Development of a DNA Vaccine for Melanoma Metastasis by Inhalation Based on an Analysis of Transgene Expression Characteristics of Naked pDNA and a Ternary Complex in Mouse Lung Tissues
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cells
2.3. Animals
2.4. Gene Expression after the Pulmonary Administration of pDNA
2.5. Preparation and Observation of Lung Sections
2.6. Tissue Clearing Method
2.7. Vaccination and Inhibition of Lung Metastasis
2.8. In Vivo Imaging
2.9. Isolation of Splenic Cells in Mice
2.10. Evaluation of Antigen-Specific Cytokine Secretion
2.11. Statistical Analysis
3. Results
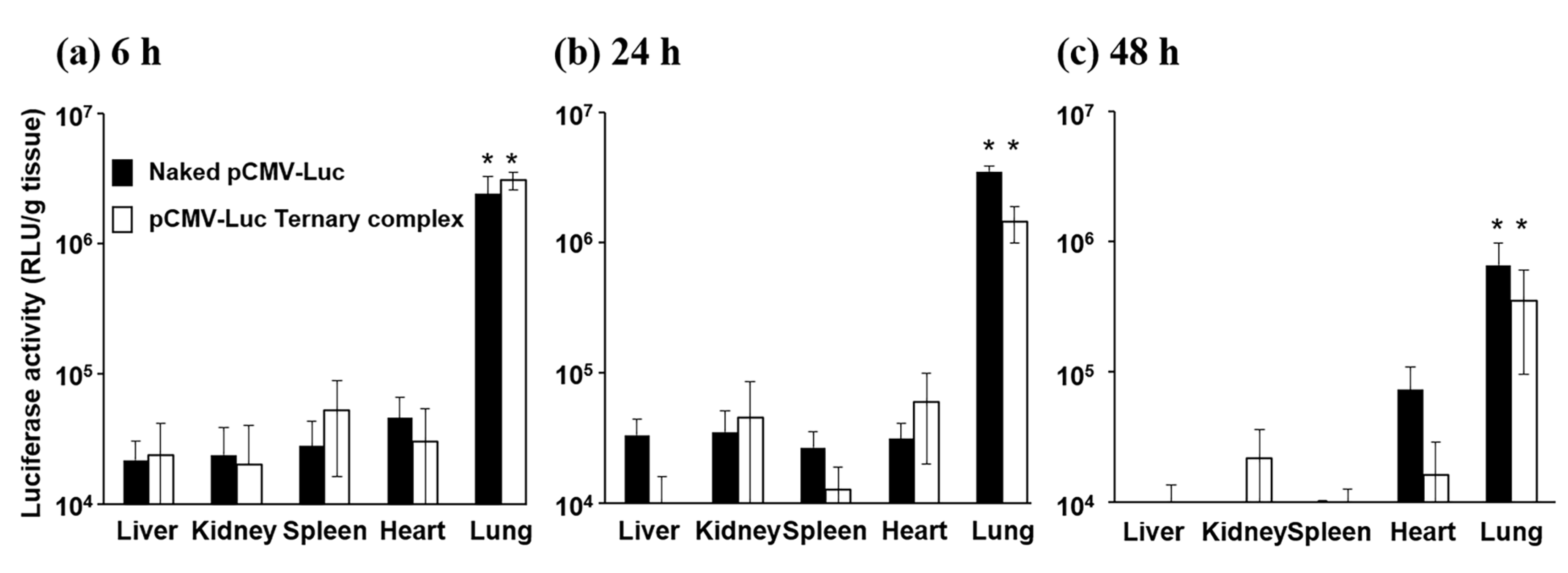
3.1. Gene Expression after the Pulmonary Administration of Naked pDNA and the Ternary Complex
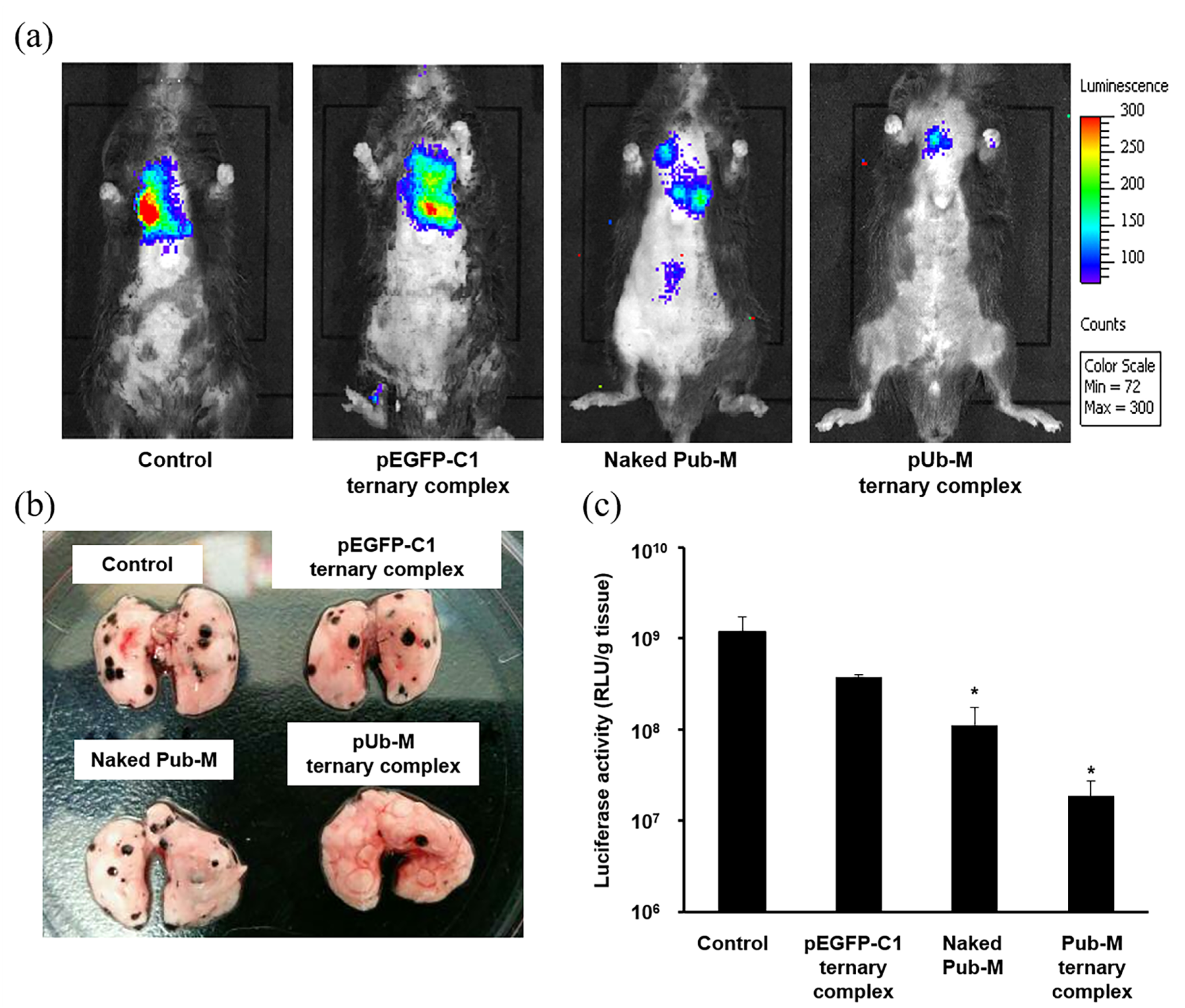
3.2. Spatial Distribution of Naked pDNA and the Ternary Complex
3.3. Suppressive Effects on Melanoma Metastasis after the Pulmonary Administration of Naked pDNA and the Ternary Complex
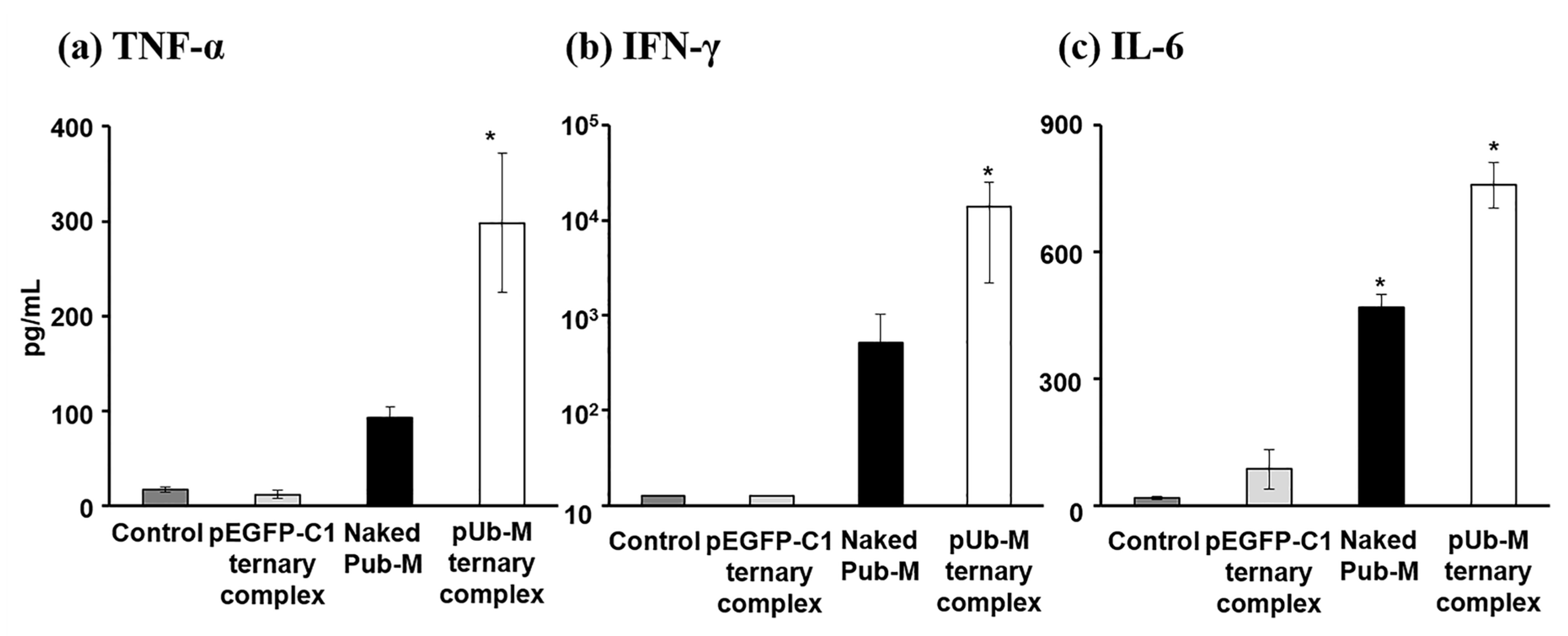
3.4. Antigen-Stimulatory Cytokine Secretion from Splenic Cells after the Pulmonary Administration of Naked pDNA and the Ternary Complex
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Patel, J.K.; Didolkar, M.S.; Pickren, J.W.; Moore, R.H. Metastatic pattern of malignant melanoma. A study of 216 autopsy cases. Am. J. Surg. 1978, 135, 807–810. [Google Scholar] [CrossRef]
- Leong, S.P.; Cady, B.; Jablons, D.M.; Garcia-Aguilar, J.; Reintgen, D.; Jakub, J.; Pendas, S.; Duhaime, L.; Cassell, R.; Gardner, M.; et al. Clinical patterns of metastasis. Cancer Metastasis Rev. 2006, 25, 221–232. [Google Scholar] [CrossRef]
- Mocellin, S.; Hoon, D.; Ambrosi, A.; Nitti, D.; Rossi, C.R. The prognostic value of circulating tumor cells in patients with melanoma: A systematic review and meta-analysis. Clin. Cancer Res. 2006, 12, 4605–4613. [Google Scholar] [CrossRef]
- Francken, A.B.; Accortt, N.A.; Shaw, H.M.; Wiener, M.; Soong, S.J.; Hoekstra, H.J.; Thompson, J.F. Prognosis and determinants of outcome following locoregional or distant recurrence in patients with cutaneous melanoma. Ann. Surg. Oncol. 2008, 15, 1476–1484. [Google Scholar] [CrossRef] [PubMed]
