Fluconazole and Lipopeptide Surfactin Interplay During Candida albicans Plasma Membrane and Cell Wall Remodeling Increases Fungal Immune System Exposure
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Growth Conditions
2.2. Determination of Azole-SU Synergy, Percentage of Viability and Fractional Inhibitory Concentration Indexes
2.3. Plasma Membrane (PM) Permeabilization
2.4. Scanning Electron Microscope (SEM) Observations
2.5. Cell Wall Staining and Microscopic Observations
2.6. Fluorescence-Activated Cell Sorting (FACS) Analyses
2.7. RNA Preparation, Reverse Transcription and Quantitative Polymerase Chain Reaction (PCR)
2.8. Computational Methodology
2.9. Statistical Analysis
3. Results
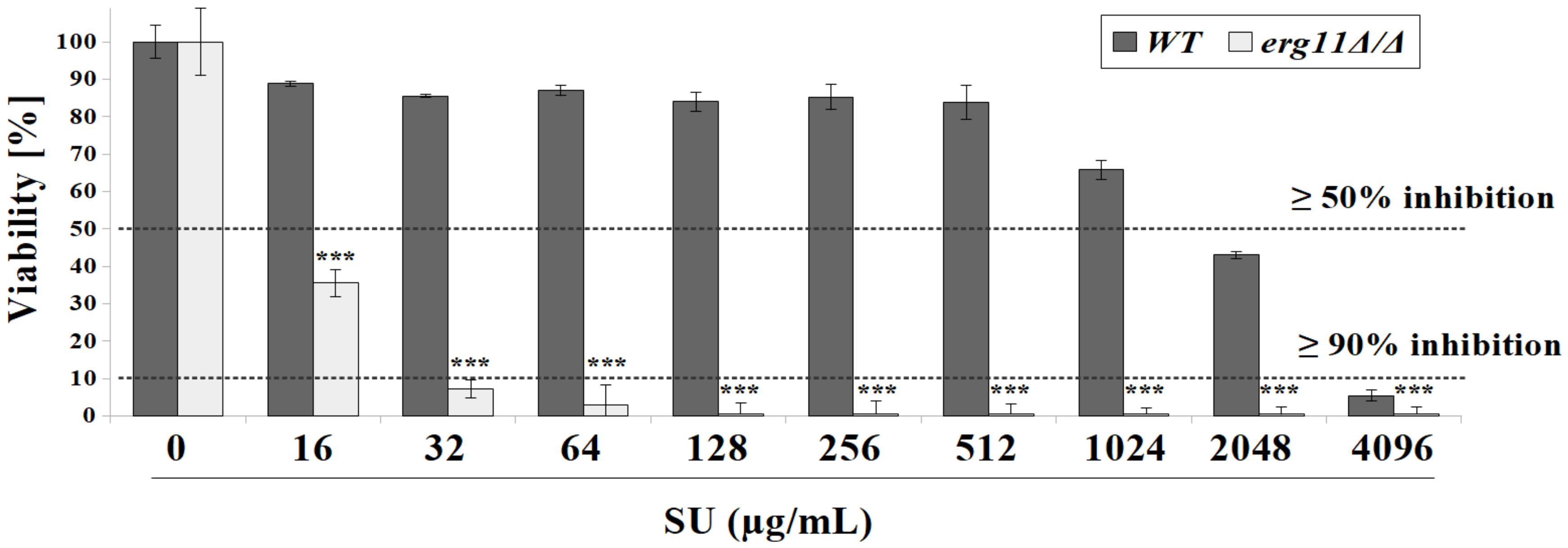
3.1. C. albicans Strain Deficient in Erg11p Are More Susceptible to Surfactin Treatment
3.2. Surfactin Acts in Synergy with Azole Compounds to Inhibit C. albicans Viability
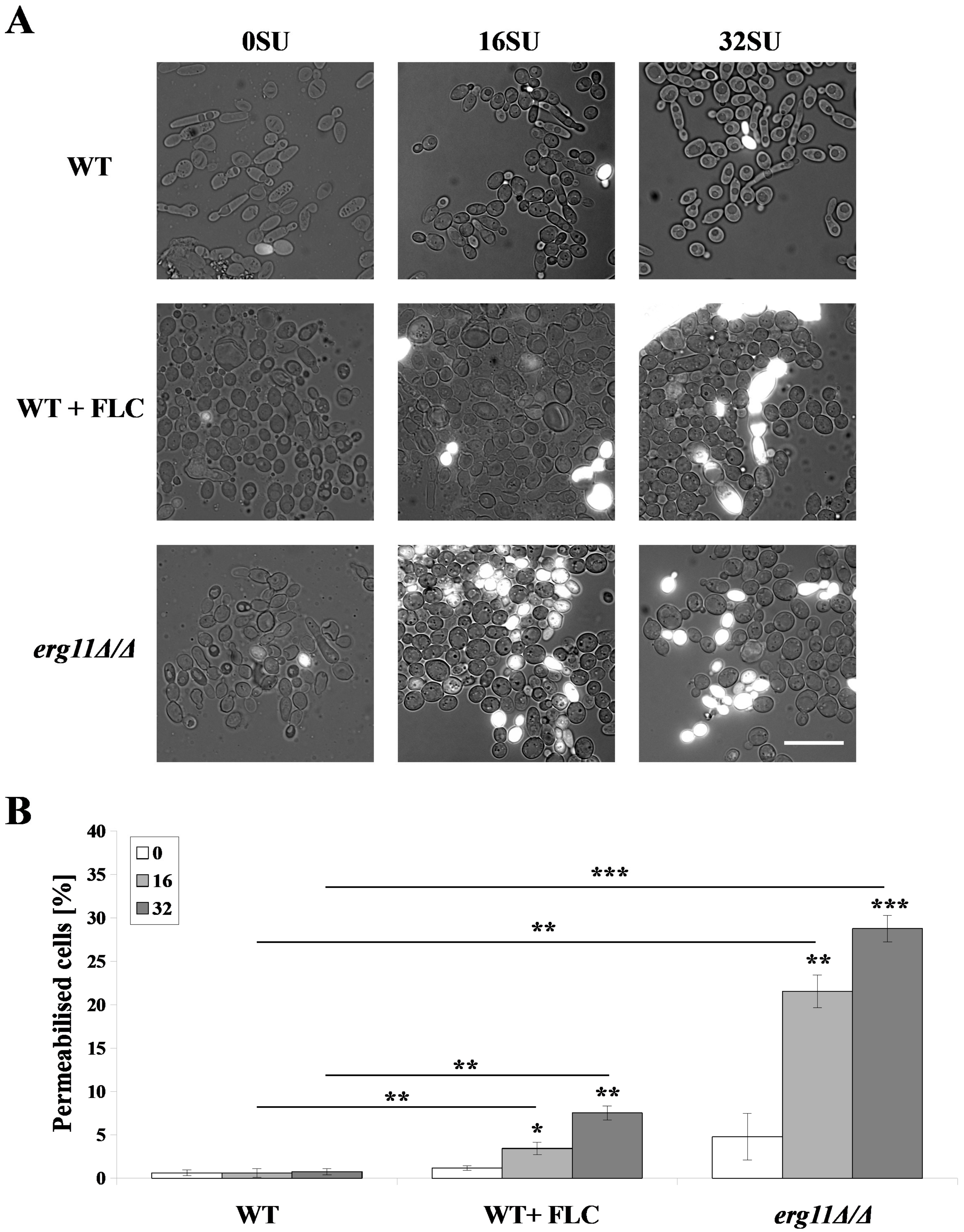
3.3. Surfactin Permeabilized the Plasma Membrane of C. albicans erg11Δ/Δ Mutant and C. albicans WT Strain after Treatment with Fluconazole
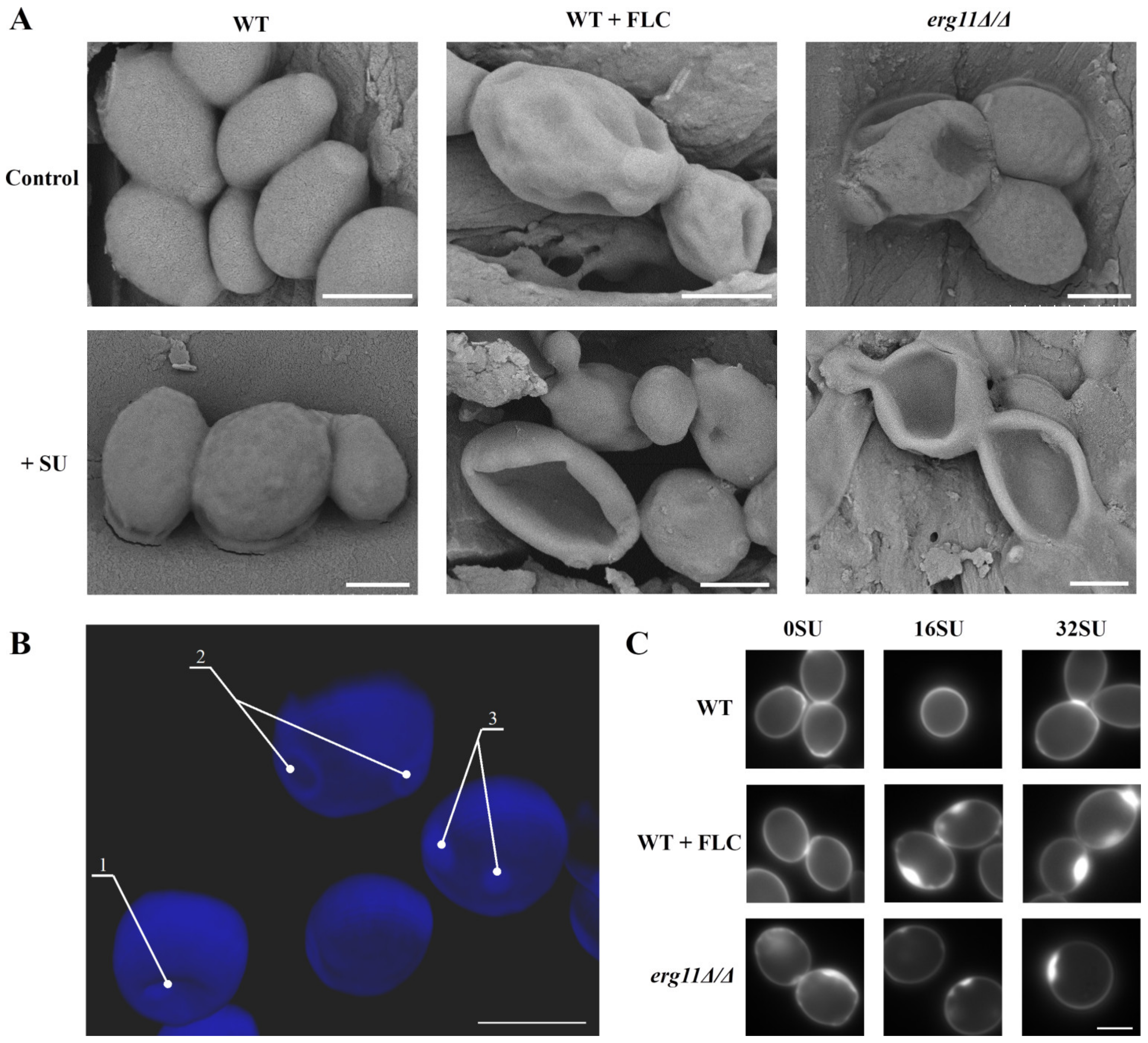
3.4. Ergosterol Depletion and Surfactin Treatment Cause Changes in Cell Shape and Local Accumulation of Chitin in the Cell Wall of C. albicans
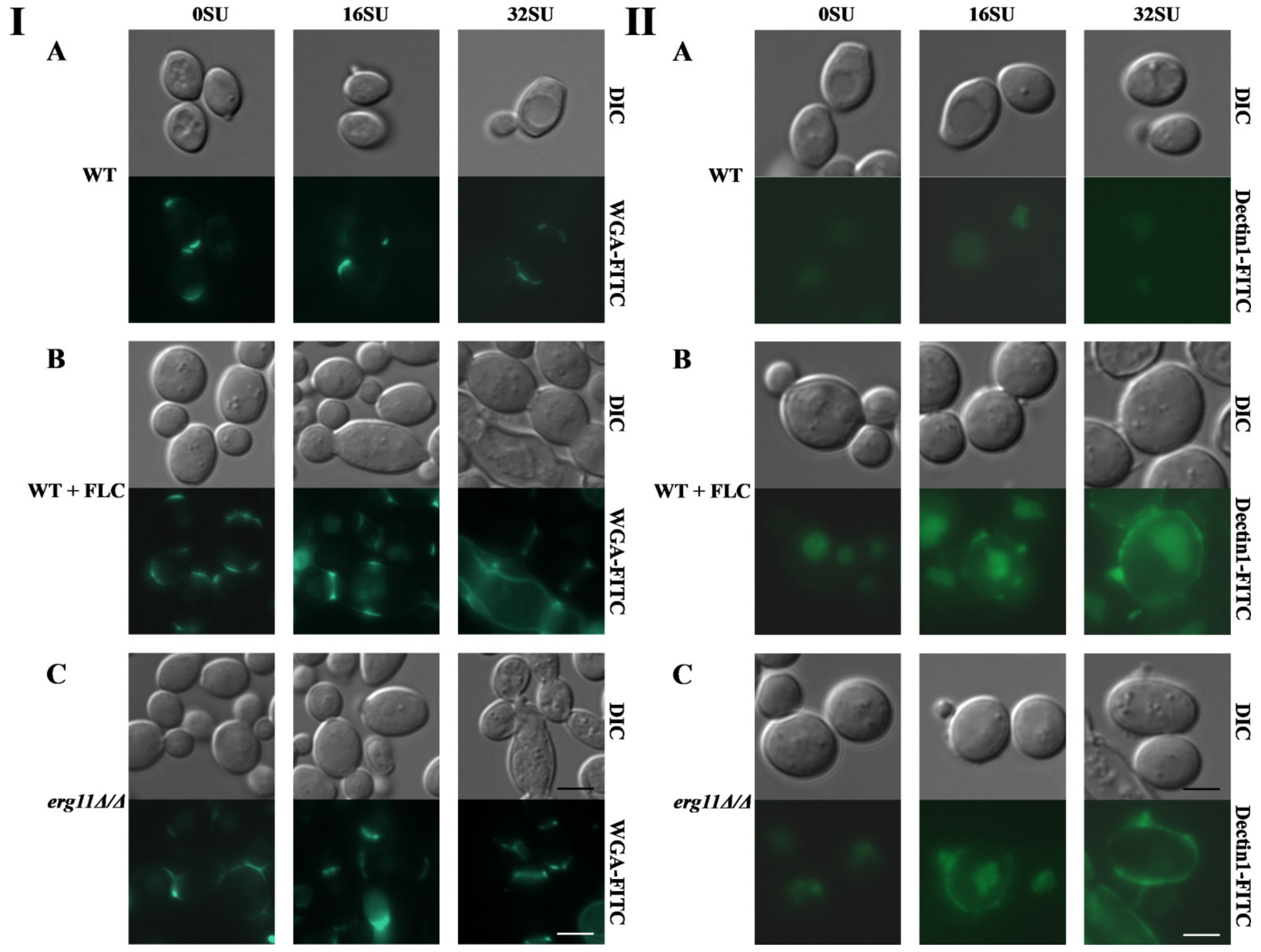
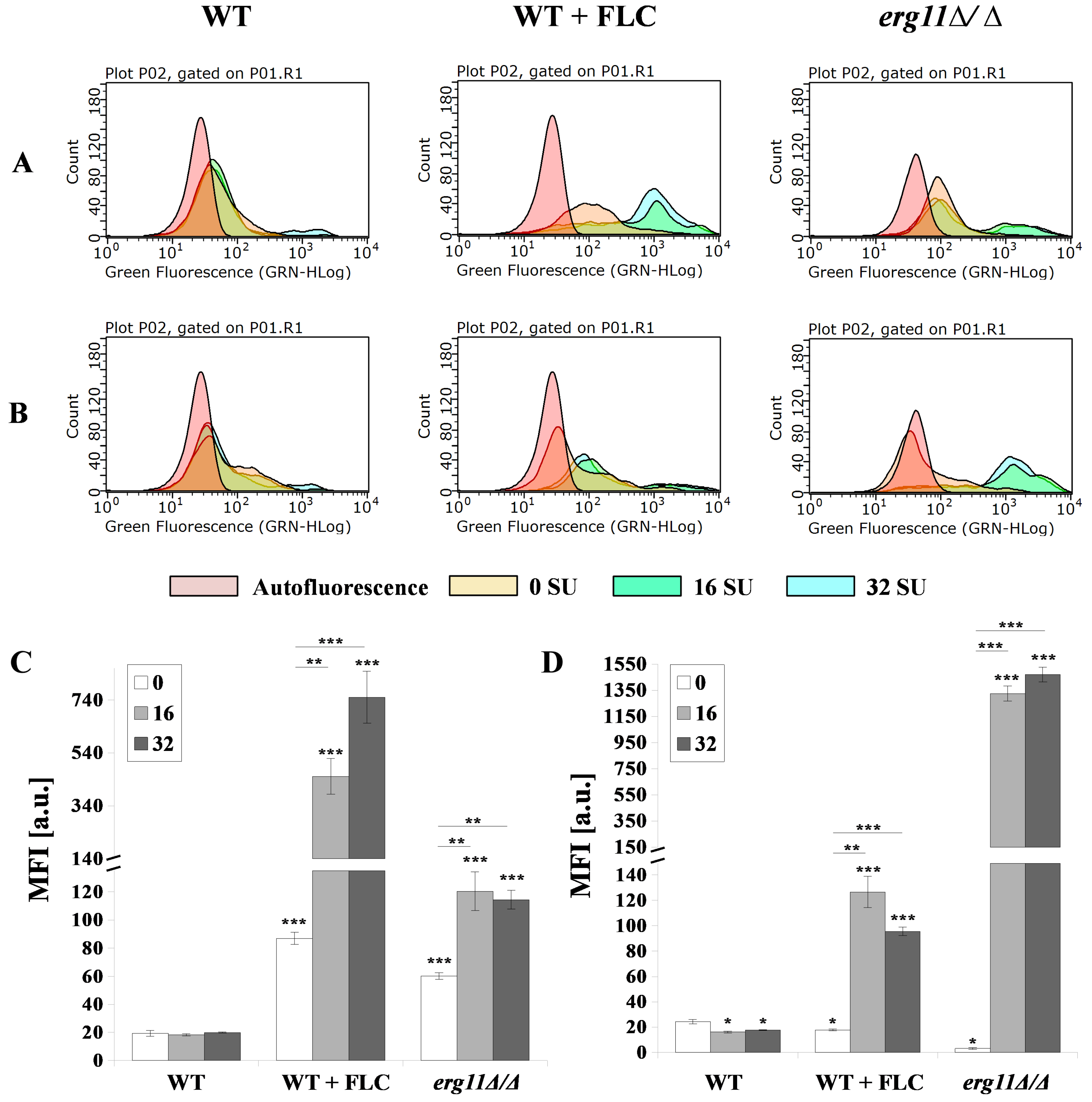
3.5. Surfactin and Fluconazole Cause Unmasking of Chitin and β-glucan in the Cell Wall of C. albicans
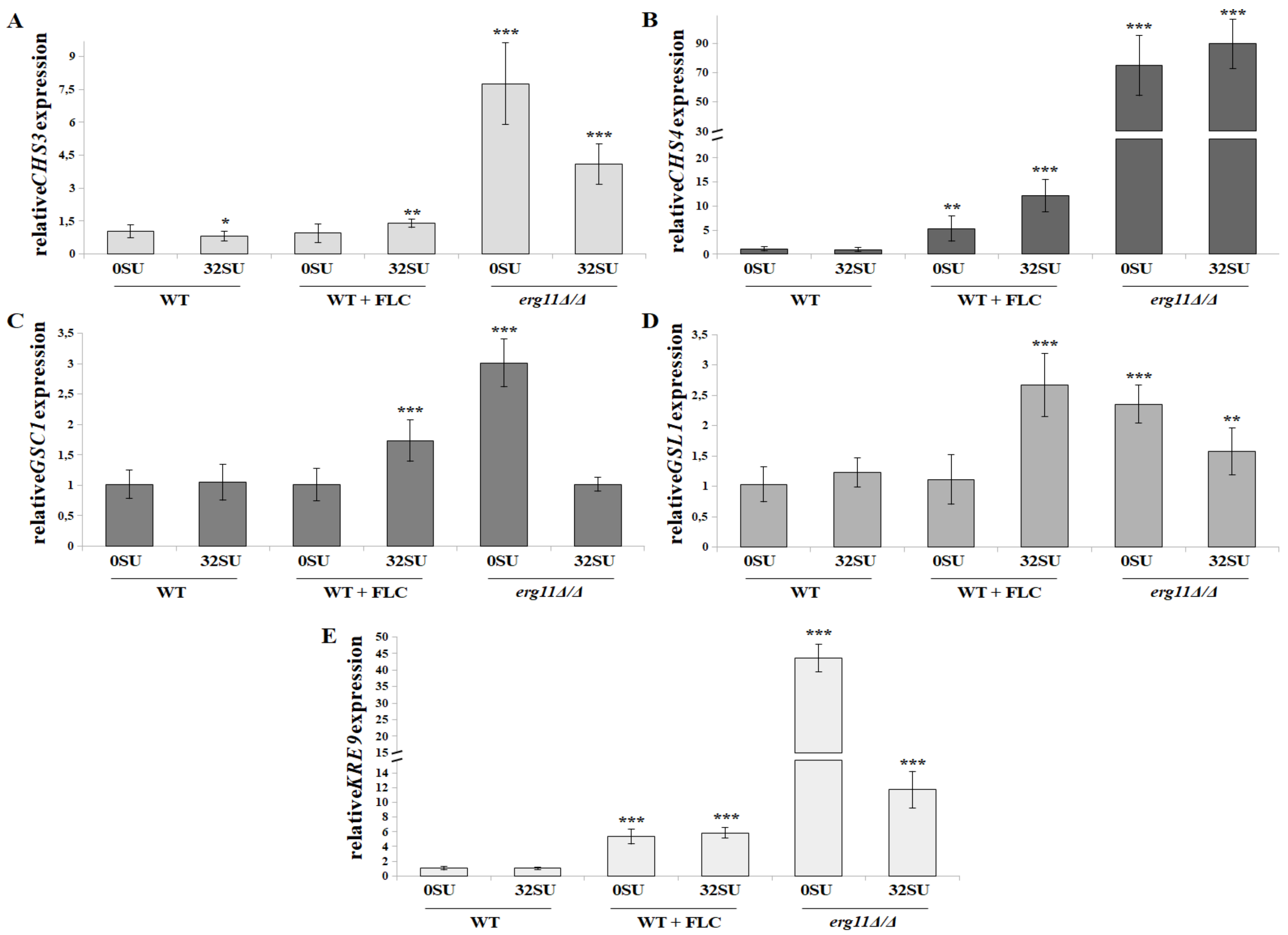
3.6. C. albicans erg11Δ/Δ Mutant Undergoes Increased Expression of Chitin and β-glucan Synthase Genes in Opposition to Surfactin Activity under these Conditions
3.7. Molecular Modeling Reveals that Surfactin Can Form Intermolecular Complexes with Chitin and β-glucan
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zaoutis, T.E.; Argon, J.; Chu, J.; Berlin, J.A.; Walsh, T.J.; Feudtner, C. The epidemiology and attributable outcomes of candidemia in adults and children hospitalized in the United States: A propensity analysis. Clin. Infect Dis. 2005, 41, 1232–1239. [Google Scholar] [CrossRef] [PubMed]
- Charlet, R.; Pruvost, Y.; Tumba, G.; Istel, F.; Poulain, D.; Kuchler, K.; Sendid, B.; Jawhara, S. Remodeling of the Candida glabrata cell wall in the gastrointestinal tract affects the gut microbiota and the immune response. Sci. Rep. 2018, 8, 3316. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Wagner, A.S.; Tams, R.N.; Eyer, J.E.; Kauffman, S.J.; Gann, E.R.; Fernandez, E.J.; Reynolds, T.B. Lrg1 regulates β (1,3)-glucan masking in Candida albicans through the Cek1 MAP kinase pathway. mBio 2019, 10, e01767-19. [Google Scholar] [CrossRef] [PubMed]
- Woolley, W. Some biological effects produced by benzimidazole and their reversal by purines. J. Biol. Chem. 1944, 152, 225–232. [Google Scholar]
- Gubbins, P.O.; Anaissie, J.E. Clinical Mycology, 2nd ed.; Churchill Livingstone: London, UK, 2009. [Google Scholar]
