Review: Local Tumor Necrosis Factor-α Inhibition in Inflammatory Bowel Disease
Abstract
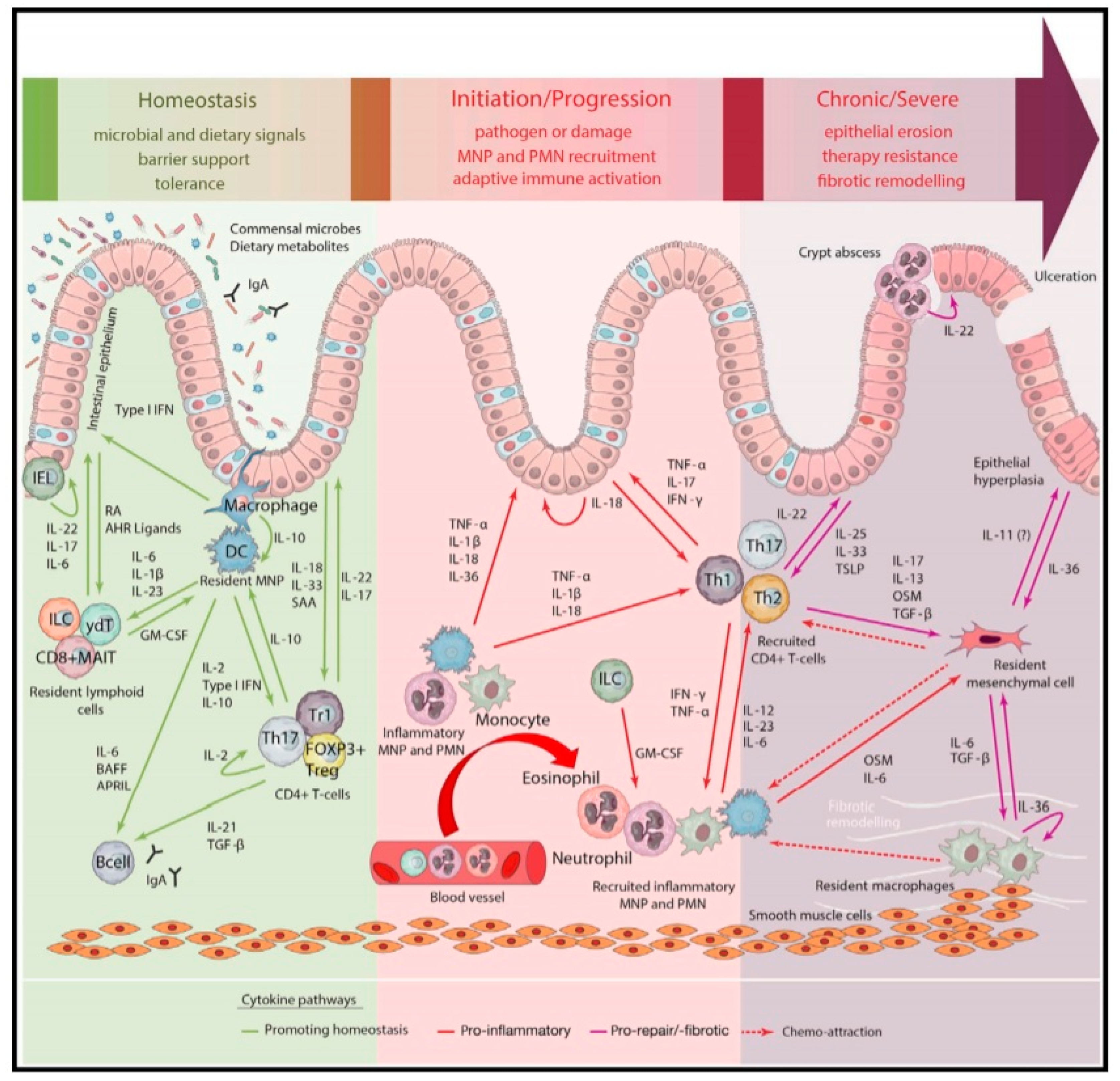
1. Introduction
2. Methods
3. Preclinical Studies on Local TNF-α Inhibition
3.1. Considerations
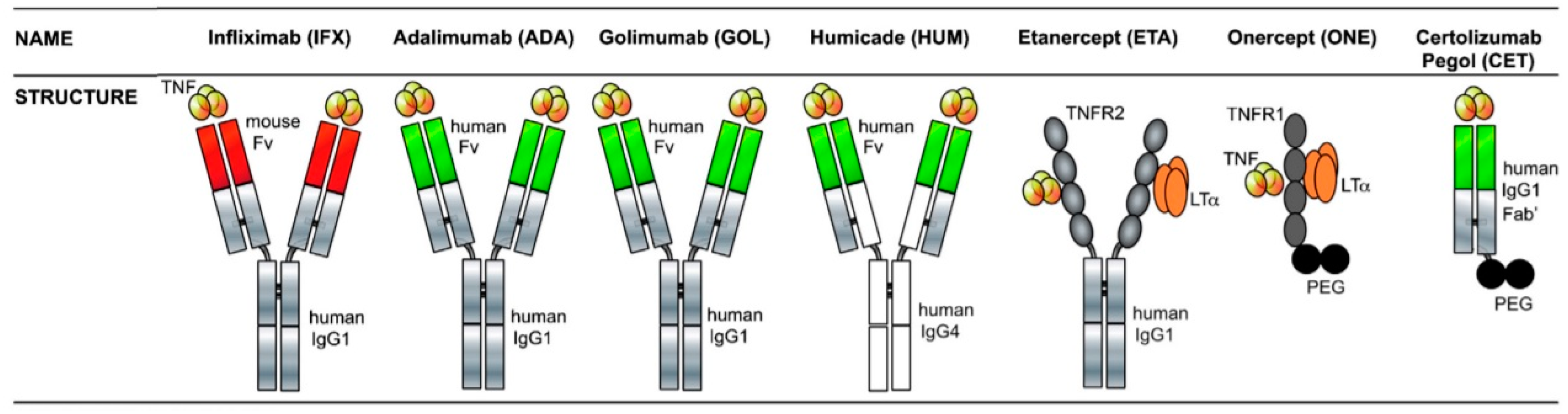
3.2. Antibodies
3.3. Antisense Oligonucleotides
3.4. microRNA
3.5. Small Interfering RNA
3.6. Prokaryotes
3.7. Eukaryotes
4. Clinical Studies on Local TNF-α Inhibition
4.1. Considerations
4.2. Local Injections
4.3. Topical Treatment
4.4. Oral Therapy
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ha, F.; Khalil, H. Crohn’s disease: A clinical update. Therap. Adv. Gastroenterol. 2015, 8, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J.-F. Ulcerative colitis. Lancet 2017, 389, 1756–1770. [Google Scholar] [CrossRef]
- Baumgart, D.C.; Sandborn, W.J. Crohn’s disease. Lancet 2012, 380, 1590–1605. [Google Scholar] [CrossRef]
- Friedrich, M.; Pohin, M.; Powrie, F. Cytokine Networks in the Pathophysiology of Inflammatory Bowel Disease. Immunity 2019, 50, 992–1006. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N. Epidemiology and risk factors for IBD. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 205–217. [Google Scholar] [CrossRef]
- De Souza, H.S.P.; Fiocchi, C. Immunopathogenesis of IBD: Current state of the art. Nat. Publ. Gr. 2015, 13, 13–27. [Google Scholar] [CrossRef]
- Geremia, A.; Biancheri, P.; Allan, P.; Corazza, G.R.; Di Sabatino, A. Innate and adaptive immunity in inflammatory bowel disease. Autoimmun. Rev. 2014, 13, 3–10. [Google Scholar] [CrossRef]
- Li, Y.; Soendergaard, C.; Bergenheim, F.H.; Aronoff, D.M.; Milne, G.; Riis, L.B.; Seidelin, J.B.; Jensen, K.B.; Nielsen, O.H. COX-2-PGE2 Signaling Impairs Intestinal Epithelial Regeneration and Associates with TNF Inhibitor Responsiveness in Ulcerative Colitis. EBioMedicine 2018, 36, 497–507. [Google Scholar] [CrossRef]
- Saini, S.; Liu, T.; Yoo, J. TNF-α stimulates colonic myofibroblast migration via COX-2 and Hsp27. J. Surg. Res. 2016, 204, 145–152. [Google Scholar] [CrossRef]
- Weinberg, J.B. Nitric Oxide Synthase 2 and Cyclooxygenase 2 Interactions in Inflammation. Immunol. Res. 2000, 22, 319–342. [Google Scholar] [CrossRef]
- Perner, A.; Rask-Madsen, J. The potential role of nitric oxide in chronic inflammatory bowel disorders. Aliment. Pharmacol. Ther. 1999, 13, 135–144. [Google Scholar] [CrossRef]
- Delgado, M.E.; Brunner, T. The many faces of tumor necrosis factor signaling in the intestinal epithelium. Genes Immun. 2019, 20, 609–626. [Google Scholar] [CrossRef] [PubMed]
- Mitoma, H.; Horiuchi, T.; Tsukamoto, H.; Ueda, N. Molecular mechanisms of action of anti-TNF-α agents–Comparison among therapeutic TNF-α antagonists. Cytokine 2018, 101, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Leppkes, M.; Roulis, M.; Neurath, M.F.; Kollias, G.; Becker, C. Pleiotropic functions of TNF- in the regulation of the intestinal epithelial response to inflammation. Int. Immunol. 2014, 26, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Kalliolias, G.D.; Ivashkiv, L.B. TNF biology, pathogenic mechanisms and emerging therapeutic strategies. Nat. Rev. Rheumatol. 2016, 12, 49–62. [Google Scholar] [CrossRef]
- Sedger, L.M.; McDermott, M.F. TNF and TNF-receptors: From mediators of cell death and inflammation to therapeutic giants–past, present and future. Cytokine Growth Factor Rev. 2014, 25, 453–472. [Google Scholar] [CrossRef] [PubMed]
- Levin, A.D.; Wildenberg, M.E.; van den Brink, G.R. Mechanism of Action of Anti-TNF Therapy in Inflammatory Bowel Disease. J. Crohn’s Colitis 2016, 10, 989–997. [Google Scholar] [CrossRef]
- Peake, S.T.C.; Bernardo, D.; Mann, E.R.; Al-Hassi, H.O.; Knight, S.C.; Hart, A.L. Mechanisms of Action of Anti–tumor Necrosis Factor α Agents in Crohn’s Disease. Inflamm. Bowel Dis. 2013, 19, 1546–1555. [Google Scholar] [CrossRef]
- Naviglio, S.; Giuffrida, P.; Stocco, G.; Lenti, M.V.; Ventura, A.; Corazza, G.R.; Di Sabatino, A. How to predict response to anti-tumour necrosis factor agents in inflammatory bowel disease. Expert Rev. Gastroenterol. Hepatol. 2018, 12, 797–810. [Google Scholar] [CrossRef]
- Slevin, S.M.; Egan, L.J. New Insights into the Mechanisms of Action of Anti–Tumor Necrosis Factor-α Monoclonal Antibodies in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2015, 21, 2909–2920. [Google Scholar] [CrossRef]
- Adegbola, S.O.; Sahnan, K.; Warusavitarne, J.; Hart, A.; Tozer, P. Anti-TNF Therapy in Crohn’s Disease. Int. J. Mol. Sci. 2018, 19, 2244. [Google Scholar] [CrossRef]
- Olesen, C.M.; Coskun, M.; Peyrin-Biroulet, L.; Nielsen, O.H. Mechanisms behind efficacy of tumor necrosis factor inhibitors in inflammatory bowel diseases. Pharmacol. Ther. 2016, 159, 110–119. [Google Scholar] [CrossRef]
- Torres, J.; Bonovas, S.; Doherty, G.; Kucharzik, T.; Gisbert, J.P.; Raine, T.; Adamina, M.; Armuzzi, A.; Bachmann, O.; Bager, P.; et al. ECCO Guidelines on Therapeutics in Crohn’s Disease: Medical Treatment. J. Crohn’s Colitis 2020, 14, 4–22. [Google Scholar] [CrossRef]
- Harbord, M.; Eliakim, R.; Bettenworth, D.; Karmiris, K.; Katsanos, K.; Kopylov, U.; Kucharzik, T.; Molnár, T.; Raine, T.; Sebastian, S.; et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 2: Current management. J. Crohn’s Colitis 2017, 11, 769–784. [Google Scholar] [CrossRef]
- Lichtenstein, L.; Ron, Y.; Kivity, S.; Ben-Horin, S.; Israeli, E.; Fraser, G.M.; Dotan, I.; Chowers, Y.; Confino-Cohen, R.; Weiss, B. Infliximab-Related Infusion Reactions: Systematic Review. J. Crohns. Colitis 2015, 9, 806–815. [Google Scholar] [CrossRef]
- O’Meara, S.; Nanda, K.S.; Moss, A.C. Antibodies to Infliximab and Risk of Infusion Reactions in Patients With Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2014, 20, 1–6. [Google Scholar] [CrossRef]
- Melo, F.J.; Magina, S. Clinical management of Anti-TNF-alpha-induced psoriasis or psoriasiform lesions in inflammatory bowel disease patients: A systematic review. Int. J. Dermatol. 2018, 57, 1521–1532. [Google Scholar] [CrossRef]
- Sacco, R.; Shah, S.; Leeson, R.; Moraschini, V.; de Almeida Barros Mourão, C.F.; Akintola, O.; Lalli, A. Osteonecrosis and osteomyelitis of the jaw associated with tumour necrosis factor-alpha (TNF-α) inhibitors: A systematic review. Br. J. Oral Maxillofac. Surg. 2020, 58, 25–33. [Google Scholar] [CrossRef]
- Brijs, K.; Miclotte, I.; Vermeire, S.; Darche, V.; Politis, C. Osteonecrosis of the jaw in patients with inflammatory bowel disease treated with tumour necrosis factor alpha inhibitors. Int. J. Oral Maxillofac. Surg. 2020, 49, 317–324. [Google Scholar] [CrossRef]
- García-De LaTorre, I.; García-Valladares, I. Antinuclear antibody (ANA) testing in patients treated with biological DMARDs: Is it useful? Curr. Rheumatol. Rep. 2015, 17, 23. [Google Scholar] [CrossRef]
- Atzeni, F.; Turiel, M.; Capsoni, F.; Doria, A.; Meroni, P.; Sarzi-Puttini, P. Autoimmunity and anti-TNF-alpha agents. Ann. N. Y. Acad. Sci. 2005, 1051, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Atzeni, F.; Talotta, R.; Salaffi, F.; Cassinotti, A.; Varisco, V.; Battellino, M.; Ardizzone, S.; Pace, F.; Sarzi-Puttini, P. Immunogenicity and autoimmunity during anti-TNF therapy. Autoimmun. Rev. 2013, 12, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Leandro, M.J. Anti-tumour necrosis factor therapy and B cells in rheumatoid arthritis. Arthritis Res. Ther. 2009, 11, 128. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.C.; Peyrin-Biroulet, L. Opportunistic Infections With Anti-Tumor Necrosis Factor-α Therapy in Inflammatory Bowel Disease: Meta-Analysis of Randomized Controlled Trials. Am. J. Gastroenterol. 2013, 108, 1268–1276. [Google Scholar] [CrossRef] [PubMed]