- Quintana, E.; Shackleton, M.; Foster, H.R.; Fullen, D.R.; Sabel, M.S.; Morrison, S.J. Phenotypic heterogeneity among tumorigenic melanoma cells from patients that is reversible and not hierarchically organized. Cancer Cell 2010, 18, 510–523. [Google Scholar] [CrossRef]
- Romano, S.; Staibano, S.; Greco, A.; Brunetti, A.; Nappo, G.; Llardi, G.; Martinelli, R.; Sorrentino, A.; Di Pace, A.; Mascolo, M.; et al. FK506 binding protein 51 positively regulates melanoma stemness and metastatic potential. Cell Death Dis. 2013, 4, e578. [Google Scholar] [CrossRef] [PubMed]
- Parmiani, G. Melanoma antigens and their recognition by T cells. Keio J. Med. 2001, 50, 86–90. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hodi, F.S. Well-defined melanoma antigens as progression markers for melanoma: Insights into differential expression and host response based on stage. Clin. Cancer Res. 2006, 12, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Plotkin, S.A. Vaccines: Past, present and future. Nat. Med. 2005, 11, S5. [Google Scholar] [CrossRef]
- Karch, C.P.; Burkhard, P. Vaccine technologies: From whole organisms to rationally designed protein assemblies. Biochem. Pharmacol. 2016, 120, 1–14. [Google Scholar] [CrossRef]
- Kutzler, M.A.; Weiner, D.B. DNA vaccines: Ready for prime time? Nat. Rev. Genet. 2008, 9, 776. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Hong, Y.; Chen, W.; Wang, C. Polymers for DNA vaccine delivery. ACS Biomater. Sci. Eng. 2016, 3, 108–125. [Google Scholar] [CrossRef]
- Hobernik, D.; Bros, M. DNA vaccines-how far from clinical use? Int. J. Mol. Sci. 2018, 19, 3605. [Google Scholar] [CrossRef] [PubMed]
- McCluskie, M.J.; Brazolot Millan, C.L.; Gramzinski, R.A.; Robinson, H.L.; Santoro, J.C.; Fuller, J.T.; Widera, G.; Haynes, J.R.; Purcell, R.H.; Davis, H.L. Route and method of delivery of DNA vaccine influence immune responses in mice and non-human primates. Mol. Med. 1999, 5, 287–300. [Google Scholar] [CrossRef] [PubMed]