- Sanglard, D.; Ischer, F.; Parkinson, T.; Falconer, D.; Bille, J. Candida albicans mutations in the ergosterol biosynthetic pathway and resistance to several antifungal agents. Antimicrob. Agents Chemother. 2003, 47, 2404–2412. [Google Scholar] [CrossRef] [PubMed]
- Prasad, R.; Shah, A.H.; Rawal, M.K. Antifungals: Mechanism of action and drug resistance. Adv. Exp. Med. Biol. 2016, 892, 327–349. [Google Scholar] [CrossRef] [PubMed]
- Sanglard, D.; Coste, A.; Ferrari, S. Antifungal drug resistance mechanisms in fungal pathogens from the perspective of transcriptional gene regulation. FEMS Yeast Res. 2009, 9, 1029–1050. [Google Scholar] [CrossRef]
- Vandeputte, P.; Ferrari, S.; Coste, A.T. Antifungal resistance and new strategies to control fungal infections. Int. J. Microbiol. 2012, 2012, 713687. [Google Scholar] [CrossRef]
- Perlin, D.S. Current perspectives on echinocandin class drugs. Future Microbiol. 2011, 6, 441–457. [Google Scholar] [CrossRef]
- Shapiro, R.S.; Robbins, N.; Cowen, L.E. Regulatory circuitry governing fungal development, drug resistance, and disease. Microbiol. Mol. Biol. Rev. 2011, 75, 213–267. [Google Scholar] [CrossRef]
- Gow, N.A.; Hube, B. Importance of the Candida albicans cell wall during commensalism and infection. Curr. Opin. Microbiol. 2012, 15, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Gow, N.A.; van de Veerdonk, F.; Brown, A.; Netea, M.G. Candida albicans morphogenesis and host defence: Discriminating invasion from colonization. Nat. Rev. Microbiol. 2012, 10, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Cortés, J.C.G.; Curto, M.G.; Carvalho, V.S.D.; Pérez, P.; Ribas, J.C. The fungal cell wall as a target for the development of new antifungal therapies. Biotechnol. Adv. 2019, 37, 107352. [Google Scholar] [CrossRef] [PubMed]
- Biniarz, P.; Baranowska, G.; Feder-Kubis, J.; Krasowska, A. The lipopeptide pseudofactin II effectively decreased Candida albicans adhesion and hydrophobicity. Antonie van Leeuwenhoek 2015, 108, 343–353. [Google Scholar] [CrossRef][Green Version]
- Milewski, S.; Mignini, F.; Borowski, E. Synergistic action of nikkomycin X/Z with azole antifungals on Candida albicans. J. Gen. Microbiol. 1991, 137, 2155–2161. [Google Scholar] [CrossRef]
- Walker, L.A.; Munro, C.A.; de Brujin, I.; Lenardon, M.D.; McKinnon, A.; Gow, N.A. Stimulation of chitin synthesis rescues Candida albicans from echinocandins. PLoS Pathog. 2008, 4, e1000040. [Google Scholar] [CrossRef]
- McKenzie, C.G.J.; Koser, U.; Lewis, L.E.; Bain, J.M.; Mora-Montes, H.M.; Barker, R.N.; Gow, N.A.R.; Erwig, L.P. Contribution of Candida albicans cell wall components to recognition by and escape from murine macrophages. Infect. Immun. 2010, 78, 1650–1658. [Google Scholar] [CrossRef]
- Gow, N.A.R.; Netea, M.G.; Munro, C.A.; Ferwerda, G.; Bates, S.; Mora-Montes, H.M.; Walker, L.; Jansen, T.; Jacobs, L.; Tsoni, V.; et al. Immune recognition of Candida albicans beta-glucan by dectin-1. J. Infect. Dis. 2007, 196, 1565–1571. [Google Scholar] [CrossRef]
- Netea, M.G.; Brown, G.D.; Kullberg, B.J.; Gow, N.A.R. An integrated model of the recognition of Candida albicans by the innate immune system. Nat. Rev. Microbiol. 2008, 6, 67–78. [Google Scholar] [CrossRef]