- Bonovas, S.; Fiorino, G.; Allocca, M.; Lytras, T.; Nikolopoulos, G.K.; Peyrin-Biroulet, L.; Danese, S. Biologic Therapies and Risk of Infection and Malignancy in Patients With Inflammatory Bowel Disease: A Systematic Review and Network Meta-analysis. Clin. Gastroenterol. Hepatol. 2016, 14, 1385–1397.e10. [Google Scholar] [CrossRef] [PubMed]
- Kedia, S.; Mouli, V.P.; Kamat, N.; Sankar, J.; Ananthakrishnan, A.; Makharia, G.; Ahuja, V. Risk of Tuberculosis in Patients With Inflammatory Bowel Disease on Infliximab or Adalimumab Is Dependent on the Local Disease Burden of Tuberculosis: A Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 2020, 115, 340–349. [Google Scholar] [CrossRef]
- Yang, C.; Huang, J.; Huang, X.; Huang, S.; Cheng, J.; Liao, W.; Chen, X.; Wang, X.; Dai, S. Risk of Lymphoma in Patients With Inflammatory Bowel Disease Treated With Anti-tumour Necrosis Factor Alpha Agents: A Systematic Review and Meta-analysis. J. Crohns. Colitis 2018, 12, 1042–1052. [Google Scholar] [CrossRef]
- Moss, A.C.; Fernandez-Becker, N.; Jo Kim, K.; Cury, D.; Cheifetz, A.S. The impact of infliximab infusion reactions on long-term outcomes in patients with Crohn’s disease. Aliment. Pharmacol. Ther. 2008, 28, 221–227. [Google Scholar] [CrossRef]
- Vaisman-Mentesh, A.; Rosenstein, S.; Yavzori, M.; Dror, Y.; Fudim, E.; Ungar, B.; Kopylov, U.; Picard, O.; Kigel, A.; Ben-Horin, S.; et al. Molecular Landscape of Anti-Drug Antibodies Reveals the Mechanism of the Immune Response Following Treatment With TNFα Antagonists. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef]
- Thomas, S.S.; Borazan, N.; Barroso, N.; Duan, L.; Taroumian, S.; Kretzmann, B.; Bardales, R.; Elashoff, D.; Vangala, S.; Furst, D.E. Comparative Immunogenicity of TNF Inhibitors: Impact on Clinical Efficacy and Tolerability in the Management of Autoimmune Diseases. A Systematic Review and Meta-Analysis. BioDrugs 2015, 29, 241–258. [Google Scholar] [CrossRef]
- Boehncke, W.-H.; Brembilla, N.C. Immunogenicity of biologic therapies: Causes and consequences. Expert Rev. Clin. Immunol. 2018, 14, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Hozumi, H.; Hokari, R.; Kurihara, C.; Narimatsu, K.; Sato, H.; Sato, S.; Ueda, T.; Higashiyama, M.; Okada, Y.; Watanabe, C.; et al. Endoscopic finding of spontaneous hemorrhage correlates with tumor necrosis factor alpha expression in colonic mucosa of patients with ulcerative colitis. Int. J. Colorectal Dis. 2013, 28, 1049–1055. [Google Scholar] [CrossRef]
- Kobori, A.; Yagi, Y.; Imaeda, H.; Ban, H.; Bamba, S.; Tsujikawa, T.; Saito, Y.; Fujiyama, Y.; Andoh, A. Interleukin-33 expression is specifically enhanced in inflamed mucosa of ulcerative colitis. J. Gastroenterol. 2010, 45, 999–1007. [Google Scholar] [CrossRef]
- Matsuda, R.; Koide, T.; Tokoro, C.; Yamamoto, T.; Godai, T.; Morohashi, T.; Fujita, Y.; Takahashi, D.; Kawana, I.; Suzuki, S.; et al. Quantitive Cytokine mRNA Expression Profiles in the Colonic Mucosa of Patients with Steroid Naïve Ulcerative Colitis During Active and Quiescent Disease. Inflamm. Bowel Dis. 2009, 15, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Olsen, T.; Goll, R.; Cui, G.; Husebekk, A.; Vonen, B.; Birketvedt, G. støa; Florholmen, J. Tissue levels of tumor necrosis factor-alpha correlates with grade of inflammation in untreated ulcerative colitis. Scand. J. Gastroenterol. 2007, 42, 1312–1320. [Google Scholar] [CrossRef] [PubMed]
- Bruno, M.E.C.; Rogier, E.W.; Arsenescu, R.I.; Flomenhoft, D.R.; Kurkjian, C.J.; Ellis, G.I.; Kaetzel, C.S. Correlation of Biomarker Expression in Colonic Mucosa with Disease Phenotype in Crohn’s Disease and Ulcerative Colitis. Dig. Dis. Sci. 2015, 60, 2976–2984. [Google Scholar] [CrossRef] [PubMed]
- León, A.J.; Gómez, E.; Garrote, J.A.; Bernardo, D.; Barrera, A.; Marcos, J.L.; Fernández-Salazar, L.; Velayos, B.; Blanco-Quirós, A.; Arranz, E. High Levels of Proinflammatory Cytokines, but Not Markers of Tissue Injury, in Unaffected Intestinal Areas from Patients with IBD. Mediators Inflamm. 2009, 2009, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sawa, Y.; Oshitani, N.; Adachi, K.; Higuchi, K.; Matsumoto, T.; Arakawa, T. Comprehensive analysis of intestinal cytokine messenger RNA profile by real-time quantitative polymerase chain reaction in patients with inflammatory bowel disease. Int. J. Mol. Med. 2003, 11, 175–179. [Google Scholar] [CrossRef]
- Yamamoto, T.; Umegae, S.; Kitagawa, T.; Matsumoto, K. Systemic and Local Cytokine Production in Quiescent Ulcerative Colitis and Its Relationship to Future Relapse: A Prospective Pilot Study. Inflamm. Bowel Dis. 2005, 11, 589–596. [Google Scholar] [CrossRef]
- Schreiber, S.; Nikolaus, S.; Hampe, J.; Hämling, J.; Koop, I.; Groessner, B.; Lochs, H.; Raedler, A. Tumour necrosis factor α and interleukin 1β in relapse of Crohn’s disease. Lancet 1999, 353, 459–461. [Google Scholar] [CrossRef]
- Baert, F.J.; D’Haens, G.R.; Peeters, M.; Hiele, M.I.; Schaible, T.F.; Shealy, D.; Geboes, K.; Rutgeerts, P.J. Tumor necrosis factor α antibody (infliximab) therapy profoundly down-regulates the inflammation in Crohn’s ileocolitis. Gastroenterology 1999, 116, 22–28. [Google Scholar] [CrossRef]
- D’Haens, G.; Van Deventer, S.; Van Hogezand, R.; Chalmers, D.; Kothe, C.; Baert, F.; Braakman, T.; Schaible, T.; Geboes, K.; Rutgeerts, P. Endoscopic and histological healing with infliximab anti–tumor necrosis factor antibodies in Crohn’s disease: A European multicenter trial. Gastroenterology 1999, 116, 1029–1034. [Google Scholar] [CrossRef]
- Rismo, R.; Olsen, T.; Ciu, G.; Paulssen, E.J.; Christiansen, I.; Florholmen, J.; Goll, R. The effect of adalimumab for induction of endoscopic healing and normalization of mucosal cytokine gene expression in Crohn’s disease. Scand. J. Gastroenterol. 2012, 47, 1200–1210. [Google Scholar] [CrossRef]
- Ferkolj, I.; Ihan, A.; Markovic, S.; Veceric, Z.; Pohar, M. Infliximab reduces the number of activated mucosal lymphocytes in patients with Crohn’s disease. J. Gastrointestin. Liver Dis. 2006, 15, 231–235. [Google Scholar] [PubMed]
- Zhang, C.; Shu, W.; Zhou, G.; Lin, J.; Chu, F.; Wu, H.; Liu, Z. Anti-TNF- α Therapy Suppresses Proinflammatory Activities of Mucosal Neutrophils in Inflammatory Bowel Disease. Mediators Inflamm. 2018, 2018, 1–12. [Google Scholar] [CrossRef]
- Di Sabatino, A.; Ciccocioppo, R.; Cinque, B.; Millimaggi, D.; Morera, R.; Ricevuti, L.; Cifone, M.G.; Corazza, G.R. Defective mucosal T cell death is sustainably reverted by infliximab in a caspase dependent pathway in Crohn’s disease. Gut 2004, 53, 70–77. [Google Scholar] [CrossRef]
- Dahlén, R.; Magnusson, M.K.; Bajor, A.; Lasson, A.; Ung, K.-A.; Strid, H.; Öhman, L. Global mucosal and serum cytokine profile in patients with ulcerative colitis undergoing anti-TNF therapy. Scand. J. Gastroenterol. 2015, 50, 1118–1126. [Google Scholar] [CrossRef] [PubMed]