- Lombry, C.; Marteleur, A.; Arras, M.; Lison, D.; Louahed, J.; Renauld, J.C.; Préat, V.; Vanbever, R. Local and systemic immune responses to intratracheal instillation of antigen and DNA vaccines in mice. Pharm. Res. 2004, 21, 127–135. [Google Scholar] [CrossRef]
- Hiremath, G.S.; Omer, S.B. A meta-analysis of studies comparing the respiratory route with the subcutaneous route of measles vaccine administration. Hum. Vaccin. 2005, 1, 30–36. [Google Scholar] [CrossRef]
- Stylianou, E.; Paul, M.J.; Reljic, R.; McShane, H. Mucosal delivery of tuberculosis vaccines: A review of current approaches and challenges. Expert Rev. Vaccines 2019, 18, 1271–1284. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, K.; Yonezawa, K.; Fumoto, S.; Miura, Y.; Hagimori, M.; Nishida, K.; Kawakami, S. Application of direct sonoporation from a defined surface area of the peritoneum: Evaluation of transfection characteristics in mice. Pharmaceutics 2019, 11, 244. [Google Scholar] [CrossRef]
- Ogawa, K.; Fuchigami, Y.; Hagimori, M.; Fumoto, S.; Maruyama, K.; Kawakami, S. Ultrasound-responsive nanobubble-mediated gene transfection in the cerebroventricular region by intracerebroventricular administration in mice. Eur. J. Pharm. Biopharm. 2019, 137, 1–8. [Google Scholar] [CrossRef]
- Suga, T.; Kato, N.; Hagimori, M.; Fuchigami, Y.; Kuroda, N.; Kodama, Y.; Sasaki, H.; Kawakami, S. Development of high-functionality and quality lipids with RGD peptide ligands: Application for PEGylated liposomes and analysis of intratumoral distribution in a murine colon cancer model. Mol. Pharm. 2018, 15, 4481–4490. [Google Scholar] [CrossRef]
- Oyama, N.; Takahashi, H.; Kawaguchi, M.; Miyamoto, H.; Nishida, K.; Tsurumaru, M.; Nakashima, M.; Yamashita, F.; Hashida, M.; Kawakami, S. Effects of tissue pressure on transgene expression characteristics via renal local administration routes from ureter or renal artery in the rat kidney. Pharmaceutics 2020, 12, 114. [Google Scholar] [CrossRef]