- Cui, J.; Ren, B.; Tong, Y.; Dai, H.; Zhang, L. Synergistic combinations of antifungals and anti-virulence agents to fight against Candida albicans. Virulence 2015, 6, 362–371. [Google Scholar] [CrossRef]
- Biniarz, P.; Łukaszewicz, M.; Janek, T. Screening concepts, characterization and structural analysis of microbial-derived bioactive lipopeptides: A review. Crit. Rev. Biotechnol. 2017, 37, 393–410. [Google Scholar] [CrossRef] [PubMed]
- Janek, T.; Krasowska, A.; Radwańska, A.; Łukaszewicz, M. Lipopeptide biosurfactant pseudofactin II induced apoptosis of melanoma a 375 cells by specific interaction with the plasma membrane. PLoS ONE 2013, 8, e57991. [Google Scholar] [CrossRef] [PubMed]
- Janek, T.; Lukaszewicz, M.; Rezanka, T.; Krasowska, A. Isolation and characterization of two new lipopeptide biosurfactants produced by Pseudomonas fluorescens BD5 isolated from water from the Arctic Archipelago of Svalbard. Bioresour. Technol. 2010, 101, 6118–6123. [Google Scholar] [CrossRef] [PubMed]
- Ceresa, C.; Rinaldi, M.; Chiono, V.; Carmagnola, I.; Allegrone, G.; Fracchia, L. Lipopeptides from Bacillus subtilis AC7 inhibit adhesion and biofilm formation of Candida albicans on silicone. Antonie van Leeuwenhoek 2016, 109, 1375. [Google Scholar] [CrossRef] [PubMed]
- Jajor, P.; Piłakowska-Pietras, D.; Krasowska, A.; Łukaszewicz, M. Surfactin analogues produced by Bacillus subtilis strains grown on rapeseed cake. J. Mol. Struct. 2016, 1126, 141–146. [Google Scholar] [CrossRef]
- Fonzi, W.A.; Irwin, M.Y. Isogenic strain construction and gene mapping in Candida albicans. Genetics 1993, 134, 717–728. [Google Scholar]
- Suchodolski, J.; Muraszko, J.; Bernat, P.; Krasowska, A. Crucial role of ergosterol in plasma membrane composition, localisation and activity of Cdr1p and H+-ATPase in Candida albicans. Microorganisms 2019, 7, 378. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (Ed.) Reference Method for Broth Dilutionantifungal Susceptibility Testing of Yeast, Approved Standard. M27-A3 28, 3rd ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008. [Google Scholar]
- Suchodolski, J.; Feder-Kubis, J.; Krasowska, A. Antifungal activity of ionic liquids based on (−)-menthol: A mechanism study. Microbiol. Res. 2017, 197, 56–64. [Google Scholar] [CrossRef]
- De Almeida, R.F.M.; Santos, F.C.; Marycz, K.; Alicka, M.; Krasowska, A.; Suchodolski, J.; Panek, J.J.; Jezierska, A.; Starosta, R. New diphenylphosphane derivatives of ketoconazole are promising antifungal agents. Sci. Rep. 2019, 9, 16214. [Google Scholar] [CrossRef]
- Suchodolski, J.; Krasowska, A. Plasma membrane potential of Candida albicans measured by Di-4-ANEPPS fluorescence depends on growth phase and regulatory factors. Microorganisms 2019, 7, 110. [Google Scholar] [CrossRef]
- Piętka-Ottlik, M.; Lewińska, A.; Jaromin, A.; Krasowska, A.; Wilk, K.A. Antifungal organoselenium compound loaded nanoemulsions stabilized by bifunctional cationic surfactants. Colloids Surf. A Physicochem. Eng. Asp. 2016, 510, 53–62. [Google Scholar] [CrossRef]