- Hassan, C.; Ierardi, E.; Burattini, O.; De Francesco, V.; Zullo, A.; Stoppino, G.; Panella, C.; Morini, S. Tumour necrosis factor alpha down-regulation parallels inflammatory regression in ulcerative colitis patients treated with infliximab. Dig. Liver Dis. 2007, 39, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Leal, R.F.; Planell, N.; Kajekar, R.; Lozano, J.J.; Ordás, I.; Dotti, I.; Esteller, M.; Masamunt, M.C.; Parmar, H.; Ricart, E.; et al. Identification of inflammatory mediators in patients with Crohn’s disease unresponsive to anti-TNFα therapy. Gut 2015, 64, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Olsen, T.; Rismo, R.; Gundersen, M.D.; Paulssen, E.J.; Johnsen, K.; Kvamme, J.-M.; Goll, R.; Florholmen, J. Normalization of mucosal tumor necrosis factor-α: A new criterion for discontinuing infliximab therapy in ulcerative colitis. Cytokine 2016, 79, 90–95. [Google Scholar] [CrossRef]
- Rismo, R.; Olsen, T.; Cui, G.; Paulssen, E.J.; Christiansen, I.; Johnsen, K.; Florholmen, J.; Goll, R. Normalization of mucosal cytokine gene expression levels predicts long-term remission after discontinuation of anti-TNF therapy in Crohn’s disease. Scand. J. Gastroenterol. 2013, 48, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Arijs, I.; De Hertogh, G.; Machiels, K.; Van Steen, K.; Lemaire, K.; Schraenen, A.; Van Lommel, L.; Quintens, R.; Van Assche, G.; Vermeire, S.; et al. Mucosal Gene Expression of Cell Adhesion Molecules, Chemokines, and Chemokine Receptors in Patients With Inflammatory Bowel Disease Before and After Infliximab Treatment. Am. J. Gastroenterol. 2011, 106, 748–761. [Google Scholar] [CrossRef] [PubMed]
- Eder, P.; Lykowska-Szuber, L.; Krela-Kazmierczak, I.; Stawczyk-Eder, K.; Zabel, M.; Linke, K. The influence of infliximab and adalimumab on the expression of apoptosis-related proteins in lamina propria mononuclear cells and enterocytes in Crohn’s disease — An immunohistochemical study. J. Crohn’s Colitis 2013, 7, 706–716. [Google Scholar] [CrossRef]
- Suenaert, P.; Bulteel, V.; Lemmens, L.; Noman, M.; Geypens, B.; Assche, G.; Van Geboes, K.; Ceuppens, J.L.; Rutgeerts, P. Anti-tumor necrosis factor treatment restores the gut barrier in Crohn’s disease. Am. J. Gastroenterol. 2002, 97, 2000–2004. [Google Scholar] [CrossRef]
- Zeissig, S.; Bojarski, C.; Buergel, N.; Mankertz, J.; Zeitz, M.; Fromm, M.; Schulzke, J.D. Downregulation of epithelial apoptosis and barrier repair in active Crohn’s disease by tumour necrosis factor alpha antibody treatment. Gut 2004, 53, 1295–1302. [Google Scholar] [CrossRef] [PubMed]
- Arijs, I.; De Hertogh, G.; Lemaire, K.; Quintens, R.; Van Lommel, L.; Van Steen, K.; Leemans, P.; Cleynen, I.; Van Assche, G.; Vermeire, S.; et al. Mucosal gene expression of antimicrobial peptides in inflammatory bowel disease before and after first infliximab treatment. PLoS ONE 2009, 4, e7984. [Google Scholar] [CrossRef]
- Aden, K.; Rehman, A.; Waschina, S.; Pan, W.-H.; Walker, A.; Lucio, M.; Nunez, A.M.; Bharti, R.; Zimmerman, J.; Bethge, J.; et al. Metabolic Functions of Gut Microbes Associate With Efficacy of Tumor Necrosis Factor Antagonists in Patients With Inflammatory Bowel Diseases. Gastroenterology 2019, 157, 1279–1292.e11. [Google Scholar] [CrossRef]
- Estevinho, M.M.; Rocha, C.; Correia, L.; Lago, P.; Ministro, P.; Portela, F.; Trindade, E.; Afonso, J.; Peyrin-Biroulet, L.; Magro, F. Features of Fecal and Colon Microbiomes Associate With Responses to Biologic Therapies for Inflammatory Bowel Diseases: A Systematic Review. Clin. Gastroenterol. Hepatol. 2019. [Google Scholar] [CrossRef]
- Dovrolis, N.; Michalopoulos, G.; Theodoropoulos, G.E.; Arvanitidis, K.; Kolios, G.; Sechi, L.A.; Eliopoulos, A.G.; Gazouli, M. The Interplay between Mucosal Microbiota Composition and Host Gene-Expression is Linked with Infliximab Response in Inflammatory Bowel Diseases. Microorganisms 2020, 8, 438. [Google Scholar] [CrossRef]
- Cornillie, F.; Shealy, D.; D’Haens, G.; Geboes, K.; Van Assche, G.; Ceuppens, J.; Wagner, C.; Schaible, T.; Plevy, S.E.; Targan, S.R.; et al. Infliximab induces potent anti-inflammatory and local immunomodulatory activity but no systemic immune suppression in patients with Crohn’s disease. Aliment. Pharmacol. Ther. 2001, 15, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Yarur, A.J.; Jain, A.; Sussman, D.A.; Barkin, J.S.; Quintero, M.A.; Princen, F.; Kirkland, R.; Deshpande, A.R.; Singh, S.; Abreu, M.T. The association of tissue anti-TNF drug levels with serological and endoscopic disease activity in inflammatory bowel disease: The ATLAS study. Gut 2016, 65, 249–255. [Google Scholar] [CrossRef]
- Yoshihara, T.; Shinzaki, S.; Kawai, S.; Fujii, H.; Iwatani, S.; Yamaguchi, T.; Araki, M.; Hiyama, S.; Inoue, T.; Hayashi, Y.; et al. Tissue Drug Concentrations of Anti-tumor Necrosis Factor Agents Are Associated with the Long-term Outcome of Patients with Crohn’s Disease. Inflamm. Bowel Dis. 2017, 23, 2172–2179. [Google Scholar] [CrossRef] [PubMed]
- Bak, A.; Ashford, M.; Brayden, D.J. Local delivery of macromolecules to treat diseases associated with the colon. Adv. Drug Deliv. Rev. 2018, 136–137, 2–27. [Google Scholar] [CrossRef]
- Rinaldi, C.; Wood, M.J.A. Antisense oligonucleotides: The next frontier for treatment of neurological disorders. Nat. Rev. Neurol. 2018, 14, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Wittrup, A.; Lieberman, J. Knocking down disease: A progress report on siRNA therapeutics. Nat. Rev. Genet. 2015, 16, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Kriegel, C.; Attarwala, H.; Amiji, M. Multi-compartmental oral delivery systems for nucleic acid therapy in the gastrointestinal tract. Adv. Drug Deliv. Rev. 2013, 65, 891–901. [Google Scholar] [CrossRef]
- Reilly, R.M.; Domingo, R.; Sandhu, J. Oral Delivery of Antibodies. Clin. Pharmacokinet. 1997, 32, 313–323. [Google Scholar] [CrossRef]
- Jasion, V.S.; Burnett, B.P. Survival and digestibility of orally-administered immunoglobulin preparations containing IgG through the gastrointestinal tract in humans. Nutr. J. 2015, 14, 22. [Google Scholar] [CrossRef]
- Wirtz, S.; Popp, V.; Kindermann, M.; Gerlach, K.; Weigmann, B.; Fichtner-Feigl, S.; Neurath, M.F. Chemically induced mouse models of acute and chronic intestinal inflammation. Nat. Protoc. 2017, 12, 1295–1309. [Google Scholar] [CrossRef] [PubMed]
- Perše, M.; Cerar, A. Dextran Sodium Sulphate Colitis Mouse Model: Traps and Tricks. J. Biomed. Biotechnol. 2012, 2012, 1–13. [Google Scholar] [CrossRef]
- Marafini, I.; Monteleone, G. Inflammatory bowel disease: New therapies from antisense oligonucleotides. Ann. Med. 2018, 50, 361–370. [Google Scholar] [CrossRef]
- Chevalier, R. siRNA Targeting and Treatment of Gastrointestinal Diseases. Clin. Transl. Sci. 2019, 12, 573–585. [Google Scholar] [CrossRef]
- Crooke, S.T. Molecular Mechanisms of Antisense Oligonucleotides. Nucleic Acid Ther. 2017, 27, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, J.; Szoka, F.C. Nucleic Acid Delivery: The Missing Pieces of the Puzzle? Acc. Chem. Res. 2012, 45, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Di Fusco, D.; Dinallo, V.; Marafini, I.; Figliuzzi, M.M.; Romano, B.; Monteleone, G. Antisense Oligonucleotide: Basic Concepts and Therapeutic Application in Inflammatory Bowel Disease. Front. Pharmacol. 2019, 10. [Google Scholar] [CrossRef]
- Gebert, L.F.R.; MacRae, I.J. Regulation of microRNA function in animals. Nat. Rev. Mol. Cell Biol. 2019, 20, 21–37. [Google Scholar] [CrossRef]
- Treiber, T.; Treiber, N.; Meister, G. Regulation of microRNA biogenesis and its crosstalk with other cellular pathways. Nat. Rev. Mol. Cell Biol. 2019, 20, 5–20. [Google Scholar] [CrossRef]
- Monteleone, G.; Neurath, M.F.; Ardizzone, S.; Di Sabatino, A.; Fantini, M.C.; Castiglione, F.; Scribano, M.L.; Armuzzi, A.; Caprioli, F.; Sturniolo, G.C.; et al. Mongersen, an oral SMAD7 antisense oligonucleotide, and Crohn’s disease. N. Engl. J. Med. 2015, 372, 1104–1113. [Google Scholar] [CrossRef] [PubMed]
