Functional Nanofibrous Biomaterials of Tailored Structures for Drug Delivery—A Critical Review
Abstract
1. Introduction
2. Variety of Polymeric Biomaterials
2.1. Natural Polymeric Biomaterials
2.1.1. Cellulose
2.1.2. Chitin and Chitosan
2.1.3. Collagen
2.1.4. Other Natural Polymeric Biomaterials
2.2. Synthetic Polymeric Biomaterials
2.2.1. Poly Lactic-co-Glycolic Acid (PLGA)
2.2.2. Polycaprolactone (PCL)
2.2.3. Polyethylene Oxide (PEO)
2.2.4. Other Synthetic Polymeric Biomaterials
3. Nanofiber Production Methods
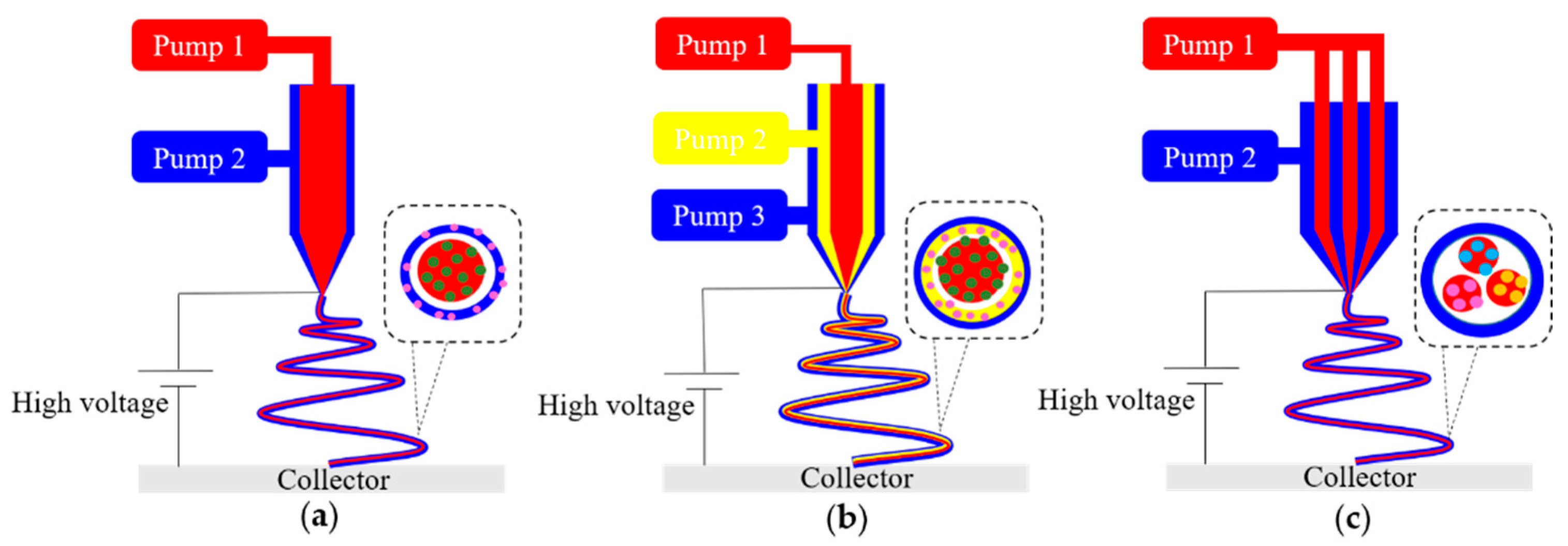
3.1. Electrospinning
3.2. Centrifugal Spinning
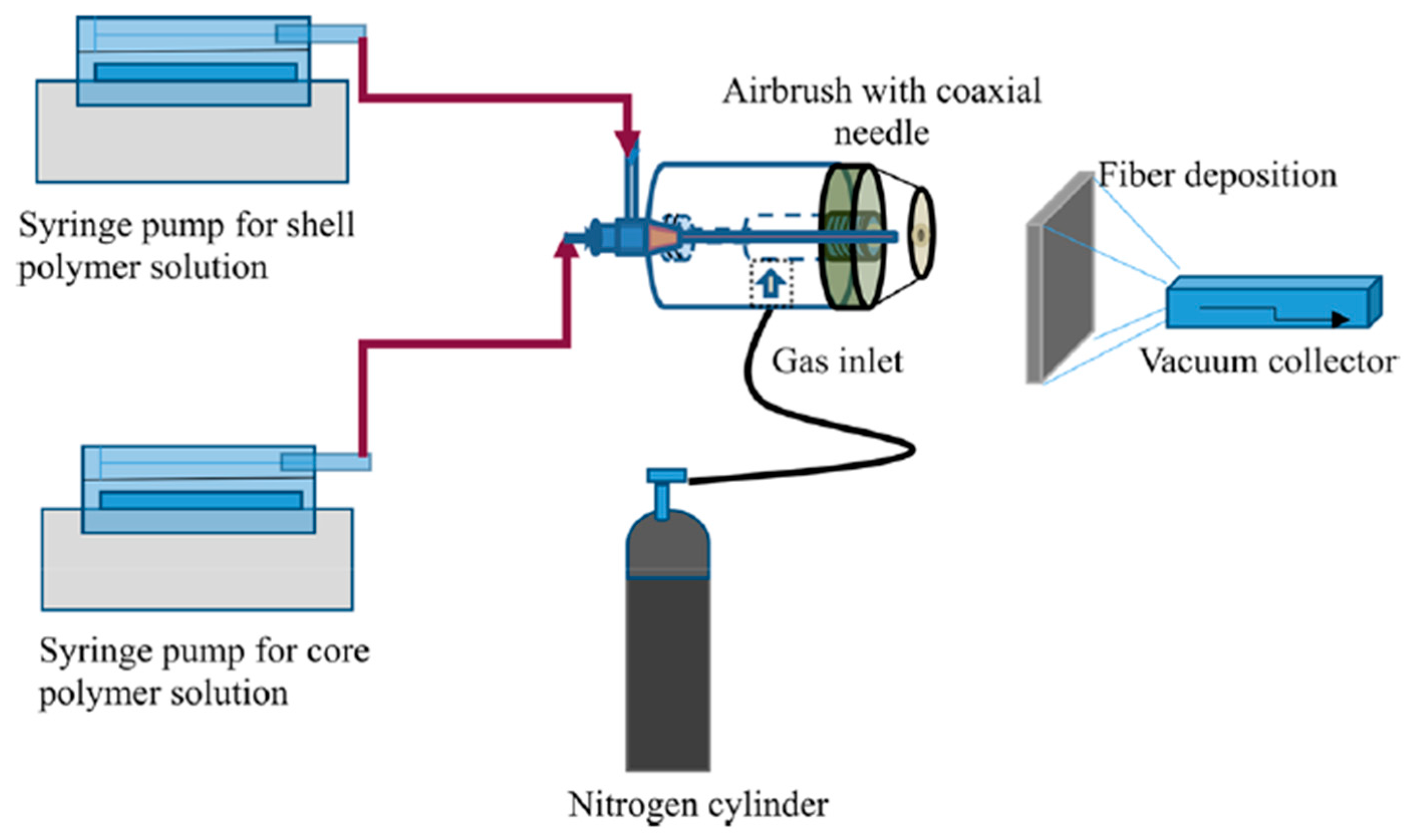
3.3. Solution Blowing
3.4. Other Nanofiber Fabrication Techniques
4. Morphologies of Nanofibers
4.1. Fiber Orientation
4.2. Fiber Cross-Section
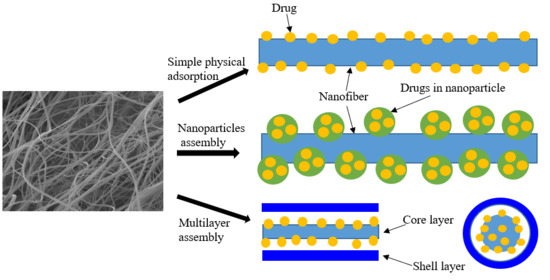
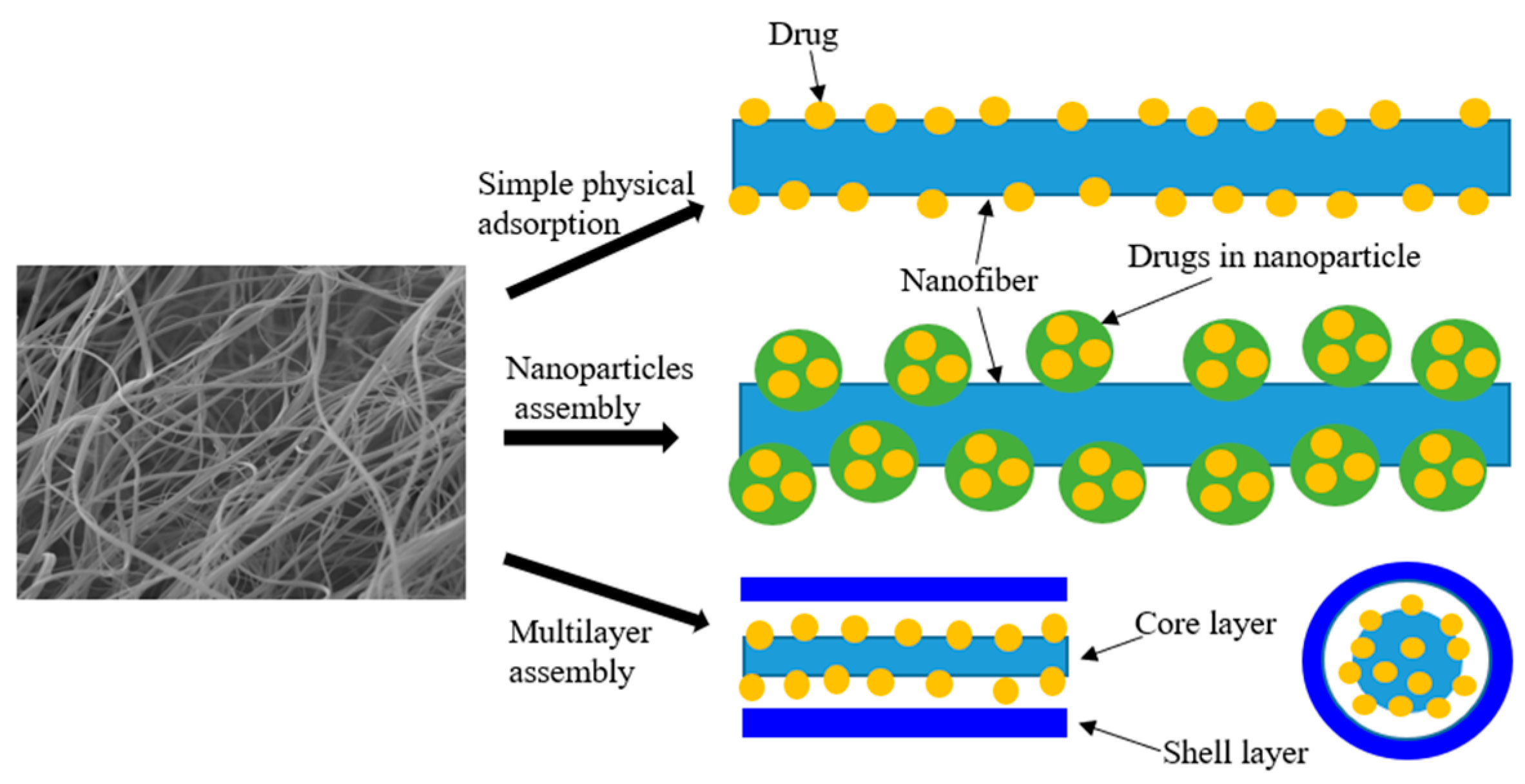
5. Drug Loading in Nanofibers
5.1. Chemical Adsorption
5.2. Physical Adsorption
6. Drug Release from Nanofibers
6.1. Drug Diffusion
6.2. Nanofiber Erosion
6.3. Drug Release Profile
7. Conclusions and Future Prospects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ketabchi, N.; Naghibzadeh, M.; Adabi, M.; Esnaashari, S.S.; Faridi-Majidi, R. Preparation and optimization of chitosan/polyethylene oxide nanofiber diameter using artificial neural networks. Neural Comput. Appl. 2017, 28, 3131–3143. [Google Scholar] [CrossRef]
- Zhang, S.; Tang, N.; Cao, L.; Yin, X.; Yu, J.; Ding, B. Highly Integrated Polysulfone/Polyacrylonitrile/Polyamide-6 Air Filter for Multilevel Physical Sieving Airborne Particles. ACS Appl. Mater. Interfaces 2016, 8, 29062–29072. [Google Scholar] [CrossRef] [PubMed]
- Yanilmaz, M.; Lu, Y.; Li, Y.; Zhang, X. SiO2/polyacrylonitrile membranes via centrifugal spinning as a separator for Li-ion batteries. J. Power Sources 2015, 273, 1114–1119. [Google Scholar] [CrossRef]
- Marano, S.; Barker, S.A.; Raimi-Abraham, B.T.; Missaghi, S.; Rajabi-Siahboomi, A.; Craig, D.Q.M. Development of micro-fibrous solid dispersions of poorly water-soluble drugs in sucrose using temperature-controlled centrifugal spinning. Eur. J. Pharm. Biopharm. 2016, 103, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Jun, Y.; Qin, J.H.; Lee, S.H. Electrospinning versus microfluidic spinning of functional fibers for biomedical applications. Biomaterials 2017, 114, 121–143. [Google Scholar] [CrossRef] [PubMed]
- Habibi, N.; Kamaly, N.; Memic, A.; Shafiee, H. Self-assembled peptide-based nanostructures: Smart nanomaterials toward targeted drug delivery. Nano Today 2016, 11, 41–60. [Google Scholar] [CrossRef]
- Zamani, M.; Prabhakaran, M.P.; Ramakrishna, S. Advances in drug delivery via electrospun and electrosprayed nanomaterials. Int. J. Nanomed. 2013, 8, 2997–3017. [Google Scholar]
- Khalf, A.; Madihally, S.V. Recent advances in multiaxial electrospinning for drug delivery. Eur. J. Pharm. Biopharm. Off. J. Arb. Fur Pharm. Verfahr. 2017, 112, 1–17. [Google Scholar] [CrossRef]
- Rasouli, R.; Barhoum, A.; Bechelany, M.; Dufresne, A. Nanofibers for Biomedical and Healthcare Applications. Macromol. Biosci. 2019, 19, 27. [Google Scholar]
- Figueiredo, P.; Lintinen, K.; Kiriazis, A.; Hynninen, V.; Liu, Z.H.; Bauleth-Ramos, T.; Rahikkala, A.; Correia, A.; Kohout, T.; Sarmento, B.; et al. In vitro evaluation of biodegradable lignin-based nanoparticles for drug delivery and enhanced antiproliferation effect in cancer cells. Biomaterials 2017, 121, 97–108. [Google Scholar] [CrossRef]
- Barnes, C.P.; Sell, S.A.; Boland, E.D.; Simpson, D.G.; Bowlin, G.L. Nanofiber technology: Designing the next generation of tissue engineering scaffolds. Adv. Drug Deliv. Rev. 2007, 59, 1413–1433. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.X.; Li, R.Q.; Li, X.R.; Xie, J.W. Electrospinning: An enabling nanotechnology platform for drug delivery and regenerative medicine. Adv. Drug Deliv. Rev. 2018, 132, 188–213. [Google Scholar] [CrossRef] [PubMed]
- Pant, B.; Park, M.; Park, S.J. Drug Delivery Applications of Core-Sheath Nanofibers Prepared by Coaxial Electrospinning: A Review. Pharmaceutics 2019, 11, 305. [Google Scholar] [CrossRef]