- Kurosaki, T.; Kitahara, T.; Nakamura, T.; Nishida, K.; Fumoto, S.; Kodama, Y.; Nakagawa, H.; Higuchi, N.; Sasaki, H. Development of effective cancer vaccine using targeting system of antigen protein to APCs. Pharm. Res. 2012, 29, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Kawakami, S.; Yamashita, F.; Hashida, M. Development of an antigen-presenting cell-targeted DNA vaccine against melanoma by mannosylated liposomes. Biomaterials 2007, 28, 3255–3262. [Google Scholar] [CrossRef] [PubMed]
- Kurosaki, T.; Kodama, Y.; Muro, T.; Higuchi, N.; Nakamura, T.; Kitahara, T.; Miyakoda, M.; Yui, K.; Sasaki, H. Secure splenic delivery of plasmid DNA and its application to DNA vaccine. Biol. Pharm. Bull. 2013, 36, 1800–1806. [Google Scholar] [CrossRef] [PubMed]
- Kodama, Y.; Noda, R.; Sato, K.; Harasawa, H.; Kurosaki, T.; Nakagawa, H.; Nakamura, T.; Kitahara, T.; Muro, T.; Sasaki, H. Methotrexate-coated complexes of plasmid dna and polyethylenimine for gene delivery. Biol. Pharm. Bull. 2018, 41, 1537–1542. [Google Scholar] [CrossRef] [PubMed]
- Xiang, R.; Lode, H.N.; Chao, T.H.; Ruehlmann, J.M.; Dolman, C.S.; Rodriguez, F.; Whitton, J.L.; Overwijk, W.W.; Restifo, N.P.; Reisfeld, R.A. An autologous oral DNA vaccine protects against murine melanoma. Proc. Natl. Acad. Sci. USA. 2000, 97, 5492–5497. [Google Scholar] [CrossRef]
- Kodama, Y.; Nakamura, T.; Kurosaki, T.; Egashira, K.; Mine, T.; Nakagawa, H.; Muro, T.; Kitahara, T.; Higuchi, N.; Sasaki, H. Biodegradable nanoparticles composed of dendrigraft poly-L-lysine for gene delivery. Eur. J. Pharm. Biopharm. 2014, 87, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Horiguchi, M.; Hirokawa, M.; Abe, K.; Kumagai, H.; Yamashita, C. Pulmonary administration of 1,25-dihydroxyvitamin D3 to the lungs induces alveolar regeneration in a mouse model of chronic obstructive pulmonary disease. J. Control. Release 2016, 233, 191–197. [Google Scholar] [CrossRef]
- Hama, H.; Hioki, H.; Namiki, K.; Hoshida, T.; Kurokawa, H.; Ishidate, F.; Kaneko, T.; Akagi, T.; Saito, T.; Saido, T.; et al. ScaleS: An optical clearing palette for biological imaging. Nat. Neurosci. 2015, 18, 1518–1529. [Google Scholar] [CrossRef]