- Malavia, D.; Lehtovirta-Morley, L.E.; Alamir, O.; Weiβ, E.; Gow, N.A.; Hube, B.; Wilson, D. Zinc limitation induces a hyper-adherent Goliath phenotype in Candida albicans. Front. Microbiol. 2017, 8, 2238. [Google Scholar] [CrossRef] [PubMed]
- Wagener, J.; MacCallum, D.M.; Brown, G.D.; Gow, N.A. Candida albicans chitin increases arginase-1 activity in human macrophages, with an impact on macrophage antimicrobial functions. mBio 2017, 8, e01820-16. [Google Scholar] [CrossRef] [PubMed]
- Szczepaniak, J.; Łukaszewicz, M.; Krasowska, A. Estimation of Candida albicans ABC transporter behavior in real-time via fluorescence. Front. Microbiol. 2015, 6, 1382. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tsan, P.; Volpon, L.; Besson, F.; Lancelin, J.M. Structure and dynamics of surfactin studied by NMR in micellar media. J. Am. Chem. Soc. 2007, 129, 1968–1977. [Google Scholar] [CrossRef] [PubMed]
- Schaftenaar, G.; Noordik, J.H. Molden: A pre- and post-processing program for molecular and electronic structures. J. Comput.-Aided Mol. Des. 2000, 14, 123–134. [Google Scholar] [CrossRef]
- Case, D.A.; Berryman, J.T.; Betz, R.M.; Cerutti, D.S.; Cheatham, T.E., III; Darden, T.A.; Duke, R.E.; Giese, T.J.; Gohlke, H.; Goetz, A.W.; et al. AMBER 2015 Reference Manual; University of California: San Francisco, CA, USA, 2015. [Google Scholar]
- Wang, J.; Wolf, R.; Caldwell, J.; Kollman, P.; Case, D. Development and testing of a general Amber force field. J. Comput. Chem. 2004, 25, 1157–1174. [Google Scholar] [CrossRef]
- Jakalian, A.; Jack, D.B.; Bayly, C.I. Fast, efficient generation of high-quality atomic charges. AM1-BCC model: II. Parameterization and validation. J. Comput. Chem. 2002, 23, 1623–1641. [Google Scholar] [CrossRef]
- Jorgensen, W.; Chandrasekhar, J.; Madura, J.; Klein, M. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Darden, T.; York, D.; Pedersen, L. Particle mesh Ewald: An N⋅log(N) method for Ewald sums in large systems. J. Chem. Phys. 1993, 98, 10089–10092. [Google Scholar] [CrossRef]
- Andersen, H.C. Molecular dynamics at constant pressure and/or temperature. J. Chem. Phys. 1980, 72, 2384–2393. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD—Visual Molecular Dynamics. J. Molec. Graph. 1996, 4, 33–38. [Google Scholar] [CrossRef]
- Pueyo, M.T.; Bloch, C.; Carmona-Ribeiro, A.M.; di Mascio, P. Lipopeptides produced by a soil Bacillus megaterium strain. Microb. Ecol. 2009, 57, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Gao, N.; Li, C.; Gao, J.; Ying, C. A newly identified amino acid substitution T123I in the 14α-demethylase (Erg11p) of Candida albicans confers azole resistance. FEMS Yeast Res. 2017, 17, fox012. [Google Scholar] [CrossRef] [PubMed]
- Cannon, R.D.; Lamping, E.; Holmes, A.R.; Niimi, K.; Baret, P.V.; Keniya, M.V.; Tanabe, K.; Niimi, M.; Goffeau, A.; Monk, B.C. Efflux-mediated antifungal drug resistance. Clin. Microbiol. Rev. 2009, 22, 291–321. [Google Scholar] [CrossRef] [PubMed]