- Wang, S.; Ju, J.P.; Wu, S.X.; Lin, M.; Sui, K.Y.; Xia, Y.Z.; Tan, Y.Q. Electrospinning of biocompatible alginate-based nanofiber membranes via tailoring chain flexibility. Carbohydr. Polym. 2020, 230, 115665. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, Y. Centrifugal Spinning: An Alternative Approach to Fabricate Nanofibers at High Speed and Low Cost. Polym. Rev. 2014, 54, 677–701. [Google Scholar] [CrossRef]
- Vocetkova, K.; Buzgo, M.; Sovkova, V.; Rampichova, M.; Staffa, A.; Filova, E.; Lukasova, V.; Doupnik, M.; Fiori, F.; Amler, E. A comparison of high throughput core-shell 2D electrospinning and 3D centrifugal spinning techniques to produce platelet lyophilisate-loaded fibrous scaffolds and their effects on skin cells. RSC Adv. 2017, 7, 53706–53719. [Google Scholar] [CrossRef]
- Kong, B.; Mi, S.L. Electrospun Scaffolds for Corneal Tissue Engineering: A Review. Materials 2016, 9, 614. [Google Scholar] [CrossRef] [PubMed]
- Badrossamay, M.R.; Balachandran, K.; Capulli, A.K.; Golecki, H.M.; Agarwal, A.; Goss, J.A.; Kim, H.; Shin, K.; Parker, K.K. Engineering hybrid polymer-protein super-aligned nanofibers via rotary jet spinning. Biomaterials 2014, 35, 3188–3197. [Google Scholar] [CrossRef]
- Lim, J.M.; Yi, G.R.; Moon, J.H.; Heo, C.J.; Yang, S.M. Superhydrophobic films of electrospun fibers with multiple-scale surface morphology. Langmuir 2007, 23, 7981–7989. [Google Scholar] [CrossRef]
- Huang, L.; Apkarian, R.P.; Chaikof, E.L. High-resolution analysis of engineered type I collagen nanofibers by electron microscopy. Scanning 2001, 23, 372–375. [Google Scholar] [CrossRef]
- Huang, L.; Nagapudi, K.; Apkarian, R.P.; Chaikof, E.L. Engineered collagen-PEO nanofibers and fabrics. J. Biomater. Sci. Polym. Ed. 2001, 12, 979–993. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.J.; Fridrikh, S.V.; Rutledge, G.C.; Kaplan, D.L. Electrospinning Bombyx mori Silk with Poly(ethylene oxide). Biomacromolecules 2002, 3, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.W.; Paras, C.B.; Weng, H.; Punnakitikashem, P.; Su, L.C.; Vu, K.; Tang, L.P.; Yang, J.; Nguyen, K.T. Dual growth factor releasing multi-functional nanofibers for wound healing. Acta Biomater. 2013, 9, 9351–9359. [Google Scholar] [CrossRef] [PubMed]
- Golecki, H.M.; Yuan, H.; Glavin, C.; Potter, B.; Badrossamay, M.R.; Goss, J.A.; Phillips, M.D.; Parker, K.K. Effect of solvent evaporation on fiber morphology in rotary jet spinning. Langmuir 2014, 30, 13369–13374. [Google Scholar] [CrossRef]
- Han, L.; Andrady, A.L.; Ensor, D.S. Chemical sensing using electrospun polymer/carbon nanotube composite nanofibers with printed-on electrodes. Sens. Actuators B Chem. 2013, 186, 52–55. [Google Scholar] [CrossRef]
- Ma, P.X.; Zhang, R.Y. Synthetic nano-scale fibrous extracellular matrix. J. Biomed. Mater. Res. 1999, 46, 60–72. [Google Scholar] [CrossRef]
- Sadeghi, A.R.; Nokhasteh, S.; Molavi, A.M.; Khorsand-Ghayeni, M.; Naderi-Meshkin, H.; Mahdizadeh, A. Surface modification of electrospun PLGA scaffold with collagen for bioengineered skin substitutes. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 66, 130–137. [Google Scholar] [CrossRef]
- Gao, J.; Huang, G.; Liu, G.; Liu, Y.; Chen, Q.; Ren, L.; Chen, C.; Ding, Z. A biodegradable antibiotic-eluting PLGA nanofiber-loaded deproteinized bone for treatment of infected rabbit bone defects. J. Biomater. Appl. 2016, 31, 241–249. [Google Scholar] [CrossRef]
- Zhang, M.; Lin, W.; Li, S.; Shi, X.-Y.; Liu, Y.; Guo, Q.; Huang, Z.; Li, L.; Wang, G.-L. Application and Effectiveness Evaluation of Electrostatic Spinning PLGA-Silk Fibroin-Collagen Nerve Conduits for Peripheral Nerve Regeneration. J. Nanosci. Nanotechnol. 2016, 16, 9413–9420. [Google Scholar] [CrossRef]
- Qiao, T.; Jiang, S.; Song, P.; Song, X.; Liu, Q.; Wang, L.; Chen, X. Effect of blending HA-g-PLLA on xanthohumol-loaded PLGA fiber membrane. Colloids Surf. B Biointerfaces 2016, 146, 221–227. [Google Scholar] [CrossRef]