- Sands, B.E.; Feagan, B.G.; Sandborn, W.J.; Schreiber, S.; Peyrin-Biroulet, L.; Frédéric Colombel, J.; Rossiter, G.; Usiskin, K.; Ather, S.; Zhan, X.; et al. Mongersen (GED-0301) for Active Crohn’s Disease: Results of a Phase 3 Study. Am. J. Gastroenterol. 2020, 115, 738–745. [Google Scholar] [CrossRef] [PubMed]
- Lopetuso, L.R.; Petito, V.; Cufino, V.; Arena, V.; Stigliano, E.; Gerardi, V.; Gaetani, E.; Poscia, A.; Amato, A.; Cammarota, G.; et al. Locally injected Infliximab ameliorates murine DSS colitis: Differences in serum and intestinal levels of drug between healthy and colitic mice. Dig. Liver Dis. 2013, 45, 1017–1021. [Google Scholar] [CrossRef] [PubMed]
- Brandse, J.F.; van den Brink, G.R.; Wildenberg, M.E.; van der Kleij, D.; Rispens, T.; Jansen, J.M.; Mathôt, R.A.; Ponsioen, C.Y.; Löwenberg, M.; D’Haens, G.R.A.M. Loss of Infliximab Into Feces Is Associated With Lack of Response to Therapy in Patients With Severe Ulcerative Colitis. Gastroenterology 2015, 149, 350–355.e2. [Google Scholar] [CrossRef] [PubMed]
- Crowe, J.S.; Roberts, K.J.; Carlton, T.M.; Maggiore, L.; Cubitt, M.F.; Clare, S.; Harcourt, K.; Reckless, J.; MacDonald, T.T.; Ray, K.P.; et al. Preclinical Development of a Novel, Orally-Administered Anti-Tumour Necrosis Factor Domain Antibody for the Treatment of Inflammatory Bowel Disease. Sci. Rep. 2018, 8, 4941. [Google Scholar] [CrossRef] [PubMed]
- Michielan, A.; D’Incà, R. Intestinal Permeability in Inflammatory Bowel Disease: Pathogenesis, Clinical Evaluation, and Therapy of Leaky Gut. Mediators Inflamm. 2015, 2015, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Crowe, J.S.; Roberts, K.J.; Carlton, T.M.; Maggiore, L.; Cubitt, M.F.; Ray, K.P.; Donnelly, M.C.; Wahlich, J.C.; Humphreys, J.I.; Robinson, J.R.; et al. Oral delivery of the anti-tumor necrosis factor α domain antibody, V565, results in high intestinal and fecal concentrations with minimal systemic exposure in cynomolgus monkeys. Drug Dev. Ind. Pharm. 2019, 45, 387–394. [Google Scholar] [CrossRef]
- Evonik Industries Eudragit Information Pamphlet. Available online: https://healthcare.evonik.com/sites/lists/NC/DocumentsHC/Evonik-Eudragit_brochure.pdf (accessed on 1 May 2020).
- Smith, H.W. Observations on the flora of the alimentary tract of animals and factors affecting its composition. J. Pathol. Bacteriol. 1965, 89, 95–122. [Google Scholar] [CrossRef]
- Chen, E.P.; Mahar Doan, K.M.; Portelli, S.; Coatney, R.; Vaden, V.; Shi, W. Gastric pH and Gastric Residence Time in Fasted and Fed Conscious Cynomolgus Monkeys Using the Bravo® pH System. Pharm. Res. 2008, 25, 123–134. [Google Scholar] [CrossRef]
- Sjögren, E.; Abrahamsson, B.; Augustijns, P.; Becker, D.; Bolger, M.B.; Brewster, M.; Brouwers, J.; Flanagan, T.; Harwood, M.; Heinen, C.; et al. In vivo methods for drug absorption–Comparative physiologies, model selection, correlations with in vitro methods (IVIVC), and applications for formulation/API/excipient characterization including food effects. Eur. J. Pharm. Sci. 2014, 57, 99–151. [Google Scholar] [CrossRef]
- Ibekwe, V.C.; Fadda, H.M.; McConnell, E.L.; Khela, M.K.; Evans, D.F.; Basit, A.W. Interplay between intestinal pH, transit time and feed status on the in vivo performance of pH responsive ileo-colonic release systems. Pharm. Res. 2008, 25, 1828–1835. [Google Scholar] [CrossRef]
- McConnell, E.L.; Fadda, H.M.; Basit, A.W. Gut instincts: Explorations in intestinal physiology and drug delivery. Int. J. Pharm. 2008, 364, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Freire, A.C.; Basit, A.W.; Choudhary, R.; Piong, C.W.; Merchant, H.A. Does sex matter? The influence of gender on gastrointestinal physiology and drug delivery. Int. J. Pharm. 2011, 415, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Ewe, K.; Schwartz, S.; Petersen, S.; Press, A.G. Inflammation does not decrease intraluminal pH in chronic inflammatory bowel disease. Dig. Dis. Sci. 1999, 44, 1434–1439. [Google Scholar] [CrossRef] [PubMed]
- Nugent, S.G.; Kumar, D.; Rampton, D.S.; Evans, D.F. Intestinal luminal pH in inflammatory bowel disease: Possible determinants and implications for therapy with aminosalicylates and other drugs. Gut 2001, 48, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Nurbhai, S.; Roberts, K.J.; Carlton, T.M.; Maggiore, L.; Cubitt, M.F.; Ray, K.P.; Reckless, J.; Mohammed, H.; Irving, P.; MacDonald, T.T.; et al. Oral Anti-Tumour Necrosis Factor Domain Antibody V565 Provides High Intestinal Concentrations, and Reduces Markers of Inflammation in Ulcerative Colitis Patients. Sci. Rep. 2019, 9, 14042. [Google Scholar] [CrossRef] [PubMed]
- Bhol, K.C.; Tracey, D.E.; Lemos, B.R.; Lyng, G.D.; Erlich, E.C.; Keane, D.M.; Quesenberry, M.S.; Holdorf, A.D.; Schlehuber, L.D.; Clark, S.A.; et al. AVX-470: A Novel Oral Anti-TNF Antibody with Therapeutic Potential in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2013, 19, 2273–2281. [Google Scholar] [CrossRef] [PubMed]
- Paiotti, A.P.R.; Miszputen, S.J.; Oshima, C.T.F.; Neto, R.A.; Ribeiro, D.A.; Franco, M. Etanercept attenuates TNBS-induced experimental colitis: Role of TNF-α expression. J. Mol. Histol. 2011, 42, 443–450. [Google Scholar] [CrossRef]
- Hartman, D.S.; Tracey, D.E.; Lemos, B.R.; Erlich, E.C.; Burton, R.E.; Keane, D.M.; Patel, R.; Kim, S.; Bhol, K.C.; Harris, M.S.; et al. Effects of AVX-470, an Oral, Locally Acting Anti-Tumour Necrosis Factor Antibody, on Tissue Biomarkers in Patients with Active Ulcerative Colitis. J. Crohn’s Colitis 2016, 10, 641–649. [Google Scholar] [CrossRef]
- Harris, M.S.; Hartman, D.; Lemos, B.R.; Erlich, E.C.; Spence, S.; Kennedy, S.; Ptak, T.; Pruitt, R.; Vermeire, S.; Fox, B.S. AVX-470, an Orally Delivered Anti-Tumour Necrosis Factor Antibody for Treatment of Active Ulcerative Colitis: Results of a First-in-Human Trial. J. Crohn’s Colitis 2016, 10, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Worledge, K.L.; Godiska, R.; Barrett, T.A.; Kink, J.A. Oral administration of avian tumor necrosis factor antibodies effectively treats experimental colitis in rats. Dig. Dis. Sci. 2000, 45, 2298–2305. [Google Scholar] [CrossRef] [PubMed]
- Baker, B.F.; Murthy, S.; Flanigan, A.; Siwkowski, A.M.; Butler, M.; Dean, N.M. Dose-dependent reduction of chronic dextransulfate sodium (DSS)-induced colitis in mice treated with TNF-a antisense oligonucleotide (ISIS 25302). Gastroenterology 2000, 118, A571. [Google Scholar] [CrossRef]
- Hotamisligil, G.; Shargill, N.; Spiegelman, B. Adipose expression of tumor necrosis factor-alpha: Direct role in obesity-linked insulin resistance. Science 1993, 259, 87–91. [Google Scholar] [CrossRef]
- Myers, K.J.; Murthy, S.; Flanigan, A.; Witchell, D.R.; Butler, M.; Murray, S.; Siwkowski, A.; Goodfellow, D.; Madsen, K.; Baker, B. Antisense Oligonucleotide Blockade of Tumor Necrosis Factor-α in Two Murine Models of Colitis. J. Pharmacol. Exp. Ther. 2003, 304, 411–424. [Google Scholar] [CrossRef] [PubMed]
- Zuo, L.; Huang, Z.; Dong, L.; Xu, L.; Zhu, Y.; Zeng, K.; Zhang, C.; Chen, J.; Zhang, J. Targeting delivery of anti-TNF oligonucleotide into activated colonic macrophages protects against experimental colitis. Gut 2010, 59, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Van Vliet, S.J.; Saeland, E.; van Kooyk, Y. Sweet preferences of MGL: Carbohydrate specificity and function. Trends Immunol. 2008, 29, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Linehan, S.A.; Martinez-Pomares, L.; Gordon, S. Mannose Receptor and Scavenger Receptor: Two Macrophage Pattern Recognition Receptors with Diverse Functions in Tissue Homeostasis and Host Defense. Biol. Pathol. Innate Immun. Mech. 2000, 479, 1–14. [Google Scholar]