- Quan, H.; Cao, Y.Y.; Xu, Z.; Zhao, J.X.; Gao, P.H.; Qin, X.F.; Jiang, Y.Y. Potent in vitro synergism of fluconazole and berberine chloride against clinical isolates of Candida albicans resistant to fluconazole. Antimicrob. Agents Chemother. 2006, 50, 1096–1099. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, T.; Xu, J.; Wu, J.; Wang, Y.; Qiu, X.; Zhang, Y.; Hou, W.; Yan, L.; An, M.; et al. The synergism of the small molecule ENOblock and fluconazole against fluconazole-resistant Candida albicans. Front. Microbiol. 2019, 10, 2071. [Google Scholar] [CrossRef]
- Zhao, Y.J.; Liu, W.D.; Shen, Y.N.; Li, D.M.; Zhu, K.J.; Zhang, H. The efflux pump inhibitor tetrandrine exhibits synergism with fluconazole or voriconazole against Candida parapsilosis. Mol. Biol. Rep. 2019, 46, 5867–5874. [Google Scholar] [CrossRef]
- Dupont, S.; Beney, L.; Ferreira, T.; Gervais, P. Nature of sterols affects plasma membrane behavior and yeast survival during dehydration. Biochim. Biophys. Acta 2011, 1808, 1520–1528. [Google Scholar] [CrossRef]
- Hsueh, Y.W.; Chen, M.T.; Patty, P.J.; Code, C.; Cheng, J.; Frisken, B.J.; Zuckermann, M.; Thewalt, J. Ergosterol in POPC membranes: Physical properties and comparison with structurally similar sterols. Biophys. J. 2007, 92, 1606–1615. [Google Scholar] [CrossRef]
- Bacia, K.; Schwille, P.; Kurzchalia, T. Sterol structure determines the separation of phases and the curvature of the liquid-ordered phase in model membranes. Proc. Natl. Acad. Sci. USA 2005, 102, 3272–3277. [Google Scholar] [CrossRef] [PubMed]
- Madhavan, P.; Jamal, F.; Pei, C.P.; Othman, F.; Karunanidhi, A.; Ng, K.P. Comparative study of the effects of fluconazole and voriconazole on Candida glabrata, Candida parapsilosis and Candida rugosa biofilms. Mycopathologia 2018, 183, 499–511. [Google Scholar] [CrossRef]
- Lee, K.K.; Maccallum, D.M.; Jacobsen, M.D.; Walker, L.A.; Odds, F.C.; Gow, N.A.; Munro, C.A. Elevated cell wall chitin in Candida albicans confers echinocandin resistance in vivo. Antimicrob. Agents Chemother. 2012, 56, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Bourgeois, C.; Majer, O.; Frohner, I.E.; Tierney, L.; Kuchler, K. Fungal attacks on mammalian hosts: Pathogen elimination requires sensing and tasting. Curr. Opin. Microbiol. 2010, 13, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Cottier, F.; Sherrington, S.; Cockerill, S.; Del Olmo Toledo, V.; Kissane, S.; Tournu, H.; Orsini, L.; Palmer, G.E.; Pérez, J.C.; Hall, R.A. Remasking of Candida albicans β-Glucan in response to environmental pH is regulated by quorum sensing. mBio 2019, 10, e02347-19. [Google Scholar] [CrossRef] [PubMed]
- Hall, R.A. Dressed to impress: Impact of environmental adaptation on the Candida albicans cell wall. Mol. Microbiol. 2015, 97, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Ballou, E.R.; Avelar, G.M.; Childers, D.S.; Mackie, J.; Bain, J.M.; Wagener, J.; Kastora, S.L.; Panea, M.D.; Hardison, S.E.; Walker, L.A.; et al. Lactate signalling regulates fungal-glucan masking and immune evasion. Nat. Microbiol. 2016, 2, 16238. [Google Scholar] [CrossRef]