- Sanaei-Rad, P.; Jafarzadeh Kashi, T.-S.; Seyedjafari, E.; Soleimani, M. Enhancement of stem cell differentiation to osteogenic lineage on hydroxyapatite-coated hybrid PLGA/gelatin nanofiber scaffolds. Biol. J. Int. Assoc. Biol. Stand. 2016, 44, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Mehrasa, M.; Asadollahi, M.A.; Nasri-Nasrabadi, B.; Ghaedi, K.; Salehi, H.; Dolatshahi-Pirouz, A.; Arpanaei, A. Incorporation of mesoporous silica nanoparticles into random electrospun PLGA and PLGA/gelatin nanofibrous scaffolds enhances mechanical and cell proliferation properties. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 66, 25–32. [Google Scholar] [CrossRef]
- Mehrasa, M.; Asadollahi, M.A.; Ghaedi, K.; Salehi, H.; Arpanaei, A. Electrospun aligned PLGA and PLGA/gelatin nanofibers embedded with silica nanoparticles for tissue engineering. Int. J. Biol. Macromol. 2015, 79, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Qi, R.-L.; Tian, X.-j.; Guo, R.; Luo, Y.; Shen, M.-W.; Yu, J.-Y.; Shi, X.-Y. Controlled release of doxorubicin from electrospun MWCNTs/PLGA hybrid nanofibers. Chin. J. Polym. Sci. 2016, 34, 1047–1059. [Google Scholar] [CrossRef]
- Bienek, D.R.; Hoffman, K.M.; Tutak, W. Blow-spun chitosan/PEG/PLGA nanofibers as a novel tissue engineering scaffold with antibacterial properties. J. Mater. Sci. Mater. Med. 2016, 27, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Blakney, A.K.; Simonovsky, F.I.; Suydam, I.T.; Ratner, B.D.; Woodrow, K.A. Rapidly Biodegrading PLGA-Polyurethane Fibers for Sustained Release of Physicochemically Diverse Drugs. ACS Biomater. Sci. Eng. 2016, 2, 1595–1607. [Google Scholar] [CrossRef]
- Chou, S.-F.; Woodrow, K.A. Relationships between mechanical properties and drug release from electrospun fibers of PCL and PLGA blends. J. Mech. Behav. Biomed. Mater. 2016, 65, 724–733. [Google Scholar] [CrossRef]
- Peschel, D.; Zhang, K.; Fischer, S.; Groth, T. Modulation of osteogenic activity of BMP-2 by cellulose and chitosan derivatives. Acta Biomater. 2012, 8, 183–193. [Google Scholar] [CrossRef]
- Rasool, A.; Ata, S.; Islam, A. Stimuli responsive biopolymer (chitosan) based blend hydrogels for wound healing application. Carbohydr. Polym. 2019, 203, 423–429. [Google Scholar] [CrossRef]
- Esmaeili, A.; Haseli, M. Optimization, synthesis, and characterization of coaxial electrospun sodium carboxymethyl cellulose-graft-methyl acrylate/poly(ethyleneoxide) nanofibers for potential drug-delivery applications. Carbohydr. Polym. 2017, 173, 645–653. [Google Scholar] [CrossRef]
- Ridolfi, D.M.; Lemes, A.P.; de Oliveira, S.; Justo, G.Z.; Palladino, M.V.; Duran, N. Electrospun poly(ethylene oxide)/chitosan nanofibers with cellulose nanocrystals as support for cell culture of 3T3 fibroblasts. Cellulose 2017, 24, 3353–3365. [Google Scholar] [CrossRef]
- Tabuchi, R.; Azuma, K.; Izumi, R.; Tanou, T.; Okamoto, Y.; Nagae, T.; Iohara, D.; Uekama, K.; Otagiri, M.; Hirayama, F.; et al. Biomaterials based on freeze dried surface-deacetylated chitin nanofibers reinforced with sulfobutyl ether beta-cyclodextrin gel in wound dressing applications. Int. J. Pharm. 2016, 511, 1080–1087. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Mei, S.; Dong, Y.; She, F.; Kong, L. High Efficiency Fabrication of Chitosan Composite Nanofibers with Uniform Morphology via Centrifugal Spinning. Polymers 2019, 11, 1550. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, R.; Prabaharan, M.; Kumar, P.T.S.; Nair, S.V.; Tamura, H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol. Adv. 2011, 29, 322–337. [Google Scholar] [CrossRef] [PubMed]
- Sell, S.A.; McClure, M.J.; Garg, K.; Wolfe, P.S.; Bowlin, G.L. Electrospinning of collagen/biopolymers for regenerative medicine and cardiovascular tissue engineering. Adv. Drug Deliv. Rev. 2009, 61, 1007–1019. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.W.; Kim, D.S.; Kang, S.Y.; Marquez, M.; Joo, Y.L. Structural studies of electrospun cellulose nanofibers. Polymer 2006, 47, 5097–5107. [Google Scholar] [CrossRef]
- Puls, J.; Wilson, S.A.; Hölter, D. Degradation of Cellulose Acetate-Based Materials: A Review. J. Polym. Environ. 2010, 19, 152–165. [Google Scholar] [CrossRef]
- Gouda, M.; Hebeish, A.A.; Aljafari, A.I. Synthesis and characterization of novel drug delivery system based on cellulose acetate electrospun nanofiber mats. J. Ind. Text. 2014, 43, 319–329. [Google Scholar] [CrossRef]