- Forde, G.M. Rapid-response vaccines-does DNA offer a solution? Nat. Biotechnol. 2005, 23, 1059–1062. [Google Scholar] [CrossRef]
- Birchall, J. Pulmonary delivery of nucleic acids. Expert Opin. Drug Deliv. 2007, 4, 575–578. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Okuda, T.; Takashima, Y.; Okamoto, H. Naked pDNA inhalation powder composed of hyaluronic acid exhibits high gene expression in the lungs. Mol. Pharm. 2019, 16, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Takeshita, F.; Mizutani, T.; Ohgi, T.; Kuwano, K.; Ochiya, T. A novel platform to enable inhaled naked RNAi medicine for lung cancer. Sci. Rep. 2013, 3, 3325. [Google Scholar] [CrossRef]
- Suarez, S.; Hickey, A.J. Drug properties affecting aerosol behavior. Respir. Care 2000, 45, 652–666. [Google Scholar] [PubMed]
- Labiris, N.R.; Dolovich, M.B. Pulmonary drug delivery. Part I: Physiological factors affecting therapeutic effectiveness of aerosolized medications. Br. J. Clin. Pharmacol. 2003, 56, 588–599. [Google Scholar] [CrossRef] [PubMed]
- Martens, J.H.; Kzhyshkowska, J.; Falkowski-Hansen, M.; Schledzewski, K.; Gratchev, A.; Mansmann, U.; Schmuttermaier, C.; Dippel, E.; Koenen, W.; Riedel, F.; et al. Differential expression of a gene signature for scavenger/lectin receptors by endothelial cells and macrophages regional metastasis. J. Pathol. 2006, 208, 574–589. [Google Scholar] [CrossRef] [PubMed]
- McCourt, P.A.; Smedsrød, B.H.; Melkko, J.; Johansson, S. Characterization of a hyaluronan receptor on rat sinusoidal liver endothelial cells and its functional relationship to scavenger receptors. Hapatology 1999, 30, 1276–1286. [Google Scholar] [CrossRef]
- Lee, W.; Lee, S.H.; Ahn, D.G.; Cho, H.; Sung, M.H.; Han, S.H.; Oh, J.W. The antiviral activity of poly-γ-glutamic acid, a polypeptide secreted by Bacillus sp., through induction of CD14-dependent type I interferon responses. Biomaterials 2013, 34, 9700–9708. [Google Scholar] [CrossRef]