- Duan, B.; Li, M.; Sun, Y.; Zou, S.; Xu, X. Orally Delivered Antisense Oligodeoxyribonucleotides of TNF-α via Polysaccharide-Based Nanocomposites Targeting Intestinal Inflammation. Adv. Healthc. Mater. 2019, 8, 1801389. [Google Scholar] [CrossRef]
- Sakisaka, H.; Takedatsu, H.; Mitsuyama, K.; Mochizuki, S.; Sakurai, K.; Sakisaka, S.; Hirai, F. Topical Therapy with Antisense Tumor Necrosis Factor Alpha Using Novel β-Glucan-Based Drug Delivery System Ameliorates Intestinal Inflammation. Int. J. Mol. Sci. 2020, 21, 683. [Google Scholar] [CrossRef]
- Schorey, J.S.; Lawrence, C. The pattern recognition receptor Dectin-1: From fungi to mycobacteria. Curr. Drug Targets 2008, 9, 123–129. [Google Scholar] [CrossRef]
- He, C.; Shi, Y.; Wu, R.; Sun, M.; Fang, L.; Wu, W.; Liu, C.; Tang, M.; Li, Z.; Wang, P.; et al. miR-301a promotes intestinal mucosal inflammation through induction of IL-17A and TNF-α in IBD. Gut 2016, 65, 1938–1950. [Google Scholar] [CrossRef]
- Ma, X.; Becker Buscaglia, L.E.; Barker, J.R.; Li, Y. MicroRNAs in NF-kappaB signaling. J. Mol. Cell Biol. 2011, 3, 159–166. [Google Scholar] [CrossRef]
- He, C.; Yu, T.; Shi, Y.; Ma, C.; Yang, W.; Fang, L.; Sun, M.; Wu, W.; Xiao, F.; Guo, F.; et al. MicroRNA 301A Promotes Intestinal Inflammation and Colitis-Associated Cancer Development by Inhibiting BTG1. Gastroenterology 2017, 152, 1434–1448.e15. [Google Scholar] [CrossRef]
- Huang, Z.; Ma, J.; Chen, M.; Jiang, H.; Fu, Y.; Gan, J.; Dong, L.; Zhang, J.; Chen, J. Dual TNF-α/IL-12p40 Interference as a Strategy to Protect Against Colitis Based on miR-16 Precursors With Macrophage Targeting Vectors. Mol. Ther. 2015, 23, 1611–1621. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Zhang, S.; Dassopoulos, T.; Harris, M.L.; Bayless, T.M.; Meltzer, S.J.; Brant, S.R.; Kwon, J.H. Identification of microRNAs associated with ileal and colonic Crohn’s disease†. Inflamm. Bowel Dis. 2010, 16, 1729–1738. [Google Scholar] [CrossRef]
- Jing, Q.; Huang, S.; Guth, S.; Zarubin, T.; Motoyama, A.; Chen, J.; Di Padova, F.; Lin, S.-C.; Gram, H.; Han, J. Involvement of MicroRNA in AU-Rich Element-Mediated mRNA Instability. Cell 2005, 120, 623–634. [Google Scholar] [CrossRef]
- Wu, F.; Zikusoka, M.; Trindade, A.; Dassopoulos, T.; Harris, M.L.; Bayless, T.M.; Brant, S.R.; Chakravarti, S.; Kwon, J.H. MicroRNAs Are Differentially Expressed in Ulcerative Colitis and Alter Expression of Macrophage Inflammatory Peptide-2α. Gastroenterology 2008, 135, 1624–1635.e24. [Google Scholar] [CrossRef] [PubMed]
- Moschen, A.R.; Tilg, H.; Raine, T. IL-12, IL-23 and IL-17 in IBD: Immunobiology and therapeutic targeting. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Chapman, C.G.; Pekow, J. The emerging role of miRNAs in inflammatory bowel disease: A review. Therap. Adv. Gastroenterol. 2015, 8, 4–22. [Google Scholar] [CrossRef]
- Kiesler, P.; Fuss, I.J.; Strober, W. Experimental Models of Inflammatory Bowel Diseases. Cell. Mol. Gastroenterol. Hepatol. 2015, 1, 154–170. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Liang, M.; Hou, X.; Zhang, Y.; Zhang, H.; Guo, Z.; Jinyu, J.; Feng, Z.; Mei, Z. The role of microRNA-16 in the pathogenesis of autoimmune diseases: A comprehensive review. Biomed. Pharmacother. 2019, 112, 108583. [Google Scholar] [CrossRef]
- Song, Y.; Kim, Y.-R.; Kim, S.M.; Ul Ain, Q.; Jang, K.; Yang, C.-S.; Kim, Y.-H. RNAi-mediated silencing of TNF-α converting enzyme to down-regulate soluble TNF-α production for treatment of acute and chronic colitis. J. Control. Release 2016, 239, 231–241. [Google Scholar] [CrossRef]
- Huang, Z.; Gan, J.; Jia, L.; Guo, G.; Wang, C.; Zang, Y.; Ding, Z.; Chen, J.; Zhang, J.; Dong, L. An orally administrated nucleotide-delivery vehicle targeting colonic macrophages for the treatment of inflammatory bowel disease. Biomaterials 2015, 48, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.-S.; Bai, G.; Tang, L.-D.; Li, Y.; Huan, Y.; Wang, H. MiR-195 alleviates ulcerative colitis in rats via MAPK signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 2640–2646. [Google Scholar]
- Cheng, W.; Tang, C.; Yin, C. Effects of particle size and binding affinity for small interfering RNA on the cellular processing, intestinal permeation and anti-inflammatory efficacy of polymeric nanoparticles. J. Gene Med. 2015, 17, 244–256. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cristofaro, P.; Silbermann, R.; Pusch, O.; Boden, D.; Konkin, T.; Hovanesian, V.; Monfils, P.R.; Resnick, M.; Moss, S.F.; et al. Engineering Mucosal RNA Interference in Vivo. Mol. Ther. 2006, 14, 336–342. [Google Scholar] [CrossRef]
- Ocampo, S.M.; Romero, C.; Aviñó, A.; Burgueño, J.; Gassull, M.A.; Bermúdez, J.; Eritja, R.; Fernandez, E.; Perales, J.C. Functionally Enhanced siRNA Targeting TNFα Attenuates DSS-induced Colitis and TLR-mediated Immunostimulation in Mice. Mol. Ther. 2012, 20, 382–390. [Google Scholar] [CrossRef]
- McCarthy, J.; O’Neill, M.J.; Bourre, L.; Walsh, D.; Quinlan, A.; Hurley, G.; Ogier, J.; Shanahan, F.; Melgar, S.; Darcy, R.; et al. Gene silencing of TNF-alpha in a murine model of acute colitis using a modified cyclodextrin delivery system. J. Control. Release 2013, 168, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Frede, A.; Neuhaus, B.; Klopfleisch, R.; Walker, C.; Buer, J.; Müller, W.; Epple, M.; Westendorf, A.M. Colonic gene silencing using siRNA-loaded calcium phosphate/PLGA nanoparticles ameliorates intestinal inflammation in vivo. J. Control. Release 2016, 222, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Schoellhammer, C.M.; Lauwers, G.Y.; Goettel, J.A.; Oberli, M.A.; Cleveland, C.; Park, J.Y.; Minahan, D.; Chen, Y.; Anderson, D.G.; Jaklenec, A.; et al. Ultrasound-Mediated Delivery of RNA to Colonic Mucosa of Live Mice. Gastroenterology 2017, 152, 1151–1160. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.S.; Dalmasso, G.; Wang, L.; Sitaraman, S.V.; Merlin, D.; Murthy, N. Orally delivered thioketal nanoparticles loaded with TNF-α–siRNA target inflammation and inhibit gene expression in the intestines. Nat. Mater. 2010, 9, 923–928. [Google Scholar] [CrossRef]
- Huang, Y.; Guo, J.; Gui, S. Orally targeted galactosylated chitosan poly(lactic-co-glycolic acid) nanoparticles loaded with TNF-α siRNA provide a novel strategy for the experimental treatment of ulcerative colitis. Eur. J. Pharm. Sci. 2018, 125, 232–243. [Google Scholar] [CrossRef]
- Kriegel, C.; Amiji, M. Oral TNF-α gene silencing using a polymeric microsphere-based delivery system for the treatment of inflammatory bowel disease. J. Control. Release 2011, 150, 77–86. [Google Scholar] [CrossRef]
- Laroui, H.; Theiss, A.L.; Yan, Y.; Dalmasso, G.; Nguyen, H.T.T.; Sitaraman, S.V.; Merlin, D. Functional TNFα gene silencing mediated by polyethyleneimine/TNFα siRNA nanocomplexes in inflamed colon. Biomaterials 2011, 32, 1218–1228. [Google Scholar] [CrossRef]
- Laroui, H.; Viennois, E.; Xiao, B.; Canup, B.S.B.; Geem, D.; Denning, T.L.; Merlin, D. Fab’-bearing siRNA TNFα-loaded nanoparticles targeted to colonic macrophages offer an effective therapy for experimental colitis. J. Control. Release 2014, 186, 41–53. [Google Scholar] [CrossRef]