- Zhang, L.F.; Menkhaus, T.J.; Fong, H. Fabrication and bioseparation studies of adsorptive membranes/felts made from electrospun cellulose acetate nanofibers. J. Membr. Sci. 2008, 319, 176–184. [Google Scholar] [CrossRef]
- Kim, C.W.; Frey, M.W.; Marquez, M.; Joo, Y.L. Preparation of submicron-scale, electrospun cellulose fibers via direct dissolution. J. Polym. Sci. Part B Polym. Phys. 2005, 43, 1673–1683. [Google Scholar] [CrossRef]
- Kulpinski, P. Cellulose nanofibers prepared by the N-methylmorpholine-N-oxide method. J. Appl. Polym. Sci. 2005, 98, 1855–1859. [Google Scholar] [CrossRef]
- Wu, X.M.; Branford-White, C.J.; Zhu, L.M.; Chatterton, N.P.; Yu, D.G. Ester prodrug-loaded electrospun cellulose acetate fiber mats as transdermal drug delivery systems. J. Mater. Sci. Mater. Med. 2010, 21, 2403–2411. [Google Scholar] [CrossRef]
- Chantarodsakun, T.; Vongsetskul, T.; Jangpatarapongsa, K.; Tuchinda, P.; Uamsiri, S.; Bamrungcharoen, C.; Kumkate, S.; Opaprakasit, P.; Tangboriboonrat, P. 6-Gingerol-loaded cellulose acetate electrospun fibers as a topical carrier for controlled release. Polym. Bull. 2014, 71, 3163–3176. [Google Scholar] [CrossRef]
- Ball, C.; Chou, S.F.; Jiang, Y.; Woodrow, K.A. Coaxially electrospun fiber-based microbicides facilitate broadly tunable release of maraviroc. Mater. Sci. Eng. C 2016, 63, 117–124. [Google Scholar] [CrossRef]
- Han, S.O.; Son, W.K.; Youk, J.H.; Lee, T.S.; Park, W.H. Ultrafine porous fibers electrospun from cellulose triacetate. Mater. Lett. 2005, 59, 2998–3001. [Google Scholar] [CrossRef]
- Wang, M.; Wang, L.; Huang, Y. Electrospun hydroxypropyl methyl cellulose phthalate (HPMCM/Erythromycin fibers for targeted release in intestine. J. Appl. Polym. Sci. 2007, 106, 2177–2184. [Google Scholar] [CrossRef]
- Frenot, A.; Henriksson, M.W.; Walkenstrom, P. Electrospinning of cellulose-based nanofibers. J. Appl. Polym. Sci. 2007, 103, 1473–1482. [Google Scholar] [CrossRef]
- Duan, B.; Huang, Y.; Lu, A.; Zhang, L.N. Recent advances in chitin based materials constructed via physical methods. Prog. Polym. Sci. 2018, 82, 1–33. [Google Scholar] [CrossRef]
- Tripathi, K.; Singh, A. Chitin, chitosan and their pharmacological actives: A review. Int. J. Pharm. Sci. Res. 2018, 9, 2626–2635. [Google Scholar]
- Mottu, F.; Laurent, A.; Rufenacht, D.A.; Doelker, E. Organic solvents for pharmaceutical parenterals and embolic liquids: A review of toxicity data. PDA J. Pharm. Sci. Technol. 2000, 54, 456–469. [Google Scholar]
- Ahsan, S.M.; Thomas, M.; Reddy, K.K.; Sooraparaju, S.G.; Asthana, A.; Bhatnagar, I. Chitosan as biomaterial in drug delivery and tissue engineering. Int. J. Biol. Macromol. 2018, 110, 97–109. [Google Scholar] [CrossRef]
- Kumar, M.; Muzzarelli, R.A.A.; Muzzarelli, C.; Sashiwa, H.; Domb, A.J. Chitosan chemistry and pharmaceutical perspectives. Chem. Rev. 2004, 104, 6017–6084. [Google Scholar] [CrossRef]
- Portero, A.; Remunan-Lopez, C.; Criado, M.T.; Alonso, M.J. Reacetylated chitosan microspheres for controlled delivery of anti-microbial agents to the gastric mucosa. J. Microencapsul. 2002, 19, 797–809. [Google Scholar] [CrossRef]
- De Campos, A.M.; Sanchez, A.; Alonso, M.J. Chitosan nanoparticles: A new vehicle for the improvement of the delivery of drugs to the ocular surface. Application to cyclosporin A. Int. J. Pharm. 2001, 224, 159–168. [Google Scholar] [CrossRef]
- Prego, C.; Garcia, M.; Torres, D.; Alonso, M.J. Transmucosal macromolecular drug delivery. J. Control. Release 2005, 101, 151–162. [Google Scholar] [CrossRef]
- Vila, A.; Sanchez, A.; Janes, K.; Behrens, I.; Kissel, T.; Jato, J.L.V.; Alonso, M.J. Low molecular weight chitosan nanoparticles as new carriers for nasal vaccine delivery in mice. Eur. J. Pharm. Biopharm. 2004, 57, 123–131. [Google Scholar] [CrossRef]
- Chen, G.K.; Fang, D.W.; Wang, K.M.; Nie, J.; Ma, G.P. Core-Shell Structure PEO/CS Nanofibers Based on Electric Field Induced Phase Separation via Electrospinning and Its Application. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 2298–2311. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, D.; Chen, X.; Xu, Q.; Lu, F.; Nie, J. Electrospun water-soluble carboxyethyl chitosan/poly(vinyl alcohol) nanofibrous membrane as potential wound dressing for skin regeneration. Biomacromolecules 2008, 9, 349–354. [Google Scholar] [CrossRef]