- Bielinska, A.U.; Makidon, P.E.; Janczak, K.W.; Blanco, L.P.; Swanson, B.; Smith, D.M.; Pham, T.; Szabo, Z.; Kukowska-Latallo, J.F.; Baker, J.R., Jr. Distinct pathways of humoral and cellular immunity induced with the mucosal administration of a nanoemulsion adjuvant. J. Immunol. 2014, 192, 2722–2733. [Google Scholar] [CrossRef]
- Uto, T.; Akagi, T.; Yoshinaga, K.; Toyama, M.; Akashi, M.; Baba, M. The induction of innate and adaptive immunity by biodegradable poly (gamma-glutamic acid) nanoparticles via a TLR4 and MyD88 signaling pathway. Biomaterials 2011, 32, 5206–5212. [Google Scholar] [CrossRef]
- Hemmi, H.; Takeuchi, O.; Kawai, T.; Kaisho, T.; Sato, S.; Sanjo, H.; Matsumoto, M.; Hoshino, K.; Wagner, H.; Takeda, K.; et al. A toll-like receptor recognizes bacterial DNA. Nature 2000, 408, 740–745. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, S.; Griesenbach, U.; Geddes, D.M.; Alton, E. Immunological hurdles to lung gene therapy. Clin. Exp. Immunol. 2003, 132, 1–8. [Google Scholar] [CrossRef]
- Sun, Y.; Guo, F.; Zou, Z.; Li, C.; Hong, X.; Zhao, Y.; Wang, C.; Wang, H.; Liu, H.; Yang, P.; et al. Cationic nanoparticles directly bind angiotensin-converting enzyme 2 and induce acute lung injury in mice. Part. Fibre Toxicol. 2015, 12, 4. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Takano, H.; Yanagisawa, R.; Hirano, S.; Kobayashi, T.; Fujitani, Y.; Shimada, A.; Yoshikawa, T. Effects of inhaled nanoparticles on acute lung injury induced by lipopolysaccharide in mice. Toxicology 2007, 238, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Liu, H.; Sun, Y.; Wang, H.; Guo, F.; Rao, S.; Deng, J.; Zhang, Y.; Miao, Y.; Guo, C.; et al. PAMAM nanoparticles promote acute lung injury by inducing autophagic cell death through the Akt-TSC2-mTOR signaling pathway. J. Mol. Cell. Biol. 2009, 1, 37–45. [Google Scholar] [CrossRef]
- Adamcakova-Dodd, A.; Stebounova, L.V.; Kim, J.S.; Vorrink, S.U.; Ault, A.P.; O’Shaughnessy, P.T.; Grassian, V.H.; Thorne, P.S. Toxicity assessment of zinc oxide nanoparticles using sub-acute and sub-chronic murine inhalation models. Part. Fibre Toxicol. 2014, 11, 15. [Google Scholar] [CrossRef]