- Sherrington, S.L.; Sorsby, E.; Mahtey, N.; Kumwenda, P.; Lenardon, M.D.; Brown, I.; Ballou, E.R.; MacCallum, D.M.; Hall, R.A. Adaptation of Candida albicans to environmental pH induces cell wall remodelling and enhances innate immune recognition. PLoS Pathog. 2017, 13, e1006403. [Google Scholar] [CrossRef]
- Wheeler, R.T.; Fink, G.R. A drug-sensitive genetic network masks fungi from the immune system. PLoS Pathog. 2006, 2, e35. [Google Scholar] [CrossRef]
- Sudoh, M.; Tatsuno, K.; Ono, N.; Ohta, A.; Chibana, H.; Yamada-Okabe, H.; Arisawa, M. The Candida albicans CHS4 gene complements a Saccharomyces cerevisiae skt5/chs4 mutation and is involved in chitin biosynthesis. Microbiology 1999, 145, 1613–1622. [Google Scholar] [CrossRef][Green Version]
- Klis, F.M.; de Groot, P.; Hellingwerf, K. Molecular organisation of the cell wall of Candida albicans. Med. Mycol. 2001, 39, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Borchani, C.; Fonteyn, F.; Jamin, G.; Destain, J.; Willems, L.; Paquot, M.; Blecker, C.; Thonart, P. Structural characterization, technological functionality, and physiological aspects of fungal β-D-glucans: A review. Crit. Rev. Food Sci. Nutr. 2016, 56, 1746–1752. [Google Scholar] [CrossRef] [PubMed]
- Salci, T.P.; Negri, M.; Abadio, A.K.R.; Svidzinski, T.I.E.; Kioshima, É.S. Targeting Candida spp. to develop antifungal agents. Drug Discov. Today 2018, 23, 802–814. [Google Scholar] [CrossRef] [PubMed]
- Mio, T.; Adachi-Shimizu, M.; Tachibana, Y.; Tabuchi, H.; Inoue, S.B.; Yabe, T.; Yamada-Okabe, T.; Arisawa, M.; Watanabe, T.; Yamada-Okabe, H. Cloning of the Candida albicans homolog of Saccharomyces cerevisiae GSC1/FKS1 and its involvement in beta-1, 3-glucan synthesis. J. Bacteriol. 1997, 179, 4096–4105. [Google Scholar] [CrossRef] [PubMed]
- Niimi, K.; Monk, B.C.; Hirai, A.; Hatakenaka, K.; Umeyama, T.; Lamping, E.; Maki, K.; Tanabe, K.; Kamimura, T.; Ikeda, F.; et al. Clinically significant micafungin resistance in Candida albicans involves modification of a glucan synthase catalytic subunit GSC1 (FKS1) allele followed by loss of heterozygosity. J. Antimicrob. Chemother. 2010, 65, 842–852. [Google Scholar] [CrossRef]
- Lussier, M.; Sdicu, A.M.; Shahinian, S.; Bussey, H. The Candida albicans KRE9 gene is required for cell wall β-1, 6-glucan synthesis and is essential for growth on glucose. Proc. Natl. Acad. Sci. USA 1998, 95, 9825–9830. [Google Scholar] [CrossRef]
- Nishimoto, A.T.; Sharma, C.; Rogers, P.D. Molecular and genetic basis of azole antifungal resistance in the opportunistic pathogenic fungus Candida albicans. J. Antimicrob. Chemother. 2019, 75, 257–270. [Google Scholar] [CrossRef]
- Prasad, R.; Nair, R.; Banerjee, A. Multidrug transporters of Candida species in clinical azole resistance. Fungal Genet. Biol. 2019, 132, 103252. [Google Scholar] [CrossRef]
- Bruno, V.M.; Kalachikov, S.; Subaran, R.; Nobile, C.J.; Kyratsous, C.; Mitchell, A.P. Control of the C. albicans cell wall damage response by transcriptional regulator Cas5. PLoS Pathog. 2006, 2, e21. [Google Scholar] [CrossRef]