- da Silva, S.B.; Krolicka, M.; van den Broek, L.A.M.; Frissen, A.E.; Boeriu, C.G. Water-soluble chitosan derivatives and pH-responsive hydrogels by selective C-6 oxidation mediated by TEMPO-laccase redox system. Carbohydr. Polym. 2018, 186, 299–309. [Google Scholar] [CrossRef]
- Shields, K.J.; Beckman, M.J.; Bowlin, G.L.; Wayne, J.S. Mechanical properties and cellular proliferation of electrospun collagen type II. Tissue Eng. 2004, 10, 1510–1517. [Google Scholar] [CrossRef]
- Kolacna, L.; Bakesova, J.; Varga, F.; Kostakova, E.; Planka, L.; Necas, A.; Lukas, D.; Amler, E.; Pelouch, V. Biochemical and biophysical aspects of collagen nanostructure in the extracellular matrix. Physiol. Res. 2007, 56, S51–S60. [Google Scholar] [PubMed]
- Parodi, R.; Carusi, G.; Santarelli, G.; Nanni, F.; Pingitore, R.; Brunel, G. Guided tissue regeneration employing a collagen membrane in a human periodontal bone defect: A histologic evaluation. Int. J. Periodontics Restor. Dent. 1997, 17, 283–291. [Google Scholar]
- Pal, P.; Dadhich, P.; Srivas, P.K.; Das, B.; Maulik, D.; Dhara, S. Bilayered nanofibrous 3D hierarchy as skin rudiment by emulsion electrospinning for burn wound management. Biomater. Sci. 2017, 5, 1786–1799. [Google Scholar] [CrossRef]
- Zhang, M.; Li, Z.Q.; Jiang, P.; Lin, T.; Li, X.Q.; Sun, D.H. Characterization and cell response of electrospun Rana chensinensis skin collagen/poly(l-lactide) scaffolds with different fiber orientations. J. Appl. Polym. Sci. 2017, 134, 45109. [Google Scholar] [CrossRef]
- Brown, J.H.; Das, P.; DiVito, M.D.; Ivancic, D.; Tan, L.P.; Wertheim, J.A. Nanofibrous PLGA electrospun scaffolds modified with type I collagen influence hepatocyte function and support viability in vitro. Acta Biomater. 2018, 73, 217–227. [Google Scholar] [CrossRef]
- Luo, J.N.; Tong, Y.W. Self-Assembly of Collagen-Mimetic Peptide Amphiphiles into Biofunctional Nanofiber. ACS Nano 2011, 5, 7739–7747. [Google Scholar] [CrossRef]
- Numata, K.; Kaplan, D.L. Silk-based delivery systems of bioactive molecules. Adv. Drug Deliv. Rev. 2010, 62, 1497–1508. [Google Scholar] [CrossRef]
- Ang, S.L.; Shaharuddin, B.; Chuah, J.A.; Sudesh, K. Electrospun poly(3-hydroxybutyrate-co-3-hydroxyhexanoate)/silk fibroin film is a promising scaffold for bone tissue engineering. Int. J. Biol. Macromol. 2020, 145, 173–188. [Google Scholar] [CrossRef]
- Barnes, C.P.; Pemble, C.W.; Brand, D.D.; Simpson, D.G.; Bowlin, G.L. Cross-linking electrospun type II collagen tissue engineering scaffolds with carbodiimide in ethanol. Tissue Eng. 2007, 13, 1593–1605. [Google Scholar] [CrossRef]
- Passerini, N.; Craig, D.Q.M. An investigation into the effects of residual water on the glass transition temperature of polylactide microspheres using modulated temperature DSC. J. Control. Release 2001, 73, 111–115. [Google Scholar] [CrossRef]
- Siegel, S.J.; Kahn, J.B.; Metzger, K.; Winey, K.I.; Werner, K.; Dan, N. Effect of drug type on the degradation rate of PLGA matrices. Eur. J. Pharm. Biopharm. 2006, 64, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Pinzon-Garcia, A.D.; Cassini-Vieira, P.; Ribeiro, C.C.; Jensen, C.E.D.; Barcelos, L.S.; Cortes, M.E.; Sinisterra, R.D. Efficient cutaneous wound healing using bixin-loaded PCL nanofibers in diabetic mice. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 1938–1949. [Google Scholar] [CrossRef] [PubMed]
- Zarghami, A.; Irani, M.; Mostafazadeh, A.; Golpour, M.; Heidarinasab, A.; Haririan, I. Fabrication of PEO/Chitosan/PCL/Olive Oil Nanofibrous Scaffolds for Wound Dressing Applications. Fibers Polym. 2015, 16, 1201–1212. [Google Scholar] [CrossRef]
- Xue, J.J.; He, M.; Liu, H.; Niu, Y.Z.; Crawford, A.; Coates, P.D.; Chen, D.F.; Shi, R.; Zhang, L.Q. Drug loaded homogeneous electrospun PCL/gelatin hybrid nanofiber structures for anti-infective tissue regeneration membranes. Biomaterials 2014, 35, 9395–9405. [Google Scholar] [CrossRef]
- Ferrand, A.; Eap, S.; Richert, L.; Lemoine, S.; Kalaskar, D.; Demoustier-Champagne, S.; Atmani, H.; Mely, Y.; Fioretti, F.; Schlatter, G.; et al. Osteogenetic Properties of Electrospun Nanofibrous PCL Scaffolds Equipped With Chitosan-Based Nanoreservoirs of Growth Factors. Macromol. Biosci. 2014, 14, 45–55. [Google Scholar] [CrossRef]