- Dredge, K.; Marriott, J.B.; Todrysk, S.M.; Dalgleish, A.G. Adjuvants and the promotion of Th1-type cytokines in tumour immunotherapy. Cancer Immunol. 2002, 51, 521–531. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kodama, Y.; Nakashima, M.; Nagahara, T.; Oyama, N.; Hashizume, J.; Nakagawa, H.; Harasawa, H.; Muro, T.; Kurosaki, T.; Yamashita, C.; et al. Development of a DNA Vaccine for Melanoma Metastasis by Inhalation Based on an Analysis of Transgene Expression Characteristics of Naked pDNA and a Ternary Complex in Mouse Lung Tissues. Pharmaceutics 2020, 12, 540. https://doi.org/10.3390/pharmaceutics12060540
Kodama Y, Nakashima M, Nagahara T, Oyama N, Hashizume J, Nakagawa H, Harasawa H, Muro T, Kurosaki T, Yamashita C, et al. Development of a DNA Vaccine for Melanoma Metastasis by Inhalation Based on an Analysis of Transgene Expression Characteristics of Naked pDNA and a Ternary Complex in Mouse Lung Tissues. Pharmaceutics. 2020; 12(6):540. https://doi.org/10.3390/pharmaceutics12060540
Chicago/Turabian StyleKodama, Yukinobu, Mikiro Nakashima, Tadayuki Nagahara, Natsuko Oyama, Junya Hashizume, Hiroo Nakagawa, Hitomi Harasawa, Takahiro Muro, Tomoaki Kurosaki, Chikamasa Yamashita, and et al. 2020. "Development of a DNA Vaccine for Melanoma Metastasis by Inhalation Based on an Analysis of Transgene Expression Characteristics of Naked pDNA and a Ternary Complex in Mouse Lung Tissues" Pharmaceutics 12, no. 6: 540. https://doi.org/10.3390/pharmaceutics12060540
APA StyleKodama, Y., Nakashima, M., Nagahara, T., Oyama, N., Hashizume, J., Nakagawa, H., Harasawa, H., Muro, T., Kurosaki, T., Yamashita, C., Hashida, M., Kitahara, T., Sasaki, H., Kawakami, S., & Nakamura, T. (2020). Development of a DNA Vaccine for Melanoma Metastasis by Inhalation Based on an Analysis of Transgene Expression Characteristics of Naked pDNA and a Ternary Complex in Mouse Lung Tissues. Pharmaceutics, 12(6), 540. https://doi.org/10.3390/pharmaceutics12060540