- Liu, T.T.; Lee, R.E.; Barker, K.S.; Lee, R.E.; Wei, L.; Homayouni, R.; Rogers, P.D. Genome-wide expression profiling of the response to azole, polyene, echinocandin, and pyrimidine antifungal agents in Candida albicans. Antimicrob. Agents Chemother. 2005, 49, 2226–2236. [Google Scholar] [CrossRef]
- Plaine, A.; Walker, L.; Da Costa, G.; Mora-Montes, H.M.; McKinnon, A.; Gow, N.A.R.; Gaillardin, C.; Munro, C.A.; Richarda, M.L. Functional analysis of Candida albicans GPI-anchored proteins: Roles in cell wall integrity and caspofungin sensitivity. Fungal Genet. Biol. 2008, 45, 1404–1414. [Google Scholar] [CrossRef] [PubMed]
- Toral, L.; Rodríguez, M.; Béjar, V.; Sampedro, I. Antifungal activity of lipopeptides from Bacillus XT1 CECT 8661 against Botrytis cinerea. Front. Microbiol. 2018, 9, 1315. [Google Scholar] [CrossRef] [PubMed]
- Fira, D.; Dimkić, I.; Berić, T.; Lozo, J.; Stanković, S. Biological control of plant pathogens by Bacillus species. J. Biotechnol. 2018, 285, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Liu, G.; Zhou, S.; Sha, Z.; Sun, C. Characterization of antifungal lipopeptide biosurfactants produced by marine bacterium Bacillus sp. CS30. Mar. Drugs 2019, 17, 199. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, C.; Dong, B.; Ma, X.; Hou, L.; Cao, X.; Wang, C. Anti-inflammatory activity and mechanism of surfactin in lipopolysaccharide-activated macrophages. Inflammation 2015, 38, 756–764. [Google Scholar] [CrossRef]
- Vollenbroich, D.; Ozel, M.; Vater, J.; Kamp, R.M.; Pauli, G. Mechanism of inactivation of enveloped viruses by the biosurfactant surfactin from Bacillus subtilis. Biologicals 1997, 25, 289–297. [Google Scholar] [CrossRef]
- Bossche, H.V.; Marichal, P.; Gorrens, J.; Bellens, D.; Verhoeven, H.; Coene, M.C.; Lauwers, W.; Janssen, P.A.J. Interaction of azole derivatives with cytochrome P-450 isozymes in yeast, fungi, plants and mammalian cells. Pestic. Sci. 1987, 21, 289–306. [Google Scholar] [CrossRef]
- Hargrove, T.Y.; Friggeri, L.; Wawrzak, Z.; Qi, A.; Hoekstra, W.J.; Schotzinger, R.J.; York, J.D.; Guengerich, F.P.; Lepesheva, G.I. Structural analyses of Candida albicans sterol 14 α-demethylase complexed with azole drugs address the molecular basis of azole-mediated inhibition of fungal sterol biosynthesis. J. Biol. Chem. 2017, 292, 6728–6743. [Google Scholar] [CrossRef]
- Hitchcock, C.A. Cytochrome P-450-dependent 14α-sterol demethylase of Candida albicans and its interaction with azole antifungals. Biochem. Soc. Trans. 1991, 19, 782–787. [Google Scholar] [CrossRef]
- Bossche, H.V. Biochemical targets for antifungal azole derivatives: Hypothesis on the mode of action. Curr. Top. Med. Mycol. 1985, 1, 313–351. [Google Scholar]
- Torosantucci, A.; Bromuro, C.; Chiani, P.; De Bernardis, F.; Berti, F.; Galli, C.; Norelli, F.; Bellucci, C.; Polonelli, L.; Costantino, P.; et al. A novel glyco-conjugate vaccine against fungal pathogens. J. Exp. Med. 2005, 202, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.D.; Gordon, S. Immune recognition of fungal beta-glucans. Cell. Microbiol. 2005, 7, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Denning, D.W. Echinocandin antifungal drugs. Lancet 2003, 362, 1142–1151. [Google Scholar] [CrossRef]