- Duda, S.; Dreyer, L.; Behrens, P.; Wienecke, S.; Chakradeo, T.; Glasmacher, B.; Haastert-Talini, K. Outer Electrospun Polycaprolactone Shell Induces Massive Foreign Body Reaction and Impairs Axonal Regeneration through 3D Multichannel Chitosan Nerve Guides. Biomed Res. Int. 2014, 2014, 1–16. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, H.B.; Andrade, J.D. Blood compatibility of polyethylene oxide surfaces. Prog. Polym. Sci. 1995, 20, 1043–1079. [Google Scholar] [CrossRef]
- Buddhiranon, S.; DeFine, L.A.; Alexander, T.S.; Kyu, T. Genistein-Modified Poly(ethylene oxide)/Poly(d,l-lactic acid) Electrospun Mats with Improved Antioxidant and Anti-inflammatory Properties. Biomacromolecules 2013, 14, 1423–1433. [Google Scholar] [CrossRef]
- Qu, F.N.; Lin, J.M.G.; Esterhai, J.L.; Fisher, M.B.; Mauck, R.L. Biomaterial-mediated delivery of degradative enzymes to improve meniscus integration and repair. Acta Biomater. 2013, 9, 6393–6402. [Google Scholar] [CrossRef]
- Spasova, M.; Stoilova, O.; Manolova, N.; Rashkov, I.; Altankov, G. Preparation of PLIA/PEG nanofibers by electrospinning and potential applications. Bioact. Compat. Polym. 2007, 22, 62–76. [Google Scholar] [CrossRef]
- Sadri, M.; Karimi-Nazari, E.; Hosseini, H.; Emamgholi, A. New Chitosan/Poly(ethylene oxide)/Thyme Nanofiber Prepared by Electrospinning Method for Antimicrobial Wound Dressing. J. Nanostruct. 2016, 6, 322–328. [Google Scholar]
- Koosha, M.; Mirzadeh, H. Electrospinning, mechanical properties, and cell behavior study of chitosan/PVA nanofibers. J. Biomed. Mater. Res. Part A 2015, 103, 3081–3093. [Google Scholar] [CrossRef]
- Liu, X.L.; Xu, Y.J.; Wu, Z.Q.; Chen, H. Poly(N-vinylpyrrolidone)-Modified Surfaces for Biomedical Applications. Macromol. Biosci. 2013, 13, 147–154. [Google Scholar] [CrossRef]
- Ding, B.; Kimura, E.; Sato, T.; Fujita, S.; Shiratori, S. Fabrication of blend biodegradable nanofibrous nonwoven mats via multi-jet electrospinning. Polymer 2004, 45, 1895–1902. [Google Scholar] [CrossRef]
- Um, I.C.; Fang, D.F.; Hsiao, B.S.; Okamoto, A.; Chu, B. Electro-spinning and electro-blowing of hyaluronic acid. Biomacromolecules 2004, 5, 1428–1436. [Google Scholar] [CrossRef]
- Formhals, A. Process and Apparatus for Preparing Artificial Threads. U.S. Patent 1,975,504, 2 October 1934. [Google Scholar]
- Taylor, G. Electrically driven jets. Proc. R. Soc. Lond. Ser. A Math. Phys. Sci. 1969, 313, 453. [Google Scholar]
- Li, D.; Xia, Y.N. Electrospinning of nanofibers: Reinventing the wheel? Adv. Mater. 2004, 16, 1151–1170. [Google Scholar] [CrossRef]
- Theron, S.A.; Yarin, A.L.; Zussman, E.; Kroll, E. Multiple jets in electrospinning: Experiment and modeling. Polymer 2005, 46, 2889–2899. [Google Scholar] [CrossRef]
- Thoppey, N.M.; Bochinski, J.R.; Clarke, L.I.; Gorga, R.E. Unconfined fluid electrospun into high quality nanofibers from a plate edge. Polymer 2010, 51, 4928–4936. [Google Scholar] [CrossRef]
- Wu, D.; Huang, X.; Lai, X.; Sun, D.; Lin, L. High Throughput Tip-Less Electrospinning via a Circular Cylindrical Electrode. J. Nanosci. Nanotechnol. 2010, 10, 4221–4226. [Google Scholar] [CrossRef]
- Niu, H.; Wang, X.; Lin, T. Needleless Electrospinning: Developments and Performances. Nanofibers Prod. Prop. Funct. Appl. 2011, 2011, 17–36. [Google Scholar]
- Wei, L.; Sun, R.; Liu, C.; Xiong, J.; Qin, X. Mass production of nanofibers from needleless electrospinning by a novel annular spinneret. Mater. Des. 2019, 179, 107885. [Google Scholar] [CrossRef]
- Jonsson, J.L. For Centrifugal Machines. U.S. Patent 581,423, 27 April 1897. [Google Scholar]
- Buzgo, M.; Rampichova, M.; Vocetkova, K.; Sovkova, V.; Lukasova, V.; Doupnik, M.; Mickova, A.; Rustichelli, F.; Amler, E. Emulsion centrifugal spinning for production of 3D drug releasing nanofibres with core/shell structure. RSC Adv. 2017, 7, 1215–1228. [Google Scholar] [CrossRef]
- She, F.; Tan, L.; Kong, L. A Multifunction Centrifugal Spinning Device; China Intellectual Property Office (C.I.P. Office), Ed.; C.I.P.: Beijing, China, 2015.