- Kriegel, C.; Amiji, M.M. Dual TNF-α/Cyclin D1 Gene Silencing With an Oral Polymeric Microparticle System as a Novel Strategy for the Treatment of Inflammatory Bowel Disease. Clin. Transl. Gastroenterol. 2011, 2, e2. [Google Scholar] [CrossRef]
- Xiao, B.; Chen, Q.; Zhang, Z.; Wang, L.; Kang, Y.; Denning, T.; Merlin, D. TNFα gene silencing mediated by orally targeted nanoparticles combined with interleukin-22 for synergistic combination therapy of ulcerative colitis. J. Control. Release 2018, 287, 235–246. [Google Scholar] [CrossRef]
- Chiabai, M.J.; Almeida, J.F.; de Azevedo, M.G.D.; Fernandes, S.S.; Pereira, V.B.; de Castro, R.J.A.; Jerônimo, M.S.; Sousa, I.G.; de Souza Vianna, L.M.; Miyoshi, A.; et al. Mucosal delivery of Lactococcus lactis carrying an anti-TNF scFv expression vector ameliorates experimental colitis in mice. BMC Biotechnol. 2019, 19, 38. [Google Scholar] [CrossRef]
- Vandenbroucke, K.; de Haard, H.; Beirnaert, E.; Dreier, T.; Lauwereys, M.; Huyck, L.; Van Huysse, J.; Demetter, P.; Steidler, L.; Remaut, E.; et al. Orally administered L. lactis secreting an anti-TNF Nanobody demonstrate efficacy in chronic colitis. Mucosal Immunol. 2010, 3, 49–56. [Google Scholar] [CrossRef]
- Ilan, Y.; Gingis-Velitski, S.; Ben Ya’aco, A.; Shabbat, Y.; Zolotarov, L.; Almon, E.; Shaaltiel, Y. A plant cell-expressed recombinant anti-TNF fusion protein is biologically active in the gut and alleviates immune-mediated hepatitis and colitis. Immunobiology 2017, 222, 544–551. [Google Scholar] [CrossRef]
- O’Mahony, A.M.; Ogier, J.; Desgranges, S.; Cryan, J.F.; Darcy, R.; O’Driscoll, C.M. A click chemistry route to 2-functionalised PEGylated and cationic β-cyclodextrins: Co-formulation opportunities for siRNA delivery. Org. Biomol. Chem. 2012, 10, 4954. [Google Scholar] [CrossRef]
- Yan, Y.; Kolachala, V.; Dalmasso, G.; Nguyen, H.; Laroui, H.; Sitaraman, S.V.; Merlin, D. Temporal and Spatial Analysis of Clinical and Molecular Parameters in Dextran Sodium Sulfate Induced Colitis. PLoS ONE 2009, 4, e6073. [Google Scholar] [CrossRef]
- Roy, I.; Mitra, S.; Maitra, A.; Mozumdar, S. Calcium phosphate nanoparticles as novel non-viral vectors for targeted gene delivery. Int. J. Pharm. 2003, 250, 25–33. [Google Scholar] [CrossRef]
- Sokolova, V.; Kozlova, D.; Knuschke, T.; Buer, J.; Westendorf, A.M.; Epple, M. Mechanism of the uptake of cationic and anionic calcium phosphate nanoparticles by cells. Acta Biomater. 2013, 9, 7527–7535. [Google Scholar] [CrossRef]
- Hines, D.J.; Kaplan, D.L. Poly(lactic-co-glycolic) Acid-Controlled-Release Systems: Experimental and Modeling Insights. Crit. Rev. Ther. Drug Carrier Syst. 2013, 30, 257–276. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Kovochich, M.; Liong, M.; Meng, H.; Kabehie, S.; George, S.; Zink, J.I.; Nel, A.E. Polyethyleneimine Coating Enhances the Cellular Uptake of Mesoporous Silica Nanoparticles and Allows Safe Delivery of siRNA and DNA Constructs. ACS Nano 2009, 3, 3273–3286. [Google Scholar] [CrossRef] [PubMed]
- Alonso, A. Ultrasound-Induced Blood-Brain Barrier Opening for Drug Delivery. In Translational Neurosonology; Karger Publishers: Basel, Switzerland, 2014; pp. 106–115. [Google Scholar]
- Peruzzi, G.; Sinibaldi, G.; Silvani, G.; Ruocco, G.; Casciola, C.M. Perspectives on cavitation enhanced endothelial layer permeability. Colloids Surfaces B Biointerfaces 2018, 168, 83–93. [Google Scholar] [CrossRef]
- Zhou, Q.-L.; Chen, Z.-Y.; Wang, Y.-X.; Yang, F.; Lin, Y.; Liao, Y.-Y. Ultrasound-Mediated Local Drug and Gene Delivery Using Nanocarriers. Biomed Res. Int. 2014, 2014, 1–13. [Google Scholar] [CrossRef]
- Lih-Brody, L.; Powell, S.R.; Collier, K.P.; Reddy, G.M.; Cerchia, R.; Kahn, E.; Weissman, G.S.; Katz, S.; Floyd, R.A.; McKinley, M.J.; et al. Increased oxidative stress and decreased antioxidant defenses in mucosa of inflammatory bowel disease. Dig. Dis. Sci. 1996, 41, 2078–2086. [Google Scholar] [CrossRef] [PubMed]
- Simmonds, N.J.; Allen, R.E.; Stevens, T.R.J.; Niall, R.; Van Someren, M.; Blake, D.R.; Rampton, D.S. Chemiluminescence assay of mucosal reactive oxygen metabolites in inflammatory bowel disease. Gastroenterology 1992, 103, 186–196. [Google Scholar] [CrossRef]
- Aouadi, M.; Tesz, G.J.; Nicoloro, S.M.; Wang, M.; Chouinard, M.; Soto, E.; Ostroff, G.R.; Czech, M.P. Orally delivered siRNA targeting macrophage Map4k4 suppresses systemic inflammation. Nature 2009, 458, 1180–1184. [Google Scholar] [CrossRef] [PubMed]
- Tchakarska, G.; Sola, B. The double dealing of cyclin D1. Cell Cycle 2020, 19, 163–178. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Bie, W.; Haegebarth, A.; Tyner, A.L. Differential Regulation of D-Type Cyclins in the Mouse Intestine. Cell Cycle 2006, 5, 180–183. [Google Scholar]
- Van Dekken, H.; Wink, J.C.; Vissers, K.J.; Franken, P.F.; Ruud Schouten, W.R.; Hop, W.C.; Kuipers, E.J.; Fodde, R.; van der Woude, C.J. Wnt pathway-related gene expression during malignant progression in ulcerative colitis. Acta Histochem. 2007, 109, 266–272. [Google Scholar] [CrossRef]
- Dos Anjos Cassado, A. F4/80 as a Major Macrophage Marker: The Case of the Peritoneum and Spleen. In Macrophages; Springer: Cham, Switzerland, 2017; pp. 161–179. [Google Scholar]
- Mizoguchi, A.; Yano, A.; Himuro, H.; Ezaki, Y.; Sadanaga, T.; Mizoguchi, E. Clinical importance of IL-22 cascade in IBD. J. Gastroenterol. 2018, 53, 465–474. [Google Scholar] [CrossRef]
- Song, A.A.-L.; In, L.L.A.; Lim, S.H.E.; Rahim, R.A. A review on Lactococcus lactis: From food to factory. Microb. Cell Fact. 2017, 16, 55. [Google Scholar] [CrossRef]
- Almon, E.; Khoury, T.; Drori, A.; Gingis-Velitski, S.; Alon, S.; Chertkoff, R.; Mushkat, M.; Shaaltiel, Y.; Ilan, Y. An oral administration of a recombinant anti-TNF fusion protein is biologically active in the gut promoting regulatory T cells: Results of a phase I clinical trial using a novel oral anti-TNF alpha-based therapy. J. Immunol. Methods 2017, 446, 21–29. [Google Scholar] [CrossRef]
- Chen, W.; Chen, H.; Fu, S.; Lin, X.; Zheng, Z.; Zhang, J. Microbiome characterization and re-design by biologic agents for inflammatory bowel disease insights. Bioprocess Biosyst. Eng. 2020. [Google Scholar] [CrossRef]
- Lichtiger, S. Healing of perianal fistulae by local injection of antibody to TNF. Gastroenterology 2001, 120, A621. [Google Scholar] [CrossRef]
- Asteria, C.R.; Ficari, F.; Bagnoli, S.; Milla, M.; Tonelli, F. Treatment of perianal fistulas in Crohn’s disease by local injection of antibody to TNF-α accounts for a favourable clinical response in selected cases: A pilot study. Scand. J. Gastroenterol. 2006, 41, 1064–1072. [Google Scholar] [CrossRef]
- Poggioli, G.; Laureti, S.; Pierangeli, F.; Rizzello, F.; Ugolini, F.; Gionchetti, P.; Campieri, M. Local Injection of Infliximab for the Treatment of Perianal Crohn’s Disease. Dis. Colon Rectum 2005, 48, 768–774. [Google Scholar] [CrossRef]
- Alessandroni, L.; Kohn, A.; Cosintino, R.; Marrollo, M.; Papi, C.; Monterubbianesi, R.; Tersigni, R. Local injection of infliximab in severe fistulating perianal Crohn’s disease: An open uncontrolled study. Tech. Coloproctol. 2011, 15, 407–412. [Google Scholar] [CrossRef]
- Poggioli, G.; Laureti, S.; Pierangeli, F.; Bazzi, P.; Coscia, M.; Gentilini, L.; Gionchetti, P.; Rizzello, F. Local Injection of Adalimumab for Perianal Crohn’s Disease: Better Than Infliximab? Inflamm. Bowel Dis. 2010, 16, 1631. [Google Scholar] [CrossRef]