- Munro, C.A.; Selvaggini, S.; de Bruijn, I.; Walker, L.; Lenardon, M.D.; Gerssen, B.; Milne, S.; Brown, A.J.; Gow, N.A. The PKC, HOG and Ca2+ signalling pathways co-ordinately regulate chitin synthesis in Candida albicans. Mol. Microbiol. 2007, 63, 1399–1413. [Google Scholar] [CrossRef] [PubMed]
- Campitelli, M.; Zeineddine, N.; Samaha, G.; Maslak, S. Combination Antifungal Therapy: A Review of Current Data. J. Clin. Med. Res. 2017, 9, 451–456. [Google Scholar] [CrossRef] [PubMed]

| Azole | ≥50% g.i. | ≥50% g.i. | ≥50% g.i. | ≥90% g.i. | ≥90% g.i. | ≥90% g.i. |
|---|---|---|---|---|---|---|
| 16 µg/mL SU | 32 µg/mL SU | 16 µg/mL SU | 32 µg/mL SU | |||
| Triazoles | ||||||
| Fluconazole | 2 | 0.5 | 0.5 | >256 | 1 | 0.5 |
| (FICI = 0.258) | (FICI = 0.258) | (FICI < 0.012) | (FICI < 0.010) | |||
| Itraconazole | 0.0313 | 0.0078 | 0.0039 | >8 | 0.0156 | 0.0078 |
| (FICI = 0.257) | (FICI = 0.132) | (FICI < 0.010) | (FICI < 0.009) | |||
| Imidazoles | ||||||
| Ketoconazole | 0.0039 | 0.0039 | 0.0039 | 4 | 0.0078 | 0.0078 |
| (FICI = 1.008) | (FICI = 1.008) | (FICI = 0.010) | (FICI = 0.010) | |||
| Clotrimazole | 0.0156 | 0.0156 | 0.0156 | 1 | 0.0313 | 0.0313 |
| (FICI = 1.008) | (FICI = 1.008) | (FICI = 0.039) | (FICI = 0.039) | |||
| Miconazole | 0.0156 | 0.0156 | 0.0156 | 2 | 0.0313 | 0.0156 |
| (FICI = 1.008) | (FICI = 1.008) | (FICI = 0.023) | (FICI = 0.016) | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suchodolski, J.; Derkacz, D.; Muraszko, J.; Panek, J.J.; Jezierska, A.; Łukaszewicz, M.; Krasowska, A. Fluconazole and Lipopeptide Surfactin Interplay During Candida albicans Plasma Membrane and Cell Wall Remodeling Increases Fungal Immune System Exposure. Pharmaceutics 2020, 12, 314. https://doi.org/10.3390/pharmaceutics12040314
Suchodolski J, Derkacz D, Muraszko J, Panek JJ, Jezierska A, Łukaszewicz M, Krasowska A. Fluconazole and Lipopeptide Surfactin Interplay During Candida albicans Plasma Membrane and Cell Wall Remodeling Increases Fungal Immune System Exposure. Pharmaceutics. 2020; 12(4):314. https://doi.org/10.3390/pharmaceutics12040314
Chicago/Turabian StyleSuchodolski, Jakub, Daria Derkacz, Jakub Muraszko, Jarosław J. Panek, Aneta Jezierska, Marcin Łukaszewicz, and Anna Krasowska. 2020. "Fluconazole and Lipopeptide Surfactin Interplay During Candida albicans Plasma Membrane and Cell Wall Remodeling Increases Fungal Immune System Exposure" Pharmaceutics 12, no. 4: 314. https://doi.org/10.3390/pharmaceutics12040314
APA StyleSuchodolski, J., Derkacz, D., Muraszko, J., Panek, J. J., Jezierska, A., Łukaszewicz, M., & Krasowska, A. (2020). Fluconazole and Lipopeptide Surfactin Interplay During Candida albicans Plasma Membrane and Cell Wall Remodeling Increases Fungal Immune System Exposure. Pharmaceutics, 12(4), 314. https://doi.org/10.3390/pharmaceutics12040314