- Medeiros, E.S.; Glenn, G.M.; Klamczynski, A.P.; Orts, W.J.; Mattoso, L.H.C. Solution Blow Spinning: A New Method to Produce Micro- and Nanofibers from Polymer Solutions. J. Appl. Polym. Sci. 2009, 113, 2322–2330. [Google Scholar] [CrossRef]
- Kumar, A.; Sinha-Ray, S. A Review on Biopolymer-Based Fibers via Electrospinning and Solution Blowing and Their Applications. Fibers 2018, 6, 45. [Google Scholar]
- Singh, R.; Ahmed, F.; Polley, P.; Giri, J. Fabrication and Characterization of Core-Shell Nanofibers Using a Next-Generation Airbrush for Biomedical Applications. ACS Appl. Mater. Interfaces 2018, 10, 41924–41934. [Google Scholar] [CrossRef]
- Koga, T.; Watanabe, T.; Higashi, N. Fabrication of Nucleobase-Functionalized Supramolecular Nanofiber through Peptide Self-Assembly. J. Nanosci. Nanotechnol. 2009, 9, 584–590. [Google Scholar] [CrossRef]
- Tanha, S.; Rafiee-Tehrani, M.; Abdollahi, M.; Vakilian, S.; Esmaili, Z.; Naraghi, Z.S.; Seyedjafari, E.; Javar, H.A. G-CSF loaded nanofiber/nanoparticle composite coated with collagen promotes wound healing in vivo. J. Biomed. Mater. Res. Part A 2017, 105, 2830–2842. [Google Scholar] [CrossRef]
- Yergoz, F.; Hastar, N.; Cimenci, C.E.; Ozkan, A.D.; Tekinay, T.; Guler, M.O.; Tekinay, A.B. Heparin mimetic peptide nanofiber gel promotes regeneration of full thickness burn injury. Biomaterials 2017, 134, 117–127. [Google Scholar] [CrossRef]
- Sacks, M.S.; Merryman, W.D.; Schmidt, D.E. On the biomechanics of heart valve function. J. Biomech. 2009, 42, 1804–1824. [Google Scholar] [CrossRef]
- Zhang, C.; Wen, J.H.; Yan, J.; Kao, Y.B.; Ni, Z.Q.; Cui, X.J.; Wang, H.Y. In situ growth induction of the corneal stroma cells using uniaxially aligned composite fibrous scaffolds. RSC Adv. 2015, 5, 12123–12130. [Google Scholar] [CrossRef]
- Kakade, M.V.; Givens, S.; Gardner, K.; Lee, K.H.; Chase, D.B.; Rabolt, J.F. Electric field induced orientation of polymer chains in macroscopically aligned electrospun polymer nanofibers. J. Am. Chem. Soc. 2007, 129, 2777–2782. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Steckl, A.J. Triaxial Electrospun Nanofiber Membranes for Controlled Dual Release of Functional Molecules. ACS Appl. Mater. Interfaces 2013, 5, 8241–8245. [Google Scholar] [CrossRef]
- Khatri, Z.; Wei, K.; Kim, B.S.; Kim, I.S. Effect of deacetylation on wicking behavior of co-electrospun cellulose acetate/polyvinyl alcohol nanofibers blend. Carbohydr. Polym. 2012, 87, 2183–2188. [Google Scholar] [CrossRef]
- Yu, D.G.; Yu, J.H.; Chen, L.; Williams, G.R.; Wang, X. Modified coaxial electrospinning for the preparation of high-quality ketoprofen-loaded cellulose acetate nanofibers. Carbohydr. Polym. 2012, 90, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Ortega, M.M.; Najera-Luna, A.; Rodriguez-Felix, D.E.; Encinas, J.C.; Rodriguez-Felix, F.; Romero, J.; Herrera-Franco, P.J. Preparation, characterization and release of amoxicillin from cellulose acetate and poly (vinyl pyrrolidone) coaxial electrospun fibrous membranes. Mater. Sci. Eng. C Mater. Biol. Appl. 2011, 31, 1772–1778. [Google Scholar] [CrossRef]
- Zilberman, M. (Ed.) Active Implants and Scaffolds for Tissue Regeneration. In Active Implants and Scaffolds for Tissue Regeneration; Studies in Mechanobiology, Tissue Engineering and Biomaterials; Springer: Berlin, Germany, 2011; pp. 1–514. [Google Scholar]
- Yang, Y.; Xia, T.; Zhi, W.; Wei, L.; Weng, J.; Zhang, C.; Li, X. Promotion of skin regeneration in diabetic rats by electrospun core-sheath fibers loaded with basic fibroblast growth factor. Biomaterials 2011, 32, 4243–4254. [Google Scholar] [CrossRef]
- Qian, W.; Yu, D.G.; Li, Y.; Liao, Y.Z.; Wang, X.; Wang, L. Dual Drug Release Electrospun Core-Shell Nanofibers with Tunable Dose in the Second Phase. Int. J. Mol. Sci. 2014, 15, 774–786. [Google Scholar] [CrossRef]
- Huang, Z.M.; He, C.L.; Yang, A.Z.; Zhang, Y.Z.; Hang, X.J.; Yin, J.L.; Wu, Q.S. Encapsulating drugs in biodegradable ultrafine fibers through co-axial electrospinning. J. Biomed. Mater. Res. Part A 2006, 77, 169–179. [Google Scholar] [CrossRef]
- Zhao, Y.; Cao, X.; Jiang, L. Bio-mimic multichannel microtubes by a facile method. J. Am. Chem. Soc. 2007, 129, 764–765. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, N.; Di, J.; Zhao, Y.; Song, Y.; Jiang, L. Nanowire-in-Microtube Structured Core/Shell Fibers via Multifluidic Coaxial Electrospinning. Langmuir 2010, 26, 11291–11296. [Google Scholar] [CrossRef] [PubMed]
- Sin, D.Y.; Koo, B.R.; Ahn, H.J. Hollow lithium manganese oxide nanotubes using MnO2-carbon nanofiber composites as cathode materials for hybrid capacitors. J. Alloys Compd. 2017, 696, 290–294. [Google Scholar] [CrossRef]
- Yoo, H.S.; Kim, T.G.; Park, T.G. Surface-functionalized electrospun nanofibers for tissue engineering and drug delivery. Adv. Drug Deliv. Rev. 2009, 61, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Ju, Y.M.; Son, J.S.; Ahn, K.D.; Han, D.K. Surface modification of biodegradable electrospun nanofiber scaffolds and their interaction with fibroblasts. J. Biomater. Sci. Polym. Ed. 2007, 18, 369–382. [Google Scholar] [CrossRef]
- Janjic, M.; Pappa, F.; Karagkiozaki, V.; Gitas, C.; Ktenidis, K.; Logothetidis, S. Surface modification of endovascular stents with rosuvastatin and heparin-loaded biodegradable nanofibers by electrospinning. Int. J. Nanomed. 2017, 12, 6343–6355. [Google Scholar] [CrossRef] [PubMed]
- Hassiba, A.J.; El Zowalaty, M.E.; Nasrallah, G.K.; Webster, T.J.; Luyt, A.S.; Abdullah, A.M.; Elzatahry, A.A. Review of recent research on biomedical applications of electrospun polymer nanofibers for improved wound healing. Nanomedicine 2016, 11, 715–737. [Google Scholar] [CrossRef]
- Bai, Y.; Yang, H.; Yang, W.W.; Li, Y.C.; Sun, C.Q. Gold nanoparticles-mesoporous silica composite used as an enzyme immobilization matrix for amperometric glucose biosensor construction. Sens. Actuators B Chem. 2007, 124, 179–186. [Google Scholar] [CrossRef]
- Choi, J.S.; Leong, K.W.; Yoo, H.S. In vivo wound healing of diabetic ulcers using electrospun nanofibers immobilized with human epidermal growth factor (EGF). Biomaterials 2008, 29, 587–596. [Google Scholar] [CrossRef]
