte leveraged chemotherapy (ELeCt): Nanoparticle assembly on erythrocyte surface to combat lung metastasis. Sci. Adv. 2019, 5, eaax9250. [Google Scholar] [CrossRef] [Green Version]
- Anselmo, A.C.; Kumar, S.; Gupta, V.; Pearce, A.M.; Ragusa, A.; Muzykantov, V.; Mitragotri, S. Exploiting shape, cellular-hitchhiking and antibodies to target nanoparticles to lung endothelium: Synergy between physical, chemical and biological approaches. Biomaterials 2015, 68, 1–8. [Google Scholar] [CrossRef]
- Brenner, J.S.; Pan, D.C.; Myerson, J.W.; Marcos-Contreras, O.A.; Villa, C.H.; Patel, P.; Hekierski, H.; Chatterjee, S.; Tao, J.-Q.; Parhiz, H.; et al. Red blood cell-hitchhiking boosts delivery of nanocarriers to chosen organs by orders of magnitude. Nat. Commun. 2018, 9, 2684. [Google Scholar] [CrossRef] [PubMed]
- Zelepukin, I.V.; Yaremenko, A.V.; Shipunova, V.O.; Babenyshev, A.V.; Balalaeva, I.V.; Nikitin, P.I.; Deyev, S.M.; Nikitin, M.P. Nanoparticle-based drug delivery via RBC-hitchhiking for the inhibition of lung metastases growth. Nanoscale 2019, 11, 1636–1646. [Google Scholar] [CrossRef] [PubMed]
- Anselmo, A.C.; Gupta, V.; Zern, B.J.; Pan, D.; Zakrewsky, M.; Muzykantov, V.; Mitragotri, S. Delivering Nanoparticles to Lungs while Avoiding Liver and Spleen through Adsorption on Red Blood Cells. ACS Nano 2013, 7, 11129–11137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
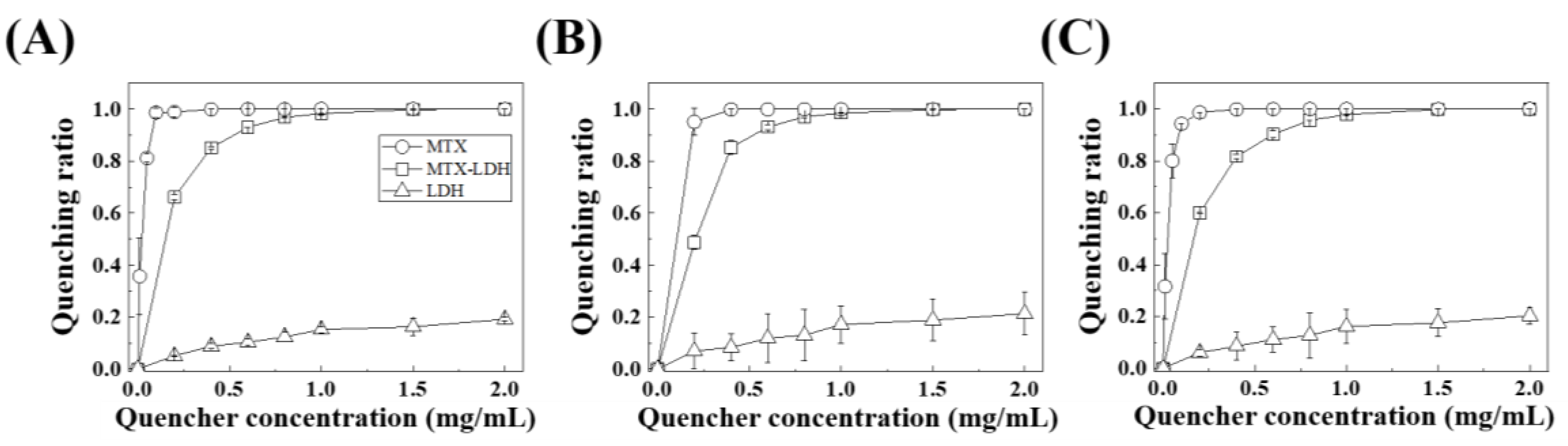
| Protein | Sample Name | n | KD | Kb | r2 |
|---|---|---|---|---|---|
| Albumin | MTX-LDH | 3.83 | 2.20 × 10−1 | 4.54 | 0.935 |
| MTX | 2.04 | 1.56 × 10−2 | 6.42 × 101 | 0.988 | |
| LDH | 1.39 | 4.5 × 10−1 | 2.21 | 0.968 | |
| Fibrinogen | MTX-LDH | 3.87 | 2.59 × 10−1 | 3.87 | 0.911 |
| MTX | 8.50 × 10−1 | 6.68 × 10−3 | 1.50 × 102 | 0.923 | |
| LDH | 1.47 | 4.19 × 10−1 | 2.39 | 0.928 | |
| Globulin | MTX-LDH | 3.89 | 2.63 × 10−1 | 3.8 | 0.971 |
| MTX | 2.45 | 2.17 × 10−2 | 4.62 × 101 | 0.953 | |
| LDH | 1.39 | 4.45 × 10−1 | 2.25 | 0.939 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, S.-Y.; Kim, H.-M.; Hwang, S.; Jeung, D.-G.; Rhee, K.-J.; Oh, J.-M. Physicochemical Properties and Hematocompatibility of Layered Double Hydroxide-Based Anticancer Drug Methotrexate Delivery System. Pharmaceutics 2020, 12, 1210. https://doi.org/10.3390/pharmaceutics12121210
Jung S-Y, Kim H-M, Hwang S, Jeung D-G, Rhee K-J, Oh J-M. Physicochemical Properties and Hematocompatibility of Layered Double Hydroxide-Based Anticancer Drug Methotrexate Delivery System. Pharmaceutics. 2020; 12(12):1210. https://doi.org/10.3390/pharmaceutics12121210
Chicago/Turabian StyleJung, Sang-Yong, Hyoung-Mi Kim, Soonjae Hwang, Do-Gak Jeung, Ki-Jong Rhee, and Jae-Min Oh. 2020. "Physicochemical Properties and Hematocompatibility of Layered Double Hydroxide-Based Anticancer Drug Methotrexate Delivery System" Pharmaceutics 12, no. 12: 1210. https://doi.org/10.3390/pharmaceutics12121210
APA StyleJung, S.-Y., Kim, H.-M., Hwang, S., Jeung, D.-G., Rhee, K.-J., & Oh, J.-M. (2020). Physicochemical Properties and Hematocompatibility of Layered Double Hydroxide-Based Anticancer Drug Methotrexate Delivery System. Pharmaceutics, 12(12), 1210. https://doi.org/10.3390/pharmaceutics12121210