- Laureti, S.; Coscia, M.; Gentilini, L.; Ugolini, F.; Vitali, G.; Vittori, L.; Rizzello, F.; Gionchetti, P.; Calabrese, C.; Calafiore, A.; et al. P393 Combination of surgical therapy and local injections of adalimumab in treatment of complex perianal Crohn’s disease. J. Crohn’s Colitis 2012, 6, S166. [Google Scholar] [CrossRef][Green Version]
- Tonelli, F.; Giudici, F.; Asteria, C.R. Effectiveness and Safety of Local Adalimumab Injection in Patients With Fistulizing Perianal Crohn’s Disease. Dis. Colon Rectum 2012, 55, 870–875. [Google Scholar] [CrossRef] [PubMed]
- Maggi, L.; Capone, M.; Giudici, F.; Santarlasci, V.; Querci, V.; Liotta, F.; Ficari, F.; Maggi, E.; Tonelli, F.; Annunziato, F.; et al. CD4+CD161+ T Lymphocytes Infiltrate Crohn’s Disease-Associated Perianal Fistulas and Are Reduced by Anti-TNF-a Local Therapy. Int. Arch. Allergy Immunol. 2013, 161, 81–86. [Google Scholar] [CrossRef]
- Biancone, L.; Cretella, M.; Tosti, C.; Palmieri, G.; Petruzziello, C.; Geremia, A.; Calabrese, E.; Pallone, F. Local injection of infliximab in the postoperative recurrence of Crohn’s disease. Gastrointest. Endosc. 2006, 63, 486–492. [Google Scholar] [CrossRef]
- Lorenzo-Zúñiga, V.; Boix, J.; Mañosa, M.; Lezcano, C.; Cabré, E.; Moreno de Vega, V.; Domènech, E. Local Injection of Infliximab in Symptomatic Isolated Mucosal Lesions. Inflamm. Bowel Dis. 2013, 19, E59–E61. [Google Scholar] [CrossRef]
- Swaminath, A.; Lichtiger, S. Dilation of colonic strictures by intralesional injection of infliximab in patients with Crohn’s colitis. Inflamm. Bowel Dis. 2008, 14, 213–216. [Google Scholar] [CrossRef]
- Teich, N.; Wallstabe, I.; Schiefke, I. Topic infliximab injection for refractory rectal stenosis in Crohn’s disease: Long-term follow-up in two patients. Int. J. Colorectal Dis. 2017, 32, 1289–1294. [Google Scholar] [CrossRef]
- Hendel, J.; Karstensen, J.G.; Vilmann, P. Serial intralesional injections of infliximab in small bowel Crohn’s strictures are feasible and might lower inflammation. United Eur. Gastroenterol. J. 2014, 2, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Molnár, T.; Farkas, K.; Nagy, F.; Németh, I.; Wittmann, T. Topically Administered Infliximab Can Work in Ulcerative Proctitis Despite the Ineffectiveness of Intravenous Induction Therapy. Am. J. Gastroenterol. 2009, 104, 1857–1858. [Google Scholar] [CrossRef]
- Adegbola, S.O.; Sahnan, K.; Tozer, P.J.; Phillips, R.K.; Faiz, O.D.; Warusavitarne, J.; Hart, A. Review of local injection of anti-TNF for perianal fistulising Crohn’s disease. Int. J. Colorectal Dis. 2017, 32, 1539–1544. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Hanauer, S.B.; Katz, S.; Safdi, M.; Wolf, D.G.; Baerg, R.D.; Tremaine, W.J.; Johnson, T.; Diehl, N.N.; Zinsmeister, A.R. Etanercept for active Crohn’s disease: A randomized, double-blind, placebo-controlled trial. Gastroenterology 2001, 121, 1088–1094. [Google Scholar] [CrossRef]
| Treatment | Formulation | ROA | Animal Model | TNF-α a | Cytokines a | Measured Effects | Histology | Reference |
|---|---|---|---|---|---|---|---|---|
| Antibodies | ||||||||
| IFX-Enema | Enema containing an IFX solution | Rec. | Mice, DSS acute colitis | – | – | Body weight, colon length, DAI, Rachmilewitz score | H&E staining | [90] |
| V565 | Anti-TNF-α single domain antibody | PO | Mice, DSS acute colitis | – | – | – | – | [92] |
| V565 tablet | Anti-TNF-α single domain antibody coated with pH sensitive polymer (pH threshold ≥6) | PO | Cynomolgus monkeys, healthy | – | – | – | – | [94] |
| Avian-anti-TNF-α | Avian antibody against TNF-α | PO | Rats, TNBS acute colitis | – | – | Colon morphology, colon weight, MPO | H&E staining, histopathology score, IgY staining | [109] |
| Rats, TNBS chronic colitis | – | – | Colon morphology, colon weight, MPO | Histopathology score, IgY staining | ||||
| AVX-470 | Bovine colostral antibody against TNF-α | PO | Mice, DSS acute colitis | – | – | Endoscopy score | – | [105] |
| Mice, DSS chronic colitis | mRNA | IL-1β, IL-6, IL-12p40 | Endoscopy score, colon length, colon weight, IHC score | Histopathology score, IHC staining | ||||
| Mice, TNBS acute colitis | – | – | Endoscopy score | – | ||||
| Antisense Oligonucleotides | ||||||||
| ISIS 25302 | ASO against TNF-α | SC | Mice, DSS chronic colitis | Protein, mRNA | – | DAI | Histopathology score | [110] |
| ISIS 25302 | ASO against TNF-α | IP | Mice, db/db | mRNA | – | - | – | [112] |
| IV | Mice, DSS acute colitis | – | – | Colon length, DAI | – | |||
| SC | Mice, DSS chronic colitis | Northern blot | – | DAI | Histopathology score | |||
| Mice, IL-10−/− colitis prophylaxis | Colon organ culture, basal and LPS stimulated | – | – | Histopathology score | ||||
| Mice, IL-10−/− colitis therapy | Colon organ culture, basal and LPS stimulated | IFN-γ | – | Histopathology score | ||||
| Gal–LMWC–ASO | Nanocomplex of ASO against TNF-α (ISIS 25302) associated with galactosylated low molecular weight chitosan | IC | Mice, TNBS acute colitis | Protein, mRNA | IFN-γ, IL-1β, IL-6, IL-12, IL-17, IL-23, | AAT, body weight, DAI, mortality, MPO | H&E staining, histopathology score, TNF-α staining | [113] |
| Mice, CD4+ CD45RBhi chronic colitis | Protein, mRNA | IFN-γ, IL-1β, IL-6, IL-12, IL-17, IL-23 | AAT, body weight, DAI, mortality, MPO | H&E staining, histopathology score. TNF-α staining | ||||
| GGG-ASO | Colon-targeted microspheres containing ASO (ISIS 25302) against TNF-α complexed with a mixture of glucomannan-gellan gum | PO | Mice, DSS acute colitis | Protein | IL-1β, IL-6, IL-12p70, IL-23 | Body weight, colon length, DAI, mortality, MPO | H&E staining, histopathology score | [131] |
| CAL-ASO | ASO against TNF-α (ISIS 25302) complexed with lentinan encapsulated in chitosan–alginate | PO | Mice, DSS acute colitis | Protein | – | Body weight, colon length, MDA, MPO, spleen size | – | [116] |
| SPG-ASO | Enema containing schizophyllan–ASO complex against TNF-α | Rec. | Mice, DSS acute colitis | mRNA | IL-1β, IL-6 | Body weight, colon length, endoscopy | H&E staining, histopathology score | [117] |
| ASO-miR-301a | Enema containing ASO against miR-301a | IC | Mice, TNBS acute colitis | mRNA | IFN-γ, IL-4, IL-10, IL17A | Body weight, colon length, DAI | H&E staining, histopathology score | [119] |
| MicroRNA | ||||||||
| Gal-LMWC-pre-miR-16 | Precursor of miR-16 complexed with galactosylated low molecular weight chitosan | IC | Mice, TNBS acute colitis | Protein, mRNA | IFN-γ, IL-1β, IL-6, IL-12p40, IL-17A, IL-23 | Body weight, DAI, mortality, MPO | H&E staining, histopathology score, IL-12p40 staining, TNF-α staining | [122] |
| miR-195 | Agomir of miR-195 | NS | Rats, TNBS acute colitis | Protein, mRNA | IL-1β, IL-6 | DAI | H&E staining | [132] |
| Small interfering RNA | ||||||||
| PACC-siRNA-TACE | Poly arginine–cysteine complex containing siRNA against TACE | IV | Mice, DSS acute colitis | Protein | IL-1β, IL-6 | Body weight, colitis score, colon length, mortality, MPO, NADPH | H&E staining, histopathology score | [130] |
| Mice, DSS chronic colitis | – | – | Body weight, colitis score, mortality | H&E staining, histopathology score | ||||
| GTC-siRNA | Nanoparticle containing siRNA against TNF-α complex with galactosylated tri-methyl-chitosan–cysteine | IC | Mice, DSS acute colitis | Protein, mRNA | – | Body weight, MPO | H&E staining | [133] |
| Lipoplex-siRNA-1 | Enema containing liposomal siRNA against TNF-α | Rec. | Mice, DSS acute colitis | mRNA | – | – | H&E staining | [134] |