- Ranjbar-Mohammadi, M.; Rabbani, S.; Bahrami, S.H.; Joghataei, M.T.; Moayer, F. Antibacterial performance and in vivo diabetic wound healing of curcumin loaded gum tragacanth/poly(epsilon-caprolactone) electrospun nanofibers. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 69, 1183–1191. [Google Scholar] [CrossRef]
- Casper, C.L.; Yamaguchi, N.; Kiick, K.L.; Rabolt, J.F. Functionalizing electrospun fibers with biologically relevant macromolecules. Biomacromolecules 2005, 6, 1998–2007. [Google Scholar] [CrossRef]
- Metwally, M.; Cheong, Y.; Li, T.C. A review of techniques for adhesion prevention after gynaecological surgery. Curr. Opin. Obstet. Gynecol. 2008, 20, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Lv, G.; Pan, C.; Song, M.; Wu, C.H.; Guo, D.D.; Wang, X.M.; Chen, B.A.; Gu, Z.Z. Poly(lactic acid) (PLA) based nanocomposites-a novel way of drug-releasing. Biomed. Mater. 2007, 2, L1–L4. [Google Scholar] [CrossRef] [PubMed]
- Rosa, R.M.; Silva, J.C.; Sanches, I.S.; Henriques, C. Simultaneous photo-induced cross-linking and silver nanoparticle formation in a PVP electrospun wound dressing. Mater. Lett. 2017, 207, 145–148. [Google Scholar] [CrossRef]
- Rujitanaroj, P.O.; Pimpha, N.; Supaphol, P. Wound-dressing materials with antibacterial activity from electrospun gelatin fiber mats containing silver nanoparticles. Polymer 2008, 49, 4723–4732. [Google Scholar] [CrossRef]
- Yang, Y.; Li, X.; Cheng, L.; He, S.; Zou, J.; Chen, F.; Zhang, Z. Core-sheath structured fibers with pDNA polyplex loadings for the optimal release profile and transfection efficiency as potential tissue engineering scaffolds. Acta Biomater. 2011, 7, 2533–2543. [Google Scholar] [CrossRef]
- Langer, R. New methods of drug delivery. Science 1990, 249, 1527–1533. [Google Scholar] [CrossRef]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef]
- Fredenberg, S.; Wahlgren, M.; Reslow, M.; Axelsson, A. The mechanisms of drug release in poly(lactic-co-glycolic acid)-based drug delivery systems—A review. Int. J. Pharm. 2011, 415, 34–52. [Google Scholar] [CrossRef]
- Webber, W.L.; Lago, F.; Thanos, C.; Mathiowitz, E. Characterization of soluble, salt-loaded, degradable PLGA films and their release of tetracycline. J. Biomed. Mater. Res. 1998, 41, 18–29. [Google Scholar] [CrossRef]
- Fu, Y.; Kao, W.J. Drug release kinetics and transport mechanisms of non-degradable and degradable polymeric delivery systems. Expert Opin. Drug Deliv. 2010, 7, 429–444. [Google Scholar] [CrossRef]
- Saindane, N.; Vavia, P. Osmotic pellet system comprising osmotic core and in-process amorphized drug in polymer-surfactant layer for controlled delivery of poorly water-soluble drug. J. Pharm. Sci. 2012, 101, 3169–3179. [Google Scholar] [CrossRef]
- Patel, H.; Patel, M.M. Formulation and evaluation of controlled porosity osmotic drug delivery system of metoprolol succinate. Int. J. Pharm. Sci. Res. 2012, 3, 1761–1767. [Google Scholar]
- Uhrich, K.E.; Cannizzaro, S.M.; Langer, R.S.; Shakesheff, K.M. Polymeric systems for controlled drug release. Chem. Rev. 1999, 99, 3181–3198. [Google Scholar] [CrossRef] [PubMed]
- Marin, E.; Briceno, M.I.; Caballero-George, C. Critical evaluation of biodegradable polymers used in nanodrugs. Int. J. Nanomed. 2013, 8, 3071–3091. [Google Scholar]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef] [PubMed]









| Polymer(s) | Solvent | Concentration | Applications | Ref. |
|---|---|---|---|---|
| Collagen-PCL | HFIP | 6 w/v% | Tissue engineering | [18] |
| Gelatin-PCL | HFIP | 6 w/v% | Tissue engineering | |
| PEO | Colloidal silica | 6–10 wt.% | Biosensors | [19] |
| Collagen-PEO | Hydrochloric acid | 1 wt.% | Wound healing, tissue engineering, and hemostatic agents | [20] |
| 1–2 wt.% | [21] | |||
| Silk-PEO | HFIP | 4.8–8.8 w/v% | Biomaterial scaffolds | [22] |
| Chitosan-PEO | Acetic acid | 2.5 w/v% | Wound healing | [23] |
| PLA | DMF | 4–9 wt.% | Tissue engineering | [24] |
| PMMA-SWCNTs | N/A | 2–5 wt.% | Biosensor | [25] |
| PAM | Colloidal silica | 6–10 wt.% | Biosensors | [19] |
| PLLA-PLGA | THF/DMF | 1–15 wt.% | Tissue engineering | [26] |
| PLGA-collagen | THF/DMF | 20 wt.% | Bioengineered skin | [27] |
| PLGA | HFIP | 24 w/v% | Tissue engineering | [28] |
| HFIP | 5wt.% | Peripheral nerve regeneration | [29] | |
| Chloroform | 5wt.% | Tissue engineering | [30] | |
| THF/DMF | 10 w/v% | Bone tissue engineering | [31] | |
| PLGA-gelatin-MSNPs | HFIP | 17 wt.% | Cell culture and tissue engineering | [32,33] |
| Dox-MWCNTs-PLGA | THF/DMF | 1–3 wt.% | Drug delivery | [34] |
| Chitosan-PEG-PLGA | Ethyl acetate | 18 wt.% | Tissue engineering | [35] |
| PLGA-PU | DCM or HFIP | 50 w/v% | Drug delivery | [36] |
| PLGA-SF-CL | HFIP | 5wt.% | Peripheral nerve regeneration | [29] |
| PCL-PLGA | HFIP | 15 w/v% | Drug delivery | [37] |
| XN-PLGA | Chloroform | 10 wt.% | Tissue engineering | [30] |
| Cellulose-Chitosan | DMF | 1 w/v% | Bone tissue engineering | [38] |
| Chitosan-PVP | Acetic acid, sodium hydroxide | 2.5 w/v% | Wound healing | [39] |
| Cellulose-TCMC-PEO | Acetone, DMF, chloroform | 3 w/v% | Drug delivery | [40] |
| Cellulose-chitosan-PEO | Sulfuric acid | 1.2 w/v% | Tissue engineering scaffolds | [41] |
| Chitin-chitosan | Sodium hydroxide and acetic acid | 0.75 w/v% | Wound dressing | [42] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Mei, S.; Dong, Y.; She, F.; Li, Y.; Li, P.; Kong, L. Functional Nanofibrous Biomaterials of Tailored Structures for Drug Delivery—A Critical Review. Pharmaceutics 2020, 12, 522. https://doi.org/10.3390/pharmaceutics12060522
Li Z, Mei S, Dong Y, She F, Li Y, Li P, Kong L. Functional Nanofibrous Biomaterials of Tailored Structures for Drug Delivery—A Critical Review. Pharmaceutics. 2020; 12(6):522. https://doi.org/10.3390/pharmaceutics12060522
Chicago/Turabian StyleLi, Zhen, Shunqi Mei, Yajie Dong, Fenghua She, Yongzhen Li, Puwang Li, and Lingxue Kong. 2020. "Functional Nanofibrous Biomaterials of Tailored Structures for Drug Delivery—A Critical Review" Pharmaceutics 12, no. 6: 522. https://doi.org/10.3390/pharmaceutics12060522
APA StyleLi, Z., Mei, S., Dong, Y., She, F., Li, Y., Li, P., & Kong, L. (2020). Functional Nanofibrous Biomaterials of Tailored Structures for Drug Delivery—A Critical Review. Pharmaceutics, 12(6), 522. https://doi.org/10.3390/pharmaceutics12060522