| Lipoplex-siRNA-2 | Enema containing liposomal siRNA against TNF-α | Rec. | Mice, DSS acute colitis | mRNA | Gene analysis of 25,000 genes | DAI, mortality, MPO, weight-over-length ratio colon | H&E staining, histopathology score | [135] |
| CycD-siRNA | Enema containing nanocomplex of cationic cyclodextrin complexed with siRNA against TNF-α | Rec. | Mice, DSS acute colitis | mRNA | IL-6 | Body weight, colon length, colon weight | – | [136] |
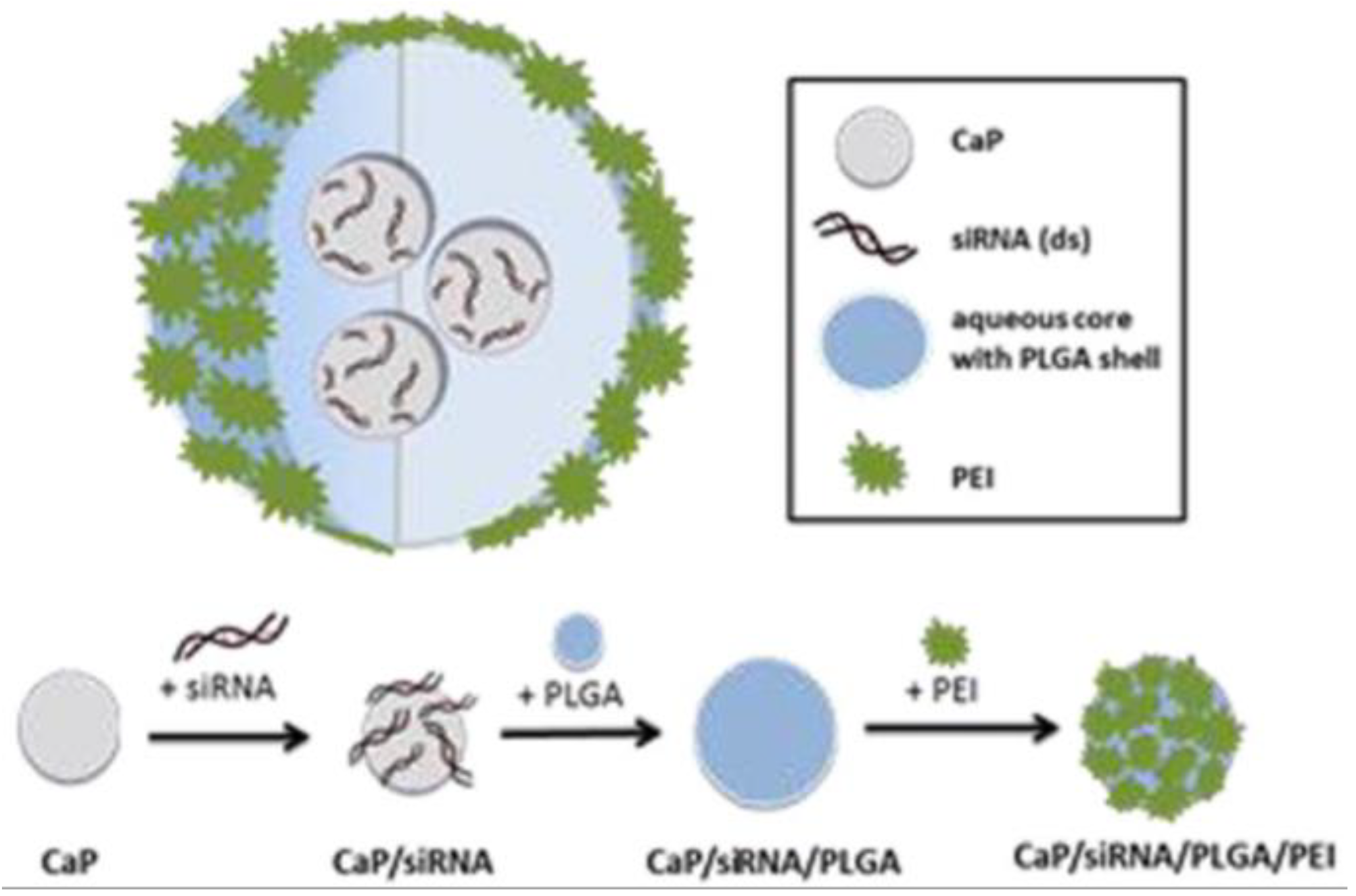
| CaP-siRNA | Enema containing nanoparticles of siRNA loaded on calcium phosphate and encapsulated in PLGA | Rec. | Mice, DSS acute colitis | Protein, mRNA | – | Body weight, colon length, DAI, hematocrit | H&E staining, histopathology score | [137] |
| US-siRNA | Enema containing siRNA against TNF-α delivered by ultrasound | Rec. | Mice, DSS acute colitis | Protein | – | Fecal score | Histopathology score | [138] |
| ROS-siRNA | Nanoparticle containing siRNA against TNF-α encapsulated in a ROS-sensitive polymer | PO | Mice, DSS acute colitis | Protein, mRNA | IFN-γ, IL-1, IL-6, IL-12 | Body weight, MPO | H&E staining | [139] |
| GalC-siRNA | Galactosylated chitosan-coated nanoparticle containing siRNA against TNF-α loaded on PLGA | PO | Mice, DSS acute colitis | Protein, mRNA | IFN-γ, IL-6 | Body weight, colon length, DAI, MPO | H&E staining | [140] |
| NiMOS-siRNA | Nanoparticle in microsphere containing siRNA against TNF-α | PO | Mice, DSS acute colitis | Protein, mRNA | GM-CSF, IFN-γ, IL-1β, IL-2, IL-5, IL-6, IL-12p70, MCP-1, MIP-1α | Body weight, colon length, MPO | H&E staining | [141] |
| CA-siRNA | Colon-targeted nanoparticle containing siRNA against TNF-α encapsulated in chitosan–alginate | PO | Mice, LPS-induced acute inflammation | Protein | – | – | – | [142] |
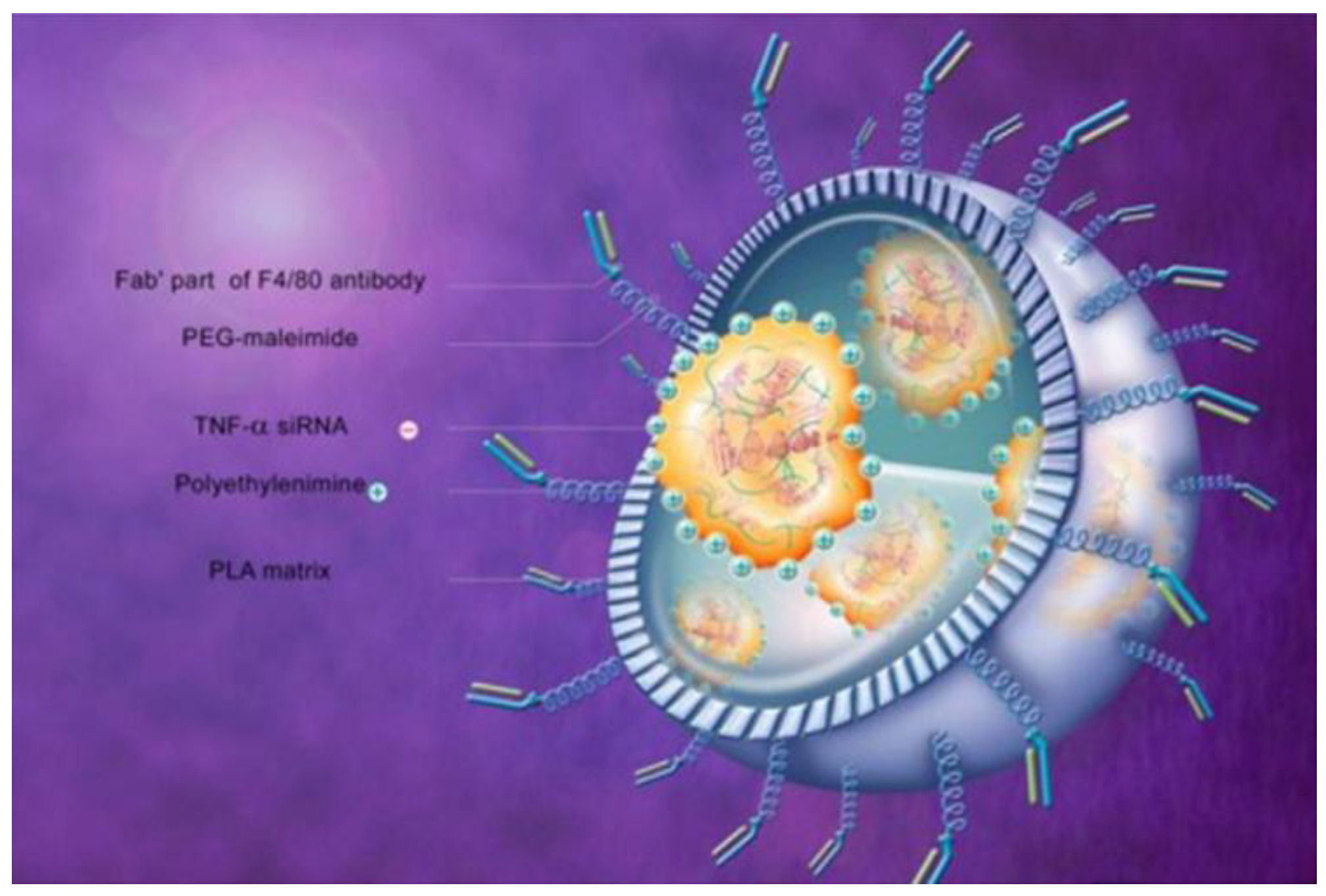
| CA-Fab’-siRNA | Colon-targeted nanoparticle containing siRNA against TNF-α bearing Fab’ of F4/80 antibody encapsulated in chitosan–alginate | PO | Mice, DSS acute colitis | – | – | Body weight, IκB-α, MPO | Ly6 g staining | [143] |
| NiMOS-siRNA-CyD1 | Nanoparticle in microsphere containing siRNA against TNF-α and CyD1 | PO | Mice, DSS acute colitis | Protein, mRNA | CyD1, GM-CSF, IFN-γ, IL-1α, IL-1β, IL-2, IL-5, IL-6, IL-17, MCP-1, MIP-1α | Body weight, colon length, MPO | H&E staining | [144] |
| Gal-siRNA-IL-22 | Nanoparticle containing IL-22 and siRNA against TNF-α in galactosylated PLGA encapsulated chitosan–alginate hydrogel | PO | Mice, DSS acute colitis | mRNA | –- | Body weight, colon length, MPO, spleen weight | H&E staining, histopathology score | [145] |
| Prokaryotes | ||||||||
| Lacto-scFv | Lactococcus lactis carrying eukaryotic vector coding for a scFv anti-TNF-α | PO | Mice, DSS acute colitis | mRNA | IL-1β, IL-6, IL-10, IL-17A, TGF-β | Body weight, colon length, CRP, DAI | H&E staining, histopathology score | [146] |
| Lacto-Nanobody | Lactococcus lactis secreting bivalent nanobodies against TNF-α | PO | Mice, DSS chronic colitis | – | – | - | H&E staining, histopathology score | [147] |
| PO | Mice, IL-10−/−, chronic colitis | – | – | MPO | H&E staining, histopathology score | |||
| Eukaryotes | ||||||||
| PRX-106 | Plant-cell expressed anti-TNF-α fusion protein consisting of sTNFR2 fused to human Fc of human IgG1 | PO | Mice, TNBS acute colitis | – | – | Body weight | H&E staining, histopathology score, IκB-α pSer32/Ser36 staining | [148] |
| Disease | Drug | ROA | Dosage | Therapy | Follow-up | Response a | Remarks | Reference |
|---|---|---|---|---|---|---|---|---|
| CD, perianal fistulas (n = 9) | IFX | Local injection | 20 mg | 0, 1, 3 weeks | 6 months | 78% | ADA did not develop during follow-up | [169] |
| CD, perianal fistulas (n = 15) | IFX | Local injection | 15–21 mg | 0, 4, 8, 12, 16, 20 weeks | 18.2 (3–30) months b | 67% | Combined with surgical treatment; included nonresponders to IV IFX and patients with contraindication for IFX | [171] |
| CD, perianal fistulas (n = 11) | IFX | Local injection | 20 mg | Every 4–16 weeks | 10.5 (7–18) months b | 73% | Including patients not responding to conventional systemic therapy | [170] |
| CD, perianal fistulas (n = 12) | IFX | Local injection | 20–25 mg | Every 4–6 weeks | 35 (19–43) months c | 88% | Combined with surgical treatment; included nonresponders to IV IFX | [172] |
| CD, perianal fistulas (n = 16) | adalimumab | Local injection | 40 mg | Every 15 days | NS | NS, but response was observed | Combined with surgical treatment and including patient who did not respond to local IFX therapy | [173] |
| CD, perianal fistulas (n = 33) | adalimumab | Local injection | 40 mg | Every 15 days | 11 (7–14) months c | 40% | Combined with surgical treatment | [174] |
| CD, perianal fistulas (n = 12) | adalimumab | Local injection | 20 mg | Every 2 weeks | 17.5 (5–30) months b | 100% | Including surgical therapy | [175] |
| CD, perianal fistulas (n = 9) | adalimumab | Local injection | 10 mg | Every 2 weeks | NS | 100% | Investigated different T cell phenotypes in peripheral blood and fistulas | [176] |
| CD, postoperative localized recurrent (n = 8) | IFX | Local injection | 8–60 mg | variable | 20 (14–21) months c | 38% | Endoscopy-guided injections into localized regions of <5 cm | [177] |
| CD, isolated symptomatic regions (n = 4) | IFX | Local injection | 20–30 mg | variable | NS | 75% | Endoscopy-guided injections into local regions | [178] |
| CD, colonic strictures (n = 3) | IFX | Local injection | 90–120 mg | variable | 5–8 months | 100% | Manual dilation in 1 patient | [179] |
| CD, rectal stenosis (n = 2) | IFX | Local injection | 25 mg | variable | NS | 100% | Combined with balloon dilation | [180] |
| CD, small bowel strictures (n = 6) | IFX | Local injection | 40 mg | 0, 2, 6 weeks | 6 months | 100% | Combined with balloon dilation | [181] |
| UC, refractory proctitis (n = 1) | IFX | Enema | 100 mg | 6 days | NS | 100% | Patient with subtotal colectomy and ileorectal anastomosis | [182] |
| Healthy volunteers (n = 14) | PRX-106 | PO | 2–16 mg | 5 days | 10 days | NA | PRX-106 was not systemically absorbed and no clear in vivo effects were seen | [167] |
| UC, colonic involvement (n = 37) | AVX-470, capsule | PO | 0.2–3.5 g | 4 weeks | 7 weeks | 14% d | Colonic biopsies were analyzed in a separate study [107] | [108] |
| CD (n = 6) | V565, enteric coated tablet | PO | 555–1665 mg | Single dose | No follow-up | NA | PK study | [104] |
| UC (n = 5) | 1110 mg | 7 days | Tissue penetration study | |||||
| Non–CD Ileostomy (n = 4) | 1665 mg | Single dose | Ileal fluid recovery study |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gareb, B.; Otten, A.T.; Frijlink, H.W.; Dijkstra, G.; Kosterink, J.G.W. Review: Local Tumor Necrosis Factor-α Inhibition in Inflammatory Bowel Disease. Pharmaceutics 2020, 12, 539. https://doi.org/10.3390/pharmaceutics12060539
Gareb B, Otten AT, Frijlink HW, Dijkstra G, Kosterink JGW. Review: Local Tumor Necrosis Factor-α Inhibition in Inflammatory Bowel Disease. Pharmaceutics. 2020; 12(6):539. https://doi.org/10.3390/pharmaceutics12060539
Chicago/Turabian StyleGareb, Bahez, Antonius T. Otten, Henderik W. Frijlink, Gerard Dijkstra, and Jos G. W. Kosterink. 2020. "Review: Local Tumor Necrosis Factor-α Inhibition in Inflammatory Bowel Disease" Pharmaceutics 12, no. 6: 539. https://doi.org/10.3390/pharmaceutics12060539
APA StyleGareb, B., Otten, A. T., Frijlink, H. W., Dijkstra, G., & Kosterink, J. G. W. (2020). Review: Local Tumor Necrosis Factor-α Inhibition in Inflammatory Bowel Disease. Pharmaceutics, 12(6), 539. https://doi.org/10.3390/pharmaceutics12060539







